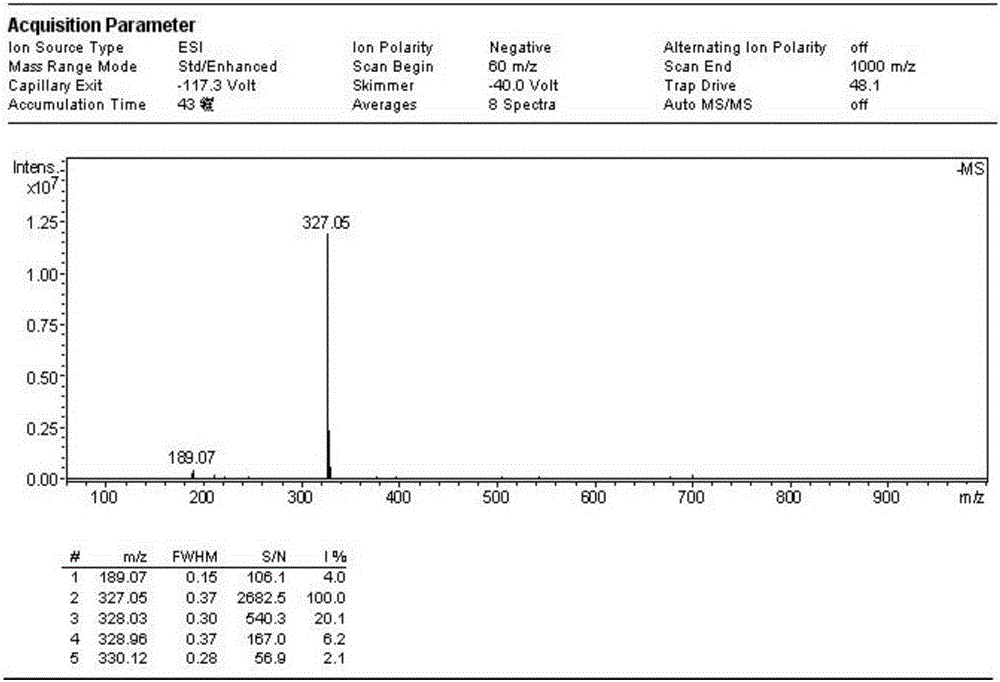
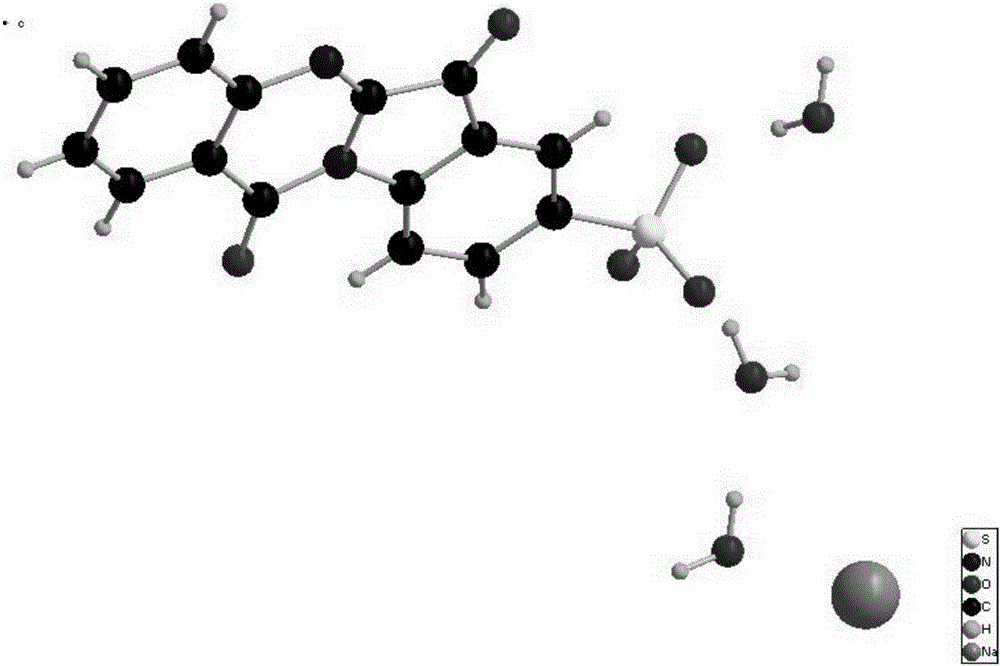
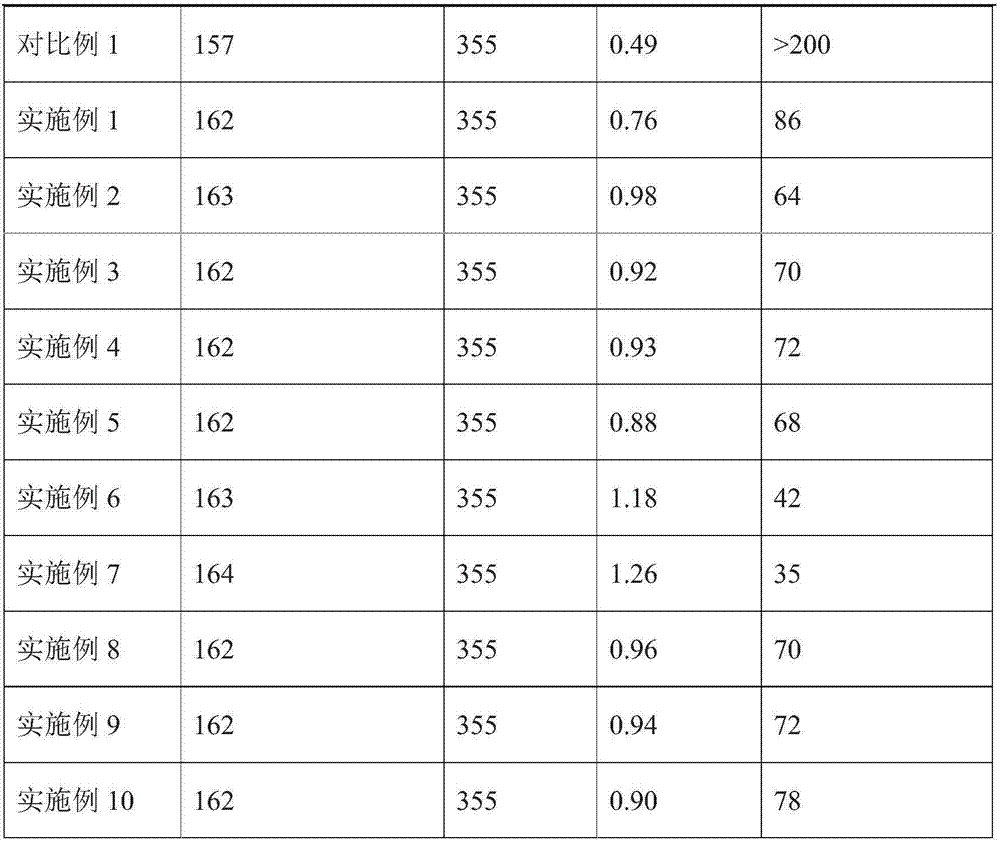
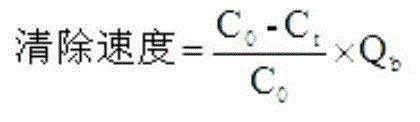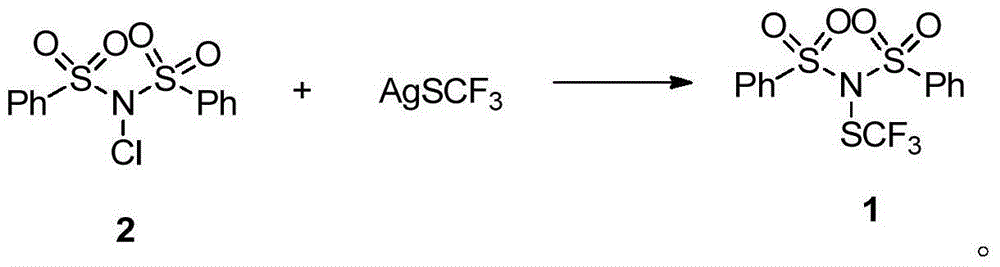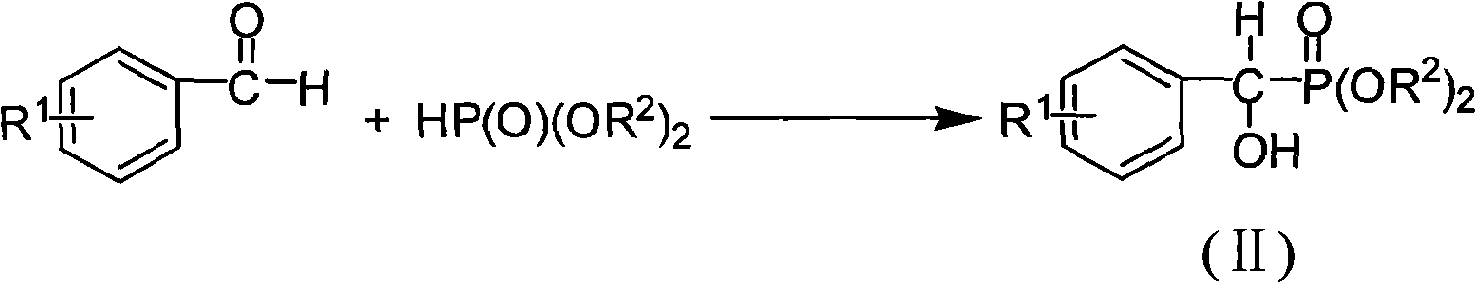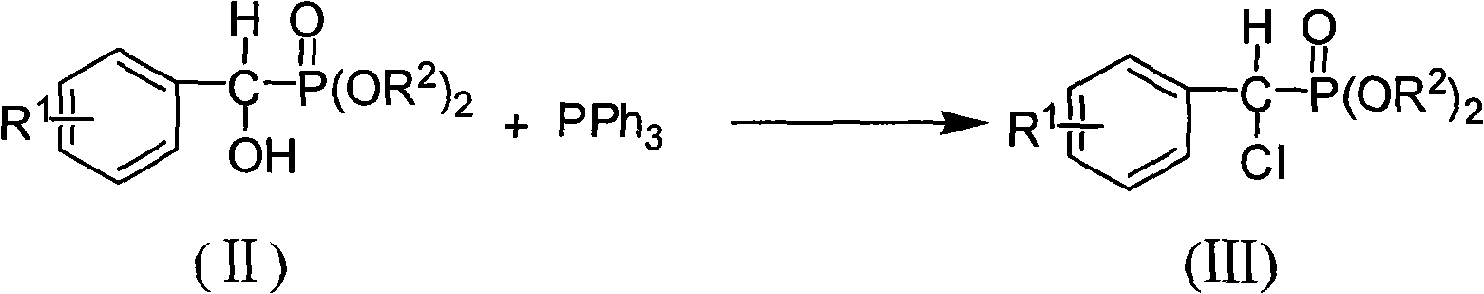Patents
Literature
173 results about "Electrophilic substitution" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
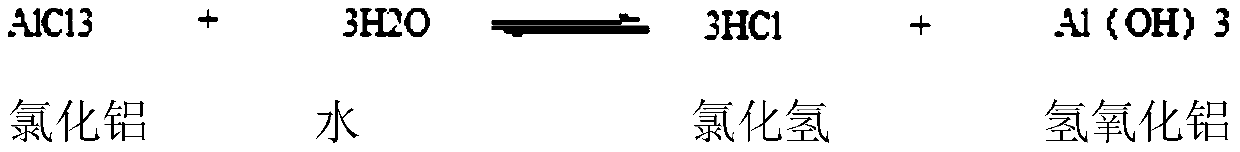
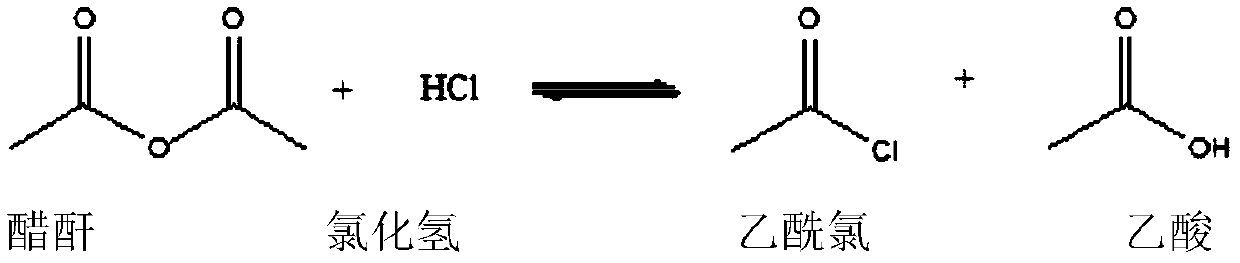
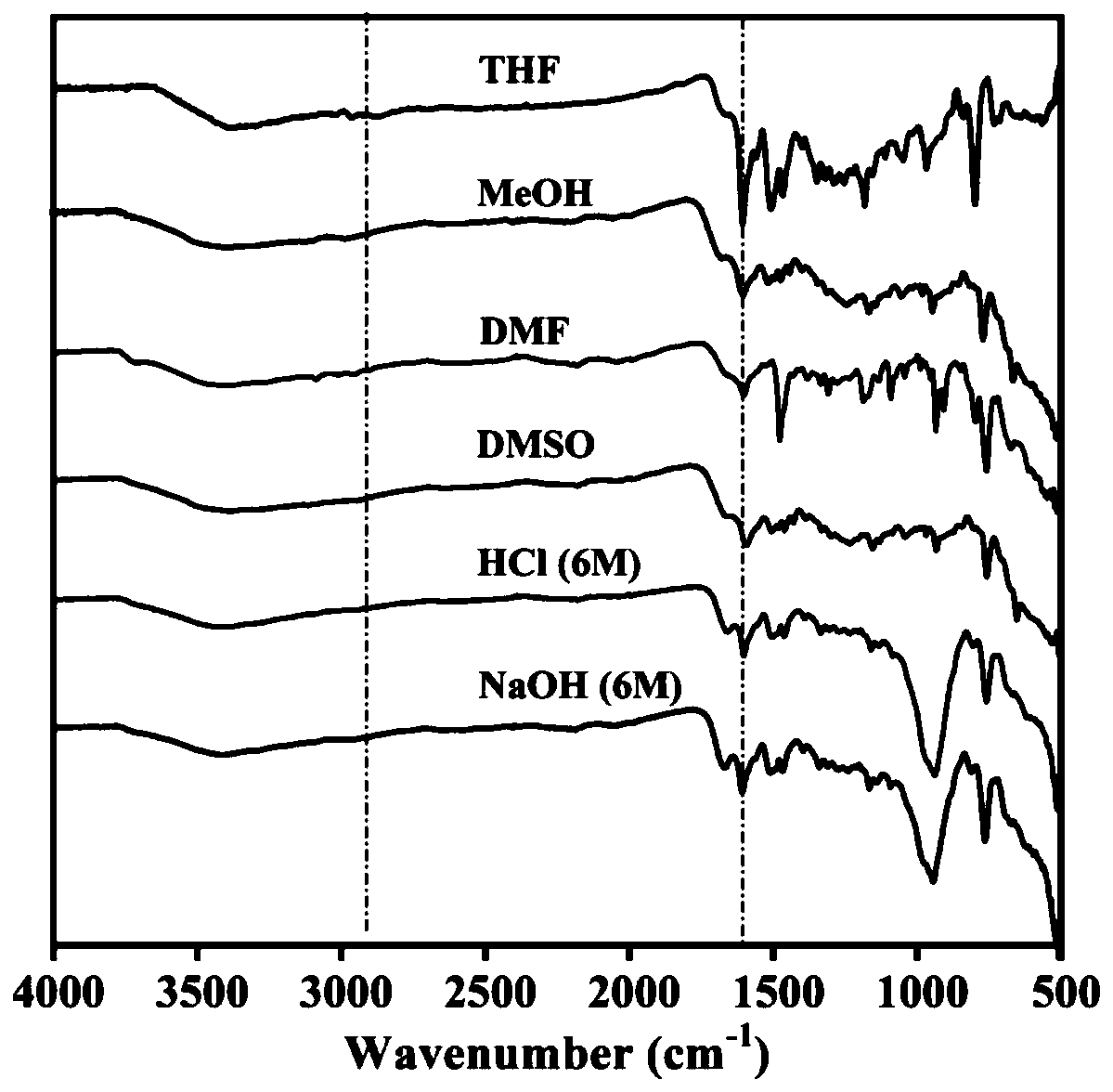
Electrophilic substitution reactions are chemical reactions in which an electrophile displaces a functional group in a compound, which is typically, but not always, a hydrogen atom. Electrophilic aromatic substitution reactions are characteristic of aromatic compounds, and are important ways of introducing functional groups of benzene rings. The other main type of electrophilic substitution reaction is an electrophilic aliphatic substitution reaction.
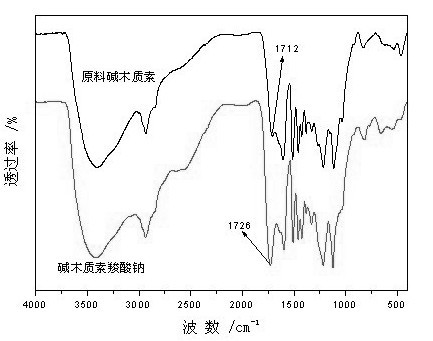
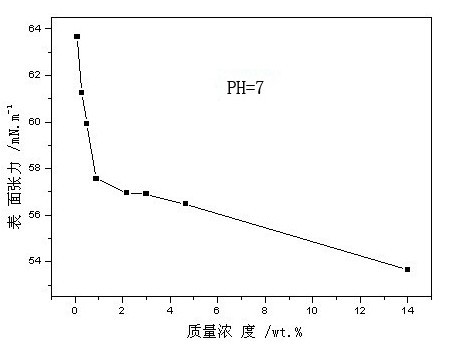
Water-soluble alkali lignin carboxylate and preparation method thereof
The invention discloses alkali lignin carboxylate and a preparation method thereof. A prescription of the modified raw material comprises the following components in parts by mass: 200 parts of alkali lignin, 20-50 parts of activating agent and 40-80 parts of carboxylic reagent; and the alkali lignin is bamboo pulp or straw pulp paper-making black liquid acid precipitating alkali lignin and has the solid content of 95 percent by mass. In the preparation, the alkali lignin is used as a main raw material, the activating agent and the carboxylic reagent are added, and the alkali lignin carboxylate is obtained by activating and electrophilic substitution reaction under basicity. By means of the preparation method, the water-soluble high molecule carboxylate is prepared by using alkali lignin with wide raw material source; and the prepared alkali lignin carboxylate has higher carboxyl content, favorable water solubility and strong surface activity. The alkali lignin carboxylate prepared by the preparation method disclosed by the invention has favorable hydrophilicity and surface activity, and the carboxyl contained in the molecules of the alkali lignin carboxylate has favorable chelating performance; and the alkali lignin carboxylate can be used as a functional auxiliary agent to be applied to a plurality of industrial fields and is a novel water-soluble lignin derivative.
Owner:SOUTH CHINA UNIV OF TECH
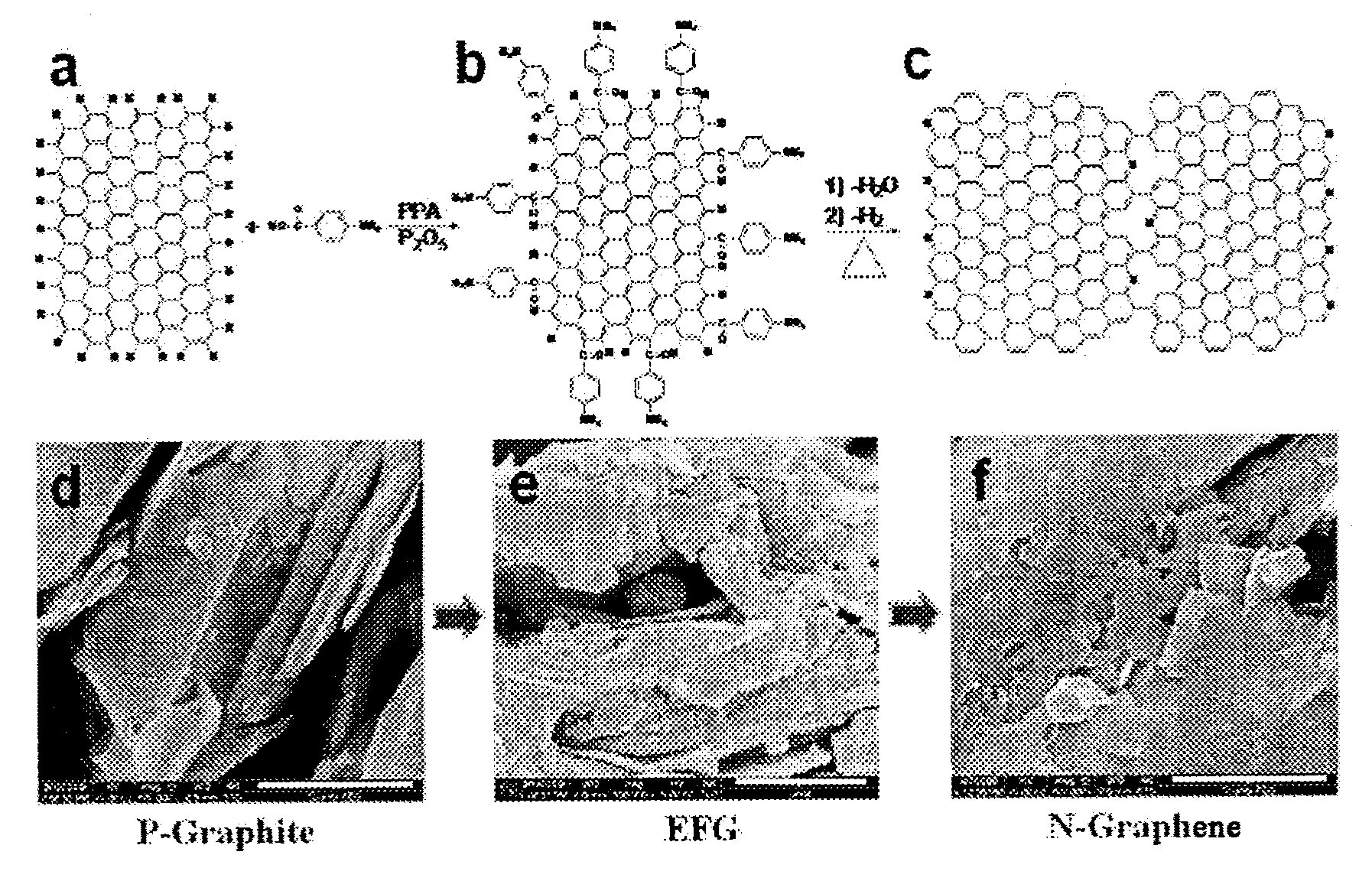
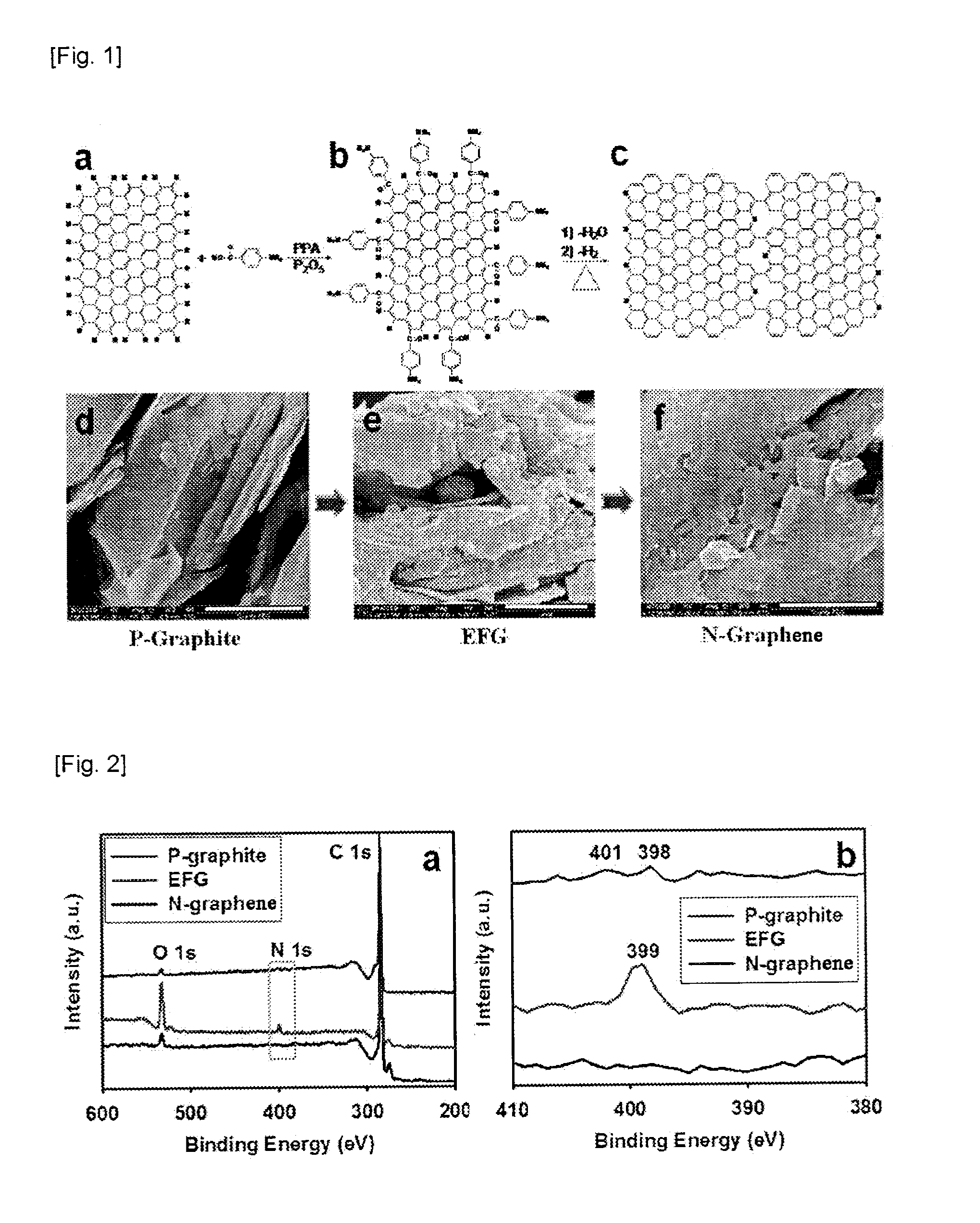
Method of preparing nitrogen-doped graphene and nitrogen-doped graphene prepared thereby
ActiveUS20120149897A1High puritySimple and inexpensive processMaterial nanotechnologyOrganic chemistryNitrogen doped grapheneElectric properties
The present invention relates to a method of preparing a nitrogen-doped graphene comprising preparing a Edge-Functionalized Graphene by binding a graphite with a organic material having amino groups and functional groups such as carboxy acid group through an electrophilic substitution reaction, and heat treating the resultant Edge-Functionalized Graphene, and a nitrogen-doped graphene prepared thereby. According to the present invention, by a more inexpensive and simpler method, a nitrogen-doped graphene can be prepared at higher purity and higher yield. The nitrogen-doped graphene obtained by the present invention has very excellent physical and electric properties, and particularly has a superior oxygen reduction capability, compared with the platinum catalyst used at cathode of a H2 / O2 fuel cell so that it will replace the platinum to lower more the cost of a H2 / O2 fuel cell or to increase its life and further to provide a new turning point for the commercialization of a H2 / O2 fuel cell.
Owner:UNIST ULSAN NAT INST OF SCI & TECH
Polyether type hyperbranched epoxy resin and preparation method thereof
ActiveCN101570592AAddresses issues such as inherent brittlenessSolve problems such as brittlenessNitrogen gasSolvent
The invention relates to polyether type hyperbranched epoxy resin and a preparation method thereof. The preparation method is as follows: the polyether type hyperbranched epoxy resin is synthesized by taking commercial dihydric phenol and polylol glycidyl ether as raw materials through a proton translocation reaction under the protection of nitrogen. All reactants are added to a reaction unit once during reaction, and in the reaction process, the hyperbranched epoxy resin is obtained according to the following steps in sequence: catalyst catalyzes the epoxy groups of a plurality of epoxy monomers to open epoxy rings, a phenolic hydroxyl group and secondary oxygen anion are processed by proton transfer and phenoxy oxygen anion is processed by electrophilic substitution. The hyperbranched epoxy polymer can be used in the fields of modification of environment-friendly bond with less solvent or no solvent, painting, traditional epoxy resin, and the like.
Owner:BEIJING UNIV OF CHEM TECH
Method for preparing high glass-transition temperature crystal type polyethylene-ketone-ketone resin material
The invention belongs to a new high performance and high polymer material synthesis field, in particular to a method for preparing a novel high glass-transition temperature crystal type polyethylene-ketone-ketone resin material in a such way that a monomer solutions of diphenyl ether and aromatic acyl chloride are dropwise added in a system, and a Friedel-Crafts low temperature solution electrophilic substitution polymerization is carried out. The viscosity of the resin materials synthesized by the method can be precisely controlled within 0.50-1.10+ / -0.10 dL / g, the glass-transition temperature can be controlled between 168 DEG C and 175 DEG C, and the melting temperature can be controlled between 310 DEG C and 360 DEG C. Compared with the FC of the same kind of already commercialized products, under the condition that the materials maintain excellent melting processing performance, the polymer has the glass-transition temperature 15 DEG C higher than that of the products, so that the invention has excellent heatproof performance.
Owner:昆山普利米斯聚合材料有限公司
Method for preparing amino acid modified polyether sulfone hematodialysis membrane
InactiveCN104984664APermanent hydrophilicityLow protein adsorptionSemi-permeable membranesSuction devicesBiocompatibility TestingAmidogen
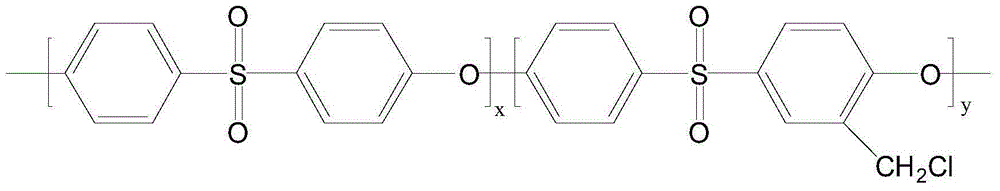
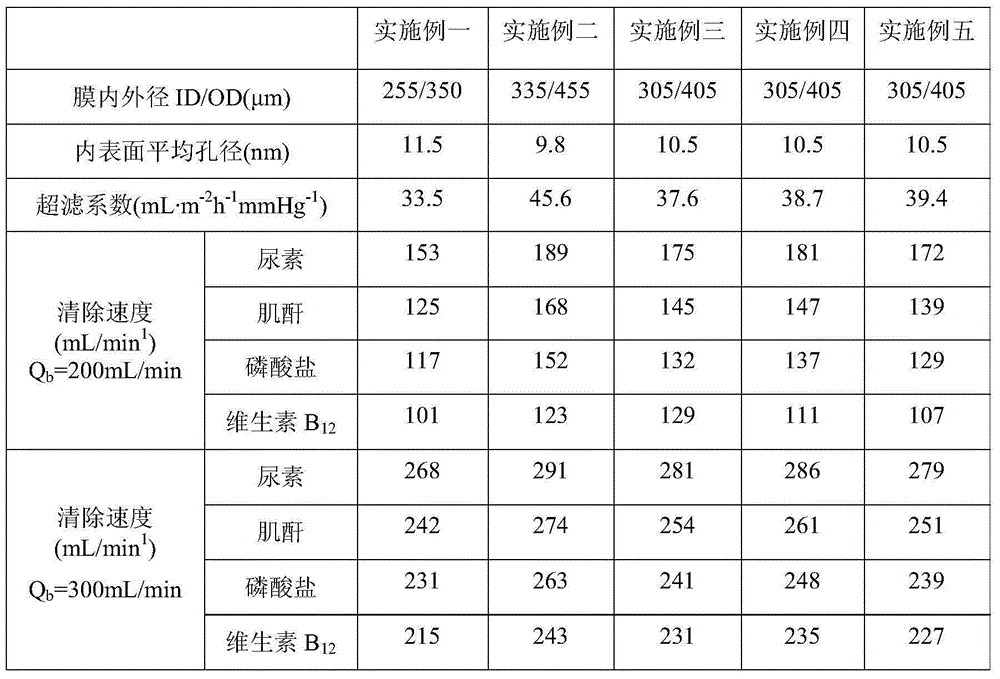
The invention relates to the technology of hematodialysis membranes, and aims at providing a method for preparing an amino acid modified polyether sulfone hematodialysis membrane. The method comprises the steps that chloromethyl group polyether sulfone, polarity aprotic organic solvent and a micromolecule pore-forming agent are taken and mixed to be stirred and dissolved to obtain a uniform and stable membrane casting solution; vacuum standing and bubble removing are carried out, and a chloromethyl group polyether sulfone hollow fiber membrane is prepared through a dry / wet phase inversion method and then immersed in hot water; finally, immersing is carried out through an ethanol solution, and drying is carried out; the chloromethyl group polyether membrane is immersed in a sodium hydroxide water solution to be reacted; the chloromethyl group polyether membrane is immersed in hot water; finally, the chloromethyl group polyether membrane is immersed in the ethanol solution, drying is carried out, and then the product is obtained. The chloromethyl group polyether sulfone hollow fiber membrane serves as a precursor material, and precise and controllable amidogen acidification modification can be carried out on the chloromethyl group polyether sulfone membrane through the electrophilic substitution policy. The polyether sulfone hematodialysis membrane modified through amino acid has the advantages of permanent hydrophilia, low-protein adsorbability and superior biocompatibility.
Owner:杭州汉膜新材料科技有限公司
Chitosan aminoethyl quaternary ammonium salt derivative and preparation method thereof
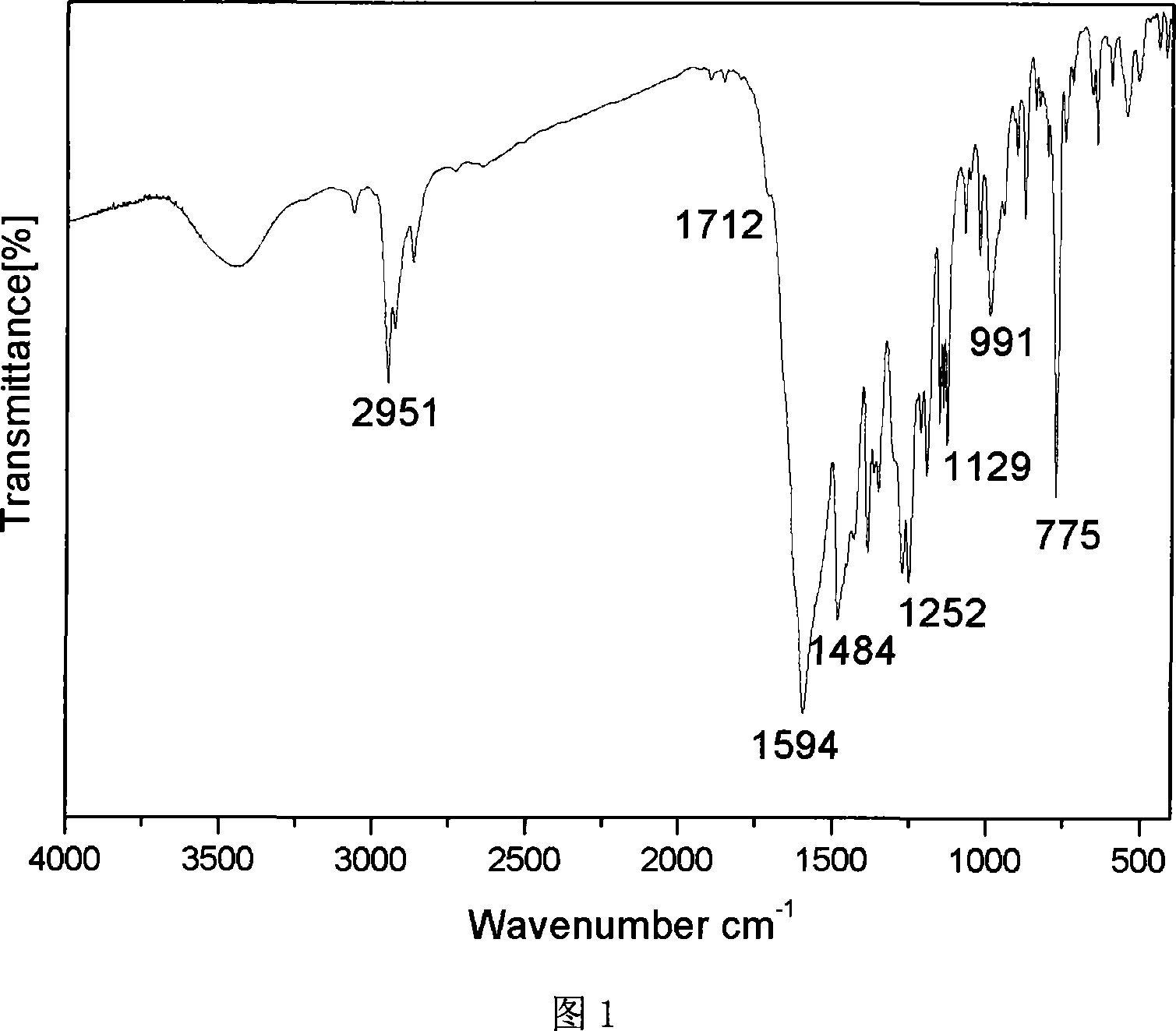
The invention belongs to ocean chemical engineering technology, and specifically relates to a chitosan aminoethyl quaternary ammonium salt derivative and a preparation method thereof. The chitosan aminoethyl quaternary ammonium salt derivative is shown as a formula (I), wherein R1 represents methyl or -H, and R2 represents -CH3, linear alkyl, branched alkyl or -Ar, and n equals to 4-4000. According to the invention, 2-chloroethylamine is subjected to an electrophilic substitution reaction with -NH2 on C2 and -OH on C6 of the chitosan to generate aminoethyl chitosan, amino on which reacts directly with iodomethane to generate N-trimethyl quaternary ammonium salt derivative, or which is subjected to a condensation reaction with aromatic aldehyde or fatty aldehyde to generate Schiff base which is reduced by sodium borohydride and quaternized under effect of iodomethane to obtain chitosan aminoethyl quaternary ammonium salt derivative. According to infrared spectroscopic analysis on the obtained derivative, chitosan and the grafted groups are effectively combined to generate aminoethyl quaternary ammonium salt. According to the invention, aminoethyl quaternary ammonium salt group is introduced into the chitosan structure to increase positive charge level of the chitosan, substantially enhance biological activity of the chitosan, such as antibiosis and sterilization, etc.
Owner:水母娘娘海洋生物科技有限公司
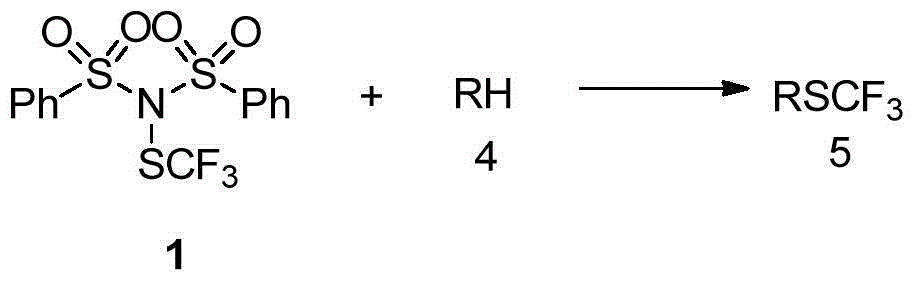
Trifluoromethylthiolation reagent, and preparation method and application thereof
ActiveCN105985266AEasy to prepareMild reaction conditionsSulfide preparationOrganic solventAlkyl substitution
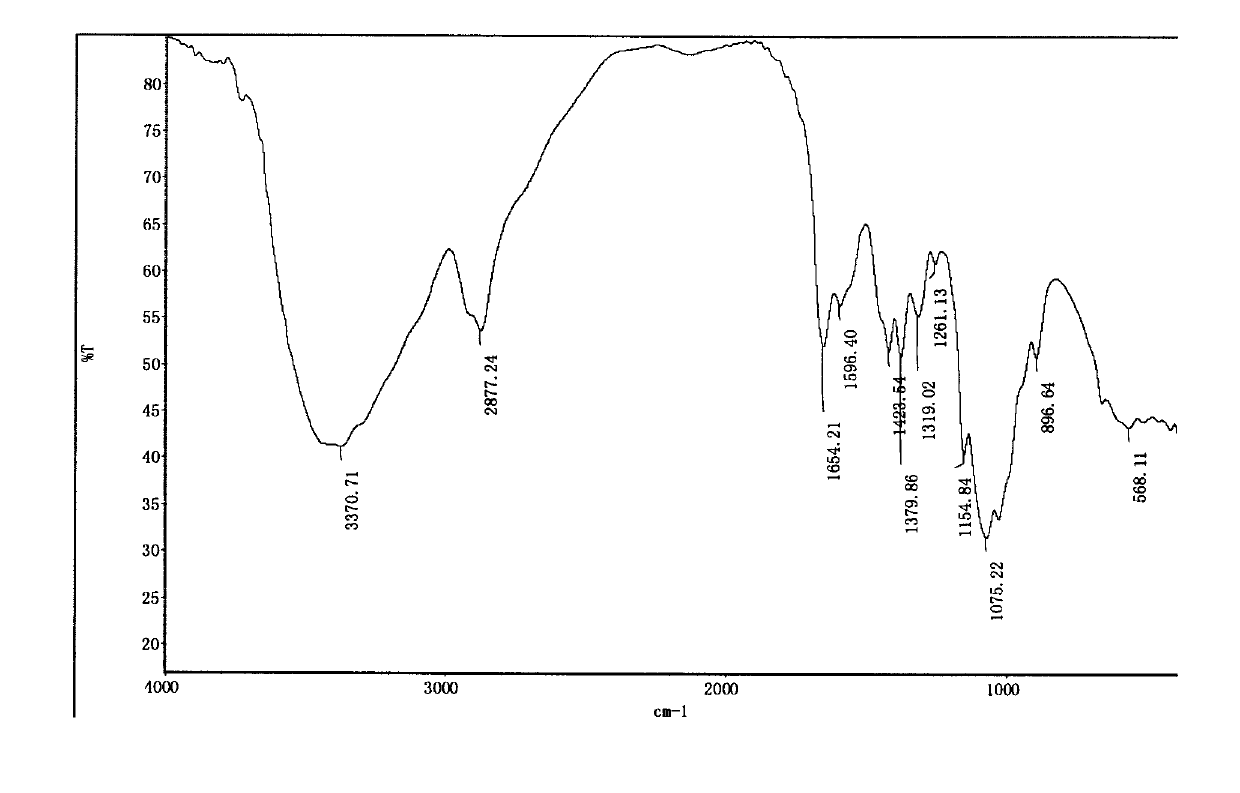
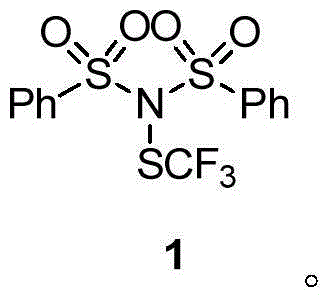
The invention discloses a trifluoromethylthiolation reagent, and a preparation method and an application thereof. The invention provides the trifluoromethylthiolation reagent represented by a formula 1, and the preparation method thereof. The method comprises the following step: in an organic solvent, a compound represented by a formula 2 is subjected to a nucleophilic substitution reaction with trifluoromethylthio silver. The invention also provides an application of the trifluoromethylthiolation reagent represented by the formula 1 in an electrophilic substitution reaction with RH for preparing a compound containing trifluoromethylthio group. The application comprises the following step: in an organic solvent, the trifluoromethylthiolation reagent represented by the formula 1 is subjected to an electrophilic substitution reaction with a compound represented by a formula 4. The trifluoromethylthiolation reagent preparation method has the advantages of simple method, mild reaction conditions, and inexpensive and easy-to-obtain raw materials. The trifluoromethylthiolation reagent provided by the invention has a wide application range, and is especially suitable for aromatic hydrocarbons. Trifluoromethylthio reaction conversion rate is high, and reaction yield. The prepared product has high purity. The reagent has a good industrialized production prospect.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
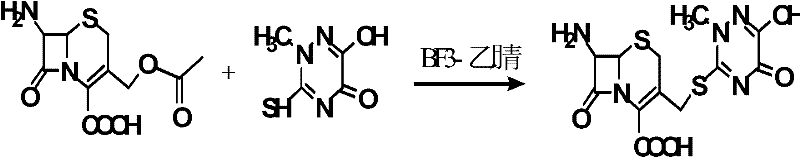
Synthetic method of ceftriaxone sodium crude salt
InactiveCN102559829AHigh purityHigh yieldOrganic chemistryFermentationChemical synthesisEnzymatic hydrolysis
The invention belongs to the field of chemical synthesis, and particularly relates to a synthetic method of a ceftriaxone sodium crude salt. The method comprises the following steps of: (1) performing electrophilic substitution on 7-ACA and a triazine ring by taking a BF3-acetonitrile solution as a catalyst to obtain 7-ACT at last; and (2) undergoing an N-acylation reaction on 7-ACT and AE-active ester in an organic phase, adding sodium iso-octoate, and undergoing a salt forming reaction to obtain the ceftriaxone sodium crude salt. The method is characterized in that: the 7-ACT is prepared with an enzymatic hydrolysis method after electrophilic substitution in the step (1); and a flocculating agent is added after the N-acylation reaction is completed in the step (2). The method has the advantages that: the product yield and purity are raised; the 7-ACT is prepared with the enzymatic hydrolysis method in the first step, and the enzymatic hydrolysis method has the characteristics of specificity and high efficiency, so that side reactions are avoided, the yield is increased by over 8 percent, and can be up to 88 percent, and the product purity is raised; and the flocculating agent is added in the second step, so that insoluble matters in a reaction liquid are removed, and a high-purity ceftriaxone sodium crude salt is obtained finally.
Owner:YIYUAN XINQUAN CHEM
Method for synthesizing aviation kerosene cycloparaffin and aroma components by utilization of wood chips
ActiveCN105623702AAbundant resourcesAchieve recyclingLiquid hydrocarbon mixture productionTreatment with hydrotreatment processesCycloparaffinsKerosene
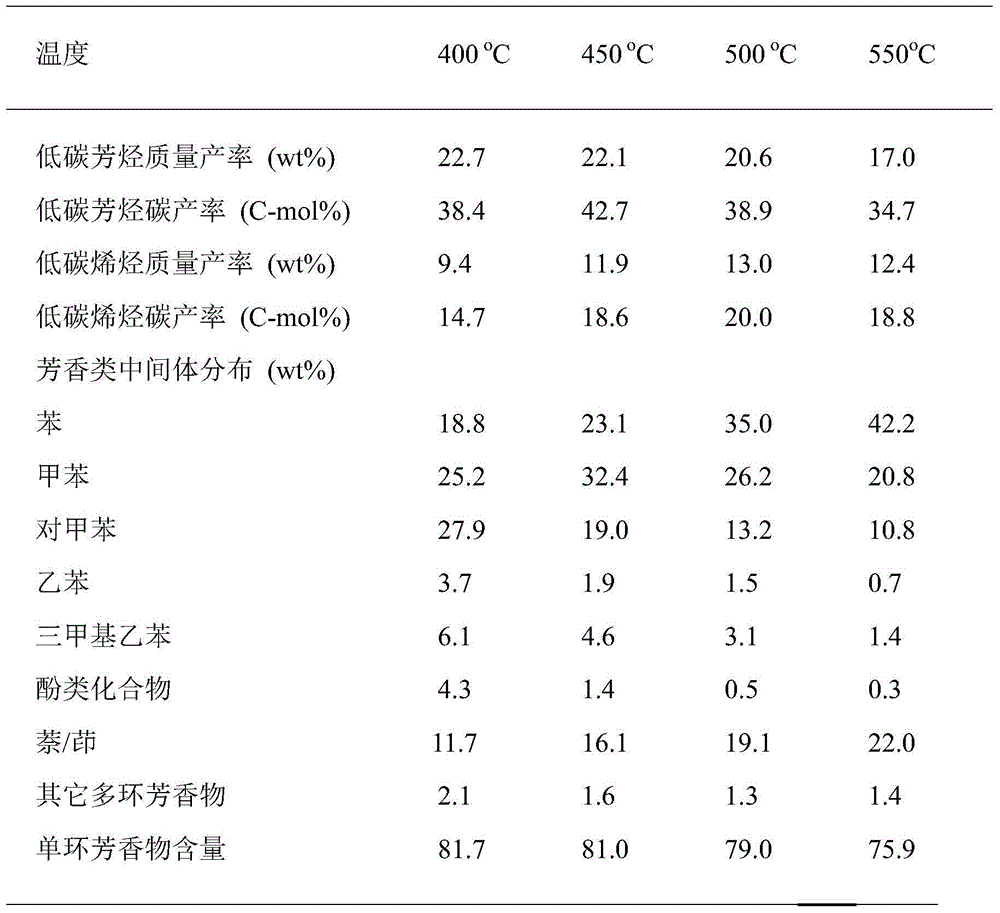
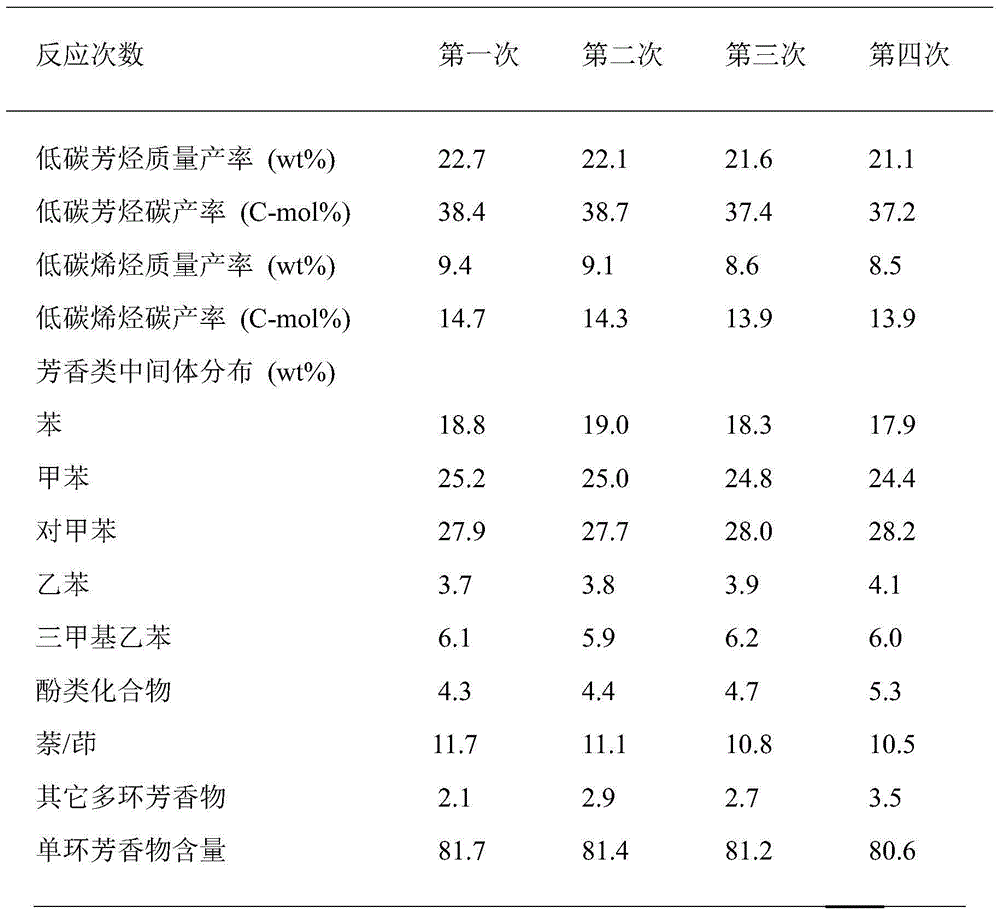
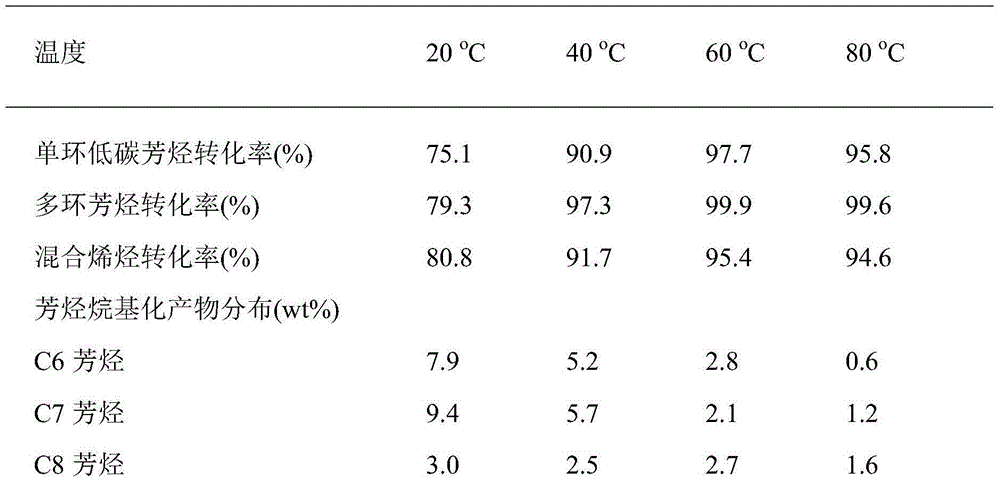
The invention provides a method for synthesizing aviation kerosene cycloparaffin and aroma components by the utilization of wood chips. The method comprises the following steps: Step 1, wood chips undergo catalytic cracking to obtain low-carbon aromatics: a catalyst is nickel / magnesium-containing modified NiO / MgO / Al-MCM-41, and low-carbon aromatics based on C6-C8 is formed after catalytic reaction of wood chips; Step 2, low-carbon aromatics is directionally converted to aromatics within the range of kerosene: an alkylating agent is low carbon olefins obtained after synchronous catalytic cracking of wood chips, a catalyst is a highly acidic xPF6-[bmim]-yAlCl3 (x,y=0.5-2) ionic liquid, and aromatics based on C9-C14 is formed after electrophilic substitution reaction of low-carbon aromatics; and Step 3, C9-C14 aromatics is directionally converted to cycloparaffin: a catalyst is palladium modified Pd / Al-MCM-22, and cycloparaffin based on C9-C14 is formed. Basic technical requirements of common aircraft fuel can be met. The method can be used for synthesizing aroma and cycloparaffin components in biological aviation kerosene.
Owner:UNIV OF SCI & TECH OF CHINA
Carbazolyl-functional bi-beta-diketo derivative and its production
A bis-beta-diketone derivative as the functional compound of carbazole is prepared from carbazole through monomolecular nucleophilic substitution reaction to obtain N-butylcarbazole, Friedel-Crafts electrophilic substitution reaction to obtain 3,6-biacetyl-N-butylcarbazole, and Claisem condensation reaction to obtain 9-butyl- 3,6-bis (1,3-butyldiketo) carbazole.
Owner:HEFEI UNIV
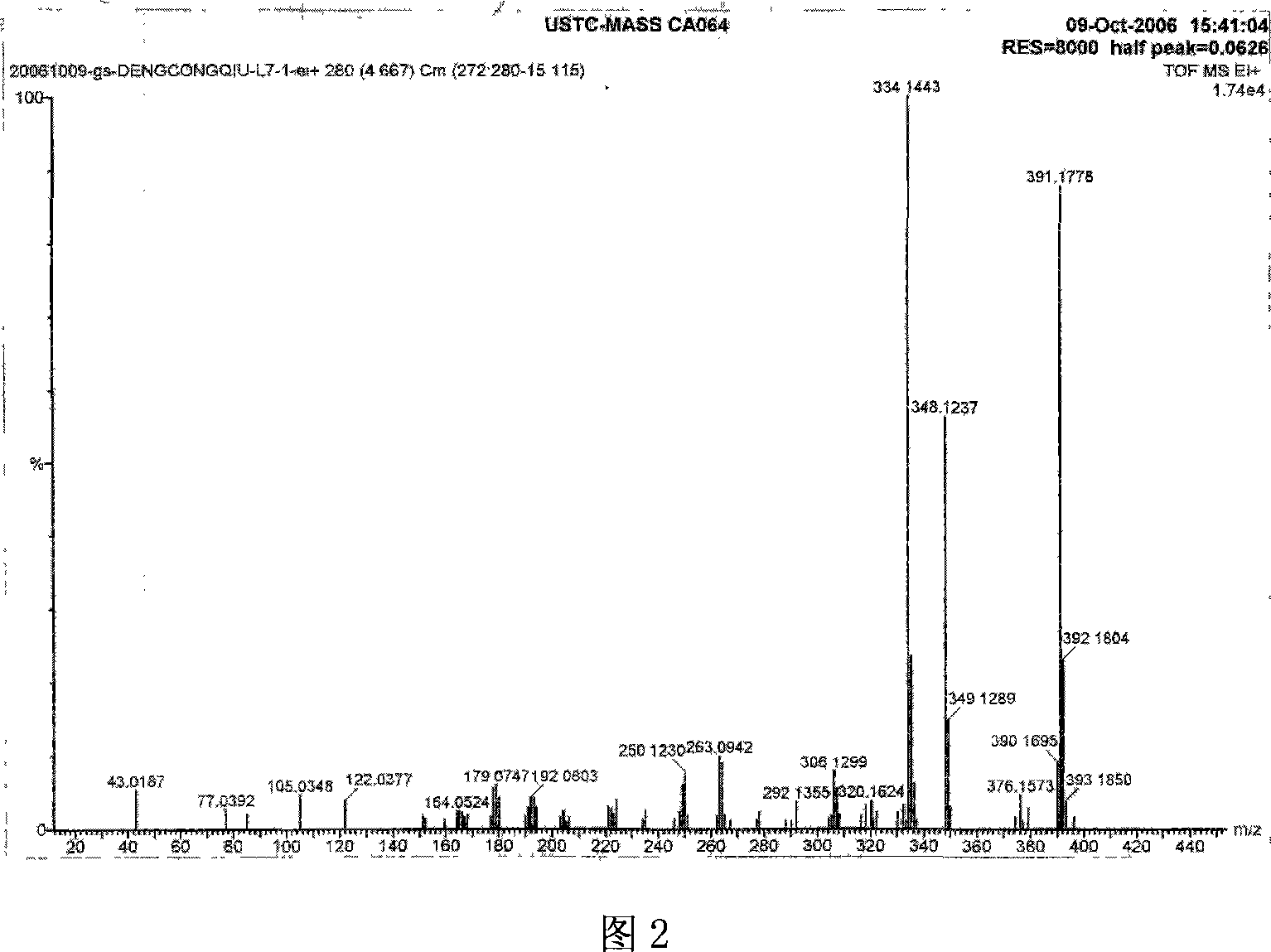
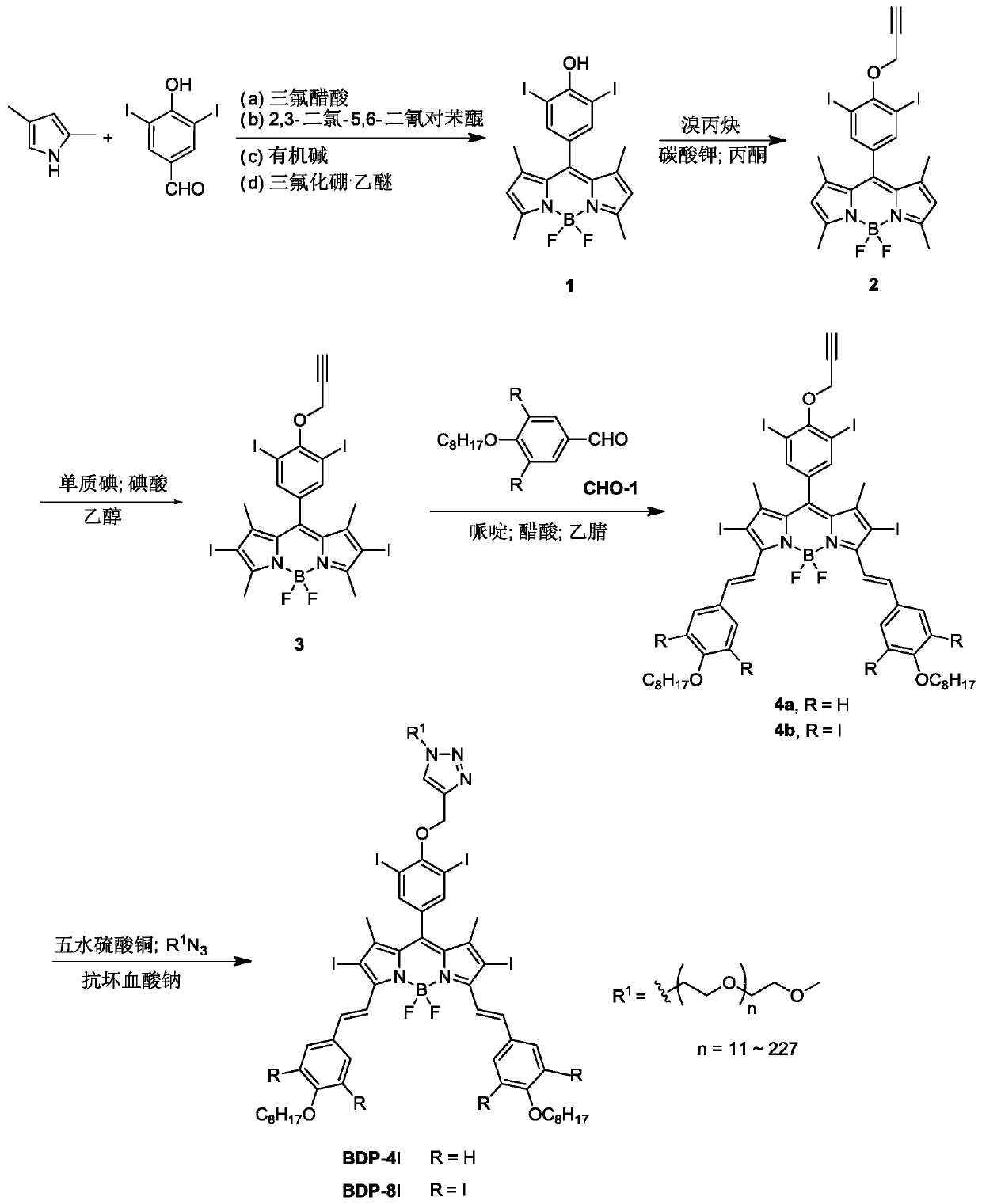
Polyiodide-modified fluoroboron dipyrrole derivative and preparation method and application thereof
ActiveCN110003461AIncreased singlet oxygen quantum yieldEnhanced photodynamic therapy activityPhotodynamic therapyAntineoplastic agentsPhotosensitizerIodine
The invention discloses a polyiodide-modified fluoroboron dipyrrole derivative. The fluoroboron dipyrrole derivative uses fluoroboron dipyrrole as a mother nucleus, extends the conjugated system of the fluoroboron dipyrrole through a condensation reaction, and is obtained by introducing a hydrophilic ethylene glycol chain at a terminal and then introducing different numbers of iodine atoms throughan electrophilic substitution reaction. The invention further discloses a preparation method and application of the fluoroboron dipyrrole derivative. The polyiodide-modified fluoroboron dipyrrole derivative has the photodynamic and photothermal therapeutic effects at the same time, and correspondingly avoids the defects of a single therapeutic effect and poor targeting performance of a clinical photosensitizer.
Owner:SUZHOU UNIV
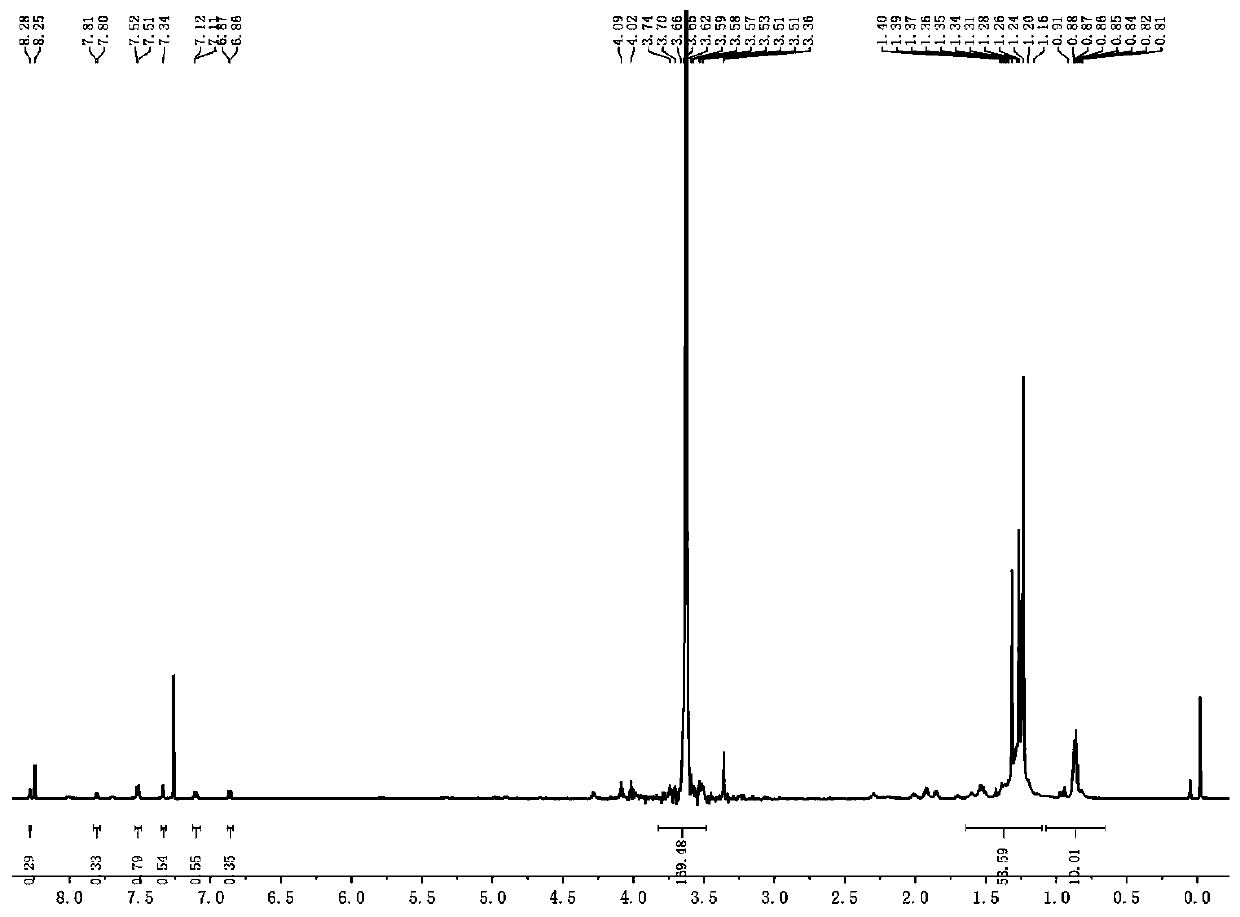
Synthesis method for orange peel cellulose-based transition metal positive ion surface imprinted polymer
ActiveCN105148866ALarge specific surface areaHydroxyl-rich surfaceOther chemical processesWater contaminantsCelluloseAlcohol
The invention relates to a synthesis method for an orange peel cellulose-based transition metal positive ion surface imprinted polymer. The synthesis method comprises the following steps: firstly, pretreating orange peels, drying, smashing and screening the orange peels to obtain orange peel powder of 100 meshes, leaching flavonoids by ethyl alcohol, and performing alkalization and alcoholization to obtain hydroxylated orange peel cellulose with a larger surface area; grafting alkyl to the surface of the orange peel cellulose by aldol condensation, and obtaining adsorption sites with a complexing effect on transition metal positive ions by electrophilic substitution reaction between the alkyl and amino; then adding target ions, complexing the target ions with the adsorption sites on the surface of the orange peel cellulose, adding a crosslinking agent for crosslinking after adsorption is balanced so as to encircle the adsorption sites and the target ions to form a semi-closed space; finally adding HCl and a thiourea solution which are mixed according to a certain ratio to prepare a regeneration agent to remove the target ions, and obtaining the surface imprinted polymer with an identification effect on the target cations.
Owner:QUZHOU UNIV +1
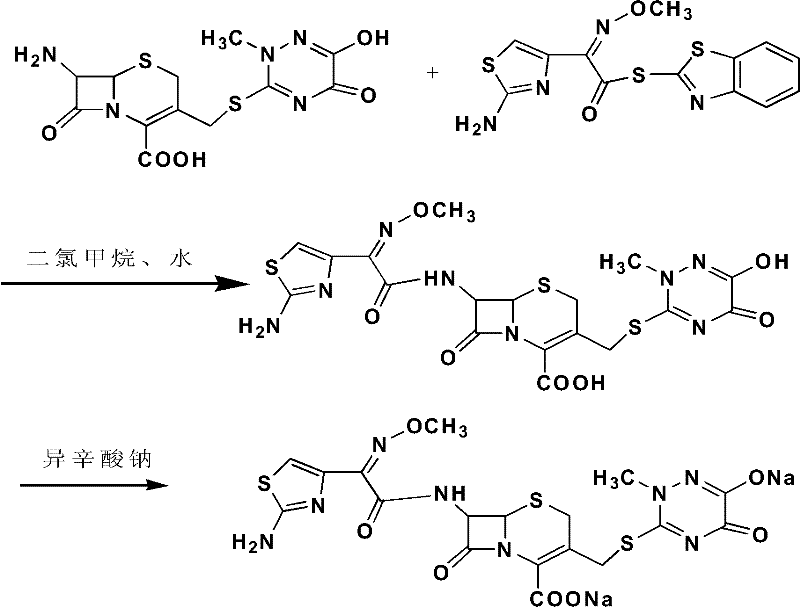
Method for synthesizing coarse salt of ceftriaxone sodium by phase transfer catalysis method
ActiveCN101747346AHighlight substantiveSignificant progressAntibacterial agentsOrganic chemistry7-ACABoron trifluoride
The invention relates to a method for synthesizing coarse salt of ceftriaxone sodium, which comprises the following steps: (1) taking 7-aminoce-phalosporanic acid (7-ACA) and a triazine ring as raw materials, adopting boron trifluoride acetonitrile as a catalyst for carrying out electrophilic substitution and finally, preparing 7-ACT by a zymohydrolysis method at a proper pH value; and (2) putting the 7-ACT and AE-active ester into an organic phase, adding a phase transfer catalyst for carrying out an N-acidylating reaction, salifying and crystallizing to obtain the coarse salt of the ceftriaxone sodium. The invention prepares the 7-ACT by the zymohydrolysis method, avoids the generation of a side reaction, enhances the yield and enhances the product purity. The reaction is carried out in the organic phase by the phase transfer catalyst, a product is transferred to a water phase, the continuous contact with a reactant is avoided, the generation of impurities with large molecular weight is lowered, and the coarse salt of the ceftriaxone sodium with high purity and high yield is finally obtained.
Owner:YIYUAN XINQUAN CHEM
Stable promotive transfer film for olefin/alkane separation and preparation method thereof
InactiveCN105771698AEnhance carrier effectImprove long-term stabilityMembranesSemi-permeable membranesAlkaneComposite film
The invention provides a stable promotive transfer film for olefin / alkane separation and a preparation method thereof. Tetracyanoethylene is introduced to an organic / inorganic composite film containing silver nanoparticles, the function of silver ions to carry olefins is improved through high electrophilic substitution of cyano groups, and separation selectivity of the film for olefins / alkanes and long-term stability of the film are improved effectively.
Owner:CHINA UNIV OF PETROLEUM (EAST CHINA)
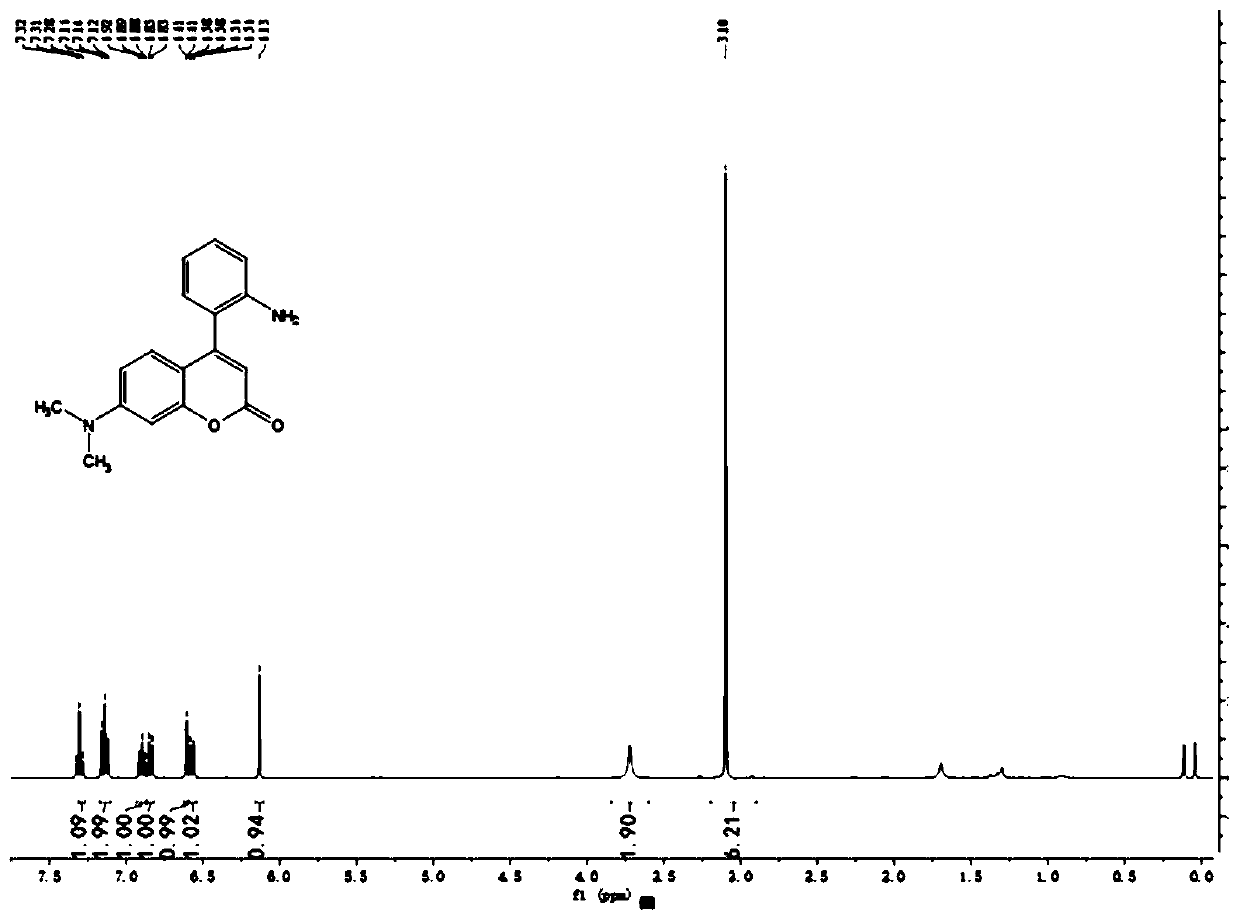
Fluorescent probe for detecting nitrite ions as well as preparation method and use method thereof
ActiveCN110483461AAchieve high sensitivityAchieving Specific DetectionOrganic chemistryFluorescence/phosphorescenceNitrite ionAniline
The invention discloses a fluorescent probe for detecting nitrite ions as well as a preparation method and a use method of the fluorescent probe. A diazotization reaction of generating a diazonium salt from aniline and nitrite under an acidic condition is utilized to connect an aniline structure to the No.4 site of coumarin. The probe itself emits green fluorescence, but under the condition that nitrite ions exist, the aniline and nitrite generate the diazonium salt, then an intramolecular electrophilic substitution reaction occurs at the No.3 site of the coumarin, a long-wavelength fluorescence product is generated, and probe molecules emit strong red fluorescence. The coumarin dye-based ratio type nitrite probe provided by the invention has good response to a nitrite solution, can realize sensitive quantitative detection of trace nitrite in a sample, and has the advantages of simplicity and convenience in operation, low cost, sensitive response, easiness in popularization and application and the like.
Owner:ZHEJIANG SCI-TECH UNIV
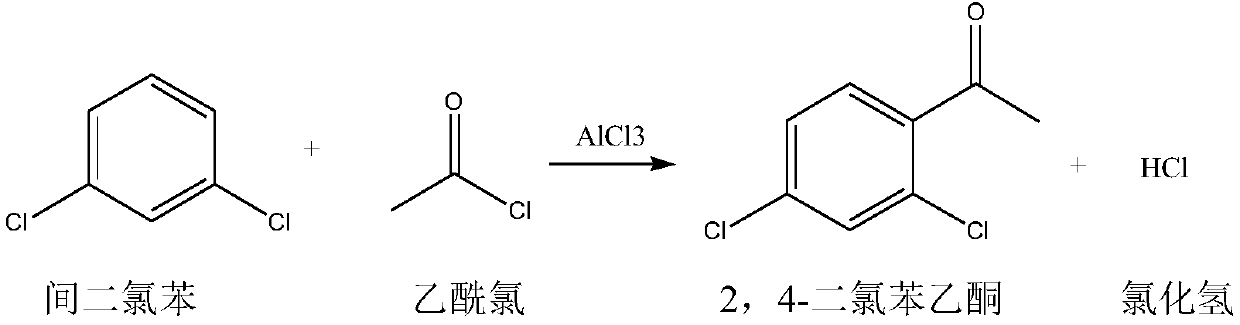
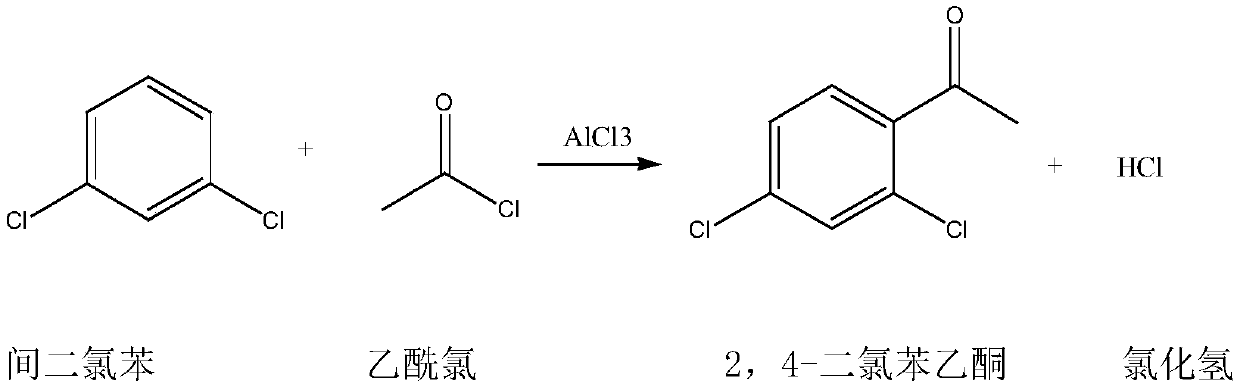
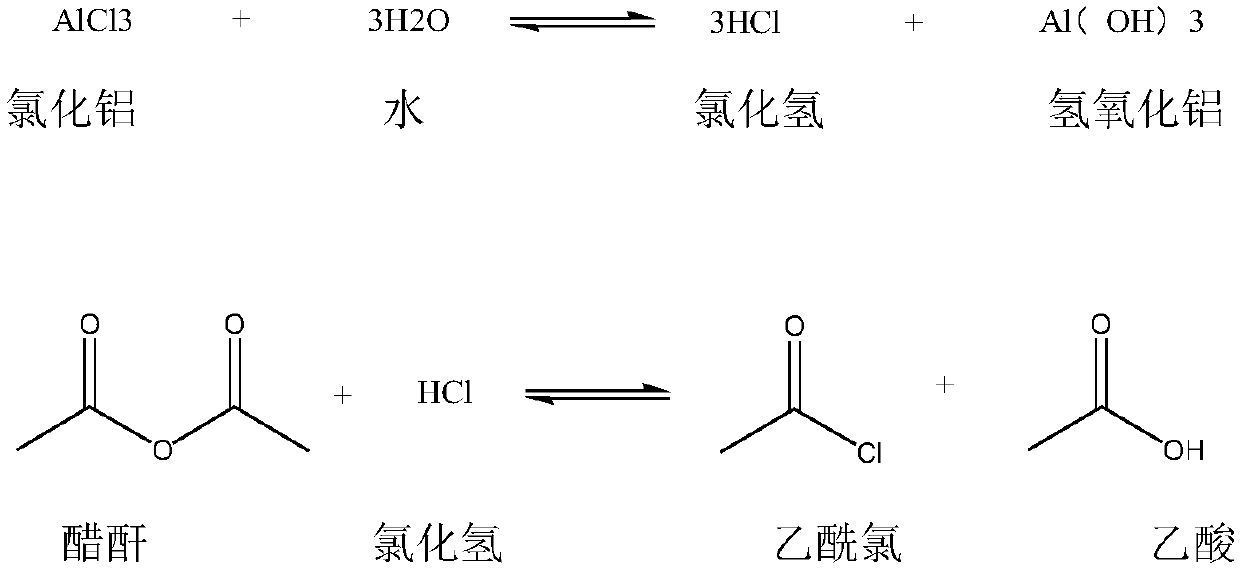
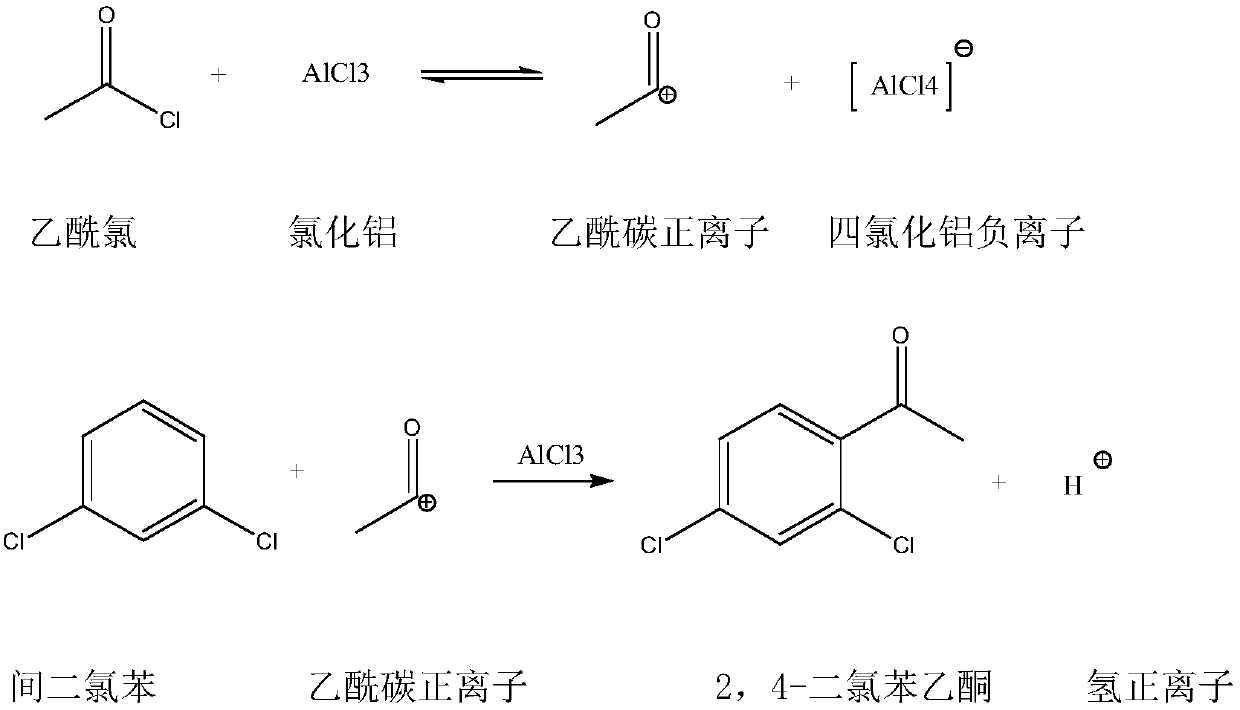
Synthetic method for 2,4-dichloroacetophenone
InactiveCN109721480AReduce unit consumptionSimple processChemical industryCarbonyl compound preparation by condensationAcetic anhydrideM-dichlorobenzene
The invention discloses a synthetic method for 2,4-dichloroacetophenone. The method comprises the step of performing an electrophilic substitution reaction by using m-dichlorobenzene and acetic anhydride as raw materials to synthesize the 2,4-dichloroacetophenone. According to the method provided by the invention, the electrophilic substitution reaction is performed by adopting a reactive rectification manner, a vacuum degree of a reaction rectification column is adjusted, temperature of the reaction rectification column is controlled, reactive rectification is performed under the optimal temperature condition, so that the catalyst unit consumption and the acetic anhydride unit consumption are reduced, and the production process is simplified.
Owner:XINCHANG COUNTY TAIRU TECH CO LTD
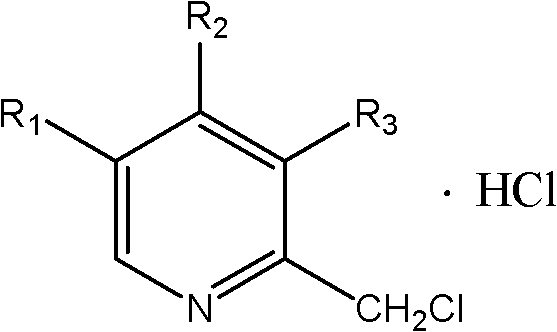
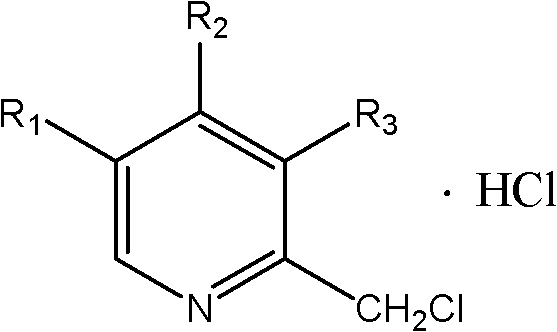
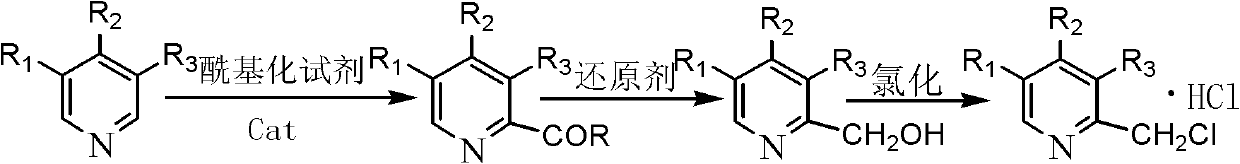
Synthetic method of chloromethylpyridine or pyridine derivative hydrochloride of chloromethylpyridine
The invention discloses a synthetic method of chloromethylpyridine or pyridine derivative hydrochloride of the chloromethylpyridine. The synthetic method is characterized in that according to the method, the positioning effect of substitutional groups is used for carrying out chloromethylation reaction, raw materials of pyridine or pyridine derivatives are subjected to Friedel-Crafts acylation reaction under the catalyst effect, No.2 sites or No.6 sites of the pyridine or the pyridine derivatives take electrophilic substitution reaction, then, hydroxymethylpyridine or hydroxymethylpyridine derivatives are obtained through reduction reaction, next, the chloromethylpyridine or the pyridine derivative hydrochloride is obtained through chlorination reaction, and the structure of the obtained product is shown as the accompanying drawing. The method has the advantages that the reaction steps are few, the cost is low, the purity is high, the yield is high, the operation is safe, and the method is suitable for large-scale industrial production.
Owner:JIANGSU ZHONGBANG PHARMA
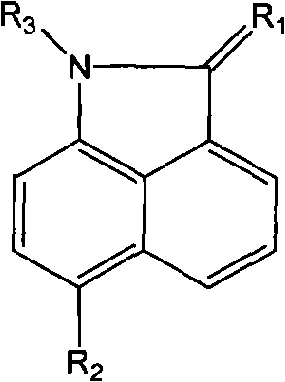
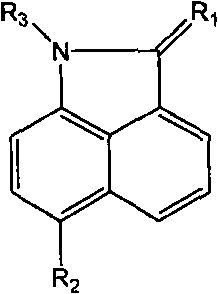
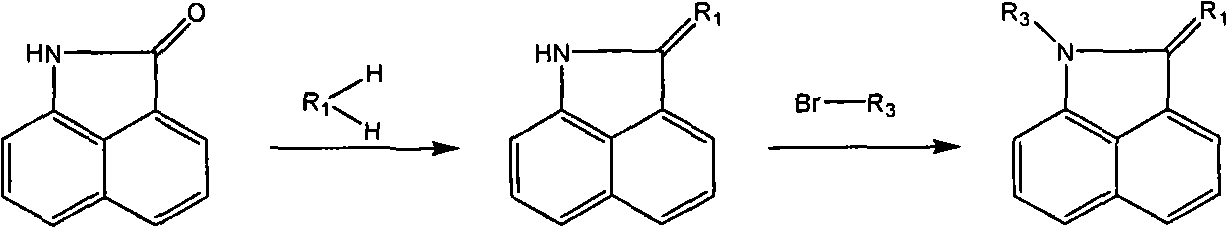
Naphthaline lactam derivative and application thereof in propagation suppression of tumor cells
InactiveCN101602707AHigh activityGood choiceOrganic active ingredientsOrganic chemistryStomach cancerDrug biological activity
The invention relates to a naphthaline lactam derivative and application thereof in the propagation suppression of tumor cells, belonging to the technical field of pharmaceutical chemistry. The naphthaline lactam derivative takes naphthaline lactam as a parent, and a derivative with high biological activity base groups, such as acetylene bonds, nitrile groups, amine groups or acylamide structures, and the like is obtained respectively by synthetic methods of firstly using different alkylogen hydrocarbon to carry out dehydrogenating condensation to two-position carbonyl and a compound with active methylene, introducing different electrophilic substitution base groups by six positions and then carrying out substitution to the active hydrogen of one-position imine. An experiment of the naphthaline lactam derivative for the propagation suppression of tumor cells selects a tetrazolium reduction method and is carried out aiming at 7721 human body liver cancer cells, MCF-7 human body mammary cancer cells, Hela human body cervical carcinoma cells or BGC-823 human body stomach cancer cells; and a result indicates that the naphthaline lactam derivative has favorable activity and selectivity in the propagation suppression of the tumor cells.
Owner:DALIAN UNIV OF TECH
Green preparation technique of 1,4-diiodo-benzene
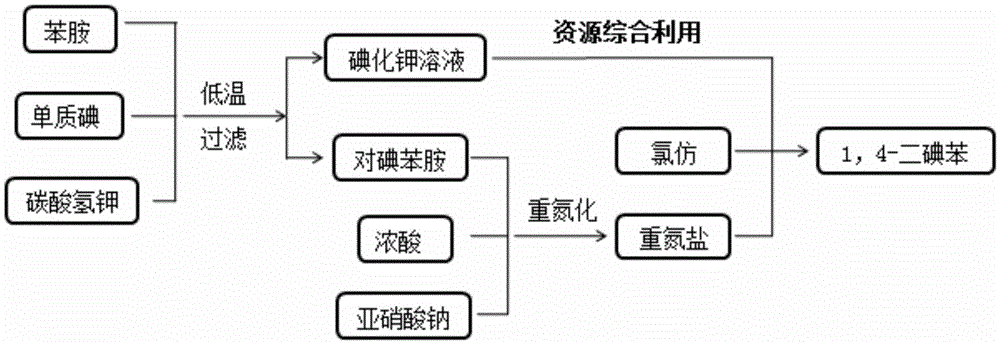
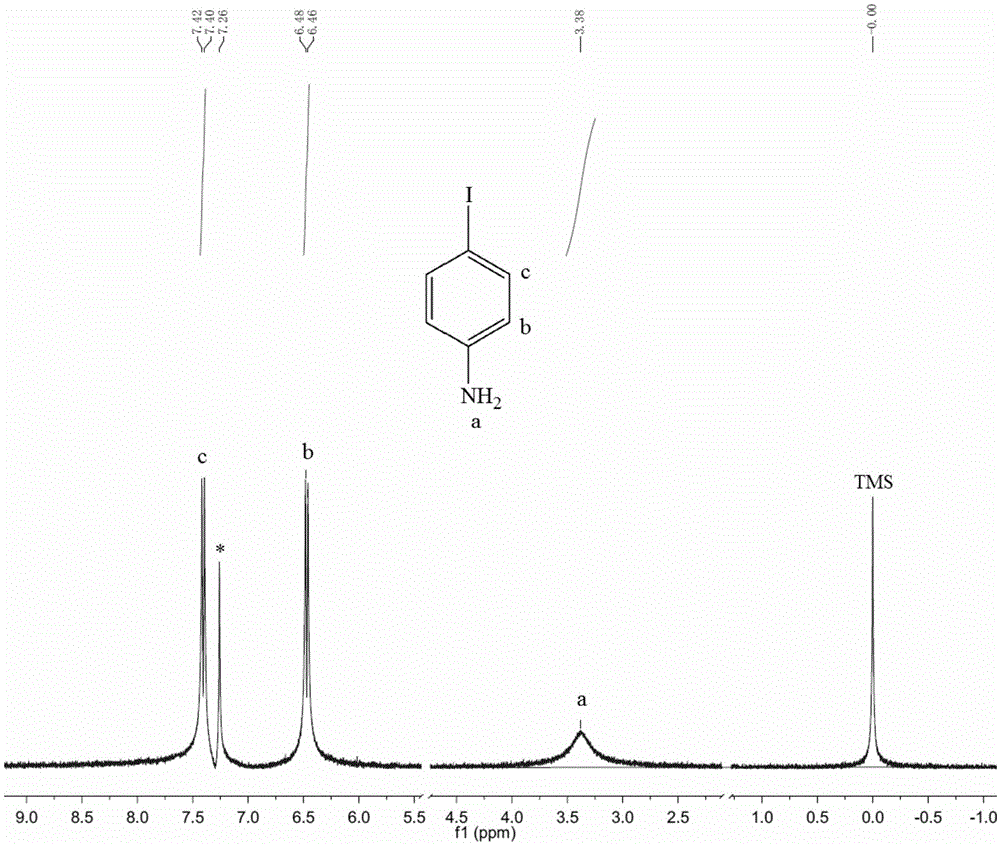
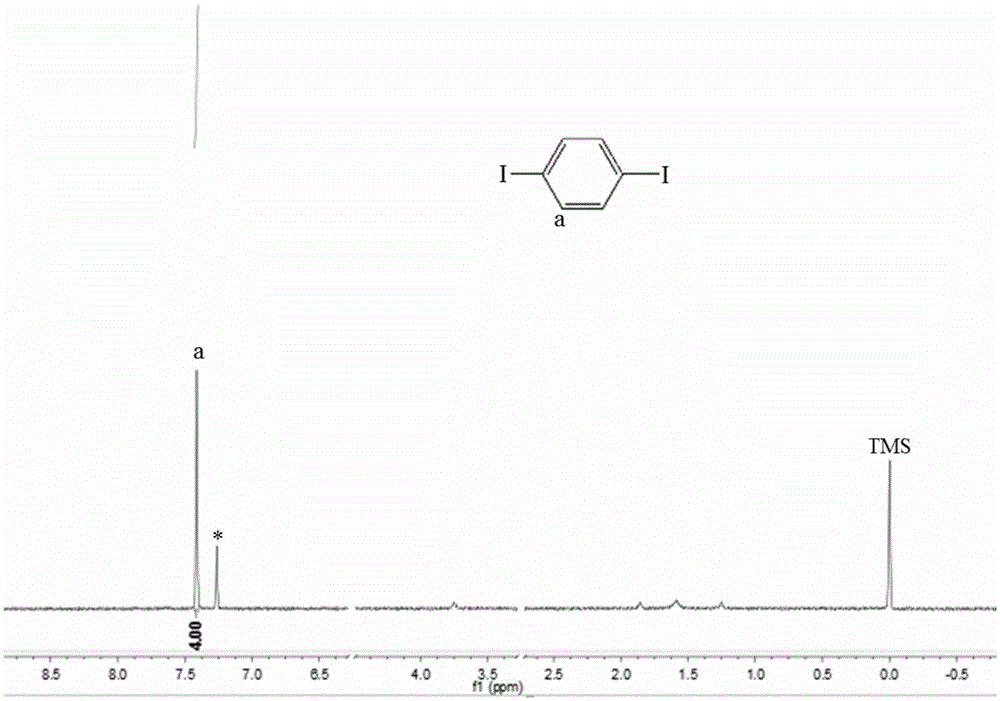
ActiveCN105669357AHigh purityReduce manufacturing costOrganic compound preparationAmino compound preparationAnilinePotassium iodine
The invention relates to a green preparation technique of 1,4-diiodo-benzene. The technique comprises the following steps: carrying out electrophilic substitution reaction on a simple substance iodine and aniline in a weakly alkaline medium solution, separating, and drying to obtain a paraiodoaniline crude product; carrying out diazo-reaction on the paraiodoaniline crude product and a sodium nitrate solution to prepare a diazonium salt, and removing excess sodium nitrate in the system; adding chloroform and a potassium iodide solution into the reaction system, reacting for 3-6 hours while keeping the temperature at -5 to -10 DEG C, slowly heating to room temperature, continuing reacting for 2-6 hours, and carrying out separation and purification on the reaction product to obtain the 1,4-diiodo-benzene. The technique has the advantage of high product purity, and obviously lowers the production cost. The technique implements comprehensive utilization of the potassium iodide waste solution resources, thereby lowering the production cost. The technique simplifies the operation engineering, and enhances the yield of the product.
Owner:WUHAN INSTITUTE OF TECHNOLOGY
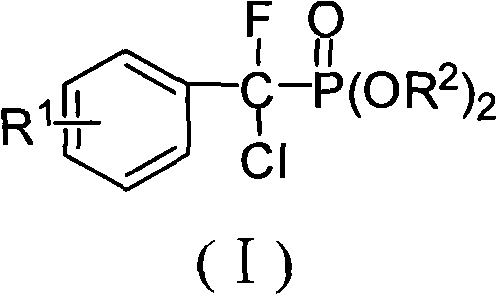
Alpha, alpha-fluorine chlorine fragrant methyl phosphonate and method for preparing the same
InactiveCN101492472AHas PTPs inhibitory activityHigh yieldGroup 5/15 element organic compoundsPhosphateSolvent
The invention discloses the alpha, alpha-chloro-fluoro-arylmethylphosphonate shown in general formula (I), the preparation method comprises the following steps: aromatic formaldehyde and phosphinate conduct the Pudovik addition reaction in a solvent and under the condition of base catalysis to generate alpha-hydroxy arylmethylphosphonate; and then the alpha-hydroxy arylmethylphosphonate and triphenylphosphine conduct nucleophilic substitution reaction in a solvent to generate alpha-chloro-arylmethylphosphonate; finally, the alpha-chloro-arylmethylphosphonate and N-fluoro bis benzene sulfonic amide conduct electrophilic substitution reaction in a solvent and under the condition of base catalysis to generate the alpha, alpha-chloro-fluoro-arylmethylphosphonate; the alpha, alpha-chloro-fluoro-arylmethylphosphonate of the invention has the potential PTPs inhibition activity, which can also be used as the synthesis intermediate of alpha-fluorine phosphate.
Owner:SOUTHWEST UNIV
Synthetic method of 2,4-dichloroacetophenone
InactiveCN109251137AEfficient separationReduce unit consumptionChemical industryCarbonyl compound preparation by condensationAcetic anhydrideElectrophilic substitution
The invention discloses a synthetic method of 2,4-dichloroacetophenone, and the 2,4-dichloroacetophenone is prepared by carrying out electrophilic substitution reaction on m-dichlorobenzene and aceticanhydride as raw materials. According to the method, the electrophilic substitution reaction is carried out by adopting a reaction rectification mode, and the vacuum degree of a reaction rectifying tower is adjusted, and the temperature of the reaction rectifying tower is controlled at optimal temperature for reaction rectification. The single consumption of a catalyst is reduced, the single consumption of the acetic anhydride is reduced, and the production process is simplified.
Owner:XINCHANG COUNTY TAIRU TECH CO LTD
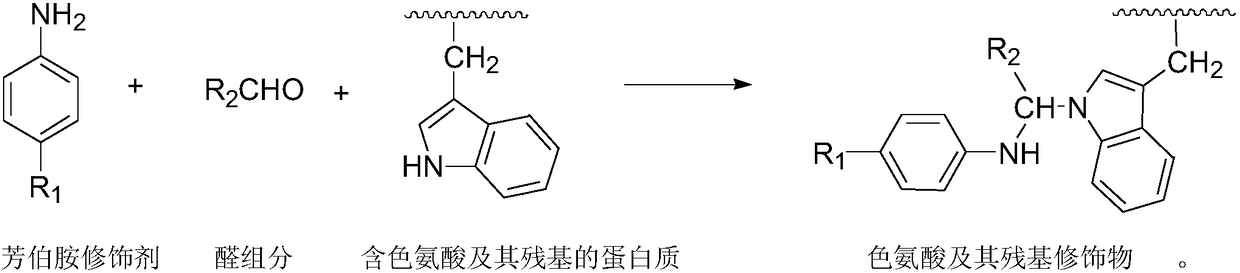
High selective chemical modification method for tryptophan and residues of tryptophan
The invention discloses a highly selective chemical modification method for tryptophan and residues of tryptophan. The method comprises the steps that: tryptophan or a substance containing a tryptophan residue is used as a modified object in water, under the action of an aldehyde component, highly selective three-component electrophilic substitution reaction are conducted on aromatic primary aminemodifiers and tryptophan / tryptophan residues in modified products, so that aromatic primary amine modifier molecules are covalently bonded to the tryptophan / tryptophan residues. In the chemical modification method, the aromatic primary amine modifiers have excellent selectivity for the tryptophan and the residues of tryptophan in proteins, and have no significant reactivity with other active amino acids and residues thereof; the covalent bonding forms among the aromatic primary amine modifier molecules and the tryptophan and the residues of tryptophan, and the durability is excellent.
Owner:ZHEJIANG SCI-TECH UNIV
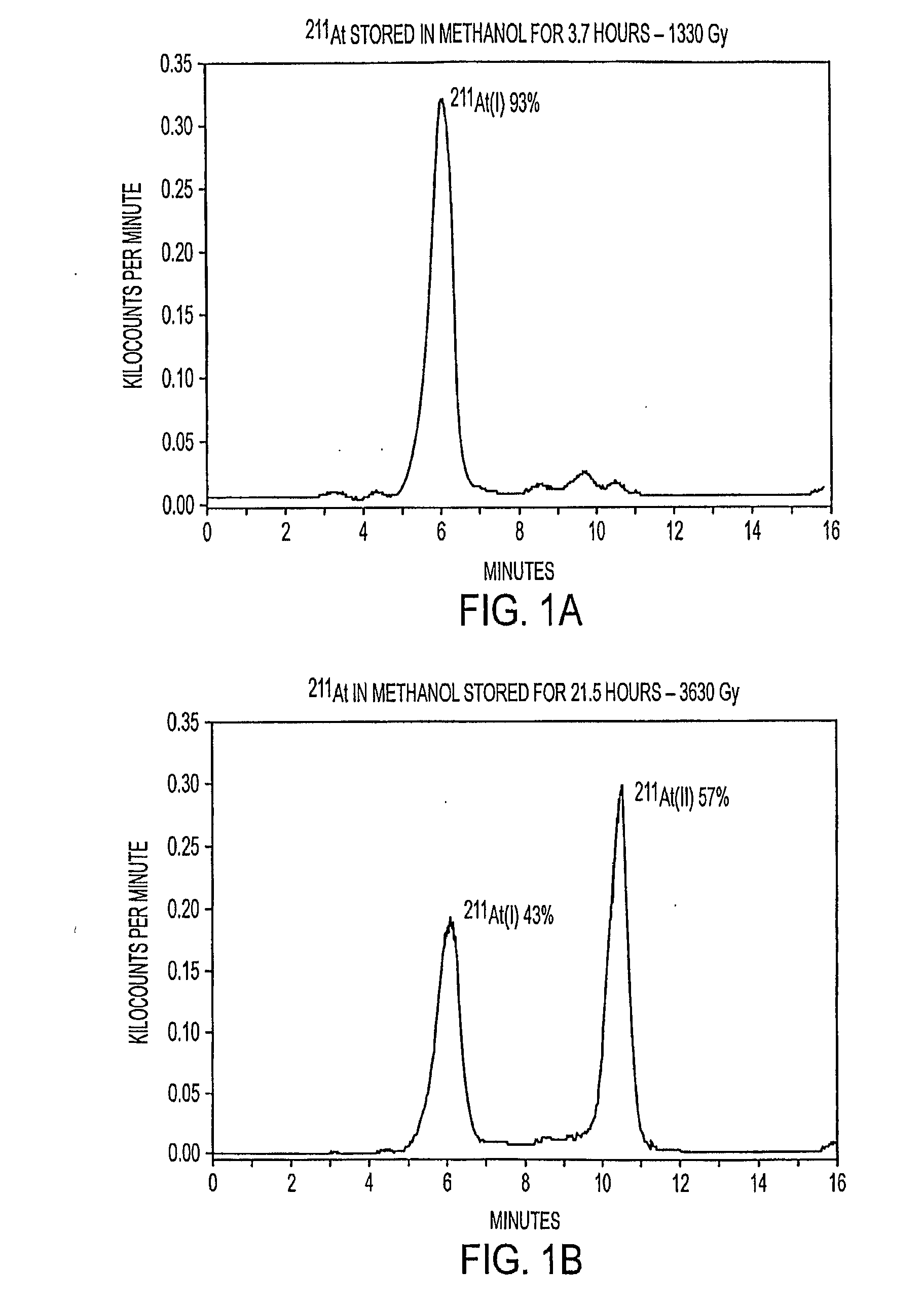
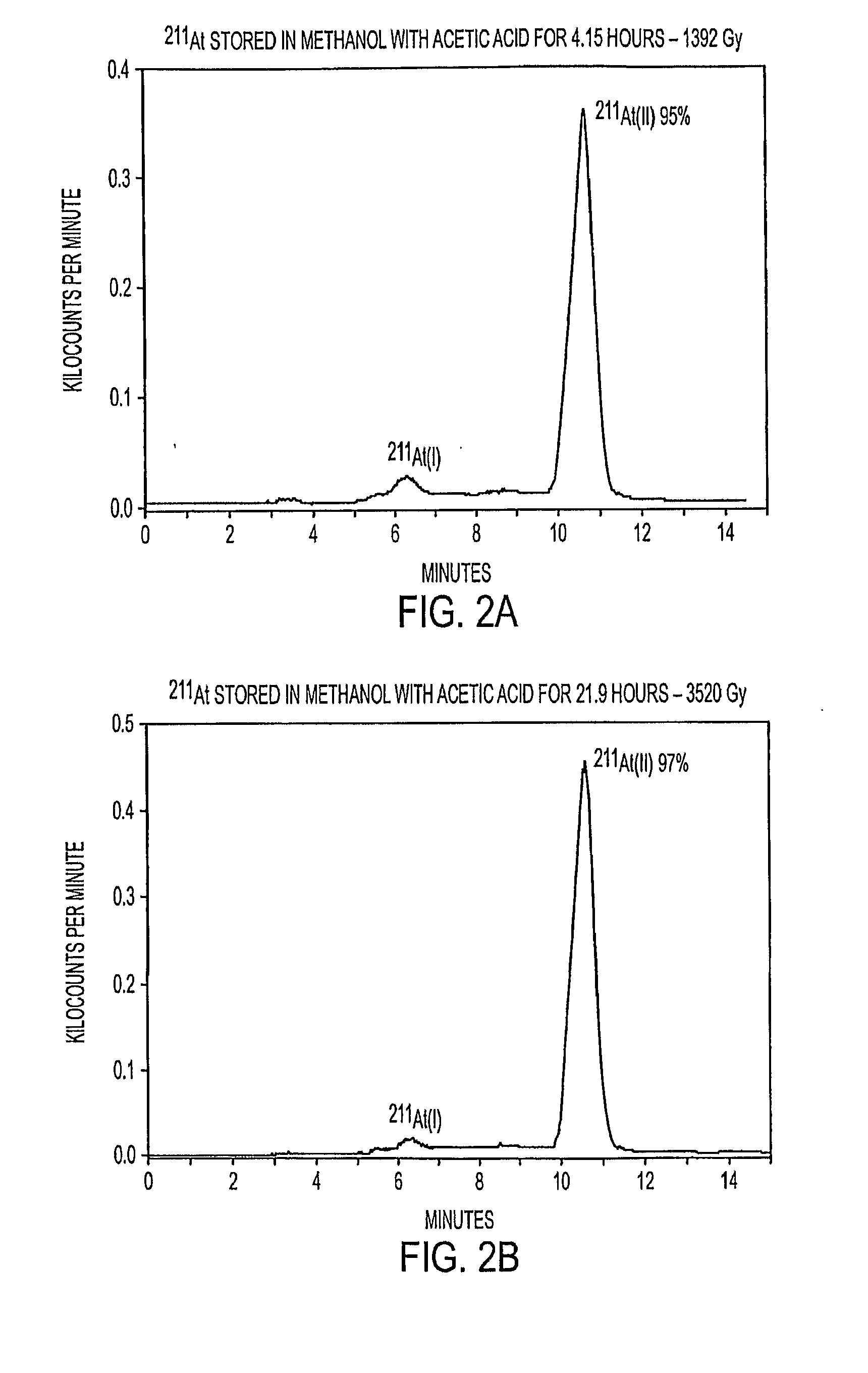
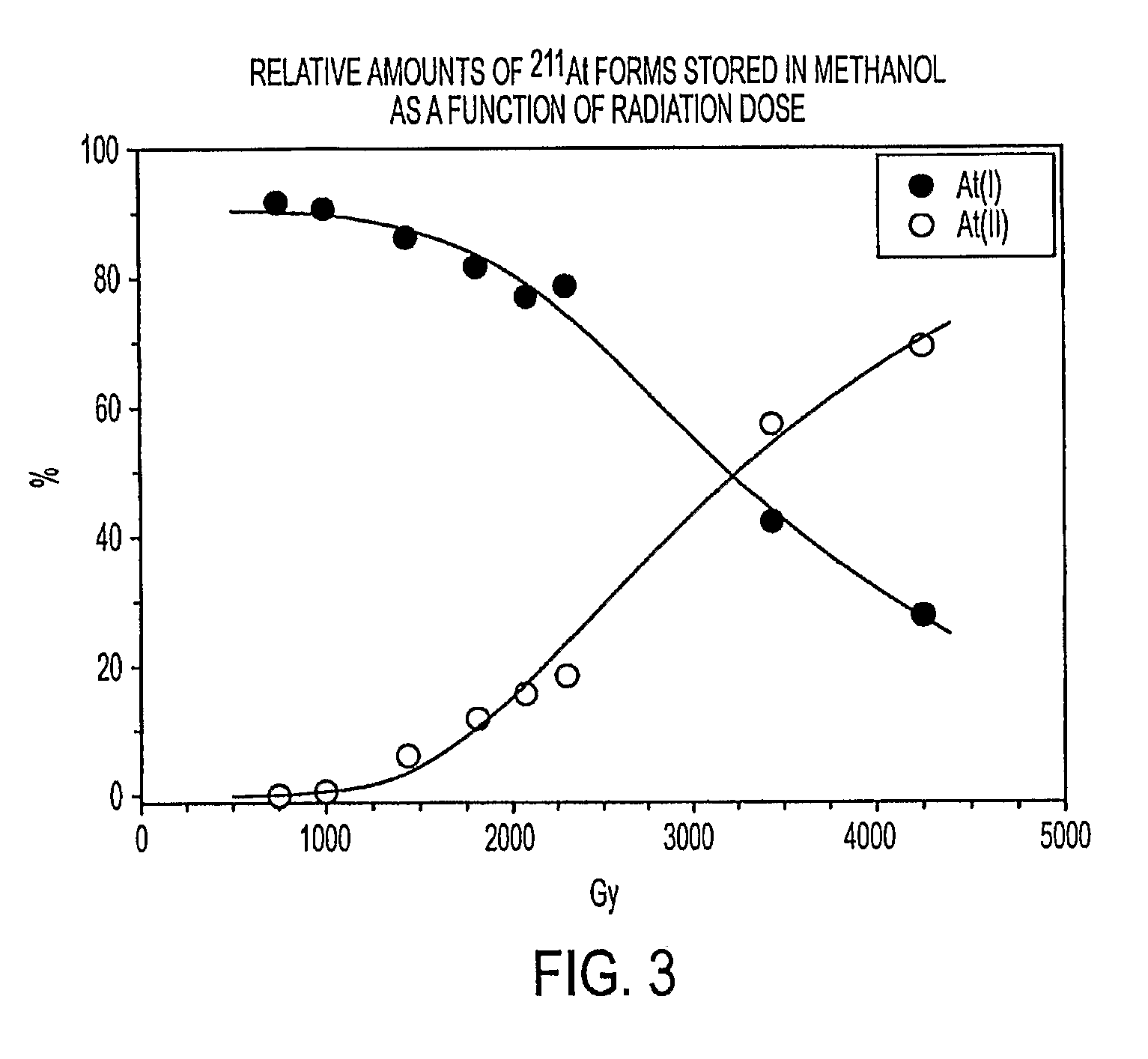
Stabilized Compositions and Methods for Radiolabeling Pharmaceuticals with Alpha-Particle Emitters
ActiveUS20090304585A1Improve radiation effectLoss of reactionOrganic compounds purification/separation/stabilisationRadioactive preparation carriersN-ChlorosuccinimideMetallic bonding
Oxidants (e.g., N-chlorosuccinimide) can be used to stabilize α-particle emitters (e.g., 211At) in solution, prior to their subsequent reaction to form α-particle emitter labeled compounds (e.g., a radiolabeled pharmaceutical or a radiolabeled pre-cursor used to prepare it). In particular, the use of an oxidant has been found to maintain the α-particle emitter in a chemical form that facilitates this reaction, which may involve a number of possible mechanisms including electrophilic substitution, nucleophilic substitution, complexation, exchange, or metallic bonding. Compounds labeled with α-particle emitters in this manner have wide-ranging therapeutic applications, particularly in the treatment of cancer.
Owner:DUKE UNIV
Tryptanthrin alkaloid salt, and preparation method and application thereof
InactiveCN105153148AStrong antiproliferative activityThe synthesis method is simpleOrganic chemistryAntineoplastic agentsSulfonateStructural formula
The invention discloses a tryptanthrin alkaloid salt, and a preparation method and application thereof, belonging to the technical field of medicine. The structural formula of the tryptanthrin alkaloid salt is as shown in a formula I which is described in the specification, and in the formula I, R is Na or K. According to the invention, tryptanthrin is used as a structural basis and undergoes electrophilic substitution reaction with fuming sulphuric acid so as to produce tryptanthrin sulfonate; and the preparation method is simple, has mild reaction conditions and uses cheap and easily available raw materials. Proliferation inhibition activity of the tryptanthrin sulfonate to seven tumor cell strains is investigated in the invention. Investigation results show that the in-vitro antitumor activity of the tryptanthrin sulfonate is selective and the tryptanthrin sulfonate has substantial proliferation inhibition activity to specific tumor cell strains, shows good potential medicinal values and is expected to be used for preparation of a variety of antitumor drugs.
Owner:YULIN NORMAL UNIVERSITY
Method for preparing high molecular weight polyaryletherketone
InactiveCN107987272AControllable degree of branchingHigh molecular weightPolymer scienceEngineering plastic
The invention belongs to the technical field of macromolecule special engineering plastics and in particular relates to a method for preparing high molecular weight polyaryletherketone. The method comprises the following steps: by taking aromatic oxalyl chloride, a polycyclic aromatic monomer with two active hydrogen atoms and a branching agent compound as polymerization monomers, enabling the components to react in the presence of lewis acid, lewis base and a polar aprotic solvent, thereby obtaining the high molecular weight polyaryletherketone. As multifunctional monomers are introduced to be polymerized, multifunctional compounds for a Friedel-Crafts acylation reaction are introduced, an aromatic electrophilic substitution route is adopted for preparation, and by using a lewis acid-lewis base co-catalysis method, the high molecular weight polyaryletherketone of which the branching degree can be controlled can be prepared under relatively gentle reaction conditions; after purification treatment, plates, rods, particles, membranes and the like can be prepared from the prepared polyaryletherketone through procedures of mold pressing, extrusion, injection molding, and the like.
Owner:SHANDONG KAISHENG NEW MATERIALS
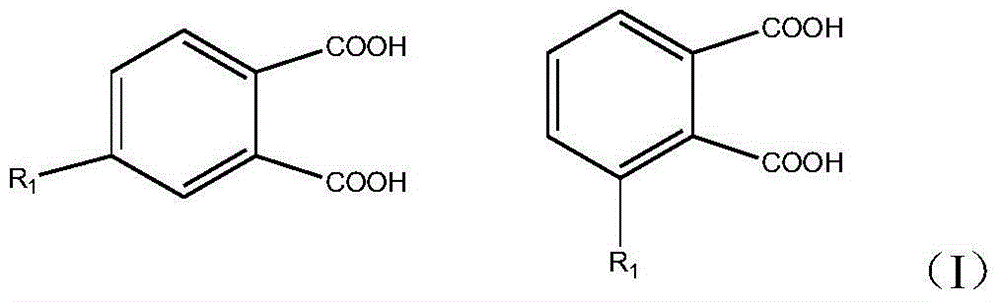
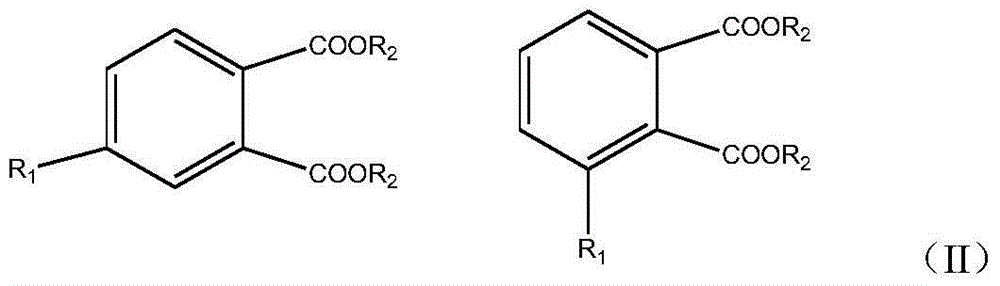
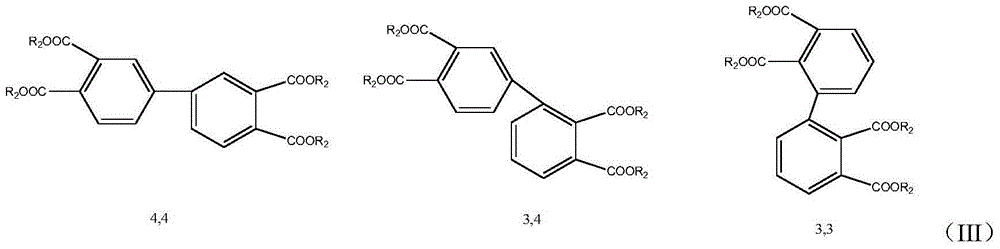
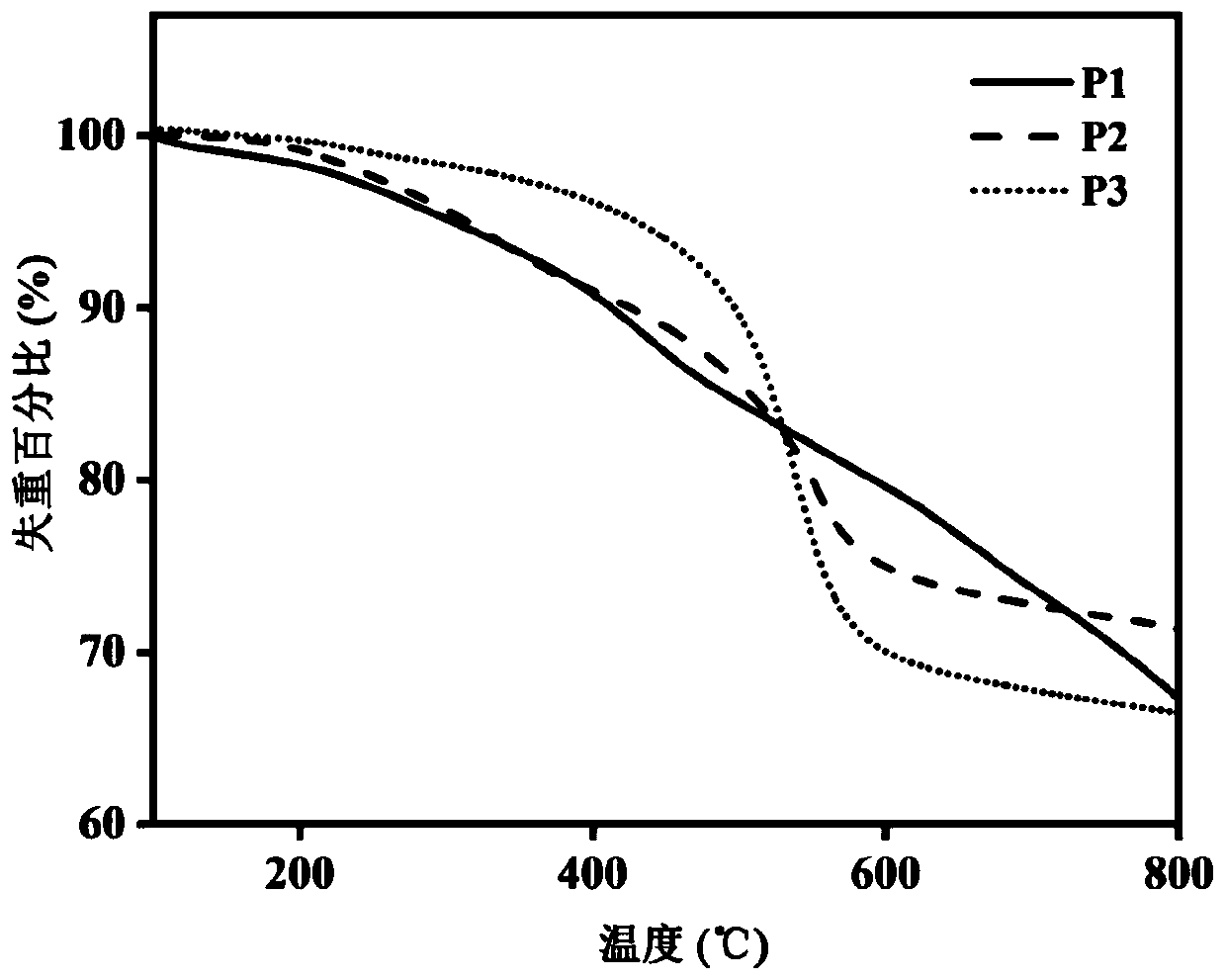
Preparation method for biphenyltetracarboxylic dianhydride mixture
The invention relates to a preparation method for a biphenyltetracarboxylic dianhydride mixture. The preparation method comprises the following steps: with phthalic acid as a starting raw material, carrying out an electrophilic substitution reaction so as to produce a phthalic acid derivative; then subjecting the derivative to esterification so as to produce phthalate; further catalyzing phthalate and carrying out a coupling reaction so as to produce a biphenyl tetraformate mixture; and finally, successively carrying out saponification and acidification so as to produce the product biphenyltetracarboxylic dianhydride mixture. The method provided by the invention uses cheap and easily phthalic acid as the starting raw material and is a novel low-cost easily-operatable environment-friendly production method for biphenyltetracarboxylic dianhydride; moreover, isomeric compounds of all the biphenyltetracarboxylic dianhydride are synthesized at one time in the invention, so efficiency is improved and the cost is reduced.
Owner:苏州亚培克生物科技有限公司
Porphyrin-based polymer-of-intrinsic-microporosity and synthesis method thereof
The invention discloses a porphyrin-based polymer-of-intrinsic-microporosity and a synthesis method thereof, and belongs to the field of functional materials, wherein the porphyrin-based polymer-of-intrinsic-microporosity is prepared by using a porphyrin motif as core through aromatic electrophilic substitution polymerization under a solvothermal condition through structural design and group regulation. According to the present invention, the porphyrin-based polymer-of-intrinsic-microporosity is formed by bridging a rigid V type troger's base structure and a porphyrin motif, the rigid skeletonstructure and the space distortion make the material have the rich permanent micropores and the large specific surface area, the pore size and the specific surface area of the polymer can be regulated through the group regulation, the material can have the excellent stability due to the covalent bond structure, and the rich porphyrin rings on the polymer skeleton can be subjected to function adjustment in different directions and different fields through the post-modification strategy, such that the material can have practical application value in the fields of light, electrocatalysis, biomimetic catalysis and the like.
Owner:JIANGNAN UNIV
Poly-halogenated benzoic acid synthesizing method
ActiveCN103772080ALow costReduce pollutionOrganic compound preparationThiol preparationBenzoic acidStrong acids
The invention provides a poly-halogenated benzoic acid synthesizing method. The method comprises the following steps: dissolving substituted benzoic acid, halogen and organic strong acid in a polar solvent for increasing temperature, after oxidation at a temperature of 50-70 DEG C, performing heat preservation at the temperature of 60-80 DEG C for 2-6 h, adding a right amount of water, and cooling to a room temperature, so as to obtain poly-halogenated benzoic acid. According to the invention, synthetic reaction is performed in an alcohols solvent, the halogen simple substance is used as a halogenating reagent, a small amount of organic strong acid is added, used as a catalyst, and used for catalyzing the electrophilic substitution reaction of the halogen to benzene rings, and hydrogen peroxide is dropwise added during the reaction to enable the halogen simple substance in the reaction system to be oxidized into halide ions so as to improve the utilization rate of the halogen and achieve the effect of atom economy.
Owner:ZHEJIANG LUOXING IND CO LTD
Aromatic amide compound comprising phosphoryl amino acid structure, preparation method of compound and application of compound taken as weed killer
InactiveCN103524552ANovel structureHigh activityBiocideGroup 5/15 element organic compoundsBristlePhenacyl
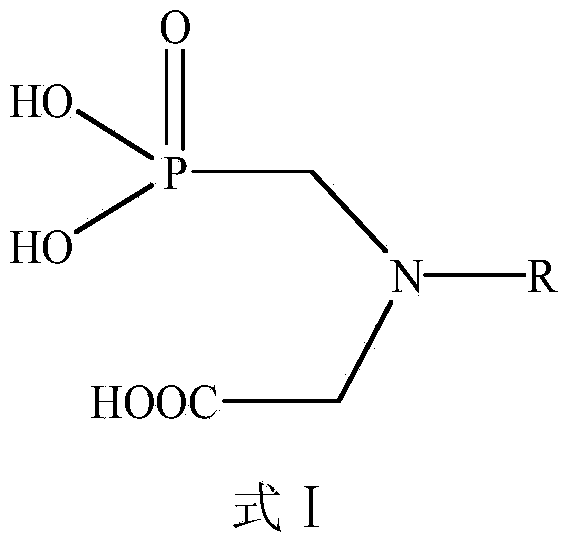
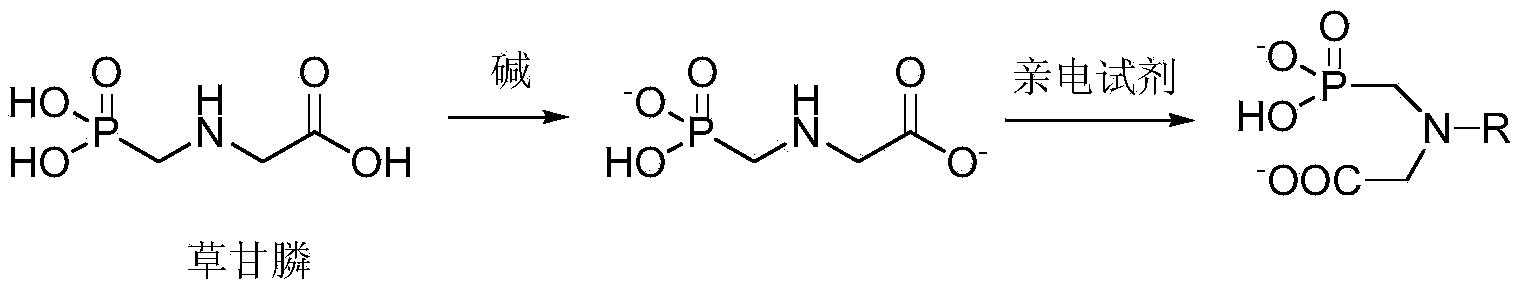
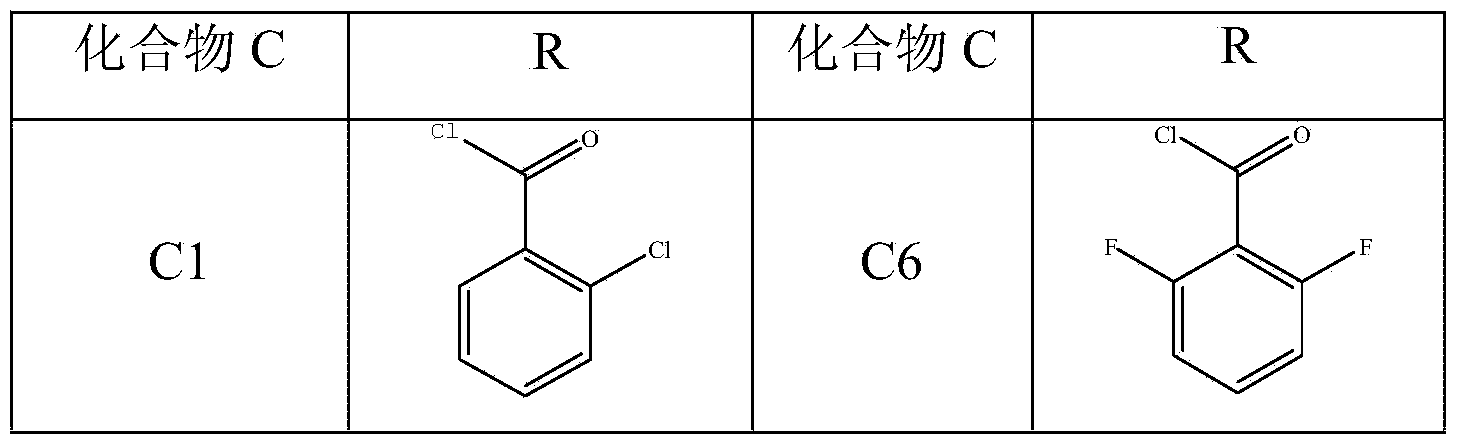
The invention discloses an aromatic amide compound comprising a phosphoryl amino acid structure, a preparation method of the compound and an application of the compound taken as a weed killer. The structural formula of the aromatic amide compound comprising the phosphoryl amino acid structure is shown in a formula I, R represents substituted or un-substituted benzoyl, substituted or un-substituted benzene oxygen acetyl or replaced propenoyl. The preparation method of the aromatic amide compound shown as the formula I and comprising the phosphoryl amino acid structure comprises the steps as follows: under the catalytic action of alkali, glyphosate and an electrophilic reagent are subjected to electrophilic substitution reaction, so that the aromatic amide compound shown in the formula I is obtained; and the electrophilic reagent is substituted or un-substituted benzoyl chloride, substituted or un-substituted phenoxyacetyl chloride or replaced acryloyl chloride. The weed killer can be used for preventing and killing weeds, green bristle grass, crab grass, barnyard grass, quinoa, summer cypress, Xanthium sibiricum and piemarker.
Owner:CHINA AGRI UNIV
Preparation method for novel SGLT2 inhibitor medicine
ActiveCN104926803AShort synthetic routeIntermediate quality is easy to controlOrganic chemistryBenzoic acidPhenacyl
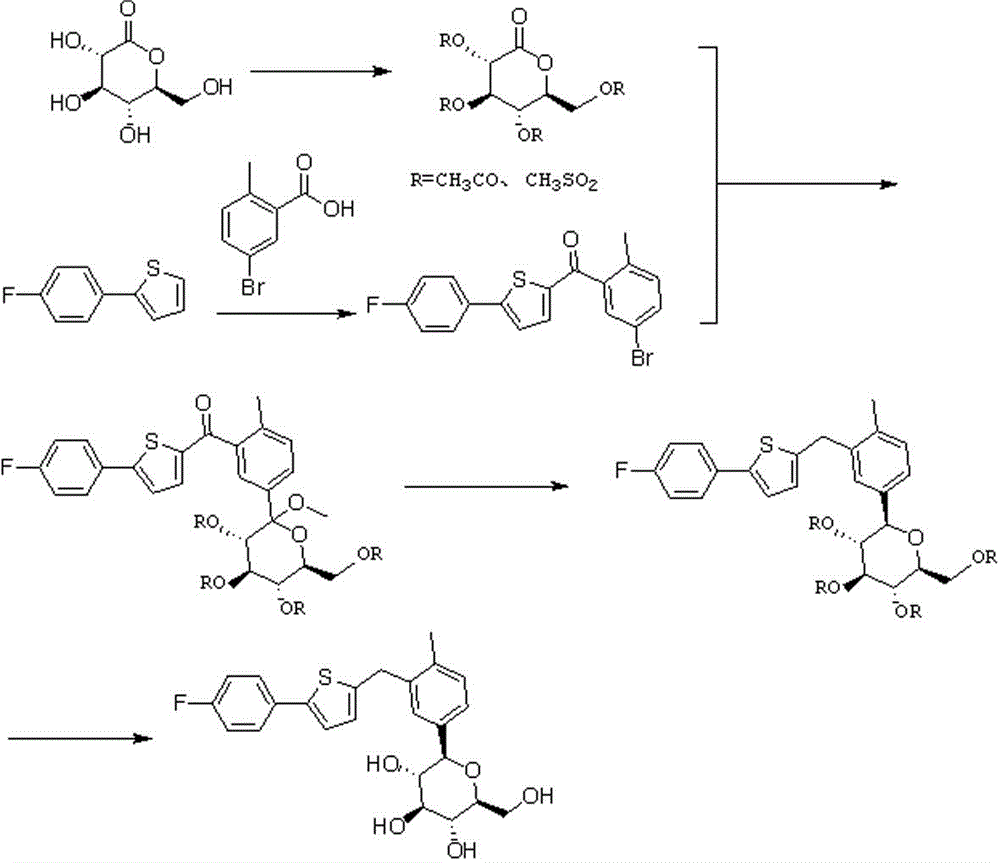
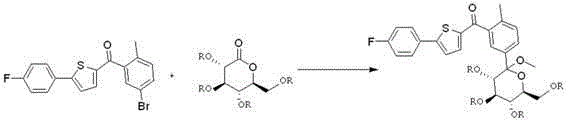
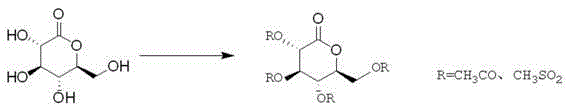
The invention discloses a preparation method for novel SGLT2 inhibitor medicine. The preparation method includes the steps that D-gluconolactone serves as an initial raw material and reacts with an acylation reagent under the action of a base to obtain a glucolactone intermediate with hydroxyl protection; 5-bromine-2-methyl benzoic acid is prepared into an acid chloride through an acylation reagent, and the acid chloride and 2-(4-fluoro-phenyl) thiophene perform friedel-Crafts acylation reaction under the action of Lewis acid; 2-(5-bromine-2-methyl-benzoyl)-5-(4-fluoro-phenyl) thiophene and the glucolactone intermediate perform electrophilic substitution reaction under the action of super-strong bases, and then etherification reaction is performed to obtain methyl etherate; the methyl etherate restores carbonyl into methylene under the action of a reducing agent, and an methoxy group is removed to form an S-configuration aromatic gluconolactone intermediate; the aromatic gluconolactone intermediate is hydrolyzed under the action of a base to obtain a canagliflozin product. The synthesis process route is short and simple, the quality of the intermediate is easy to control, the reaction yield is high, the production cost is low, and the preparation method is suitable for industrialized production.
Owner:NANTONG CHANGYOO PHARMATECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com