Patents
Literature
93 results about "N-Chlorosuccinimide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
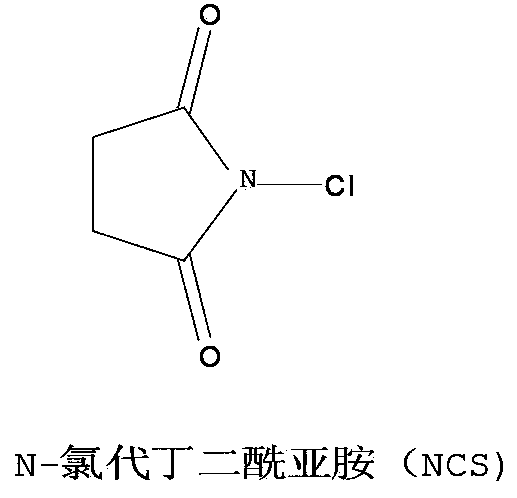
N-Chlorosuccinimide is the organic compound with the formula C₂H₄(CO)₂NCl. A white solid, it is used for chlorinations. It is also used as a mild oxidant. NCS is related to succinimide, but with NCl in place of NH. The N-Cl bond is highly reactive, and NCS functions as a source of "Cl⁺".
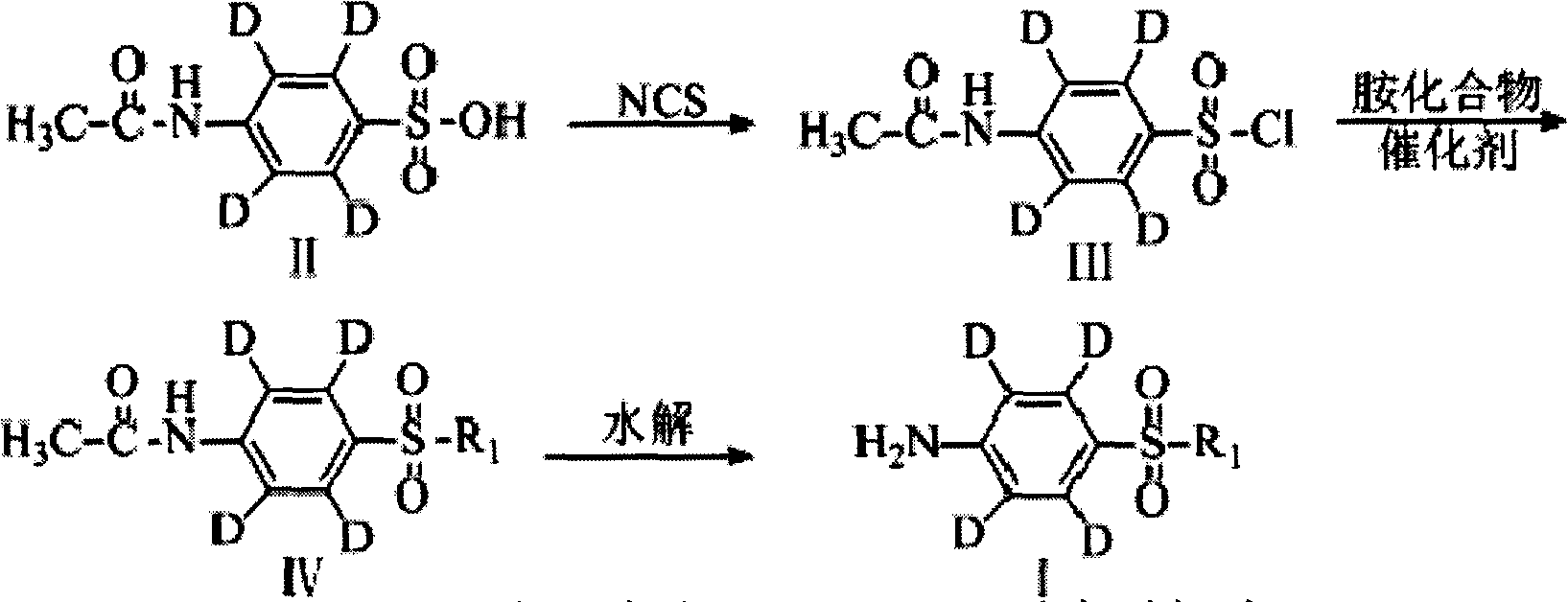
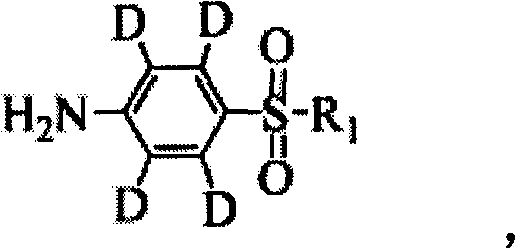
Synthetic method of deuterium-marked sulfanilamide
ActiveCN102603655ARaw materials are easy to getMild reaction conditionsOrganic chemistrySulfonyl chlorideN-Chlorosuccinimide
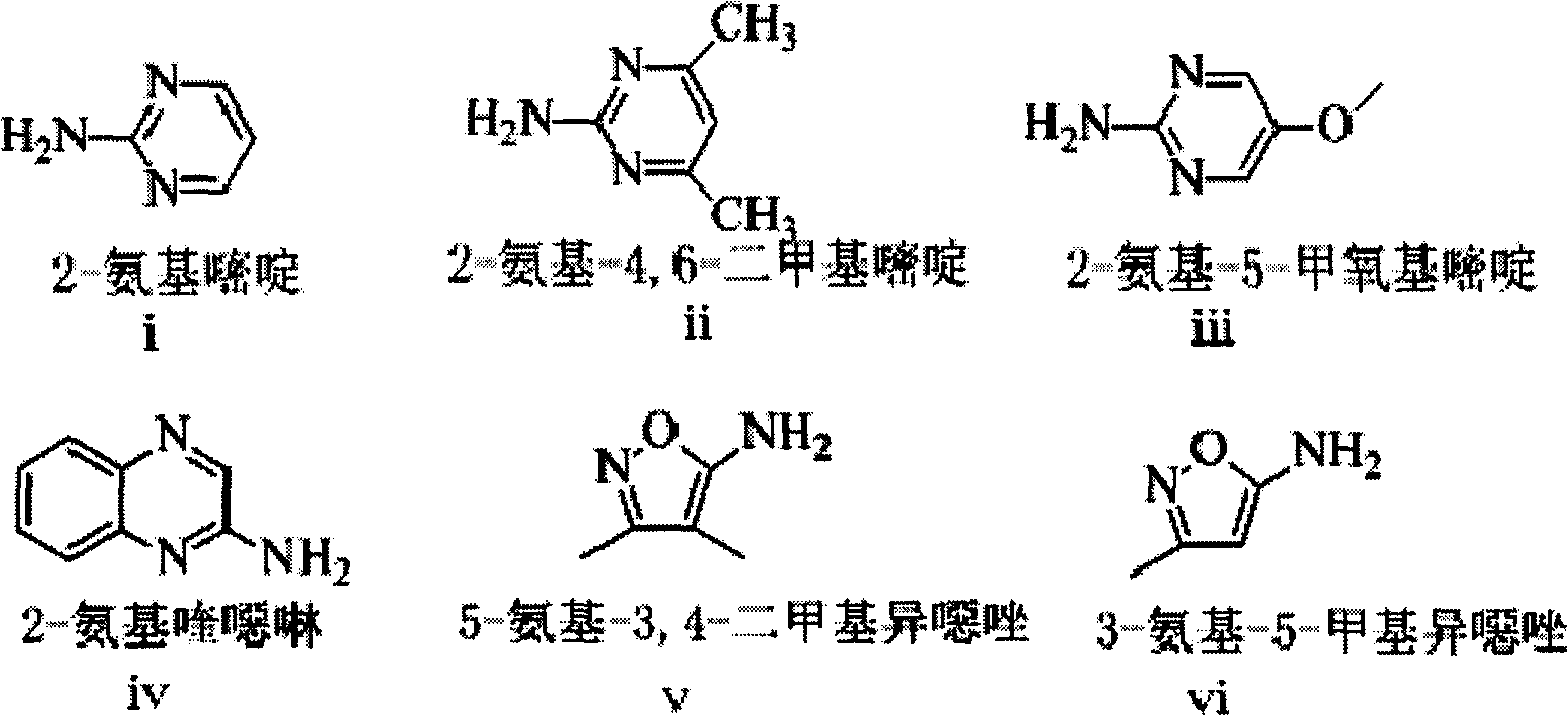
The invention relates to a synthetic method of deuterium-marked sulfanilamide. The method comprises the following steps of: reacting deuterated sulfonic acid with N-chlorosuccinimide (NCS) to obtain deuterated sulfonyl chloride which is not required to be separated; and adding amine for undergoing an amination reaction under the action of a metal catalyst to obtain deuterium-marked sulfanilamide. Compared with the prior art, the method has the advantages that: acyl chloride synthesis and the amination reaction are performed with a 'one pot process', operation is simple and convenient, the yield is high, and a synthesized product comprises sulfapyridine-benzene ring-D4, sulfadimidine-benzene ring-D4, sulfameter-benzene ring-D4, sulfaquinoxaline-benzene ring-D4, sulfisoxazole-benzene ring-D4 and sulfamethoxazole-benzene ring-D4.
Owner:SHANGHAI RES INST OF CHEM IND
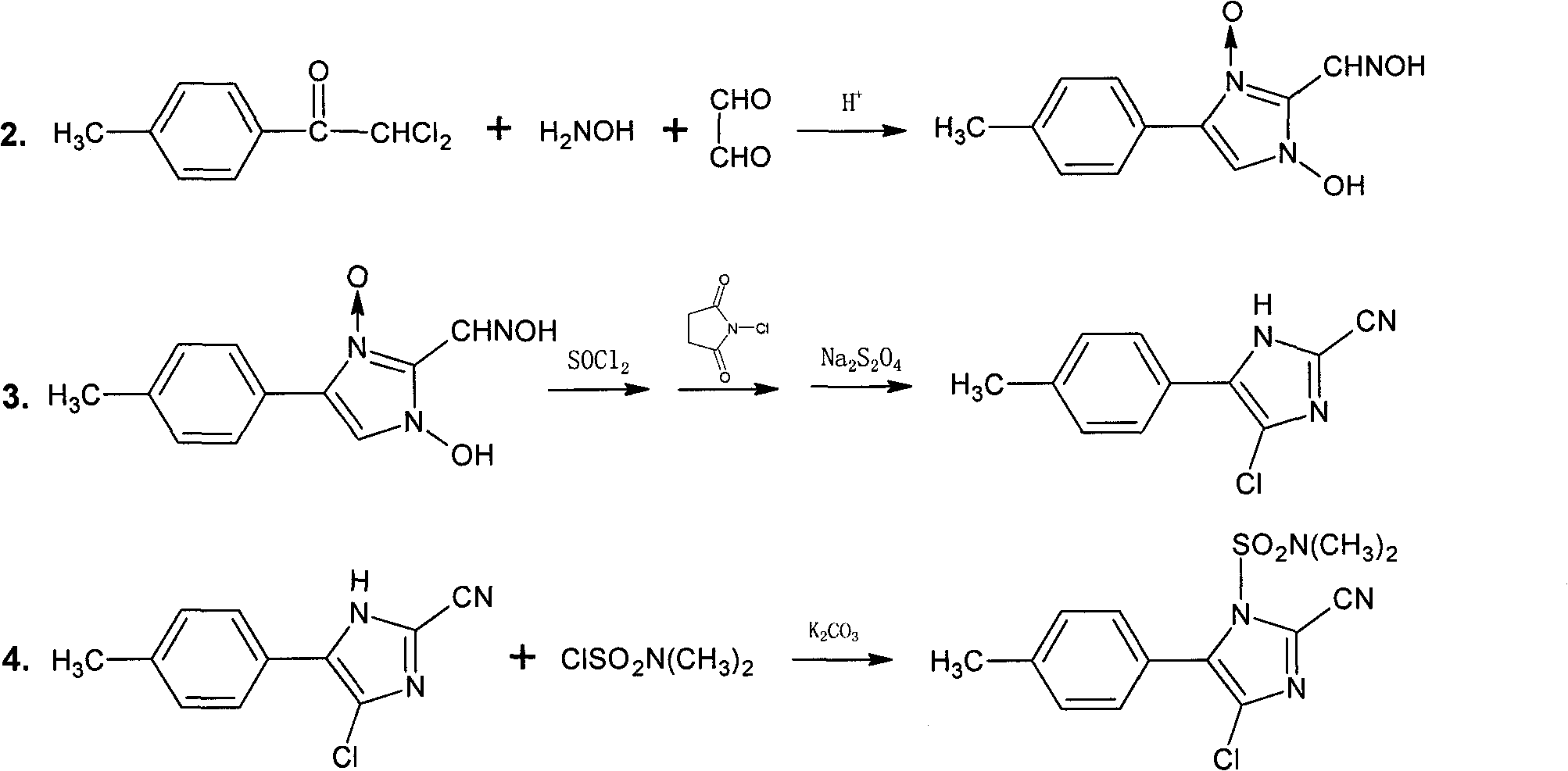
Synthesis method of 4-chloro-2-cyano-1-dimethylamino-sulfonyl-5-(4-methylphenyl)imidazo
ActiveCN102424671AEmission reductionShort reaction timeOrganic chemistrySodium dithioniteN-Chlorosuccinimide
The invention discloses a synthesis method of 4-chloro-2-cyano-1-dimethylamino-sulfonyl-5-(4-methylphenyl)imidazo, which aims to solve the technical problems of long reaction time, low yield and environment pollution of the prior art. The method is used for preparing 2,2-dichloro-4'-methyl acetophenone as an intermediate through an acylation reaction of methylbenzene and dchloroethanoyl chloride and preparing 4(5)-chloro-2-cyano-5(4)-(4-methylphenyl)imidazo as an intermediate by taking ethyl acetate and the like as solvent, N-succinchlorimide as chlorinating agent and sodium dithionite and the like as reductant. The synthesis method has short reaction time, high yield and total yield up to 61.7% and is mainly used for preparing the 4-chloro-2-cyano-1-dimethylamino-sulfonyl-5-(4-methylphenyl)imidazo.
Owner:XIAN MODERN CHEM RES INST
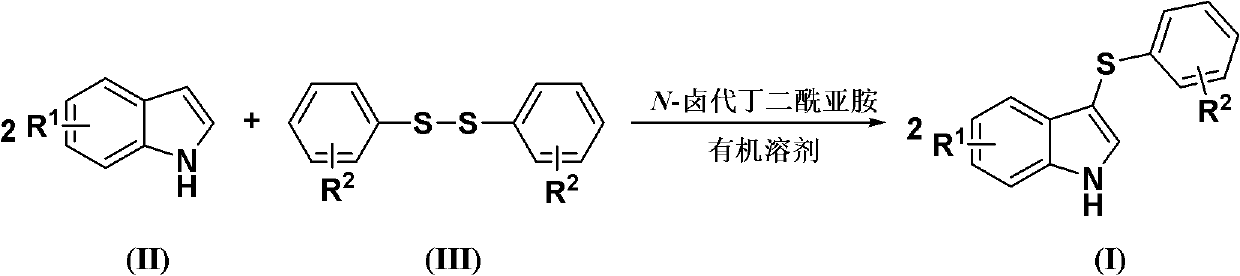
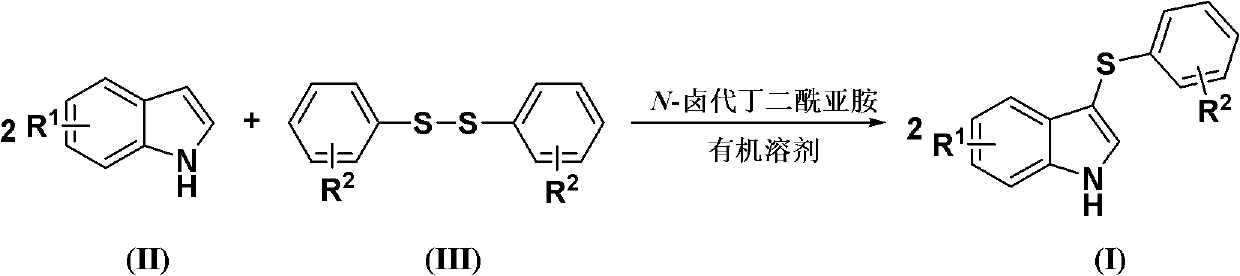
Method for synthesizing 3-aryl sulfydryl indole compound
The invention discloses a method for synthesizing a 3-aryl sulfydryl indole compound which is shown as a formula (I). According to the method for synthesizing the 3-aryl sulfydryl indole compound, an indole compound which is shown as a formula (II) and diaryl disulfides which are shown as a formula (III) are taken as raw materials, and the 3-aryl sulfydryl indole compound is obtained by fully reacting the indole compound and the diaryl disulfides in an organic solvent at the temperature of between -30 and 40 DEG C in the presence of N-halogenate succinimide; and the N-halogenate succinimide is one of N-chlorobutanimide, N-bromosuccinimide, and N-Iodosuccinimide. According to the method, a nonmetal low-toxicity reaction system is used, and cost is low; reaction selectivity and yield are high; a process route is advanced and reasonable, and reaction conditions are mild; and the method has great implementation value and social and economic benefit.
Owner:WENZHOU UNIVERSITY
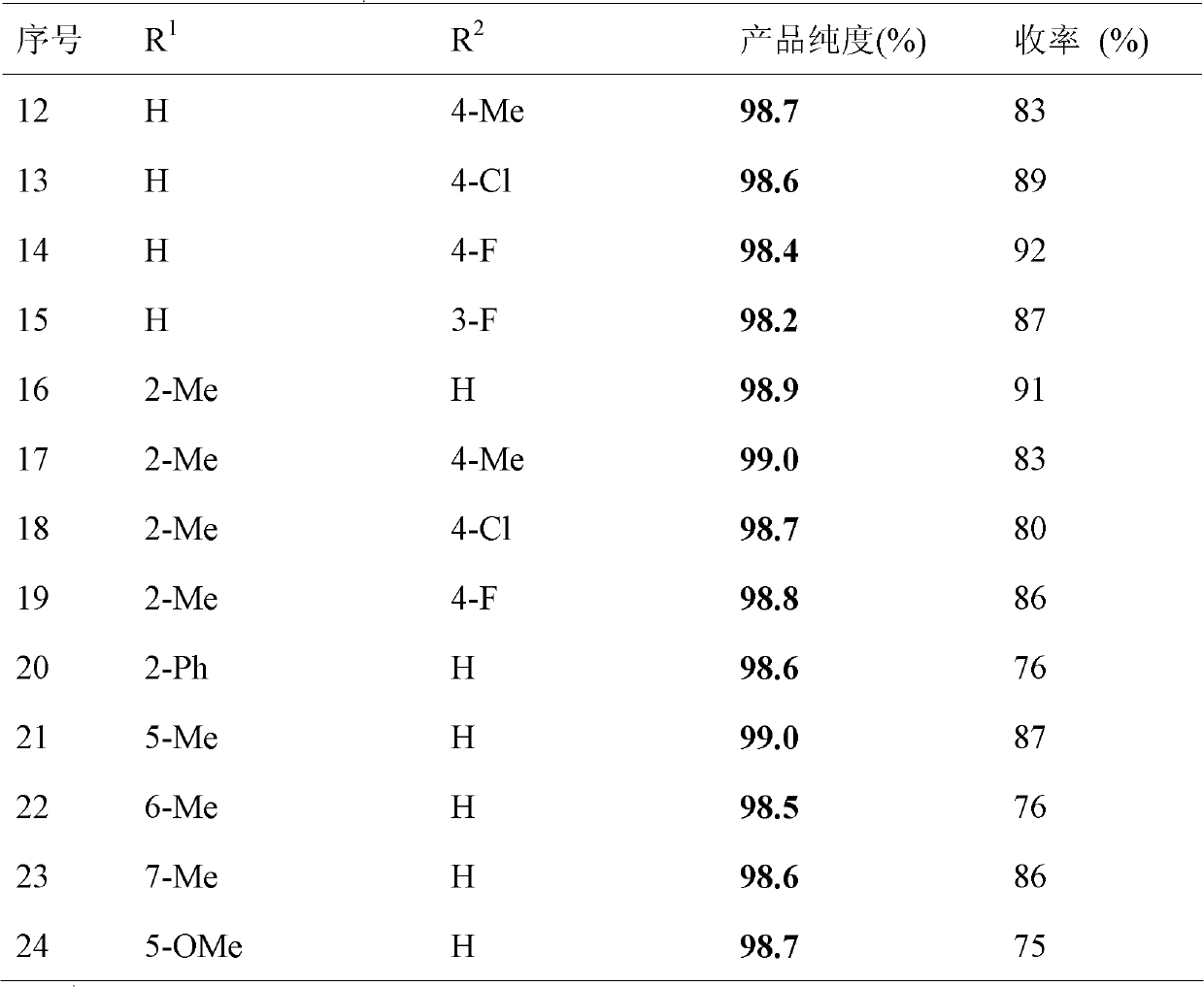
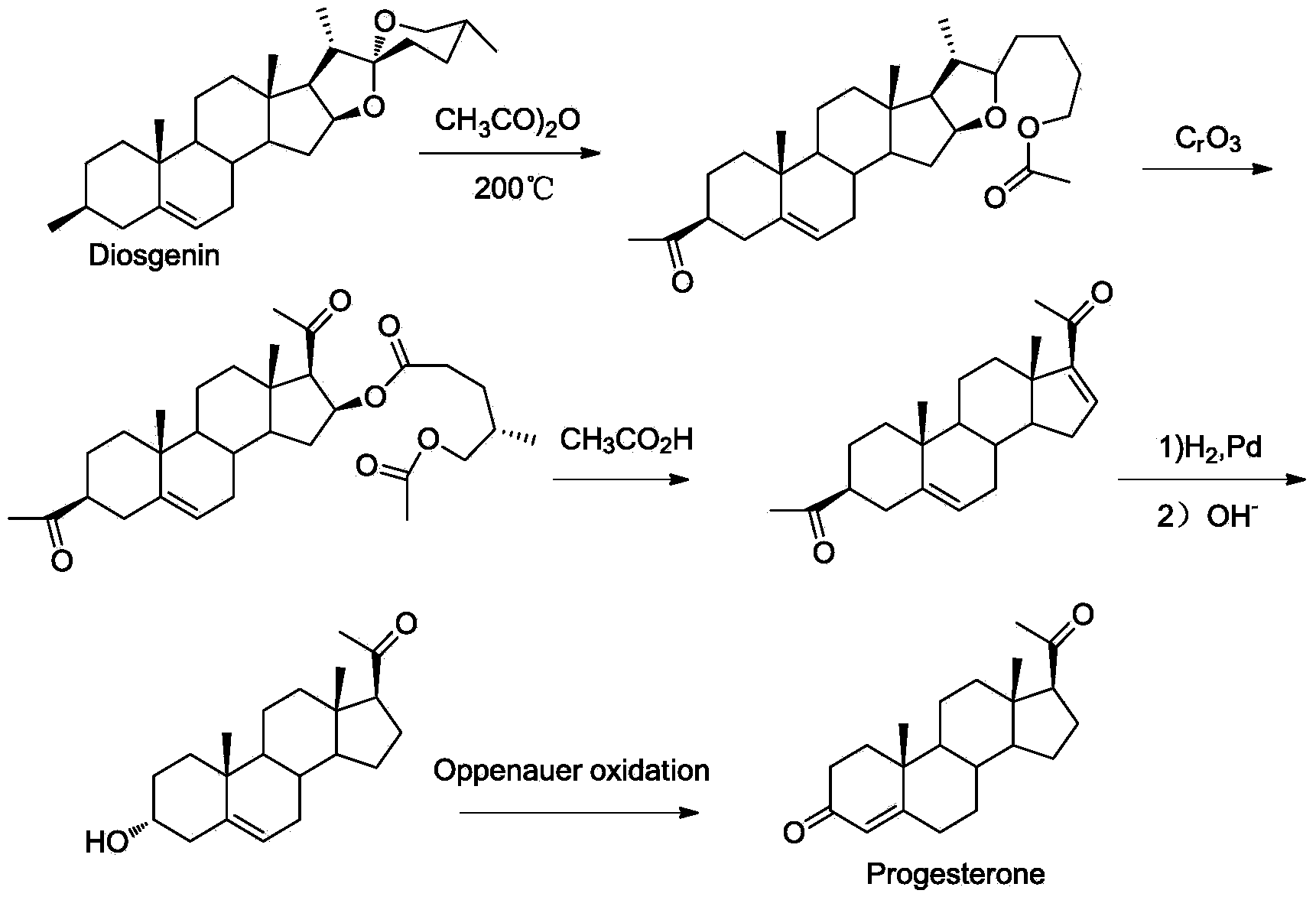
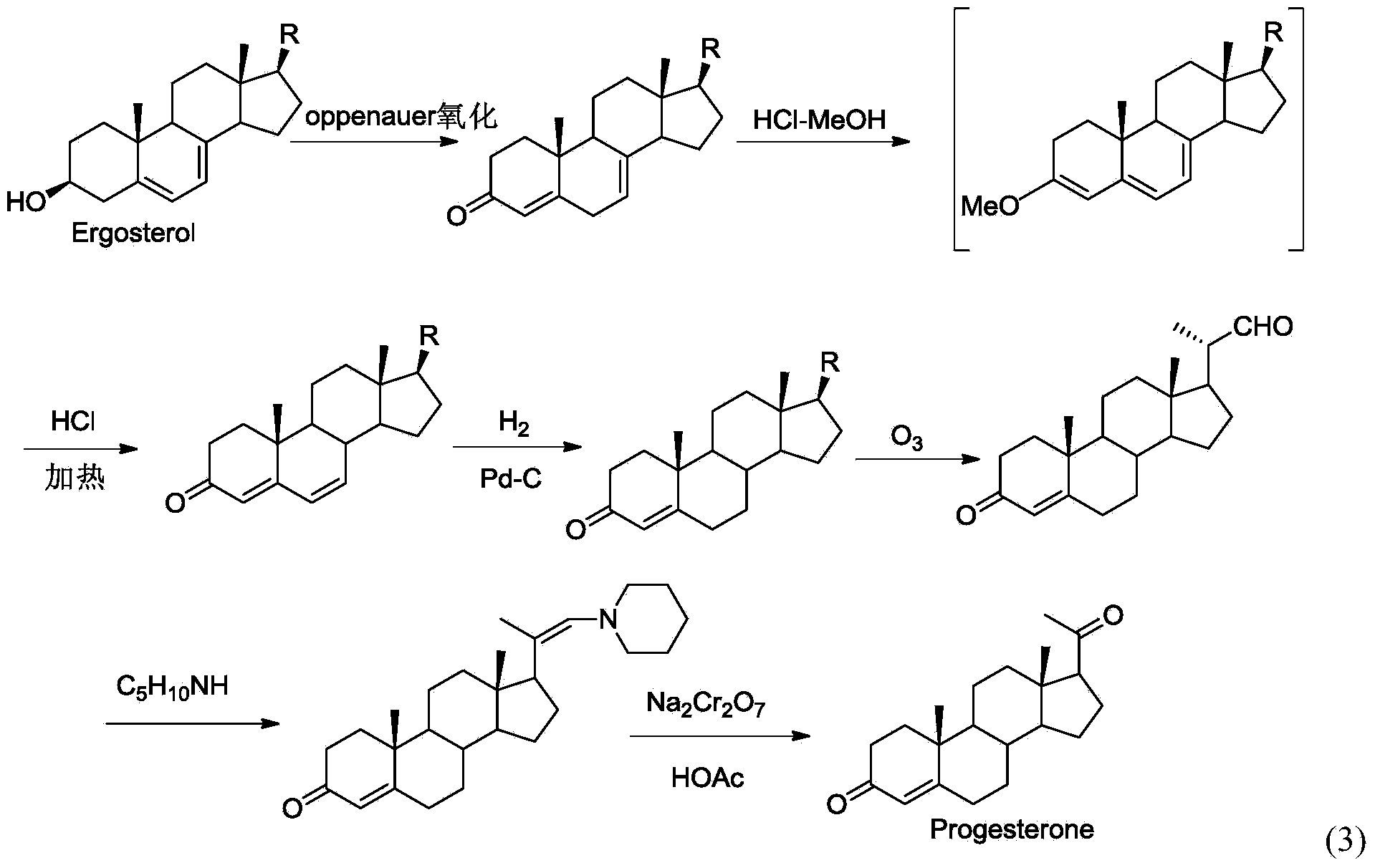
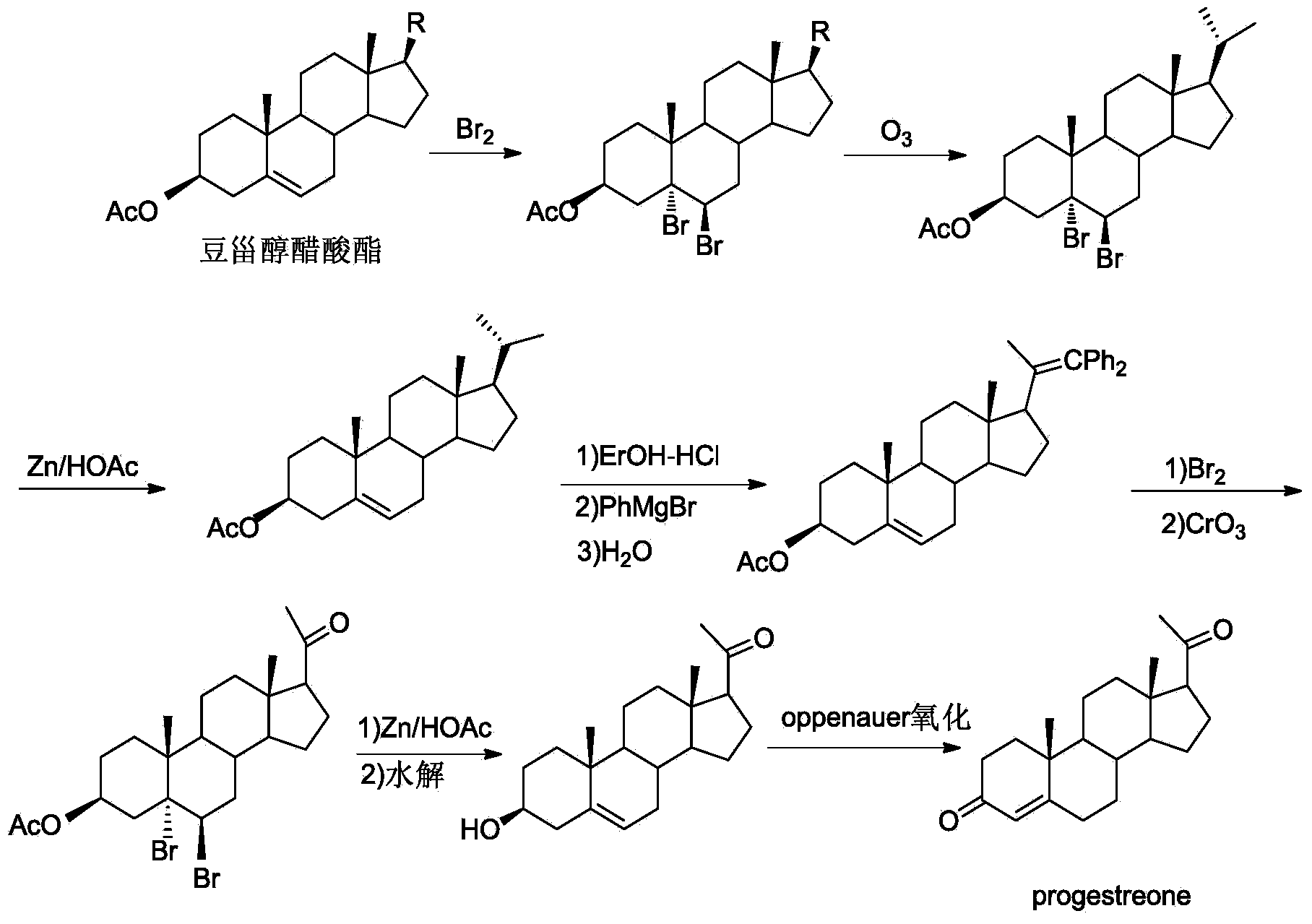
New technique for synthesizing progesterone
InactiveCN104109183ARaw materials are easy to getEasy to operateSteroidsN dimethylformamideN-Chlorosuccinimide
The invention discloses a new technique for synthesizing progesterone, which comprises the following steps: by using a phytosterin fermentation product (20S)-20-hydroxymethylpregna-4-ene-3-one as an initial raw material, oxidizing with a dimethyl sulfide / N-chlorosuccinimide mixture, and carrying out oxidative decarboxylation reaction in a DMF (N,N-dimethylformamide) solution of DBU (1,8-diazabicyclo(5.4.0)undec-7-ene), Cu(OAc)2.H2O and 2,2'-dipyridine to obtain the progesterone. The technique has the advantages of simple process, high yield (up to 80%), favorable product quality, accessible raw materials and low preparation cost.
Owner:HUBEI GEDIAN HUMANWELL PHARMACEUTICAL CO LTD
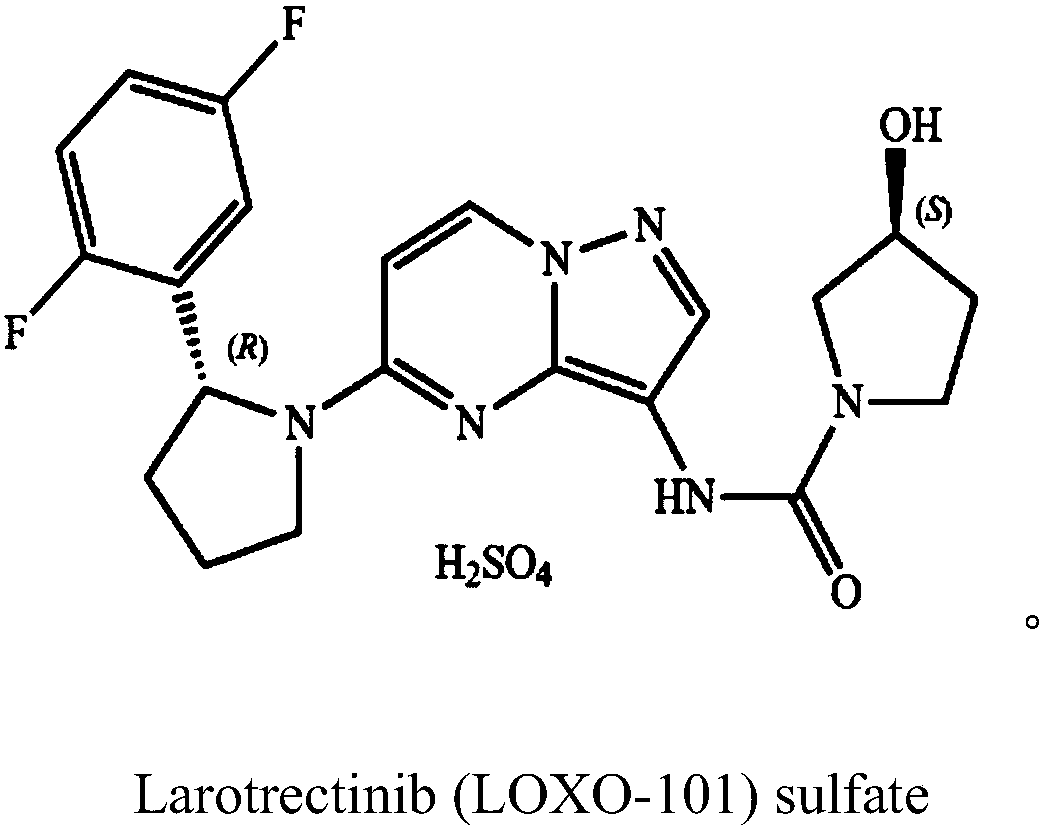
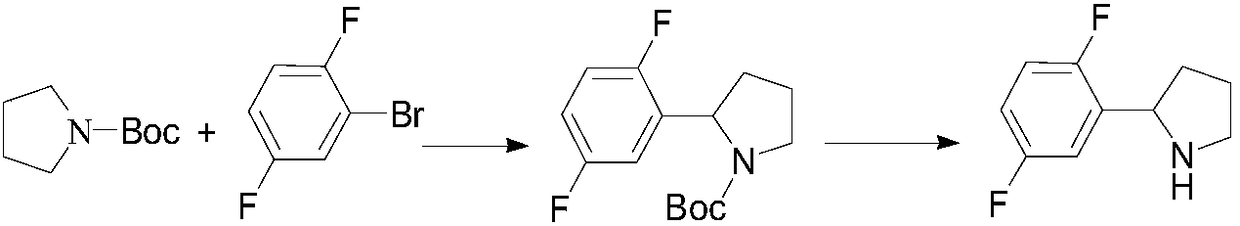
Preparation method of 2-(2,5-difluorophenyl) pyrrolidine
The invention provides a preparation method of 2-(2,5-difluorophenyl) pyrrolidine. The preparation method comprises the following steps: (1) tetrahydropyrrole reacts with N-chlorosuccinimide to generate 1-chloropyrrolidine; (2) the 1-chloropyrrolidine generated in the step (1) carries out elimination reaction in sodium methylate solution to generate 3,4-dihydro-2H-pyrrole; (3) the 3,4-dihydro-2H-pyrrole generated in the step (2) reacts with a 2,5-difluorobromobenzene Grignard reagent to obtain the 2-(2,5-difluorophenyl) pyrrolidine. The preparation method of the 2-(2,5-difluorophenyl) pyrrolidine provided by the invention has the beneficial effects that the synthetic process is reasonably designed, the reagent is cheap, the purchase is convenient, no heavy metal pollution is caused, the reaction conditions are mild, the cost of raw materials is low, no special equipment is needed, the operation is simple and convenient, and the energy consumption is low, so that the preparation methodis sutiable for industrial production.
Owner:GUANGDONG SCI FINDER PHARMA TECH CO LTD
Preparation method of 2-pyrrolidinone compound
ActiveCN108409625AImprove efficiencyAvoid Metal ResidueOrganic chemistryOrganic solventTrimethylsilyl
The invention relates to a preparation method of a 2-pyrrolidinone compound. According to the method, a 1, 6-enyne compound serves as a raw material, iodosobenzene diacetate serves as an oxidizing agent and reacts with trimethyl silicon-based azide and N-chlorosuccinimide in organic solvents, and the 2-pyrrolidinone compound is conveniently prepared by excellent yield.
Owner:NINGBO UNIV
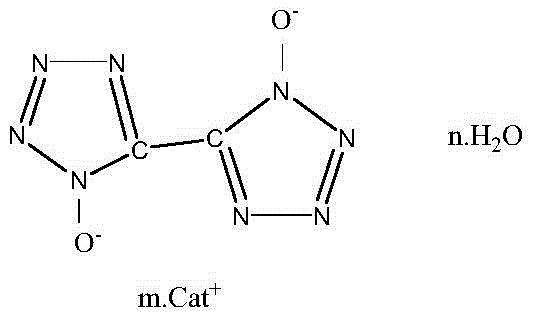
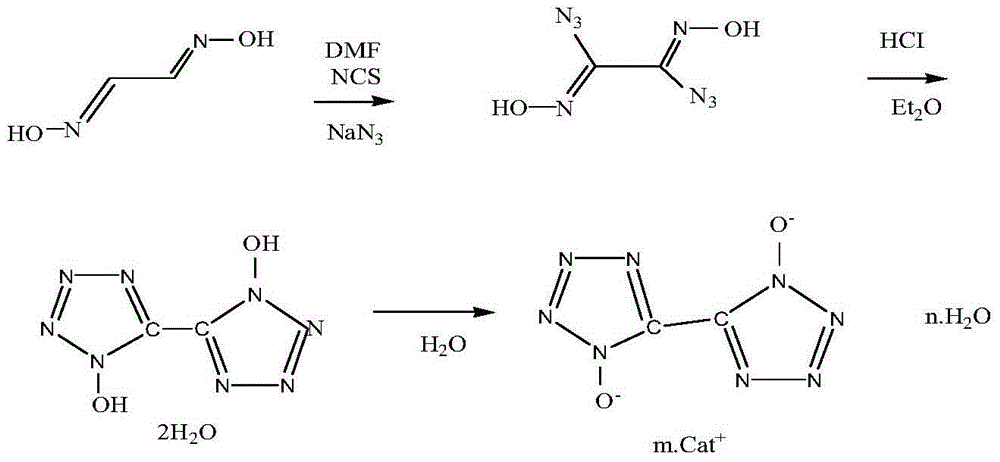
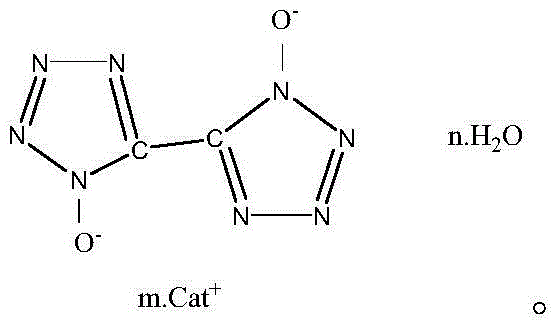
5,5'-bistetrazole-1,1'-dioxide metal salt and synthesis method thereof
InactiveCN104829549AAvoid risk factorsShort synthetic routeOrganic chemistryPressure gas generationSynthesis methodsN-Chlorosuccinimide
The present invention relates to a 5,5'-bistetrazole-1,1'-dioxide metal salt and a synthesis method thereof, and belongs to the high-energy nitrogen-rich compound synthesis in the energy-containing material field. According to the present invention, glyoxime is adopted as a raw material, N-chlorosuccinimide chlorination, sodium azide azidization and ring formation are performed to obtain a skeleton 5,5'-bistetrazole-1,1'-dihydroxy dehydrate, and the 5,5'-bistetrazole-1,1'-dihydroxy dehydrate reacts with a water-soluble metal ion compound in a water medium to obtain the different types of the 5,5'-bistetrazole-1,1'-dioxide metal salts; and the method has characteristics of less synthesis steps, simple process, and high product yield.
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
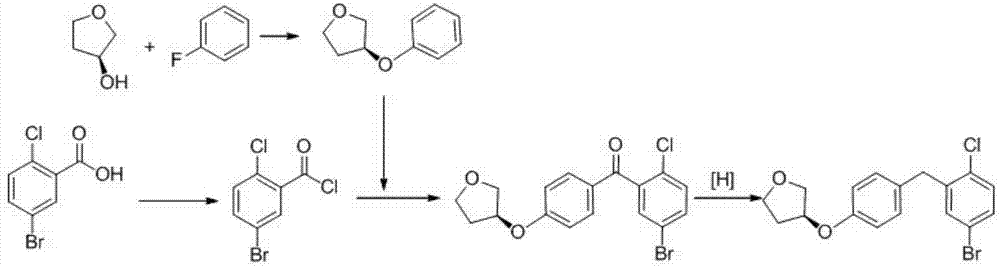
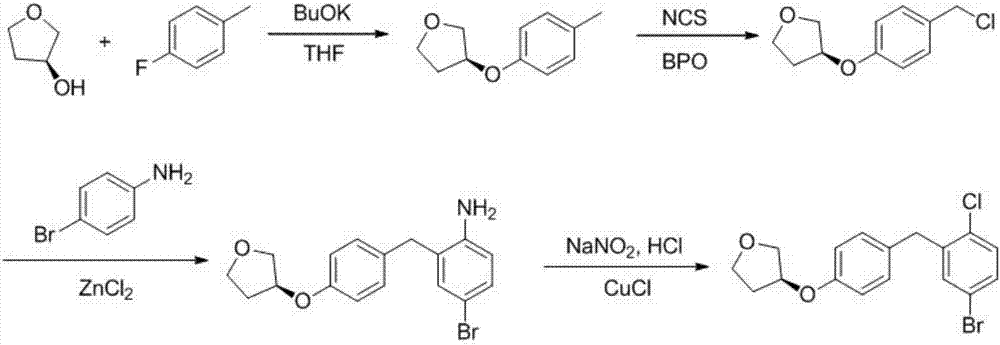
Preparation method of empagliflozin intermediate
The invention provides a preparation method of an empagliflozin intermediate. The preparation method comprises the following steps: 1), taking 4-fluorotoluene and (R)-3-hydroxytetrahydrofuran as raw materials, a polar solvent as a reaction solvent and inorganic alkali as a catalyst, performing a reaction to obtain (S)-3-p-cresyl tetrahydrofuran; 2), with N-chlorosuccinimide and the product obtained in the step 1) as raw materials, a non-polar solvent as a reaction solvent and dibenzoyl peroxide or azobisisobutyronitrile as an initiator, performing a reaction to obtain (S)-3- p-chlorophenol tetrahydrofuran; 3) dissolving 4-bromaniline and the product obtained in the step 2) into ethyl acetate, adding a catalyst Lewis acid, performing a reaction to obtain (S)-3-(4-(5-bromo-2-aminobenzyl)phenoxy) tetrahydrofuran; 4), performing a diazotization reaction on the product obtained in the step 3), and then reacting with cuprous chloride to synthesize (S)-3-(4-(5-bromo-2-chlorobenzyl)phenoxy) tetrahydrofuran. The preparation method has the advantages that the cost is low, the finished product is high in purity and the synthesis route is short.
Owner:安徽省诚联医药科技有限公司
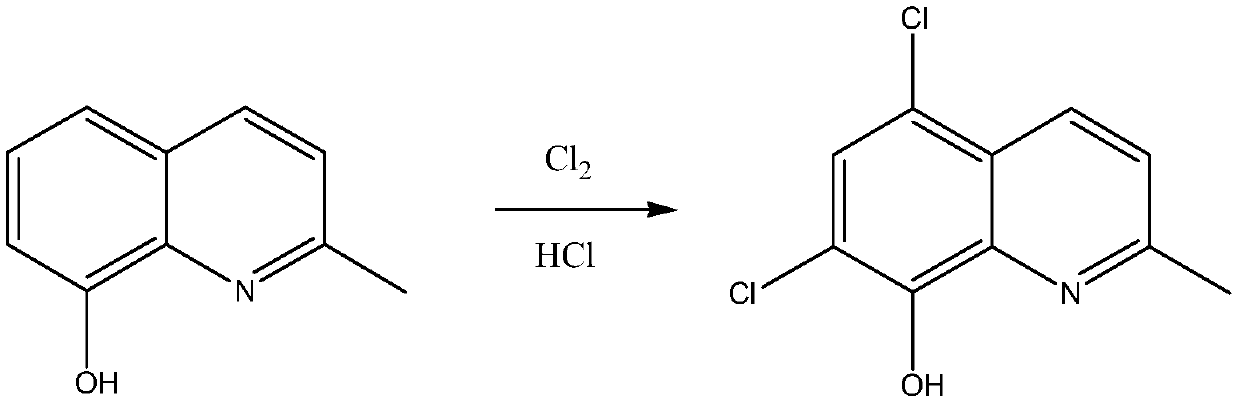
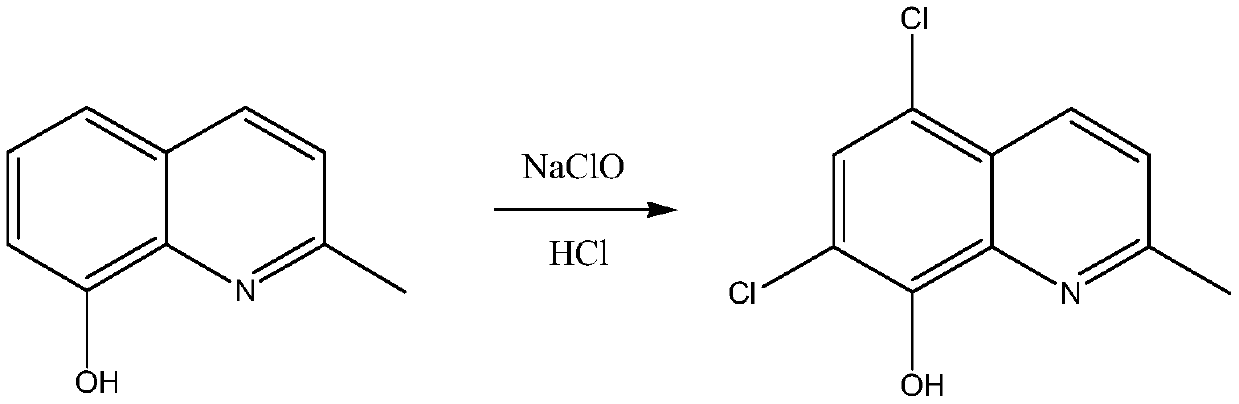
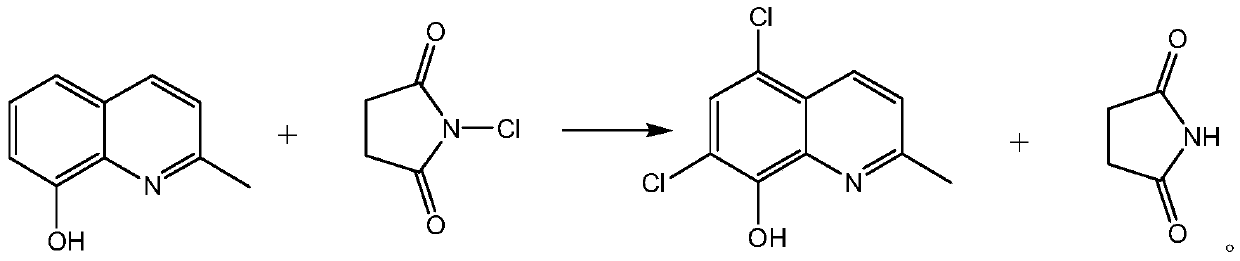
Synthetic technology of chlorquinaldol
ActiveCN110143919AReduce pollutionQuality assuranceOrganic chemistryEnvironmental resistanceN-Chlorosuccinimide
The invention belongs to the field of pharmaceutical synthesis technology and specifically relates to a synthetic technology of chlorquinaldol. By using Lewis acid as a catalyst, 8-hydroxy-2-methylquinoline and N-Chlorosuccinimide, which are used as raw materials, are subjected to a one-step chlorination reaction to generate chlorquinaldol; and after the reaction, chlorquinaldol is refined to obtain the chlorquinaldol. By using N-Chlorosuccinimide to replace chlorine as the raw material of the chlorination reaction, the selectivity is good, side reaction is reduced, conversion rate and yield are increased, yield reaches 98.2% and above, purity is 99.90% and above, quality of the chlorquinaldol is guaranteed, generation of spend liquor is decreased, environmental pollution is reduced, the cost is saved, water dissolution of the reaction product is avoided, and the yield is increased. The technology of the invention is a green and environmentally-friendly technology, and is suitable forindustrial production.
Owner:BEIJING JINCHENG TAIER PHARMA CO LTD
Reagent preparation method
InactiveCN103554175AWon't produceHigh purityGroup 5/15 element organic compoundsLiquid wastePhosphorous acid
The invention discloses a reagent preparation method, and belongs to the field of organic synthesis. The reagent preparation method comprises the following steps: adding a preset amount of N-chlorosuccinimide to a reaction flask; replacing air in the reaction flask with inert gas, and cooling the reaction flask to a preset temperature; dropwise adding phosphorous acid diester to the reaction flask; when ending the reaction, diluting by using a preset amount of anhydrous solvent, and filtering an diluted product; and concentrating the product, thus obtaining diester chlorophosphate. The reagent preparation method has the advantages that no solvent is used in an ester chlorophosphate reaction process, the reaction can be completely carried out, the reaction time is short, and liquid waste is not generated; under normal circumstances, the obtained product has enough high purity and meets the requirements of common organic synthesis, and if the purity is unsatisfactory, the product can be further distilled.
Owner:NORTHWEST A & F UNIV
Synthetic method of 1,3-dithiane structure-containing polysubstituted olefin derivatives
The invention relates to a synthetic method of 1,3-dithiane structure-containing polysubstituted olefin derivatives. The method comprises the following steps: carrying out an oxyradical coupling reaction on 1,3-dithiane and polysubstituted olefins in a solvent in the presence of a catalyst Lewis acid and an activator N-chlorosuccimide with air or oxygen as an oxidant, separating, and purifying to obtain the 1,3-dithiane structure-containing polysubstituted olefin derivatives. The method has the advantages of avoiding of use of a metal catalyst due to cheap and easily available substrates used in the invention, environmental protection, simple reaction process and mild conditions, the 1,3-dithiane structure-containing polysubstituted olefin derivatives have good function group tolerance and can be directly obtained through a one-kettle process, the method has beneficial technical effects, and can be well applied in scientific researches and industrial production.
Owner:LANZHOU UNIVERSITY
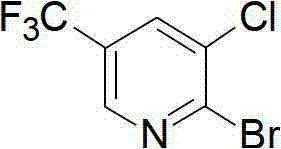
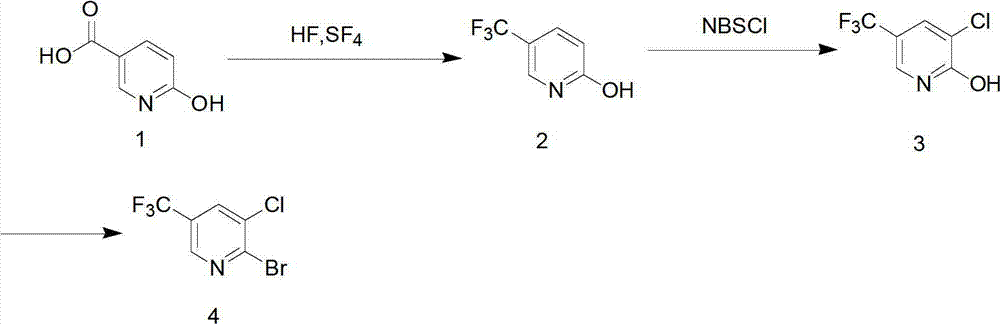
Preparation method for pyridine medical intermediate for synthesizing anti-cancer auxiliary medicines
The invention relates to the field of medical chemistry and discloses a method for synthesizing a pyridine medical intermediate, namely 2-bromo-3-chloro-5-trifluoromethyl pyridine, for synthesizing anti-cancer auxiliary medicines. The method comprises the following steps of: (1) reacting 6-hydroxynicotinic acid, hydrofluoric acid and sulfur tetrafluoride at the temperature of between 100 and 120DEG C and under the pressure of 0.1-0.3MPa, and adding water to obtain 2-hydroxy-5-trifluoromethyl pyridine; (2) reacting with N-chlorosuccinimide, and performing water precipitation to obtain 3-chloro-5-trifluoromethyl-2-hydroxypyridine; and (3) adding excessive phosphorus oxybromide, reacting at the temperature of between 145 and 160DEG C for 5 to 8 hours, cooling, violently stirring at the temperature of between -5 and 0DEG C, extracting, combining organic phases, drying, filtering, performing spin drying, and purifying by using a silica gel column. According to the method, raw materials are readily available, the cost is low, the method is suitable for industrial production and the yield exceeds 38 percent.
Owner:上海泰坦科技股份有限公司
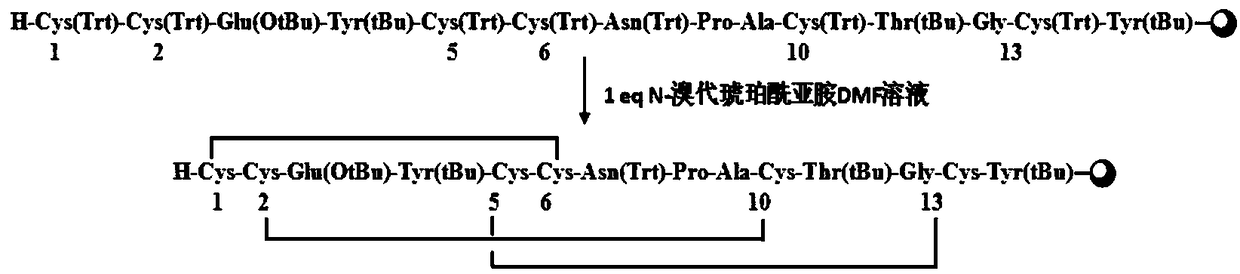
A synthetic method for linaclotide
ActiveCN109311941AAvoid repeated foldingAvoid it happening againPeptide preparation methodsBulk chemical productionN-BromosuccinimideAmino acid side chain
The invention relates to the field of pharmaceutical synthesis, and discloses a synthetic method for linaclotide. The method uses a solid phase one-step cyclization method to prepare linaclotide, andthe linaclotide linear peptide resin is directly cyclized by a N-X-substituted succinimide solution oxidation system without cleavage to obtain linaclotide resin, the resin is cleaved, purified and lyophilized to give linaclotide. The N-X-substitured succinimide is one of N-chlorosuccinimide, N-bromosuccinimide, N-iodosuccinimide, and N-hydroxy thiosuccinimide. The method has the following advantages that: 1) solid phase cyclization is adopted, firstly, the pseudo-dilution effect is achieved, repeated folding of the peptide chain is avoided, and the cyclization reaction can be carried out at ahigher concentration, which can greatly improve the production efficiency; secondly, the linear peptide resin is not cleaved before cyclization, avoiding the production of a large amount of impurities and improving the efficiency of linaclotide cyclization; 2) one-step cyclization using N-X-substituted succinimide can avoid multi-step purification of the intermediates, reduce the composition of the intermediate purification step, and improve the total yield of linaclotide; and 3) a specific amino acid side chain protecting group is adopted, thus positioning a pair of disulfide bonds in the cyclization process, reducing the formation of mismatch by-products, improving the purity of linaclotide, greatly improving production efficiency, and reducing the manufacturing cost.
Owner:SHENZHEN JYMED TECH
Synthesis of Important intermediate for mosapride citrate
InactiveCN1226295CHigh synthetic yieldImprove composite qualityOrganic compound preparationCarboxylic acid amides preparationAcetic anhydrideN-Chlorosuccinimide
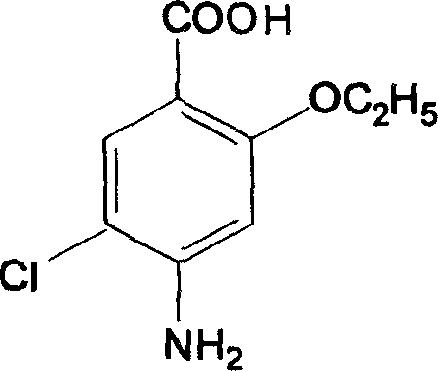
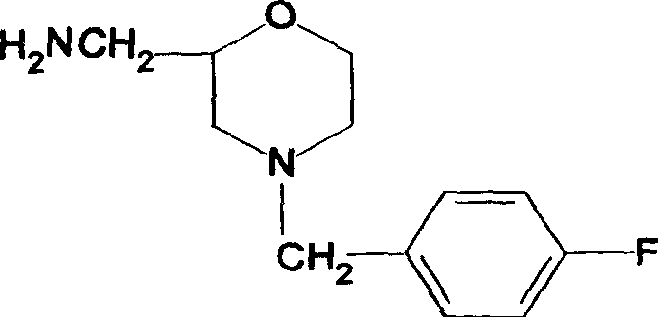
The present invention relates to the preparation process of important intermediates for Mosapride citrate, 2-oxethyl-4-actamino-5-chlorobenzoic acid and 4-(4-fluorobenzyl-2- aminomethyl morpholine. The intermediate 2-oxethyl-4-actamino-5-chlorobenzoic acid is prepared with amino salicylic acid as initial material and through acidification with hydrochloric acid, esterification with methol, acetylation with acetic anhydride, ethylation, NCS chlorination and alkali hydrolysis. The intermediate 4-(4-fluorobenzyl-2- aminomethyl morpholine is prepared with p-fluorobenzaldehyde and phthalimide as initial material and through dropping at 70-90 deg.c, maintaining at 125-145 deg.c, and post-treatment in ice bath in 2-10 deg.c while adding acetic anhydride through stirring for 10-20hr. The present invention can raise the yield of Mosapride citrate and lower its production cost.
Owner:LUNAN BETTER PHARMA
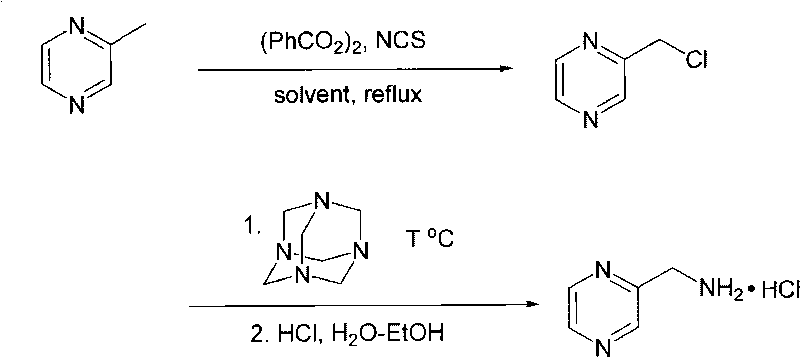
Preparation method of pharmaceutical intermediate 2-amine methylpyrazine hydrochloride
InactiveCN101698664ARaw materials are cheap and easy to getLow priceOrganic chemistryN-ChlorosuccinimideCarbon Chloride
The invention discloses a preparation method of pharmaceutical intermediate 2-amine methylpyrazine hydrochloride, comprising the following steps: (1) under the protection of nitrogen, 2-methylpyrazine, dibenzoyl peroxide and N-chlorosuccinimide at the molar ratio of 1:0.01-0.1:1-1.3 are added into anhydrous carbon tetrachloride to prepare alpha-chlorine methylpyrazine by reflux reaction; (2) after prepared alpha-chlorine methylpyrazine is mixed with potassium iodide and anhydrous methylbenzene, the mixture is cooled to be below 15 DEG C; then, hexamine is added under stirring, temperature is controlled below 28 DEG C, reaction is carried out when temperature is 0-100 DEG C after adding for 1-20 hours to obtain 2-chlorine methylpyrazine hexamine complex salt; superfluous concentrated hydrochloric acid and solvent alcohol are added into the reaction mixture to react, cool, filter, wash and dry to obtain the coarse product of the 2-amine methylpyrazine hydrochloride; and (3) recrystallization is carried out on the above coarse product in methanol-chloroform to obtain the pharmaceutical intermediate 2-amine methylpyrazine hydrochloride of the invention. The invention has cheap and abundant raw material resource, high overall yield and simple after-treatment and is suitable for industrial production.
Owner:ZAOZHUANG UNIV
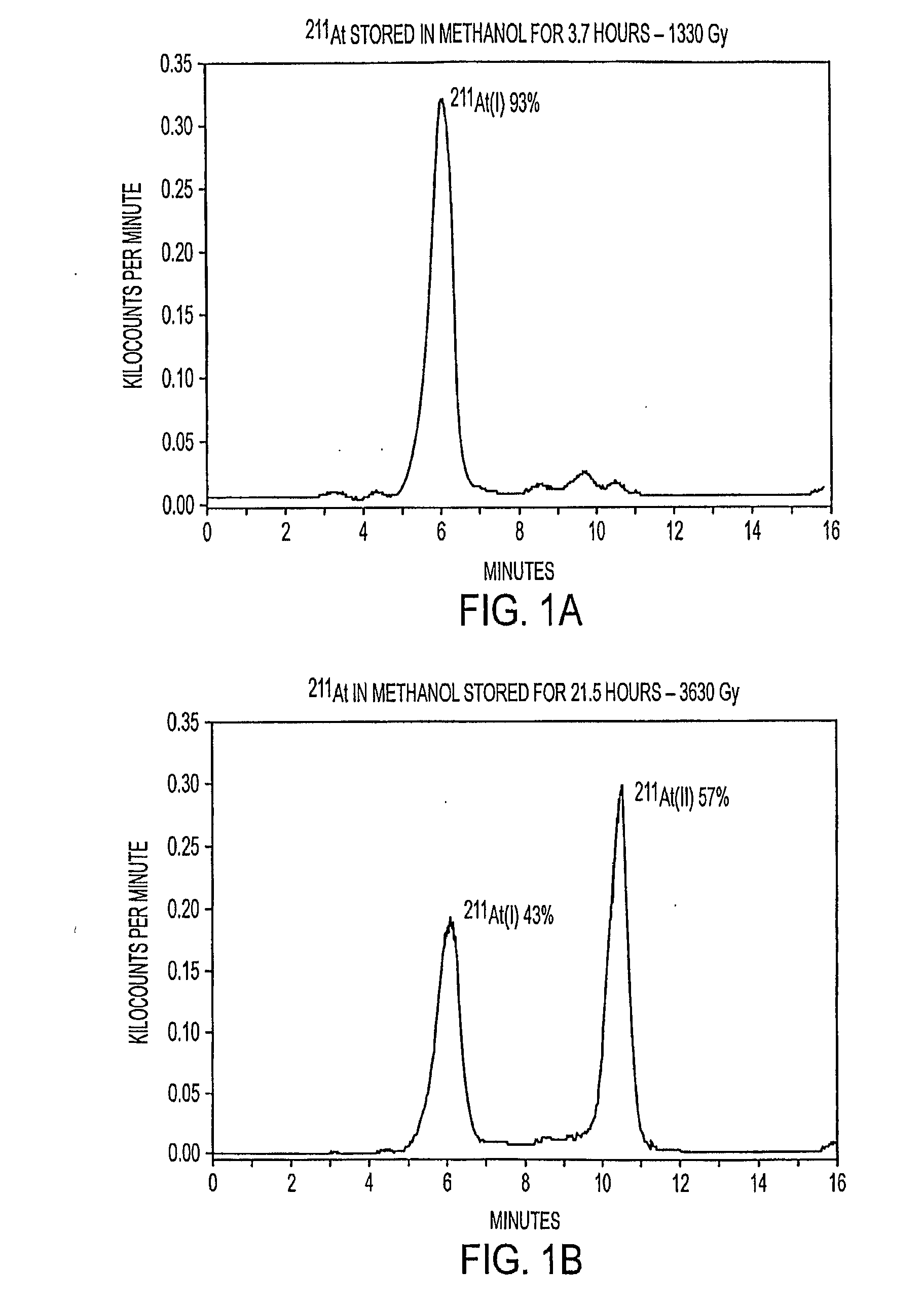
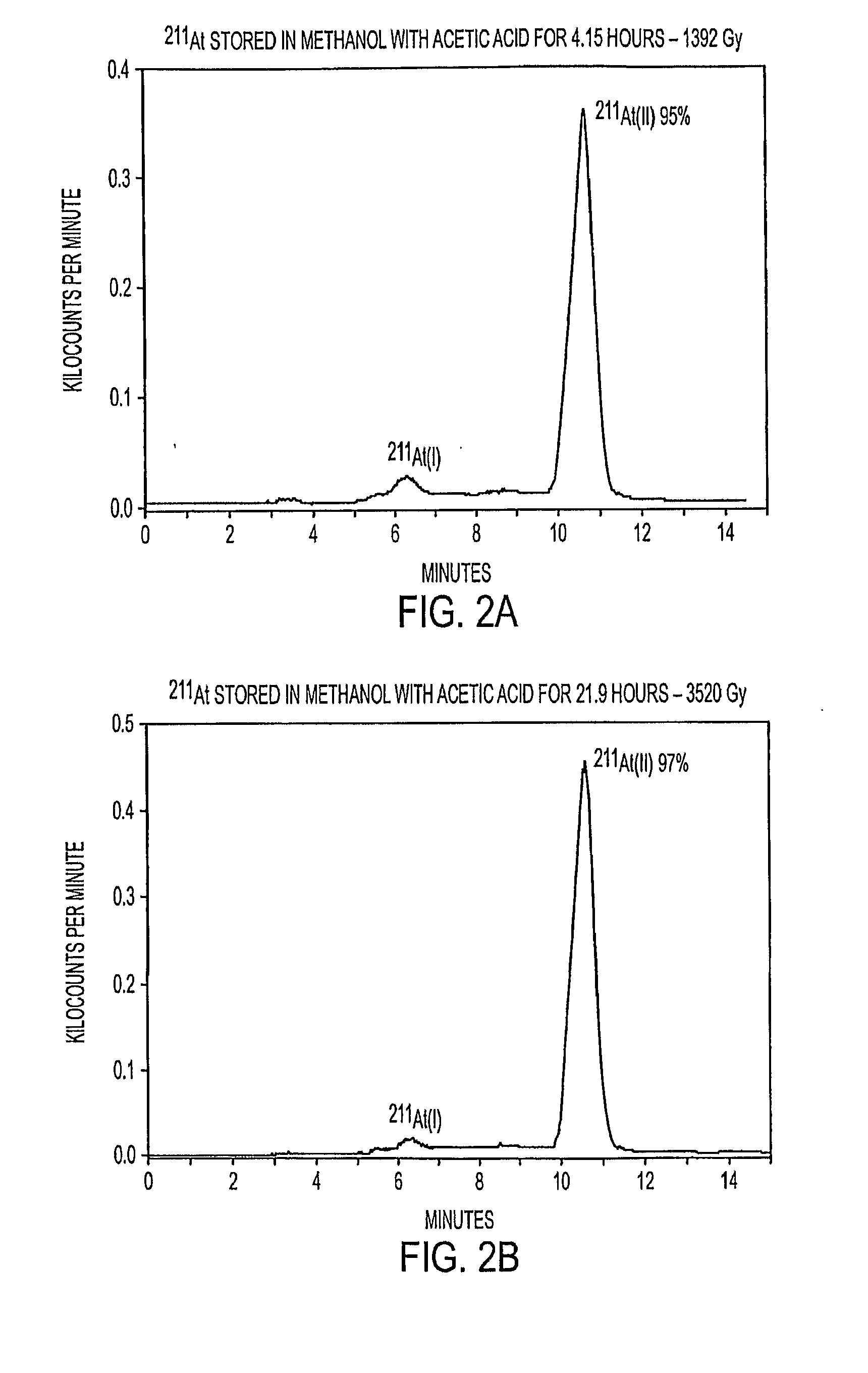
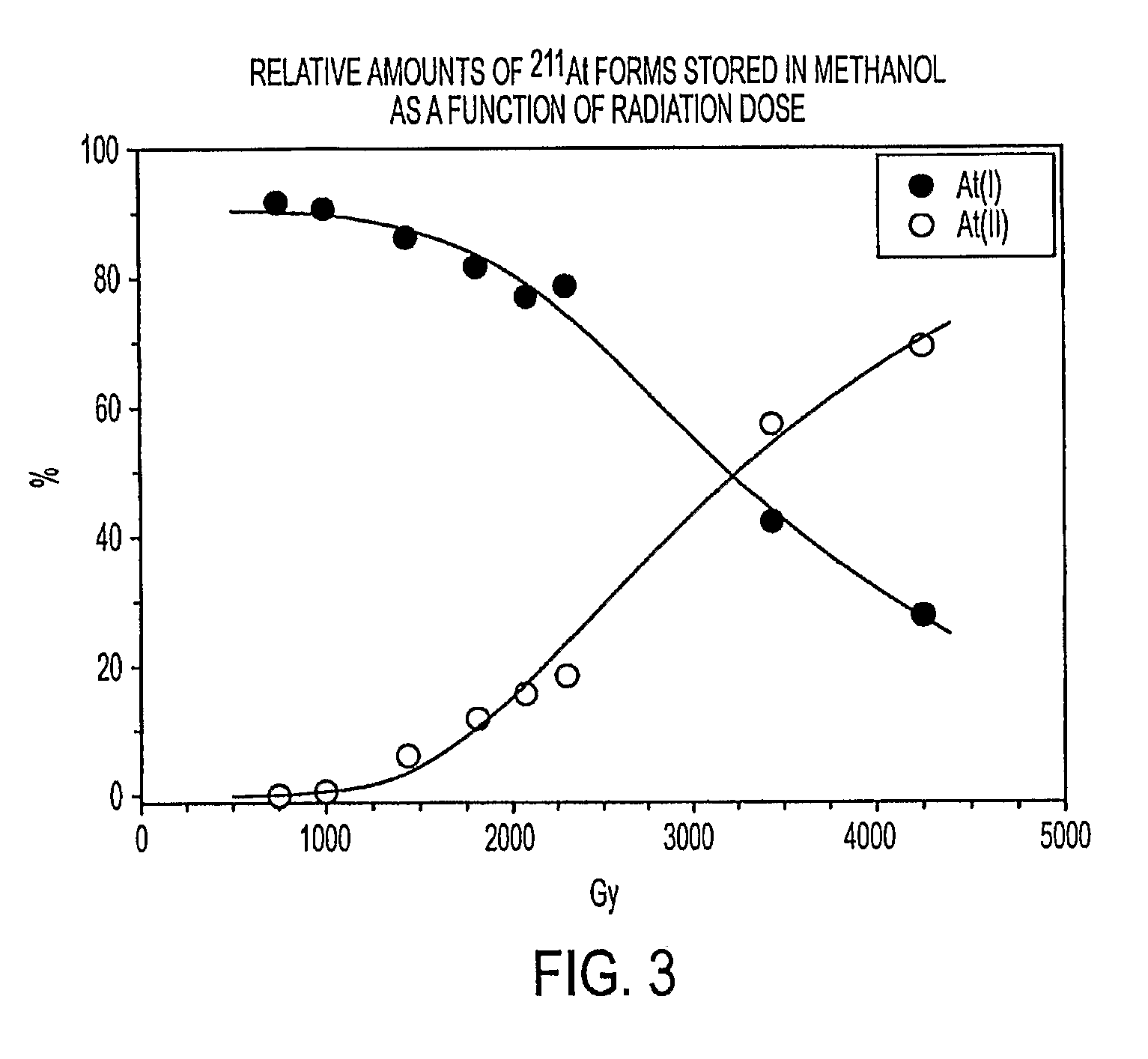
Stabilized Compositions and Methods for Radiolabeling Pharmaceuticals with Alpha-Particle Emitters
ActiveUS20090304585A1Improve radiation effectLoss of reactionOrganic compounds purification/separation/stabilisationRadioactive preparation carriersN-ChlorosuccinimideMetallic bonding
Oxidants (e.g., N-chlorosuccinimide) can be used to stabilize α-particle emitters (e.g., 211At) in solution, prior to their subsequent reaction to form α-particle emitter labeled compounds (e.g., a radiolabeled pharmaceutical or a radiolabeled pre-cursor used to prepare it). In particular, the use of an oxidant has been found to maintain the α-particle emitter in a chemical form that facilitates this reaction, which may involve a number of possible mechanisms including electrophilic substitution, nucleophilic substitution, complexation, exchange, or metallic bonding. Compounds labeled with α-particle emitters in this manner have wide-ranging therapeutic applications, particularly in the treatment of cancer.
Owner:DUKE UNIV
One-step synthesis method of 6-chlorine-3H-oxazole [4,5-b] pyridine-2-ketone
ActiveCN103709175ARaw materials are cheap and easy to getSimple and fast operationOrganic chemistryN-ChlorosuccinimideSynthesis methods
The invention belongs to the field of organic synthesis, and particularly relates to a one-step synthesis method of 6-chlorine-3H-oxazole [4,5-b] pyridine-2-ketone. The one-step synthesis method of 6-chlorine-3H-oxazole [4,5-b] pyridine-2-ketone comprises the step as follows: oxazole [4,5-b] pyridine-2(3H)-ketone reacts with NCS(N-chlorosuccinimide) in a proper solvent at proper temperature, so as to generate 6-chlorine oxazole [4,5-b] pyridine-2(3H)-ketone. The 6-chlorine-3H-oxazole [4,5-b] pyridine-2-ketone is prepared by adopting a one-step method by reaction disclosed by the invention. The 6-chlorine-3H-oxazole [4,5-b] pyridine-2-ketone is cheap and available in raw materials, the method is simple and convenient to operate, mild in reaction condition, low in production cost, and easy in achieving industrial amplification, and the yield can be up to 89%.
Owner:陕西友帮生物医药科技有限公司
Preparation method of pregabalin
ActiveCN103922950AReduce usageHigh yieldOrganic compound preparationAmino-carboxyl compound preparationN-ChlorosuccinimidePregabalin
The invention discloses a preparation method of pregabalin. According to the preparation method, (R)-(-)-3-(carbamyl methyl)-5-methylhexanol has a Hofmann degradation reaction under the action of N-chlorosuccinimide in the presence of an alkali to produce pregabalin. By adopting N-chlorosuccinimide as a reagent of Hofmann degradation reaction, the use of bromine is avoided, the reaction yield is improved and the content of impurities in the product is reduced, so that the reaction is suitable for industrial production.
Owner:ZHEJIANG MENOVO PHARMA
Synthetic method of ethyl 3-aldehyde-6-chloroimidazo[1,2-a]pyridine-8-formate
InactiveCN104402882AReaction raw materials are readily availableReasonable priceOrganic chemistryN dimethylformamideN-Chlorosuccinimide
The invention relates to a synthetic method of ethyl 3-aldehyde-6-chloroimidazo[1,2-a]pyridine-8-formate. The method comprises the following steps: carrying out a substitution reaction on ethyl 2-aminonicotinate and N-chlorosuccinimide in a certain solvent at normal temperature to prepare ethyl 2-amino-5-chloronicotinate; and reacting2-amino-5-chloronicotinate with N,N-dimethylformamide dimethyl acetal to prepare an intermediate, reacting the intermediate with chloroacetaldehyde in a certain solvent at 0-100DEG C without purifying the intermediate, cooling, and drying to obtain ethyl 3-aldehyde-6-chloroimidazo[1,2-a]pyridine-8-formate. The method has the advantages of easily available reaction raw materials, reasonable price, mild reaction conditions, easy operation, easy control and simple post-treatment, and the above obtained product has the advantages of stable quality and high purity.
Owner:SHANDONG YOUBANG BIOCHEM TECH
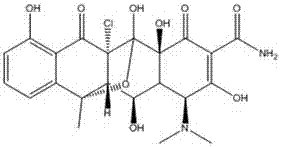
Preparation method of chlorinated terramycin
The invention discloses a preparation method of chlorinated terramycin, comprising the following steps: 1) adding methanol into a reactor with mechanical agitation, controlling temperature to 10-15 DEG C, adding terramycin into the reactor, stirring and cooling and adding N-chlorosuccinimide, fully stirring and reacting for 1h, and precipitating out crystals after the reaction; 2) injecting a mixture obtained in the step 1) into filtering equipment and filtering, washing with methanol, and discharging after 30 min; and 3) drying a product obtained in the step 2) through drying equipment. By the preparation method of chlorinated terramycin, use amount of the reactant N-chlorosuccinimide is smaller, toxic and side effect is low, and reaction yield is increased and can reach 95%. Therefore, less waste residue is produced, and the method has good practicality.
Owner:扬州联博药业有限公司
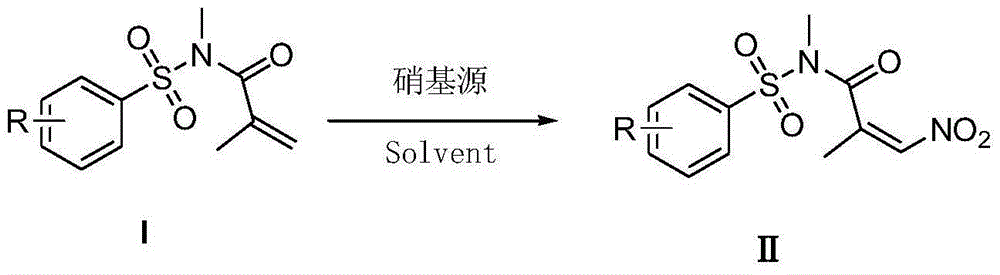
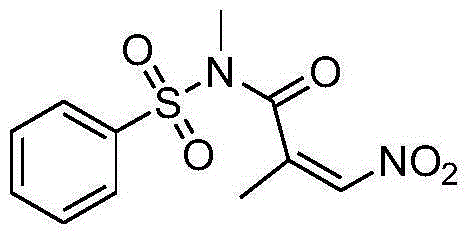
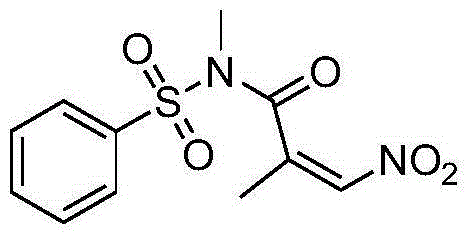
Nitro acrylamides compound synthetic method
ActiveCN106316894AReduce consumptionLow toxicitySulfonic acid amide preparationOrganic solventN-Chlorosuccinimide
The invention discloses a synthetic method of a nitro acrylamides compound shown in a formula II. The method comprises the following steps: an acrylamides compound shown in a formula I, N-chlorosuccinimide, and AgNO2 are added in an organic solvent, the materials are reacted for 5-20 hours at the temperature of 25-100 DEG C, and the nitro acrylamides compound shown in the formula II is obtained by separating and purifying an obtained reaction solution. A nitrogen source system has the advantages of low cost, easy acquisition, little toxicity, environment friendliness, mild reaction condition, good universality of the function group, and simple operation.
Owner:ZHEJIANG UNIV OF TECH
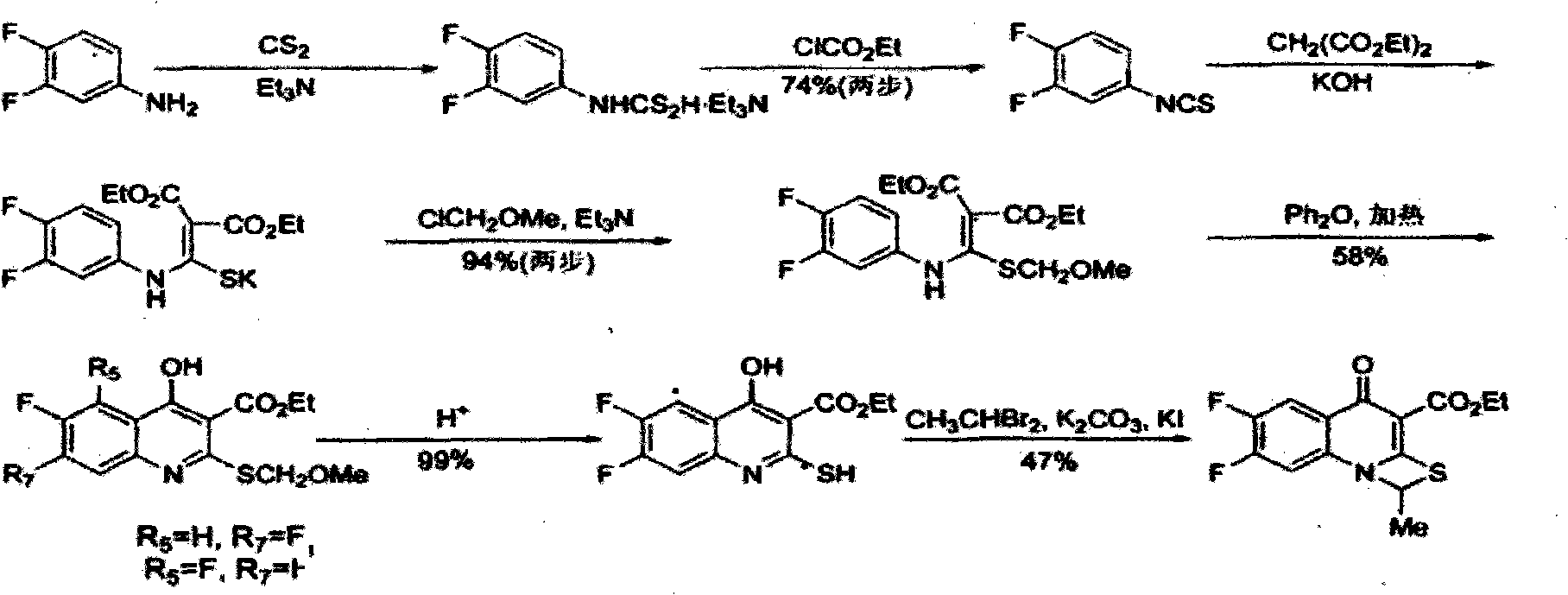
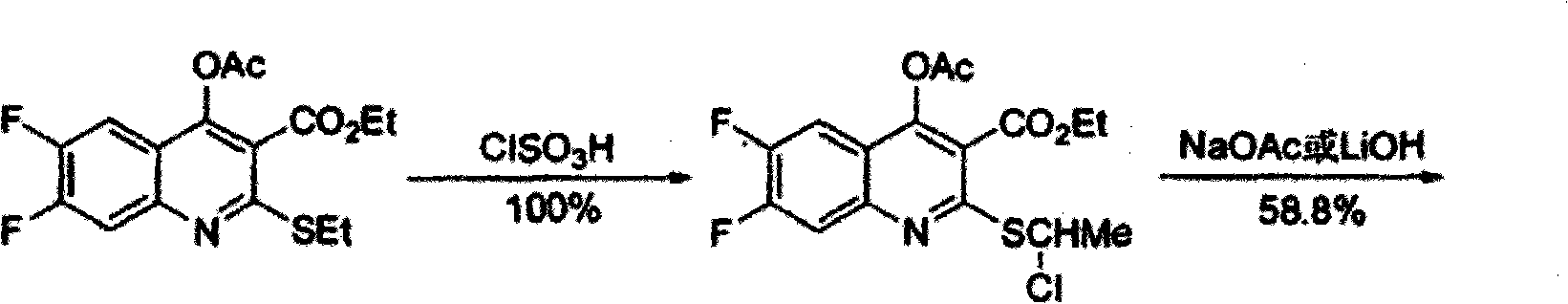
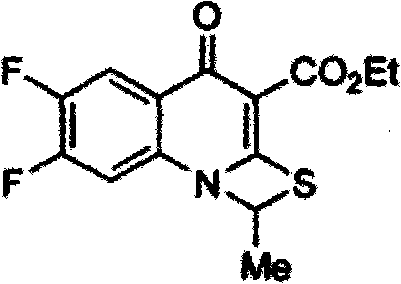
Preparation method for Prulifloxacin
Owner:湖南欧亚药业有限公司
Preparation method of (S,S)-2, 8-diazabicyclo[4,3,0] nonane
The invention relates to a preparation method of (S,S)-2, 8-diazabicyclo[4,3,0] nonane. The preparation method comprises the following steps: taking 2,3-dimethylpyridine as a raw material, performing halogenation on 2,3-dimethylphyridine through N-chloro succinimide to be in cyclization with benzylamine, performing hydrogenation on pyridine ring and removing benzyl groups, and finally performing chiral resolution to obtain the (S,S)-2, 8-diazabicyclo[4,3,0] nonane. According to the invention, the preparation method has advantages of short process flow, low cost and high yield, reaches total yield of 23% and ee value of above 99% and is safe and reliable, thereby being applicable in industrial production.
Owner:浙江凯迪药业有限公司
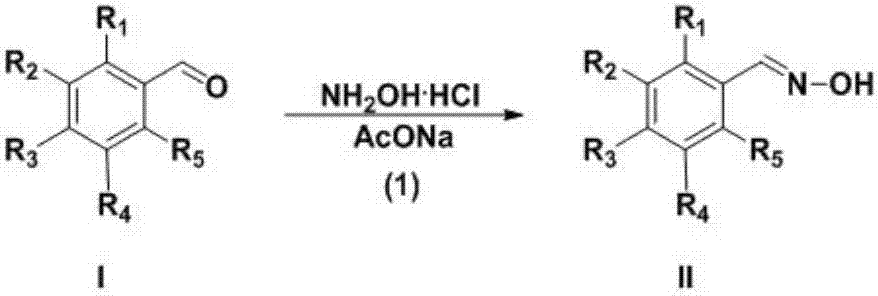
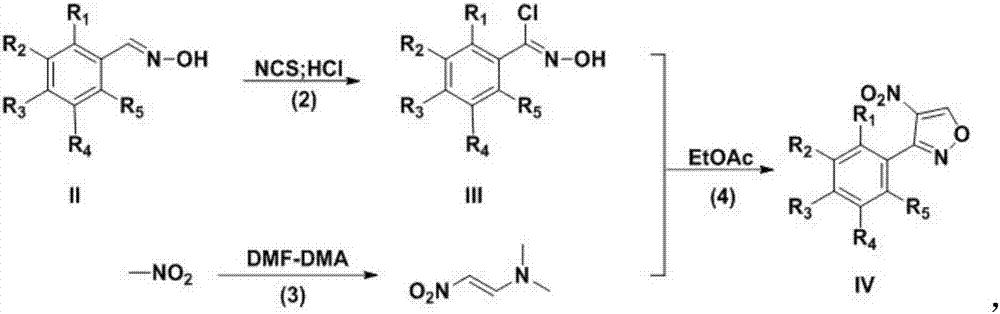
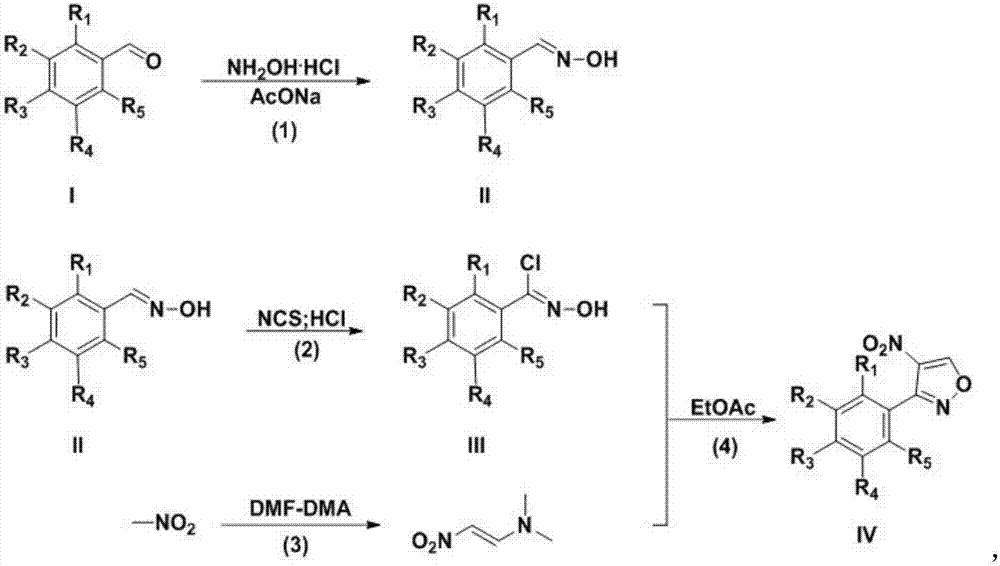
Preparation technology for 3-aryl-4-nitro isoxazole compound
InactiveCN107382892ARaw materials are cheap and easy to getEasy to purifyOrganic chemistryN dimethylformamideDimethyl acetal
The invention discloses a preparation technology for a 3-aryl-4-nitro isoxazole compound. The preparation technology comprises the following steps: synthesizing a compound shown as formula II through the nucleophilic addition of hydroxylamine hydrochloride and the compound shown as formula I used as the raw material; acquiring the compound shown as formula III through the substitution reaction of the compound shown as formula II and N-chlorosuccinimide; preparing 1-dimethyl amino-2-nitro ethylene through the reaction of N,N-dimethylformamide dimethyl acetal and nitromethane used as the raw material; and acquiring a target product 3-aryl-4-nitro isoxazole compound through the cyclization reaction of the compound shown as formula III and 1-dimethyl amino-2-nitro ethylene. The raw materials in the synthesis route are low in cost and easily acquired, the operation condition is mild and is easily controlled, the product is easily purified and the preparation technology is a new method for synthesizing the 3-aryl-4-nitro isoxazole compound.
Owner:GUIZHOU UNIV
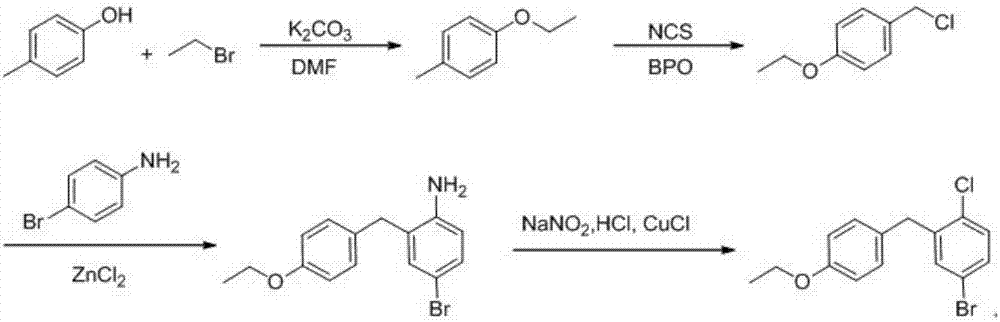
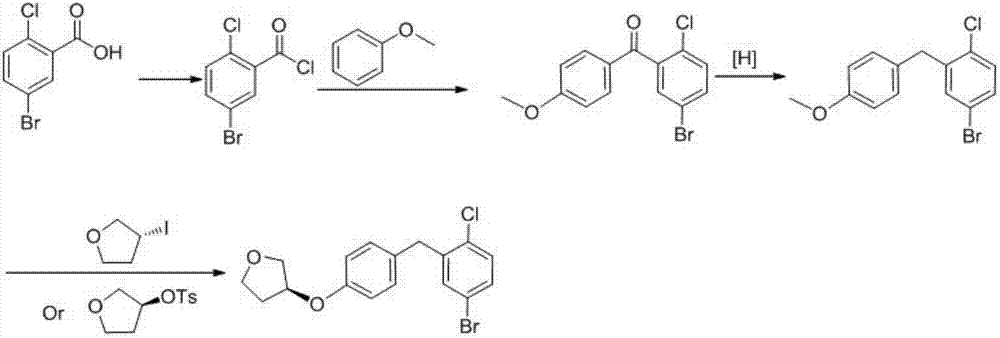
Preparation method of dapagliflozin intermediate
InactiveCN107382679AShort synthetic routeHigh purityOrganic chemistryOrganic compound preparationDiphenylmethaneN-Chlorosuccinimide
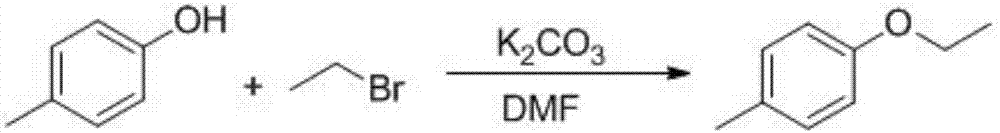
The invention provides a preparation method of a dapagliflozin intermediate. The preparation method comprises the following steps: (1) by taking 4-methylphenol and bromoethane as raw materials, a polar solvent as a reaction solvent and an inorganic base as a catalyst, carrying out reaction for preparing 4-ethyoxyl methylbenzene; (2) by taking N-chlorosuccinimide and 4-ethyoxyl methylbenzene obtained in the step (1) as raw materials, a non-polar solvent as a reaction solvent and dibenzoyl peroxide as an initiator, carrying out reaction, thus obtaining 4-ethyoxyl benzyl chloride; (3) dissolving 4-ethyoxyl benzyl chloride obtained in the step (2) and 4-bromaniline into ethyl acetate, adding a catalyst lewis acid, and carrying out reaction, thus obtaining 5-bromo-2-amino-4-ethyoxyl diphenylmethane; and (4) carrying out diazotization reaction on 5-bromo-2-amino-4-ethyoxyl diphenylmethane obtained in the step (3), and then reacting with cuprous chloride, thus synthesizing 5-bromo-2-chloro-4'-ethyoxyl diphenylmethane. The preparation method provided by the invention has the advantages of low cost, low environmental stress and short synthetic route.
Owner:安徽省诚联医药科技有限公司
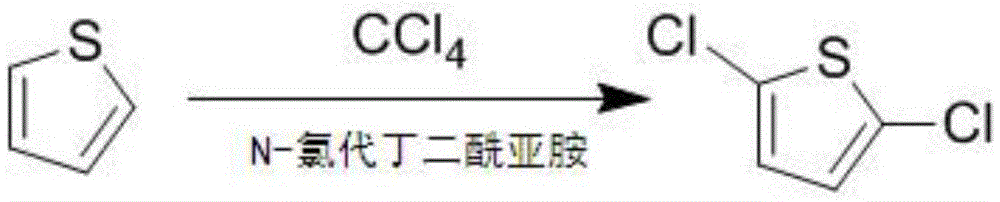
2, 5-dichloro thiophene preparation method
The present invention discloses a 2, 5-dichloro thiophene preparation method comprising the following steps: (1) an organic solvent and thiophene are added into a reaction vessel and warmed to reflux, under reflux conditions, batches of added N-chlorosuccinimide are added for refluxing for 3 to 5 hours to obtain a reaction solution; (2) the reaction solution of the step (1) is cooled to room temperature, impurities are filtered out, the organic solvent in the filtrate is concentrated and recovered to obtain the remaining reaction solution; (3) the remaining reaction solution of the step (2) is heated to 100 DEG C for vacuum rectification, a fraction of 100 DEG C is collected, namely a 2, 5-dichloro thiophene desired product is collected. The N-chlorosuccinimide and the thiophene are reacted in carbon tetrachloride, the reaction process does not produce gases polluting the environment, the reactants can be more fully reacted in the case of use of the carbon tetrachloride as a solvent, the product yield is higher, after the reaction, the carbon tetrachloride can easily be separated and recovered, and the product purity is higher.
Owner:CHONGQING TIANYI HENGHUA TECH CO LTD
Method for preparing hydroxymethyl furfural by catalytic dehydration reaction of hexose
InactiveCN102212048ALow priceEasy to getOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsN-ChlorosuccinimideSolvent
The invention provides a method for preparing hydroxymethyl furfural by catalytic dehydration reaction of hexose. The method comprises the following steps of: dissolving reaction substrates of hexose into a solvent, and adding a catalyst of halogenated succinimide to form a reaction system; and at the temperature of between 0 and 200 DEG C and under the protection of atmosphere gas, stirring the reaction system by magnetic force for dehydration reaction for 0.5 to 10 hours to prepare the hydroxymethyl furfural, wherein the catalyst of the halogenated succinimide may be N-bromosuccinimide, N-chlorosuccinimide, N-iodosuccinimide, N-fluorosuccinimide or any succinimide derivatives with substituted halogen atoms. The method has the advantages that: the catalyst of the halogenated succinimide is low in price and easily bought, and can catalyze the hexose in high efficiency as well as high selectivity to prepare the hydroxymethyl furfural; the method is environment-friendly, and the productis easily disposed; and in the whole process, only regenerated compounds such as fructose are consumed, the cost is low, the technically economical requirement is met, and the application prospect isbroad.
Owner:TIANJIN UNIVERSITY OF TECHNOLOGY
Preparation method of 5-haloacetyl propionate
InactiveCN108358789AWide variety of sourcesHigh reaction yieldOrganic compound preparationCarboxylic acid esters preparationPhosphorus tribromideN-Chlorosuccinimide
The invention discloses a preparation method of 5-haloacetyl propionate. Acetylpropionic acid or acetyl propionate and a halogenating agent are taken as raw materials, and the halogenating agent includes NBS (N-Bromosuccinimide), NCS (N-Chlorosuccinimide), ferrous bromide, iron bromide, aluminum bromide, phosphorus tribromide, copper bromide and copper chloride. The acetylpropionic acid or acetylpropionate obtained by hydrolysis of biomass is taken as a raw material, and undergoes a halogenation reaction in the presence of a halogenating agent to synthesize the 5-haloacetyl propionate. The selected raw materials are wide in sources, cheap and readily available; the reaction conditions are mild, and the reaction yield and selectivity are high; moreover, the selected halogenating agent serving as a raw material is less toxic and more environmentally friendly than liquid bromine used in the traditional mercury process, and has a good industrial application prospect.
Owner:XIAMEN UNIV
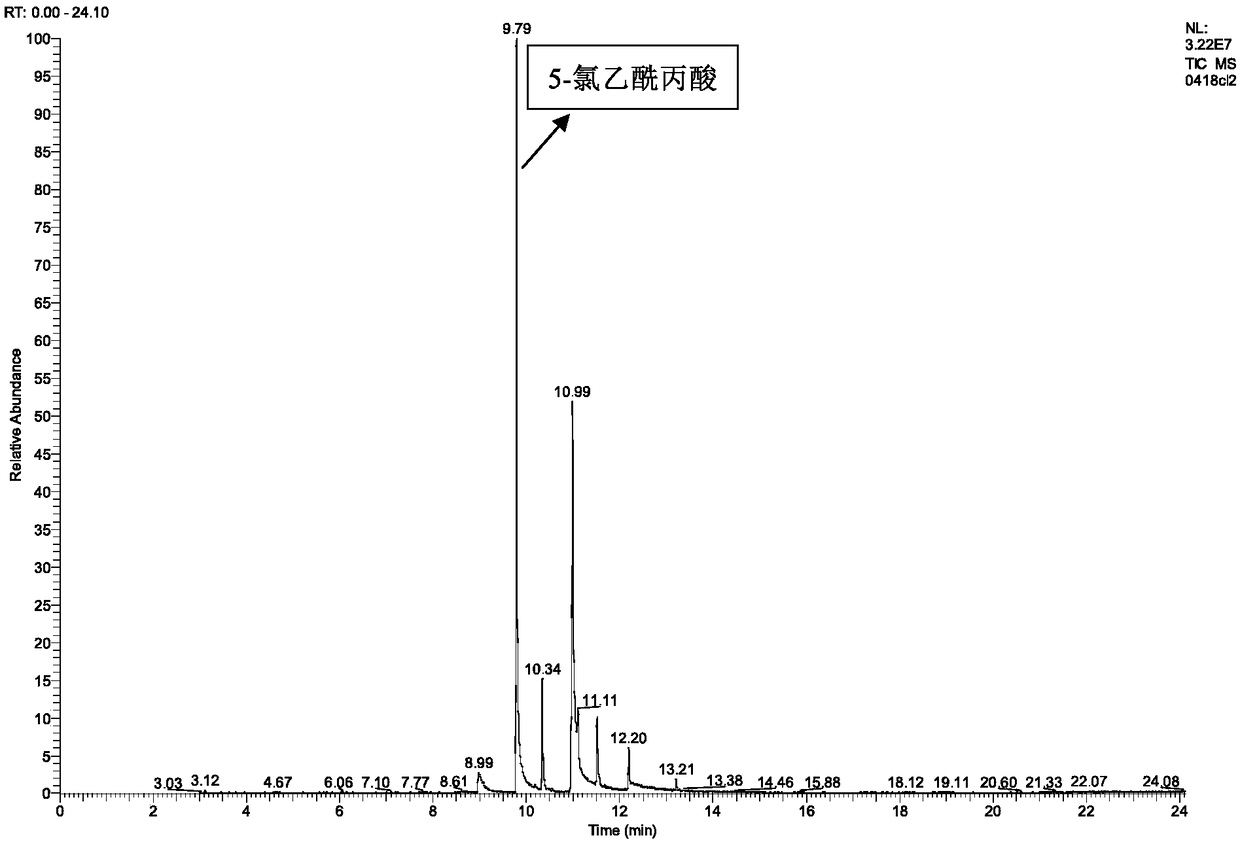
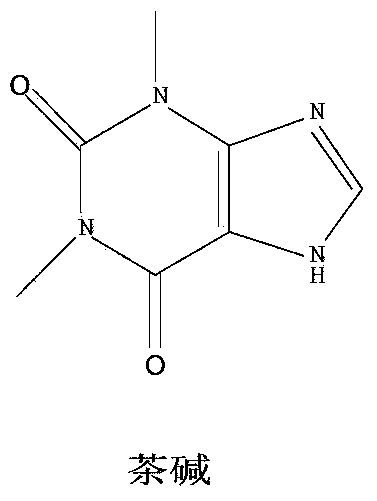
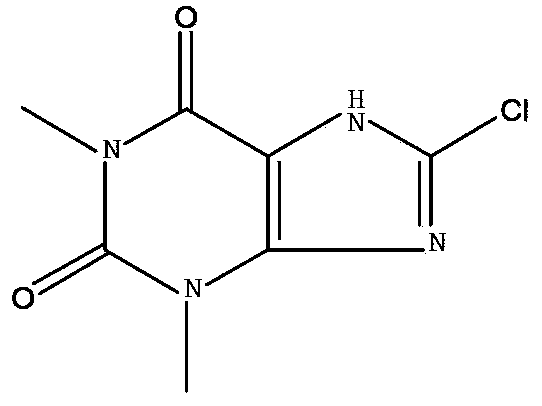
8-chlorotheophylline preparation method
ActiveCN103360394AAvoid generatingImprove protectionOrganic chemistryChemical synthesisEnvironmental resistance
The invention belongs to the chemical synthesis field, and provides a 8-chlorotheophylline synthesis method. The method is characterized in that caffeine is not used as a raw material, theophylline is used as the raw material, highly toxic reagents are abandoned, a water phase is used to substitute an organic solvent, and a chloridizing agent N-chlorosuccimide is adopted to substitute chlorine for chlorinating hydrogen in the 8th position of theophylline. The yield and the HPLC purity of the obtained product reach 88-90% and above 99% respectively under synthesis technology conditions, and the method is environmentally friendly.
Owner:SHANGHAI WANXIANG PHARMA
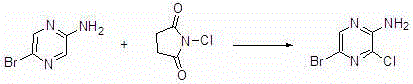
Synthesis method of 2-amino-3-chlorine-5-bromopyrazine
InactiveCN106632094AMild reaction conditionsEasy to operateOrganic chemistrySynthesis methodsN-Chlorosuccinimide
The invention relates to a synthesis method of 2-amino-3-chlorine-5-bromopyrazine. The synthesis method of the 2-amino-3-chlorine-5-bromopyrazine comprises the following steps: performing reaction on 2-amino-5-bromopyrazine and N-chlorosuccinimide which serve as raw materials under the action of a proper solvent to produce the 2-amino-3-chlorine-5-bromopyrazine, and performing recrystallization to obtain a 2-amino-3-chlorine-5-bromopyrazine pure product. According to the synthesis method of the 2-amino-3-chlorine-5-bromopyrazine, the raw materials are easily available and the price is reasonable; meanwhile, heavy metal and corrosive gas are not used in the preparation reaction, the reaction is mild, special requirements on the reaction equipment are not needed, and the common corrosion-resistant equipment can perform production; the reaction yield is high and the purity is high.
Owner:SHANDONG YOUBANG BIOCHEM TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
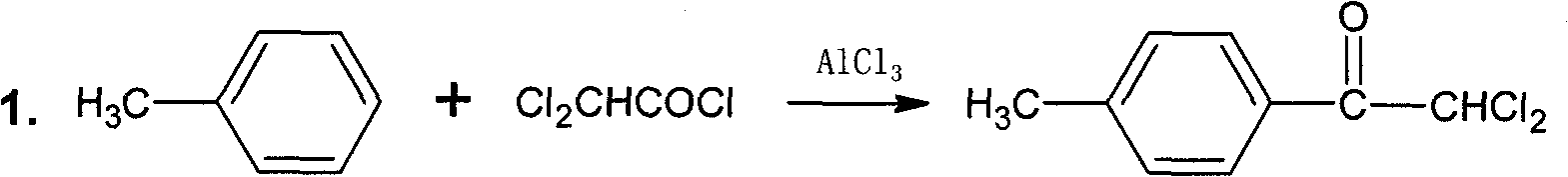


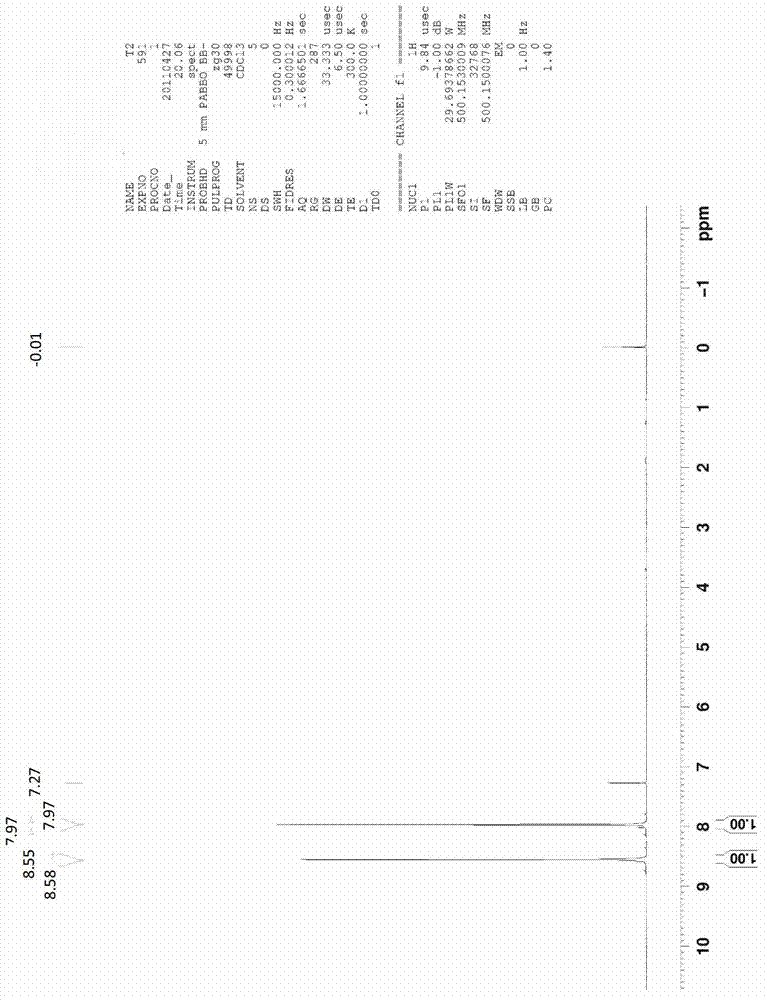
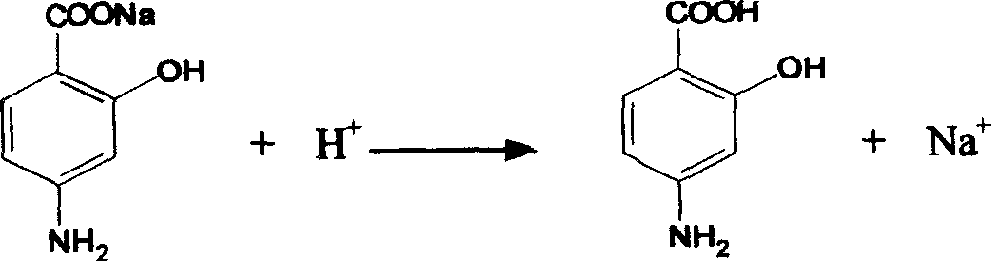
![One-step synthesis method of 6-chlorine-3H-oxazole [4,5-b] pyridine-2-ketone One-step synthesis method of 6-chlorine-3H-oxazole [4,5-b] pyridine-2-ketone](https://images-eureka.patsnap.com/patent_img/8092f8a2-d743-4aa6-a98f-a0c7fd56c036/567303DEST_PATH_IMAGE001.PNG)


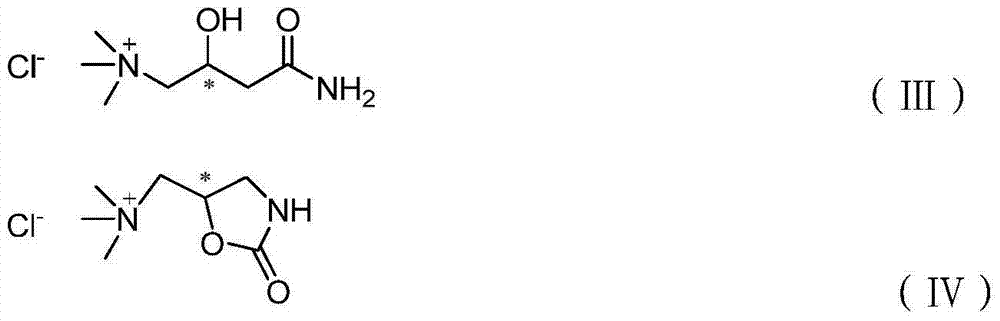
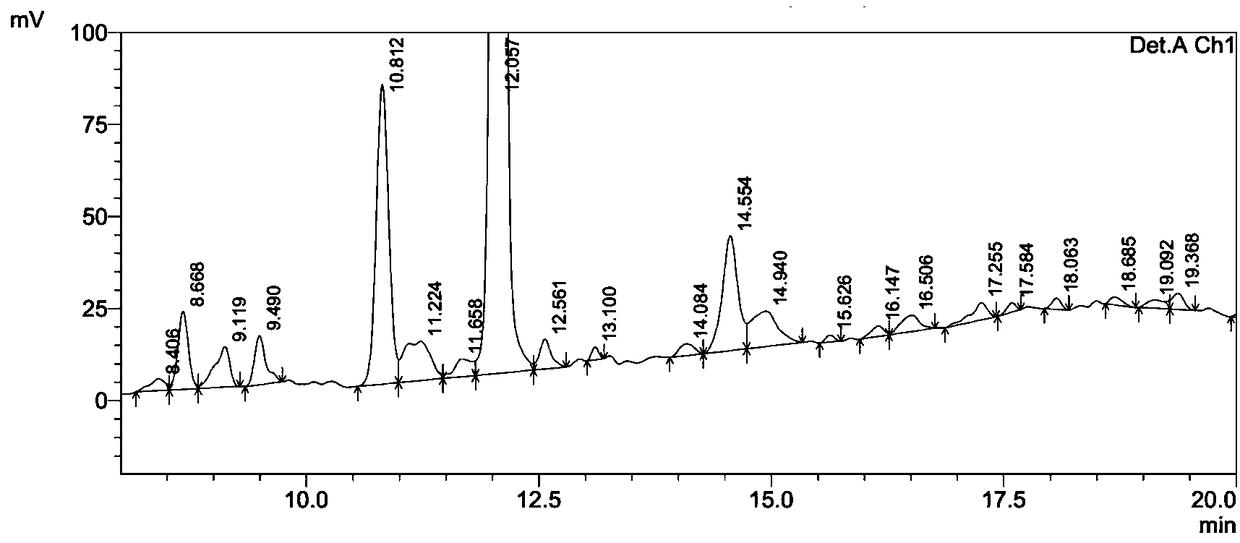
![Preparation method of (S,S)-2, 8-diazabicyclo[4,3,0] nonane Preparation method of (S,S)-2, 8-diazabicyclo[4,3,0] nonane](https://images-eureka.patsnap.com/patent_img/9aaa783c-99d9-434a-8122-426e03a79b43/HSA00000666248600011.PNG)
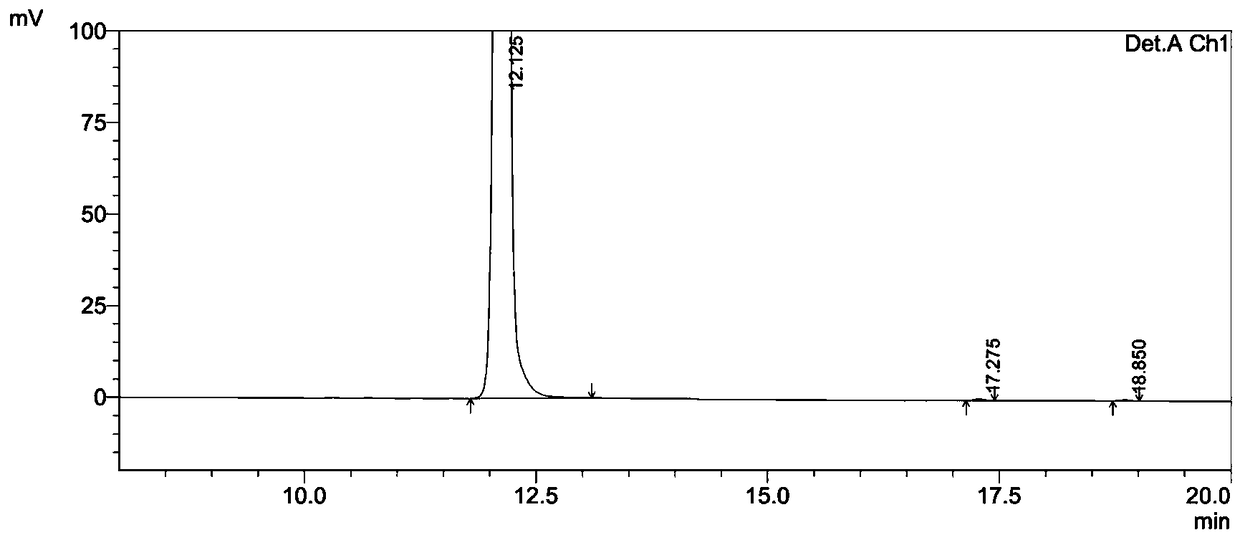
![Preparation method of (S,S)-2, 8-diazabicyclo[4,3,0] nonane Preparation method of (S,S)-2, 8-diazabicyclo[4,3,0] nonane](https://images-eureka.patsnap.com/patent_img/9aaa783c-99d9-434a-8122-426e03a79b43/BSA00000666248500011.PNG)
![Preparation method of (S,S)-2, 8-diazabicyclo[4,3,0] nonane Preparation method of (S,S)-2, 8-diazabicyclo[4,3,0] nonane](https://images-eureka.patsnap.com/patent_img/9aaa783c-99d9-434a-8122-426e03a79b43/BSA00000666248500021.PNG)