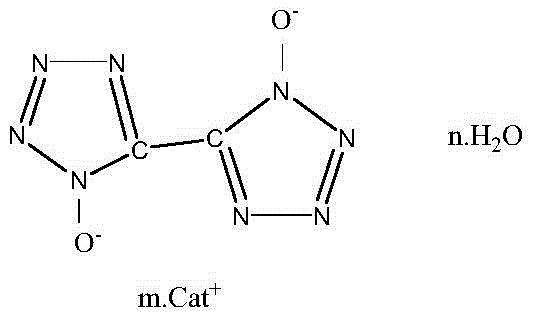
5,5'-bistetrazole-1,1'-dioxide metal salt and synthesis method thereof
A technology of metal dioxide and synthesis methods, applied in the fields of nitration explosive components, compressed gas generation, organic chemistry, etc., can solve the problems of unfavorable industrial preparation and low purity, reduce the characteristic signal of propellant, increase energy output, and synthesize The effect of short process route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
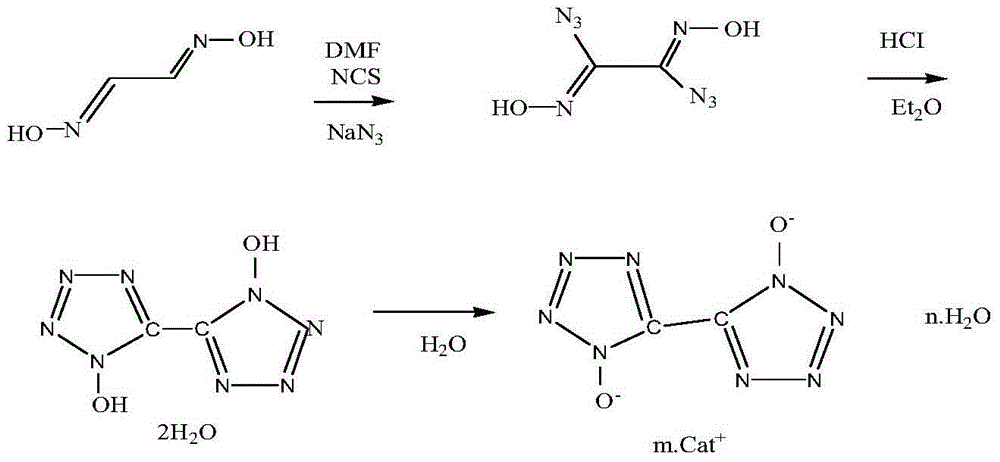
[0024] Synthesis of 5,5'-bitetrazole-1,1'-dihydroxy dihydrate
[0025] (1) Add 10 g of glyoxime to 100 mL of N-N-dimethylformamide system, slowly add 30 g of N-chlorosuccinimide (NCS) in batches at room temperature, and wait until N-chlorosuccinyl After the imine (NCS) was completely dissolved, it was stirred and kept at room temperature for 8 hours. The temperature of the reaction system was lowered to 0°C, and 16 g of sodium azide was slowly added in batches. Keep warm at 0-5°C for 60 minutes. The reaction solution was poured into 100mL of water, precipitated and filtered. The filter cake was washed once more with 200 mL of water to obtain 25 g of a wet product of diazideglyoxime, which was then dried to obtain 17.5 g.
[0026] (2) Add 4.25 g of the wet product diazideglyoxime into 65 mL of ether system, and mix the feed liquid completely and evenly. When the temperature is lowered to 0-5°C, HCI gas is introduced until the ether solution is saturated. Warm up to room te...
Embodiment 2
[0028] Synthesis of 5,5'-bitetrazole-1,1'-dihydroxy dihydrate
[0029] (1) Add 10 g of glyoxime to 150 mL of N-N-dimethylformamide system, slowly add 30 g of N-chlorosuccinimide (NCS) in batches at room temperature, and wait until N-chlorosuccinyl After the imine (NCS) was completely dissolved, it was stirred and kept at room temperature for 12 hours. The temperature of the reaction system was lowered to 0°C, and 16 g of sodium azide was slowly added in batches. 0-5 ℃ insulation 90min. The reaction solution was poured into 150mL of water, precipitated and filtered. The filter cake was washed once with 200 mL of water to obtain 25.6 g of a wet product of diazideglyoxime, which was then dried to obtain 17.9 g.
[0030] (2) Add 4.25 g of the wet product diazideglyoxime into 85 mL of ether system, and mix the feed liquid completely and evenly. When the temperature is lowered to 0-5°C, HCI gas is introduced until the ether solution is saturated. Warm up to room temperature and...
Embodiment 3
[0032] Synthesis of 5,5'-bistetrazole-1,1'-dioxodipotassium
[0033] Add 4 g of 5,5′-bitetrazole-1,1′-dihydroxy dihydrate into 100 mL of water system, start stirring, and raise the temperature to 40°C to make the liquid completely dissolved and transparent. Add 2.64 g of potassium hydroxide, keep warm for 60 min, slowly cool down to room temperature, filter, and wash the filter cake with cold water. Naturally air-dried to obtain 3.72 g of colorless 5,5′-bistetrazole-1,1′-dioxypotassium salt crystals, with a yield of 78%.
[0034] Structure Identification:
[0035] Infrared (KBr, υ / cm -1 ) 2166, 1667, 1510, 1408, 1356, 1233, 1164, 1058, 997, 732, 502.
[0036] Elemental Analysis: C 2 N 8 o 2 K 2
[0037] Theoretical value: C (9.756%), N (45.3%);
[0038] Found values: C (9.859%), N (46.51%).
[0039] Thermal decomposition temperature: Tp=383℃.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com