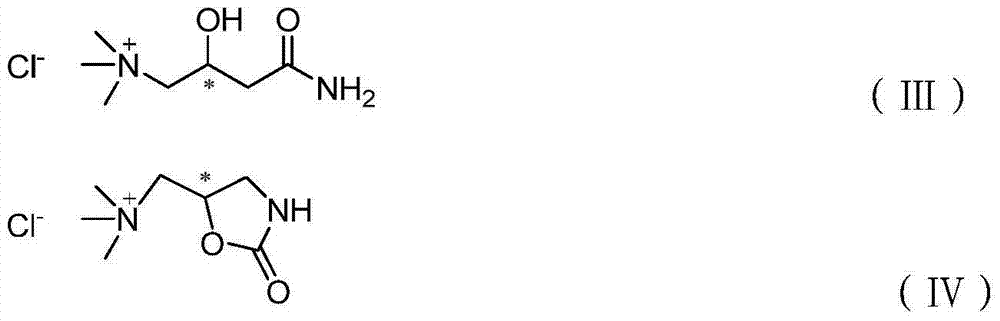Preparation method of pregabalin
A technology of pregabalin and carbamoylmethyl, applied in the field of drug synthesis, can solve the problems of reduced product quality, difficult concentration control, unstable sodium hypochlorite, etc., and achieves the effect of reducing impurity content and improving yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Add 250mL water and 55g (5equiv) sodium hydroxide to a 500mL flask, stir until dissolved, then cool to -5~0°C, add 45g (1.26equiv) N-chlorosuccinimide (NCS) in batches , Control the temperature below 0°C during the addition process. Then add 50g (0,27mol) (R)-(-)-3-(carbamoylmethyl)-5-methylhexanoic acid (CAS181289-33-8) in batches to the above mixed system. Keep the reaction temperature below 0°C. After the addition, keep the temperature at 0-10°C for reaction (take a sample for TLC to detect the reaction process). After 3 hours, TLC shows that the reaction is complete, and continue to stir at 25°C for 12 hours.
[0028] About 60 mL of concentrated hydrochloric acid was added dropwise to the obtained reaction solution to adjust the pH to 5.0-5.5, and the reaction system was slowly cooled to 0-5° C. within 2 to 3 hours, then stirred for 1 hour and then filtered to obtain a white solid. Add 75mL of water and 75mL of isopropanol to the obtained white solid, immerse at ro...
Embodiment 2
[0030] The operation of this embodiment is basically the same as that of Example 1, except that the substrate (R)-(-)-3-(carbamoylmethyl)-5-methylhexanoic acid and N-chlorobutyl The addition order of diimide is reversed, and the reaction results are shown in Table 1.
Embodiment 3
[0032] The operation of this embodiment is basically the same as that of Example 1, except that potassium hydroxide is used instead of sodium hydroxide, and the amount used is 4.0 equiv. The reaction results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com