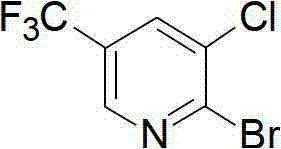
Preparation method for pyridine medical intermediate for synthesizing anti-cancer auxiliary medicines
A technology of pyridines and intermediates, applied in the field of synthesis of 2-bromo-3-chloro-5-trifluoromethylpyridine, which can solve the problem of high price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
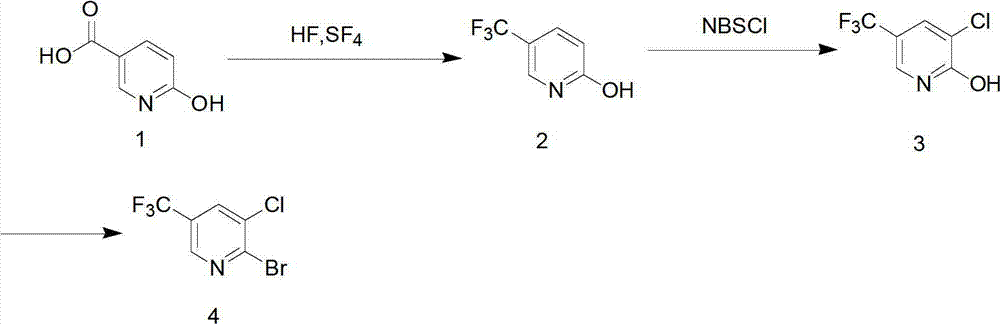
[0023] (1) Add 0.13mol of 6-hydroxynicotinic acid (18.2g), 2.6ml of anhydrous hydrofluoric acid (concentration above 95wt%) (0.13mol), and 42.1g of sulfur tetrafluoride (0.39mol) into a stainless steel pressure tank Li and heated to 100 ° C, reacted at 0.15 MPa for 12 hours, and the gas product was treated with a tail gas absorption device.
[0024] The remaining product was transferred to a polytetrafluoroethylene container and heated to 40°C to remove traces of hydrogen fluoride. The resulting product was added to 150 ml of water, adjusted to pH 6.8-7.2 with saturated sodium carbonate solution, extracted with chloroform, dried, filtered, and spin-dried to obtain 15.3 g (0.094 mol) of 2-hydroxy-5-trifluoromethylpyridine , yield 72%.
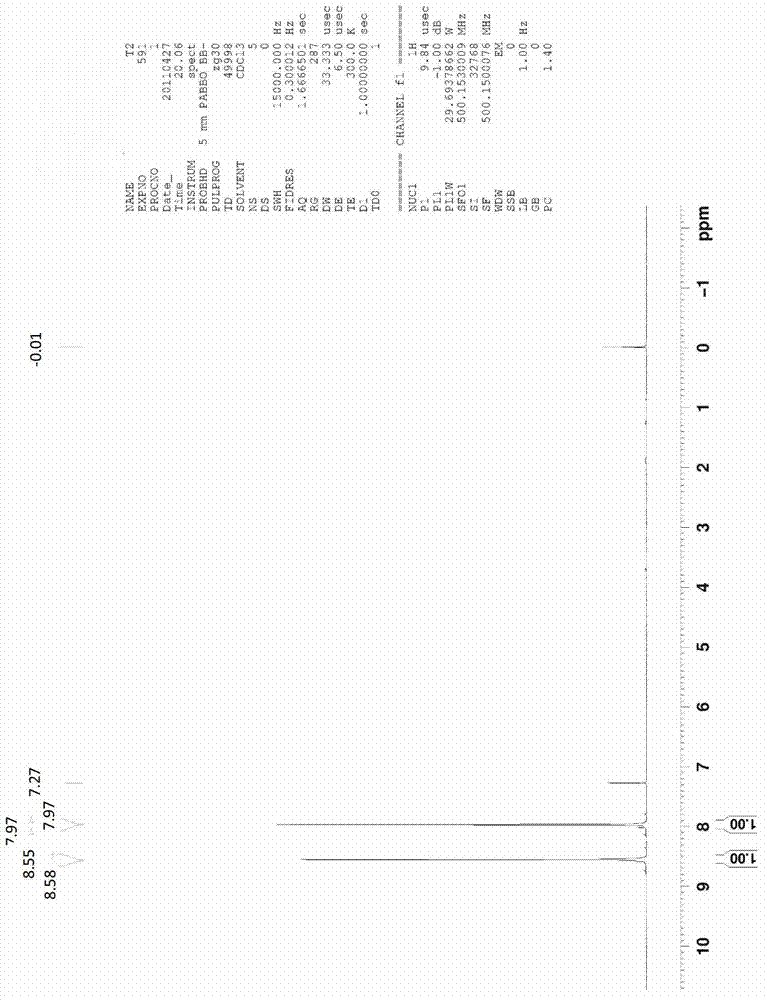
[0025] (2) Add 0.094mol of 2-hydroxy-5-trifluoromethylpyridine (15.3g) and 14.4g of N-chlorosuccinimide (0.108mol) to 50ml of anhydrous DMF / nitromethylpyrrolidone , reacted at room temperature for 8 hours under stirring conditions. The reacta...
Embodiment 2
[0029] (1) Add 0.15mol 6-hydroxynicotinic acid, anhydrous hydrofluoric acid (0.16mol content), and 0.5mol sulfur tetrafluoride into a stainless steel pressure tank and heat to 120°C, and react at 0.2MPa for 12 hours; The gaseous product is treated with a tail gas absorption device. Transfer the remaining product to a polytetrafluoroethylene container and heat it to about 40°C to remove traces of hydrogen fluoride. The resulting product was added to 150 ml of water, adjusted to pH 6.5-7.4 with saturated sodium carbonate solution, extracted with chloroform, dried, filtered, and spin-dried to obtain 17.8 grams (0.109 mol) of 2-hydroxy-5-trifluoromethylpyridine , yield 72.6%.
[0030] (2) Add 17.8 g of 2-hydroxy-5-trifluoromethylpyridine and 0.12 mol of N-chlorosuccinimide (133.54 molar ratio of 1:1.1 to 1.3) to 50 ml of anhydrous DMF / nitrogen In the base pyrrolidone. The reactant was slowly added to 250 ml of water, and a pale yellow precipitate precipitated out. The resultin...
Embodiment 3
[0034] (1) The 2-bromo-3-chloro-5-trifluoromethylpyridine prepared in Example 1 or Example 2 is used to prepare compound A1 according to the record of US2009 / 186879A1, and the reaction formula is as follows:
[0035]
[0036] Among them, the compound A1 has good drug activity and can be used as an auxiliary anticancer drug.
[0037] (2) The 2-bromo-3-chloro-5-trifluoromethylpyridine prepared in Example 1 or Example 2 was prepared according to the record of WO2005 / 4606 to prepare compound A2, the reaction formula is as follows:
[0038]
[0039] Among them, the compound A2 has good antifungal bacteria and anti-infection activities, and can be used as an anti-cancer auxiliary drug.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com