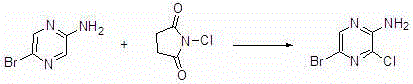
Synthesis method of 2-amino-3-chlorine-5-bromopyrazine
A synthesis method and technology of bromopyrazine are applied in the synthesis field of 2-amino-3-chloro-5-bromopyrazine, can solve the problems of intense reaction, high cost of raw materials, long reaction time and the like, and achieve mild reaction conditions, The effect of stable product quality and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Add 2-amino-5-bromopyrazine (3.48g, 20mmol), N-chlorosuccinimide (2.67g, 20mmol) and 20ml of methanol into a 50mL single-necked round bottom flask. The mixture in the reaction flask was stirred and reacted at 35°C for 4 hours. TLC confirmed the completion of the reaction. After the reaction, the solvent was removed by rotary evaporation to obtain a crude product, which was recrystallized to obtain the pure product 2-amino-3-chloro-5-bromopyrazine. After drying, the calculated yield was 76.15%, and the purity was 98.42% (HPLC).
Embodiment 2
[0022] Add 2-amino-5-bromopyrazine (3.48g, 20mmol), N-chlorosuccinimide (3.20g, 24mmol) and methanol 20ml into a 50mL single-necked round bottom flask. The mixture in the reaction flask was stirred and reacted at 35°C for 4 hours. TLC confirmed the completion of the reaction. After the reaction, the solvent was removed by rotary evaporation to obtain a crude product, which was recrystallized to obtain the pure product 2-amino-3-chloro-5-bromopyrazine. After drying, the calculated yield was 83.40%, and the purity was 98.75% (HPLC).
Embodiment 3
[0024] Add 2-amino-5-bromopyrazine (52.20 g, 300 mmol), N-chlorosuccinimide (48.07 g, 360 mmol) and 350 ml of acetonitrile into a 1000 mL single-necked round bottom flask. The mixture in the reaction flask was stirred and reacted at 35°C for 4 hours. TLC confirmed the completion of the reaction. After the reaction, the solvent was removed by rotary evaporation to obtain a crude product, which was recrystallized to obtain the pure product 2-amino-3-chloro-5-bromopyrazine. After drying, the calculated yield was 87.00%, and the purity was 99.00% (HPLC).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com