Preparation method of 5-haloacetyl propionate
A technology of halolevulinate and levulinate, which is applied in the field of preparation of 5-halolevulinate, can solve the problems of low bromination efficiency, target product selectivity, low yield, complicated operation, etc., and achieve High reaction yield and selectivity, good industrial application prospects, mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
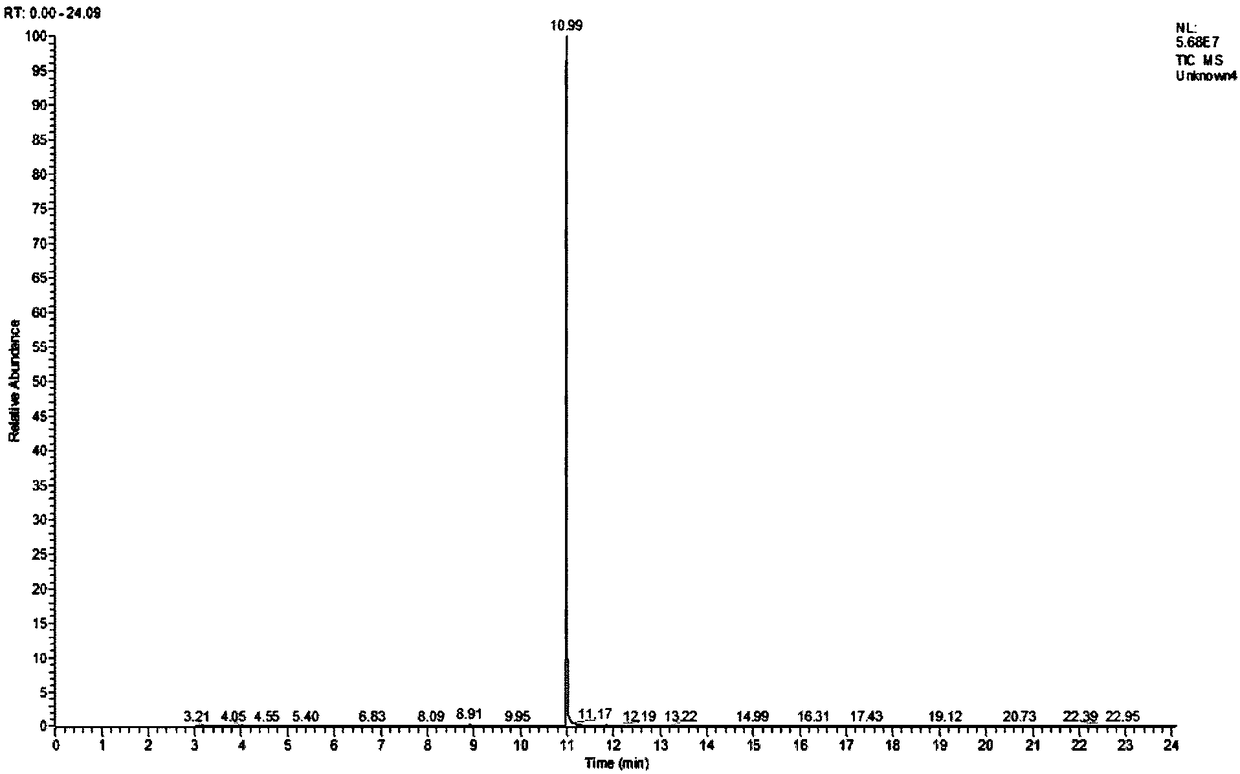
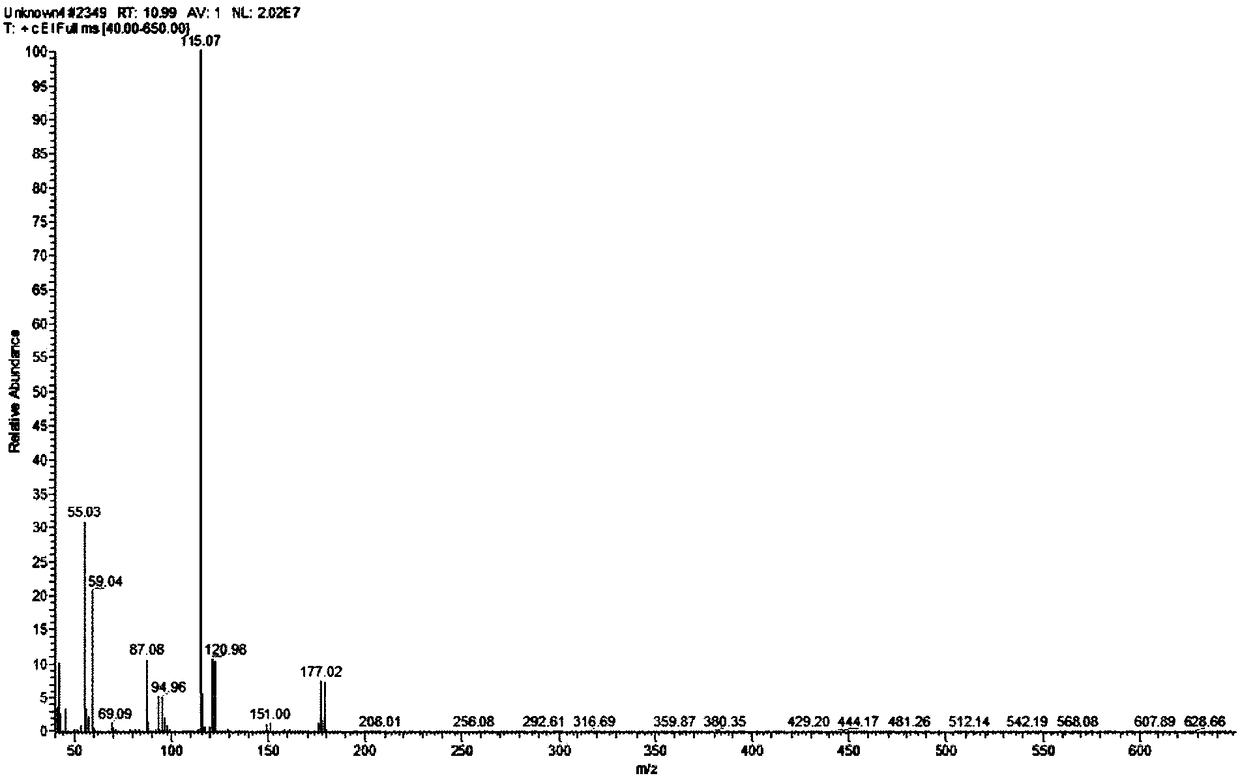
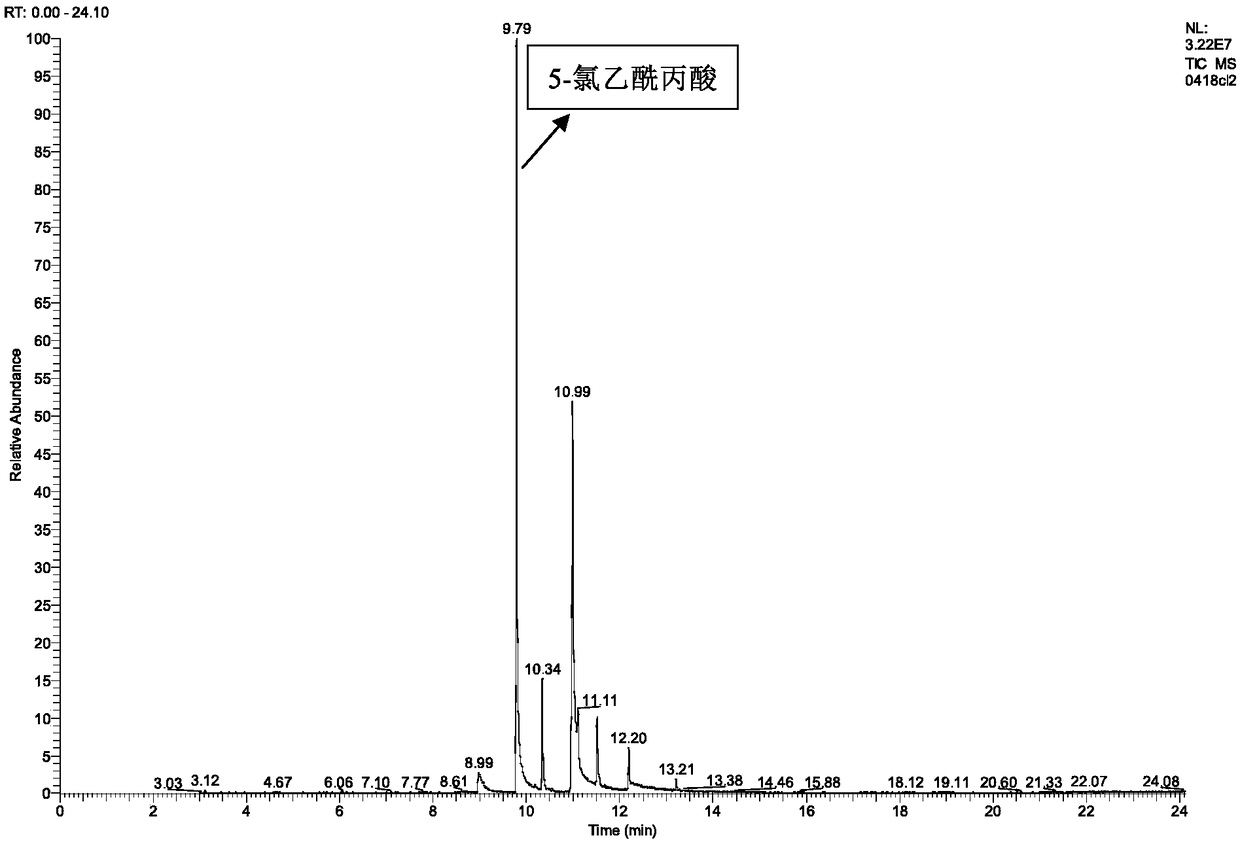
[0025] Add 0.33 g (0.0025 mol) of methyl levulinate and 20 mL of methanol into a 100 mL three-necked flask (the concentration of methyl levulinate is 0.125 mol / L). Connect the condenser and place it in a water bath at 60°C, add a total of 1.67g of copper bromide (the molar ratio of methyl levulinate to copper bromide is 0.33:1) for reaction, and react for 2 hours. After the reaction, the product is distilled under reduced pressure . Add 20 mL of methanol to the residual liquid to dissolve and filter. After the filtrate is diluted 5 times, the target product is qualitatively detected by gas chromatography-mass spectrometry (GC-MS), and quantitatively calculated by the external standard method. The measured volume concentration of the product methyl 5-bromolevulinate was 0.0079g / mL, and the yield was 29.97%.
[0026] Distill the product dissolved in methanol again under reduced pressure, add a small amount of deionized water to dissolve the rotary steamed product, then add an a...
Embodiment 2
[0028] Add 0.33 g (0.0025 mol) of methyl levulinate into a 100 mL three-necked flask, and a total of 20 mL of an equal volume of methanol and chloroform mixture (the concentration of methyl levulinate is 0.125 mol / L). Connect the condenser and place it in a water bath at 55°C, add a total of 1.67g of copper bromide and potassium bromide (the molar ratio of methyl levulinate to copper bromide and potassium bromide is about 0.33:1) for reaction, and react for 4 hours After the reaction, the product was distilled under reduced pressure. Add 20mL of methanol to the residual liquid to dissolve and filter. After the filtrate is diluted 5 times, the target product is qualitatively detected by gas chromatography-mass spectrometry (GC-MS), and quantitatively calculated by external standard method. The concentration of the product methyl 5-bromolevulinate was 0.0081 g / mL, and the yield was 30.73%.
[0029] Distill the product dissolved in methanol again under reduced pressure, add a sm...
Embodiment 3
[0031] Add 0.33 g (0.0025 mol) of methyl levulinate into a 100 mL three-necked flask, and a total of 20 mL of an equal volume of methanol and chloroform mixture (the concentration of methyl levulinate is 0.125 mol / L). Connect the condenser tube and place it in a 40°C water bath, add a total of 1.67g of copper bromide (the molar ratio of methyl levulinate to copper bromide is 0.33:1) for reaction, and react for 10 hours. After the reaction, the product is distilled under reduced pressure . Add 20 mL of methanol to the residual liquid to dissolve and filter. After the filtrate is diluted 5 times, the target product is qualitatively detected by gas chromatography-mass spectrometry (GC-MS), and quantitatively calculated by the external standard method. The measured volume concentration of the product methyl 5-bromolevulinate was 0.0093 g / mL, and the yield was 35.28%.
[0032] Distill the product dissolved in methanol again under reduced pressure, add a small amount of deionized w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com