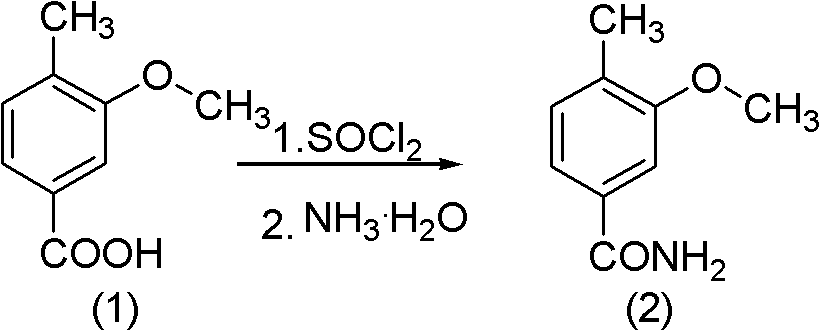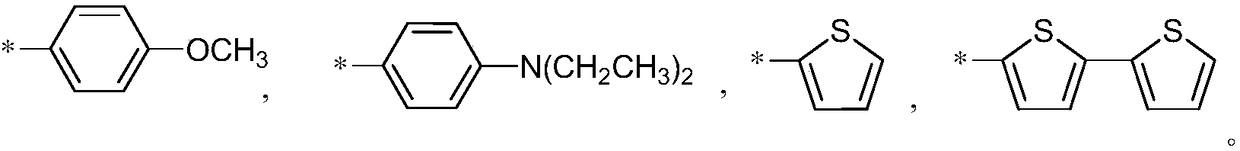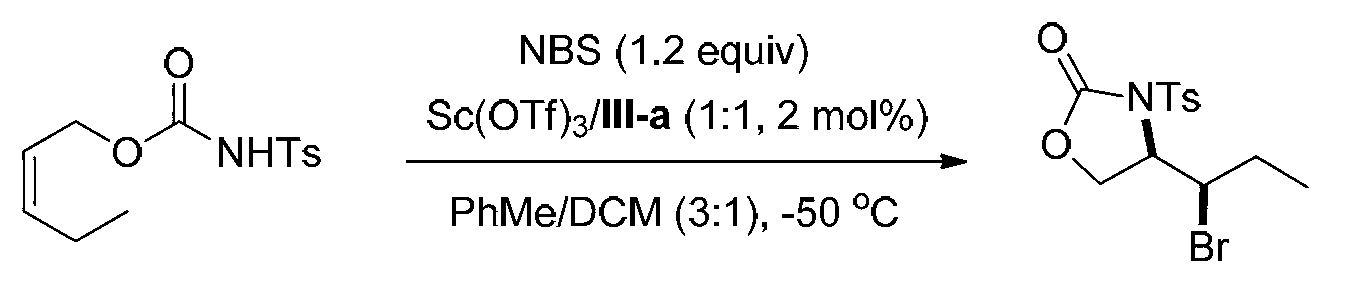Patents
Literature
236 results about "N-Bromosuccinimide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
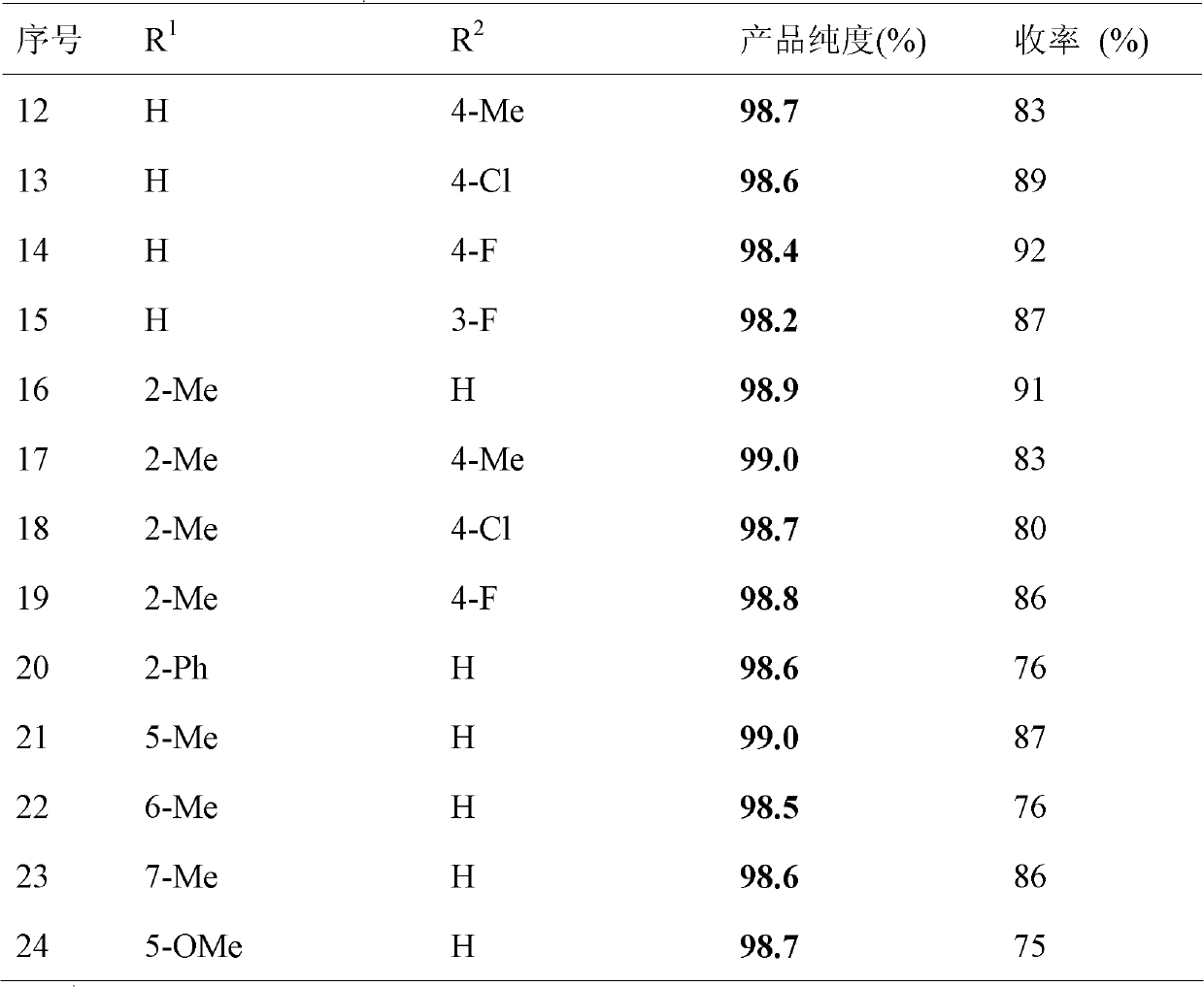
Patent Status
Application Year
Inventor
N-Bromosuccinimide or NBS is a chemical reagent used in radical substitution, electrophilic addition, and electrophilic substitution reactions in organic chemistry. NBS can be a convenient source of Br•, the bromine radical.
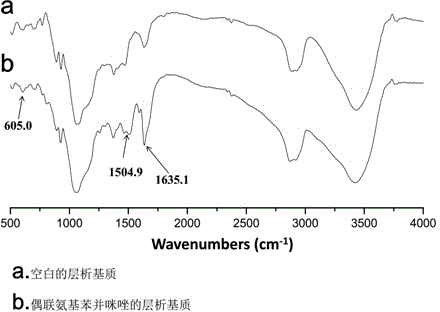
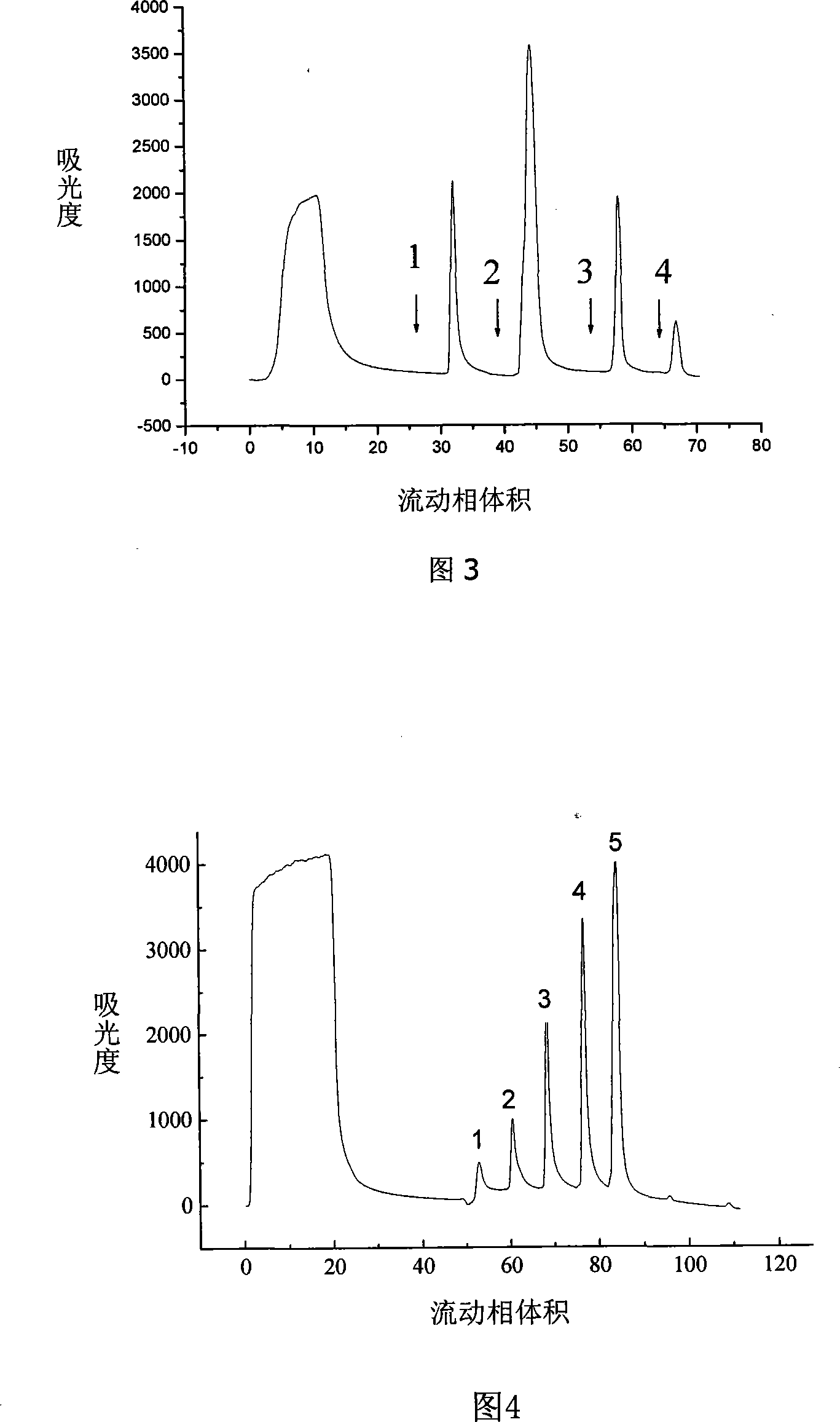
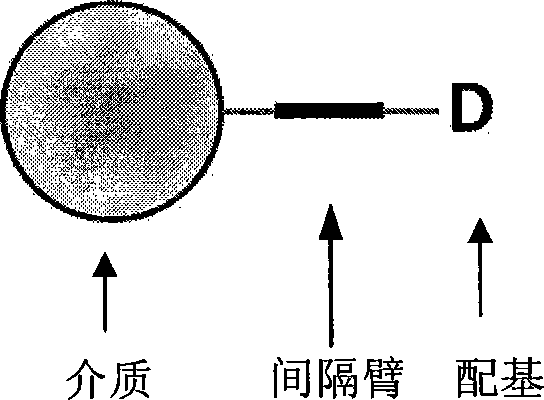
Chromatographic medium using amino benzimidazole as function ligand and preparation method thereof
ActiveCN104096544ALarge adsorption capacityReduce adverse effectsOther chemical processesPeptide preparation methodsChromatographic separationDesorption
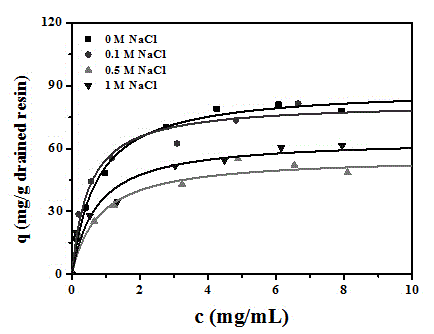
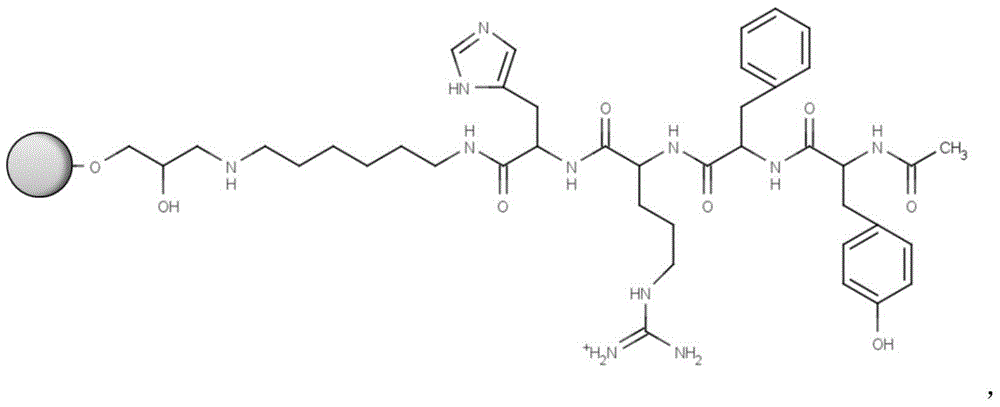
The invention discloses a chromatographic medium using amino benzimidazole as a function ligand and a preparation method thereof. Hydrophilic porous microspheres are used as a chromatographic medium, activated by allyl bromide, and coupled with the amino benzimidazole to obtain a medium using the amino benzimidazole as the function ligand; dimethyl sulfoxide and the allyl bromide are sequentially added into a chromatographic matrix for activation; the activated chromatographic matrix is reacted with N-bromo-succinimide for bromo-alcoholization; the bromo-alcoholized chromatographic matrix is mixed with an amino benzimidazole solution for coupling the amino benzimidazole ligand; finally an aqueous ethanol amine solution is used for sealing unreacted bromo-alcoholized ends to obtain a hydrophobic charge induced chromatographic medium using the amino benzimidazole as the function group. The new chromatographic medium is simple in preparation process and high in antibody adsorption capacity, and has the characteristics of non salt dependent adsorption, can realize desorption and recovery by changing the solution pH to weak acid, and can be used for hydrophobic charge induction chromatographic separation of antibodies.
Owner:ZHEJIANG UNIV
Preparation method of 5-bromine-2-chlorine-4'-ethyoxyl diphenylmethane
InactiveCN104478670ALow water requirementMild reaction conditionsOrganic chemistryOrganic compound preparationDiphenylmethaneAlkyl transfer
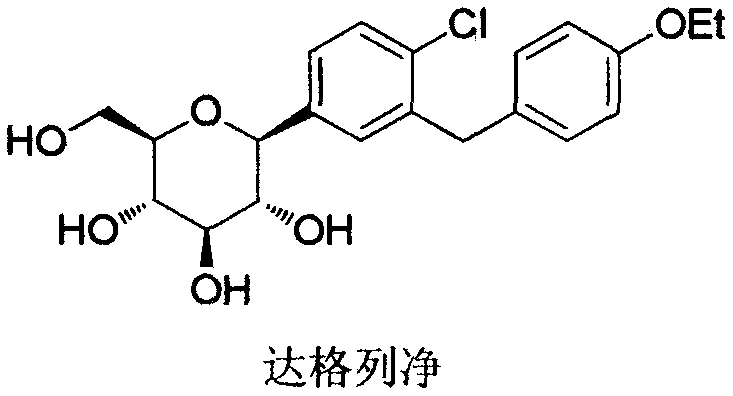
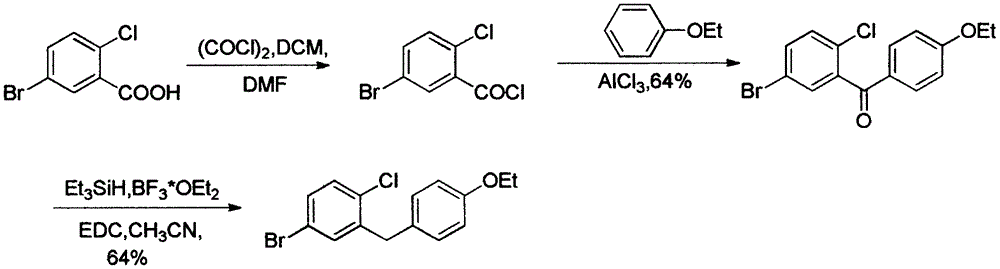
The invention relates to the chemical field and particularly relates to a novel synthesis method for preparing a key intermediate 5-bromine-2-chlorine-4'-ethyoxyl diphenylmethane of a drug dapagliflozin for treating diabetes mellitus II. The preparation method comprises the following steps: enabling a starting raw material ortho-toluidine to firstly perform bromization and then perform chlorination after diazotization on a benzene ring with N-bromo-succinimide; then, in the presence of a halogenating agent, performing halogenating reaction of beta-position; and finally, performing Friedel-Crafts alkylation synthesis with phenetole, thereby obtaining the key intermediate. The preparation method is simple and convenient, economical and relatively high in reaction yield in each step, and suitable for industrial production.
Owner:CHINA PHARM UNIV
Affinity chromatography medium employing tetrapeptide as functional ligand and preparation method of affinity chromatography medium
ActiveCN104645949AHigh affinityGood choiceOther chemical processesSolid sorbent liquid separationSodium acetateAcetic anhydride
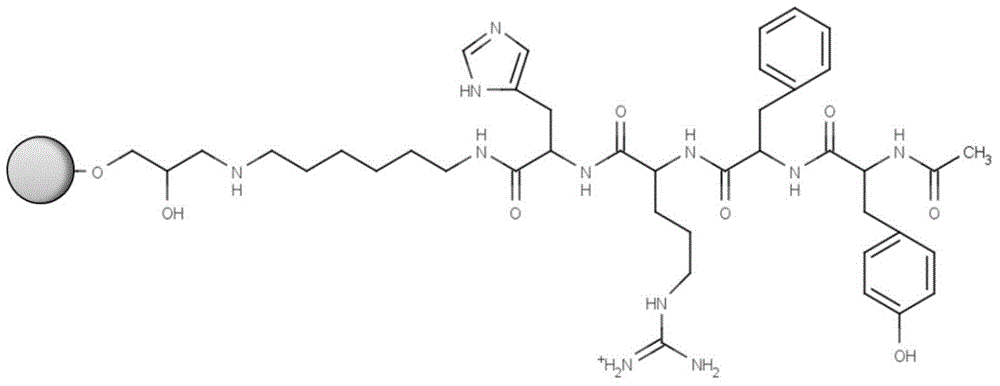
The invention discloses an affinity chromatography medium employing tetrapeptide as a functional ligand and a preparation method of the affinity chromatography medium. The method comprises the following steps: adding dry matrix and allyl bromide to a dimethyl sulfoxide solution, activating, and reacting activating matrix with N-bromo succinimide; enabling bromo alcoholized matrix to react with hexamethylendiamine to obtain amino activating matrix; sequentially washing with deionized water, absolute ethyl alcohol and anhydrous N,N-dimethyl formamide, adding an N,N-dimethyl formamide solution containing tetrapeptide, 2-(7-azobenzotriazole)-N,N,N',N'-te-tramethyluronium hexafluorophosphate and N,N-diisopropylethylamine, and coupling a tetrapeptide ligand; and putting a medium coupled to tetrapeptide in a mixed liquid of sodium acetate and acetic anhydride to obtain the affinity chromatography medium employing tetrapeptide as the functional ligand. According to the novel chromatography medium developed by the method, a functional group is tetrapeptide composed of tyrosine, phenylalanine, arginine and histidine, and is designed on the basis of a protein A binding site of an antibody Fc segment; the antibody binding selectivity is greatly improved; and the affinity chromatography medium can be applied to efficient separation of an antibody.
Owner:ZHEJIANG UNIV
Soluble branch substituted anthracene molecule blue material as well as preparation method and uses thereof
InactiveCN101200634AEasy to synthesizeEasy to purifySolid-state devicesSemiconductor/solid-state device manufacturingSolubilityN-Bromosuccinimide
The present invention discloses soluble branch substituted anthracene molecular blue light-emitting material and a preparation method and an application thereof. The anthracene molecular blue light-emitting material considers anthracene as a center, and soluble branch group Dendron and rigid group Ar1 are respectively accessed at the two ends of the anthracene to ensure that the prepared emitting material with an asymmetrical structure has certain solubility and can be purified by solution method. When the anthracene molecular blue light-emitting material is prepared, 9-bromoanthracene or 9-anthracene boric acid ester is used as reaction raw material, the soluble branch group Dendron is introduced by the palladium catalysis Suzuki coupling reaction, then N-bromosuccinimide is used for bromizing at 10-position of the anthracene to obtain an anthracene molecular bromide; the Ar1 is introduced into the obtained bromide or boric acid prepared from the bromide by the palladium catalysis Suzuki coupling reaction to obtain a target product. The material has the advantages of synthesis and purification and has important application prospect at electroluminescence display, illumination and laser.
Owner:GUANG ZHOU NEW VISION OPTO ELECTRONICS TECH
Synthesis method of 5-bromo-2-chloro benzoic acid
ActiveCN105622382AEasy to operateHigh purityOrganic compound preparationCarboxylic compound preparationBenzoic acidChlorobenzilate
The invention provides a synthesis method of 5-bromo-2-chloro benzoic acid.The method includes the following steps of A, making 2-chlorine benzotrichloride and bromide reagents react under the effect of a catalyst to obtain 2-chloro-5-bromine benzotrichloride, wherein bromide reagents include one or more of bromine, N-bromosuccinimide, dibromohydantoin and hydrobromic acid; B, conducting hydrolysis reaction on 2-chloro-5-bromine benzotrichloride in the step A under the acid condition to obtain 5-bromo-2-chloro benzoic acid.According to the method, 2-chlorine benzotrichloride which is low in price and easy to obtain is adopted as the raw material, operation is easy, intermediates do not need to be purified, 5-bromo-2-chloro benzoic acid is synthesized through a one-pot method, purity is high, yield is high, three-waste emission is little, and production cost is low.It is shown through experiment results that 2-chloro-5-benzoic acid obtained according to the synthesis method has yield larger than 95% and purity of 80-92%.
Owner:苏州正济药业有限公司
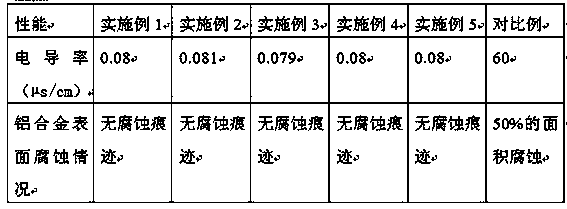
Organic fuel cell anti-freeze cooling liquid with low conductivity and ultra-long acting and preparation method of anti-freeze cooling liquid
ActiveCN108102616AImprove conductivityReduce conductivityHeat-exchange elementsFuel cellsOrganic fuel8-Hydroxyquinoline
The invention discloses an organic fuel cell anti-freeze cooling liquid with low conductivity and ultra-long acting and a preparation method of the anti-freeze cooling liquid in the field of anti-freeze cooling fluids. The anti-freeze liquid is prepared from components in percentage by weight as follows: 10wt%-70wt% of ethylene glycol, 0.001wt%-0.01wt% of 8-hydroxyquinoline, 0.005wt%-0.02wt% of uracil, 0.01wt%-0.03wt% of 4-acetaminophen, 0.01wt%-0.05wt% of benzotriazole octadecylamine, 0.005wt%-0.05wt% of N-bromosuccinimide, 0.001wt%-0.01wt% of inosine and the balance of deionized water. The preparation method of the anti-freeze liquid comprises the steps as follows: all the components of the anti-freeze cooling fluid are put in a reaction kettle in percentage by mass; the components are stirred and mixed for 30-90 min, so that all components are fully dissolved and mixed uniformly, the mixed solution passes through anion and cation mixed exchange resin by the aid of a pressure pump, and the fuel cell anti-freeze cooling fluid is obtained. The organic fuel cell anti-freeze cooling liquid with low conductivity and ultra-long acting is weakly alkaline, is anti-freezing and anti-boiling and has performance of low conductivity, ultra-long acting and high corrosion inhibition.
Owner:扬州中德汽车零部件有限公司
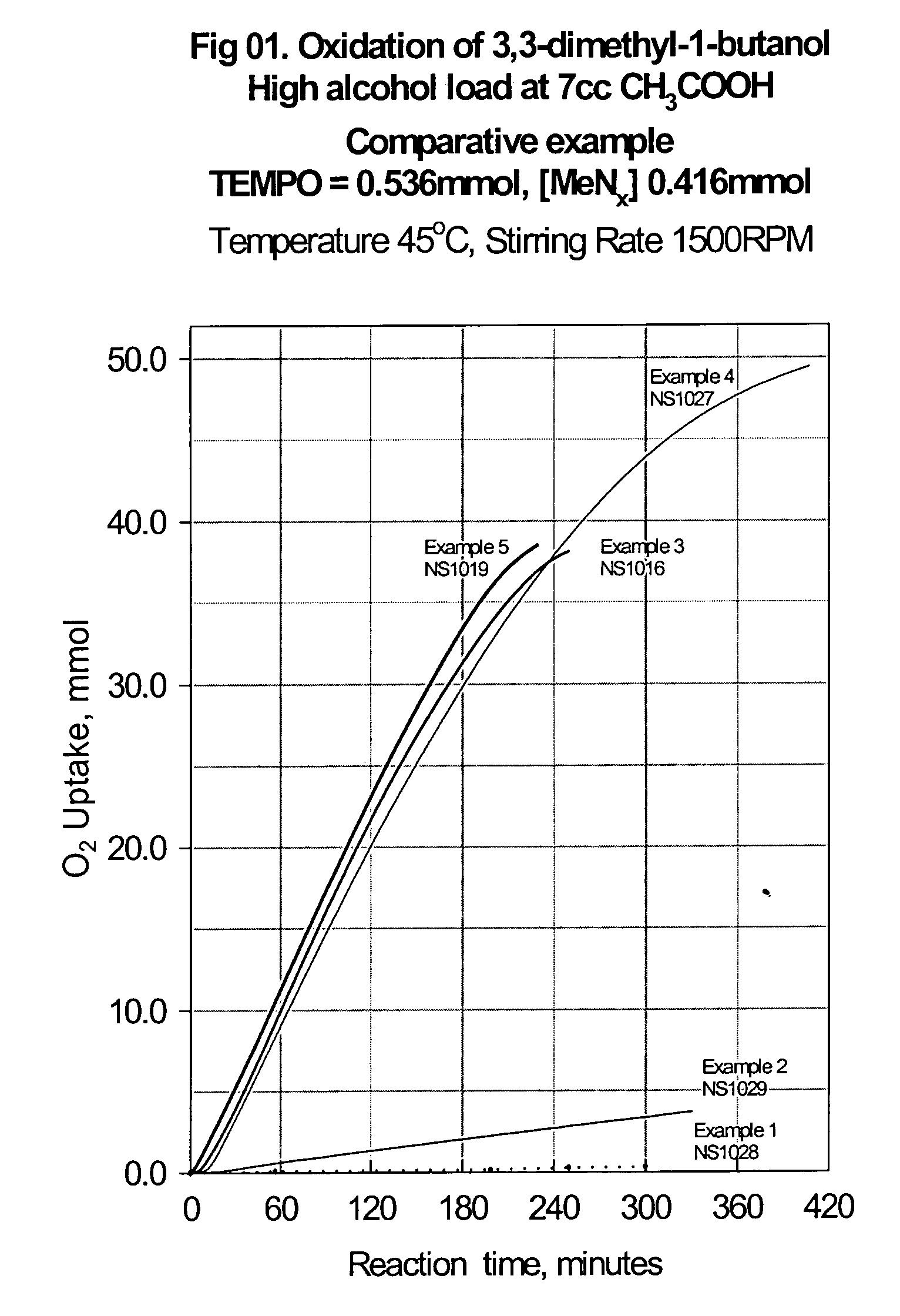
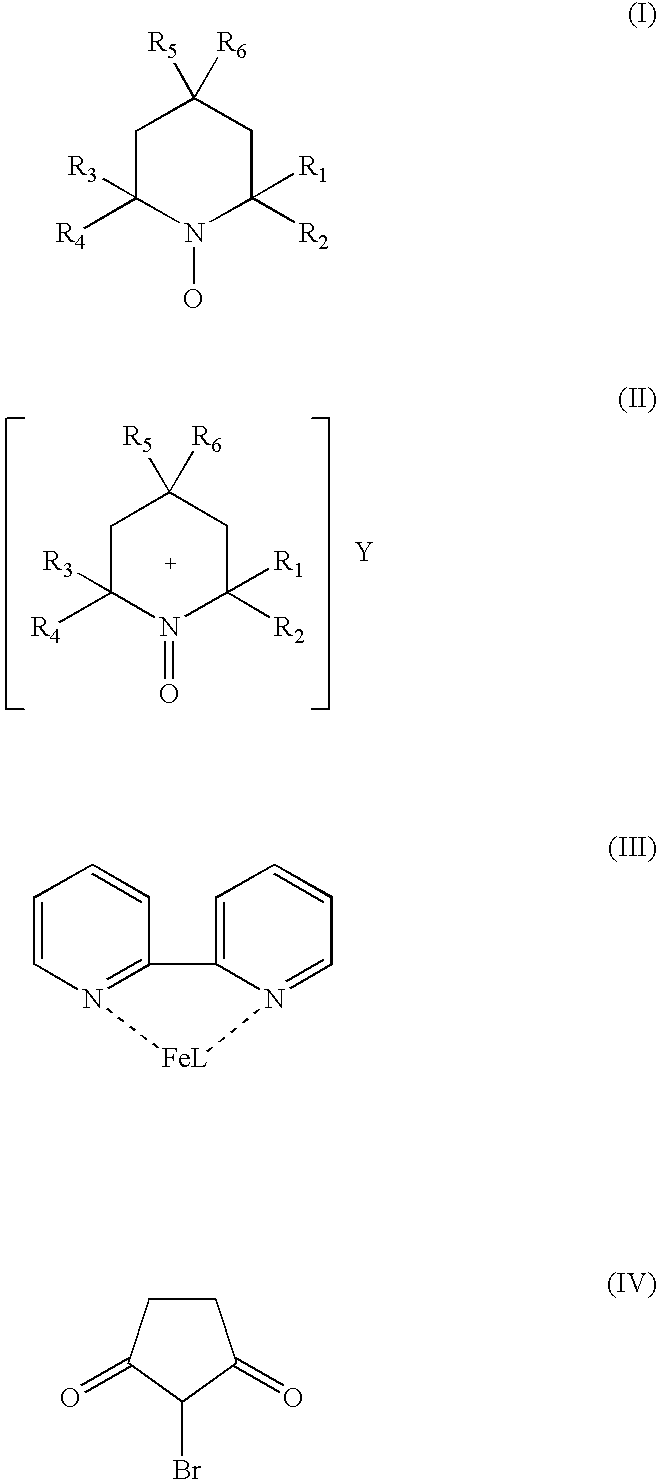
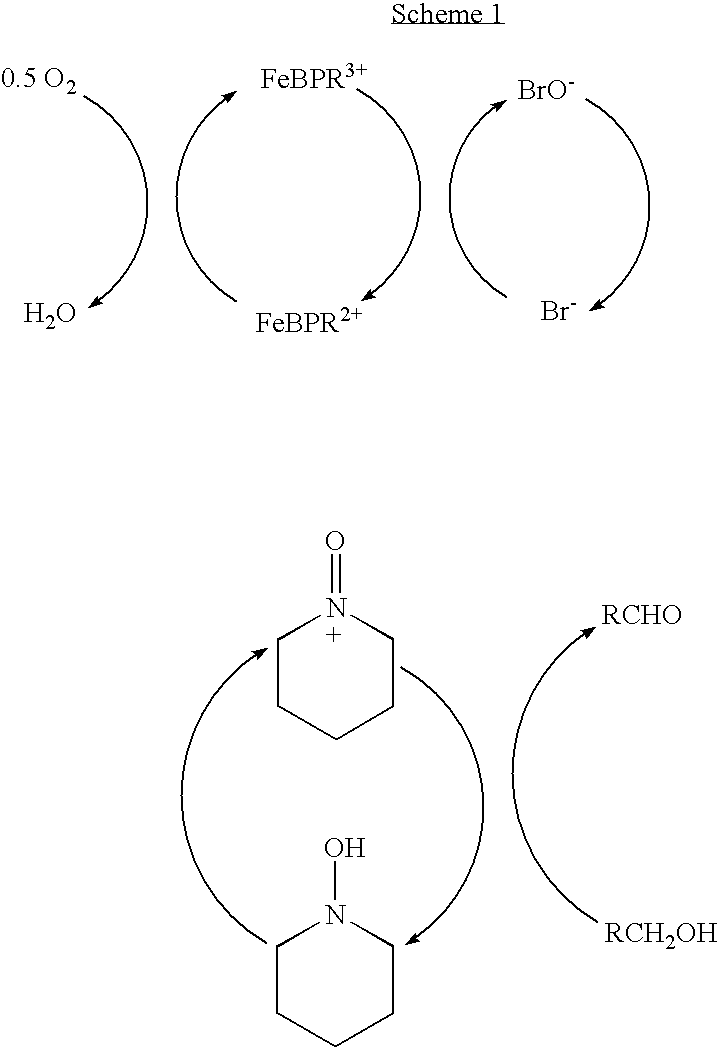
Catalyst system for aerobic oxidation of primary and secondary alcohols
InactiveUS20070078284A1Organic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsAcetic acidN-Bromosuccinimide
The present invention relates to a process of oxidation of alcohols selectively to aldehydes or ketones with molecular oxygen using a TEMPO based catalyst, Fe-bipyridyl or Fe-phenantroline co-catalyst and N-bromosuccinimide promoter in acetic acid solvent. The oxidation takes places at high rates and high aldehyde selectivity at temperatures in the range 45-50° C. and oxygen or air pressures of 0-15 psi. The alcohol conversion of 95-100% and aldehyde selectivity higher than 95% are achieved over 3-4 hours reaction time. Aldehydes such as 3,3-dimethyl-1-butanal can be produced efficiently using the present invention.
Owner:NUTRASWEET PROPERTY HLDG
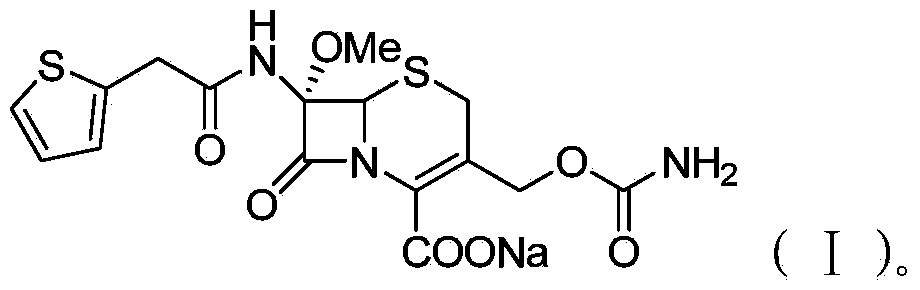
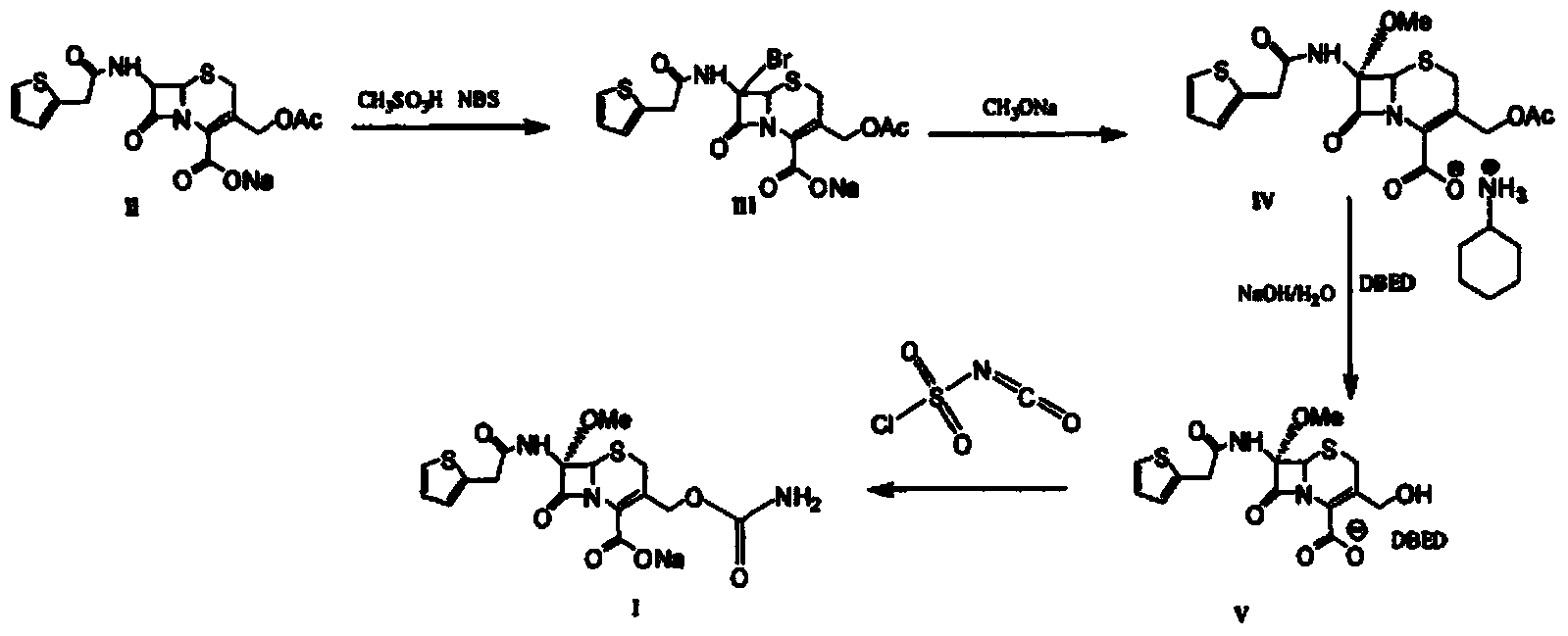
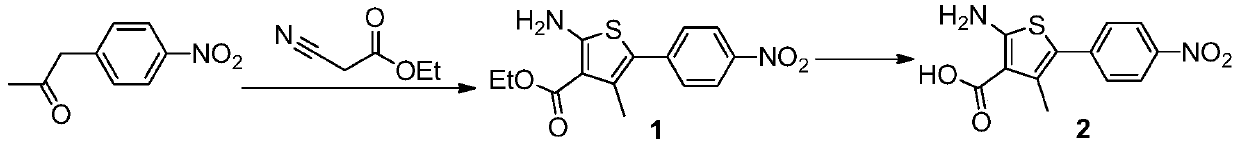
Preparation method of cefoxitin sodium
The invention discloses a preparation method of cefoxitin sodium. The preparation method comprises the following steps: (1) bromizing, for example, the site 7 of a main nucleus of cefalotin by using an NBS (N-bromosuccinimide) reagent to form a bromination compound; performing nucleophilic substitution at the site 5 by using a methoxyl group to generate an intermediate IV; (2) performing acyl group hydrolysis on the site 3 of the intermediate IV to obtain an intermediate V; (3) substituting hydrogen atoms on the hydroxyl group by using chloriosulfonyl isocyanate, and then hydrolyzing to obtain the cefoxitin sodium. The method has the advantages of simple process, high product yield, high purity and high reaction selectivity; no special equipment is used in the production; the preparation method is suitable for industrial production.
Owner:HAINAN HULUWA PHARMA GRP CO LTD
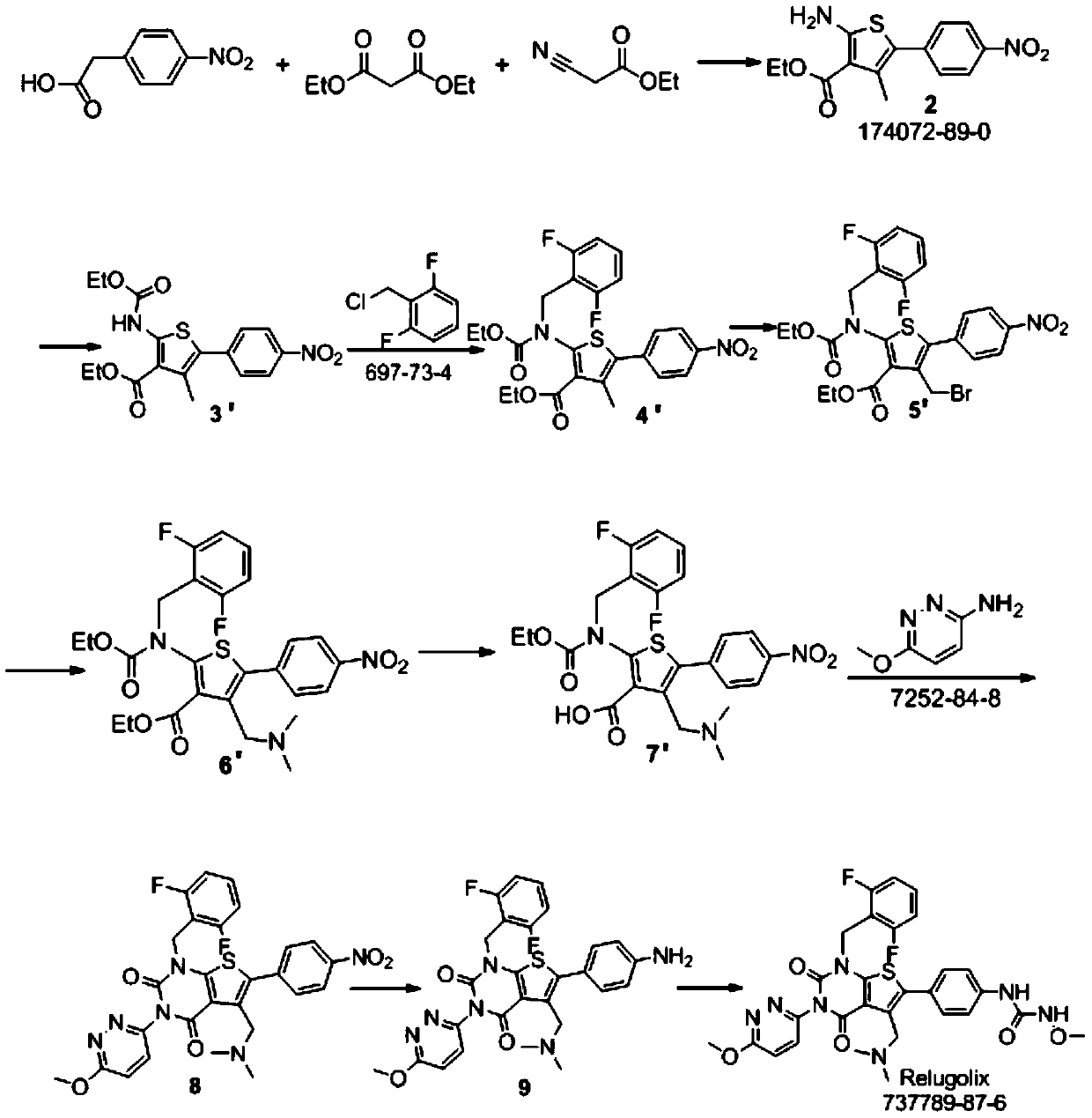
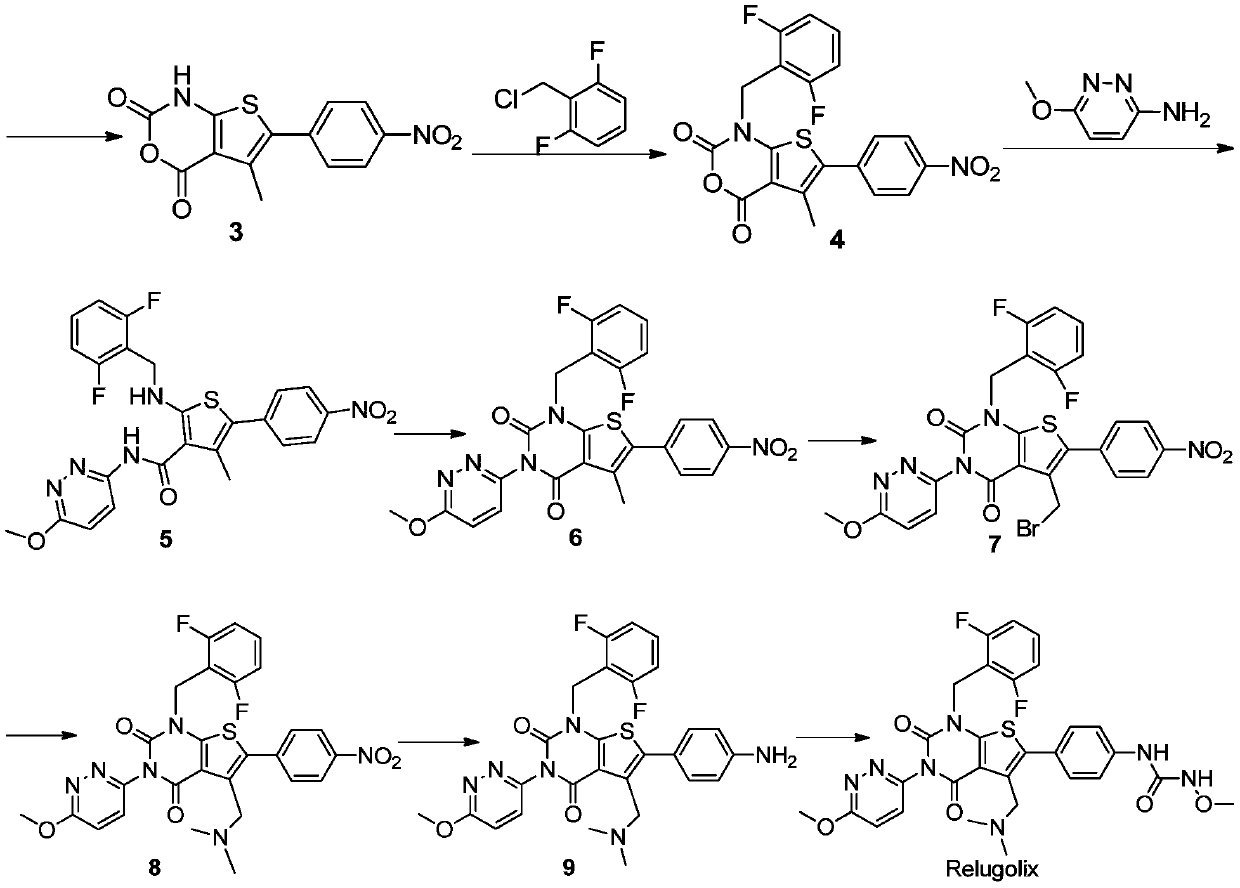
Relugolix synthesis method
The present invention provides a method for preparing a relugolix intermediate compound 8. The method comprises: (a) carrying out a reaction on a compound 2 and N,N'-carbonyldiimidazole to obtain a compound 3; (b) carrying out a reaction on the compound 3 and 2,6-difluorobenzyl chloride to obtain a compound 4; (c) carrying out a reaction on the compound 4 and 3-amino-6-methoxypyridazine to obtaina compound 5; (d) carrying out a reaction on the compound 5 and N,N'-carbonyldiimidazole to obtain a compound 6; (e) carrying out a reaction on the compound 6, N-bromosuccinimide and azobisisobutyronitrile to obtain a compound 7; and (f) carrying out a reaction on the compound 7 and dimethylamine hydrochloride to obtain a compound 8. The invention further provides a relugolix preparation method, which comprises: (g) carrying out a reaction on the compound 8 obtained by the method and hydrogen under a catalyst to obtain a compound 9; and (h) carrying out a reaction on the compound 9, N,N'-carbonyldiimidazole and methoxy amine hydrochloride to obtain relugolix. According to the present invention, the method adopts the route sequentially comprising loop closing and coupling, such that the method has characteristics of simple operation, less side-reaction, mild reaction condition, high yield, high product purity and easy product purification, and is suitable for commercial scale production.
Owner:四川伊诺达博医药科技有限公司
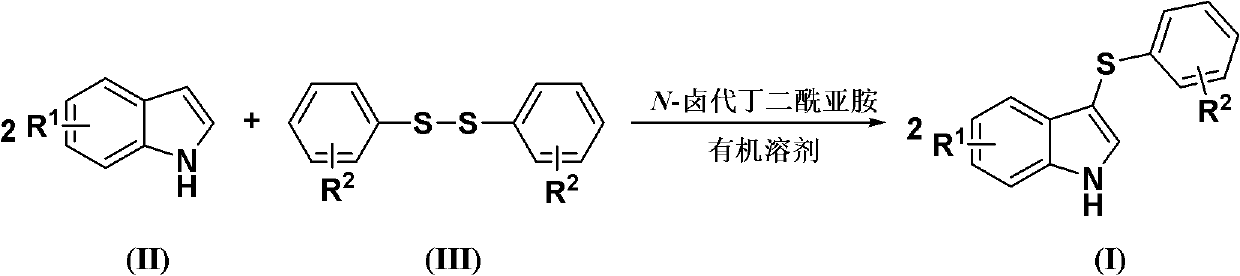
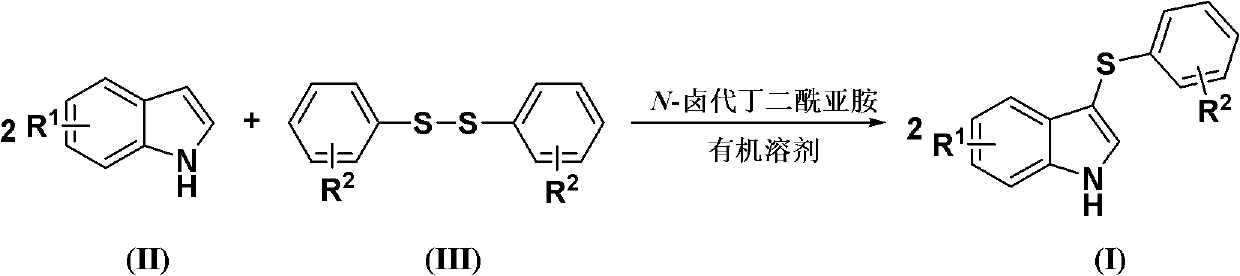
Method for synthesizing 3-aryl sulfydryl indole compound
The invention discloses a method for synthesizing a 3-aryl sulfydryl indole compound which is shown as a formula (I). According to the method for synthesizing the 3-aryl sulfydryl indole compound, an indole compound which is shown as a formula (II) and diaryl disulfides which are shown as a formula (III) are taken as raw materials, and the 3-aryl sulfydryl indole compound is obtained by fully reacting the indole compound and the diaryl disulfides in an organic solvent at the temperature of between -30 and 40 DEG C in the presence of N-halogenate succinimide; and the N-halogenate succinimide is one of N-chlorobutanimide, N-bromosuccinimide, and N-Iodosuccinimide. According to the method, a nonmetal low-toxicity reaction system is used, and cost is low; reaction selectivity and yield are high; a process route is advanced and reasonable, and reaction conditions are mild; and the method has great implementation value and social and economic benefit.
Owner:WENZHOU UNIVERSITY
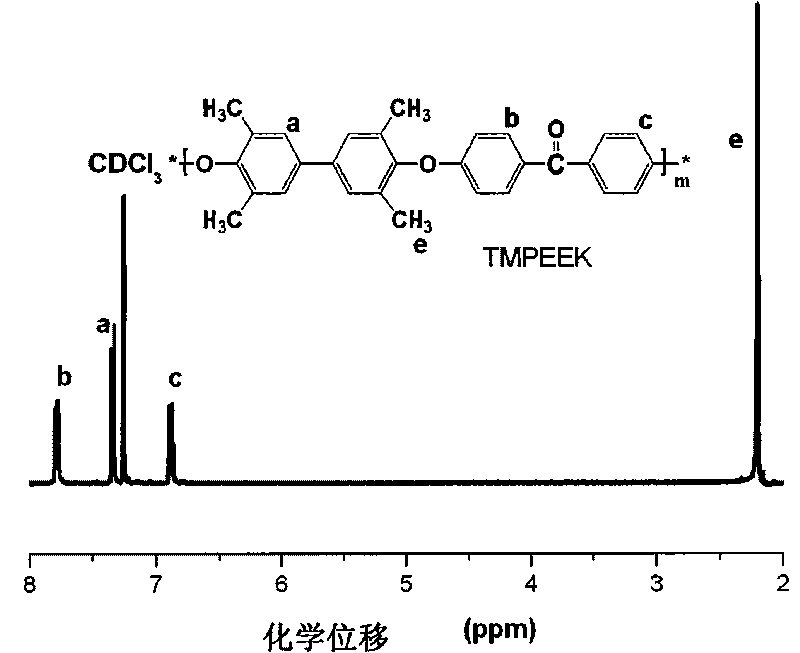
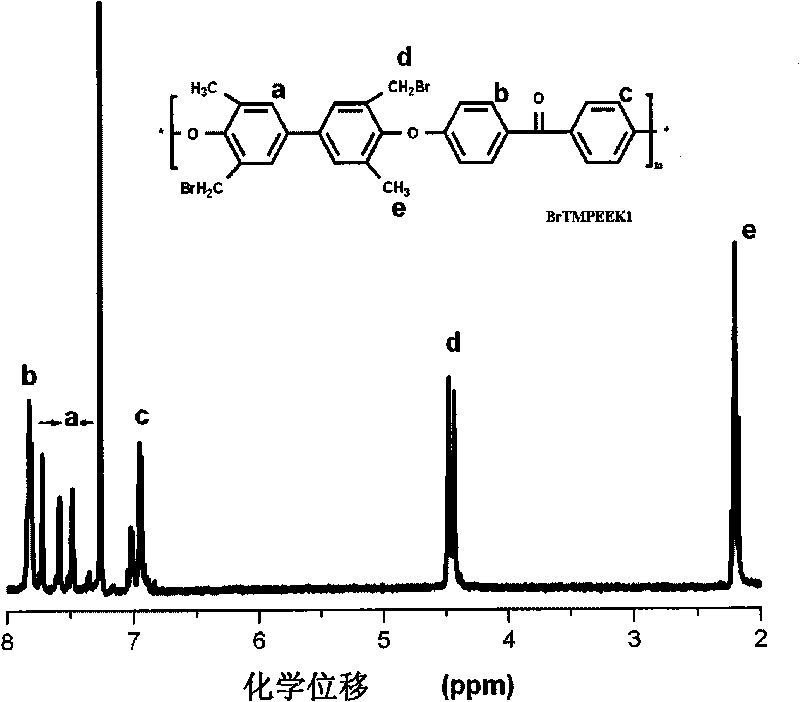
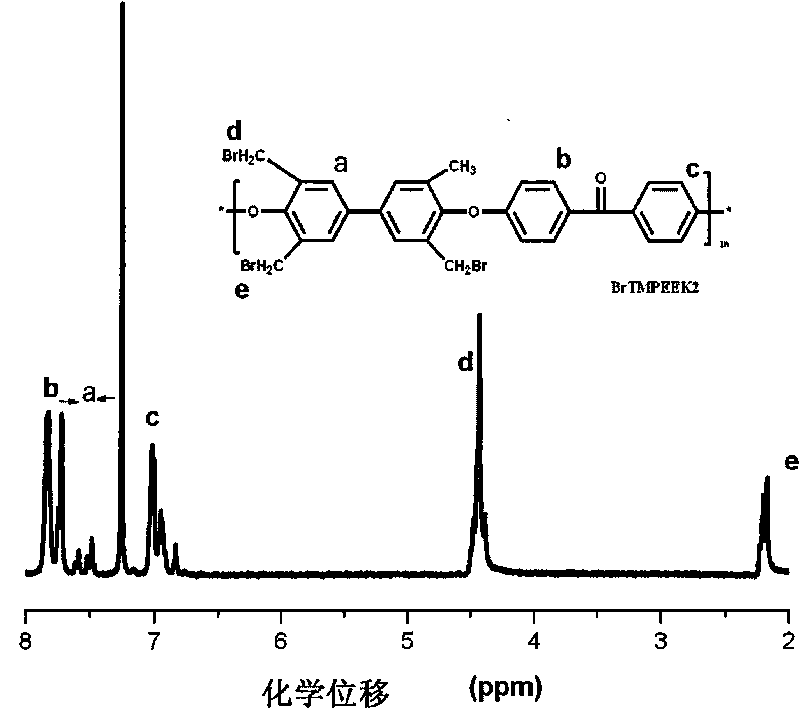
Tetramethyl-diphenol type polyarylether ketone (polyarylether sulphone) containing bromine at phenmethyl position and preparation method thereof
The invention belongs to the field of high-molecular materials, in particular to bromo-polyarylether ketone / polyarylether sulphone the phenmethyl position of which is substituted by bromine and a preparation method thereof. The bromo-polyarylether ketone / polyarylether sulphone is prepared by taking tetramethyl-diphenol type polyarylether ketone (polyarylether sulphone) containing phenacyl as a polymer main body and N-bromosuccinimide as raw materials and introducing a bromine group to the main chain of the polymer. The bromination degree can be effectively controlled by regulating reaction time and the ratio of monomer to the polymer, laying foundations for further functionalizing the polymer. Meanwhile, the introduction of a polar functional group can reduce crystals of the polymer, thereby effectively improving the processability of the polymer.
Owner:JILIN UNIV
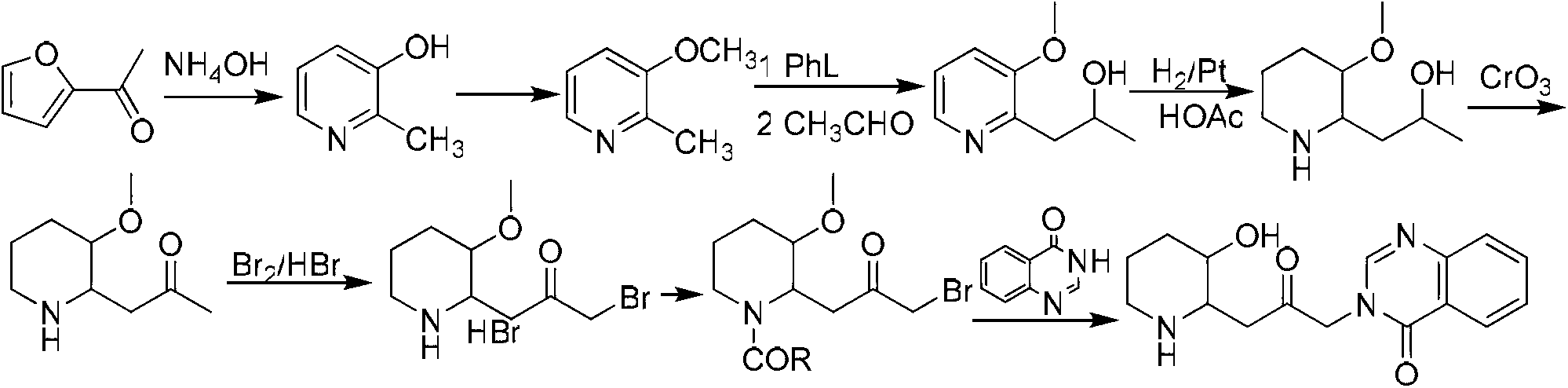
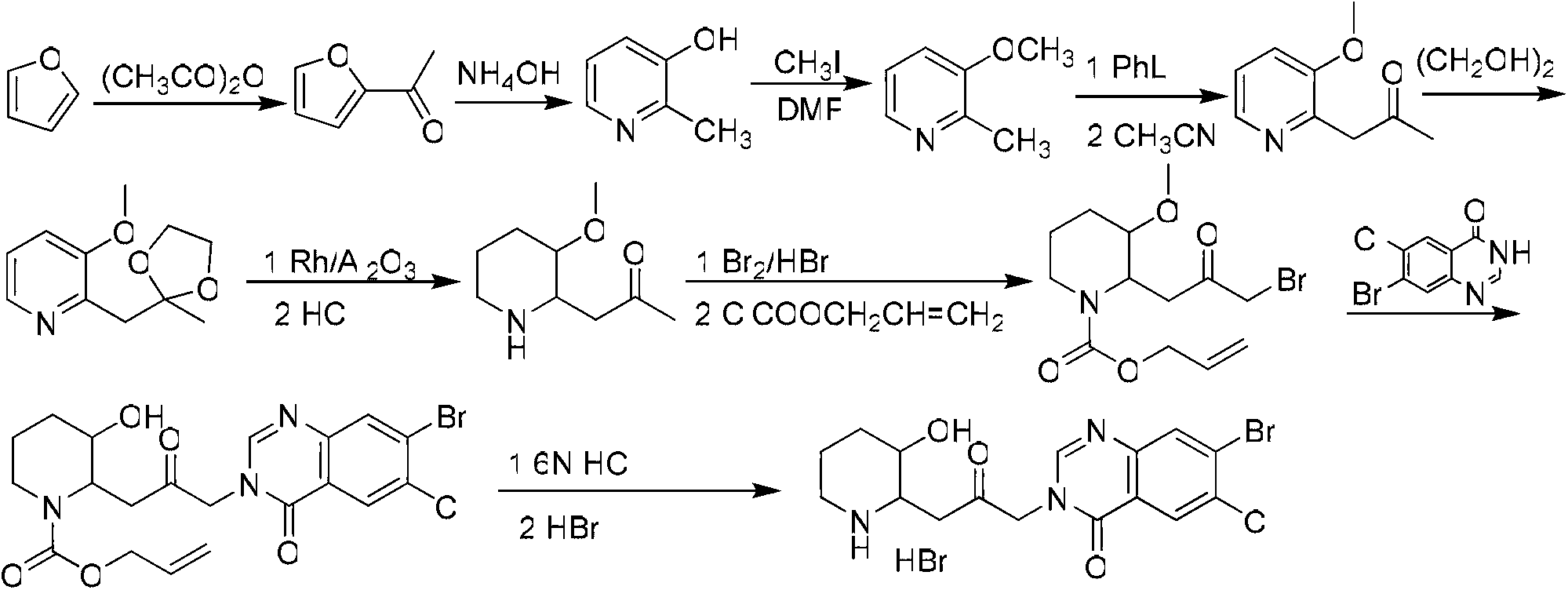
Method for preparing halofuginone hydrobromide
The invention discloses a method for preparing halofuginone hydrobromide, which comprises the following steps: by using N-benzyl-3-piperidone or hydrochloride thereof, organic or inorganic base, halide of alkali metal and beta, gamma-dihaloalkene or allyl haloalkane as initial raw materials, performing Steven rearrangement reaction to obtain an intermediate VIII; performing Von Braun reaction on the intermediate VIII to obtain an intermediate VII; performing reduction reaction on the intermediate VII to obtain an intermediate VI; obtaining an intermediate V after reaction of the intermediate VI and NBS (N-bromosuccinimide); obtaining an intermediate IV after the reaction of the intermediate V and a compound 7-bromine-6-chlorine-4(3H) quinazolinone; performing deprotection on the intermediate IV to obtain an intermediate III; performing backflow processing on the intermediate III in ethanol to obtain an intermediate II; and obtaining the halofuginone hydrobromide after the salt forming reaction of intermediate II. The invention develops an efficient and simple method for synthesizing the halofuginone hydrobromide. The total yield can reach over 50 percent, cost is greatly reduced and the product has high purity.
Owner:CHONGQING WEIPENG PHARMA
Synthesis methods of firocoxib and firocoxib intermediate
ActiveCN107686471AMild reaction conditionsShort reaction timeOrganic compound preparationCarbonyl compound preparation by condensationN-BromosuccinimideSynthesis methods
The invention relates to the field of medicine synthesis and provides synthesis methods of firocoxib and a firocoxib intermediate. The synthesis method of the firocoxib intermediate takes 4'-bromopropiophenone as a starting raw material and a condition that thioether which is not environmentally friendly is used as the starting raw material is avoided; the 4'-bromopropiophenone and a methylating reagent are subjected to methylating reaction to obtain a first intermediate; the first intermediate is subjected to sulphination to directly obtain a second intermediate; sulfidation reaction and oxidization reaction are shortened to one-step substitution reaction, so that reaction steps are extremely simplified, the reaction time is shortened and the reaction efficiency is improved. Meanwhile, anNBS (N-Bromosuccinimide) system is also adopted so that the second intermediate is subjected to hydroxylation reaction to directly obtain the firocoxib intermediate, so that the synthesis method is more environmentally friendly and is suitable for large-scale production, and the reaction speed is improved. Furthermore, the synthesis method of the firocoxib comprises the synthesis method of the firocoxib intermediate and the firocoxib can be synthesized by a manner which has more moderate reaction conditions and is more environmentally friendly.
Owner:CHENGDU EASTON BIOPHARMACEUTICALS CO LTD +1
Fluorochrome for cytolysosome positioning and preparation method and application thereof
InactiveCN105315988AEasy to operateRaw materials are easy to getOrganic chemistryMicrobiological testing/measurementMorpholineCytotoxicity
The invention discloses fluorochrome for cytolysosome positioning and a preparation method and application thereof. The preparation method of the fluorochrome compound includes the steps that a benzophenone derivative containing methyl substituents is reacted under the catalysis of Zn / TiC14, an obtained product and N-bromosuccinimide are subjected to a bromination reaction, then a product is reacted with morpholine, and the tetraphenyl ethylene derivative stain containing morpholine groups is obtained. The preparation method is easy to implement, raw materials are easy to get, and reaction conditions are mild. The obtained fluorochrome contains tetraphenyl ethylene fluorescence groups capable of gathering the activity of inducing fluorescence emission and can be used for performing specific staining on all kinds of cytolysosome. The fluorochrome has the advantages of being small in cytotoxicity, stable in cell metabolism resistance, good in photobleaching resisting effect and the like and hardly affects normal physiological activities of cells, and a new method is provided for observing the morphologic changes of cytolysosome for a long time. The fluorochrome can be widely applied to monitoring the morphology of cytolysosome, observing the apoptosis process and the like.
Owner:CENT SOUTH UNIV
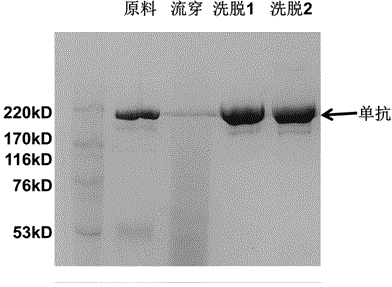
Chromatogram medium for immunoglobulin class protein separation purification and preparation method thereof
InactiveCN101185881AImprove adsorption capacityHigh purityOther chemical processesPeptide preparation methodsCell culture supernatantChromatography column
The invention relates to a chromatography which is used for isolating and purifying immunoglobulin protein and a preparation method and belongs to the preparation technology of isolating and purifying a chromatography medium of the immunoglobulin. The chromatography medium refers to the end of a space arm that is coupled with a double-loop compound molecular which has an imidazol group and a benzene ring as a chromatography medium matrix. The preparation method uses an allylic chromatography medium and a bromide alchoholization reaction of N-bromosuccinimide to get an activated chromatography medium which reacts with the amino of the double-loop compound to complete the coupling of the matrix in dimethyl sulfoxide solution. The chromatography medium has higher dynamic adsorption capacity to the antibody within the ionic strength scope from 0.02 mol / L to 2.0 mol / L and is directly applied to the recycling of the antibody in the solid to liquid; at he same time, the medium has more moderate elution condition and can purify the antibody from serum, ascites, cell culture supernatant and other solid to liquid having the antibody. The chromatography medium has the wide application prospect in the process of antibody preparation.
Owner:TIANJIN UNIV
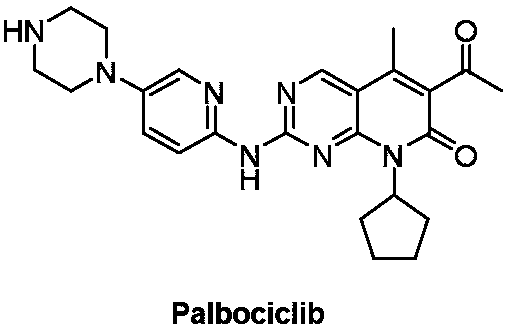
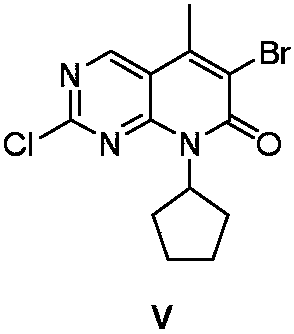
Preparation method of Palbociclib intermediate
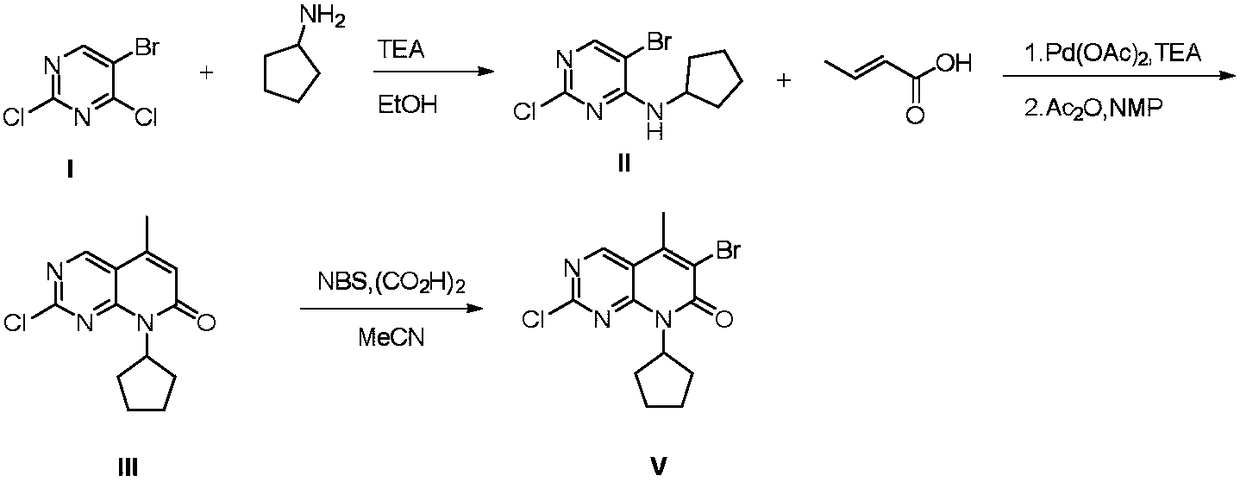
The invention relates to the field of medicine synthesis, and discloses a preparation method of a Palbociclib intermediate. In the preparation process, 5-bromine-2,4-dichloropyrimidine is used as a starting raw material; through ammoniation substitution reaction, green solvent PEG (polyethylene glycol) promotion palladium catalysis coupled reaction, BTC (triphosgene) promotion cyclization reactionand NBS (N-bromosuccinimide) bromination reaction, a target compound V is finally obtained; through aftertreatment improvement, the HPLC purity of the final product can reach 99 percent or higher. Compared with a traditional process, the preparation method has the main beneficial effects that the reaction conditions are mild; the operation is simple and convenient; the palladium catalyst consumption is low; the yield is high; the cost is low; the three-waste quantity is small; the industrialization is easy; high implementation values and socioeconomic benefits are realized.
Owner:HANGZHOU FST PHARMA +1
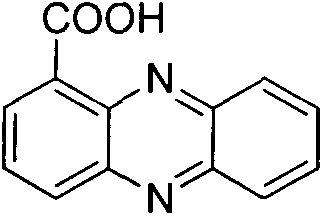
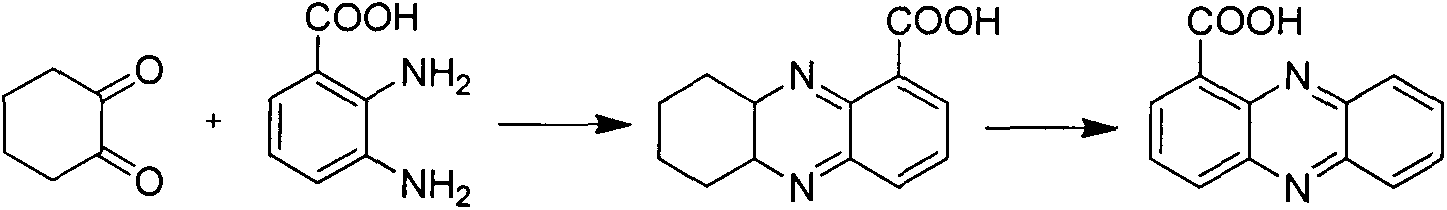
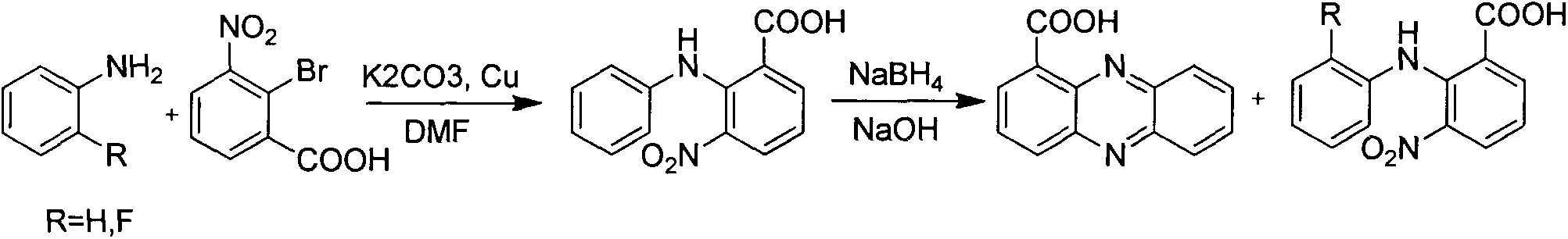
Method for synthesizing phenazine-1-carboxylic acid
The invention provides a method for synthesizing phenazine-1-carboxylic acid. The method comprises the steps of carrying out bromination reaction on 1-methyl-phenazine as a starting material and N-bromosuccinimide in the presence of benzoyl peroxide as a catalyst to produce 1-bromomethylphenazine, further hydrolyzing 1-brominemethylphenazine to obtain 1-hydroxymethyl phenazine and finally oxidizing to obtain phenazine-1-carboxylic acid, namely, shenqinmycin. According to the method disclosed by the invention, disadvantages of low yield, relatively high production cost, high treatment cost of three wastes and the like are solved, and the method for synthesizing phenazine-1-carboxylic acid with the advantages of simple synthetic steps, high yield, low cost and environment friendliness is provided.
Owner:NANJING TECH UNIV
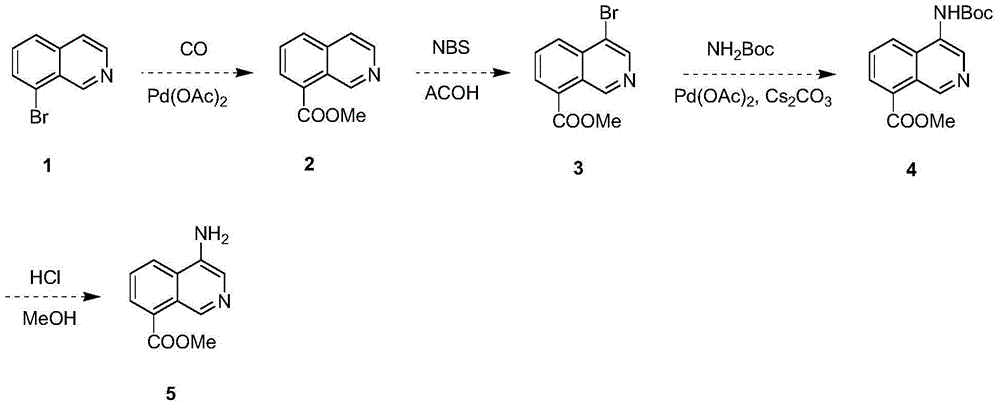
Synthesis method of 4-aminoisoquinoline-8-methyl formate
ActiveCN104447547AGood choiceHigh yieldOrganic chemistryBulk chemical productionCarbamateSynthesis methods
The invention provides a synthesis method of 4-aminoisoquinoline-8-methyl formate. According to the synthesis method, 8-bromoisoquinoline reacts with carbon monoxide in methanol in the presence of palladium acetate to generate 8-isoquinoline methyl formate; 8-isoquinoline methyl formate reacts with N-bromosuccinimide in acetic acid to generate 4-bromoisoqunoline-8-methyl formate; the compound reacts with tert-butyl carbamate in the presence of the palladium acetate and cesium carbonate to generate amino protected 4-t-butoxycarbonylaminoisoquinoline-8-methyl formate, and finally, the protecting group of 4-t-butoxycarbonylaminoisoquinoline-8-methyl formate is removed in a mixed solvent of hydrochloric acid and methanol to obtain 4-aminoisoquinoline-8-methyl formate. The synthesis method of 4-aminoisoquinoline-8-methyl formate is good in selectivity, high in total yield (71%), convenient to operate, simple in after-treatment and suitable for large-scale production.
Owner:SUZHOU KANGRUN PHARMA
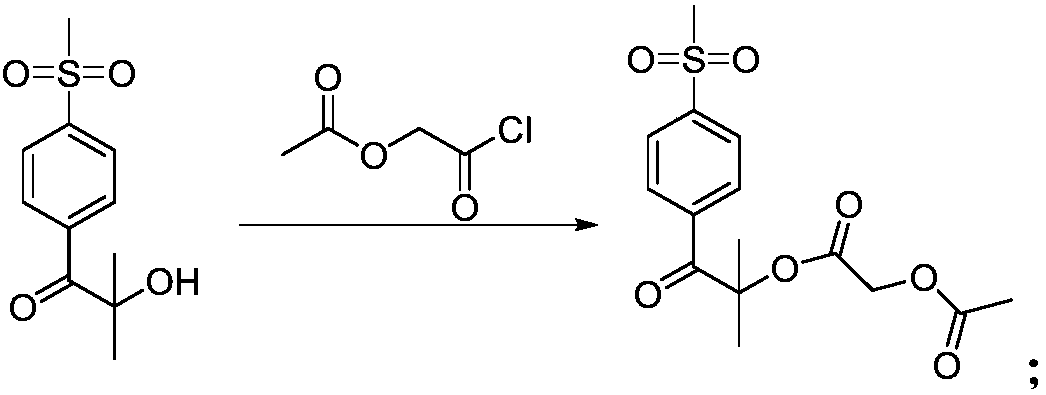
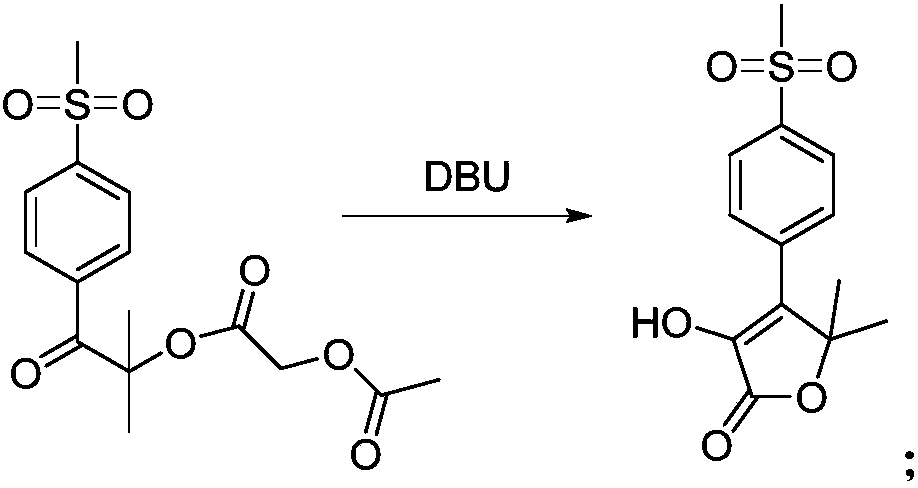
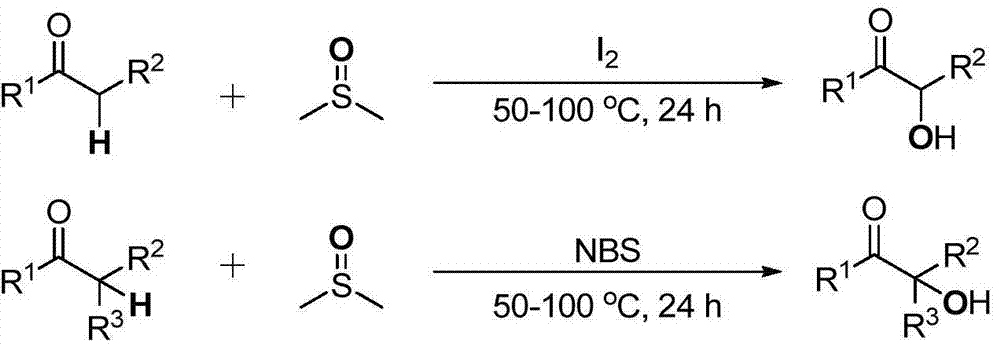
Cheap and efficient synthesis method of alpha-hydroxyketone compound
ActiveCN104710256AEmission reductionHigh yieldOrganic compound preparationHydroxy group formation/introductionSynthesis methodsEthyl acetate
The invention discloses a cheap and efficient synthesis method of an alpha-hydroxyketone compound. The synthesis method is characterized in that a carbonyl compound undergoes an oxidation hydroxylation reaction at 10-120DEG C under normal pressure with iodine simple substance, N-bromosuccimide, copper bromide, bromine simple substance, hydrogen bromide, N-iodosuccimide or hydrogen iodide as a catalyst, sulfoxide as an oxidant, water or sulfoxide as a hydroxy source and sulfoxide, ethyl acetate, N,N-dimethyl formamide, acetonitrile, toluene, 1,4-dioxane, 1,2-dichloroethane, tetrahydrofuran or H2O as a solvent, and converts into the alpha-hydroxyketone compound in a high selectivity manner. Compared with traditional synthesis methods, the method disclosed in the invention has the advantages of simple operation, high yield, simple conditions, easy purification, small waste discharge amount, simple reaction apparatus, and easy industrial production. The method has wide applicability and can be used for synthesizing various alpha-hydroxyketone compounds.
Owner:QINGDAO RUIJI MEDICAL TECH CO LTD
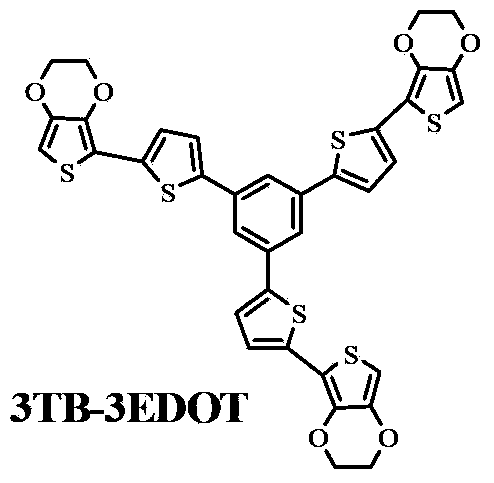
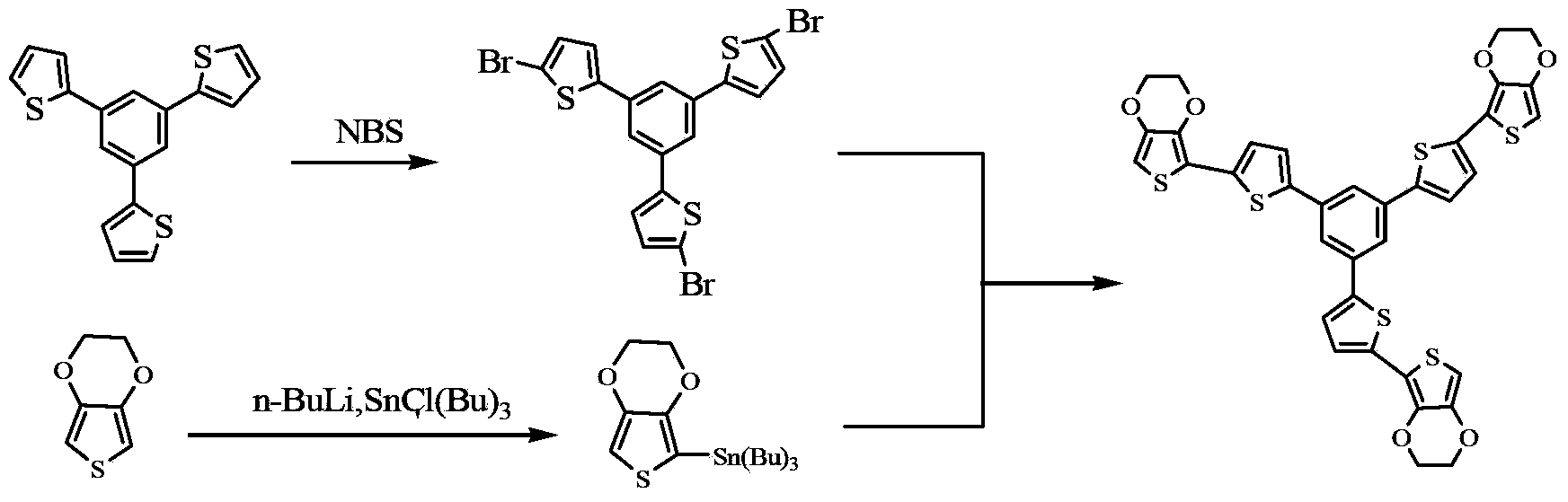
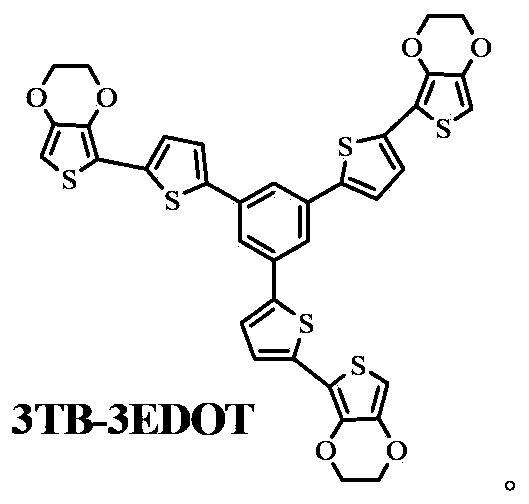
Oligothiophene derivative and preparation method thereof
The invention relates to an oligothiophene derivative and a preparation method thereof. The preparation method of the oligothiophene derivative mainly comprises the following steps of: dissolving 3TB into dichloromethane, adding NBS (N-bromosuccinimide), stirring and reacting so as to obtain 3TB-3Br; adding EDOT (3,4-ethylenedioxythiophene) and tetrahydrofuran into a three-neck flask, controlling the reaction temperature to be minus 78 DEG C, dropwise adding n-butyllithium, gradually raising to be room temperature after the dropwise adding, and continuously reacting; subsequently decreasing the reaction temperature to be minus 78 DEG C, dropwise adding tributyl stannic chloride, gradually raising to be the room temperature after the dripping, and reacting to obtain an EDOT tin reagent; under the protection of nitrogen, adding methylbenzene into the three-neck flask, further adding the 3TB-3Br and the EDOT tin reagent, carrying out backflow reaction so as to obtain a 3TB-3Br crude product, thus obtaining pure 3TB-3EDOT through separation. As cation (or anion) free radicals generated in electrochemical doping are stable, the oligothiophene derivative can be used as a novel organic electrochromic material.
Owner:SOUTH CHINA UNIV OF TECH
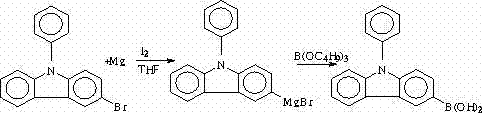
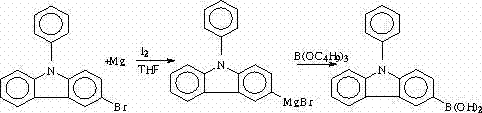
Synthesis method of N-phenyl-3(4-bromophenyl) carbazole
The invention discloses a synthesis method of N-phenyl-3(4-bromophenyl) carbazole. The synthesis method is characterized in that carbazole is selected as a raw material and is subjected to Ullmann reaction with iodobenzene to prepare N-phenyl carbazole; N-phenyl-3-bromine carbazole is synthesized by NBS (N-bromosuccinimide) bromination; N-phenyl-3-boric acid base carbazole is prepared through Grignard coupling; and N-phenyl-3-boric acid base carbazole and p-bromoiodobenzene are subjected to cross coupling to obtain N-phenyl-3(4-bromophenyl) carbazole. The method provided by the invention has the characteristics of high yield, low production cost, less three-waste emission and high target compound selectivity and yield, a high-efficiency loaded catalyst is used, and isomer is reduced.
Owner:山东盛华电子新材料有限公司
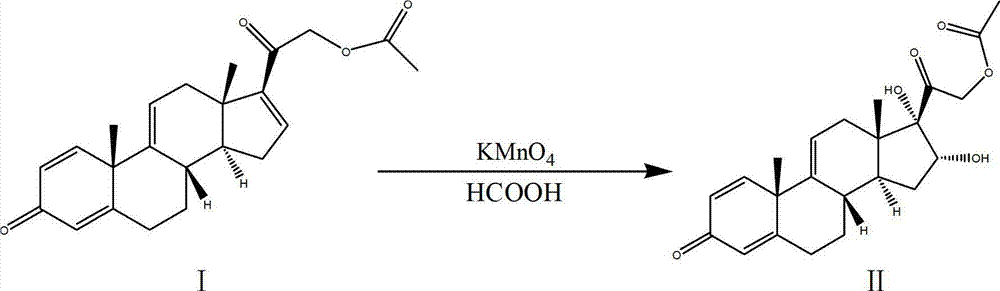
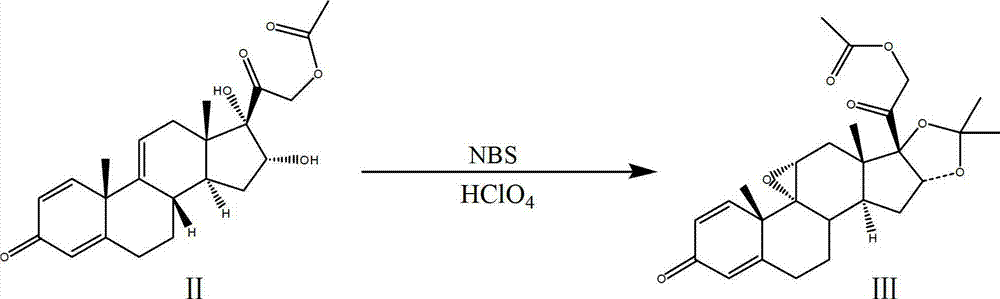
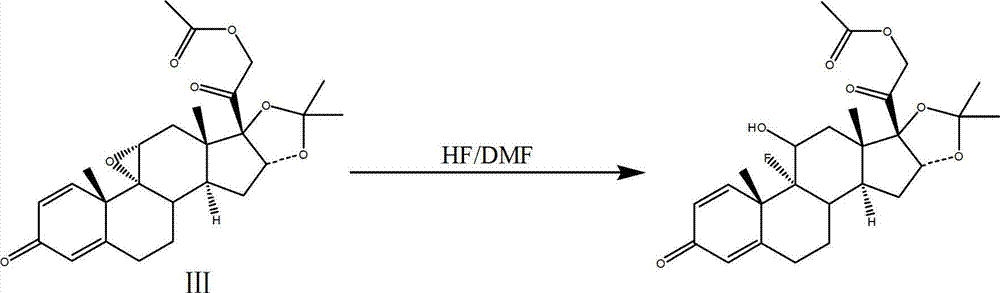
Process for synthesizing triamcinolone acetonide acetate
The invention discloses a process for synthesizing triamcinolone acetonide acetate. The process comprises the steps of firstly, taking an acetic acid tetraene material as a raw material, and obtaining an oxide through an oxidation reaction under the effect of methanoic acid and potassium permanganate; secondly, taking the oxide as a raw material, and obtaining a ring-reducing material through a ring-reducing reaction under the effect of perchloric acid and N-bromosuccinimide; thirdly, taking the ring-reducing material as a raw material, and conducting a fluorination reaction under the effect of fluorine hydride and dimethylformamide to obtain a triamcinolone acetonide acetate crude product; and fourthly, purifying the triamcinolone acetonide acetate crude product to obtain the triamcinolone acetonide acetate. The process is mild in reaction condition, easy to control, low in toxicity and dangerousness of used auxiliary materials, low in pollution and applicable to industrial production, the triamcinolone acetonide acetate which is synthesized by the process is subjected to high performance liquid chromatography (HPLC) detection, the purity can exceed 99%, and the yield is high.
Owner:BAOJI KANGLE BIOTECH
Preparation method for 5-benzyl-7(S)-t-butyloxycarborylamino-5-aza-spiro[2,4]heptanes
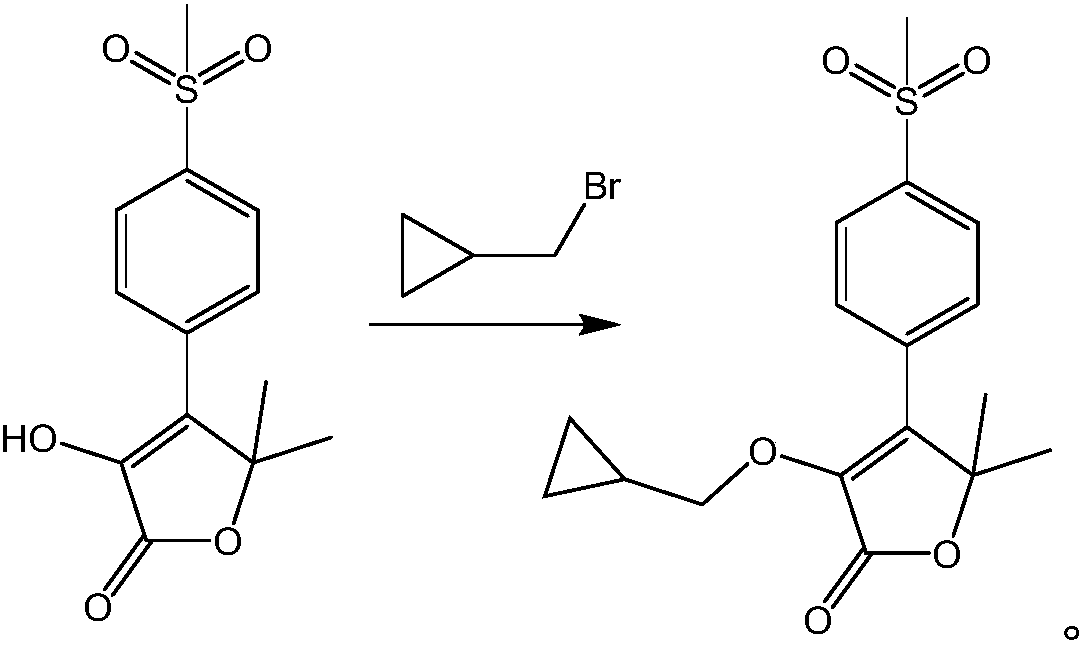
ActiveCN103420896APromote safe productionEasy to operateOrganic chemistryTert-Butyloxycarbonyl protecting groupKetone
The invention discloses a preparation method for 5-benzyl-7(S)-t-butyloxycarborylamino-5-aza-spiro[2,4]heptane. The method comprises the following steps: reacting benzoylamide acetoacetate as a raw material with 1,2-dichloroethane to obtain 3-cyclopropyl benzoylamide acetoacetate, brominating 3-cyclopropyl benzoylamide acetoacetate by NBS (n-bromosuccinimide) to obtain 1-bromo-3-cyclopropyl benzoylamide acetoacetate, cyclizing under alkaline conditions to obtain 5-benzyl-5-aza-spiro[2,4]heptane-4,7-diketone, further reacting with hydroxylamine hydrochloride to form an oxime compound-4-oxo-5-benzyl-7-oximido-5-aza-spiro[2,4]heptane, reducing by NaBH4 and boron trifluoride diethyl etherate to obtain 5-benzyl-7-amino-5-aza-spiro[2,4]heptane, performing chiral resolution by a resolving agent-L-camphorsulfonic acid to obtain 5-benzyl-7(S)-amino-5-aza-spiro[2,4]heptane, and reacting with di-tert-butyl dicarbonate ester to obtain 5-benzyl-7(S)-t-butyloxycarborylamino-5-aza-spiro[2,4]heptane. According to the method, an intermediate body-carbonyl does not need protection, raw materials are easy to get, a process route is simple, and the method is suitable for industrial production.
Owner:苏州楚凯药业有限公司
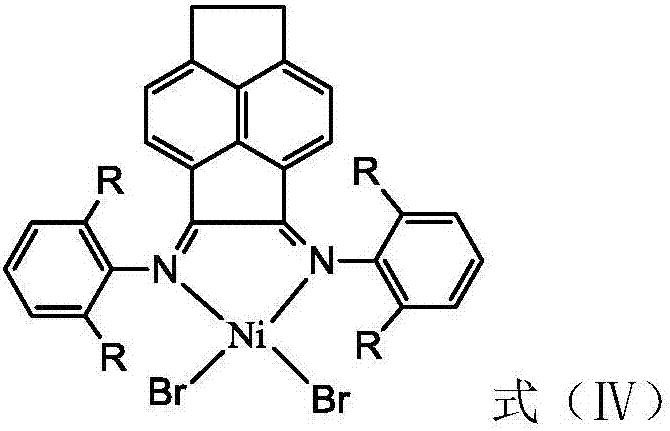
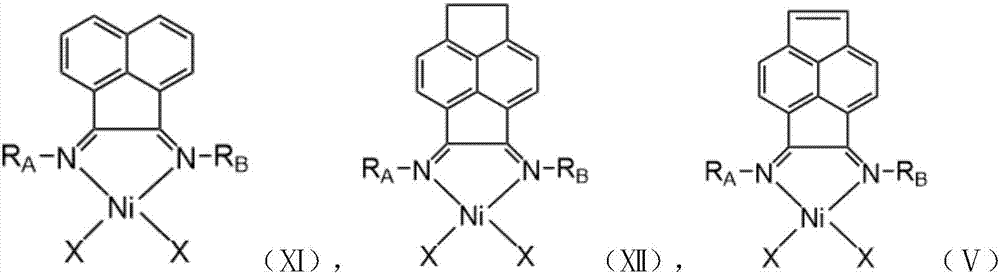
Vinylene acenaphthene (alpha-diimine) nickel olefin catalyst, and preparation method and application thereof
ActiveCN108003259AHigh activityImprove thermal stabilityNickel organic compoundsBulk chemical productionKetoneAniline
The invention relates to the field of olefin catalytic polymerization, and aims at providing a vinylene acenaphthene (alpha-diimine) nickel olefin catalyst, and preparation and an application thereof.The preparation comprises the typical synthetic steps: carrying out a diacylation reaction of acenaphthene to obtain a compound C1; carrying out a bromination reaction of the compound C1 and N-bromosuccinimide (NBS) to obtain a compound C2; carrying out an elimination reaction of the compound C2 to obtain a compound C3; carrying out ketone amine condensation reaction of the compound C3 with symmetric aniline, to obtain alpha-diimine ligands C4-C8; and under anhydrous and anaerobic conditions, complexing the alpha-diimine ligands C4-C8 with ethylene glycol dimethyl ether nickel dibromide to obtain a final product. The catalyst has higher activity and better thermal stability, and can catalyze ethylene with high activity at the temperature of greater than or equal to 60 DEG C to obtain high-molecular-weight hyperbranched polyethylene. Under the same polymerization conditions, ethylene can be catalyzed to polymerize to obtain branched polyethylene with higher molecular weight, so as to meet more application requirements. The cost of raw materials is low, the reaction yield is high and industrialized production can be achieved.
Owner:ZHEJIANG UNIV
Preparation method for di-thiophene-[2, 3-b; 3', 2'-d] thiophene
The invention relates to a preparation method of dithiophene-[2, 3-b; 3`, 2`-d] thiophene, comprising the following steps of: (1) preparing [3, 3`]-bithiophene through the bromine-lithium exchange to 3-bromo-thiophene and the oxidative coupling to copper chloride; (2) preparing 2, 2`-dibromo-[3, 3`] bithiophene with high yield through the bromation to the prepared [3, 3`]-bithiophene with N-bromosuccinimide NBS; (3) producing 2, 2`-dibromo-5, 5`-bis(trimethylsilyl)-[3, 3`]-bithiophene by protecting the 2, 2`-dibromo-[3, 3`] bithiophene with a TMS group; (4) preparing 2, 5-bis(trimethylsilyl)-dithiophene-[2, 3-b; 3`, 2`-d] thiophene by implementing the bromine-lithium exchange and sulfo-ring closure to the 2, 2`-dibromo-5, 5`-bis(trimethylsilyl)-[3, 3`]-bithiophene; and (5) producing dithiophene-[2, 3-b; 3`, 2`-d] thiophene through the de-protection to the 2, 5- bis(trimethylsilyl)-dithiophene-[2, 3-b; 3`, 2`-d] thiophene with trifluoroacetic acid. The method adopts a group TMS assistant in dissolving to protect Alpha active sites of thiophene rings, thereby effectively reducing the generation of side reaction, and increasing the preparation yield of dithiophene-[2, 3-b: 3`, 2`-d] thiophene compounds by 40 to 47 percent.
Owner:HENAN UNIVERSITY
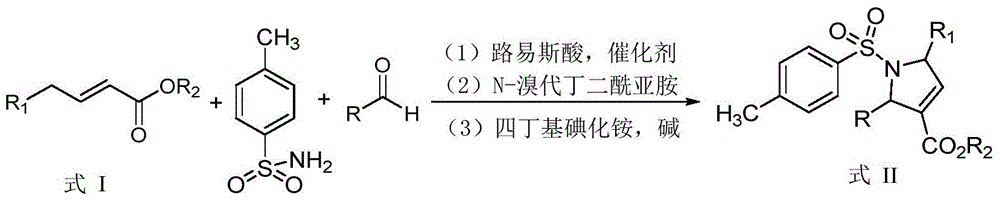
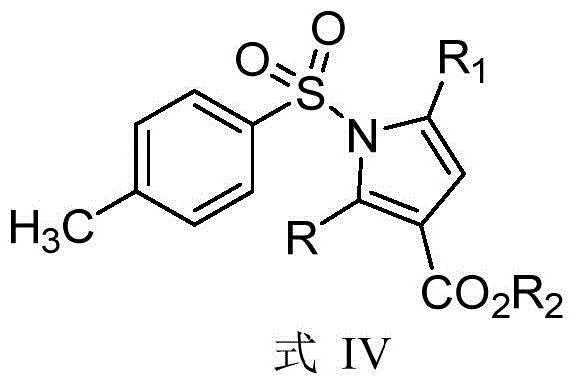
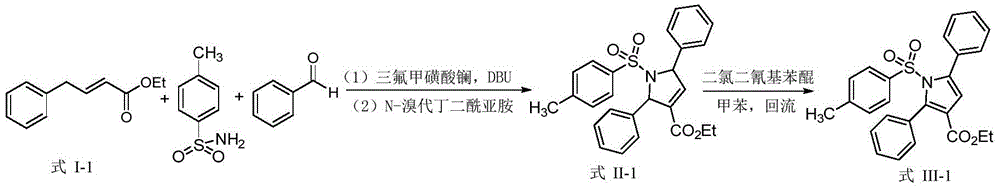
Synthetic method of dihydropyrrole and pyrrole compounds
InactiveCN105712922AHigh yieldHigh stereoselectivityOrganic chemistryIntramolecular cyclizationBenzoquinone
The invention discloses a synthetic method of dihydropyrrole and pyrrole compounds. The method comprises: reacting Alpha,Beta-unsaturated ester as raw material with para toluene sulfonamide and aldehydes under the action of an organic small-molecular catalyst 1,8-diazabicycloundercane-7-ene or 1,5-diazabycyclo[4,3,0]one-5-ene without separating an intermediate product, directly adding N-bromosuccinimide and alkali, and enabling intramolecular cyclization under mild reaction conditions so as to produce multi-group substituted dihydropyrrole compounds difficult to prepare by a conventional method, with high yield and good stereoselectivity. The synthetic method is simple, environment-friendly and high in atomic economy, enables dihydropyrrole compounds to be quickly and efficiently synthesized with one kettle, and the synthesized dihydropyrrole compounds are dehydrogenized under the action of dichloro dicyane benzoquinone to directly obtain pyrrole compounds.
Owner:SHAANXI NORMAL UNIV
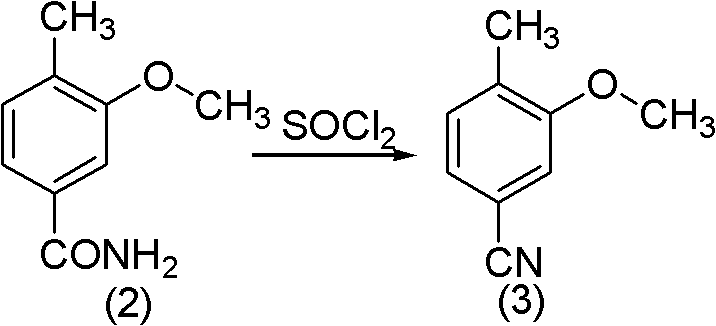
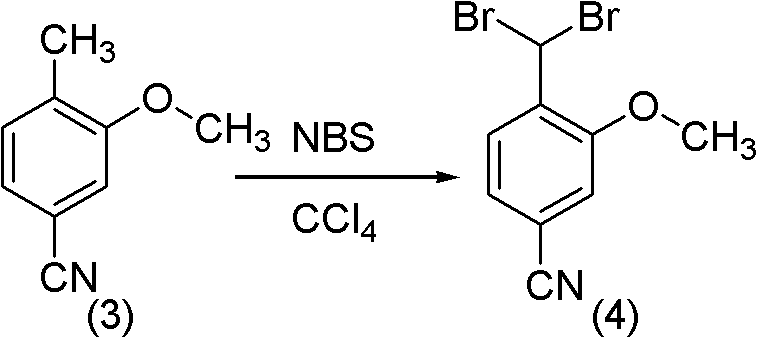
Method for synthesizing 2-methoxy-4-cyano benzaldehyde
PendingCN102020587ACarboxylic acid nitrile preparationOrganic compound preparationN-BromosuccinimideBenzaldehyde
The invention relates to a method for synthesizing 2-methoxy-4-cyano benzaldehyde which servers as an important medicinal intermediate. The method comprises the following steps: causing 3-methoxy-4-methyl benzoate to react with thionyl chloride react under the heating condition to generate 3-methoxy-4-methyl benzoyl chloride; causing the 3-methoxy-4-methyl benzoyl chloride to react with aqueous ammonia to generate 3-methoxy-4-methyl benzoyl amide; dehydrating the 3-methoxy-4-methyl benzoyl amide to generate 3-methoxy-4-methyl benzonitrile; brominating the 3-methoxy-4-methyl benzonitrile by N-bromosuccinimide (NBS) to generate 3-methoxy-4-benzylene bromide benzonitrile; and hydrolyzing the 3-methoxy-4-benzylene bromide benzonitrile to obtain the target product of 2-methoxy-4-cyano benzaldehyde. The method provided by the invention has the outstanding advantages that the reaction condition is mild, the reactions are rapid, the process is simple, and the operation is easy, thereby being suitable for industrial production.
Owner:大连凯飞精细化工有限公司
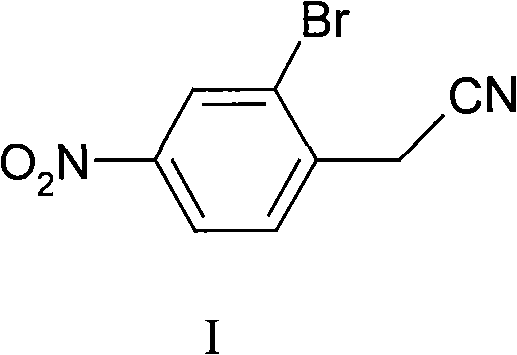
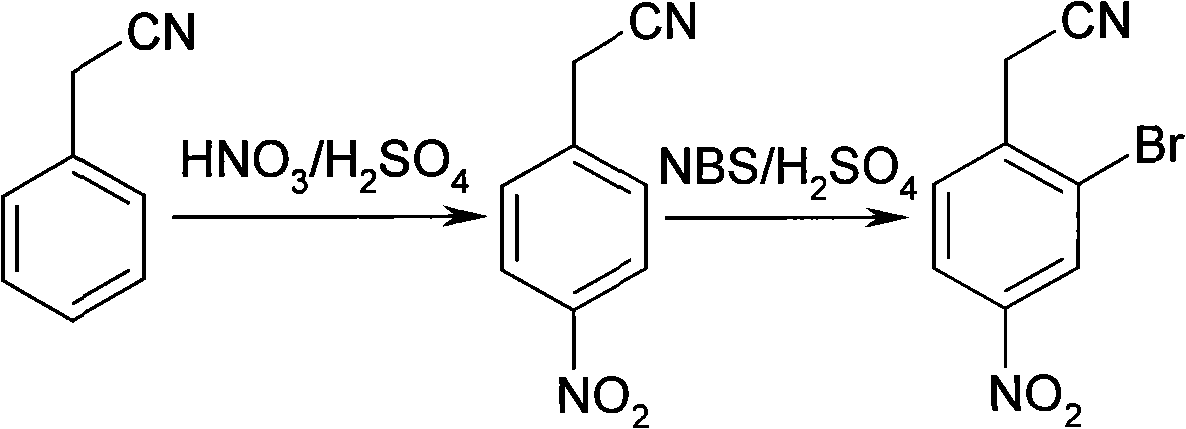
Synthesis of 2-bromine-4-nitrobenzene ethane nitrile
InactiveCN101402589AHigh yieldLow costCarboxylic acid nitrile preparationOrganic compound preparationN-BromosuccinimideIce water
The invention discloses a synthesis method for 2-bromine-4-nitryl phenylacetonitrile. The synthesis method uses phenylacetonitrile as a main starting material and comprises the following: 1) a step of nitrifying: stirring the phenylacetonitrile in mixed acid consisting of concentrated sulfuric acid and concentrated nitric acid to react, putting the obtained reaction product in ice water, separating out solid for filtering to obtain precipitate, and then washing the precipitate by water and drying the precipitation in turn to obtain n-nitro-phenylacetonitrile; and 2) a step of bromizing: dissolving the n-nitro-phenylacetonitrile in the concentrated sulfuric acid, adding N-bromosuccinimide to bromize, putting the obtained reaction mixture in the ice water, separating out solid for filtering to obtain precipitate, and then washing the precipitate by water and drying the precipitate in turn to obtain the 2-bromine-4-nitryl phenylacetonitrile. The 2-bromine-4-nitryl phenylacetonitrile synthesized by the method has the characteristics of simple technique, low cost and high yield.
Owner:ZHEJIANG UNIV
Aggregation-induced emission near-infrared emission diketopyrrolopyrrole compound and preparation method thereof
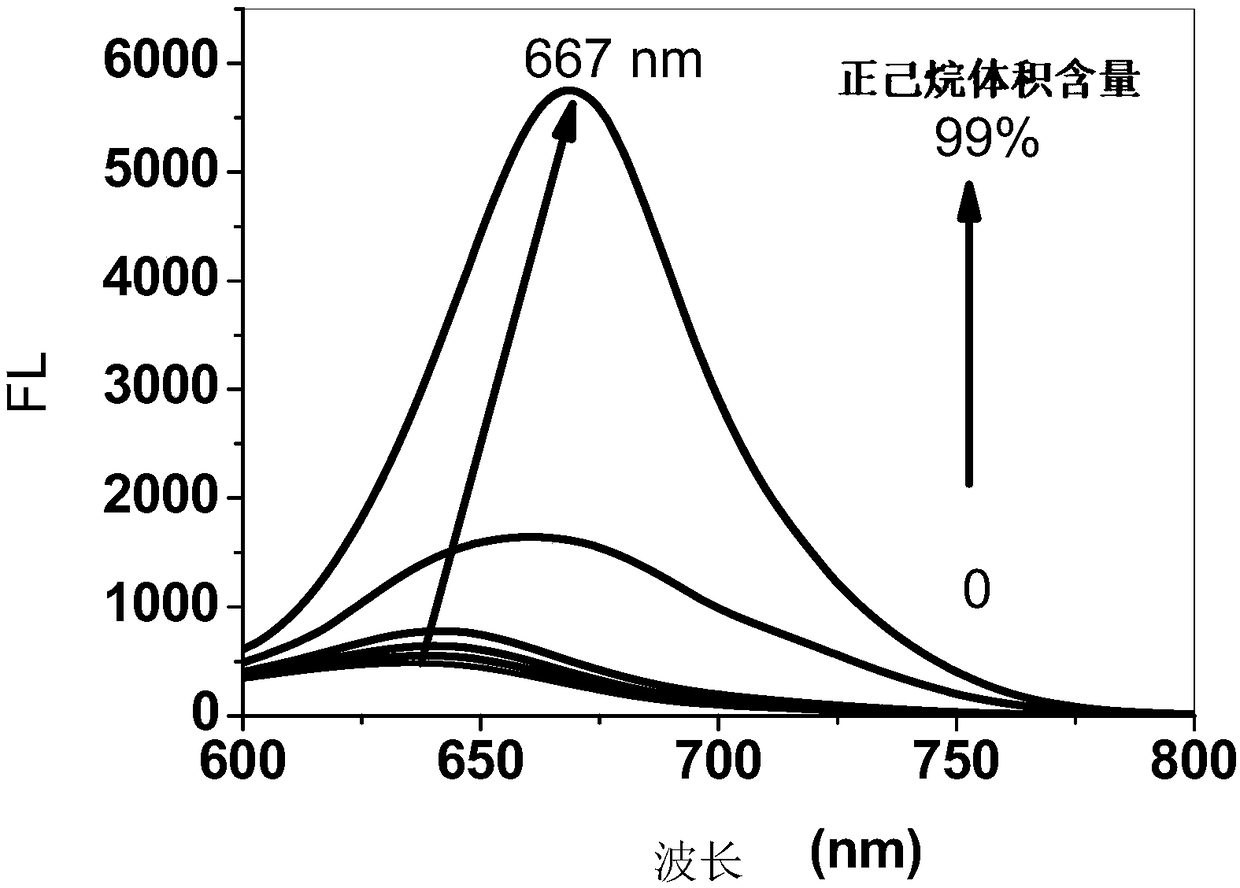
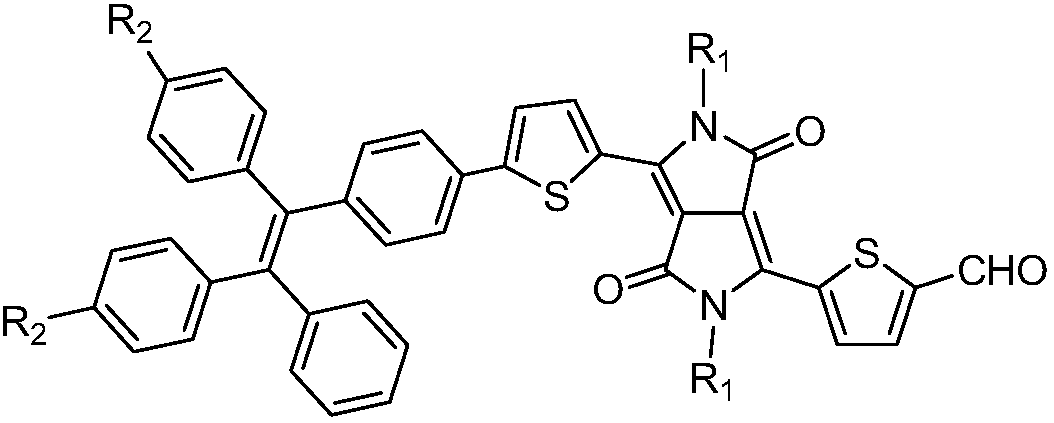
InactiveCN108912126AAdjustment rangeAdjust the fluorescence intensityOrganic chemistryLuminescent compositionsN dimethylformamideKetone
The invention discloses an aggregation-induced emission near-infrared emission diketopyrrolopyrrole compound and a preparation method thereof. The method comprises the steps that 3,6-dithiophene diketopyrrolopyrrole reacts with twice molar weight of alkyl bromide so as to obtain 2,5-dialkyl-3,6-dithienopyrrolopyrrole, then the 2,5-dialkyl-3,6-dithienopyrrolopyrrole sequentially undergoes Vilsmeierreaction with phosphorus oxychloride / N,N-dimethylformamide and undergoes substitution reaction with N-bromosuccinimide so as to obtain 2,5-dialkyl-3-(5-formyl)thienyl-6-(5-bromine)thienyl diketopyrrolopyrrole, and then the 2,5-dialkyl-3-(5-formyl)thienyl-6-(5-bromine)thienyl diketopyrrolopyrrole undergoes Suzuki reaction with tetraphenyl ethylene boric acid ester so as to obtain 2,5-dialkyl-3(4-((E)-2-phenyl-1,2-disubstituted styryl)phenyl)thienyl-6-(5-formyl)thienyl diketopyrrolopyrrole. The compound has the emission range greater than 650 nm and has the aggregation-induced emission property.
Owner:SOUTH CHINA UNIV OF TECH
Derivative containing halogen light active oxazolidinone and preparation method thereof
The invention discloses a derivative containing halogen light active oxazolidinone and a preparation method thereof. The structural formula of the compound is shown as the formula I. N-Bromosuccinimide serves as a bromine source, a complex of chiral phosphine ligand and lewis acid serves as a catalyst, olefin substrates with different catalyzing structures are subjected to asymmetric bromine amine cyclization reaction, and the derivative containing halogen light active oxazolidinone is synthesized and prepared through a simple column chromatography post-processing step. An ee (enantiomer excess) value can achieve highest to 97%. The reaction can be amplified to a gram level, the ee value of products after recrystallization can achieve 99%, and a potential industrial application value is provided. Reaction products can be further derived into various useful compounds, and product application values are achieved.
Owner:INST OF CHEM CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
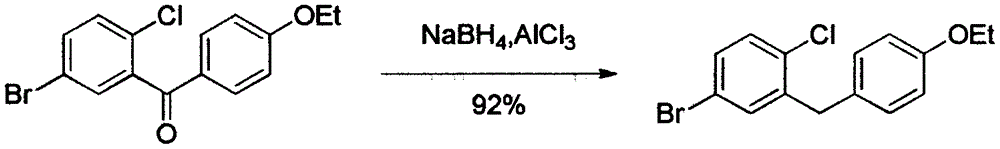





![Preparation method for 5-benzyl-7(S)-t-butyloxycarborylamino-5-aza-spiro[2,4]heptanes Preparation method for 5-benzyl-7(S)-t-butyloxycarborylamino-5-aza-spiro[2,4]heptanes](https://images-eureka.patsnap.com/patent_img/c4e5bc10-490b-4118-8859-0b9646c67500/BSA0000093282790000011.png)
![Preparation method for 5-benzyl-7(S)-t-butyloxycarborylamino-5-aza-spiro[2,4]heptanes Preparation method for 5-benzyl-7(S)-t-butyloxycarborylamino-5-aza-spiro[2,4]heptanes](https://images-eureka.patsnap.com/patent_img/c4e5bc10-490b-4118-8859-0b9646c67500/BSA0000093282790000021.png)
![Preparation method for 5-benzyl-7(S)-t-butyloxycarborylamino-5-aza-spiro[2,4]heptanes Preparation method for 5-benzyl-7(S)-t-butyloxycarborylamino-5-aza-spiro[2,4]heptanes](https://images-eureka.patsnap.com/patent_img/c4e5bc10-490b-4118-8859-0b9646c67500/BSA0000093282790000031.png)
![Preparation method for di-thiophene-[2, 3-b; 3', 2'-d] thiophene Preparation method for di-thiophene-[2, 3-b; 3', 2'-d] thiophene](https://images-eureka.patsnap.com/patent_img/6b56c5c0-03f8-4976-9255-f1d9a8c30b84/a20071005483400161.PNG)
![Preparation method for di-thiophene-[2, 3-b; 3', 2'-d] thiophene Preparation method for di-thiophene-[2, 3-b; 3', 2'-d] thiophene](https://images-eureka.patsnap.com/patent_img/6b56c5c0-03f8-4976-9255-f1d9a8c30b84/a20071005483400162.PNG)
![Preparation method for di-thiophene-[2, 3-b; 3', 2'-d] thiophene Preparation method for di-thiophene-[2, 3-b; 3', 2'-d] thiophene](https://images-eureka.patsnap.com/patent_img/6b56c5c0-03f8-4976-9255-f1d9a8c30b84/a20071005483400171.PNG)