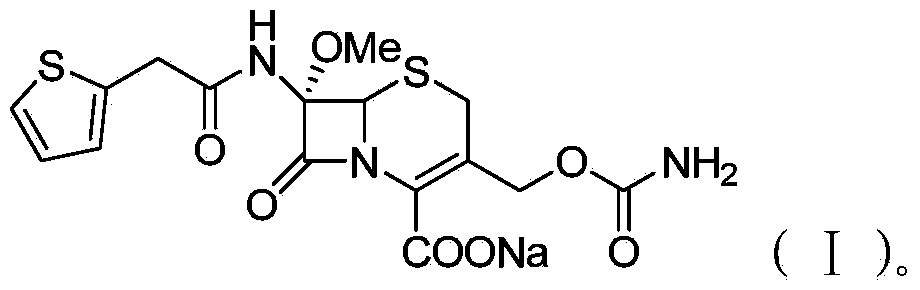
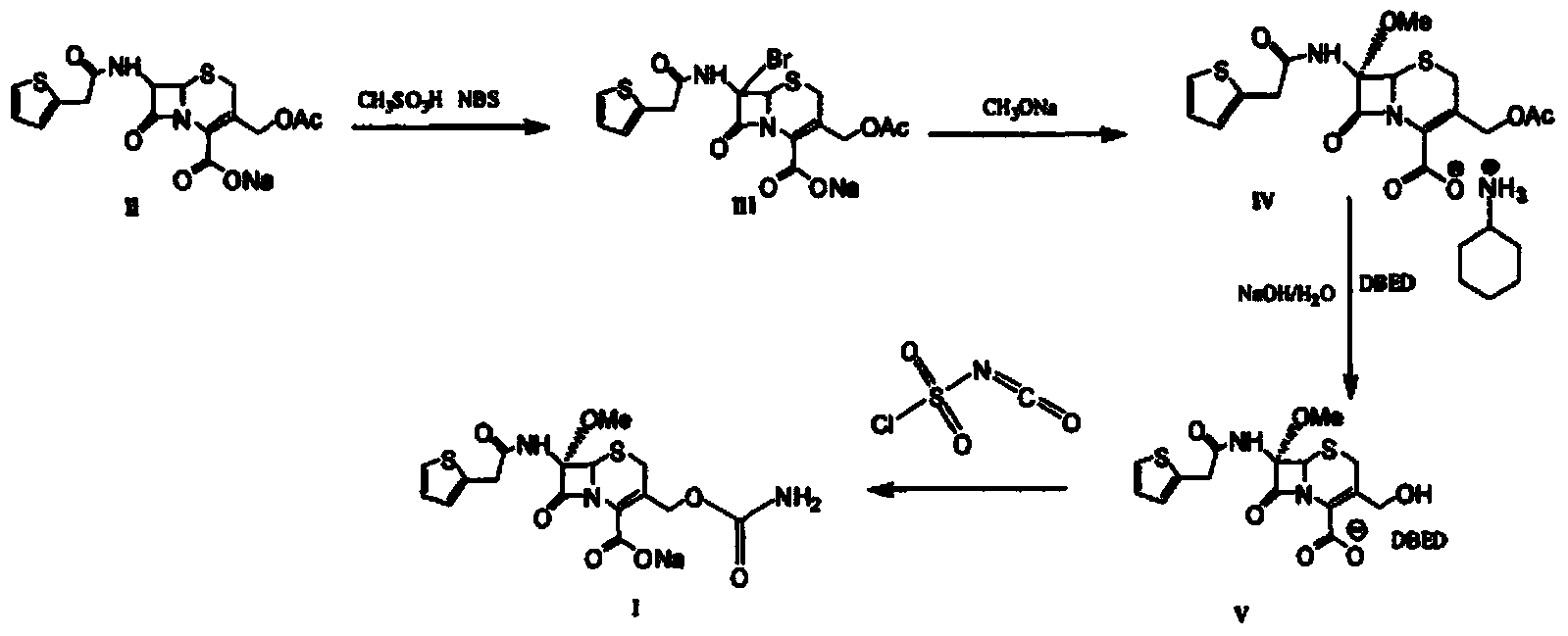
Preparation method of cefoxitin sodium
A technology of cefoxitin sodium and cephalosporanic acid, applied in the direction of organic chemistry, can solve the problems of cumbersome process, difficult to obtain, long reaction steps, etc., and achieve the effect of simple operation process, high yield and purity, and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Synthesis of 7-α-methoxy-7-[(2-thienyl)acetamido]-4-cephalosporanic acid cyclohexylamine salt (IV)
[0036] (1) Add 324ml of dichloromethane, 36ml of methanol, and 360g of cephalothin into a three-neck reaction flask, and cool down to -20°C. Stir well. Add 10.12g of methanesulfonic acid, cool, add NBS (N-bromosuccinimide) (256g) in batches, add sodium methoxide (1163g), fully stir the reaction for 2h, after the reaction is complete, add 8.24g of sodium sulfite, acetic acid 60ml, saturated sodium chloride solution. After reacting for 3 hours, heat up to 0°C, adjust the pH value to 2 with 2mol / L hydrochloric acid, separate the liquid, wash the organic layer with water, add cyclohexylamine solution to pH 6.5, add isopropyl ether, and stir at 0°C for 2h , precipitated white crystals, stood overnight, filtered, washed the filter cake with acetone, and dried to obtain 43.5g of compound IV (97.9% yield, 97.6% purity);
[0037] The above compound was subjected to elemental a...
Embodiment 2
[0040] Synthesis of 3-Hydroxymethyl-7-α-[(2-thienyl)acetamido]-4-cephalosporanic acid benzathine acetate salt (IV)
[0041] (2) Add 130.4ml of water and 146.4ml of methanol into a three-neck reaction flask, cool down, add 410g of compound IV, and cool down. Slowly add pre-cooled 4mol / L sodium hydroxide solution to adjust the pH value to 10, stir well until complete reaction. Adjust the pH with acetic acid, raise the temperature to adjust the pH, raise the temperature to 25°C, add ethyl acetate and 16g of benzathine acetate, and keep stirring at 25°C for 2h. Filter and filter cake with water in turn. Rinse with ethyl acetate and dry to obtain compound V287.11g (96% yield, 97.63% purity).
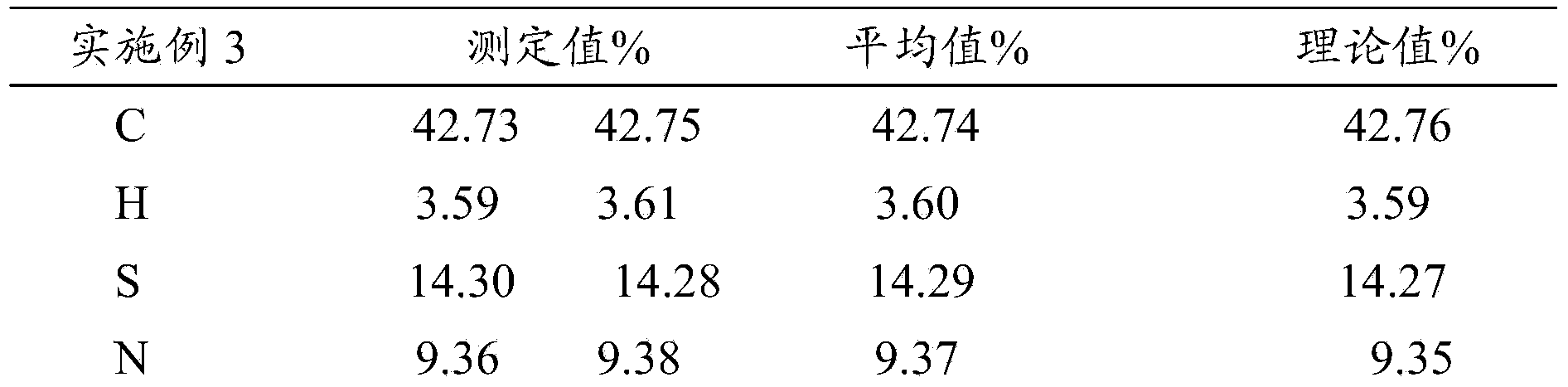
Embodiment 3
[0043] Synthesis of Cefoxitin Sodium (I)
[0044] (3) Add 287.11 g of compound IV and THF into a three-neck reaction flask, cool, add 158.97 g of pre-cooled chlorosulfonyl isocyanate in batches, and fully stir until the reaction is complete. Pour the reaction solution into pre-cooled distilled water, stir for 2 hours, add ethyl acetate, filter the contents, and add 10% sodium chloride solution to the filtrate. Stir for 10 min. Separate the liquid, wash the organic layer with 10% sodium chloride solution, adjust the pH value to 2.0 with 2 mol / L hydrochloric acid, precipitate white crystals, let stand overnight, filter, wash the filter cake with ethyl acetate, and dry in vacuo. Get cefoxitin. Add acetone solution, add 2.0 g of sodium lactate at 30°C, stir for 10 min, then add acetone, filter and dry the obtained solid in vacuum to obtain 319.59 g of cefoxitin sodium (95% yield, 99.5% purity).
[0045] The above compound was subjected to elemental analysis, and the results wer...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com