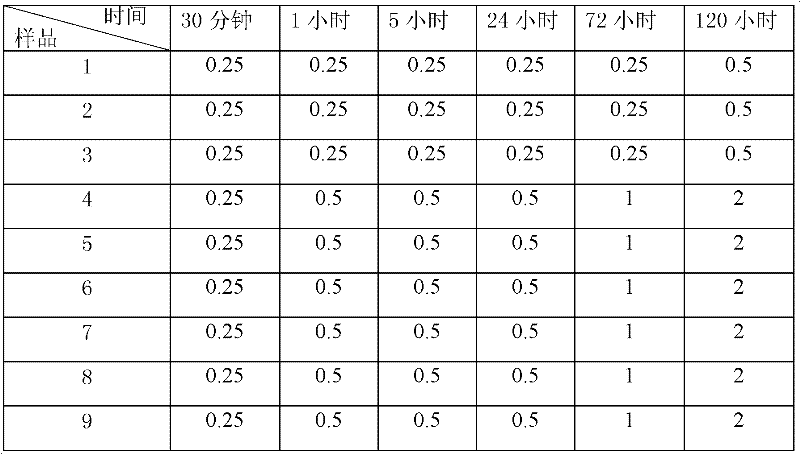
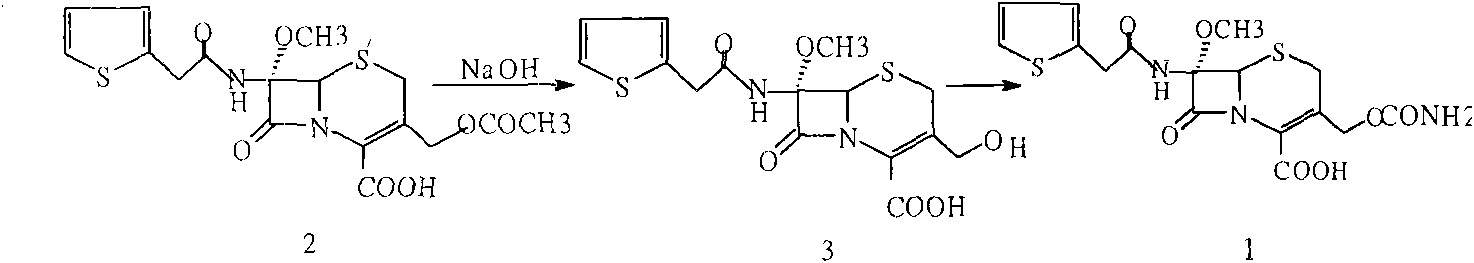
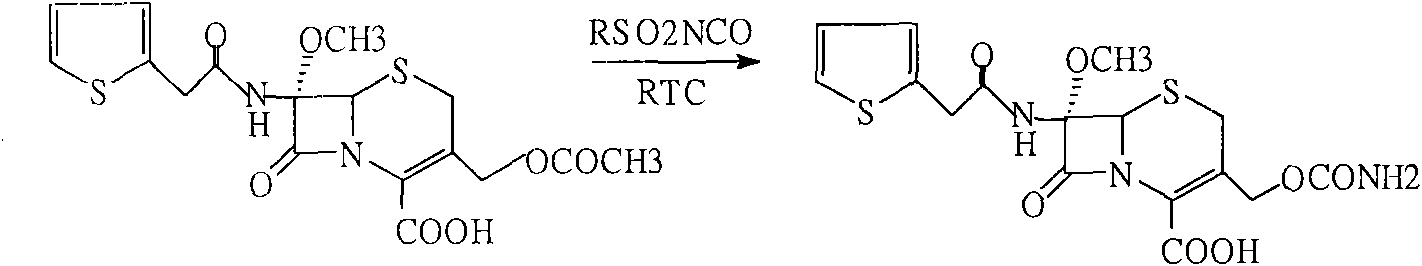
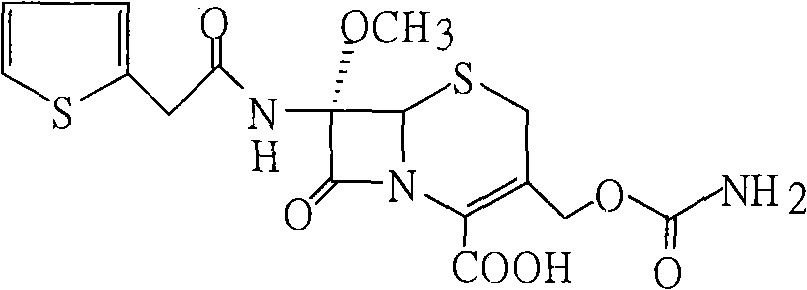
Patents
Literature
67 results about "Cefoxitin Sodium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
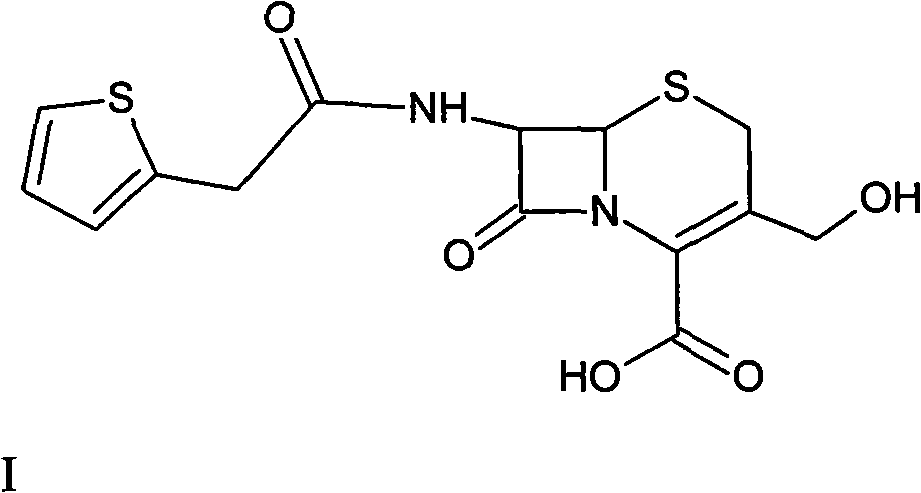
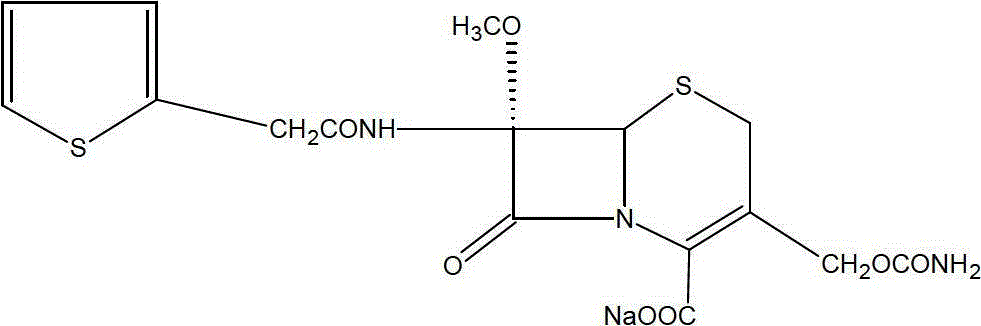
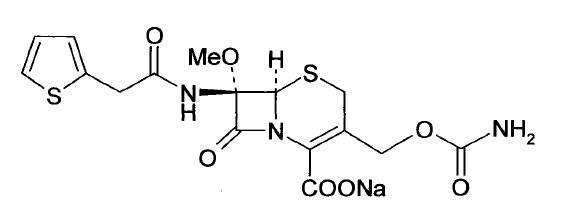
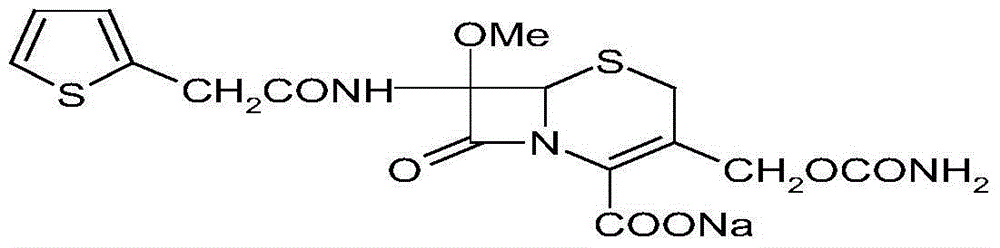
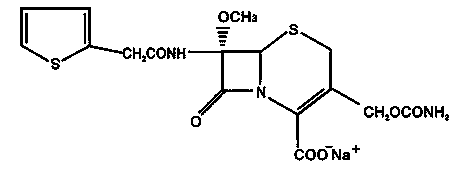
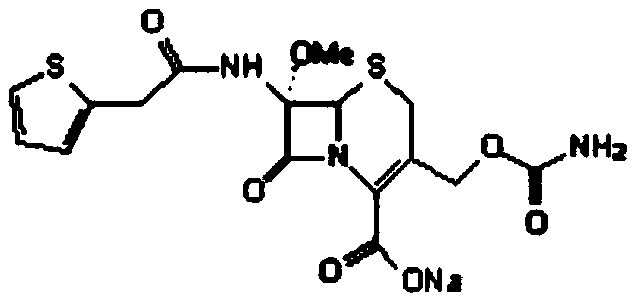
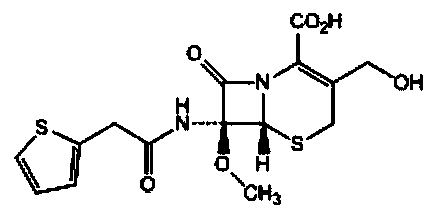
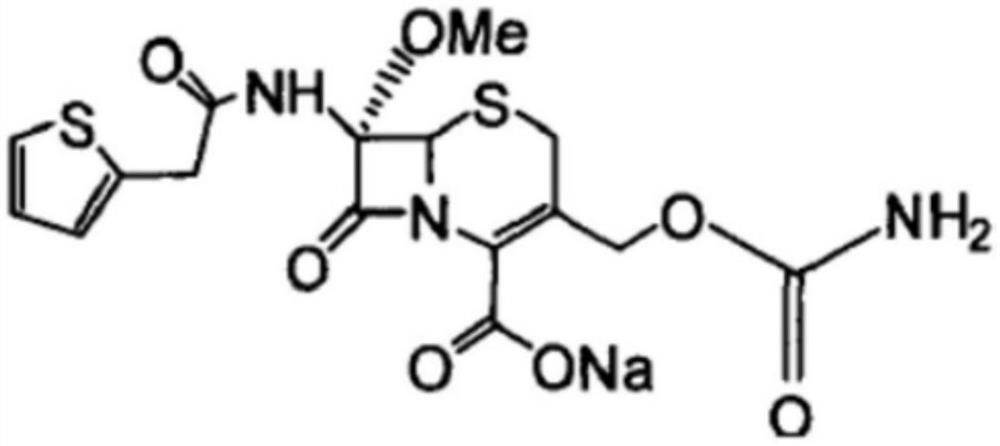
The sodium salt form of cefoxitin, a beta-lactam, second-generation cephalosporin antibiotic with bactericidal activity. Cefoxitin sodium binds to and inactivates penicillin-binding proteins (PBP) located on the inner membrane of the bacterial cell wall. PBPs participate in the terminal stages of assembling the bacterial cell wall, and in reshaping the cell wall during cell division. Inactivation of PBPs interferes with the cross-linkage of peptidoglycan chains necessary for bacterial cell wall strength and rigidity. This results in the weakening of the bacterial cell wall and causes cell lysis.
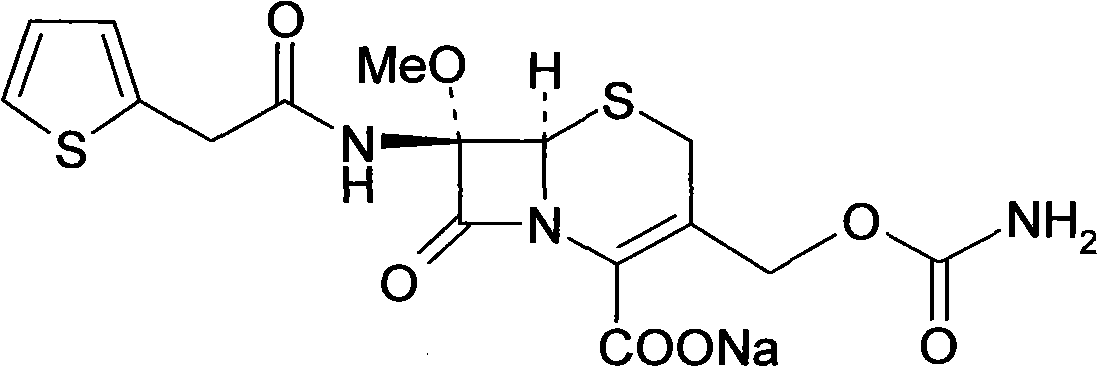
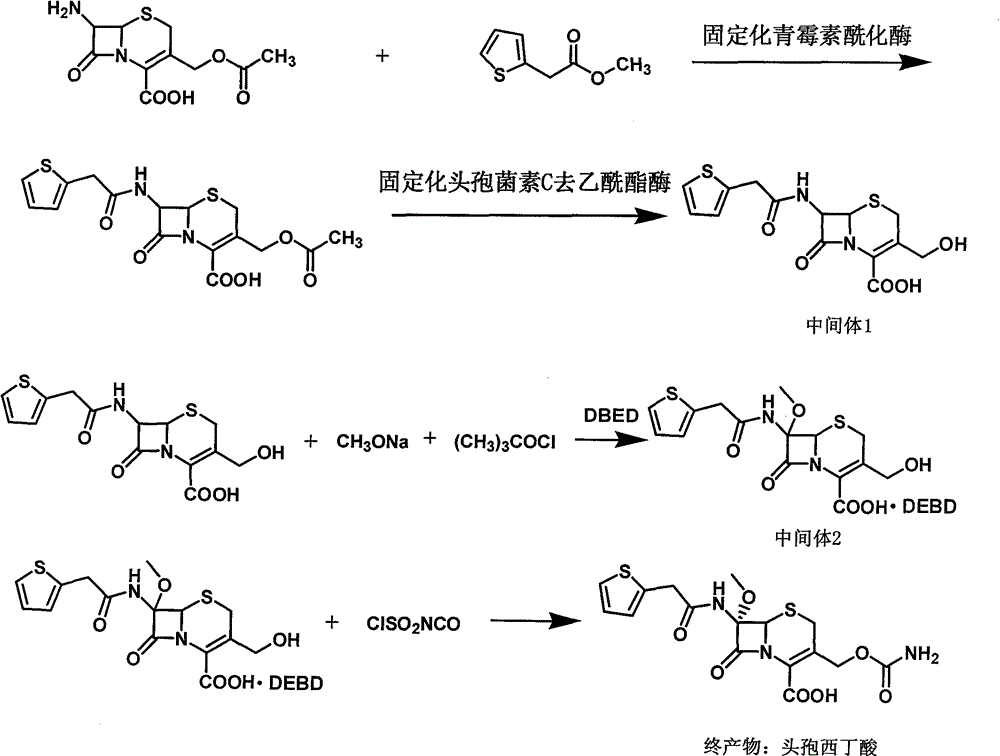
Method for preparing cefoxitin sodium
ActiveCN101613361ASimplify production stepsHigh yieldOrganic chemistryAntiinfectivesLithium methoxideOrganic solvent
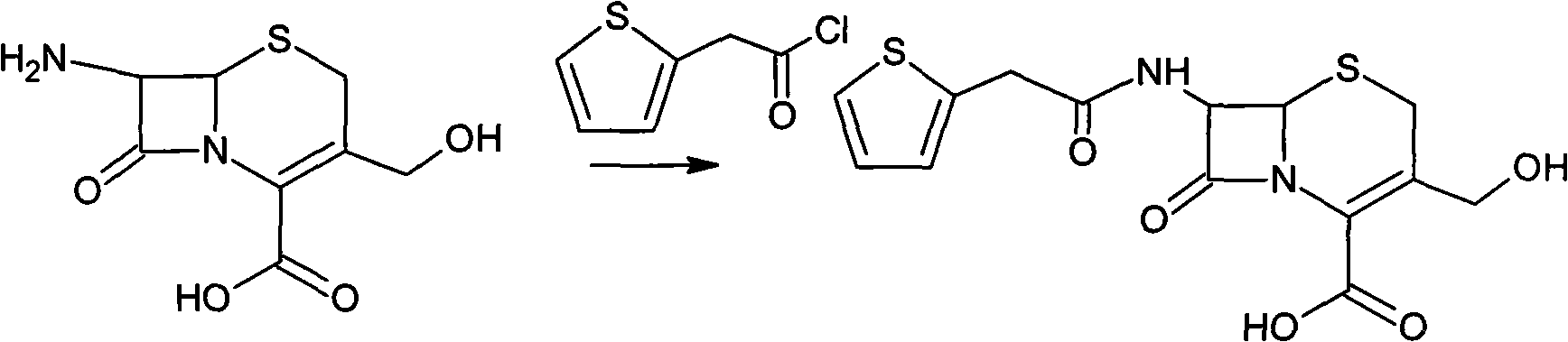
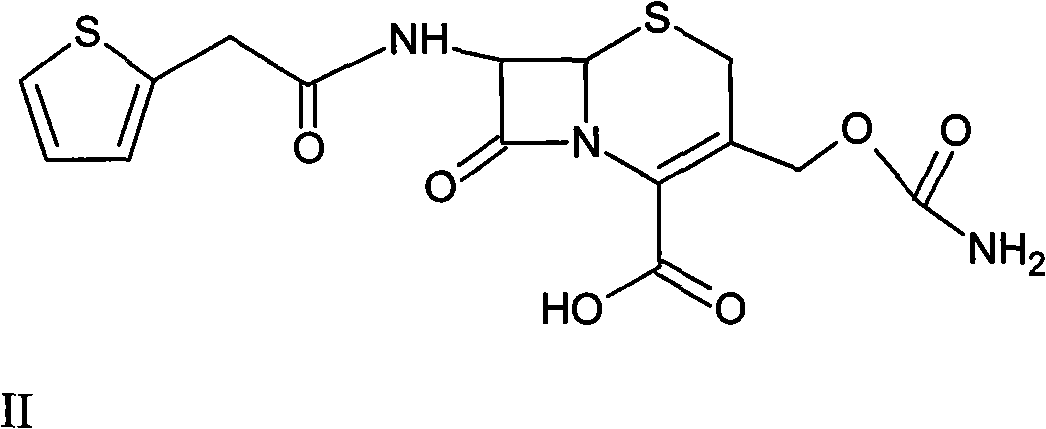
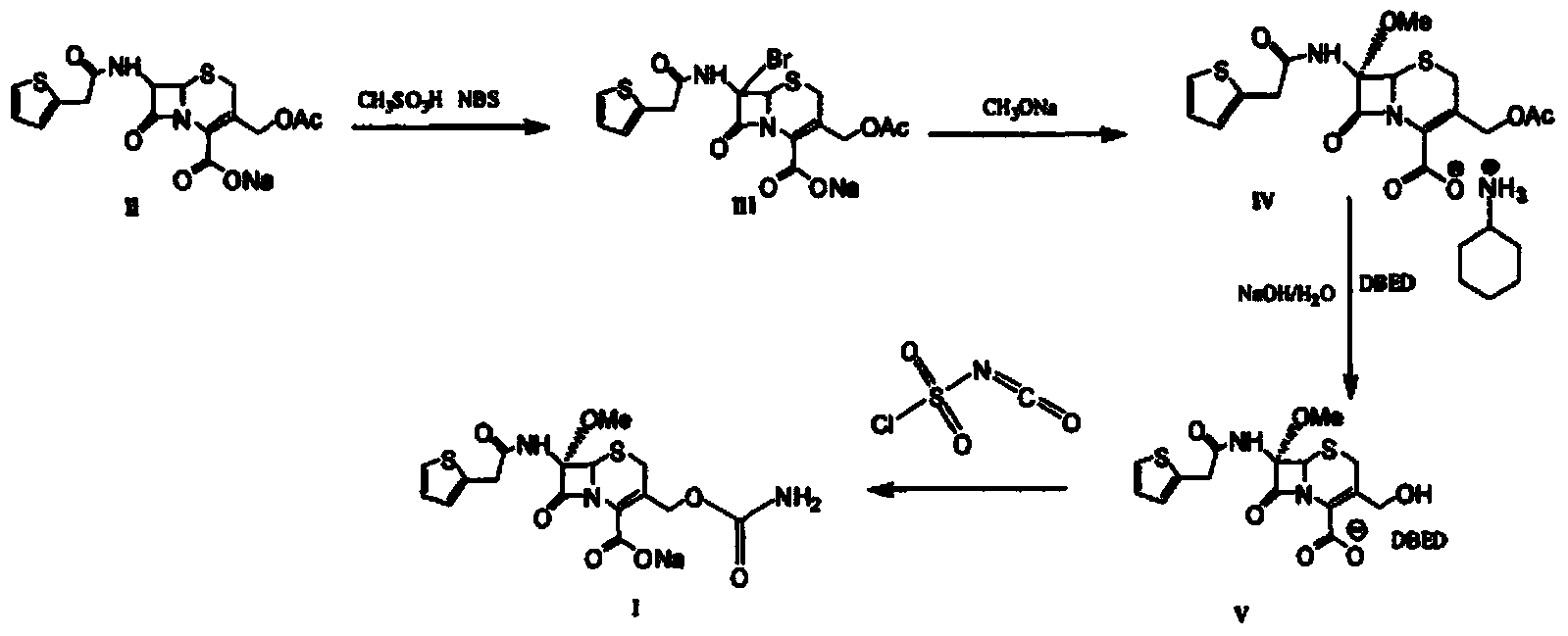
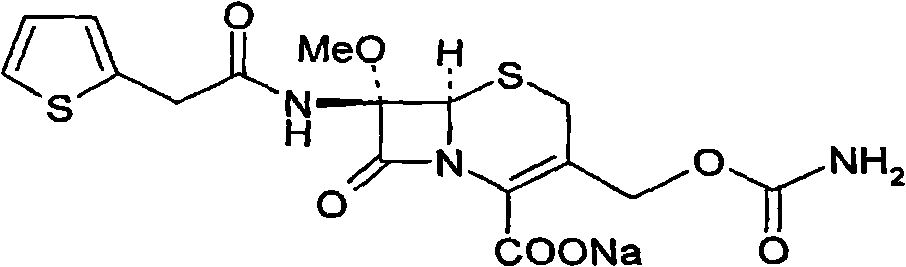
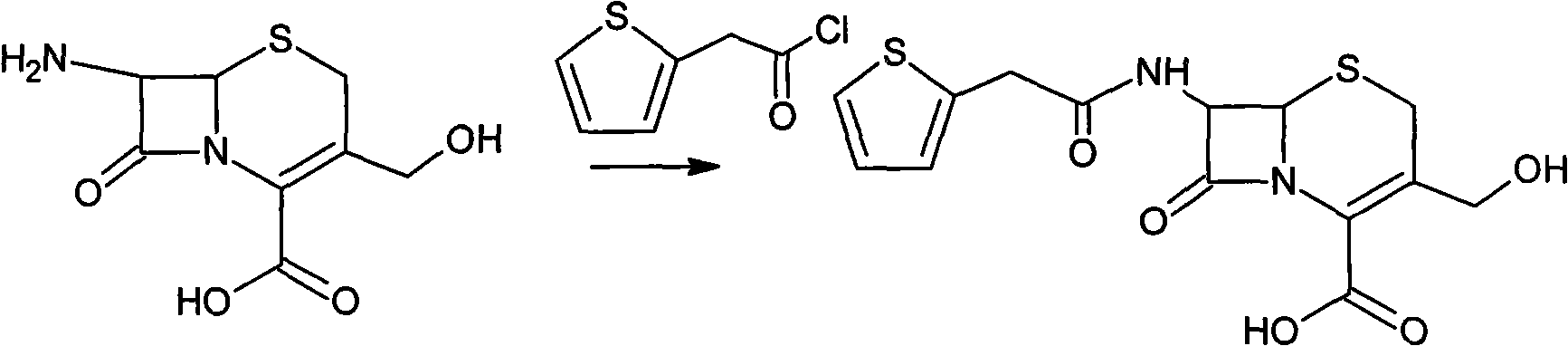
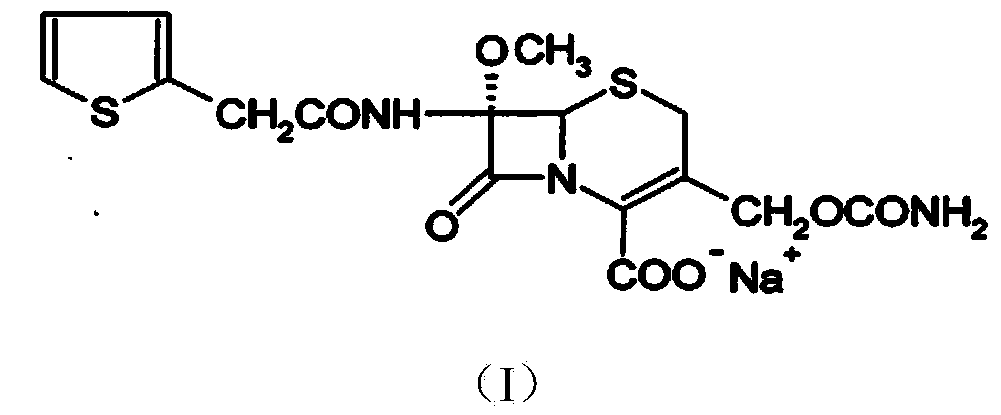
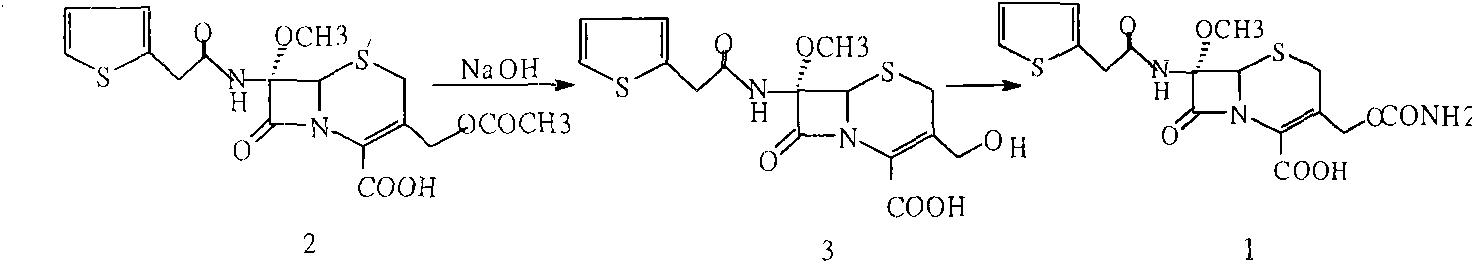
The invention provides a method for preparing cefoxitin sodium, which comprises the following steps: 1, using deacetyl7-ACA as a raw material, and introducing thiopheneacetyls to obtain an deacetylthiophene acid I; 2, introducing carbamoymethoxyls to the positions 3 of molecules of deacetylthiophene acid I to obtain an intermediate of (6R, 7S)-3-carbamoymethoxyl-7-[2-(2-theiophene) acetamino]-8-oxo-5-thia-1-azabicyclo[4.2.0]octa-2-en-2-methanoic acid II; 3, reacting the intermediate II with lithium methoxide to introduce methoxyls into positions 7 of the molecules of the intermediate to obtain cefoxitin acid III; and 4, adding ethanol solution of a sodium salt into an organic solvent containing the cefoxitin acid and obtaining the cefoxitin sodium IV through suction filtration and drying.
Owner:哈药集团股份有限公司 +1
Preparation method of cefoxitin sodium
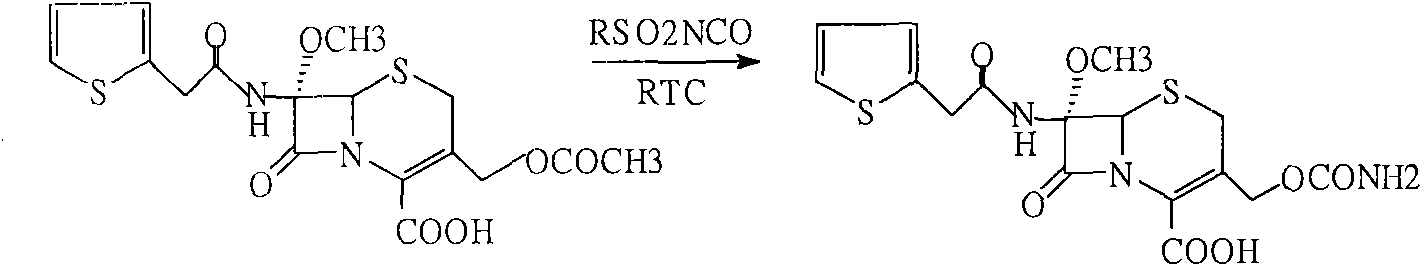
The invention discloses a preparation method of cefoxitin sodium. The preparation method comprises the following steps: (1) bromizing, for example, the site 7 of a main nucleus of cefalotin by using an NBS (N-bromosuccinimide) reagent to form a bromination compound; performing nucleophilic substitution at the site 5 by using a methoxyl group to generate an intermediate IV; (2) performing acyl group hydrolysis on the site 3 of the intermediate IV to obtain an intermediate V; (3) substituting hydrogen atoms on the hydroxyl group by using chloriosulfonyl isocyanate, and then hydrolyzing to obtain the cefoxitin sodium. The method has the advantages of simple process, high product yield, high purity and high reaction selectivity; no special equipment is used in the production; the preparation method is suitable for industrial production.
Owner:HAINAN HULUWA PHARMA GRP CO LTD
Cefoxitin sodium crystal compound and cefoxitin sodium composition powder injection
ActiveCN102358744AImprove solubilitySolubility stabilityAntibacterial agentsPowder deliveryCefoxitin SodiumSodium benzoate
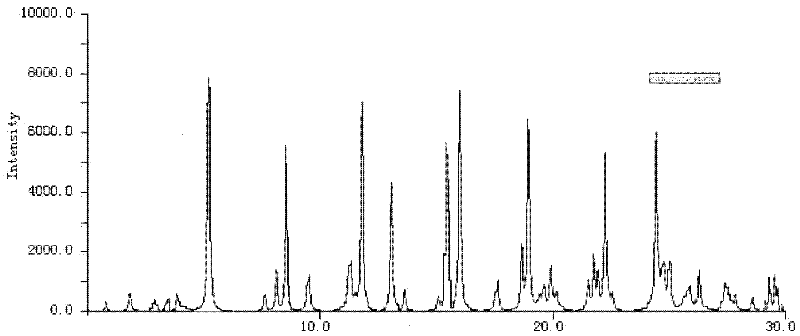
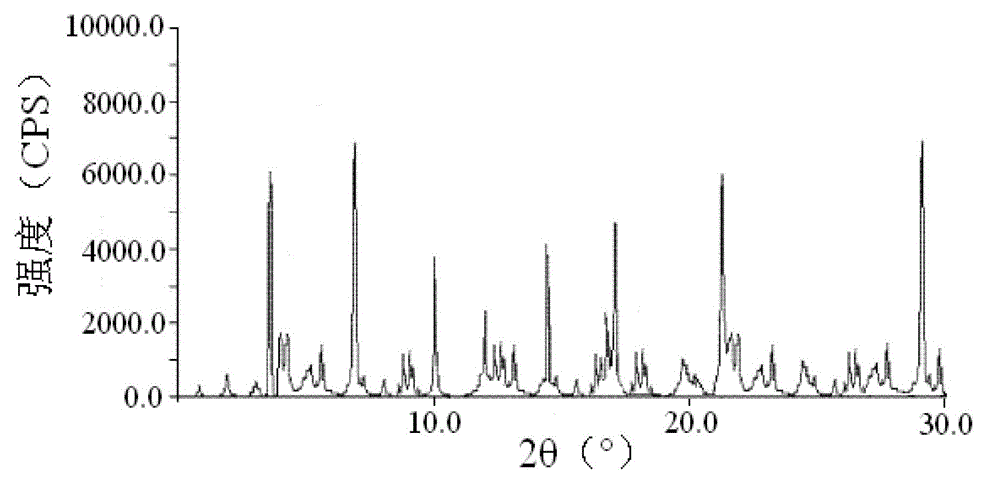
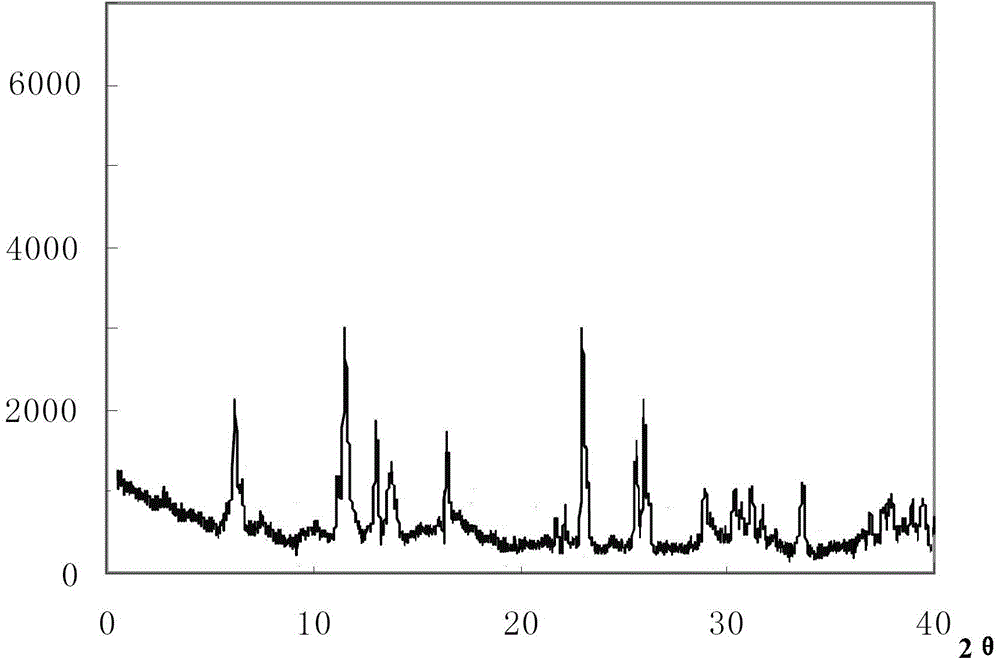
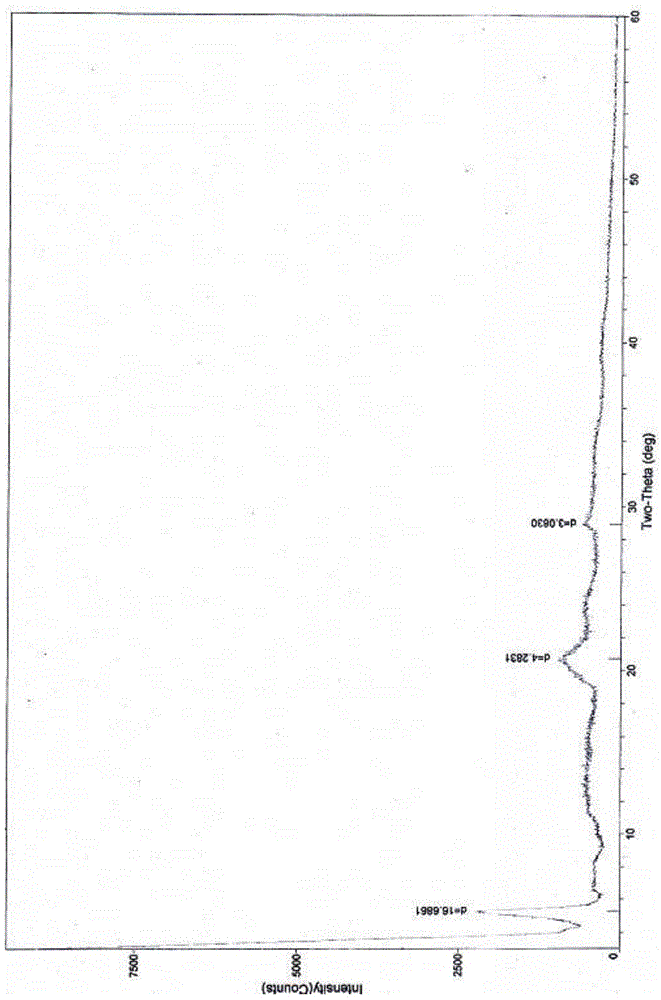
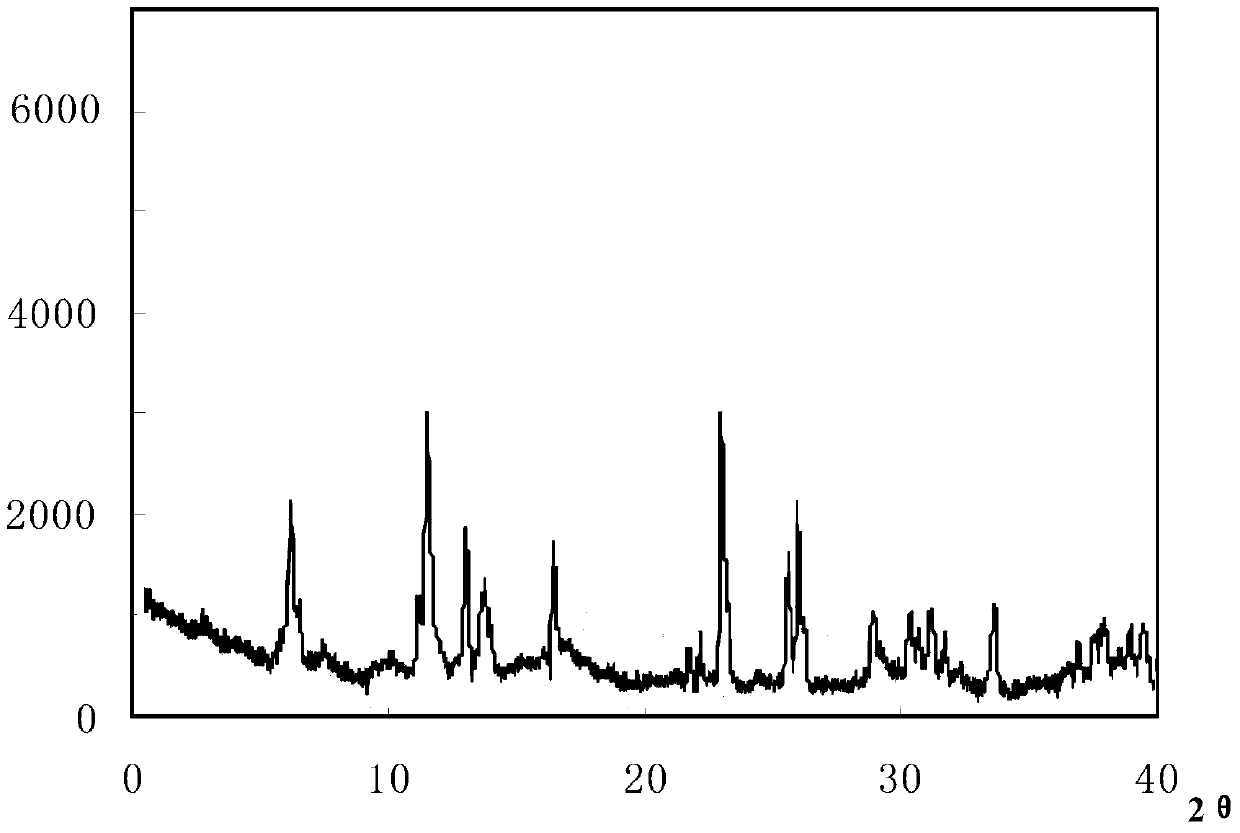
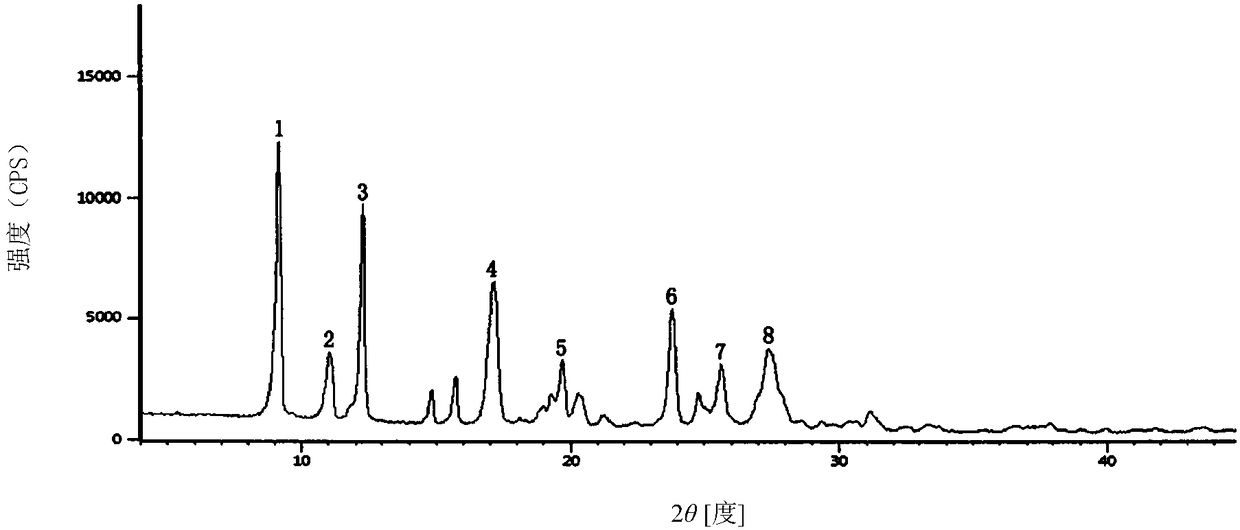
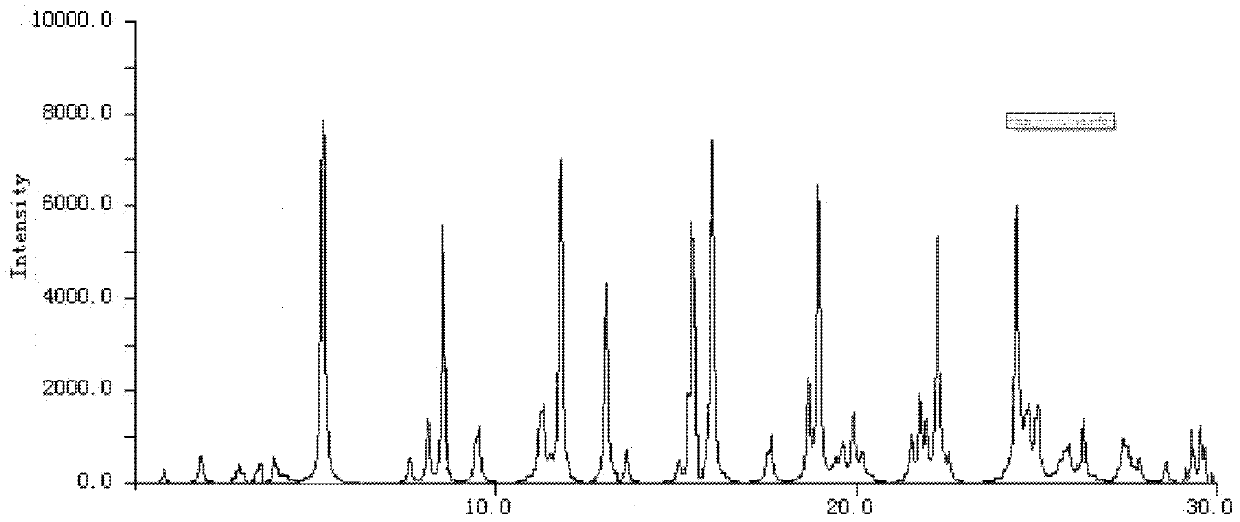
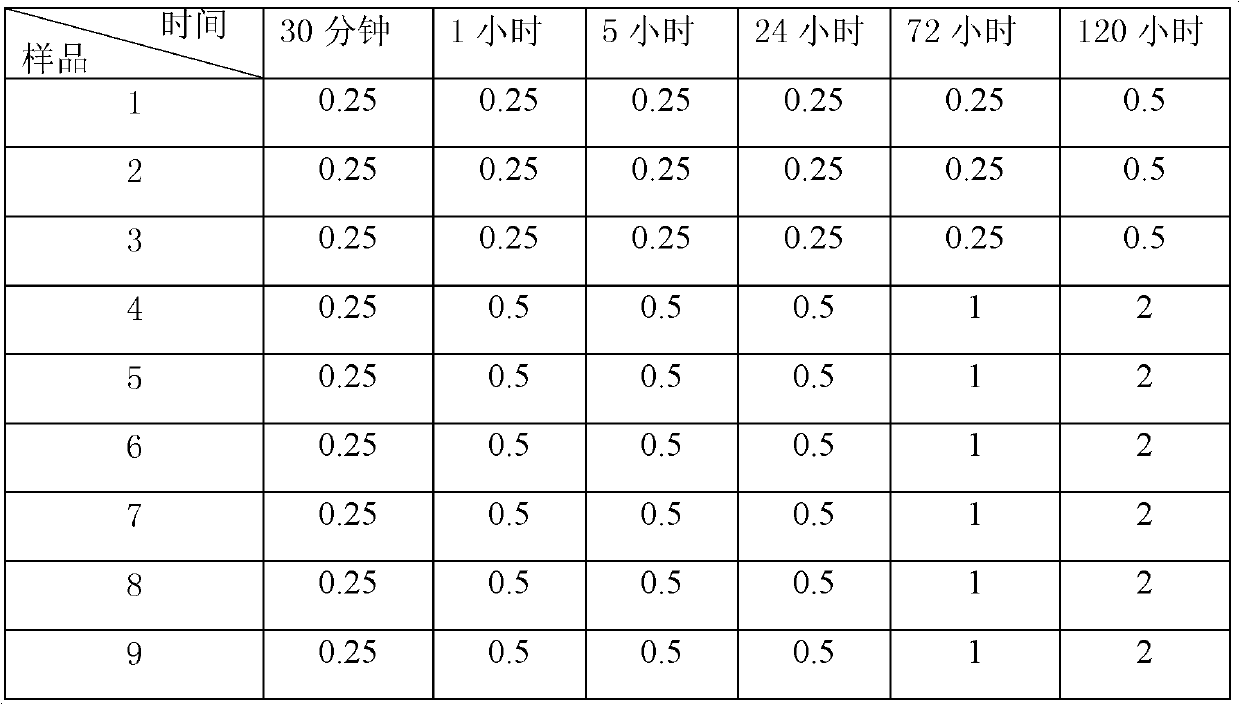
The invention relates to a cefoxitin sodium crystal compound. The method of powder X-ray diffractometry is utilized to determine the cefoxitin sodium crystal compound, and an X-ray powder diffraction pattern represented by the diffraction angle of 2theta+-0.2 degrees has characteristic diffraction peaks at 5.3 degrees, 8.6 degrees, 11.9 degrees, 13.2 degrees, 15.5 degrees, 16.1 degrees, 19.0 degrees, 22.3 degrees and 24.4 degrees. The invention also relates to a cefoxitin sodium composition powder injection containing the cefoxitin sodium crystal compound, and the cefoxitin sodium composition powder injection comprises 95 to 100 parts of the cefoxitin sodium crystal compound and 0.1 to 1 part of sodium benzoate.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD +2
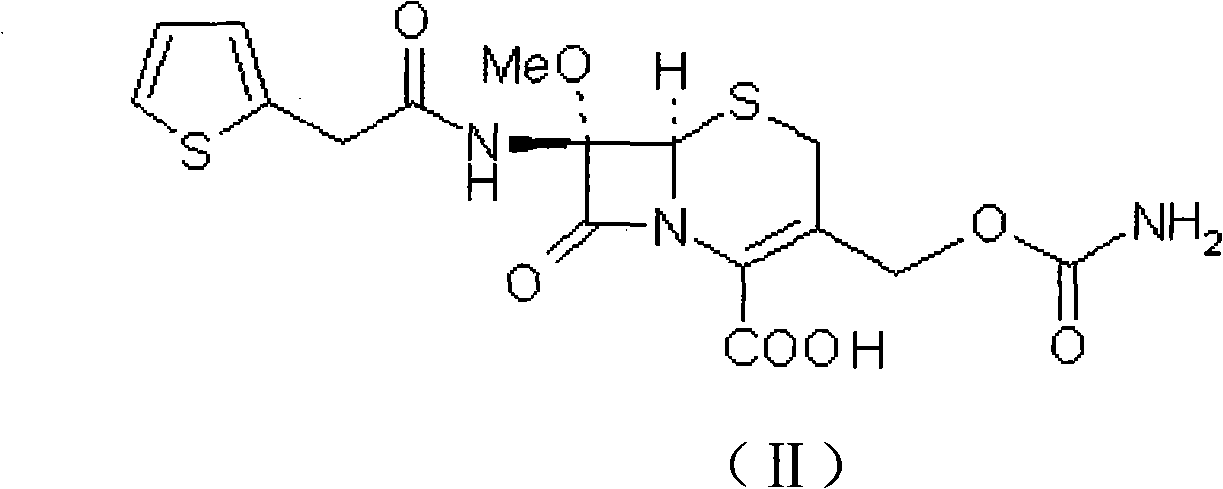
Method for preparing cefoxitin acid
ActiveCN102153567AIncrease costIncrease production costOrganic chemistryCefoxitin SodiumRaw material
The invention relates to a method for preparing cefoxitin acid serving as the key raw material of cefoxitin sodium, and belongs to the field of chemical Chinese herbal medicines. In the method, 7-a-methoxyl cefalotin is subjected to deacetylation and carbamylation directly and simultaneously by isocyanide ester, and the processes of independent 3-position deacetylation and carbamylation are savedso as to obtain the cefoxitin acid directly. The cefoxitin sodium is obtained by the known method. A production process is simplified, the yield is high and the cost is low. In addition, the production link is reduced obviously and the cost is saved.
Owner:重庆天地药业有限责任公司
Cefoxitin sodium liposome injection
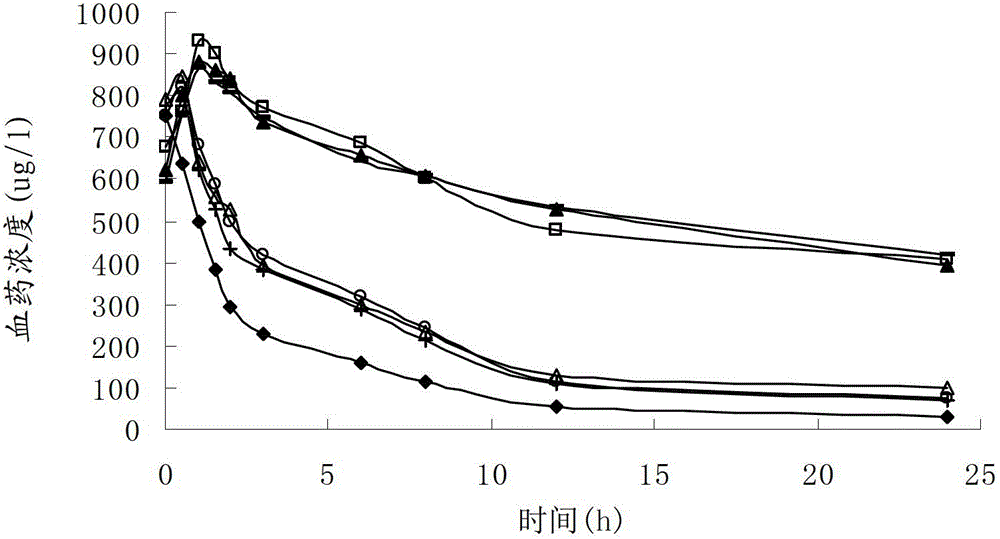
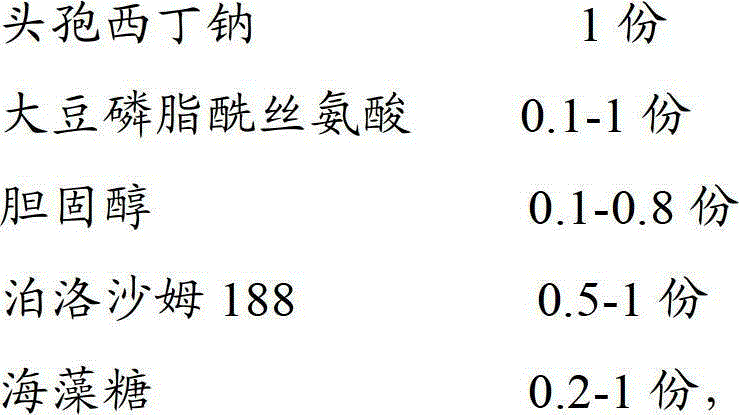
The invention discloses a cefoxitin sodium liposome injection and a preparation method thereof. The cefoxitin sodium liposome injection which is high in quality is prepared from cefoxitin sodium, phosphatidylserine, cholesterol, poloxamer 188, and trehalose in a specific weight ratio. Compared with the conventional preparation, the preparation has the advantages that the stability and the bioavailability of the preparation are greatly improved, the medicine is released stably, the quality of the preparation product is improved, the toxic and side effects are reduced, and the injection is obvious in curative effect.
Owner:灵康药业集团股份有限公司
Method for preparing cefoxitin sodium for injection
ActiveCN102895182AReduce in quantityQuality improvementAntibacterial agentsOrganic active ingredientsForeign matterCLARITY
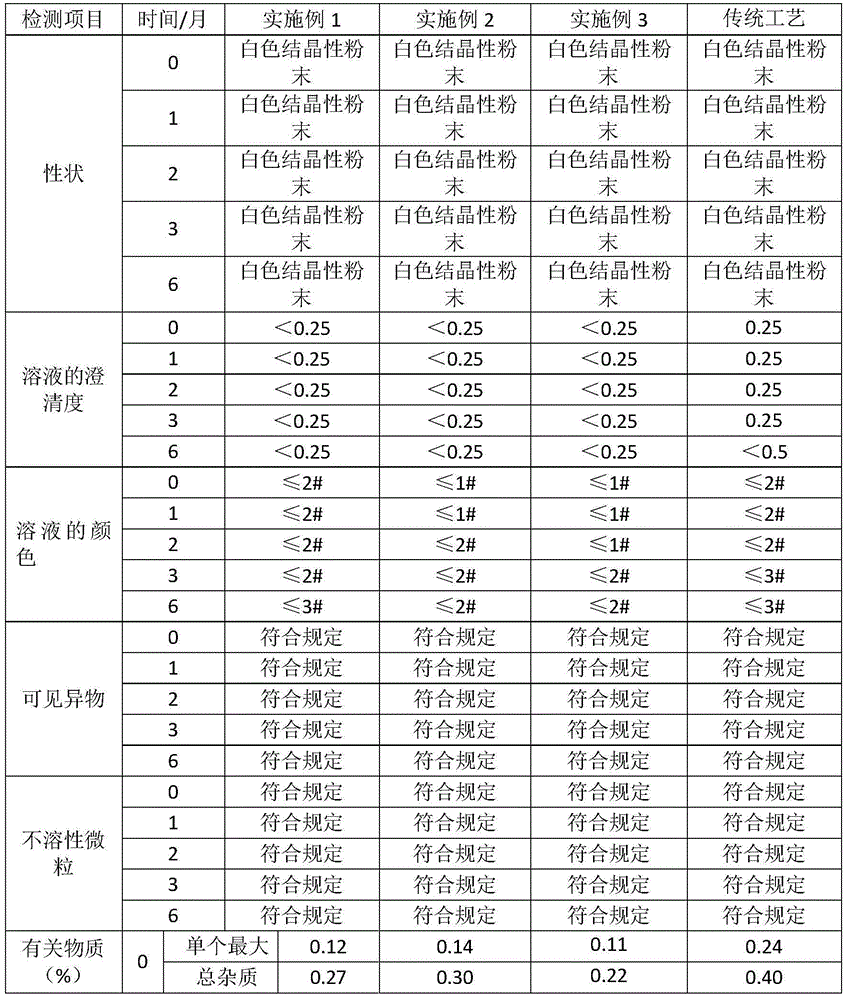
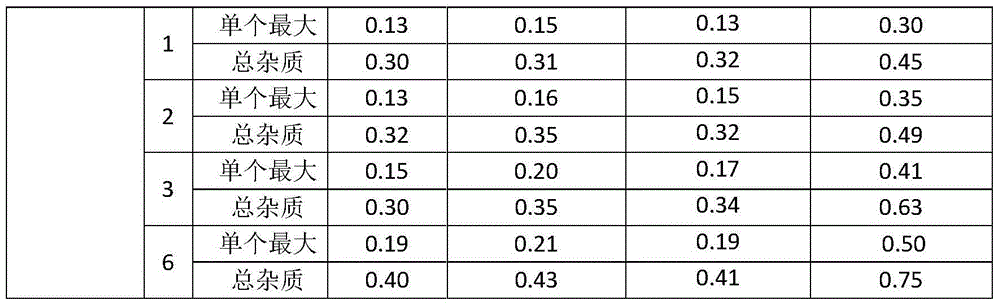
The invention relates to a method for preparing cefoxitin sodium for injection, comprising the steps of bottle washing, rubber plug treating, aluminium cap treating, cefoxitin sodium separate loading and capping to obtain cefoxitin sodium for the injection. The preparation method disclosed by the invention has the advantages that through the reasonable design of the process and the control of a plurality of parameters in the process, the pH value range of prepared cefoxitin sodium for the injection can be controlled between 6.7-7.0; the clarity, the visible foreign matter and the sterile determination of prepard cefoxitin sodium for the injection all meet the requirements; the impurity content, the endotoxin content and the quantity of insoluble particles are remarkably lower than the requirements established by the conventional standard; and the product quality is further increased and the injection safety of cefoxitin sodium for the injection is enhanced.
Owner:SICHUAN PHARMA
Pharmaceutical packaging composition for injection and preparation method thereof
InactiveCN102670400AGuaranteed sterilityTotal quality stabilityPharmaceutical containersMedical packagingPolyesterCephazolin sodium
The invention provides a pharmaceutical packaging composition for injection, which comprises the combination of an aluminum-plastic composition cover containing a coating plastic plug, a sterile antibiotic glass bottle and pharmaceutical sterile powder for injection, wherein the coating material of the coating plastic plug is polydimethylsiloxane coating, polyparaxylene coating, polytetrafluoroethylene coating, ethylene-tetrafluoroethylene copolymer coating, polyester coating, polyethylene coating or polypropylene coating; and the pharmaceutical sterile powder for injection is cephalo-type pharmaceutical sterile powder, such as cefuroxime sodium, cefoxitin sodium, cephazolin sodium and ceftriaxone sodium. The pharmaceutical packaging composition for injection is compatible with the pharmaceutical sterile powder for injection, so that the transition incidence rate is lower, and the quality risks of unqualified solution clarity due to compatibility and addition of related substances are reduced. The invention further provides a preparation method for the pharmaceutical packaging composition for injection, so that the obtained pharmaceutical packaging composition for injection can solve the compatibility problem very well and guarantee the stability of pharmaceutical quality.
Owner:SINOPHARM ZHIJUN (SHENZHEN) PHARMA CO LTD
Preparation method of high-purity cefoxitin sodium
ActiveCN101941983AEnsure safetyEasy to operateOrganic chemistryCefoxitin SodiumPharmaceutical technology
The invention provides a preparation method of high-purity cefoxitin sodium (I), belonging to the field of chemical pharmaceutical technology. The method comprises the following steps: taking cefoxitin acid as a raw material, adsorbing and purifying the cefoxitin acid with a resin column, and allowing the purified cefoxitin acid to react with sodium salt at the temperature of -20-20 DEG C to obtain the high-purity cefoxitin sodium.
Owner:ZHEJIANG WHITESON PHARMA
Synthesis technology of cefoxitin acid
ActiveCN104447800AEmission reductionMild reaction conditionsOrganic chemistrySynthesis methodsTwo step
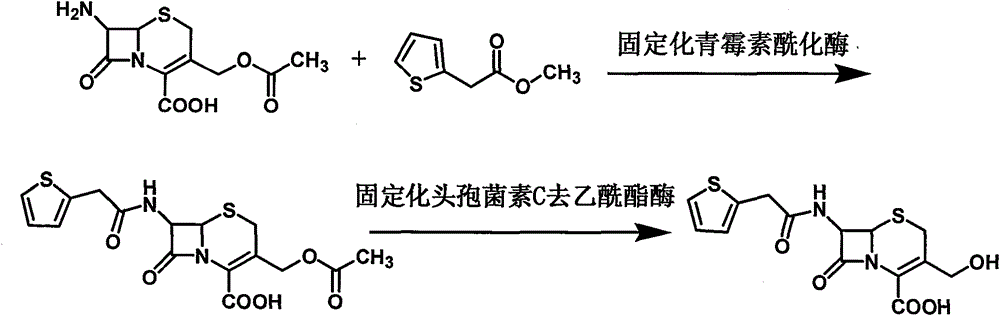
The invention provides a novel synthesis method of cefoxitin acid. Cefoxitin acid is used as a raw material for synthesizing cefoxitin sodium and belongs to second-generation cephalosporin. The cefoxitin acid has balanced antibacterial spectra and has a strong antibacterial effect on gram-negative bacteria. Due to the existence of 7alpha methoxy in the cefoxitin acid, the hydrolysis action of the cefoxitin acid on beta-lactamase can be reduced greatly, so that the beta-lactamase can exist stably in the cefoxitin acid. In the invention, 3-deacetylase cefoxitin acid which is an intermediate is produced by adopting an enzyme process through two-step continuous reaction, materials react in a mild reaction condition, and the process is simple and is convenient to operate. By adopting the novel synthesis method, time and labor can be saved, and the yield and quality of the product can be improved. Because the two-step reaction is carried out in a water phase at room temperature, the consumption of energy and the discharge of organic wastewater can be reduced greatly. The novel synthesis method meets the requirements of large-scale industrial production.
Owner:LIAONING TIANHUA CHEM
Cefoxitin sodium compound-containing pharmaceutical composition
ActiveCN102942577AImprove stabilitySimple methodAntibacterial agentsOrganic active ingredientsX-rayDiethyl ether
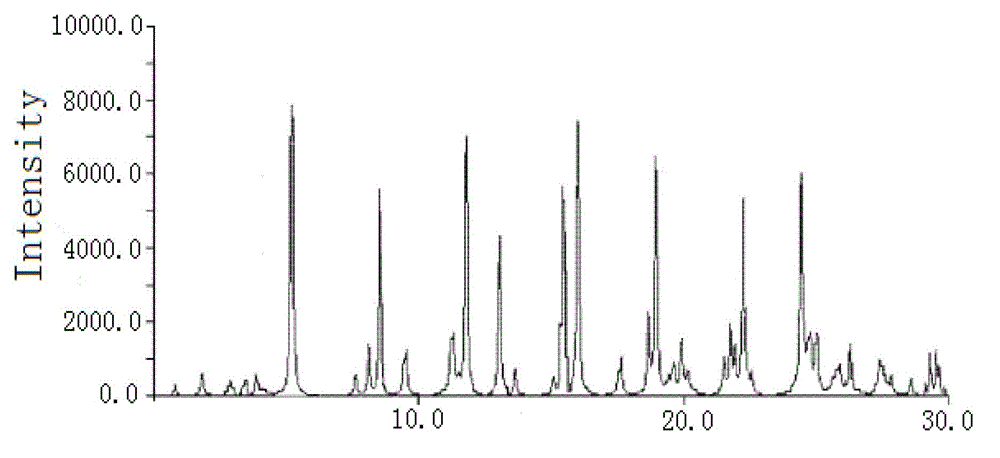
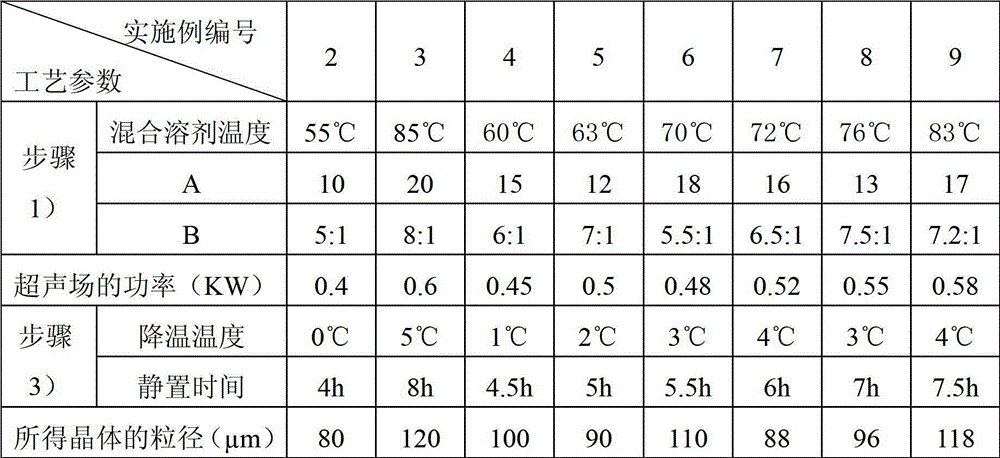
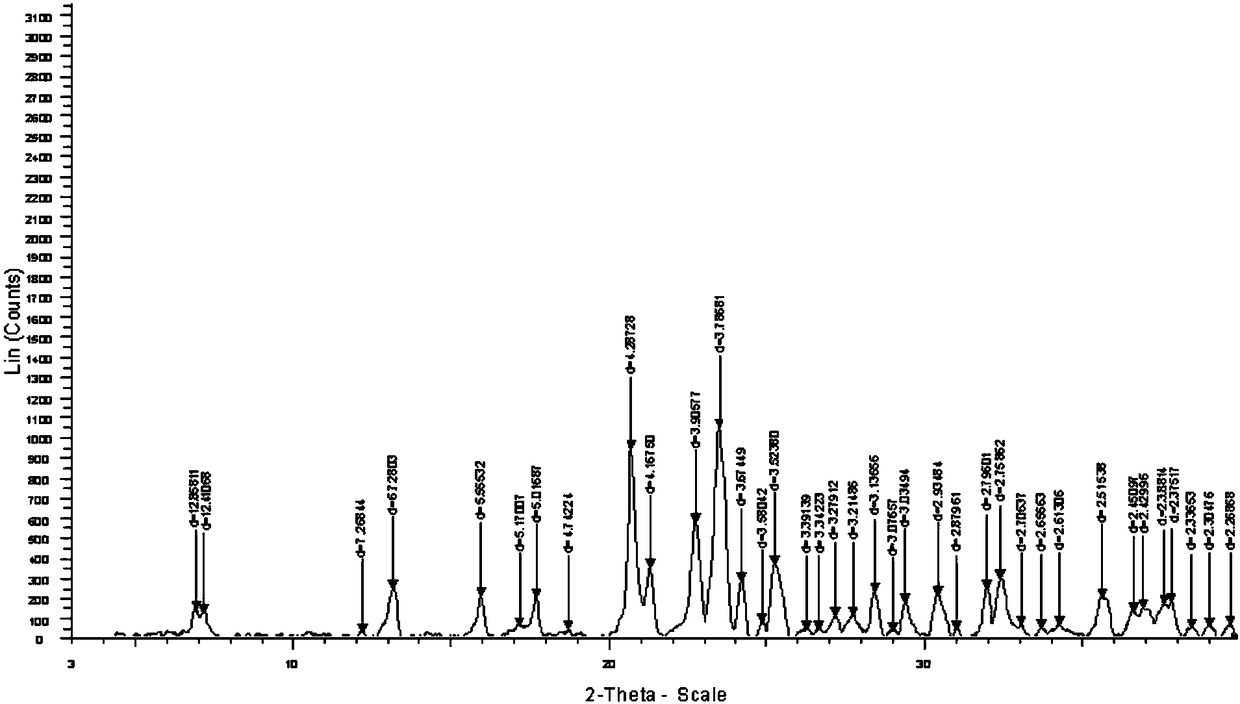
The invention relates to a pefoxitin sodium compound-containing pharmaceutical composition. The pefoxitin sodium compound is crystal and has X-ray diffraction pattern shown by figure 1. A preparation method of the pharmaceutical composition comprises the steps of: 1) adding mixed solvent of n-butyl alcohol and tetrahydrofuran with 55-85 DEG C into pefoxitin sodium crude product, and stirring for dissolving to obtain the n-butyl alcohol and tetrahydrofuran solution of the pefoxitin sodium; 2) dripping diethyl ether into the n-butyl alcohol and tetrahydrofuran solution of the pefoxitin sodium obtained in the step 1) under the ultrasonic field until the crystal is separated out; and 3) closing the ultrasonic field, cooling, standing still, filtering, washing a filter cake by ethanol, and drying to obtain the pefoxitin sodium compound crystal. The pefoxitin sodium compound crystal has better stability and basically does not absorb moisture.
Owner:罗诚
Cefoxitin sodium medicinal composition, powder injection and preparation method thereof
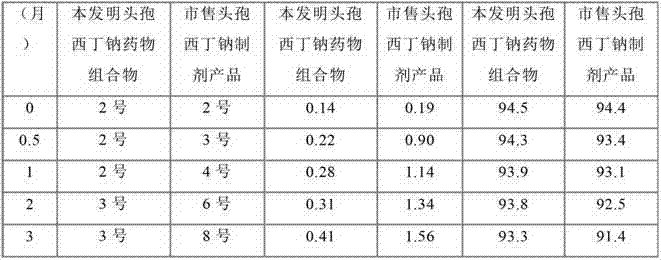
InactiveCN102755325ALow content of related substancesQuality improvementAntibacterial agentsOrganic active ingredientsSide effectCefoxitin Sodium
The invention relates to a cefoxitin sodium medicinal composition, powder injection and a preparation method thereof. The medicinal composition consists of cefoxitin sodium, histidine and hydroxypropyl-beta-cyclodextrin, wherein the weight of the histidine is 3 to 8 percent of that of the cefoxitin sodium, and the weight of the hydroxypropyl-beta-cyclodextrin is 5 to 10 percent of that of the cefoxitin sodium. The medicinal composition is high in stability, related medicament content of the medicinal composition is further lower than that of a commercial cefoxitin sodium product, gastrointestinal side effect is low, the quality of the medicaments is greatly promoted, and the safety of the medicaments is improved.
Owner:SHENZHEN SALUBRIS PHARMA CO LTD
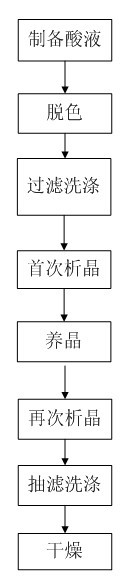
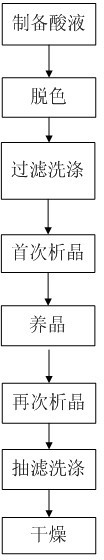
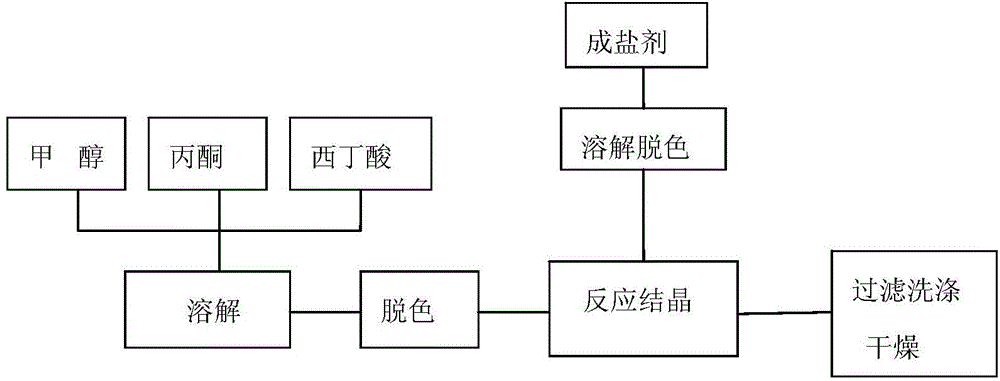
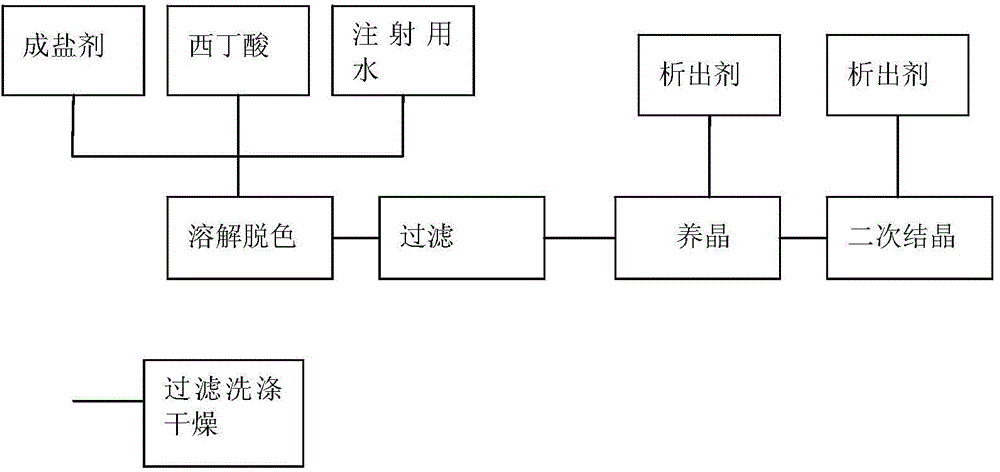
Preparation method for Cefoxintin sodium
The invention discloses a preparation method for Cefoxintin sodium. The preparation method particularly comprises the steps of preparing acidic solution, decoloring, filtering and washing, performing crystallization for the first time, cultivating a crystal, performing crystallization again, performing suction filtration and washing, and drying. The preparation method for the Cefoxintin sodium is simple and practicable. The prepared finished product has few solvent residues, high content and high color grade.
Owner:苏州盛达药业有限公司
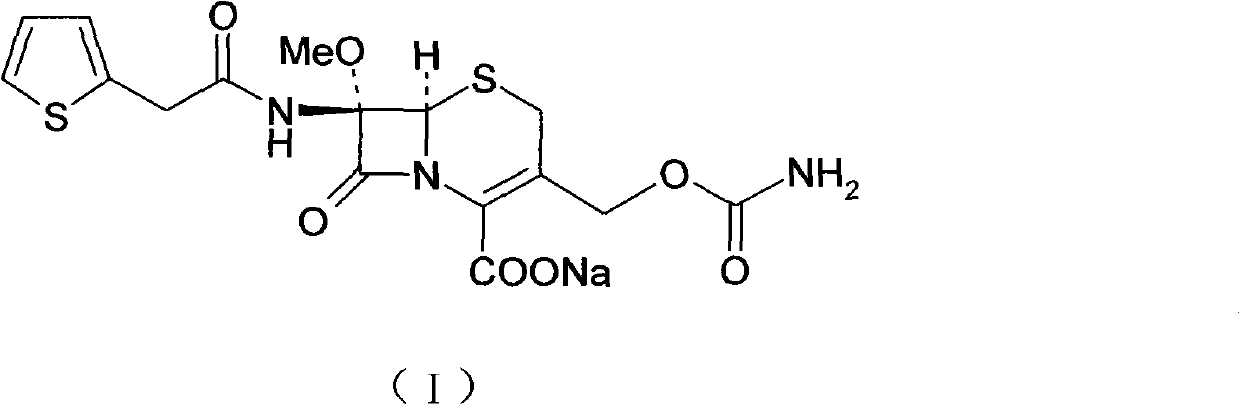
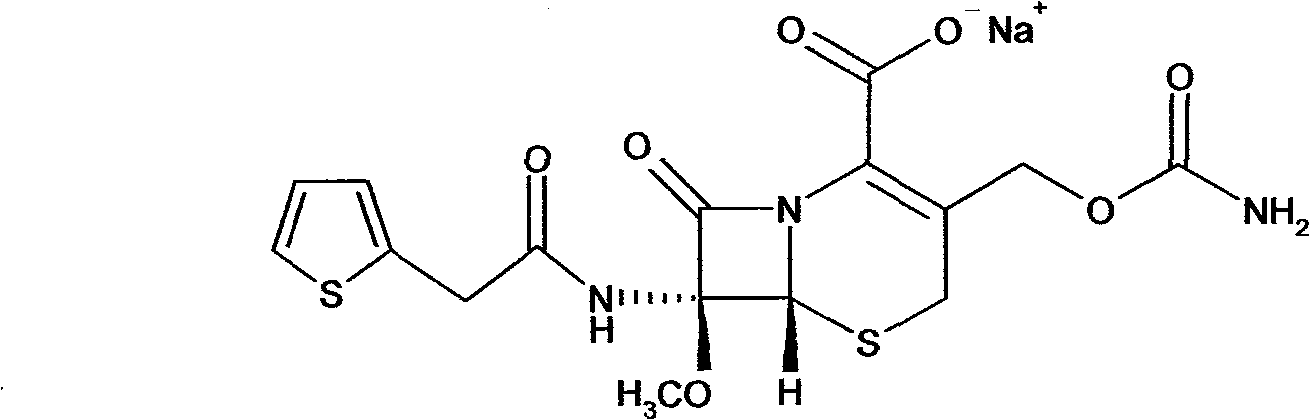
Cefoxitin sodium compound and preparation method thereof
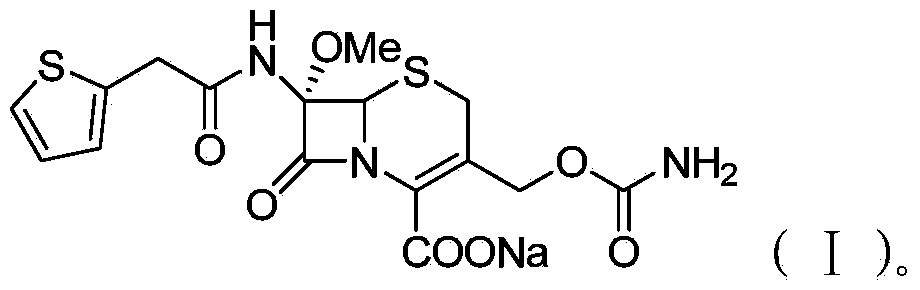
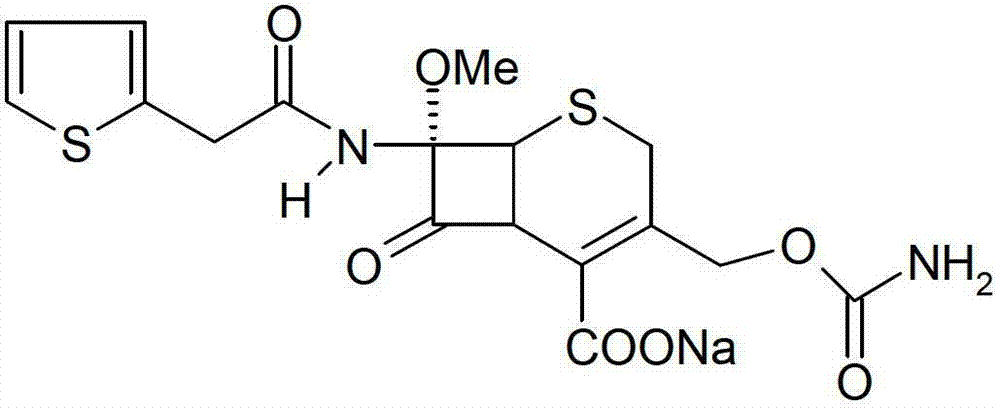
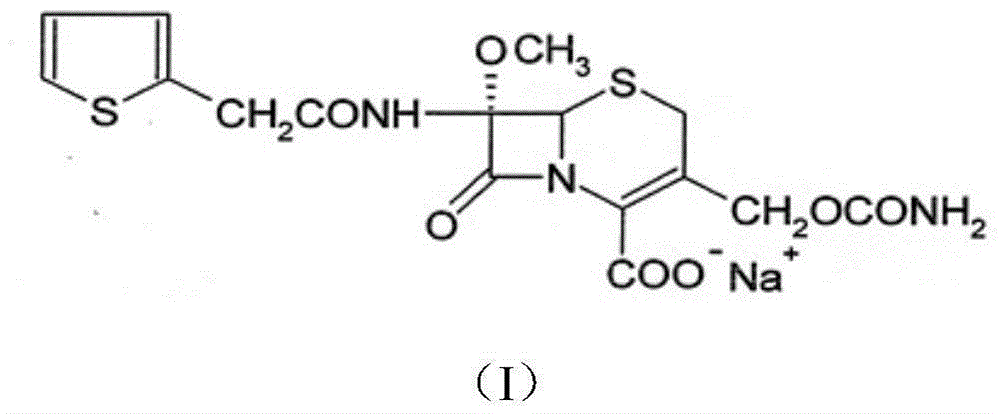
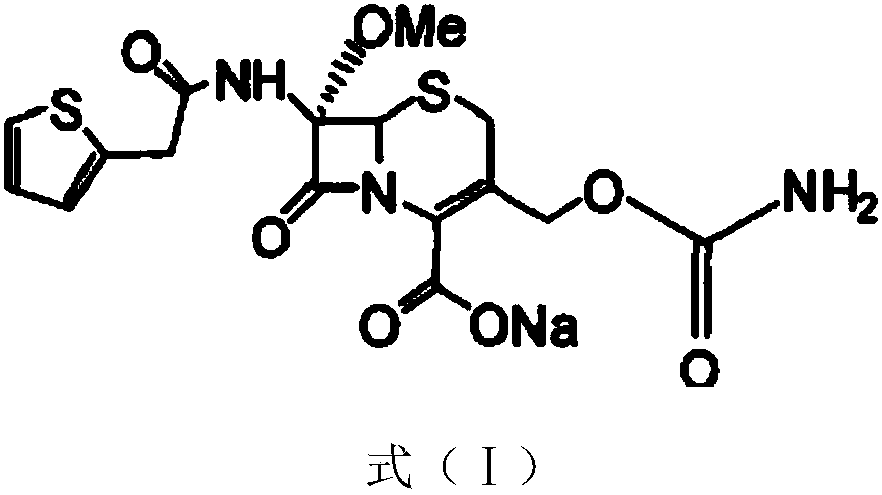
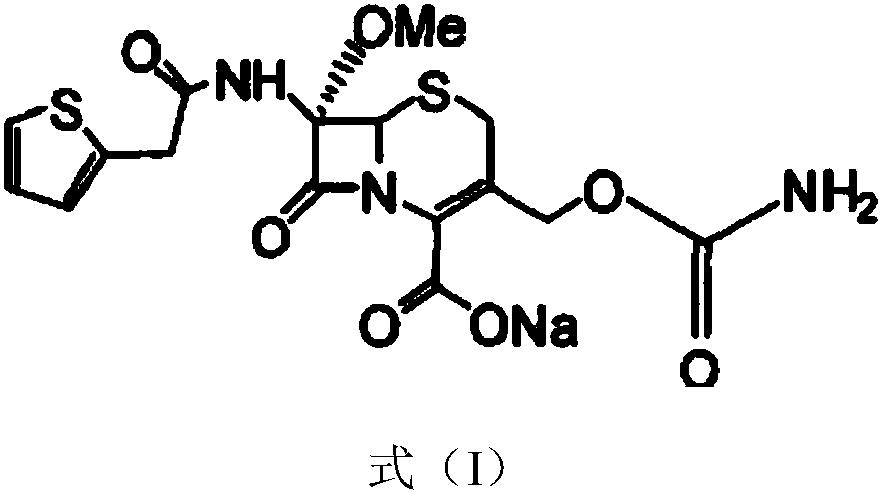
InactiveCN103601739AModerate granularityHigh yieldOrganic chemistryCefoxitin SodiumStructural formula
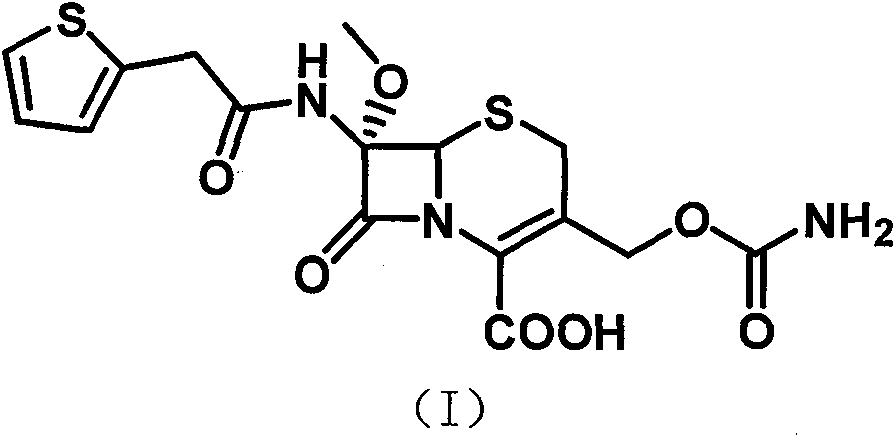
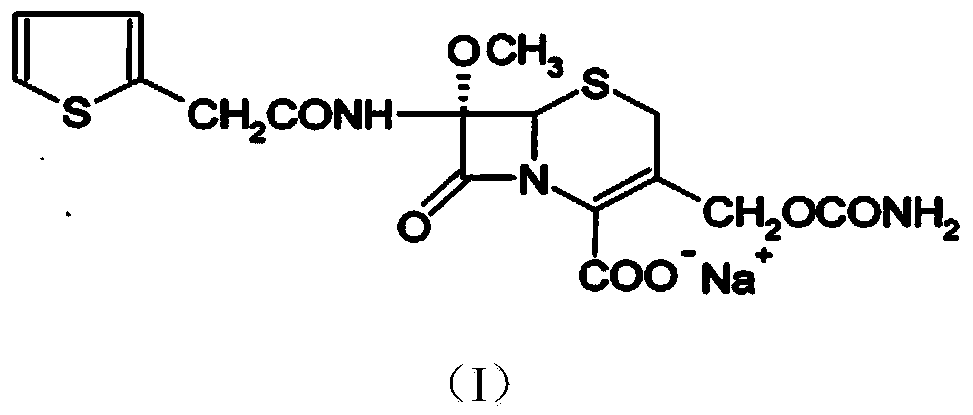
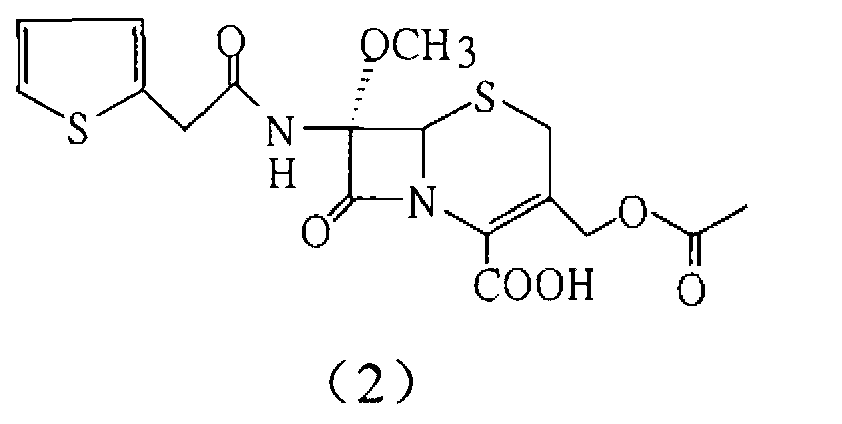
The invention relates to the field of medicines, and particularly relates to a cefoxitin sodium crystal compound and a preparation method thereof. The structural formula of the cefoxitin sodium crystal compound is shown as formula (I). The cefoxitin sodium crystal compound has high purity, is not easy to absorb water, has good stability, and is safe and reliable for clinical application.
Owner:YOUCARE PHARMA GROUP
Clostridium difficile chromogenic medium and application thereof
InactiveCN105838774AShort training periodThe culture cycle is shortened from the separation time of the existing technologyMicrobiological testing/measurementMicroorganism based processesSulfite saltSolvent
The invention discloses a Clostridium difficile chromogenic medium and an application thereof. The chromogenic medium consists of 1-20g / L of tryptone, 1-20g / L of digested animal tissue, and 0.1-5.5g / L of glucose. L, yeast extract 0.2‑5.5g / L, sodium chloride 0.5‑15g / L, sodium sulfite 0.05‑1g / L, escin 0.1‑20g / L, iron 0.1‑5g / L, amino acid 0.2‑25g / L , agar powder 5-20g / L, cycloserine 0.1-2.5g / L and cefoxitin sodium 0.01-0.15g / L, the solvent is deionized water, pH value 7.0~7.6; the medium culture period of the present invention is 24 hours, Colonies are specifically black, and C. difficile isolates can be accurately obtained from clinical stool samples by color when using this medium.
Owner:ZHEJIANG CENT FOR DISEASE CONTROL & PREVENTION
Cefoxitin sodium powder preparation for injection
ActiveCN104622695ALess impuritiesImprove stabilityAntibacterial agentsOrganic active ingredientsShape optimizationSolvent
The invention discloses a cefoxitin sodium powder preparation for injection. The cefoxitin sodium powder preparation is prepared by the following steps: (1) controlling the temperature, and preparing a sodium-forming agent solution; (2) adding acetone and methyl alcohol to a reaction tank, cooling, stirring under nitrogen protection, adding cefoxitin acid until dissolving, adding activated carbon, decolorizing, filtering, mixing a solvent and washing; (3) controlling the temperature of a crystallization tank, the stirring speed and the nitrogen pressure by molecular assembly and shape optimization techniques for crystal products in a grain process by Hebei Huamin Pharmaceutical Co.,Ltd. in North China, dropwise adding the sodium-forming agent solution, and adding acetone according to a feeding rate form; (4) filtering and drying crystalline liquid, detecting and discharging; and (5) carrying out sub-package of preparations with different specifications, and controlling the environment temperature and humidity to obtain cefoxitin sodium for injection. The cefoxitin sodium for injection, prepared by the preparation method, has good hydrodynamics performance, and is perfect in crystalline form and uniform in particle size distribution; and the color grade, the clarity, the purity and the stability are greatly improved.
Owner:NORTH CHINA PHARMA HEBEI HUAMIN PHARMA +1
Cefoxitin sodium superfine-powder preparation and preparation method thereof
InactiveCN104045656AHigh purityLess impuritiesAntibacterial agentsOrganic active ingredientsActivated carbonPhysical chemistry
The invention discloses a preparation method of cefoxitin sodium superfine-powder preparations. The method comprises the following steps: 1, at room temperature, adding 7-alpha-methoxy-3-deacetyl cefalotin benzathine to acetone; 2, pouring reaction liquid obtained after reaction into ionized water, stirring the obtained mixture to carry out a hydrolysis reaction at 0-25 DEG C; 3, adding ethyl acetate to the obtained hydrolysis liquid; 4, adding activated carbon into an organic phase, stirring for 40 minutes, filtering the obtained product; 5, dissolving sodium iso-octoate into an ethanol solution; and 6, crushing dried cefoxitin sodium into superfine powder by using an air flow. The invention also discloses a cefoxitin sodium superfine-powder preparation which comprises special cefoxitin sodium superfine powder. According to the preparation method, the purity of cefoxitin sodium is greatly improved, so that the cefoxitin sodium has the advantages of high purity, less impurities, small particles, large specific surface area, good solubility, good activity and the like.
Owner:杭州长典老一元健康管理有限公司
Production technology and detection method of cefoxitin sodium preparation
InactiveCN102283811AExtended service lifeImprove the experimental effectAntibacterial agentsPowder deliverySubstance useFiller Excipient
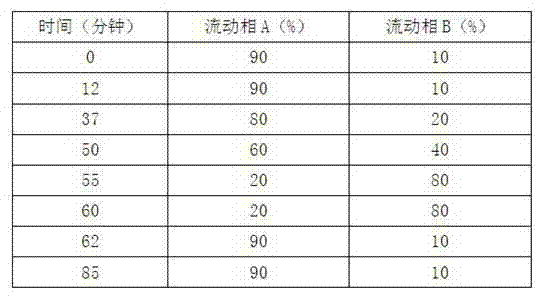
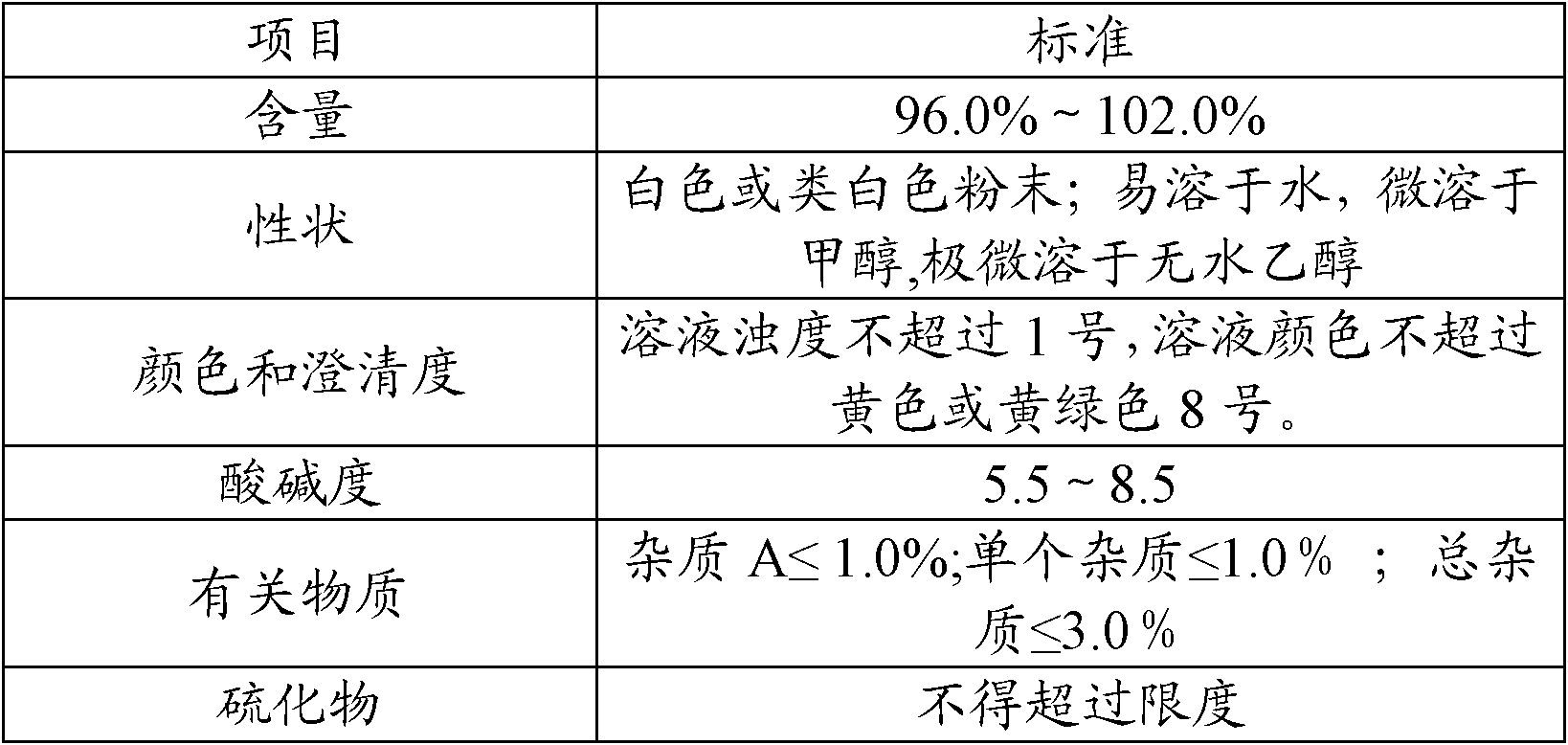
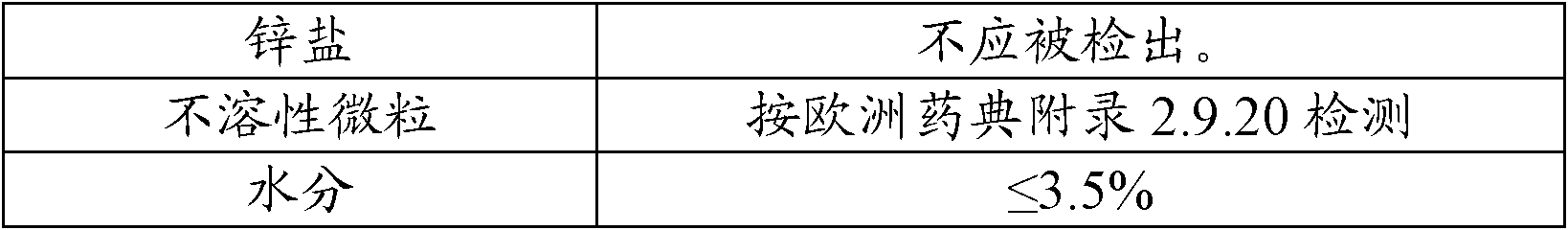
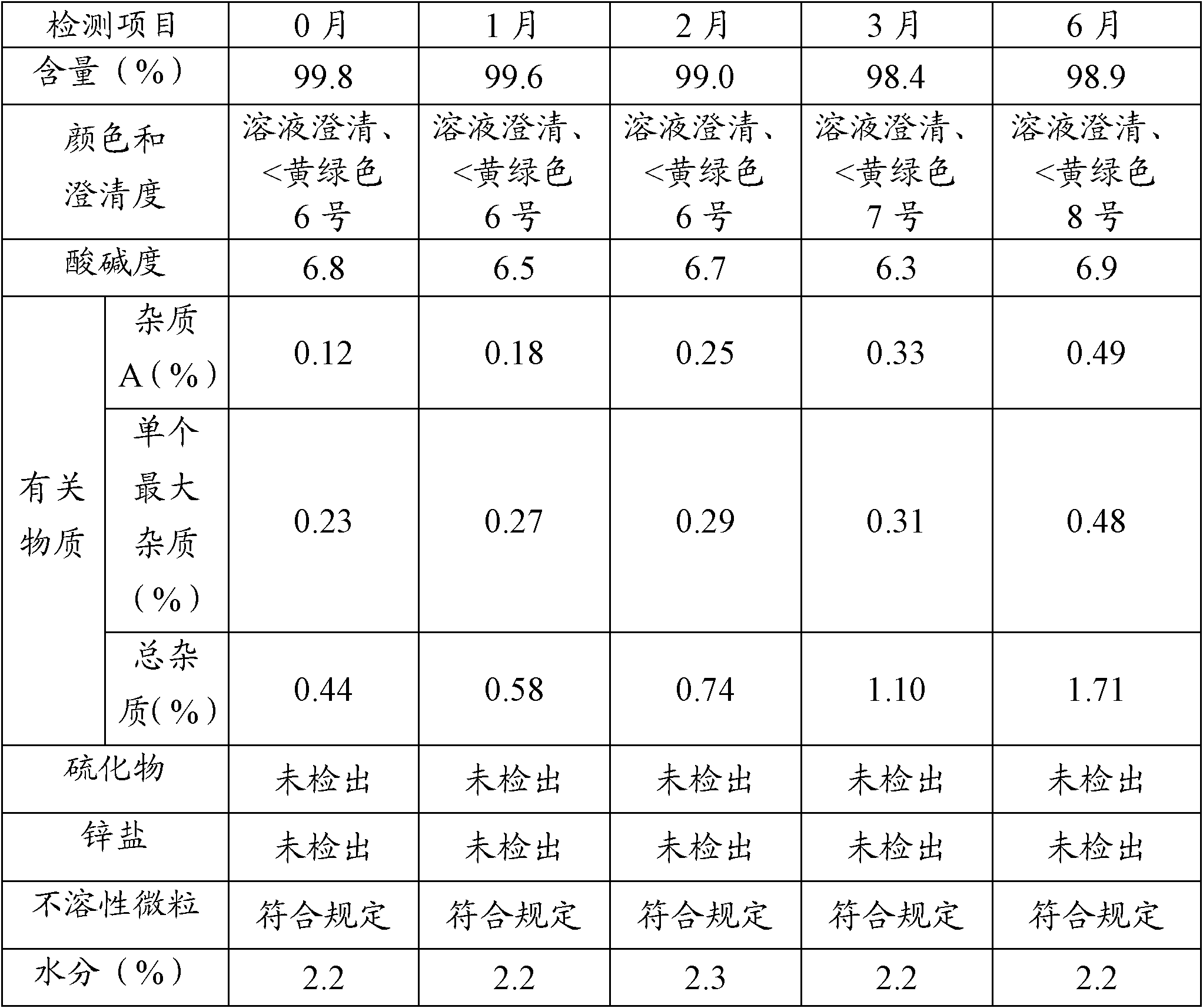
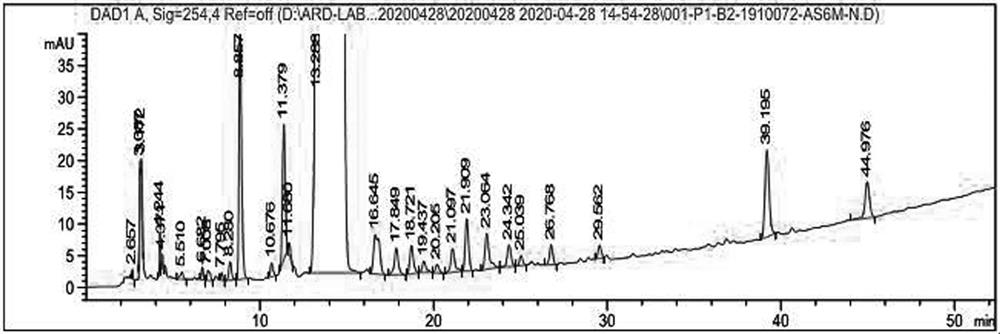
The invention provides a production process of cefoxitin sodium for injection with simple production process, stable and reliable quality, and a method for determining related substances in cefoxitin sodium by high performance liquid chromatography. The method adopts a chromatographic column with phenylsilane-bonded silica gel as the filler, the mobile phase A is water (adjusted to pH 2.7 with formic acid), and the mobile phase B is acetonitrile; linear gradient elution, high performance liquid chromatography for the determination of cefoxitin related substances in sodium. The specificity of this law is good, and the relevant substances are basically completely detected.
Owner:SUZHOU ERYE PHARMA CO LTD
Cefoxitin sodium, preparation method and uses thereof
ActiveCN105315300AGood uniformity of crystal formImprove liquidityOrganic active ingredientsOrganic chemistryFiltrationCefoxitin Sodium
According to the present invention, on the process basis of the conventional cefoxitin sodium preparation method using a methanol and acetone mixing solvent or similar mixing solvent to carry out reaction crystallization, C1-C4 alkanol or tetrahydrofuran is adopted as a precipitating agent, and solvent crystallization is performed to prepare cefoxitin sodium; the obtained cefoxitin sodium has characteristics of good crystal form uniformity, good fluidity, stable quality, easy filtration, and easy drying; and the method is suitable for industrial production.
Owner:HAIKOU PHARMA FACTORY +1
Cefoxitin sodium drug combination
InactiveCN101822681AImprove antibacterial propertiesReduce adverse reactionsAntibacterial agentsOrganic active ingredientsAdditive ingredientCurative effect
The invention discloses a cefoxitin sodium drug combination, which consists of the following effective ingredients in part by weight: 1000 to 2000 parts of cefoxitin sodium, 100 to 200 parts of lidocaine, 50 to 120 parts of reduced glutathione and 80 to 120 parts of diammonium glycyrrhizinate. The drug combination cannot cause adverse reactions, and has a high curative effect, and moreover, the preparation method is simple and environment-friendly.
Owner:邓学峰
Composition of cefoxitin acid
InactiveCN101669956ASolve the problem of insoluble in waterSolve instabilityAntibacterial agentsOrganic active ingredientsGeneration rateArginine
The invention relates to a cefoxitin acid composition which is characterized by comprising the combination of cefoxitin acid and arginine. The preparation method of the composition mainly comprises the following steps: weighing the aseptic raw material medicines of the cefoxitin acid and the arginine according to the proportion of a recipe in an aseptic environment, mixing evenly and then subpackaging. The composition solves the problem that the active component of the cefoxitin acid is not dissolved in water, also solves the problem that the original medicine of cefoxitin sodium is unstable,can effectively lower the impurity content and obviously enhance the stability of a product, thereby reducing the generation rate of side reactions, such as anaphylactic reactions, effectively; a safety testing result shows that compared with the original pharmaceutical preparation, the preparation has smaller local irritability and very important clinical application value.
Owner:CHANGSHA KINGDAY BIO PHARMA TECH
Method for preparing cefoxitin sodium
ActiveCN101613361BSimplify production stepsHigh yieldOrganic chemistryAntiinfectivesLithium methoxideOrganic solvent
The invention provides a method for preparing cefoxitin sodium, which comprises the following steps: 1, using deacetyl7-ACA as a raw material, and introducing thiopheneacetyls to obtain an deacetylthiophene acid I; 2, introducing carbamoymethoxyls to the positions 3 of molecules of deacetylthiophene acid I to obtain an intermediate of (6R, 7S)-3-carbamoymethoxyl-7-[2-(2-theiophene) acetamino]-8-oxo-5-thia-1-azabicyclo[4.2.0]octa-2-en-2-methanoic acid II; 3, reacting the intermediate II with lithium methoxide to introduce methoxyls into positions 7 of the molecules of the intermediate to obtain cefoxitin acid III; and 4, adding ethanol solution of a sodium salt into an organic solvent containing the cefoxitin acid and obtaining the cefoxitin sodium IV through suction filtration and drying.
Owner:哈药集团股份有限公司 +1
Cefoxitin sodium compound entity and composition and uses thereof
InactiveCN104402908AEasy to slidePromote dissolutionAntibacterial agentsOrganic chemistryUpper urinary tract infectionChronic pyonephrosis
The invention discloses a cefoxitin sodium new chemical compound entity and a composition thereof, the cefoxitin sodium new chemical compound storage has better stability less hygroscopicity, and is suitable for preparation of drugs for the treatment or prevention of human or animal respiratory tract infection, endocarditis, peritonitis, pyelonephritis, urinary tract infection, blood poisoning and bone, joint, skin and soft tissue infection and the like caused by gram positive or negative bacteria sensitive bacteria.
Owner:胡梨芳
Cefoxitin sodium compound and preparation method thereof
ActiveCN103804397AImprove stabilityImprove bioavailabilityOrganic chemistryCefoxitin SodiumBioavailability
The invention relates to the field of medicines, and particularly relates to a cefoxitin sodium compound and a preparation method thereof. A structural formula of the cefoxitin sodium compound is shown as a formula (I). The cefoxitin sodium compound disclosed by the invention is high in purity, difficulty in absorbing moisture, good in stability, high in bioavailability and safe and reliable in clinical application. The formula (I) is described in the specification.
Owner:YOUCARE PHARMA GROUP +1
Method for preparing cefoxitin acid
ActiveCN102153567BIncrease production costSimple production processOrganic chemistryCefoxitin SodiumRaw material
Owner:重庆天地药业有限责任公司
Cefoxitin sodium agent and preparation method thereof
InactiveCN103271877AImprove stabilityThe prescription process is simpleAntibacterial agentsPowder deliveryAdditive ingredientFreeze-drying
The invention relates to a cefoxitin sodium agent and preparation method thereof, especially relates to a cefoxitin sodium injection for treating microbe infection, preferably relates to a freeze-drying powder injection. The cefoxitin sodium injection of the present invention is mainly composed of cefoxitin and accessory mannitol. The solvent in the injection of the present invention is injection water; the excipient in the freeze-drying powder injection is mannitol, and sodium hydroxide or hydrochloric acid are used for adjusting pH valve.
Owner:张宏民
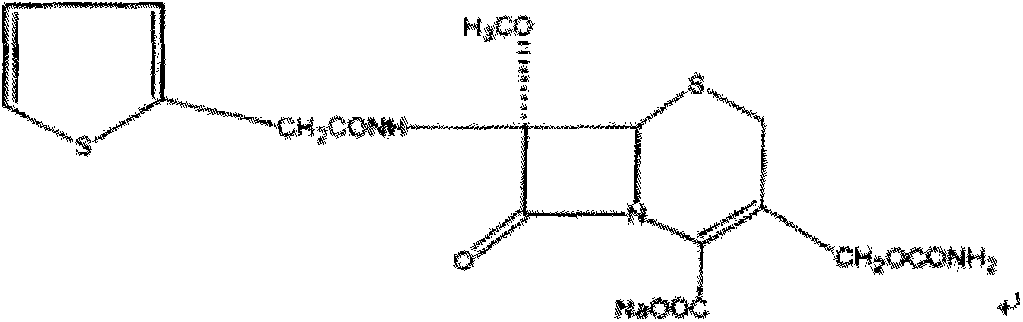
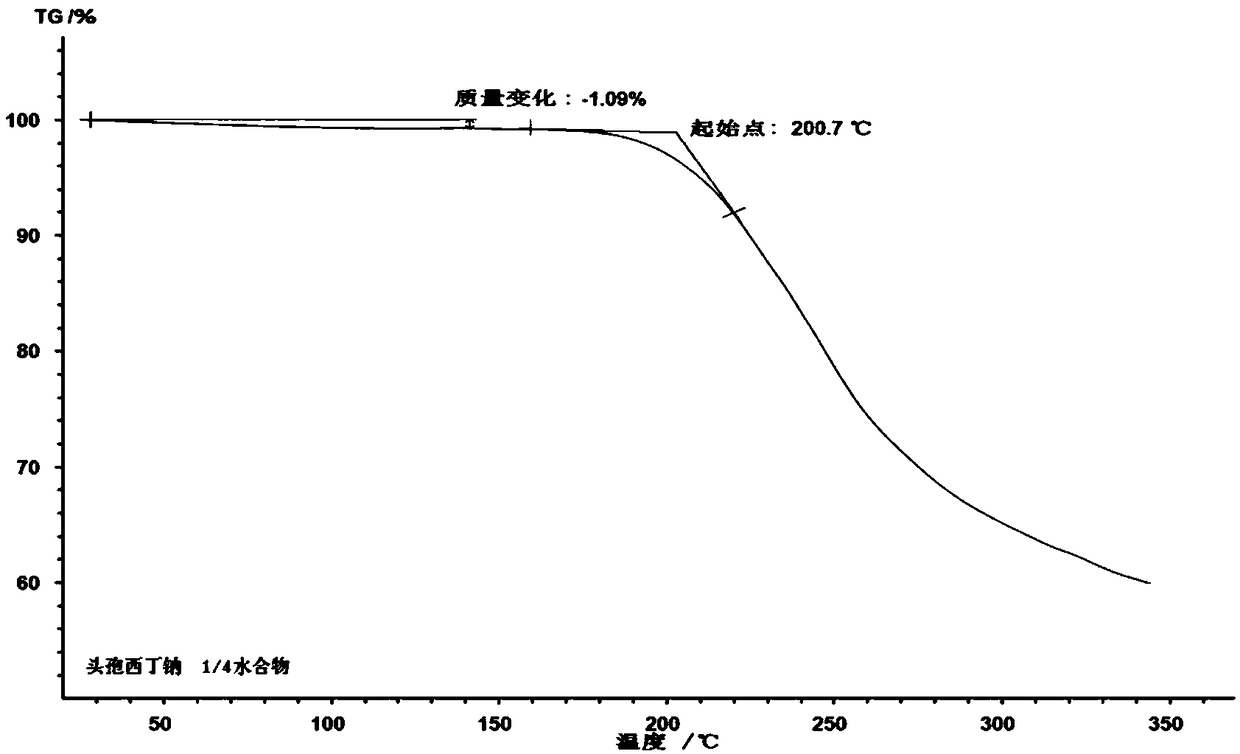
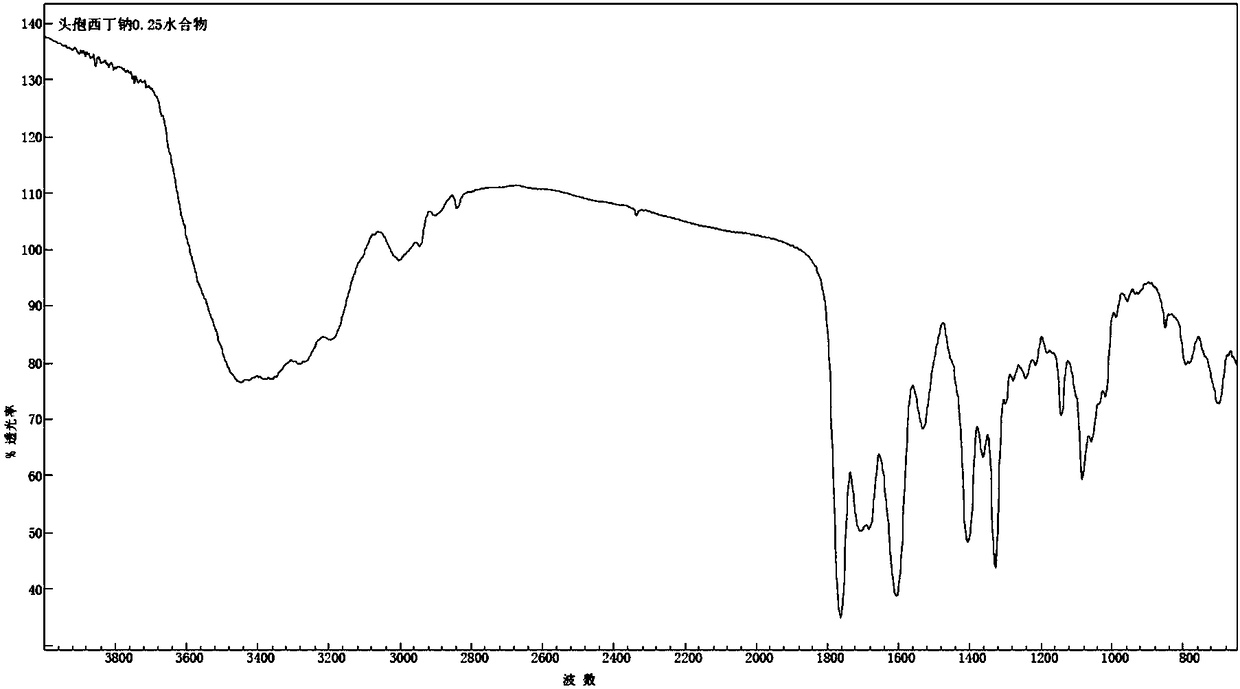
1/4 water cefoxitin sodium compound
The invention discloses a 1 / 4 water cefoxitin sodium compound and a preparation method thereof. Each mole of the cefoxitin sodium contains 1 / 4 mole of water. The preparation method is simple, reactants are easy to obtain, reaction conditions are mild, and the yield is high. The 1 / 4 water cefoxitin sodium compound has good particle size distribution, good fluidity, low impurity content, thermodynamic stability and wide application prospects.
Owner:梁怡
Synthesis method of cefoxitin sodium
ActiveCN109651403AAvoid it happening againEasy to operateOrganic chemistrySynthesis methodsCefoxitin Sodium
The invention provides a synthesis method of cefoxitin sodium. The synthesis method is characterized in that cefalotin acid is taken as the raw material to sequentially synthesize 7-a-methoxyl cefalotin cyclohexane salt, 7-a-methoxyl-3-deacetyl cefalotin benzathine salt, and cefoxitin acid to obtain the target product; cefalotin acid reacts with tert.-butyl hypochloric acid to obtain 7-a-methoxylcefalotin cyclohexane salt, and after reactions, the reaction product is purified by a post treatment. The provided synthesis method can largely reduce the happening rate of side reactions, hydrolysis, and degradation, reduces the impurities, improves the product quality, and increases the yield. Moreover, the product quality is stable, the operation is simple, and the synthesis method is suitablefor industrial production.
Owner:SHANGHAI NEW ASIA PHARMA
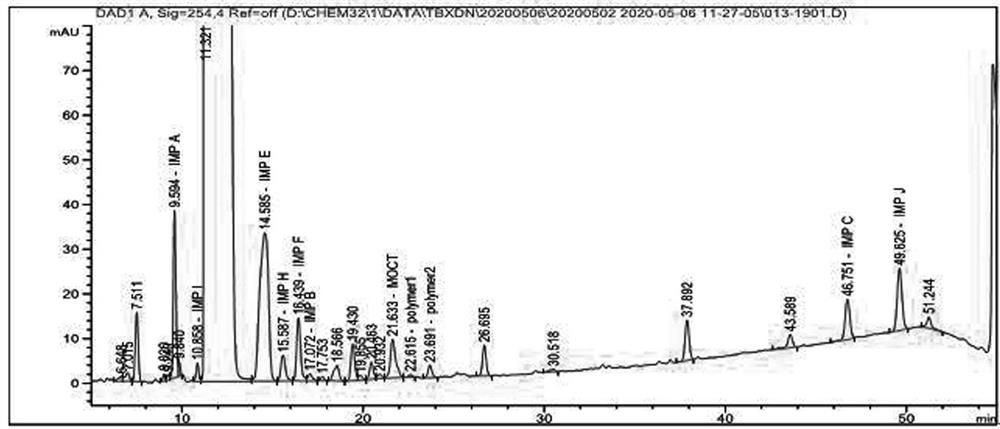
Method for detecting polymer in cefoxitin sodium for injection
PendingCN113281445AReduce sensitivityHigh sensitivityComponent separationFluid phaseBiochemical engineering
The invention belongs to the technical field of pharmaceutical analysis, and particularly relates to a method for detecting polymers in cefoxitin sodium for injection. The method comprises three steps of detection condition, solution preparation, system applicability requirement and limit. According to the present invention, the high performance liquid chromatography is adopted, such that the operation is simple, the sensitivity is high, the specificity is good, the content of the single polymer and the total polymer in the product can be accurately controlled, the product quality can be effectively controlled, and the clinical use safety can be ensured.
Owner:SHENYANG SANJIU PHARMA
Cefoxitin sodium crystal compound and cefoxitin sodium composition powder injection
ActiveCN102358744BGood dissolution stabilityImprove bioavailabilityAntibacterial agentsPowder deliveryCefoxitin SodiumSodium benzoate
The invention relates to a cefoxitin sodium crystal compound. The method of powder X-ray diffractometry is utilized to determine the cefoxitin sodium crystal compound, and an X-ray powder diffraction pattern represented by the diffraction angle of 2theta+-0.2 degrees has characteristic diffraction peaks at 5.3 degrees, 8.6 degrees, 11.9 degrees, 13.2 degrees, 15.5 degrees, 16.1 degrees, 19.0 degrees, 22.3 degrees and 24.4 degrees. The invention also relates to a cefoxitin sodium composition powder injection containing the cefoxitin sodium crystal compound, and the cefoxitin sodium composition powder injection comprises 95 to 100 parts of the cefoxitin sodium crystal compound and 0.1 to 1 part of sodium benzoate.
Owner:SHANDONG LUOXIN PHARMA GRP CO LTD +2
Cefoxitin sodium crystalline compound
InactiveCN108218894AImprove thermal stabilityAntibacterial agentsOrganic active ingredientsRoom temperatureX-ray
The invention belongs to the technical field of medicine and particularly relates to a cefoxitin sodium crystalline compound shown as formula (I) in the description. An X-ray powder diffraction pattern of the compound under measurement with Cu-Kalpha rays is shown as figure 1. The cefoxitin sodium crystalline compound has better stability and can be stored at room temperature, and storage cost andtransport cost of the drug are reduced effectively.
Owner:BEIJING RED SUN PHARMA +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com