Preparation method of high-purity cefoxitin sodium
A technology of cefoxitin sodium and cefoxitin acid, which is applied in the direction of organic chemistry to achieve the effect of simple operation and improved product quality
Active Publication Date: 2011-01-12
ZHEJIANG WHITESON PHARMA
View PDF3 Cites 9 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
The first step: resin adsorption purification
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
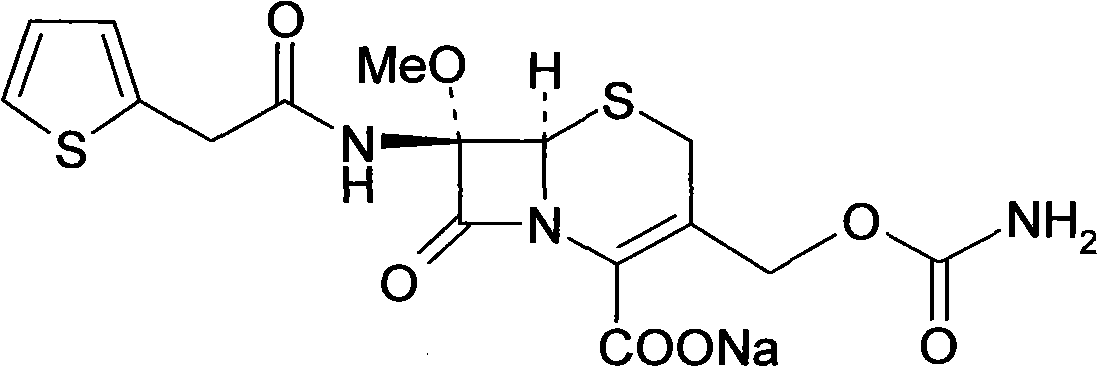
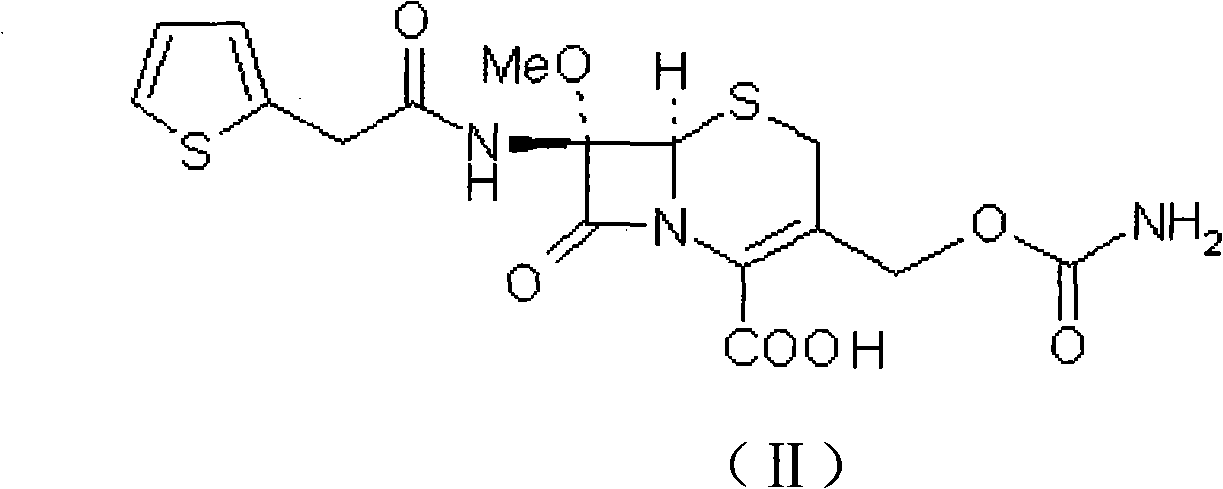
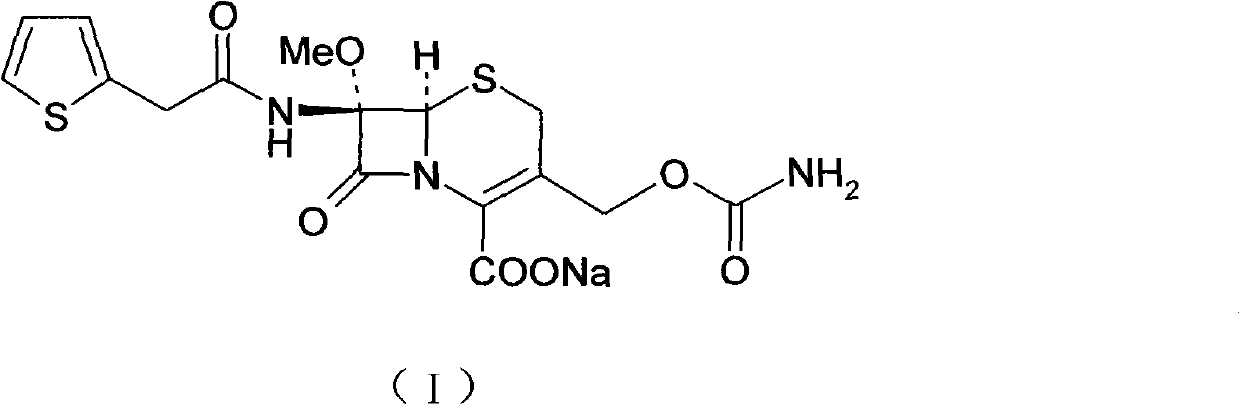
The invention provides a preparation method of high-purity cefoxitin sodium (I), belonging to the field of chemical pharmaceutical technology. The method comprises the following steps: taking cefoxitin acid as a raw material, adsorbing and purifying the cefoxitin acid with a resin column, and allowing the purified cefoxitin acid to react with sodium salt at the temperature of -20-20 DEG C to obtain the high-purity cefoxitin sodium.
Description
technical field The invention relates to a preparation method of a high-purity compound, in particular to a preparation method of cephalosporin compound cefoxitin sodium. It belongs to the technical field of chemical pharmacy. Background technique Cefoxitin sodium (Cefoxitin sodium), chemical name: (6R, 7S)-3-carbamoyloxymethyl-7-methoxy-8-oxo-7-[2-(2-thiazolyl) Acetamido]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid sodium salt. Its chemical structural formula is as follows: It is a second-generation cephalosporin antibiotic developed by Merck Company of the United States. It has a balanced antibacterial spectrum and is stable to β-lactamase. At present, due to the increasing bacterial resistance to cephalosporins, cefoxitin, which is different from the first-generation and third-generation cephalosporins, has once again attracted people's attention. Contents of the invention The invention adopts a new production process, uses cefoxitin acid as a raw mate...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): C07D501/57C07D501/04
Inventor 芦红代王超魏曾光
Owner ZHEJIANG WHITESON PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com