Method for preparing cefoxitin sodium
A technology of cefoxitin sodium and cefoxitin acid, which is applied in the directions of organic chemistry, anti-infective drugs, and drug combinations to achieve the effects of stabilizing product quality, reducing production costs, and improving product yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
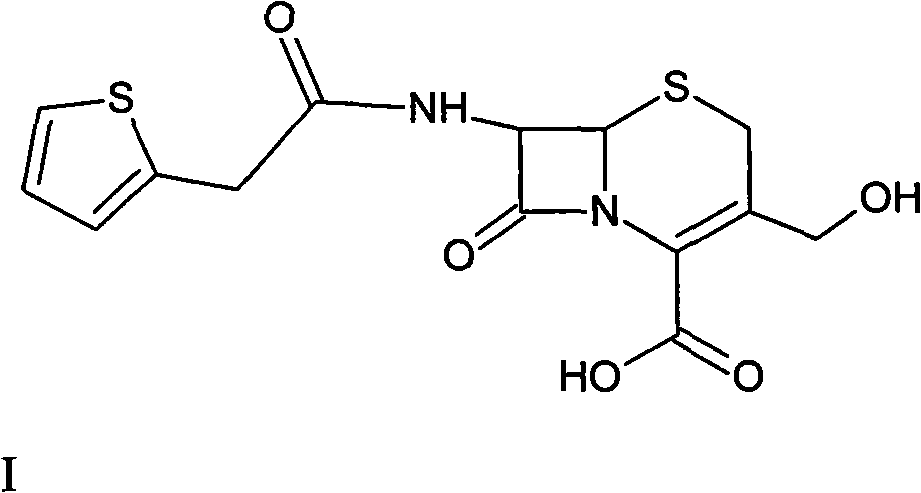
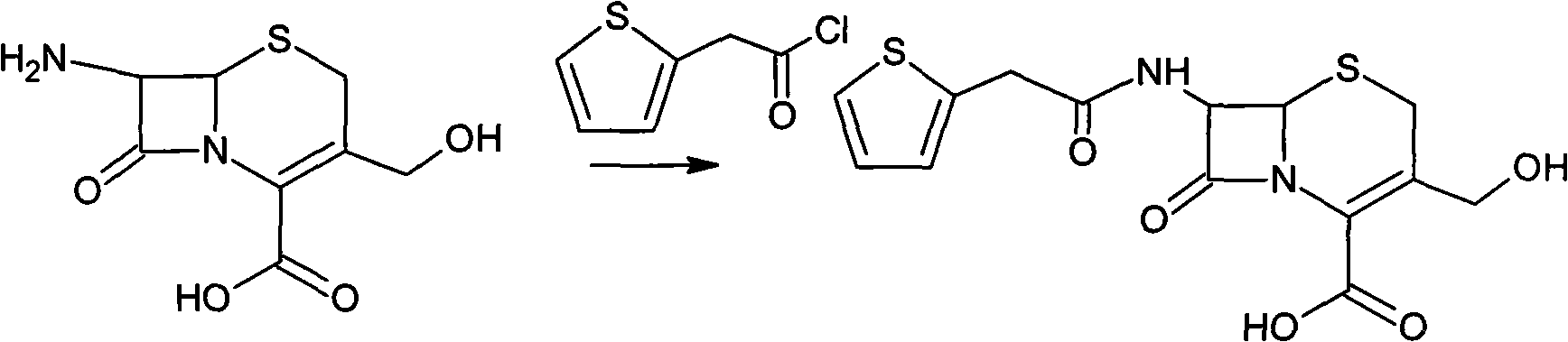
[0041] Preparation of deacetylthiophene acid I
[0042] At room temperature, add 120 g of deacetylated 7-ACA into a mixed solution of 1.2 L of acetone and 0.6 L of water, stir rapidly, add 70 g of thiophene acetyl chloride dropwise, and react for 30 minutes after the drop is complete, evaporate the acetone in vacuum at 40°C, and the remaining 0.7L concentrate. Add 10% HCL dropwise to the concentrated solution at room temperature to stabilize the pH value at 2.5, grow the crystal for 30 minutes, filter with suction, wash the filter cake three times with 0.9L of water, and dry it under vacuum at 30°C. Get deacetylthiophene acid 158g, yield 131.7%
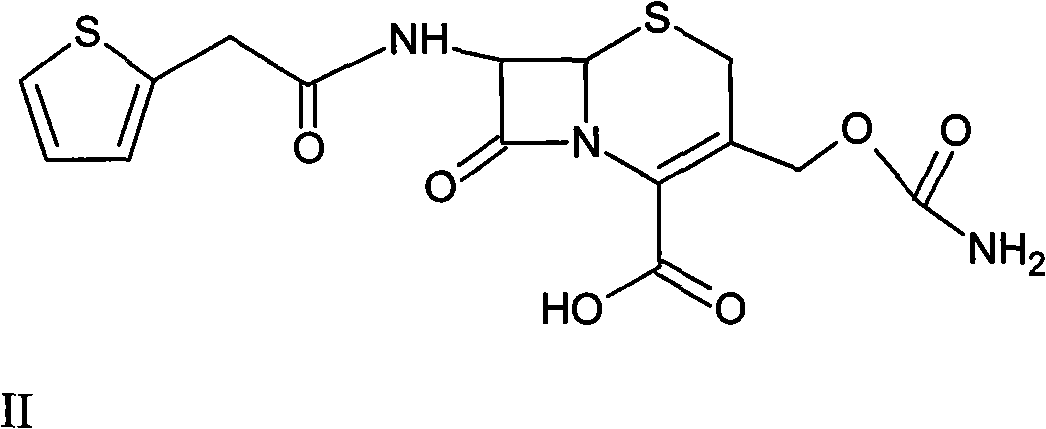
[0043] Intermediate (6R, 7S)-3-carbamoylmethoxy-7-[2-(2-thienyl)acetamido]-8-oxo-5-thia-1-azabicyclo[4.2. 0] Preparation of Oct-2-ene-2-carboxylic acid II
[0044] At minus 30°C, add 150 g of deacetylthiophene acid into 1 L of tetrahydrofuran, and stir until completely dissolved. Add 72 g of carbamyl reagent (chlorosulfonyl isocya...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com