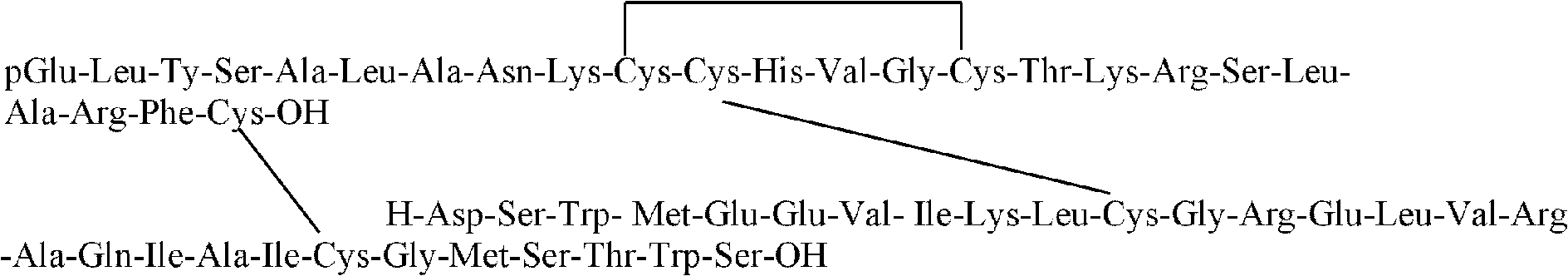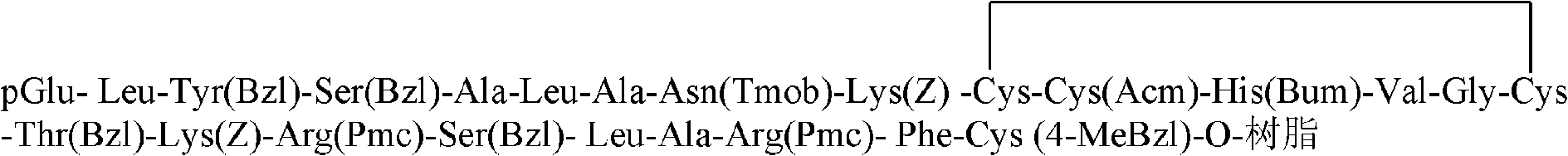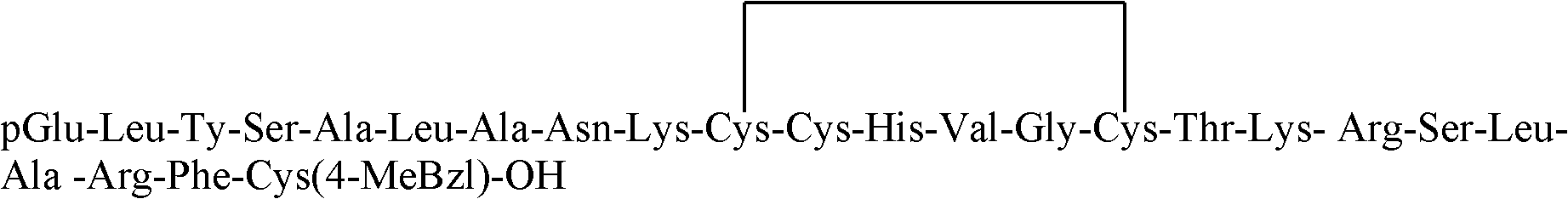Solid-phase synthesis method of human relaxin-2
A technology of solid-phase synthesis and relaxin, which is applied in the field of synthesis of human relaxin-2, can solve the problems of complex B chain synthesis process, unfavorable industrial production, and low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] pGlu-Leu-Tyr(Bzl)-Ser(Bzl)-Ala-Leu-Ala-Asn(Tmob)-Lys(Z)-Cys(Trt)-Cys(Acm)-His(Bum)-Val-Gly-Cys Solid Phase Synthesis of (Trt)-Thr(Bzl)-Lys(Z)-Arg(Pmc)-Ser(Bzl)-Leu-Ala-Arg(Pmc)-Phe-Cys(4-MeBzl)-O-Resin
[0044] (1) Swell 50 mmol Wang resin in dichloromethane, and wash the resin with N-methylpyrrolidone.
[0045] (2) After dissolving 60mmol Fmoc-Cys(4-MeBzl)-OH with an appropriate amount of N,N-dimethylformamide, add the solution to the resin.
[0046] Get 60mmol 1-oxygen-3-bisdimethylaminocarbonylbenzotriazole boron tetrafluoride salt, 180mmol 1-hydroxybenzotriazole and 60mmol N, N-diisopropylethylamine to prepare a solution, then The solution was slowly added dropwise into the mixed system of resin and Fmoc-Cys(4-MeBzl)-OH, and stirred at room temperature for 3 hours to obtain Fmoc-Cys(4-MeBzl)-O-resin.
[0047] (3) The Fmoc-Cys(4-MeBzl)-O-resin was sucked dry, and washed three times with dichloromethane, using 5ml of dichloromethane for each gram of resin in each wa...
Embodiment 2
[0051] The cyclization reaction of embodiment 2 linear A chains
[0052] pGlu-Leu-Tyr(Bzl)-Ser(Bzl)-Ala-Leu-Ala-Asn(Tmob)-Lys(Z)-Cys(Trt)-Cys(Acm)-His(Bum)-Val-Gly- Cys(Trt)-Thr(Bzl)-Lys(Z)-Arg(Pmc)-Ser(Bzl)-Leu-Ala-Arg(Pmc)-Phe-Cys(4-MeBzl)-O-resin with hydrogen bromide In the mixed solution that acetic acid is made into by volume ratio 3: 7, the mixed solution consumption of hydrogen bromide and acetic acid is that every gram of resin uses 4-9ml, reacts 20 minutes and removes the Trt protecting group of Cys (Trt) at room temperature, will After washing the resin with dichloromethane, pGlu-Leu-Tyr(Bzl)-Ser(Bzl)-Ala-Leu-Ala-Asn(Tmob)-Lys(Z)-Cys(Trt)-Cys(Acm )-His(Bum)-Val-Gly-Cys(Trt)-Thr(Bzl)-Lys(Z)-Arg(Pmc)-Ser(Bzl)-Leu-Ala-Arg(Pmc)-Phe-Cys( Add water, acetonitrile and dimethyl sulfoxide to the 4-MeBzl)-O-resin to prepare a mixed solution of 5:4:1 by volume, and stir at room temperature for 4 hours to carry out the cyclization reaction, wherein the mixed solution of hydrog...
Embodiment 3
[0054] Embodiment 3A chain side chain protecting group and the excision of resin
[0055] Wash the peptide-linked resin obtained in Example 2 and add it to the mixed solution prepared by bromotrimethylsilane, thioanisole, methylene chloride and trifluoroacetic acid in a volume ratio of 3:2:4.5:0.5 , standing at a constant temperature of 10°C for 2 hours, then washing and drying the resin linked to the polypeptide, adding trifluoroacetic acid, dichloromethane, N-methylmorpholine and triethylsilane in a volume ratio of 5.5:2.0:1.5:1.0 to prepare In the mixed solution, it was kept at a constant temperature of 0°C for 2 hours. Then add the resin connecting the peptide chains to a mixed solution prepared by volume 70:8:10:5:7 of trifluoroacetic acid, thioanisole, phenol, dimercaptoethanol and water, and let it stand for 2 hours to form the following structural formula: A chain of single rings exists within the chain:
[0056]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com