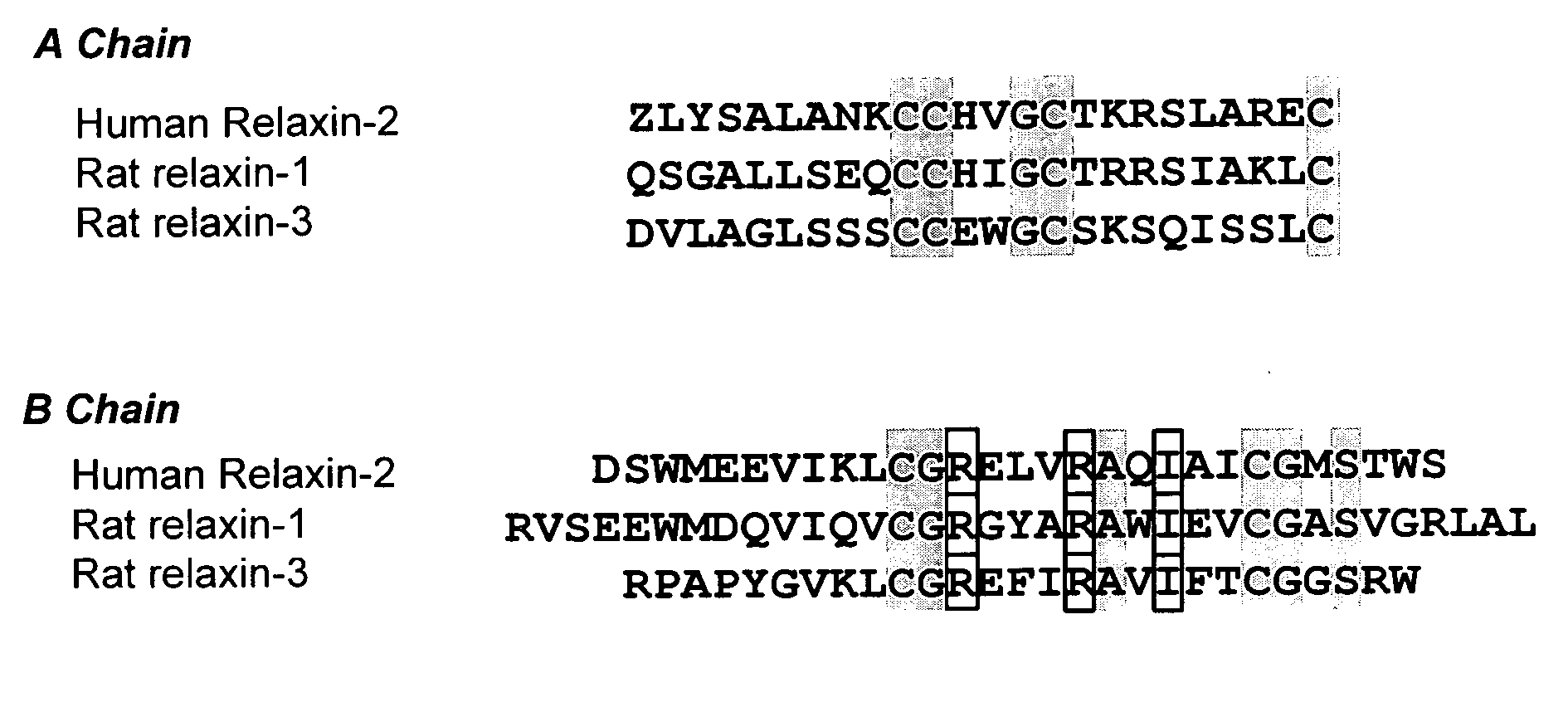Patents
Literature
75 results about "Relaxin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
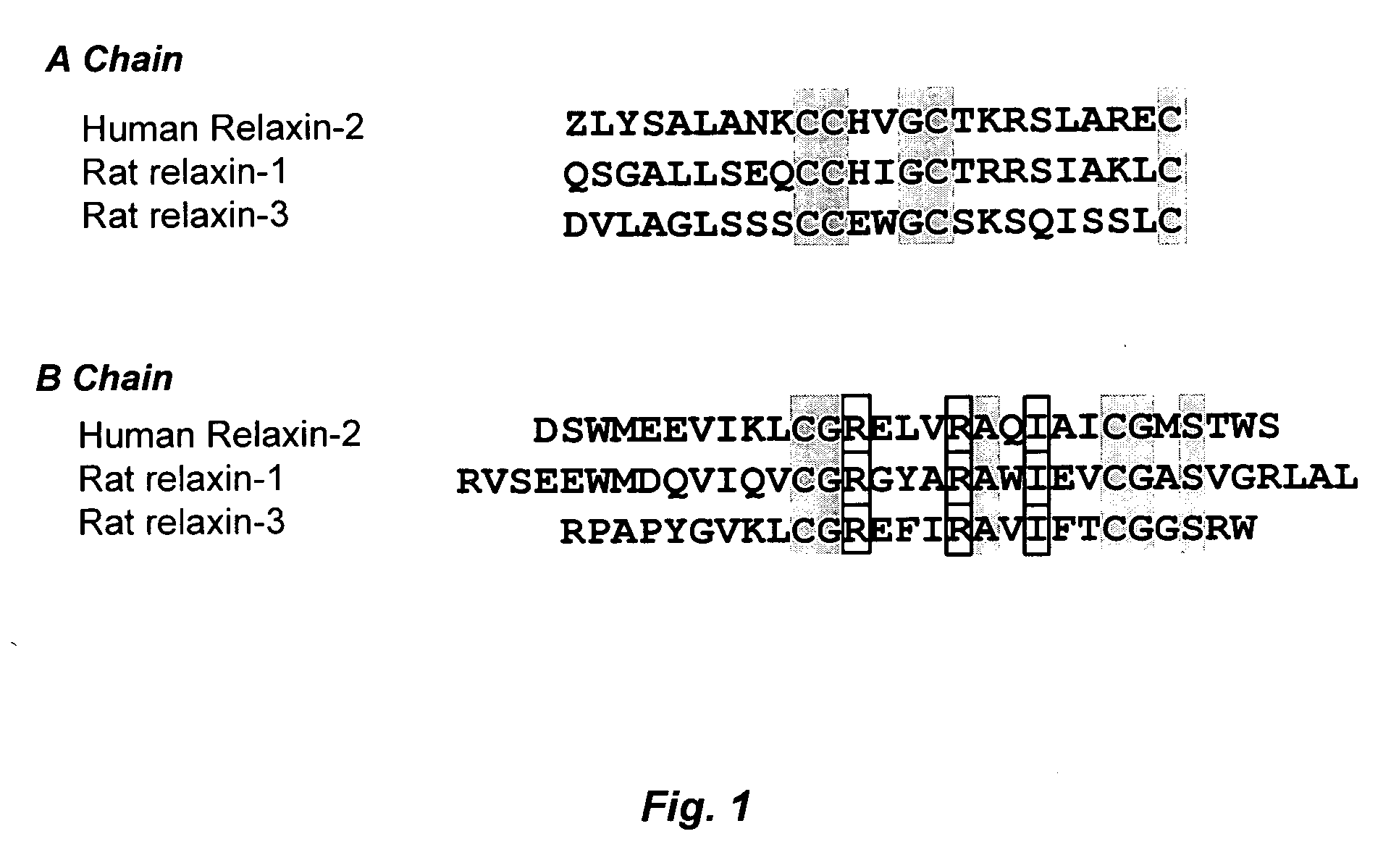
Relaxin is a protein hormone of about 6000 Da first described in 1926 by Frederick Hisaw. The relaxin-like peptide family belongs in the insulin superfamily and consists of 7 peptides of high structural but low sequence similarity; relaxin-1 (RLN1), 2 (RLN2) and 3 (RLN3), and the insulin-like (INSL) peptides, INSL3, INSL4, INSL5 and INSL6. The functions of relaxin-3, INSL4, INSL5, INSL6 remain uncharacterised.
Method of promoting angiogenesis using relaxin
InactiveUS6211147B1Organic active ingredientsPeptide/protein ingredientsRelaxinAngiogenesis growth factor
Relaxin is useful for promoting angiogenesis and the treatment of infections or ischemic wounds where the injury results from lack of oxygen due to poor circulation.
Owner:CORTHERA INC
Use of relaxin treat diseases related to vasoconstriction
InactiveUS6723702B2Safety profileImprove securityElectrotherapyPeptide/protein ingredientsArteriolar VasoconstrictionVascular disease
The invention related to methods of treating disease related to vasoconstriction that is a major factor in hypertensive vascular diseases and vasodilation, generally comprising administering to an individual an effective amount of a pharmaceutically active relaxin. Relaxin functions to increase both vasodilation and angiogenesis in males as well as females, and is useful in treating a wide variety of diseases relating to vasoconstriction.
Owner:RUTGERS THE STATE UNIV +2
Modified relaxin polypeptides and their uses
ActiveUS20120046229A1Increased pharmacokineticIncreased pharacodynamicAntibacterial agentsFungiRelaxinChemistry
Owner:AMBRX
Gene Delivery System Containing Relaxin Gene And Pharmaceutical Composition Using Relaxin
InactiveUS20070202080A1High transduction efficiencyEnhance transduction (or spreading) efficiencyBiocidePeptide/protein ingredientsBiotechnologyGene delivery
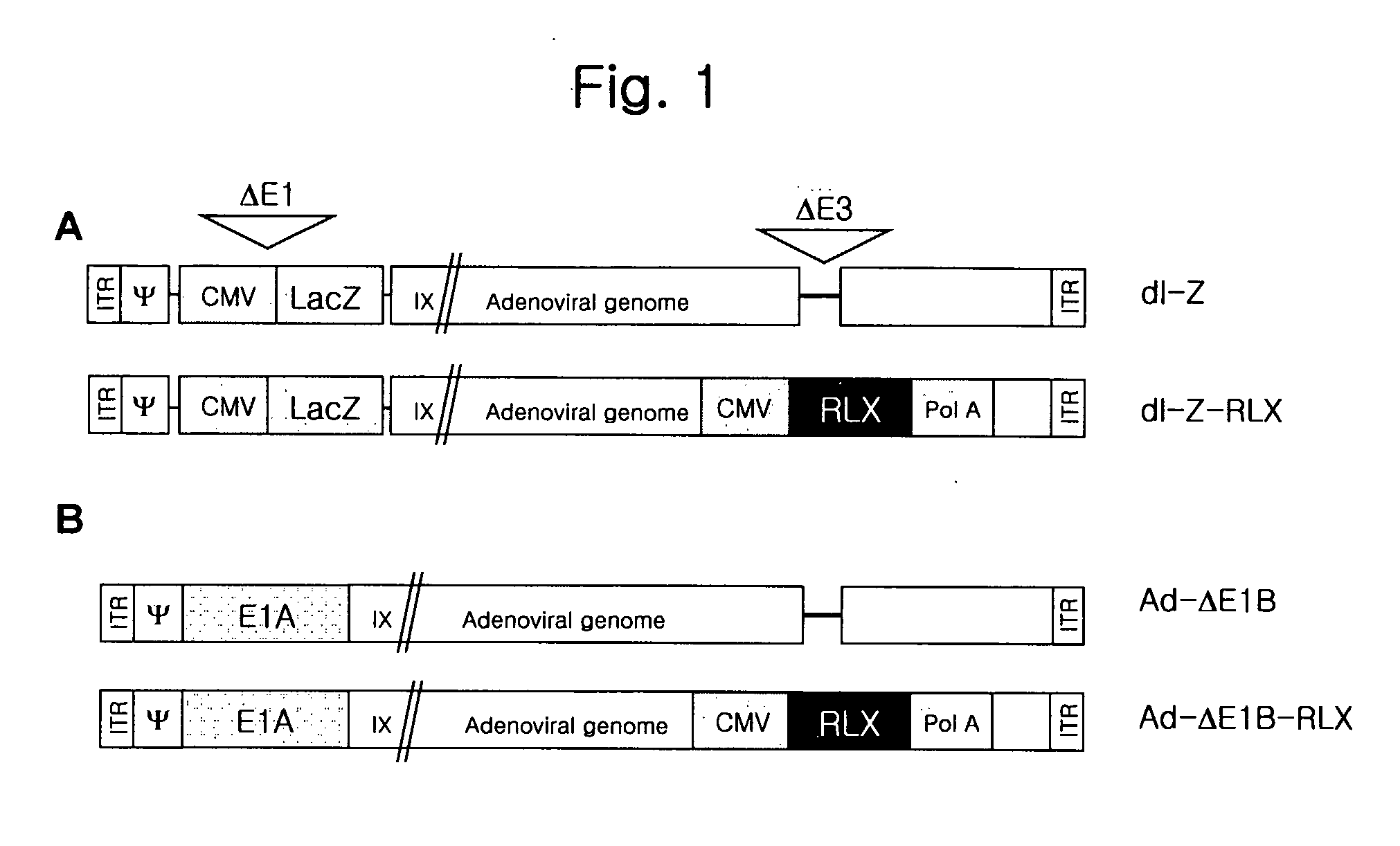
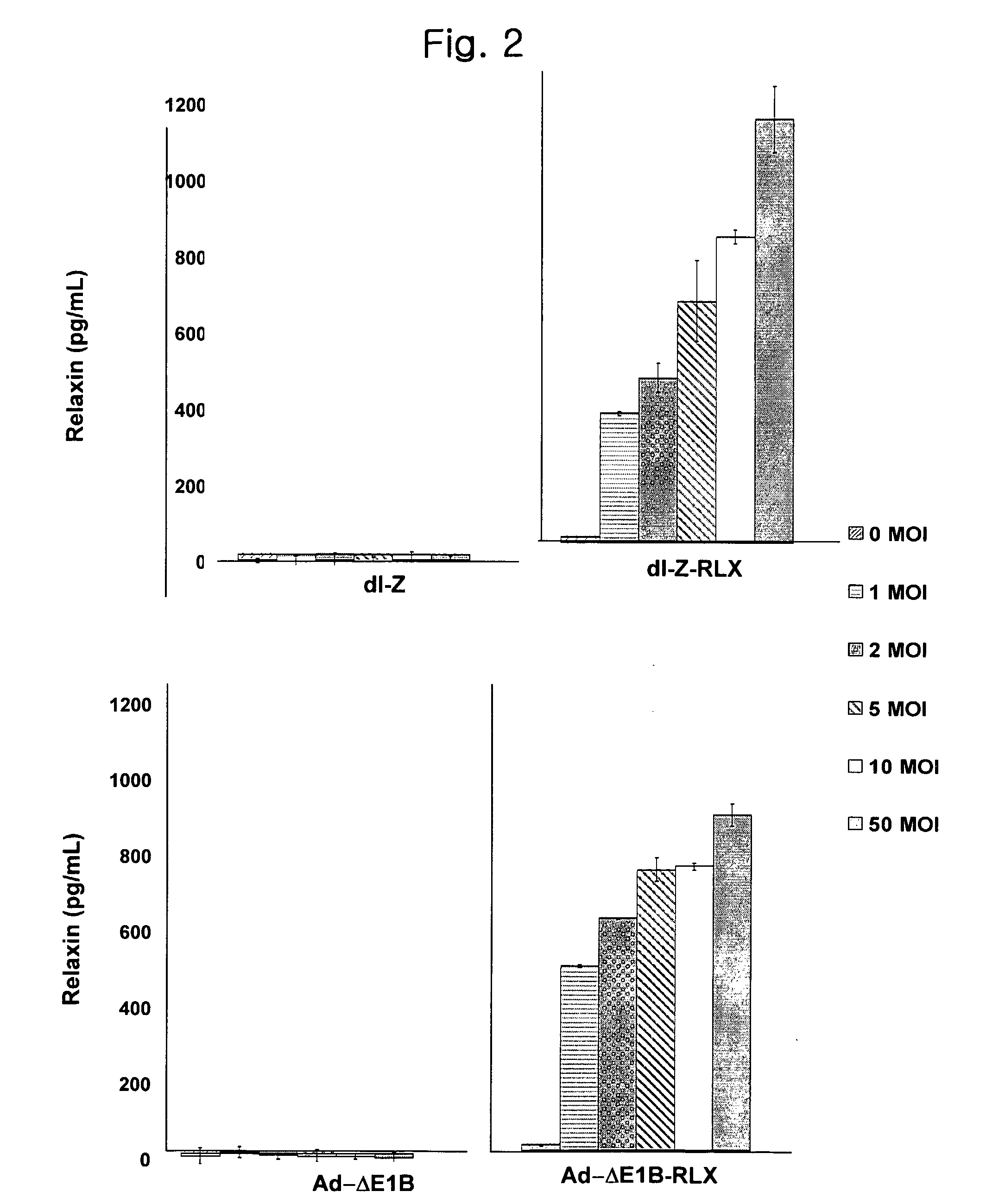
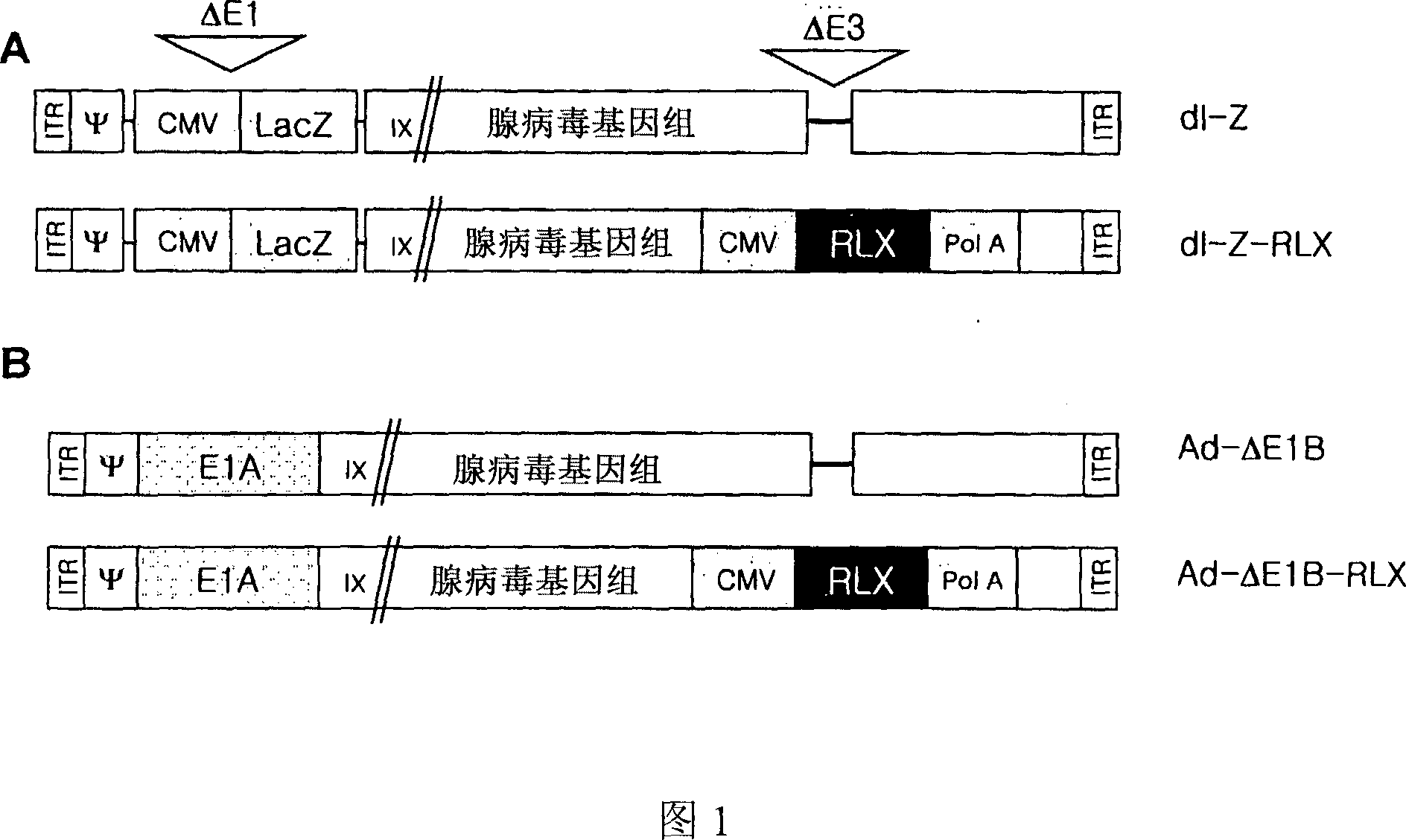
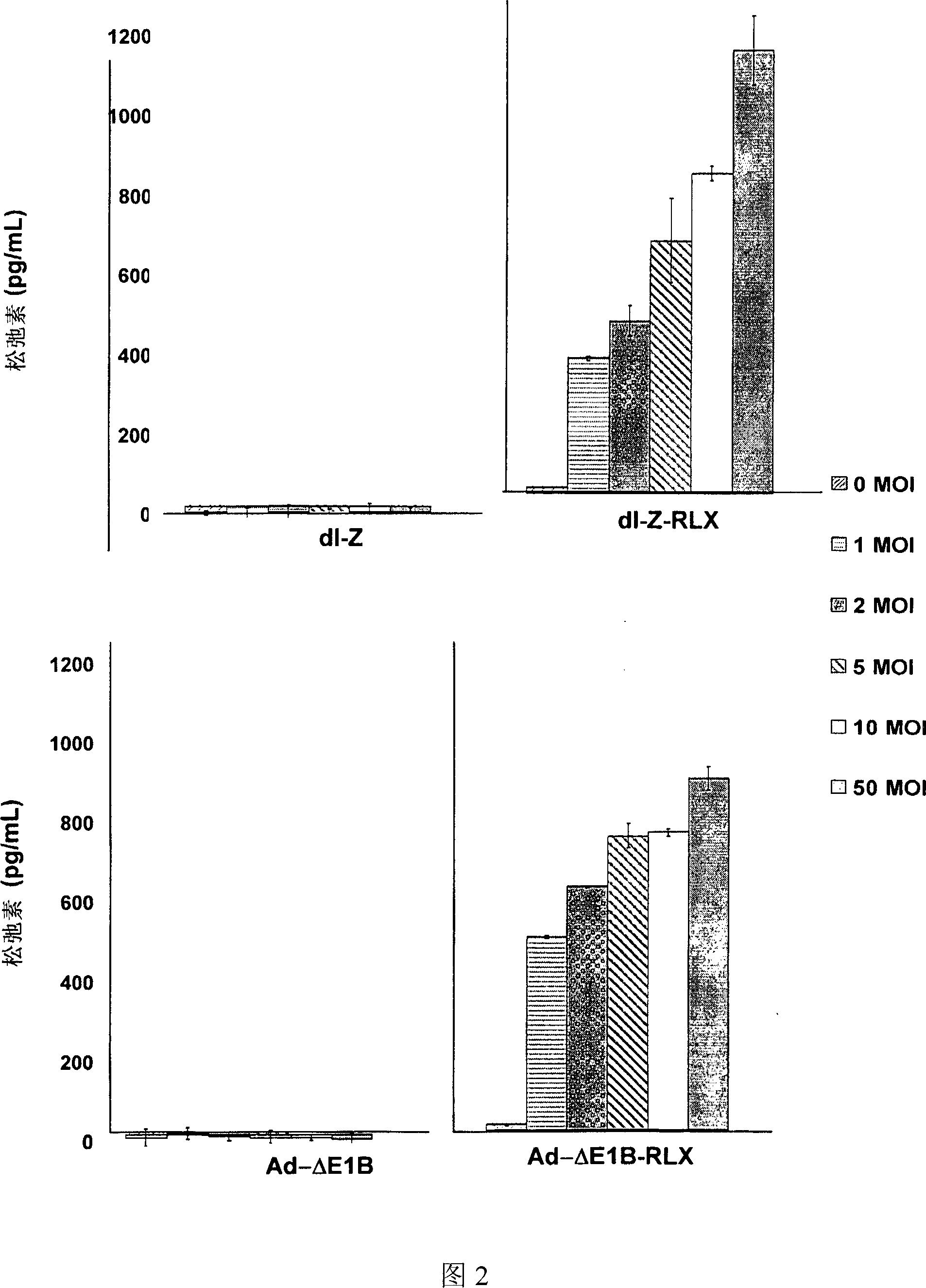
The present invention relates to a novel gene delivery system and recombinant adenovirus comprising the relaxin-encoding sequence to enhance transduction efficiency of transgenes, a pharmaceutical anti-tumor composition comprising the recombinant adenovirus, a pharmaceutical composition having improved tissue penetration potency and a pharmaceutical composition for treating a disease or disorder associated with accumulation of excess extracellular matrix.
Owner:IND ACADEMIC CORP FOUND YONSEI UNIV
Use of relaxin to treat diseases related to vasoconstriction
InactiveUS20040266685A1Safety profileImprove securityPeptide/protein ingredientsRelaxinsArteriolar VasoconstrictionDisease
The invention relates to methods of treating diseases related to vasodilation, generally comprising administering to an individual an effective amount of a pharmaceutically active relaxin. Relaxin functions to increase both vasodilation and angiogenesis in males as well as females, and is therefore useful in treating a wide variety of diseases relating to vasoconstriction.
Owner:THE UNIVERSITY OF MEDICINE & DENTISTRY OF NEW JERSEY ROBERT WOOD JOHNSON MEDICAL SCHOOL +2
Prevention of fibrosis following cardiac injury
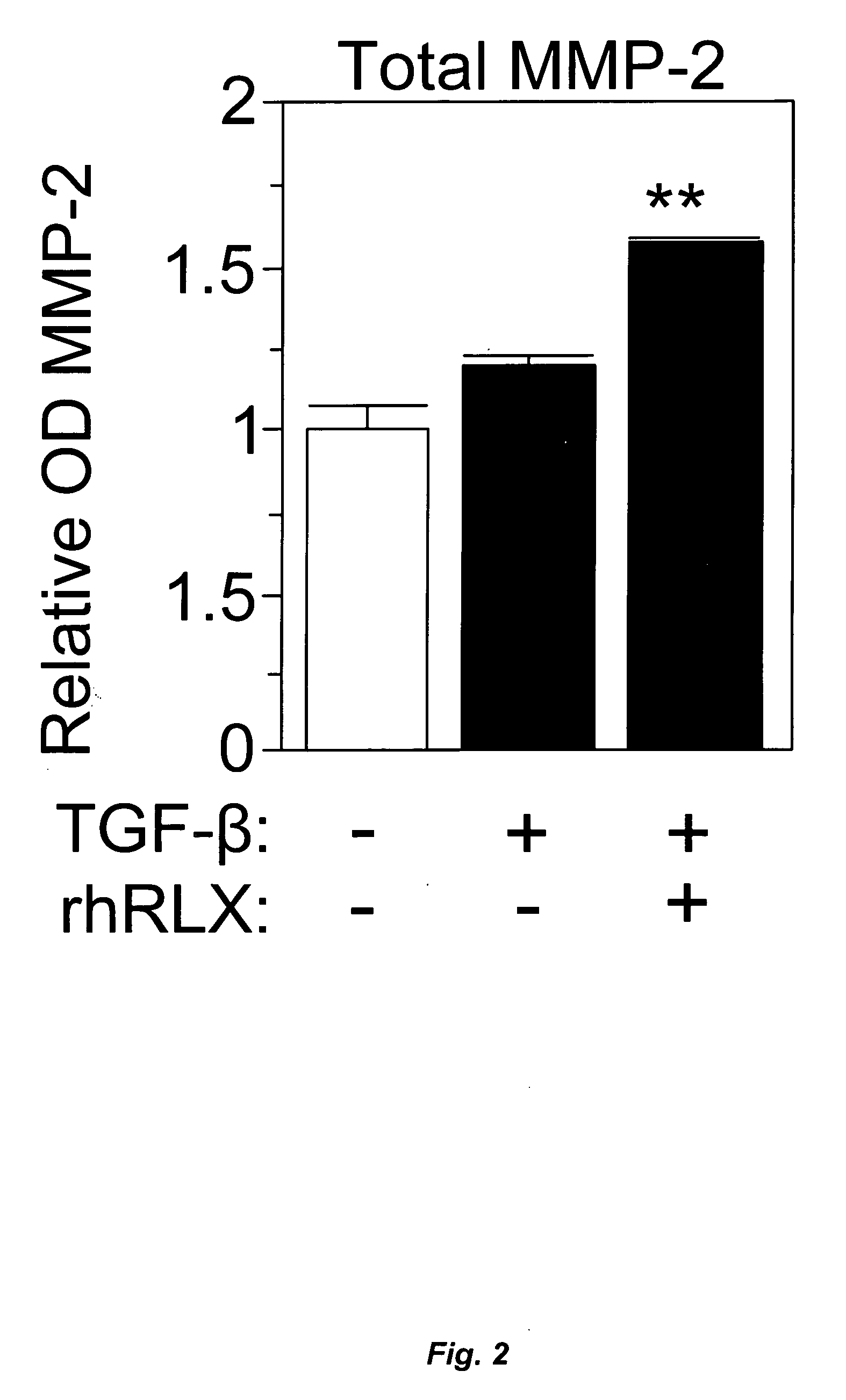
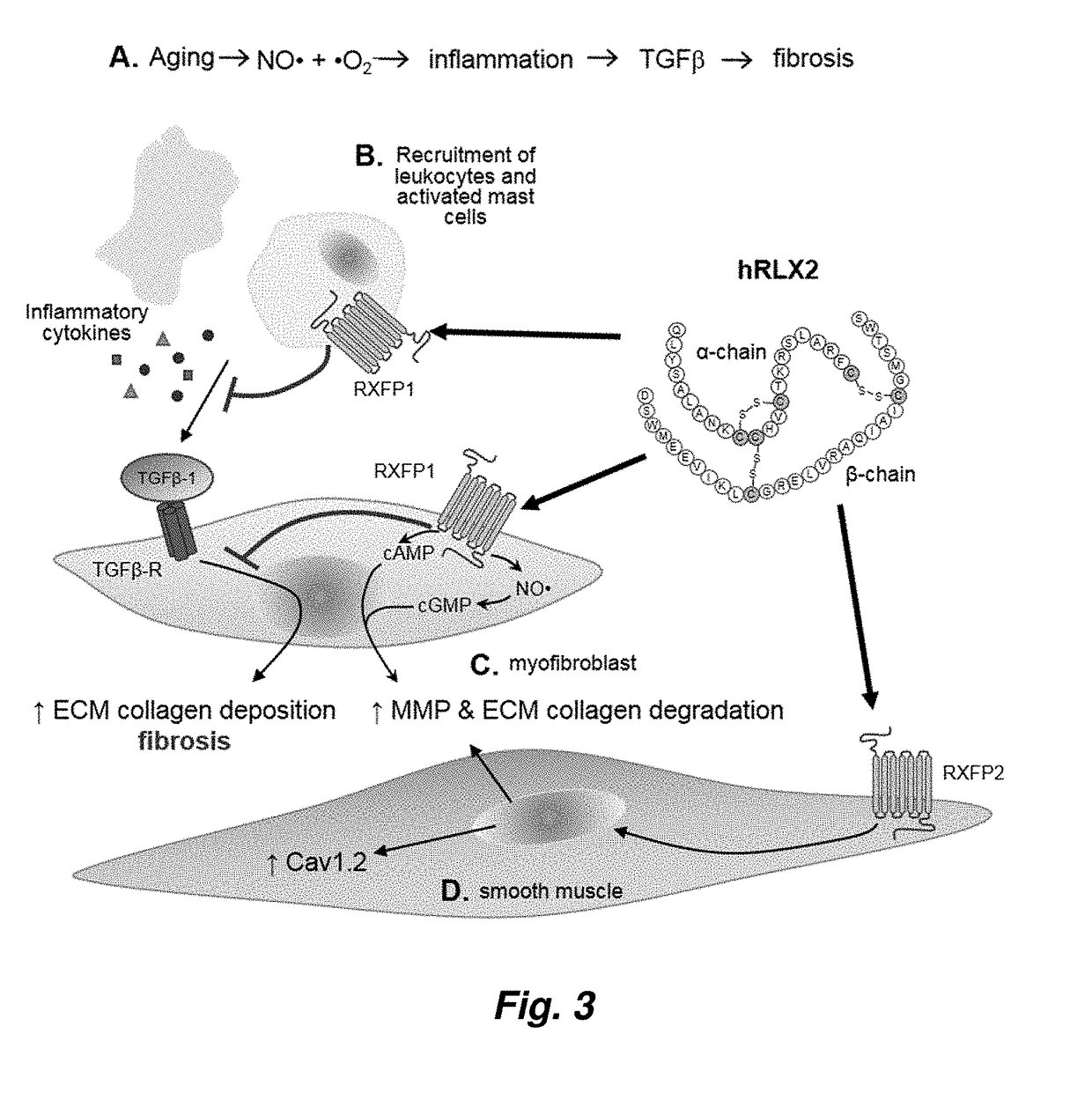
InactiveUS20060264367A1Reduce fibrosisReduce activationHormone peptidesPeptide/protein ingredientsCardiac fibrosisRelaxin
A method for treating cardiac fibrosis resulting from injury of a mammalian heart is described comprising the step of contacting a therapeutically effective amount of relaxin and / or an LGR7 activating agent with cardiac cells following the injury in an amount sufficient to reduce the fibrosis. Also described are methods for protecting the heart following an ischemic event, inhibiting the proliferation of activated fibroblasts and antagonising collagen secretion or deposition in a mammalian heart.
Owner:BAKER MEDICAL RES INST +1
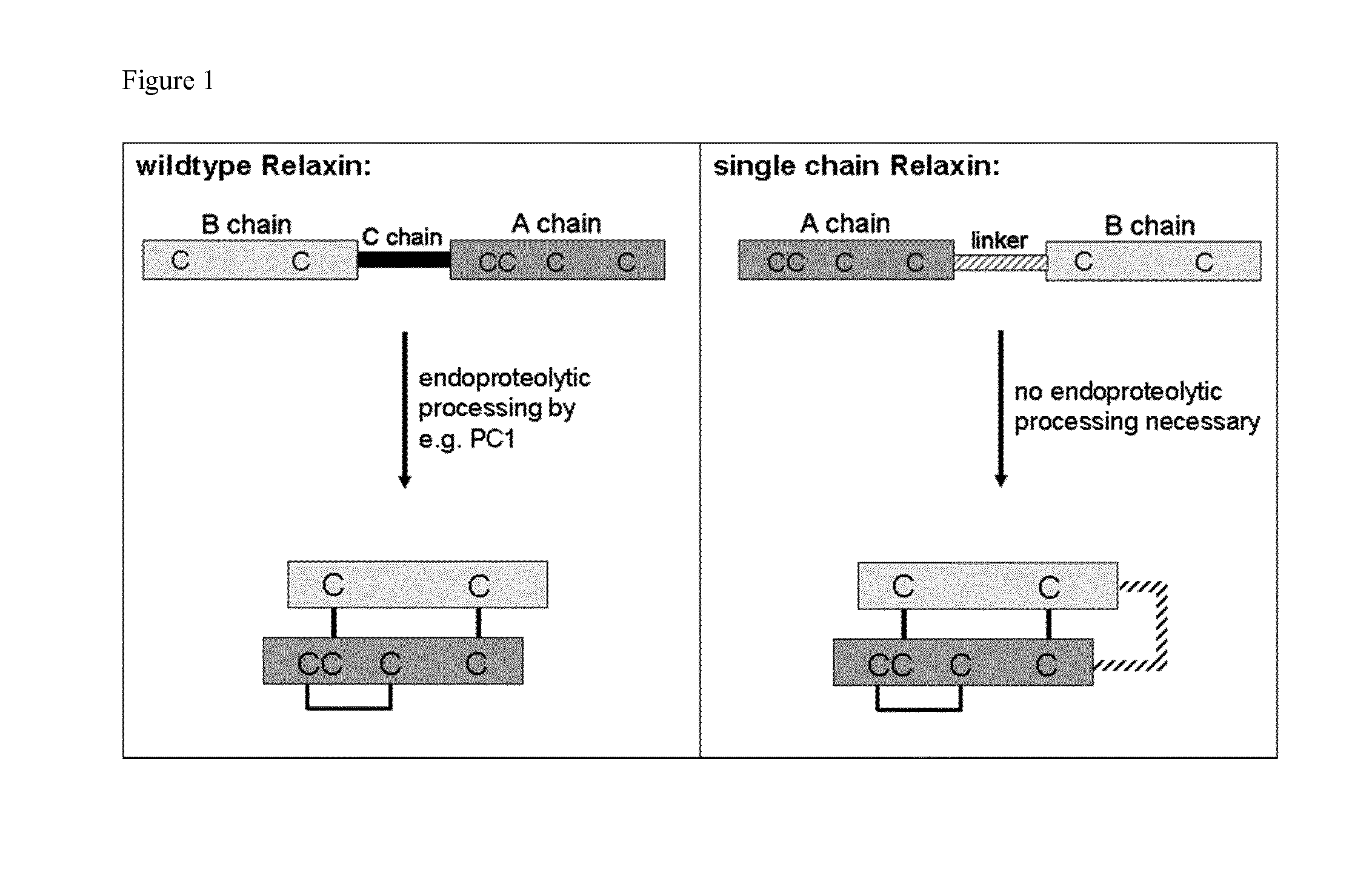
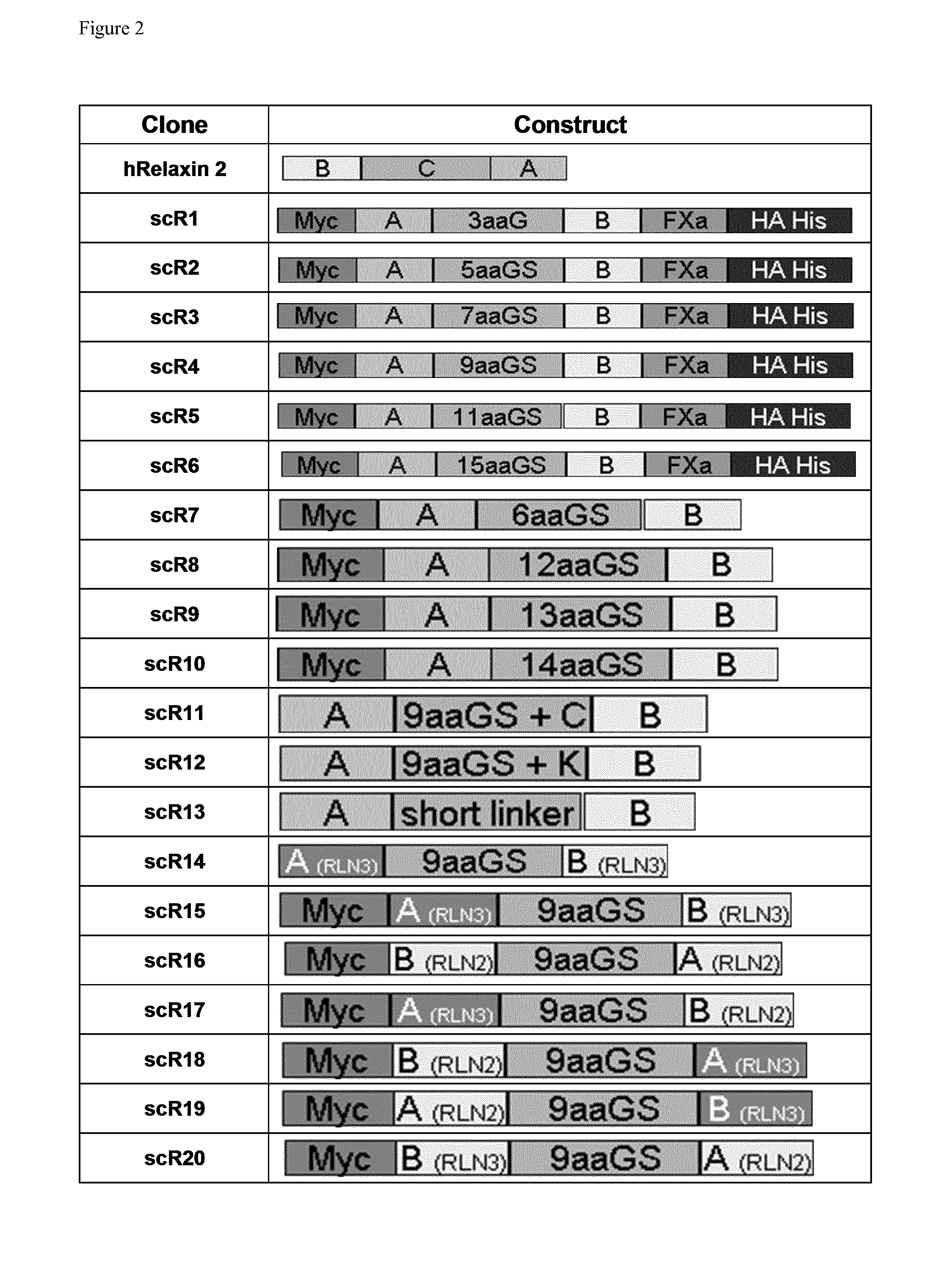
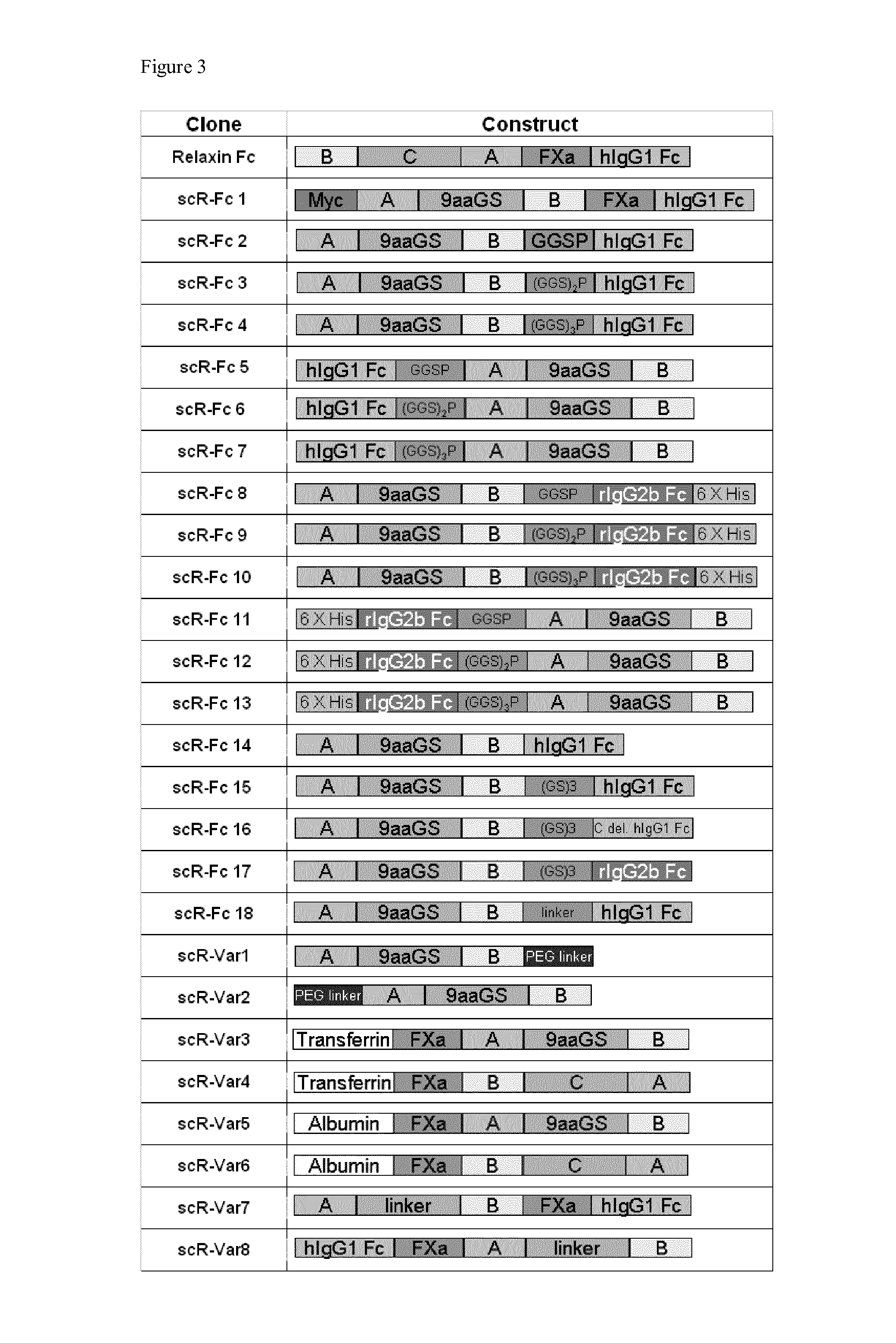
Relaxin fusion polypeptides and uses thereof
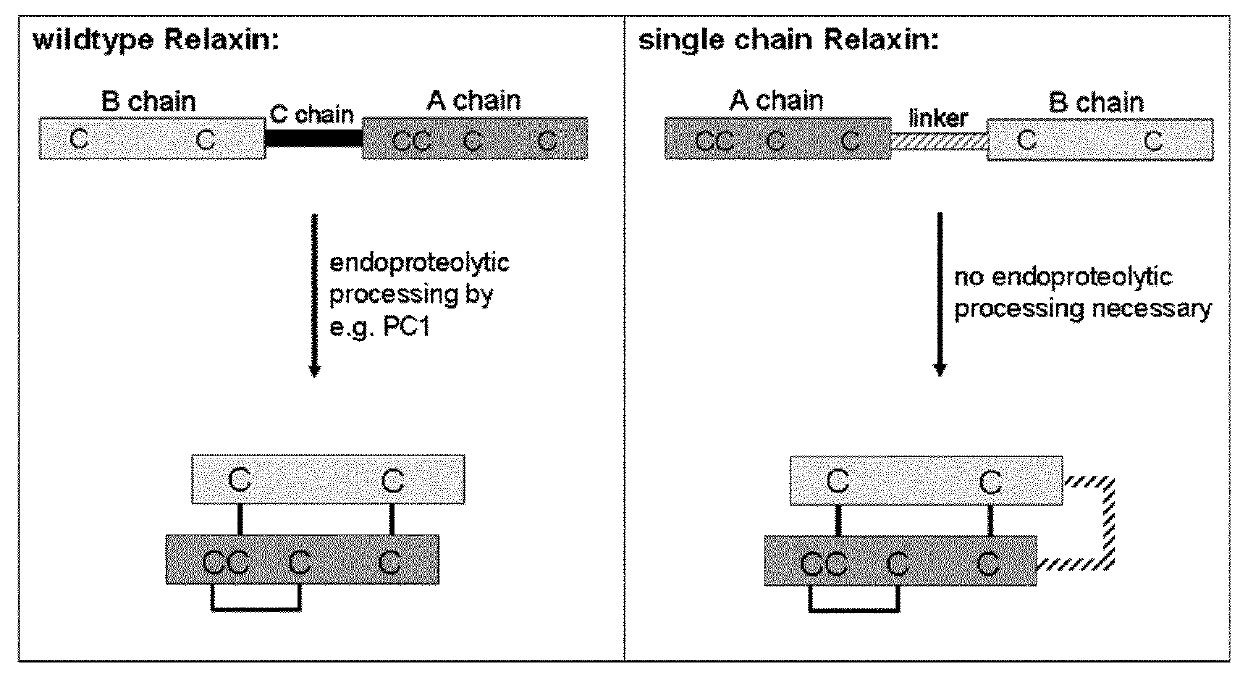
The present invention provides Relaxin fusion polypeptides A-L-B with a non-wild type array of the Relaxin A-chain and Relaxin B-chain, wherein the A- and B-chains are connected by a linker peptide. The invention further provides Relaxin fusion polypeptides with extended half-life. Furthermore, the invention provides nucleic acid sequences encoding the foregoing fusion polypeptides, vectors containing the same, pharmaceutical compositions and medical use of such fusion polypeptides.
Owner:BAYER INTELLECTUAL PROPERTY GMBH
Gene delivery system containing relaxin gene and pharmaceutical composition using relaxin
ActiveCN1968717AIncrease lethalityStrong penetrating powerPeptide/protein ingredientsDigestive systemGene deliveryDisease
The present invention relates to a novel gene delivery system and recombinant adenovirus comprising the relaxin-encoding sequence to enhance transduction efficiency of transgenes, a phamaceutical anti-tumor composition comprising the reconvinant adenovirus, a phamaceutical composition having improve tissue penetration potency and a phamaceutical composition for treating a desease or disorder associated with accumulation of excess extarcellular matrix.
Owner:GENEMEDICINE CO LTD
Relaxin fusion polypeptides and uses thereof
Owner:BAYER INTELLECTUAL PROPERTY GMBH
Method and compositions for the treatment of diabetes and related complications
InactiveUS20060281669A1Improve the situationNervous disorderPeptide/protein ingredientsDiabetes mellitusDisease
Owner:SKY BIOHEALTH SOLUTIONS
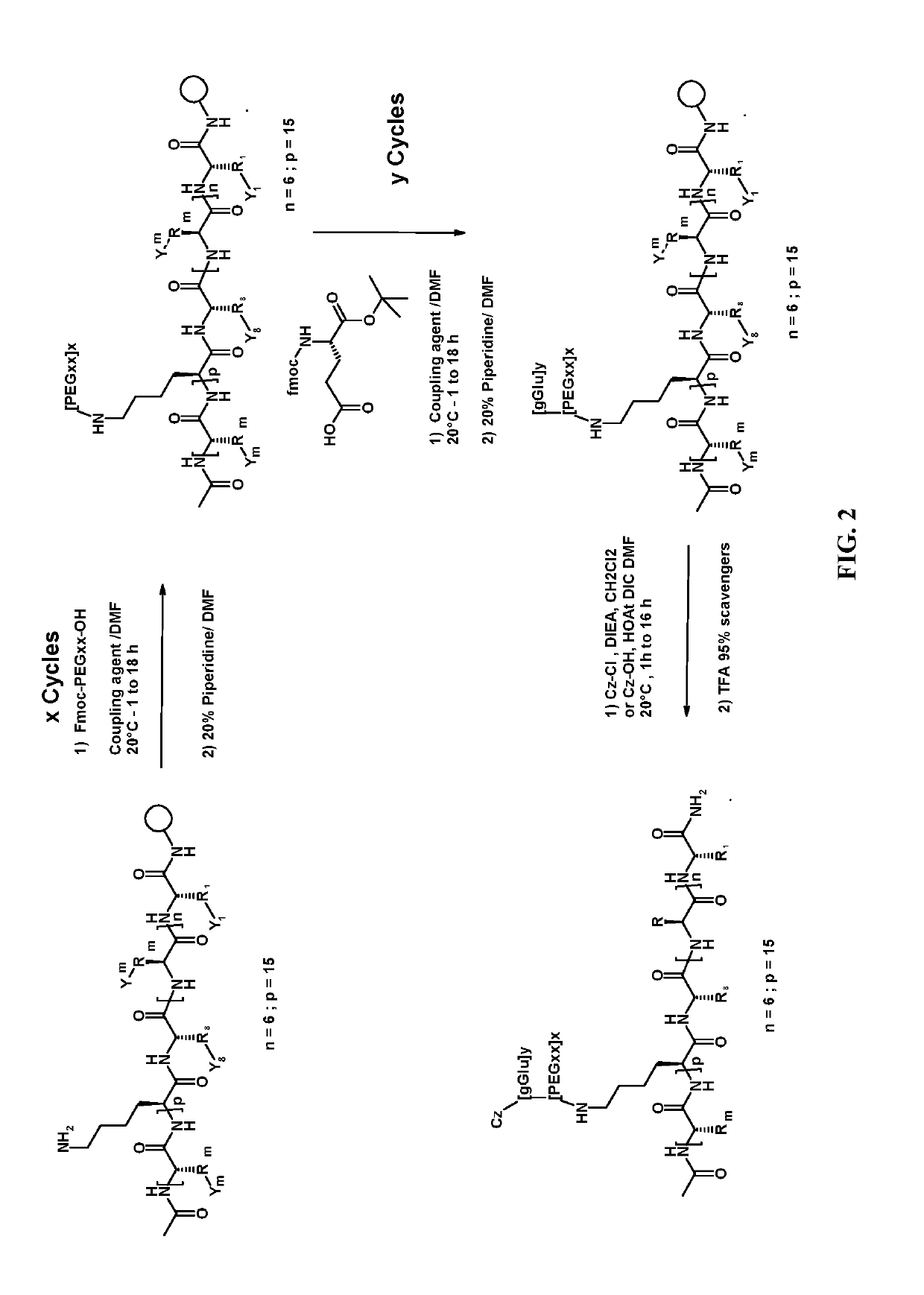
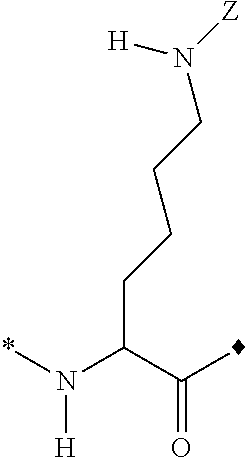
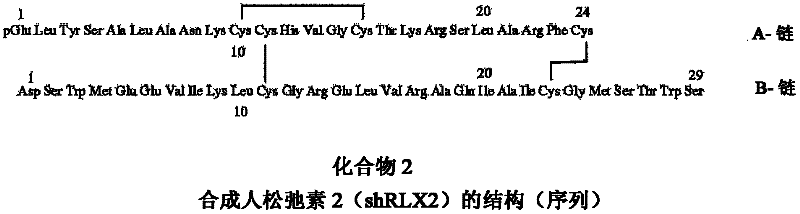
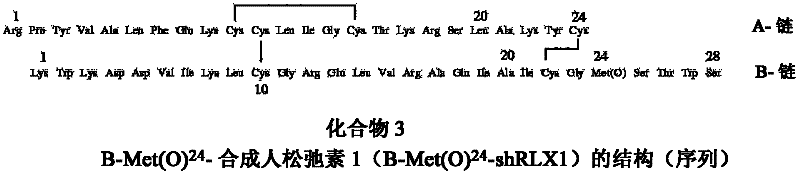
Peptide Synthesis
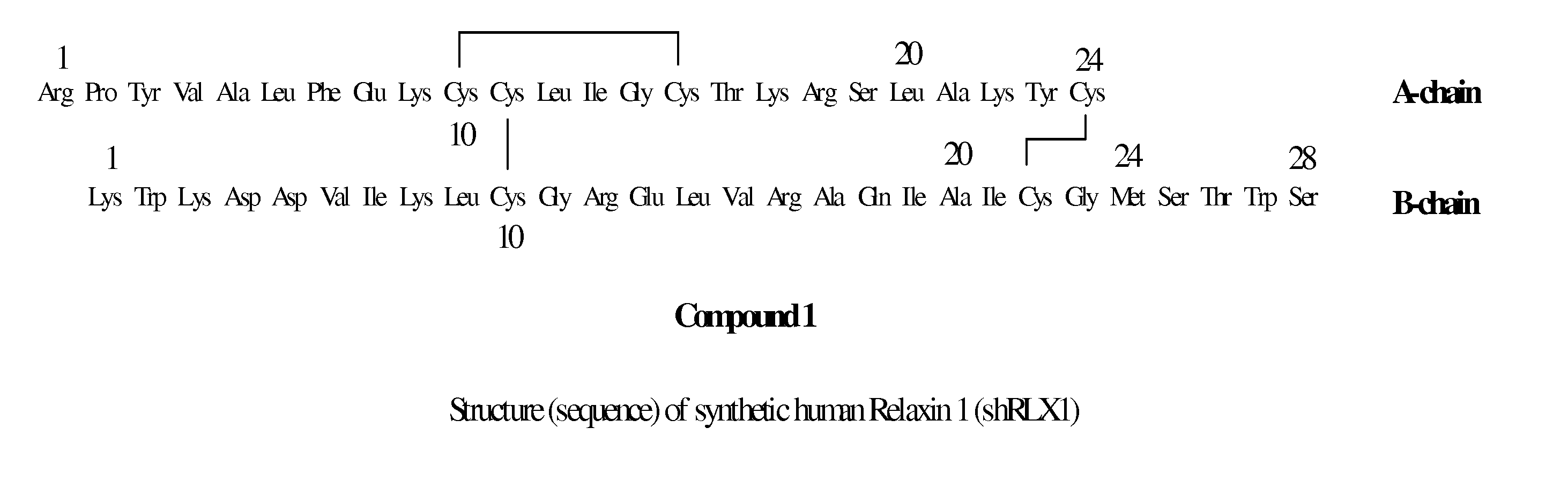
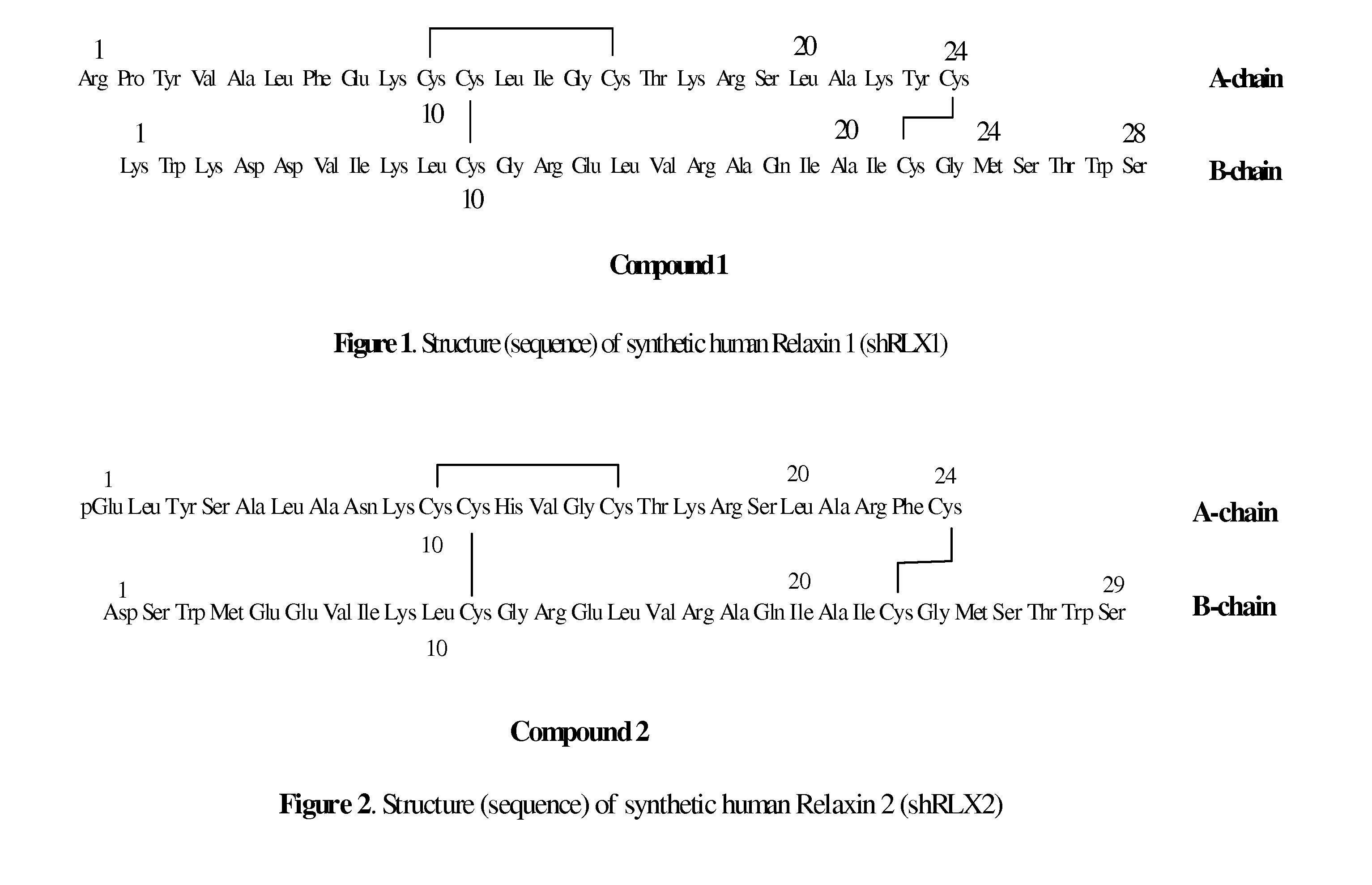
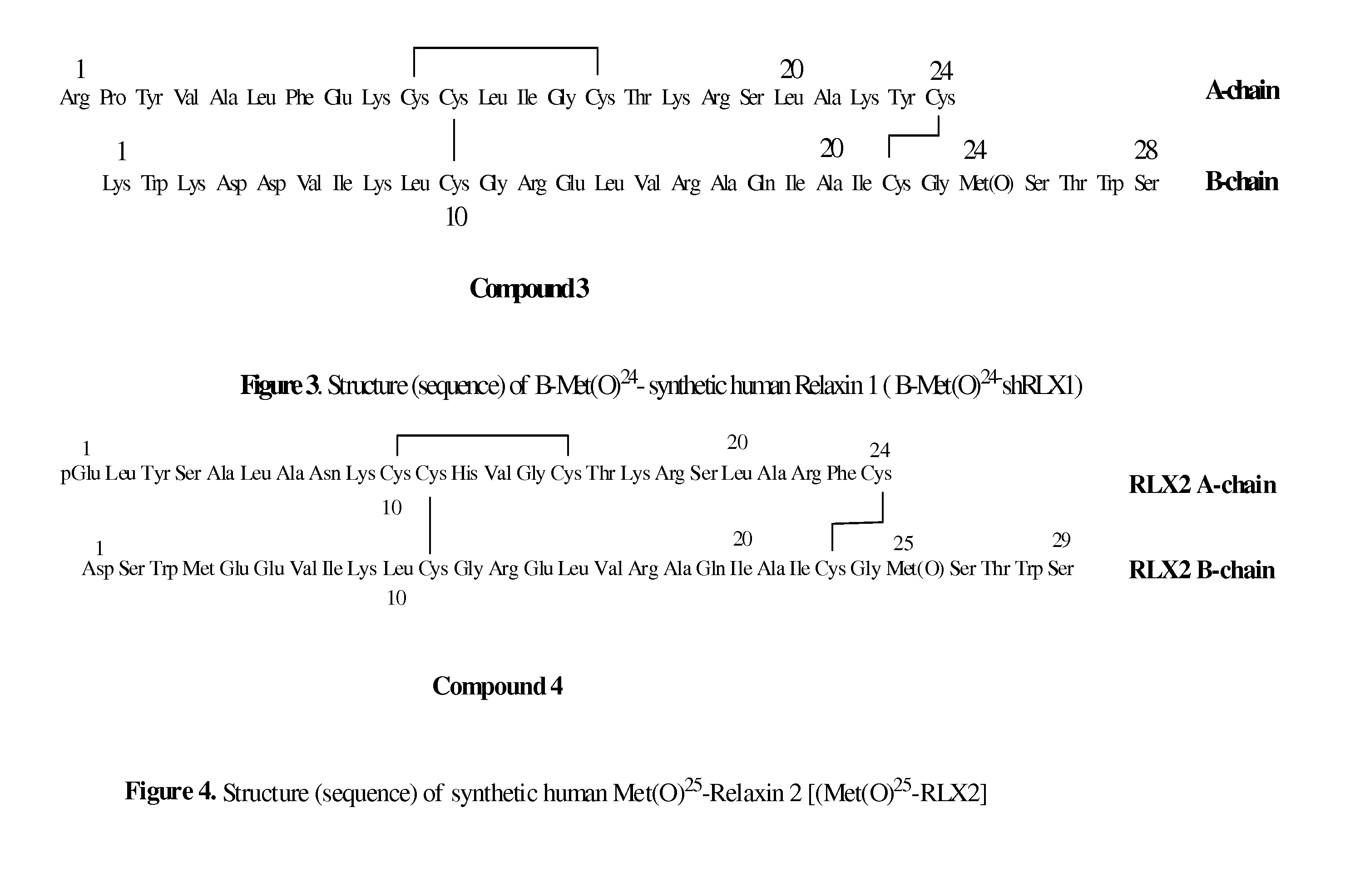
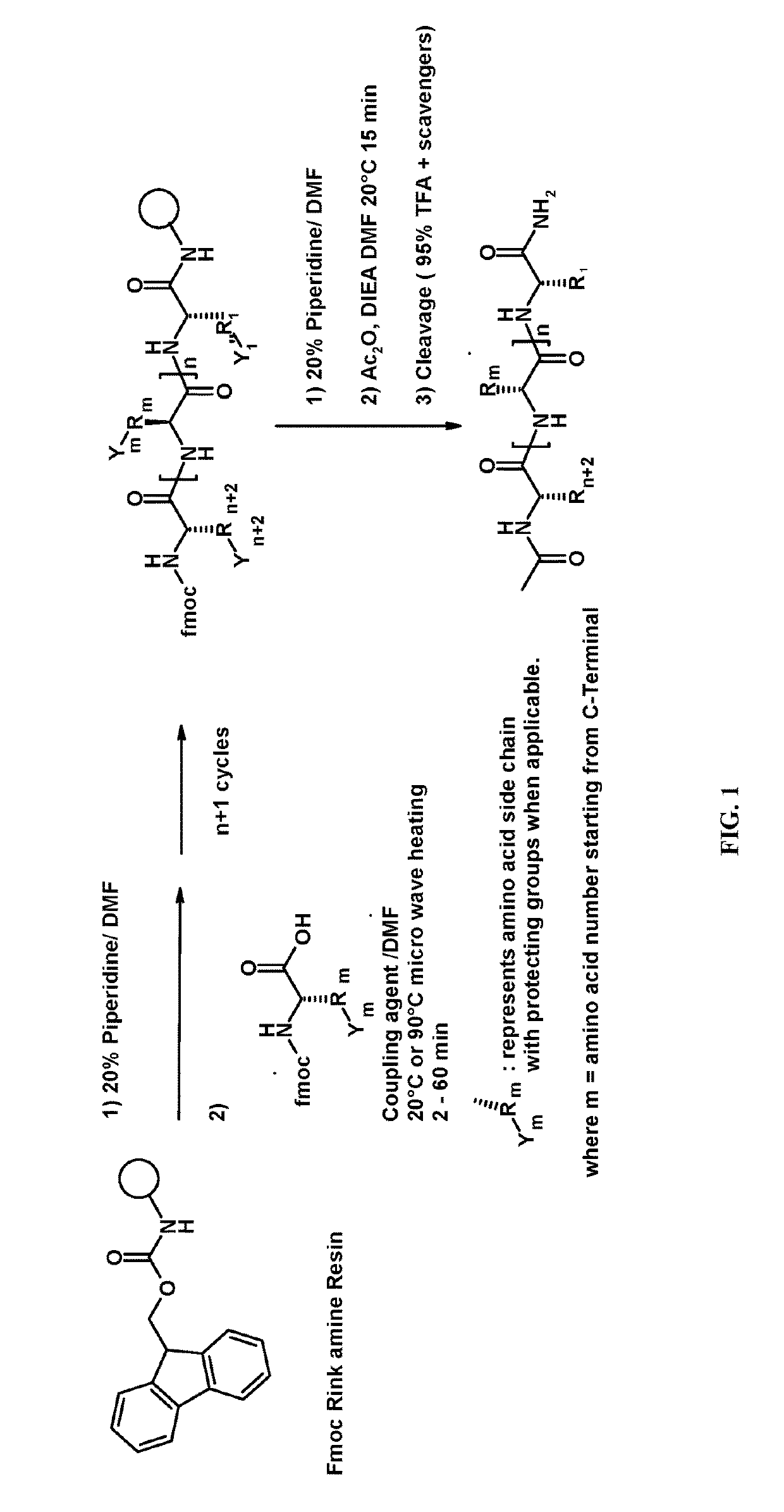
ActiveUS20110039778A1Improve solubilityGood flexibilityPeptide/protein ingredientsMuscular disorderSolubilityRelaxin
A process for producing an insulin type peptide, for example a relaxin, involving oxidizing a methionine residue on a B-chain having cysteine residues and combining the B chain with an A chain having cysteine residues to form a peptide having intermolecular disulphide links and biological activity. Novel synthetic relaxin 1 and methionine oxidized relaxins and Met(O) B-chains having enhanced solubility are disclosed.
Owner:CHEM & BIOPHARML LAB OF PATRAS
Use of relaxin as adjuvant in the differentiation of stem cells for the reconstruction of tissues
InactiveUS20050143299A1Promote rapid maturationRapidly organizationBiocideNervous disorderAdjuvantSmell loss
On the basis of the experimental results it has emerged that relaxin has a pro-differentiating effect on stem cells. It is therefore suggested the use of this hormone as principal component of a drug for the treatment of all those situations that find or will find benefit from the use of stem cells for the reconstruction of tissues damaged by traumatic events or by ischemic-inflammatory-degenerative diseases. It is also suggested the use of relaxin for the treatment of syndromes deriving from the missing activation of stem cells during fetal development (at subsequent somatic-functional maturation), such as for example hypogonotropic hypogonadism with anosmia (Kallman's syndrome).
Owner:BIGAZZI MARIO
Methods of modulating apoptosis by administration of relaxin agonists or antagonists
InactiveUS20050032683A1Increase and decreases functional activity of moleculeNervous disorderPeptide/protein ingredientsRelaxinApoptosis
The present invention relates to the discovery that relaxin is associated with the development or maturation of body tissues. Knockouts of the gene encoding relaxin result in various abnormalities in the development of various tissues. The present invention provides methods of modulating apoptosis by administering a relaxin agonist or antagonist to a subject.
Owner:MOLECULAR MEDICINE RES INST
Novel insulin/IGF/relaxin family polypeptides and DNAs thereof
InactiveUS20060073567A1Promotes and inhibits activityOrganic active ingredientsFungiDiseaseDisulfide bond
The present invention relates to (1) a polypeptide containing the same or substantially the same amino acid sequence as the amino acid sequence represented by SEQ ID NO:7 (A chain), (2) a polypeptide containing the same or substantially the same amino acid sequence as the amino acid sequence represented by SEQ ID NO:8 (B chain), (3) a polypeptide wherein the A and B chains are bonded to each other via disulfide bonds (2 chains) or its salt, (4) DNAs encoding the same, etc. These polypeptides and DNAs encoding the same can be used for the diagnosis, treatment, prevention, etc. for diseases, including abnormalities (e.g., diabetes mellitus, etc.) in metabolic regulation (sugar metabolism, lipid metabolism, etc.) of energy sources such as carbohydrates.
Owner:TAKEDA PHARMA CO LTD
Modified lipidated relaxin b chain peptides and their therapeutic use
ActiveUS20190233494A1Improve solubilityLong retentionPeptide/protein ingredientsMetabolism disorderRelaxin-3Stereochemistry
The present invention relates to a biologically active single chain Relaxin peptide having the following formula (I) (SEQ ID NO 105):Nter-Ac-X10-E-G-R-E-X15-V-R-X18-X19-I-X21-X22-E-G-X25-S-X27-X28-X29-X30—X31—X32—X33—NH2—Cter or a salt or solvate thereof.It also concerns a pharmaceutical composition comprising at least one peptide of the invention, and the peptide or the pharmaceutical composition for its use as a medicament.
Owner:SANOFI SA
Anti cancer controlled release formulation of containing interstitial hydrolytic agent
InactiveCN1957912AInhibitionAvoid damagePeptide/protein ingredientsPharmaceutical delivery mechanismTreatment effectMicrosphere
A slow-release anticancer medicine in the form of injection or implant is disclosed. Said slow-release injection is composed of a special solvent containing suspending aid and the slow-release microballs consisting anticance medicine, iterstitial hydrolyte and slow-releasing auxiliary. Said anticancer medicine is chosen from taxane, alkalating angent and vegetative alkaloide. Said interstitial hydrolyte is chosen from collagenase, relaxin, etc. Said slow-releasing auxiliary is chosen from polylactic acid copolymer, EVAc, polyethanediol, etc.
Owner:SHANDONG LANJIN PHARMA +1
Anti-cancer sustained-released agent
InactiveCN101396341AInhibition formationSpeed up entryPeptide/protein ingredientsSolution deliveryDepressantSuspending Agents
The invention relates to an anti-cancer sustained release injection, consisting of sustained release microspheres and menstruum, wherein, the sustained release microspheres comprise sustained release auxiliary material, angiogenesis inhibitor and / or proteolytic enzyme; and the menstruum contains suspending agent. The angiogenesis inhibitor is selected from gefitinib, erlotinib, lapatinib, vatalanib, pelitinib, endostatin, imatinib, semaxanib, dasatinib, avastin, sorafenib, sunitinib, telcyta or panitumumab; the proteolytic enzyme is selected from one or the combination of collagenase, hyaluronidase, relaxin and plasmase; the sustained release auxiliary material is selected from polifeprosan, decanedioic acid copolymer, EVAc, polylactic acid, the mixture or the copolymer thereof, and the like; and the suspending agent is selected from carboxymethyl cellulose and the like with the viscosity of 100cp to 3000cp (under the temperature of 25 DEG C to 30 DEG C). The injection can also be made into a sustained release implant. The sustained release injection is injected or arranged in or around the tumour and can improve the local drug concentration selectively, reduce the general reaction of the drug, inhibit the growth of tumour cell and blood vessel and enhance the treatment effect of non-operative treatments, such as radiotherapy, chemotherapy, etc.
Owner:SHANDONG LANJIN PHARMA +1
Relaxin peptide synthesis
A process for producing an insulin type peptide, for example a relaxin, involving oxidizing a methionine residue on a B-chain having cysteine residues and combining the B chain with an A chain having cysteine residues to form a peptide having intermolecular disulphide links and biological activity. Novel synthetic relaxin 1 and methionine oxidized relaxins and Met(O) B-chains having enhanced solubility are disclosed.
Owner:PATRAS CHEM & BIOPHARMACEUTICAL LAB
Modified relaxin b chain peptides and their therapeutic use
ActiveUS20190233495A1Improve solubilityRetain efficacyPeptide/protein ingredientsRelaxinsRelaxinPharmaceutical drug
The present invention relates to a biologically active single chain Relaxin peptide having the following formula (I) (SEQ ID NO:65):Nter-X-(E)a-X10-E-G-R-E-X15-V-R-X18-X19-I-X21-X22-E-G-X25-S-X27-X28-X29-X30-R-(X32)b-(X33)c-(X34)d-NH2-Cter It also concerns a pharmaceutical composition comprising at least one peptide of the invention, or a pharmaceutically acceptable salt or a solvate thereof, and the peptide, a pharmaceutically acceptable salt or solvate thereof, or the pharmaceutical composition, for its use as a medicament.
Owner:SANOFI SA
Composition of anti cancer medication prepared from carried platinum category compound and consubstantial hydrolytic agent
InactiveCN1957913AEasy to operateGood repeatabilityPeptide/protein ingredientsPharmaceutical delivery mechanismCarboplatinMicrosphere
A slowly-release anticancer medicine in the form of injection or implant is disclosed. Said slowly-release injection is composed of a special solvent containing suspending aid and the slowly-release microballs consisting Pt compound, iterstitial hydrolyte and slowly-releasing auxiliary. Said Pt compound is chosen from cisplatin, carboplatin, etc. Said interstitial hydrolyte is chosen from collagenase, relaxin, etc. Said slowly-releasing auxiliary is chosen from polylactic acid, polyethanediol, etc.
Owner:SHANDONG LANJIN PHARMA +1
Controlled release agent of containing fluorouracil and synergis
InactiveCN1957922AEasy injectionIncrease drug concentrationOrganic active ingredientsSolution deliveryTreatment effectWhole body
A slowly-release anticancer medicine in the form of injection or implant is disclosed. Said slowly-release injection is composed of a special solvent containing suspending aid and the slowly-release microballs consisting anticance medicine, iterstitial hydrolyte and slowly-releasing auxiliary. Said anticancer medicine is chosen from 5-FU, triptoreline, etc. Said interstitial hydrolyte is chosen from collagenase, relaxin, etc. Said slowly-releasing auxiliary is chosen from biocompatible, biodegradable and absorptive high-molecular polymers, polyethanediol, etc.
Owner:SHANDONG LANJIN PHARMA +1
Method and Compositions for the Treatment of Diabetes and Related Complications
Owner:SKY BIOHEALTH SOLUTIONS
Therapy for Lower Urinary Tract Dysfunctions
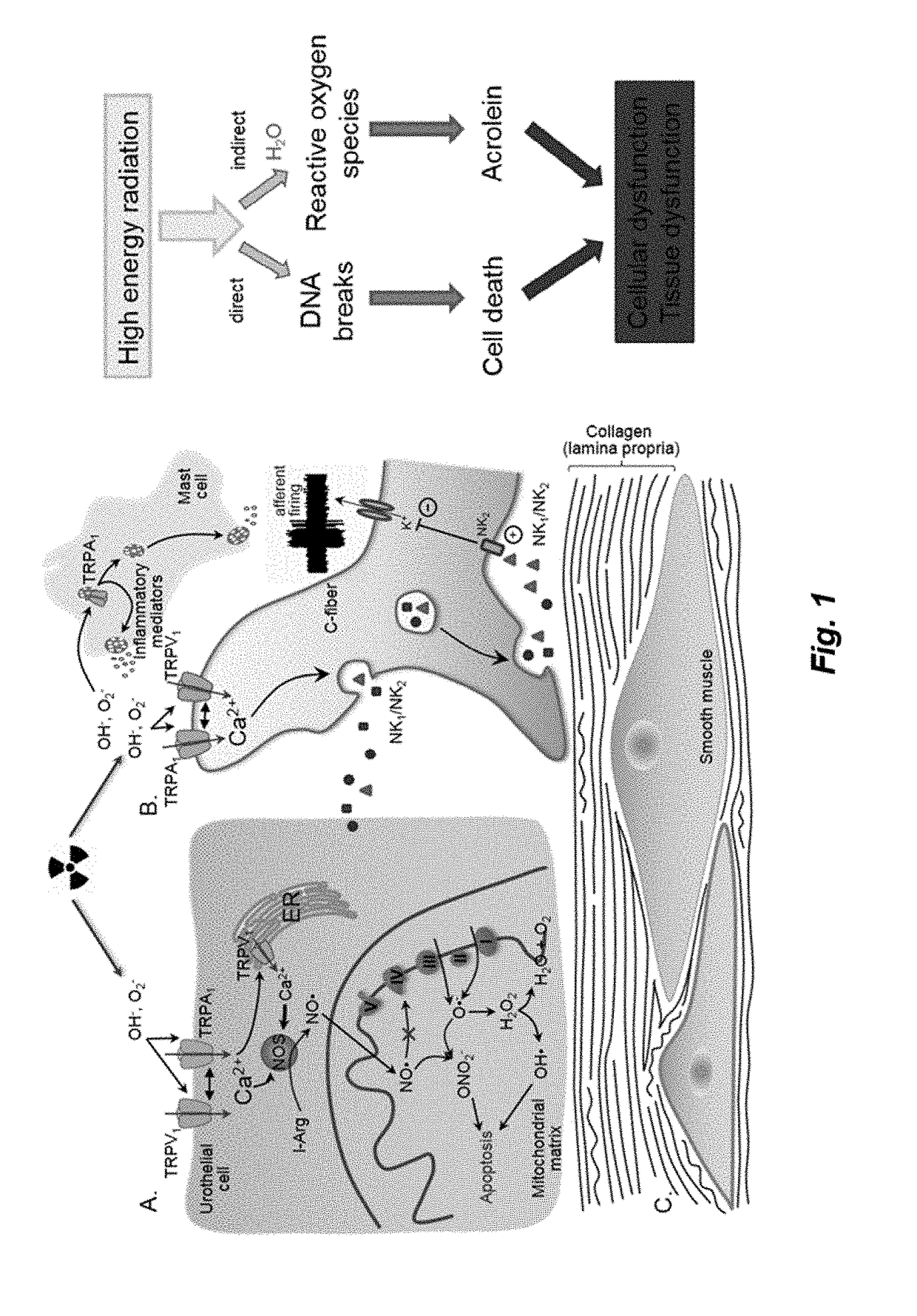
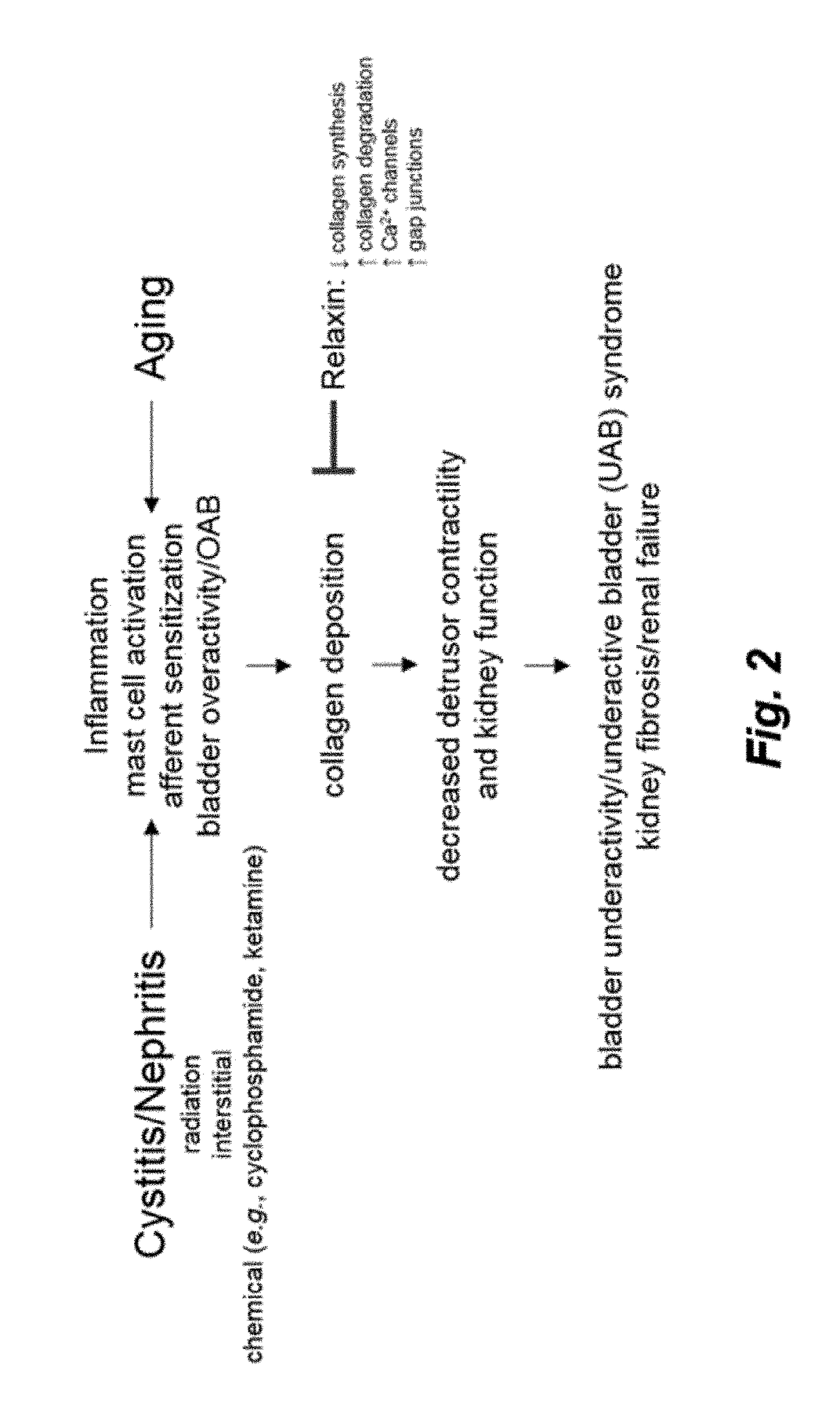
InactiveUS20180371046A1Treatment safetyEffective treatmentPeptide/protein ingredientsRelaxinsRelaxinFunctional disorder
The present invention relates to the use of relaxin for the treatment of lower urinary tract dysfunctions.
Owner:UNIVERSITY OF PITTSBURGH
An anticancer sustained releasing agent
InactiveCN1961861AInhibition formationSpeed up entryPeptide/protein ingredientsPharmaceutical delivery mechanismDepressantHyaluronidase
Disclosed is an anti-cancer slow release agent comprising slow release microspheres and dissolvent, wherein the slow release microspheres include slow release auxiliary materials, anti-angiogenesis agent and / or proteolytic enzyme, the dissolvent comprises suspension adjuvant. The anti-angiogenesis agent is selected from gefinitib, erlotinib, lapatinib, pelitinib, endostatin, imatinib, smasnib, avastin, sorafenib, sunitinib, oersted or panitoma, the proteolytic enzyme is selected from collagenase, hyaluronidase, relaxin and thrombase or the combination of them, the slow release auxiliary materials are selected from Polifeprosan, sebacylic acid copolymer, EVAc, polylactic acid and their mixture of copolymer, the viscosity of the suspension adjuvant is 100-3000cp (at 25-30 deg C), and is selected from sodium carboxymethylcellulose. The agent can also be prepared into implanting agent for injection or placement in or around tumor with the effects of selectively increasing local concentration, lowering general reaction of the drugs, suppressing growth of tumor cells and blood vessel, and improving the treatment effect of the non-operative treatment methods such as chemotherapy.
Owner:SHANDONG LANJIN PHARMA +1
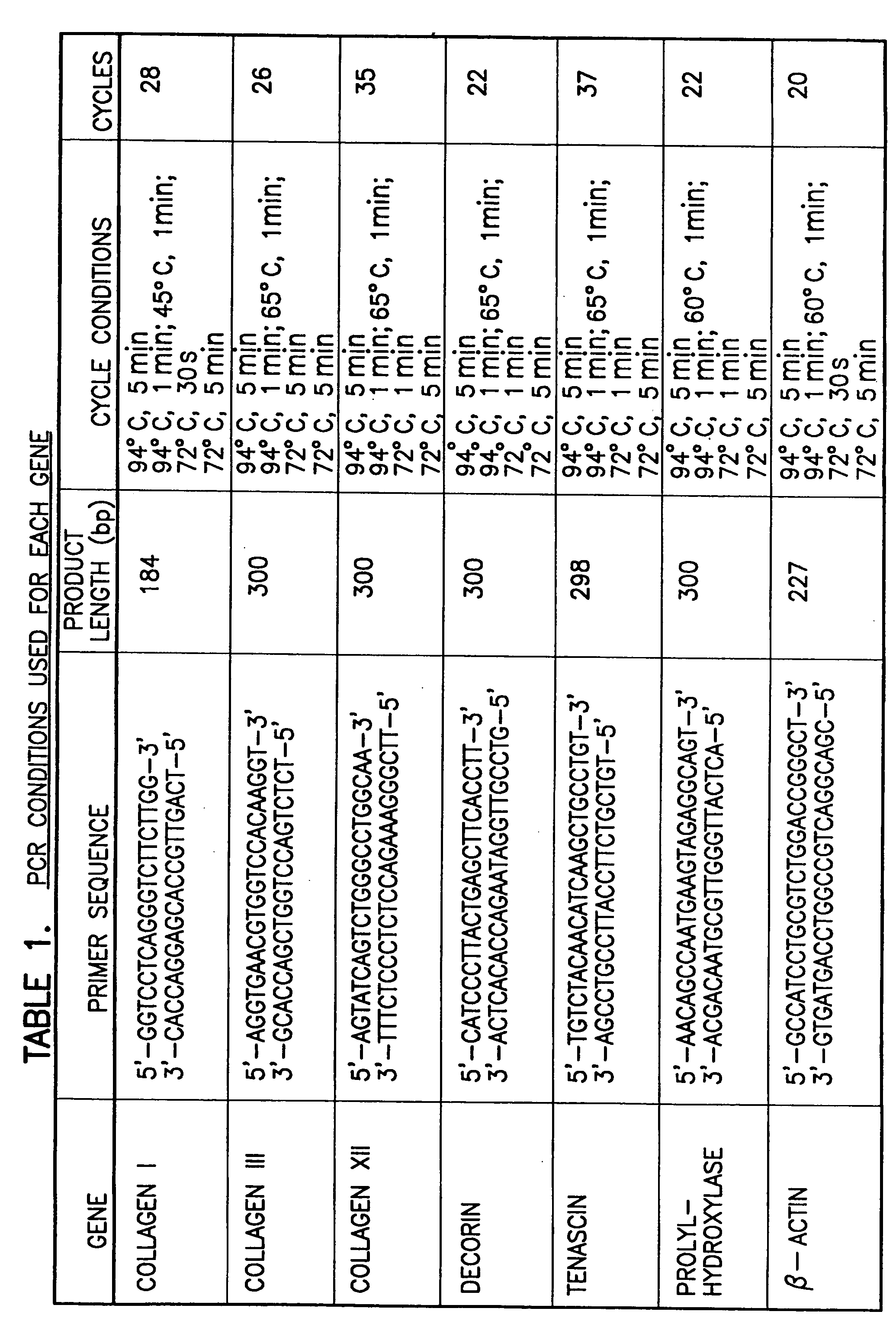
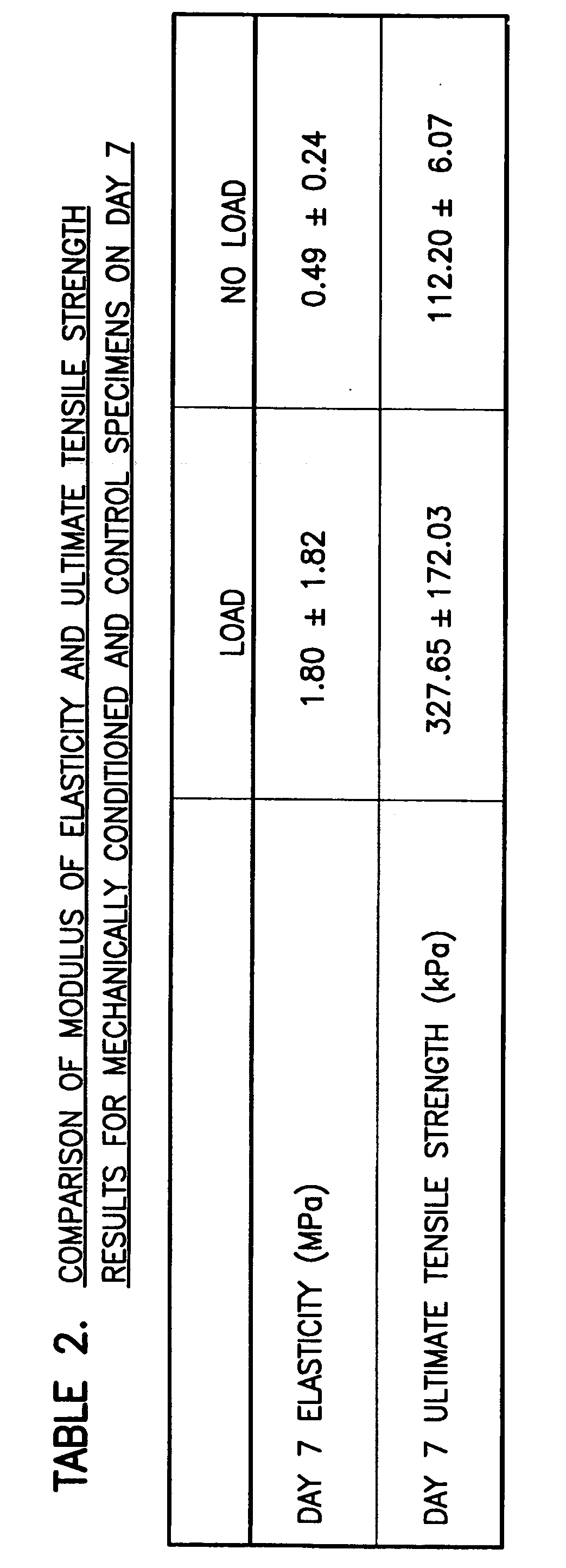
Modulation of cell intrinsic strain to control matrix synthesis, secretion, organization and remodeling
InactiveUS20070077653A1Reduce expressionArtificial cell constructsSkeletal/connective tissue cellsBinding siteIn vivo
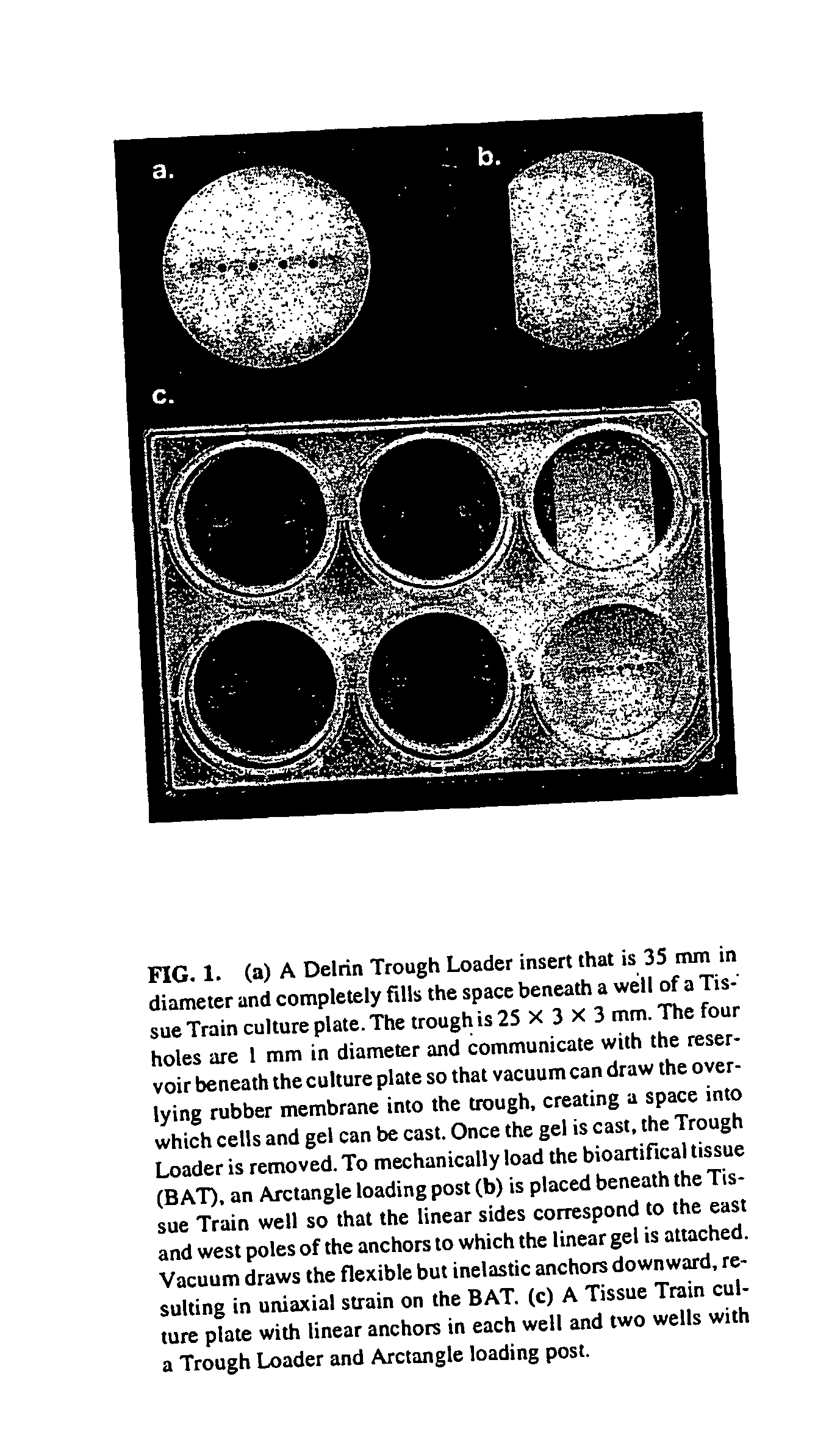
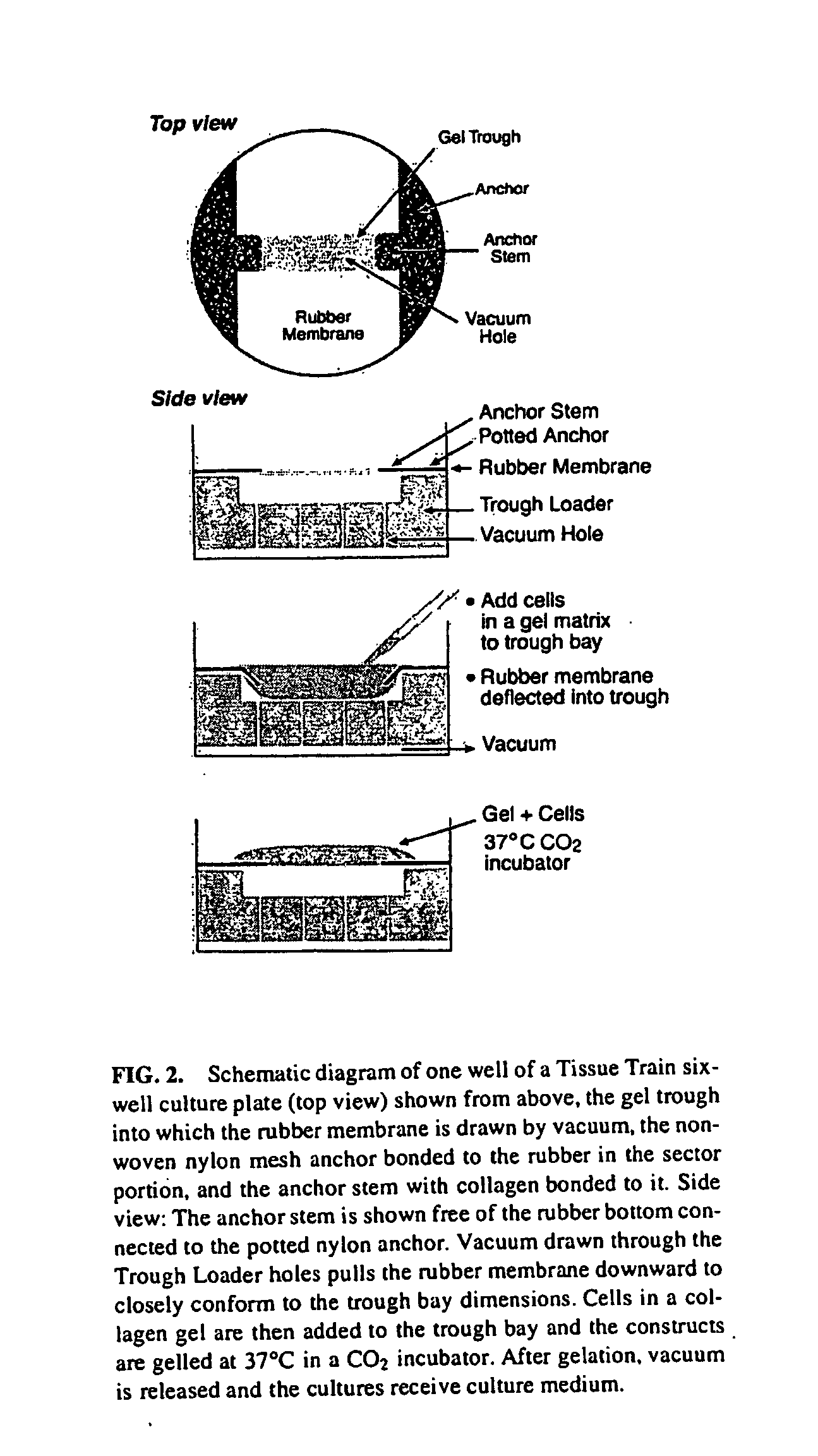
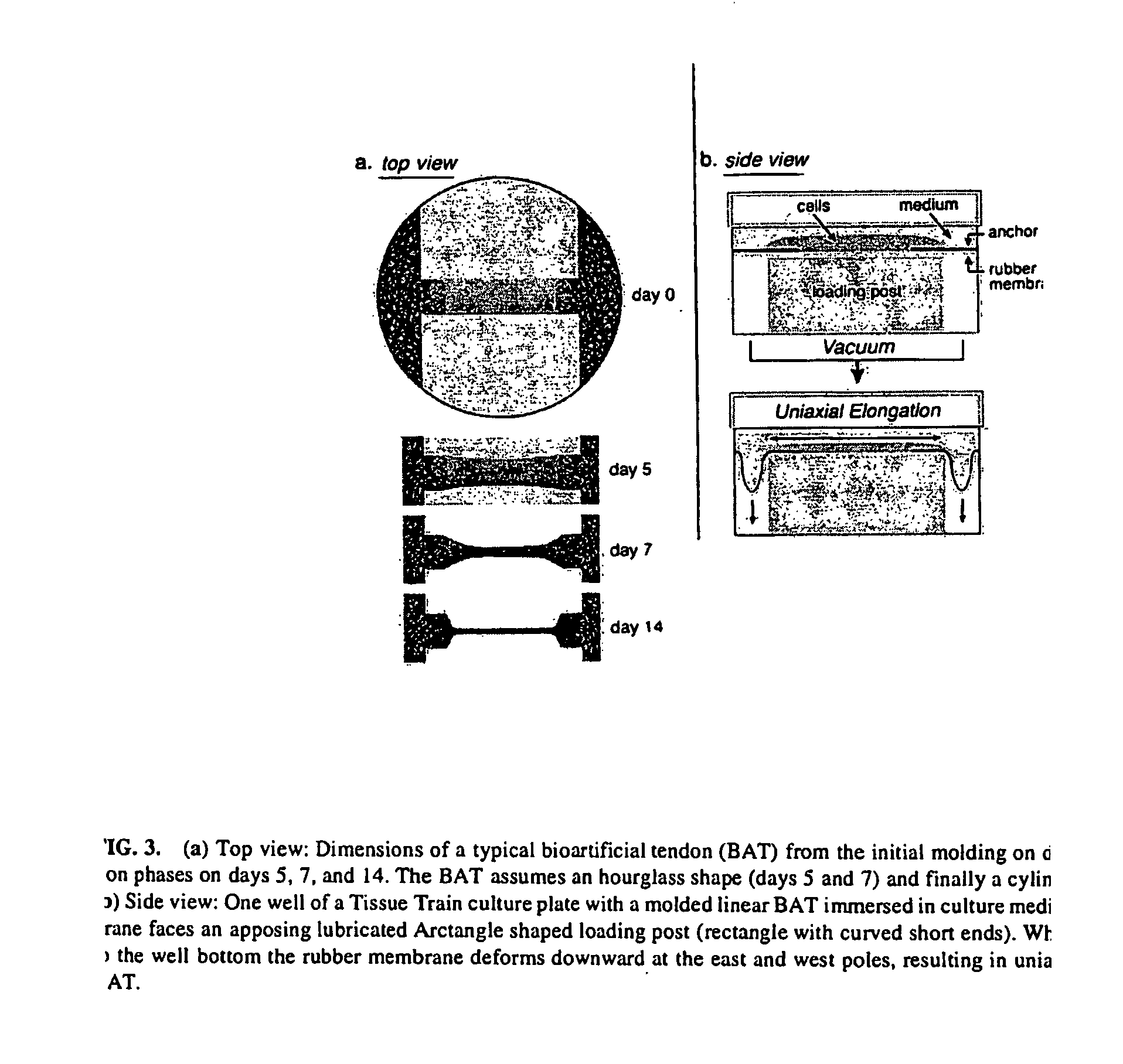
The present invention provides methods for manipulating the intrinsic strain of cells by treating tissue engineered constructs or native tissue with compounds which affect the intrinsic strain setpoint of the cells in order to modulate matrix synthesis, secretion, organization and / or remodeling so that the tissues withstand in vivo mechanical forces and have the structural characteristics of host tissue which has been permanently altered by injury, atrophy or disease. The compounds include binding site peptides, ATP, UTP and related analogues, IL-1&bgr; TGF-&agr; cytochalasin D, hyaluronic acid, nocodazole and others. Also provided are methods for applying a mechanical external strain to the tissues, as well as methods for modulating the expression of cytoskeletal genes that transcribe cytoskeletal proteins which regulate a cell's intrinsic strain setpoint.
Owner:MEDTRAIN TECH
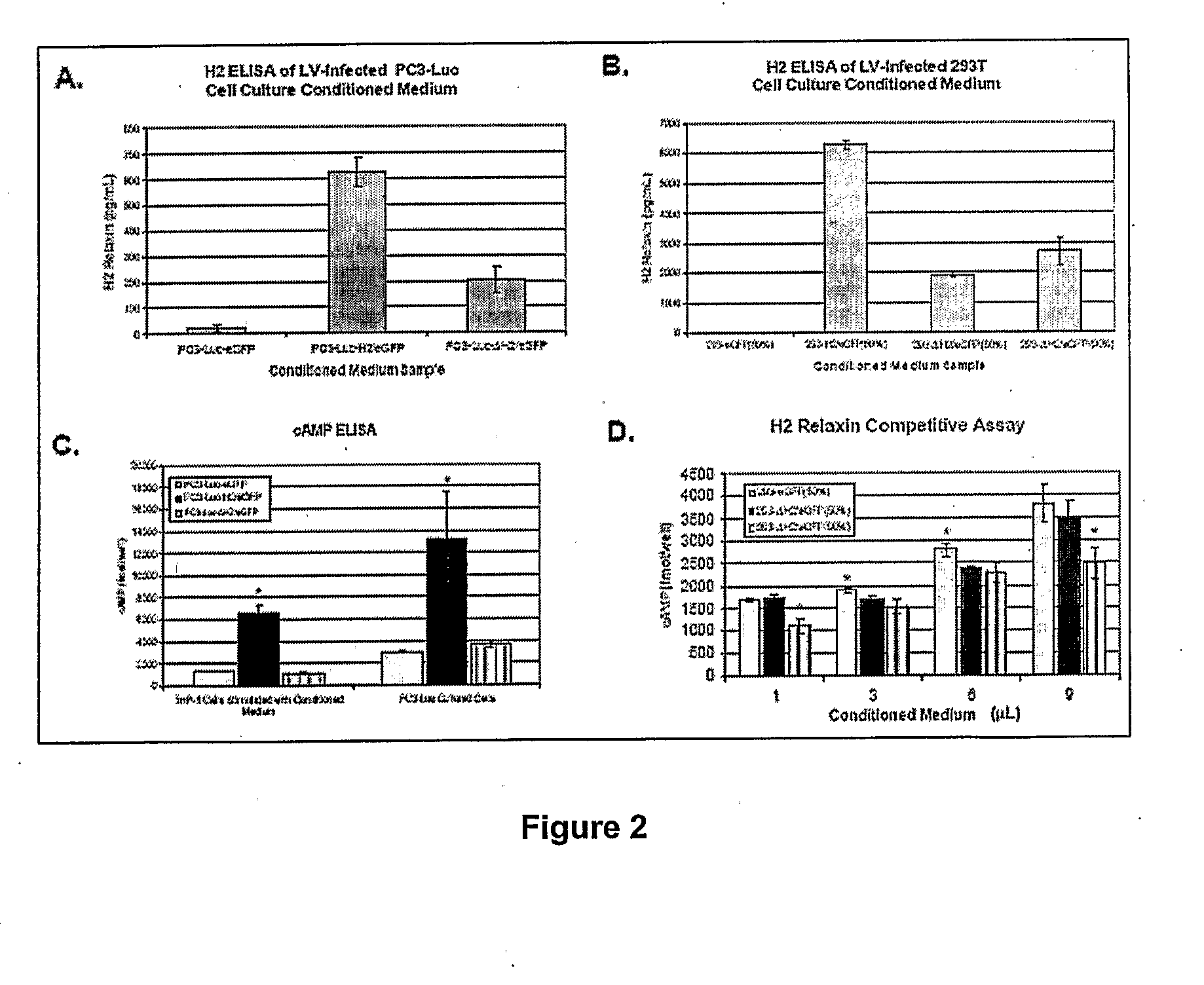
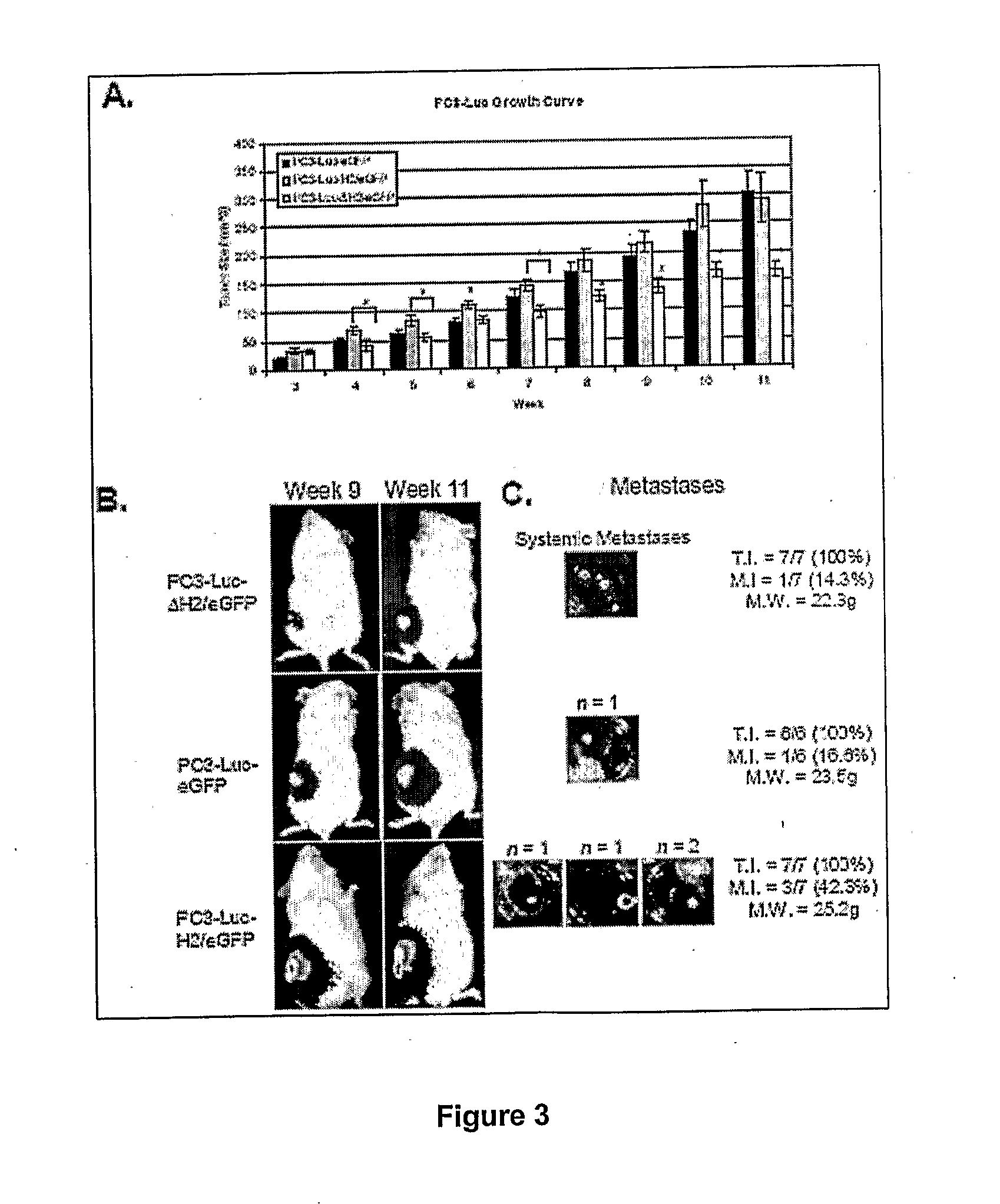
Modified h2 relaxin for tumor suppression
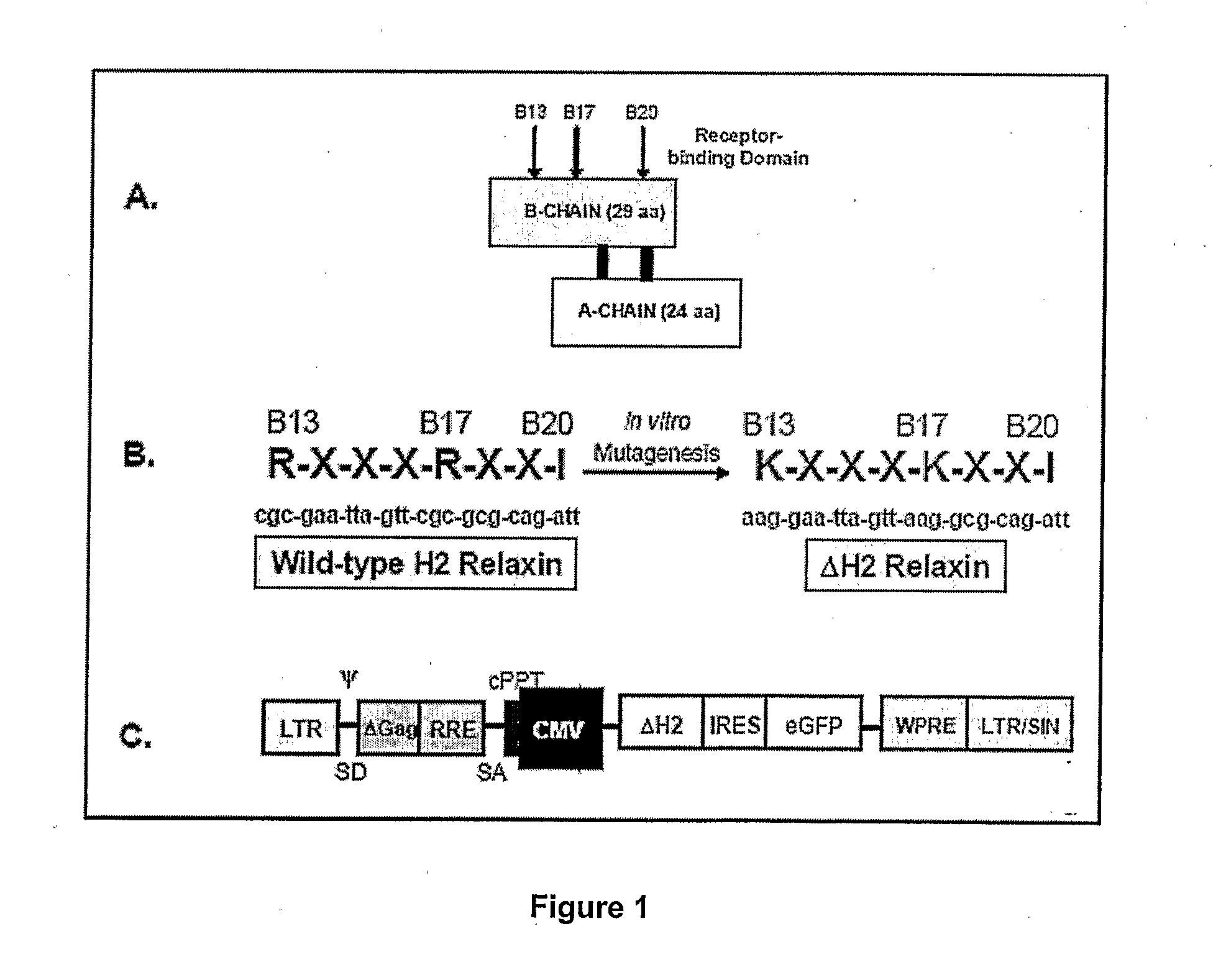
Modified H2 relaxms which act as antagonists of the relaxm receptor in cells and tissues, in particular, modified H2 relaxms comprising one or more alterations of the ammo acid sequence at positions B 13, B17 and B20 located in the receptor binding domain The antagonists retain affinity to the receptor, but do not substantially activate the receptor once bound thereto The H2 relaxm antagonists are used in compositions and methods for the treatment of cancers wherein a relaxm receptor is expressed
Owner:ARMOR THERAPEUTICS
A kind of controlled release injection of carried fluorouracil and synergis
InactiveCN1957923AEasy injectionIncrease drug concentrationSolution deliveryPharmaceutical non-active ingredientsTreatment effectMicrosphere
A slowly-release anticancer medicine in the form of injection or implant is disclosed. Said slowly-release injection is composed of a special solvent containing suspending aid and the slowly-release microballs consisting anticance medicine, iterstitial hydrolyte and slowly-releasing auxiliary. Said anticancer medicine is chosen from 5-FU, oxaliplatin, etc. Said interstitial hydrolyte is chosen from collagenase, relaxin, etc. Said slowly-releasing auxiliary is chosen from polylactic acid, FAD, polyethanediol, etc.
Owner:SHANDONG LANJIN PHARMA +1
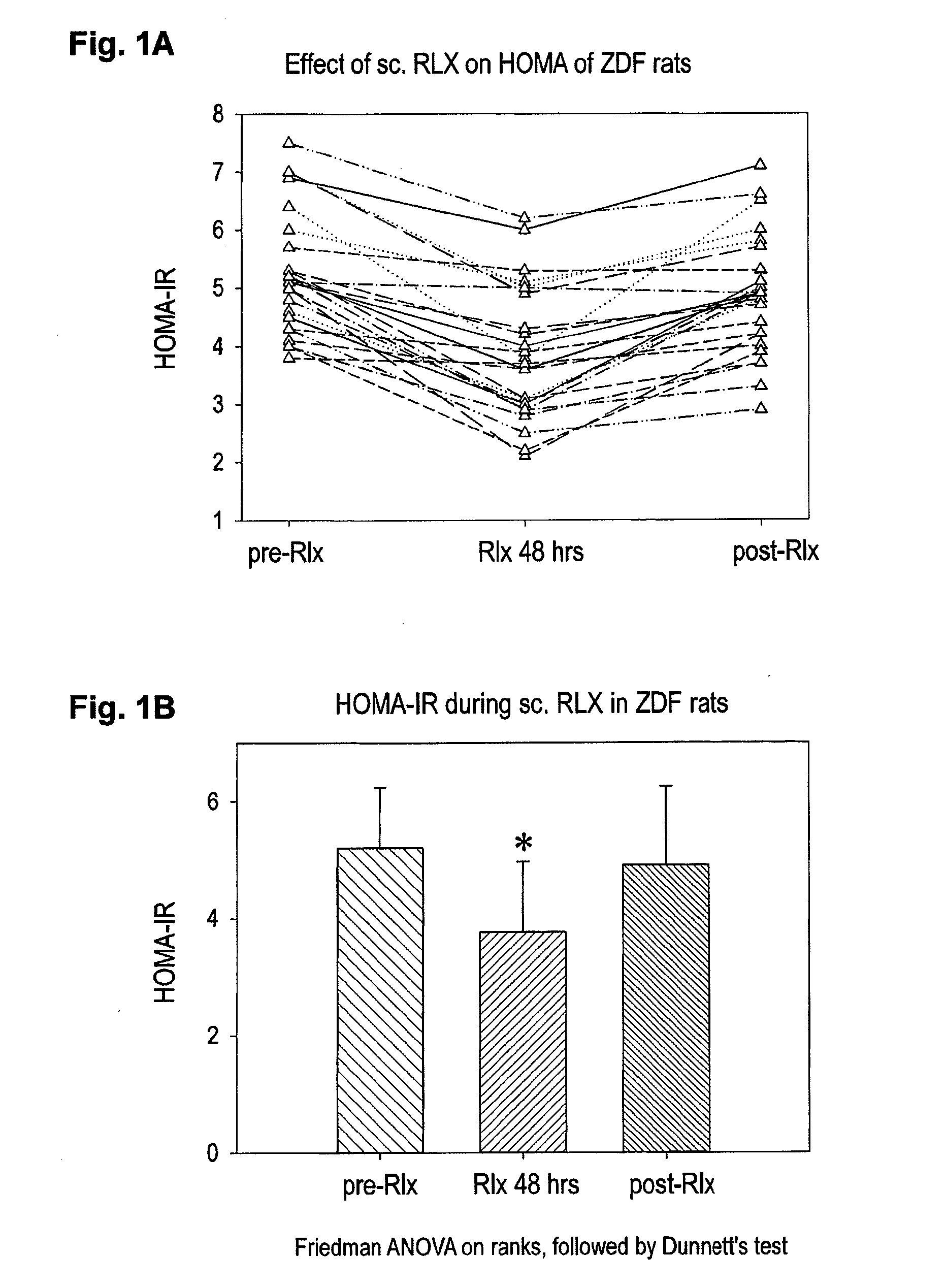
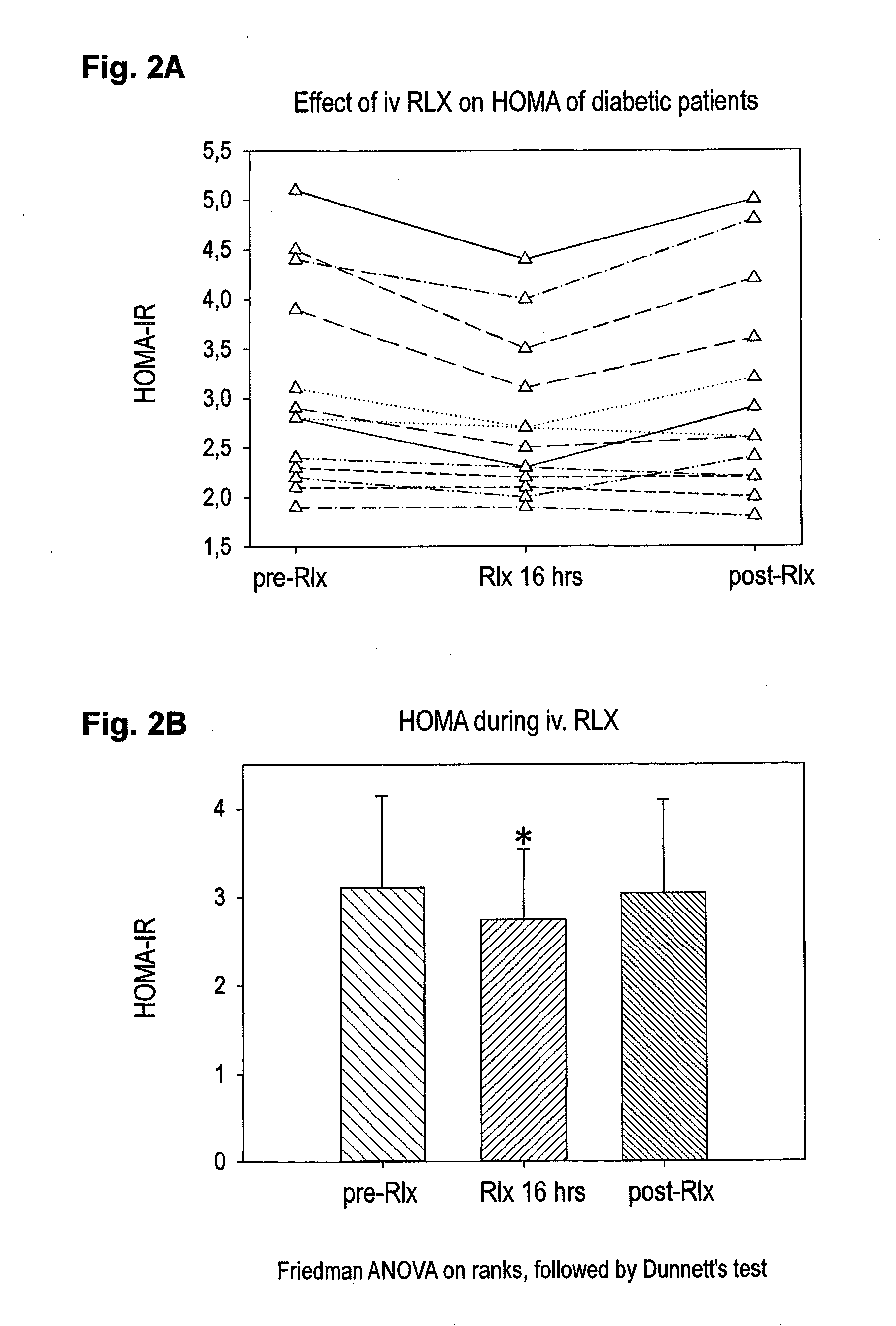
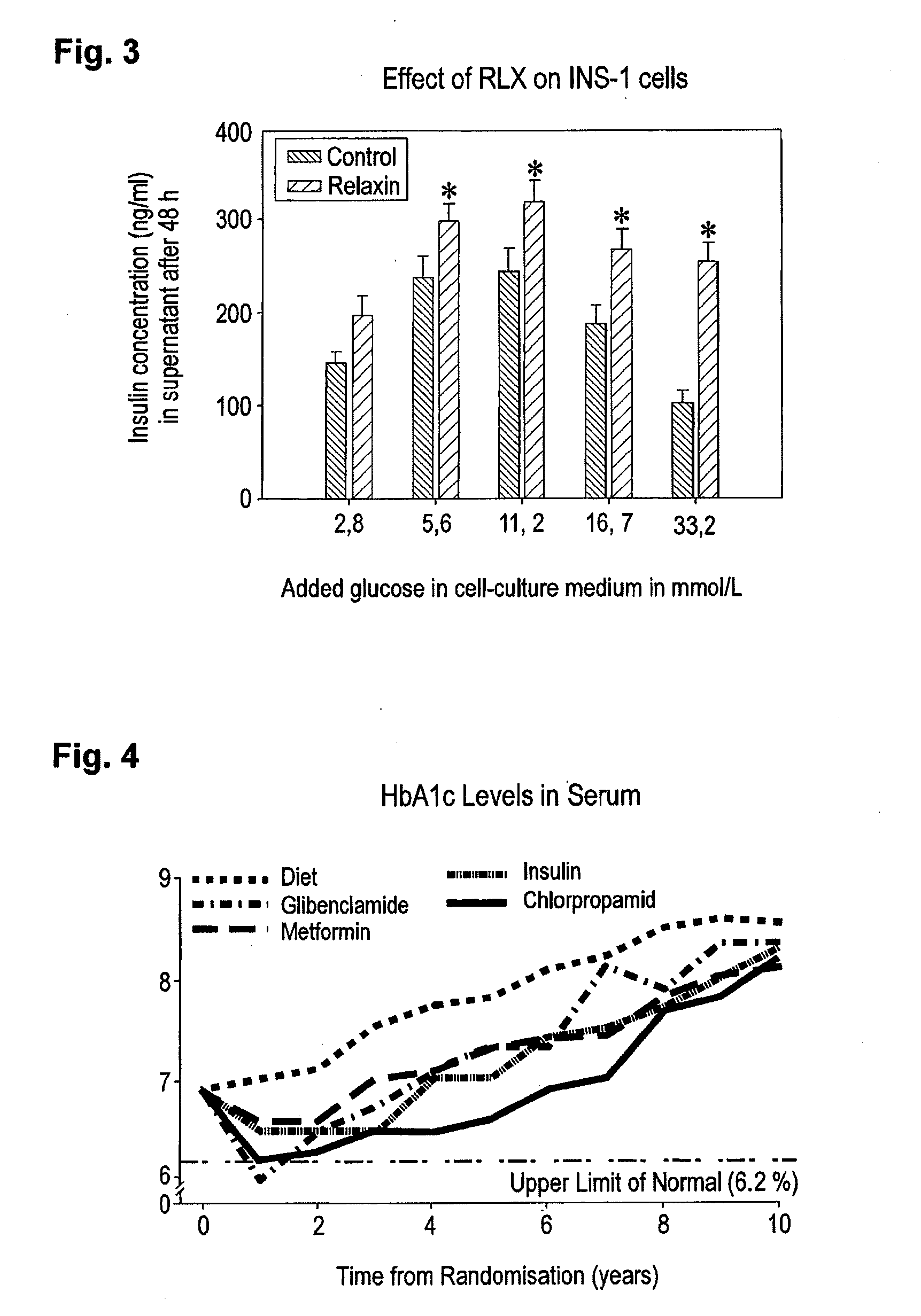
Relaxin for treating patients afflicted of impaired glucose tolerance
ActiveUS20150038411A1Patient compliance is goodGood treatment effectPeptide/protein ingredientsMetabolism disorderIGT - Impaired glucose toleranceBeta-cell Function
A pharmaceutical composition for treatment for of r afflicted or in risk of becoming of impaired glucose tolerance (IGT) and / or type-2 it comprising an effective amount of relaxin for protecting beta-cells and beta-cell function against the effects of high blood glucose (glucotoxicity). Treatment of persons of disglycaemias, and protection of beta cells of the islets of Langerhans and beta-cell function in patients having type-2 diabetes, is diclosed.
Owner:IMMUNDIAGNOSTIK
Modulation of cell intrinsic strain to control cell modulus, matrix synthesis, secretion, organization, material properties and remodeling of tissue engineered constructs
The present invention provides methods for manipulating the intrinsic strain of cells by treating tissue engineered constructs or native tissue with compounds which affect the intrinsic strain setpoint of the cells in order to modulate matrix synthesis, secretion, organization and / or remodeling so that the tissues withstand in vivo mechanical forces and have the structural characteristics of host tissue which has been permanently altered by injury, atrophy or disease. The compounds include binding site peptides, ATP, UTP and related analogues, IL-1β, TGF-α, cytochalasin D, hyaluronic acid, nocodazole and others. Also provided are methods for applying a mechanical external strain to the tissues, as well as methods for modulating the expression of cytoskeletal genes that transcribe cytoskeletal proteins which regulate a cell's intrinsic strain setpoint.
Owner:MEDTRAIN TECH
Compound anticancer sustained releasing agent containing mesenchyme hydrolytic reagent
InactiveCN1961862AInhibitionAvoid damagePeptide/protein ingredientsPharmaceutical delivery mechanismTherapeutic effectHyaluronidase
Owner:JINAN KANGQUAN PHARMA TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com