Use of relaxin to treat diseases related to vasoconstriction
a technology of vasoconstriction and relaxin, which is applied in the direction of anti-noxious agents, drug compositions, peptide/protein ingredients, etc., can solve the problems of reducing cardiac output and other problems, and achieve the effect of safe relaxation profile and beneficial therapeutic
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
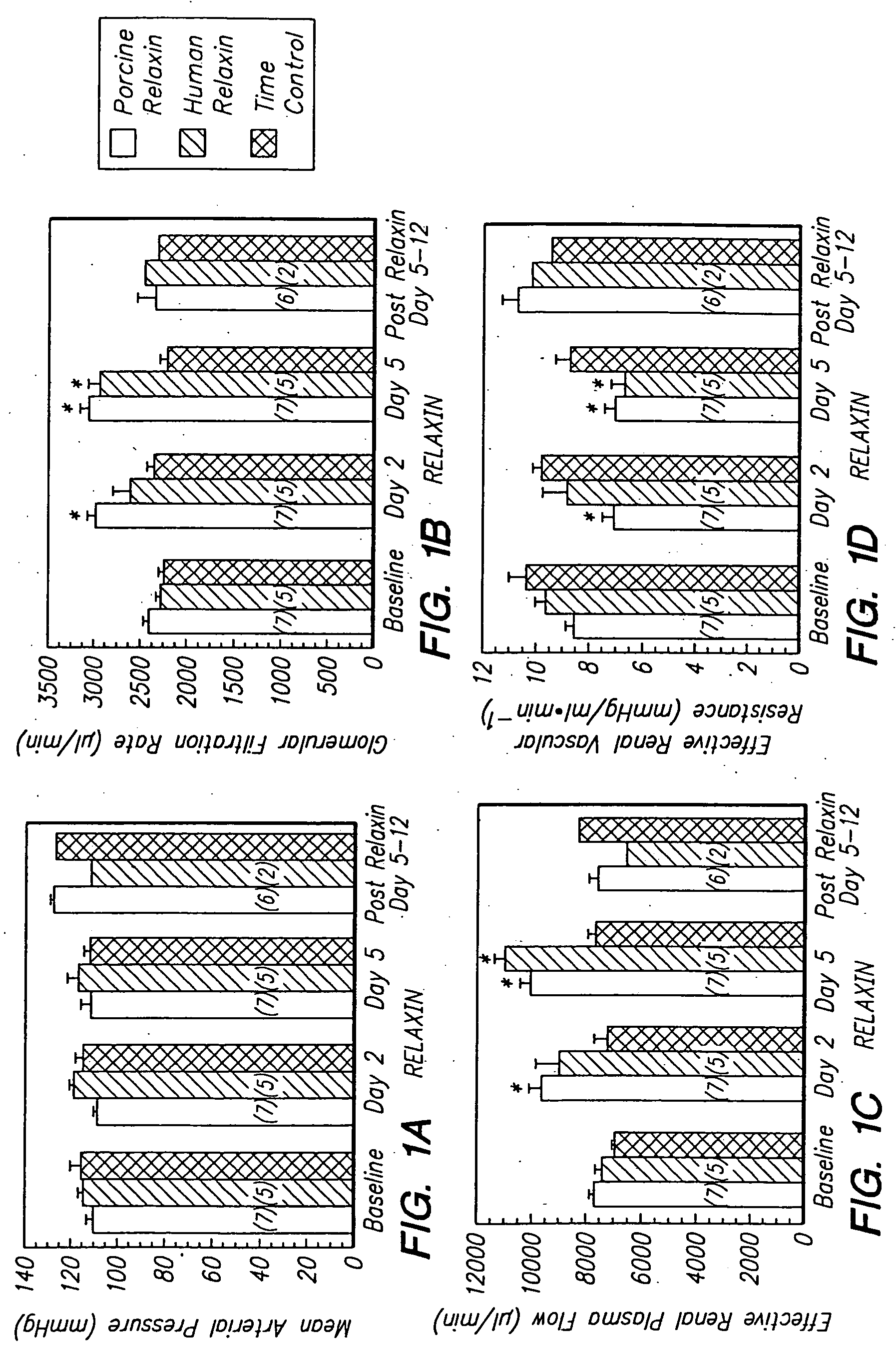
Relaxin is a Potent Renal Vasodilator in Conscious Rats
[0090] Materials and Methods
[0091] Animal Preparation
[0092] Long-Evans female rats aged 10-14 weeks were purchased from Harlan Sprague-Dawley (Frederick, Md.). They were fed PROLAB RMH 2000 diet containing 0.48% sodium (PME Feeds Inc., St. Louis, Mo.) and provided water ad libitum. To prepare the rats for experimental procedures, they were trained for several hours in a Plexiglas restraining cage (Braintree Scientific Co., Braintree, Mass.) on at least five different occasions before surgical intervention. These cages afforded sufficient space for grooming of the face and front paws while preventing the rat from turning around. Thus, accurate timed-urine collections and blood samplings were made possible from the chronically implanted bladder and vascular catheters, respectively. Rats failing to habituate to the cage were eliminated from the study (<1%). All animal procedures were approved by the Institutional Animal Care and Us...
example 2
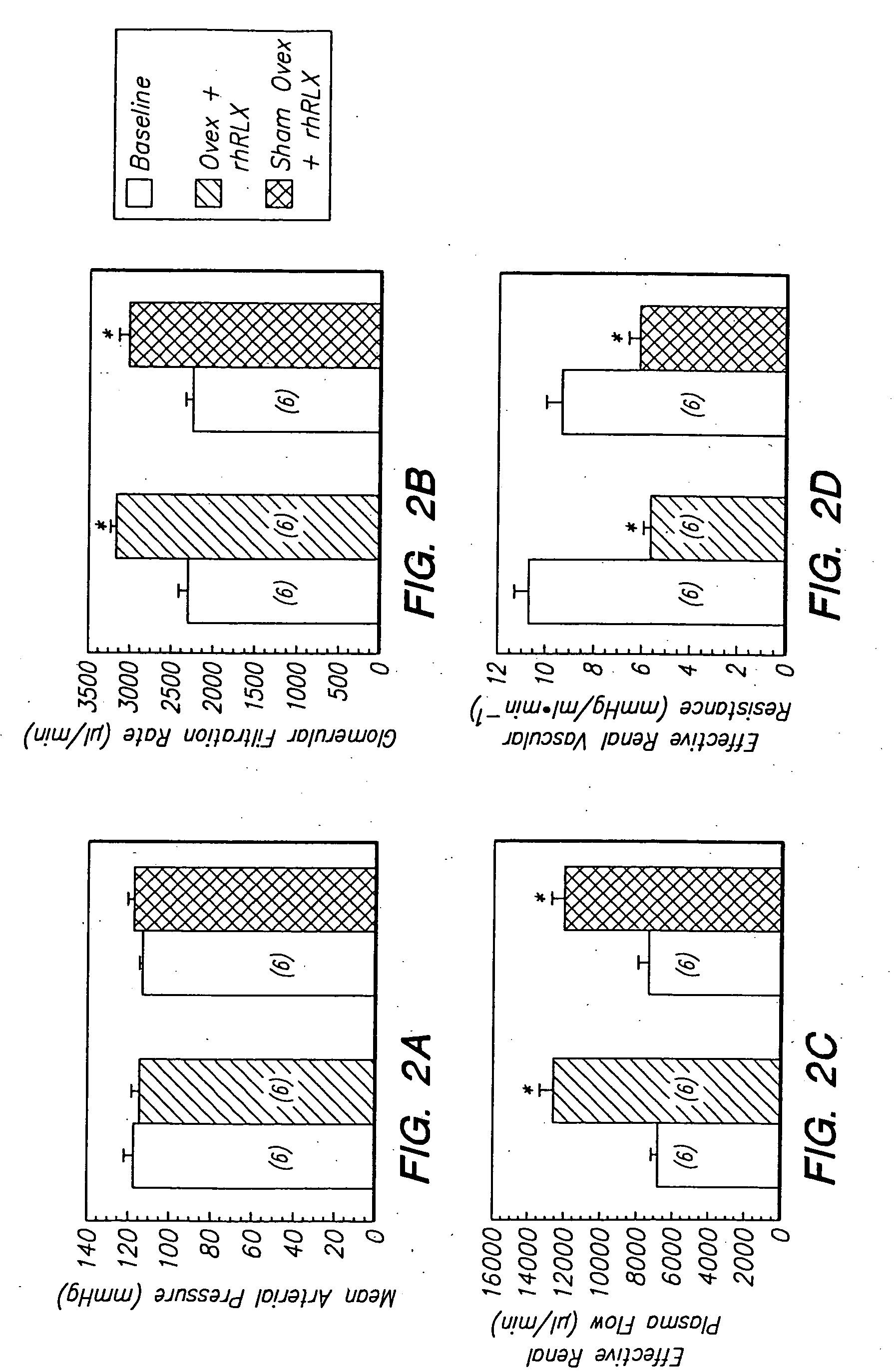
Impact of Gender and Endothelin on Renal Vasodilation and Hyperfiltration Induced by Relaxin in Conscious Rats
[0113] Methods
[0114] Animal preparation. Long Evans female and male rats of 10-14 weeks of age were used. Those animals studied at the University of New Mexico were purchased from Harlan Sprague-Dawley (Indianapolis, Ind.) and were fed PROLAB RMH 2500 diet containing 0.40% sodium (PME Feeds Inc., St. Louis, Mo.). The rats investigated at the Magee-Womens Research Institute were purchased from Harlan Sprague-Dawley (Frederick, Md.) and they were fed PROLAB RMH 2000 diet containing 0.48% sodium (PME Feeds Inc., St. Louis Mo.). The rats were maintained on a 12 hour light / dark cycle in fully accredited Animal Resource Facilities approved by the Association for Assessment and Accreditation of Laboratory Animal Care. All experiments were approved by the Institutional Animal Care and Use Committee of the University of New Mexico School of Medicine or the Magee-Womens Research Insti...
example 3
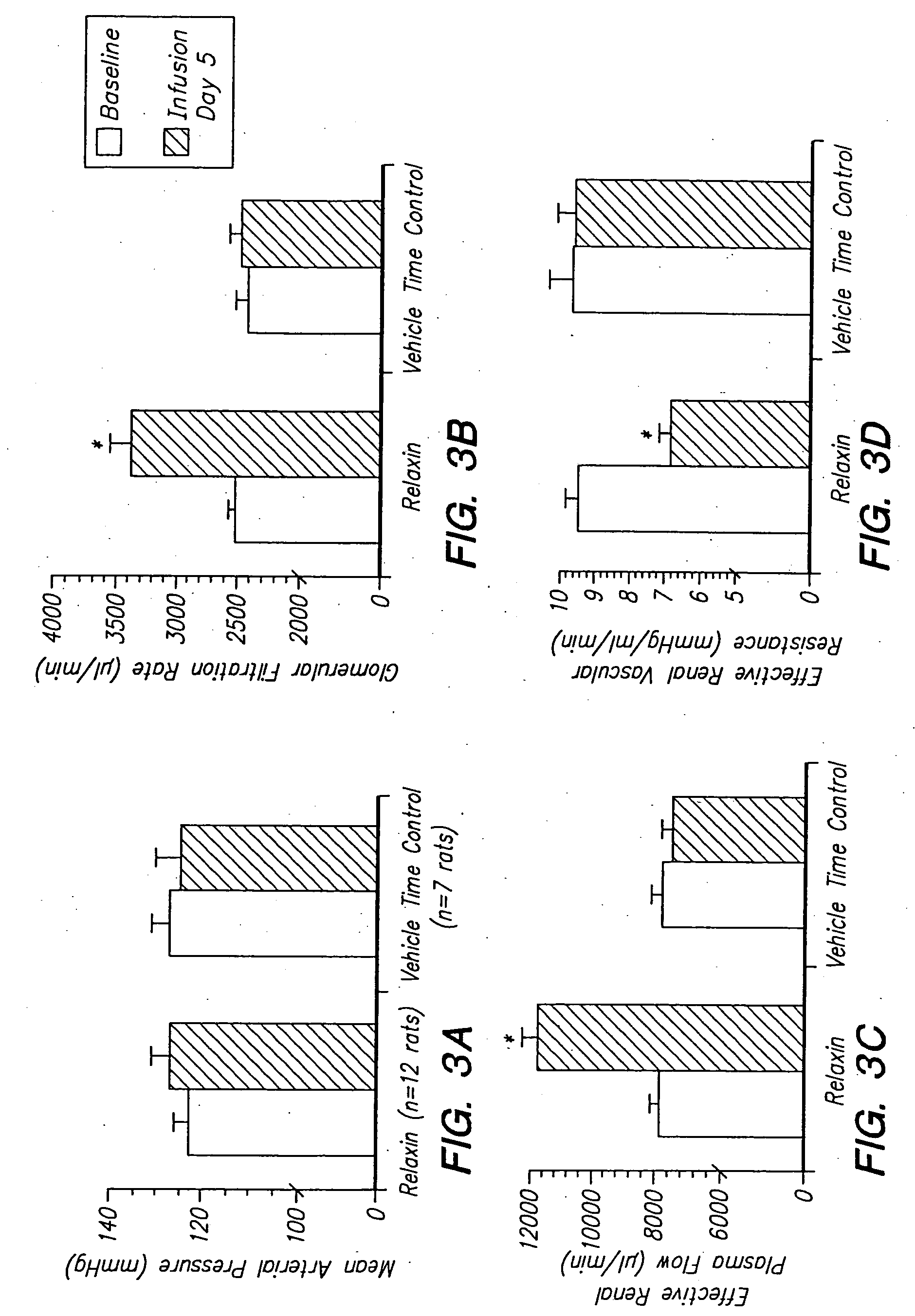
[0134] Example 3
Systemic Relaxin Administration Stimulates Angiogenic / Vasbdilatory Cytokine Expression and Vessel Formation in a Rat Myocardial Infarct Model
[0135] Female Sprague Dawley rats, approximately 12-weeks of age were used. Rats were anesthetized by intraperitoneal (i.p.) injection of up to 1 ml / kg ketamine / medetomidine (6:4). Following exteriorization of the heart, the left coronary artery was ligated near its origin with a silk suture. In sham surgery control animals, the suture was placed superficially into the muscle adjacent to the coronary artery. Post-closure EKG's were monitored for S-T segment elevation in LCAL animals to confirm the outcome of ligation. Immediately following cardiac surgery, a primed mini-osmotic pump containing relaxin or vehicle (20 mM acetate, pH 5.0) was aseptically implanted into a subcutaneous (s.c.) pocket on the dorsal interscapular region. Vehicle or relaxin (0.1 mg / kg / day) was delivered as a continuous s.c. infusion for 7 or 21 days to e...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com