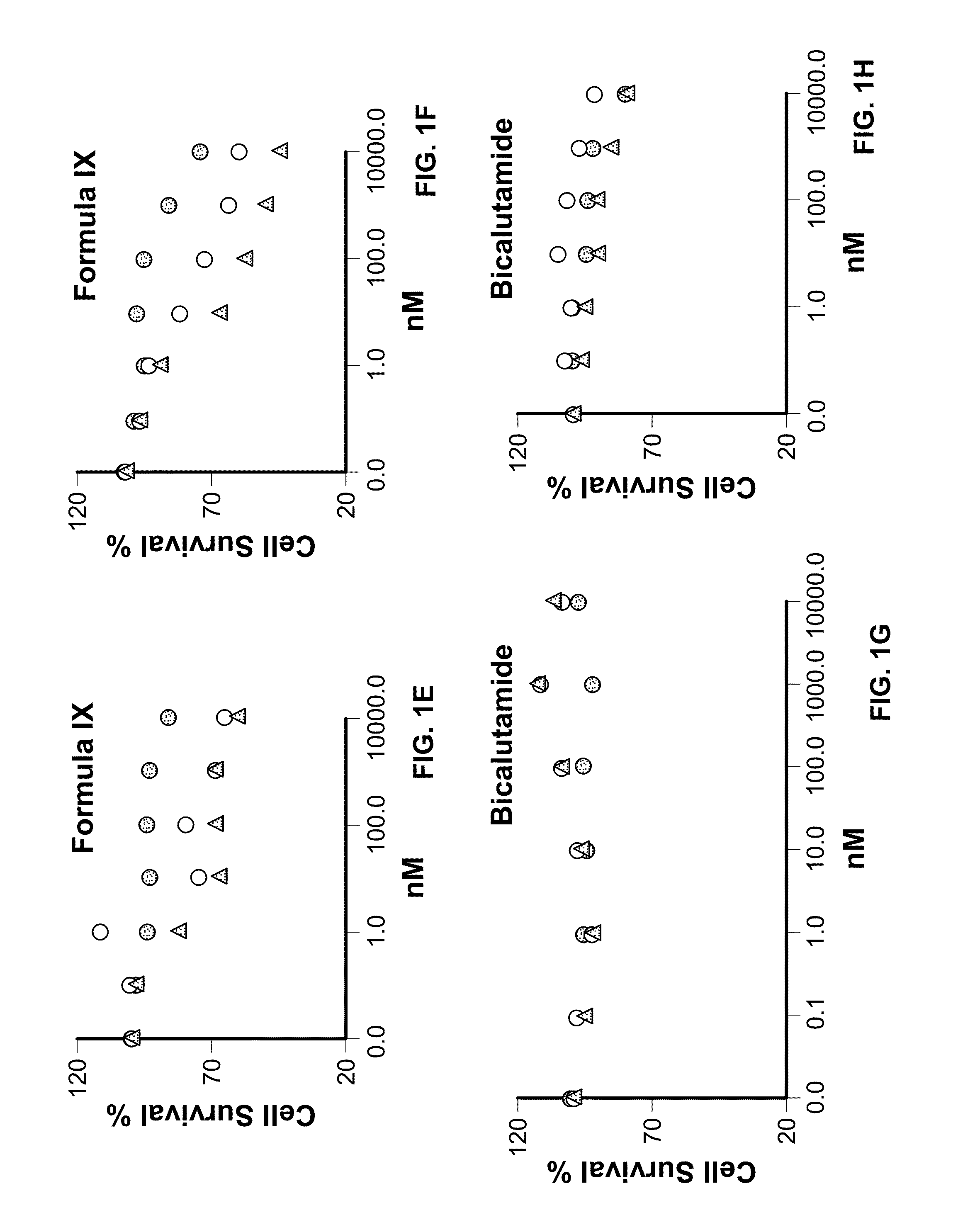
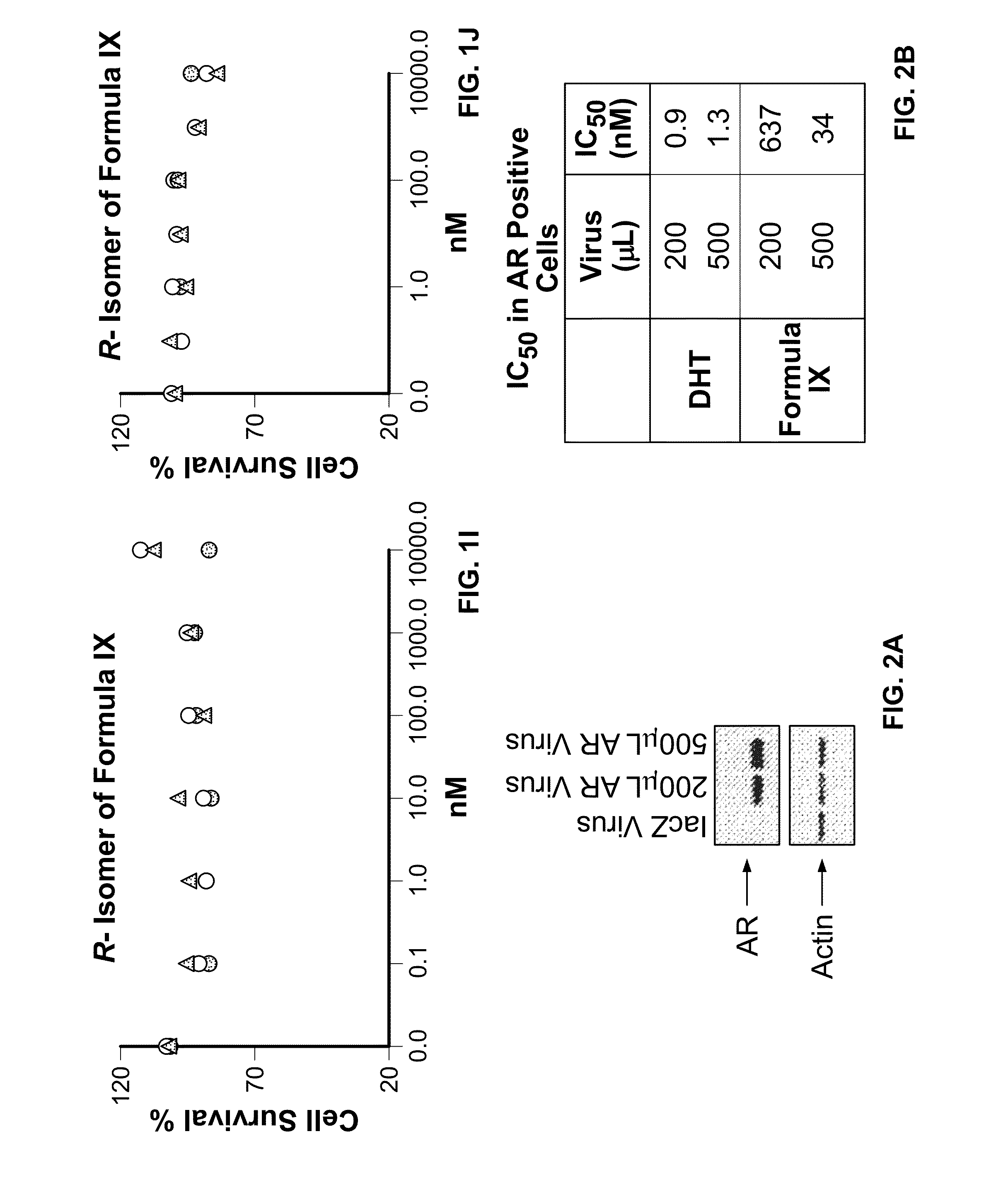
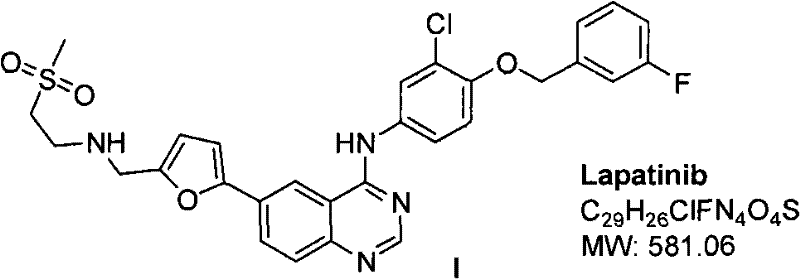
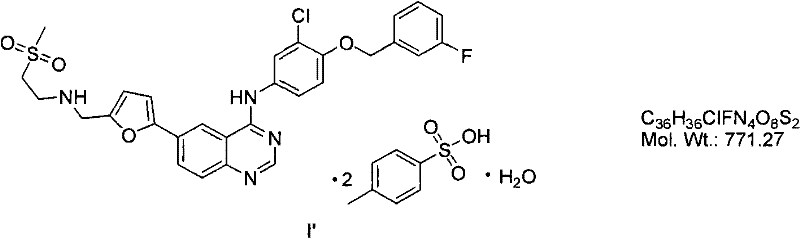
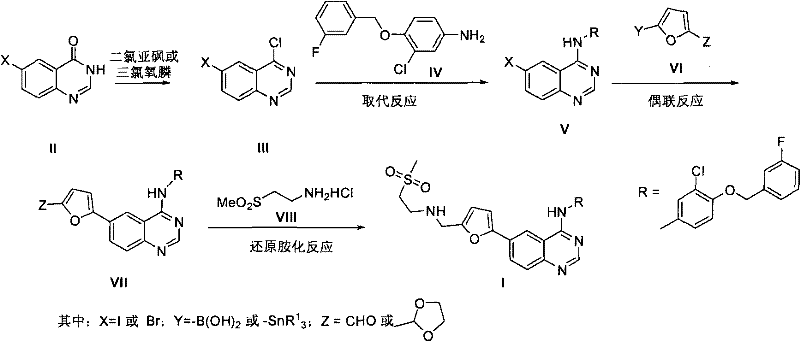
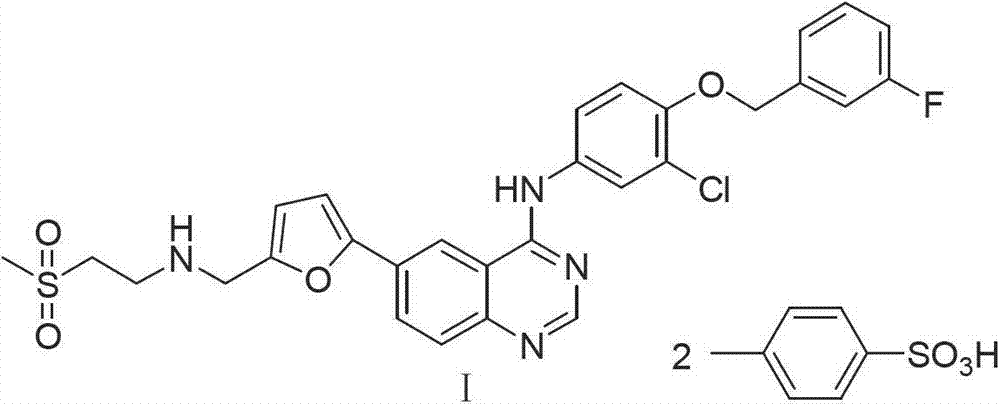
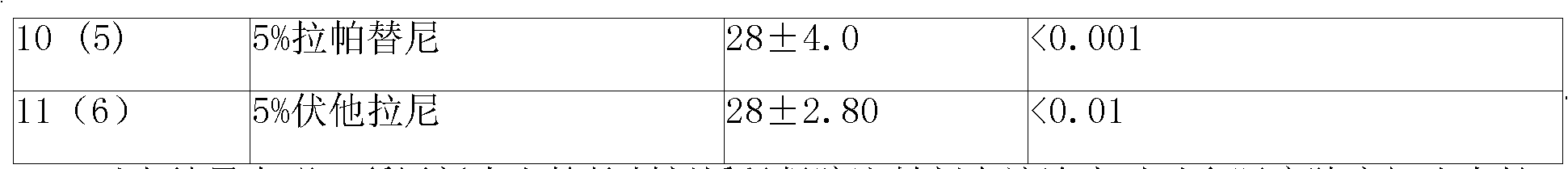Patents
Literature
135 results about "Lapatinib" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
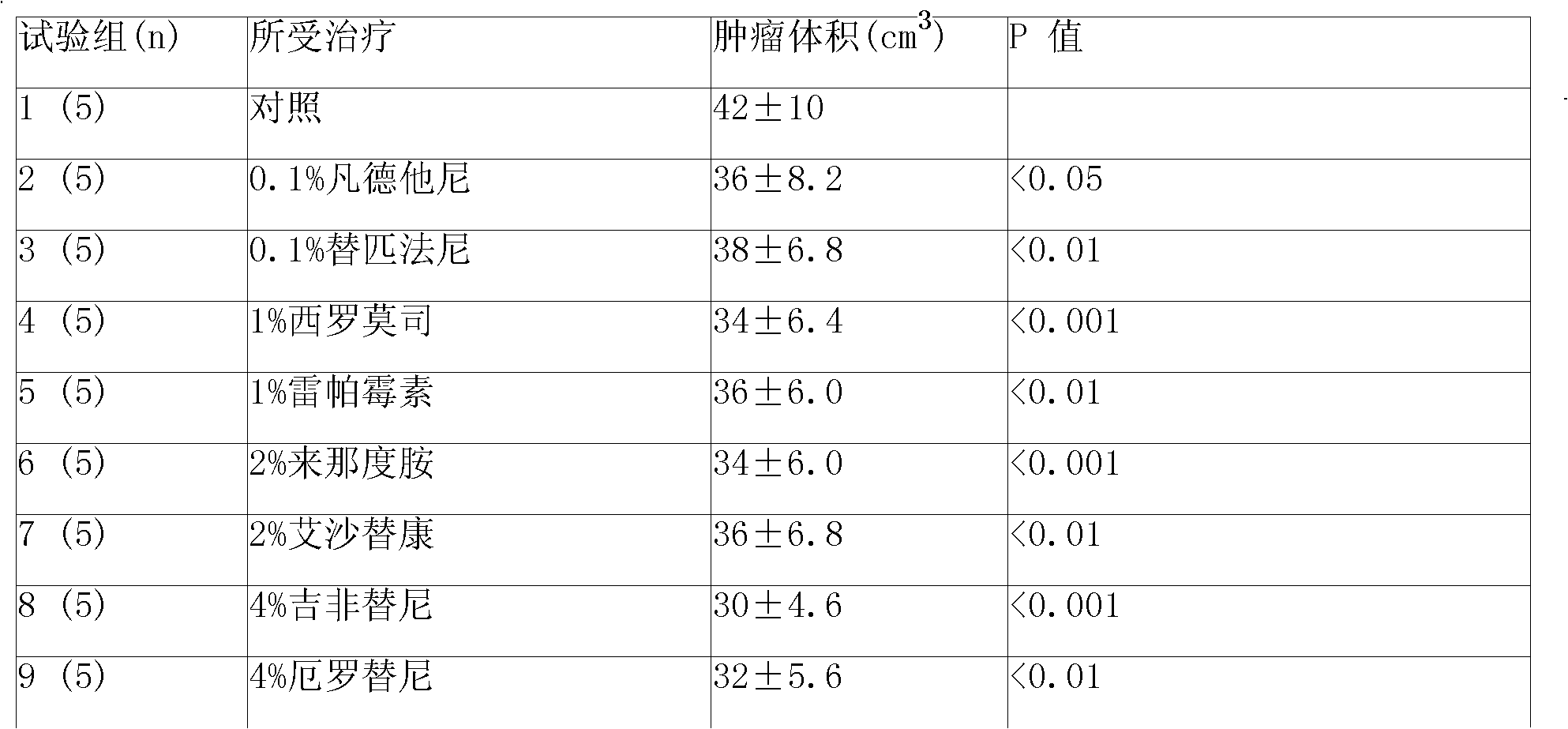
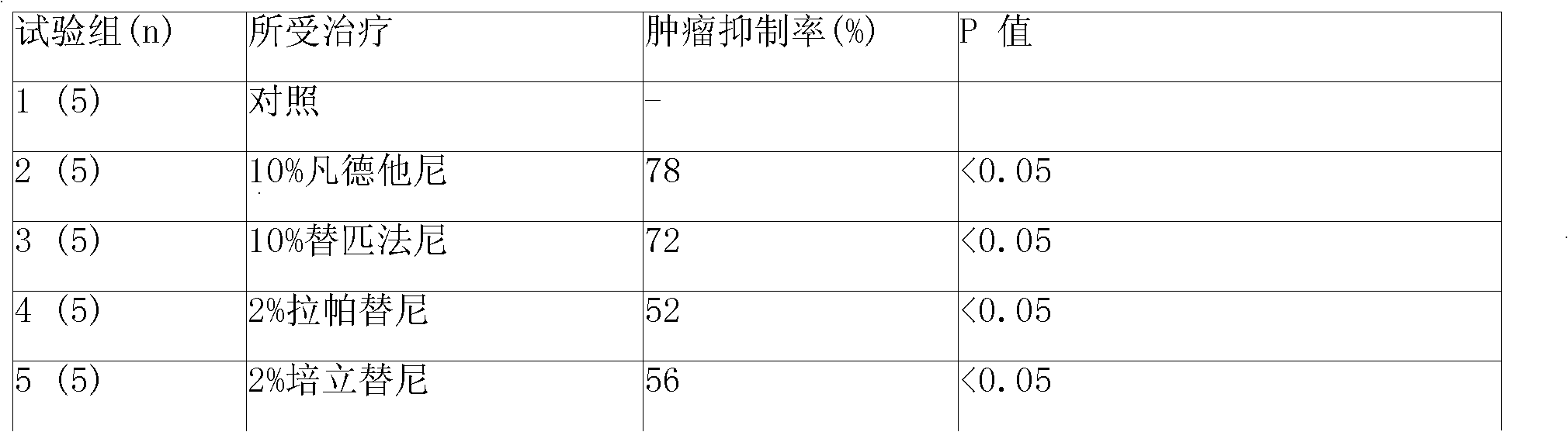
Lapatinib is used together with another medication (capecitabine) to treat a certain type of breast cancer (HER2-positive) that has not responded to the standard treatment. Lapatinib may also be used together with another medication (letrozole) to treat HER2-positive breast cancer in women after menopause.
Compounds for immunopotentiation
InactiveUS20100226931A1Reduce capacityLow production costSsRNA viruses positive-senseSnake antigen ingredientsPyridazineErlotinib
Methods of stimulating an immune response and treating patients responsive thereto with 3,4-di(1H-indol-3-yl)-1H-pyrrole-2,5-diones, staurosporine analogs, derivatized pyridazines, chromen-4-ones, indolinones, quinazolines, nucleoside analogs, and other small molecules are disclosed. In a preferred embodiment benzopyrimidine derivatives such as ZD-6474, MLN-518, lapatinib, gefitinib or erlotinib are used.
Owner:NOVARTIS VACCINES & DIAGNOSTICS INC
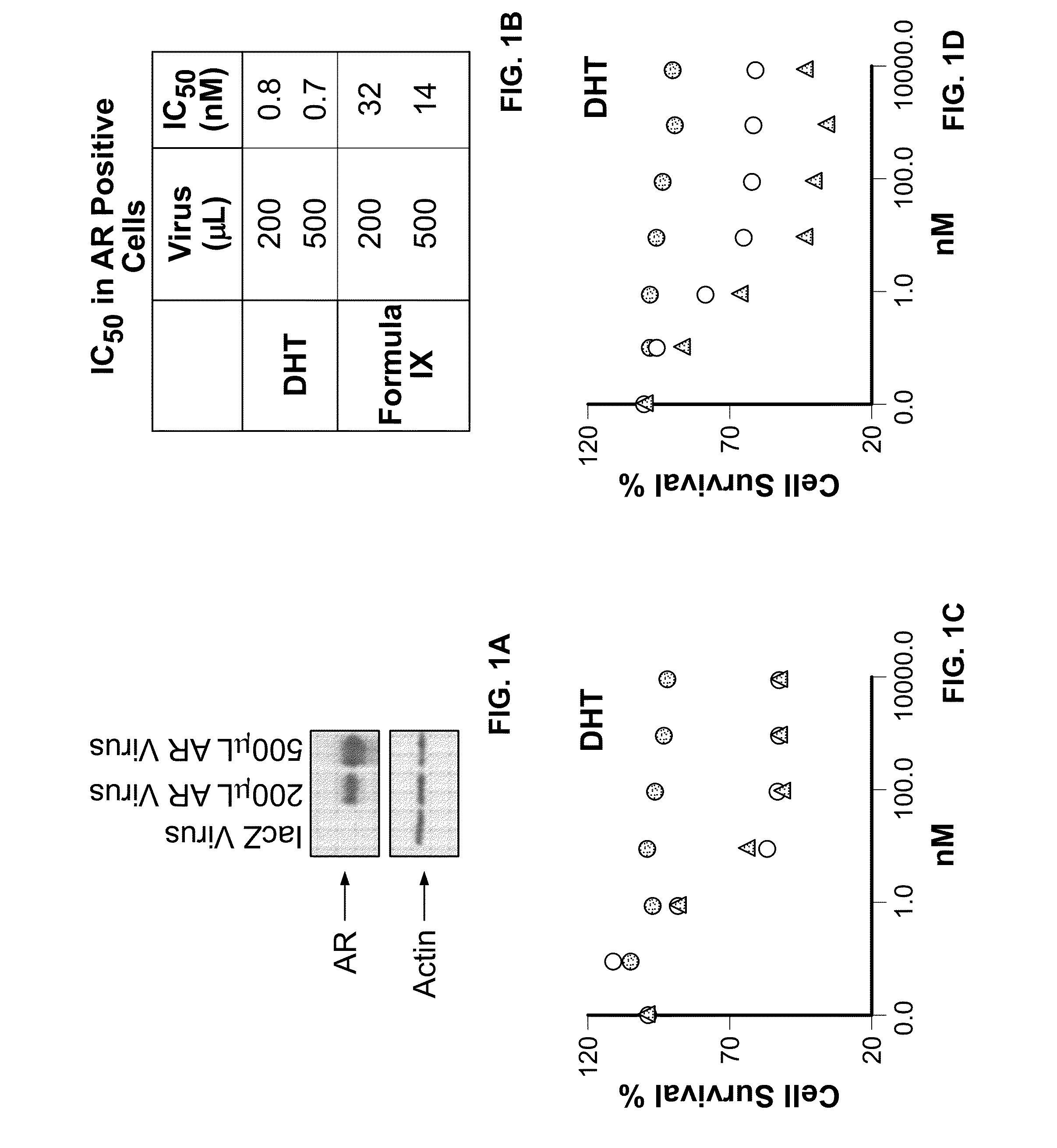
Method of treating estrogen receptor (ER) -positive breast cancers with selective androgen receptor modulator (SARMS)
This invention relates to the treatment of androgen receptor-positive breast cancer in a subject, for example a female subject. Accordingly, this invention provides methods of: a) treating a subject suffering from breast cancer; b) treating a subject suffering from metastatic breast cancer; c) treating a subject suffering from refractory breast cancer; d) treating a subject suffering from AR-positive breast cancer; e) treating a subject suffering from AR-positive refractory breast cancer; f) treating a subject suffering from AR-positive metastatic breast cancer; g) treating a subject suffering from AR-positive and ER-positive breast cancer; h) treating a subject suffering from triple negative breast cancer; i) treating a subject suffering from advanced breast cancer; j) treating a subject suffering from breast cancer that has failed SERM (tamoxifen, toremifene), aromatase inhibitor, trastuzumab (Herceptin, ado-trastuzumab emtansine), pertuzumab (Perjeta), lapatinib, exemestane (Aromasin), bevacizumab (Avastin), and / or fulvestrant treatments; k) treating, preventing, suppressing or inhibiting metastasis in a subject suffering from breast cancer; l) prolonging survival of a subject with breast cancer, and / or m) prolonging the progression-free survival of a subject with breast cancer; comprising administering to the subject a therapeutically effective amount of a selective androgen receptor modulator (SARM) compound, comprising administering to the subject a therapeutically effective amount of a SARM compound of this invention.
Owner:UNIV OF TENNESSEE RES FOUND
Method of treating androgen receptor (AR) -positive breast cancers with selective androgen receptor modulator (SARMS)
ActiveUS20140350102A1Treating and preventing and suppressing and inhibiting metastasisProlonged progression-free survivalBiocideOrganic chemistryToremifeneLymphatic Spread
This invention relates to the treatment of breast cancer in a subject, for example a female subject. Including methods of: treating metastatic breast cancer; refractory breast cancer; AR-positive breast cancer; AR-positive refractory breast cancer; AR-positive metastatic breast cancer; AR-positive and ER-positive breast cancer; triple negative breast cancer advanced breast cancer; breast cancer that has failed SERM (tamoxifen, toremifene), aromatase inhibitor, trastuzumab (Herceptin, ado-trastuzumab emtansine), pertuzumab (Perjeta), lapatinib, exemestane (Aromasin), bevacizumab (Avastin), and / or fulvestrant treatments; metastasis in a subject suffering from breast cancer; comprising administering to the subject a therapeutically effective amount of a selective androgen receptor modulator (SARM) compound.
Owner:UNIV OF TENNESSEE RES FOUND
Eutectic complex composed of resveratrol and protein kinase inhibitor, and composition comprising eutectic complex
InactiveCN110283052AHydroxy compound active ingredientsOrganic chemistry methodsLenvatinibPTK Inhibitors
The invention provides a eutectic complex composed of resveratrol and a protein kinase inhibitor, and a composition comprising the eutectic complex. The eutectic complex is characterized in that the protein kinase inhibitor is selected from one of imatinib, gefitinib, erlotinib, sunitinib, sorafenib, dasatinib, lapatinib, nilotinib, pazopanib, afatinib, crizotinib, axitinib, regorafenib, ibrutinib, lenvatinib, palbociclib, osimertinib and alectinib or pharmaceutically acceptable salt thereof. The eutectic complex provided by the invention can produce synergistic effects of inhibiting histamine release.
Owner:黄泳华
Anticancer sustained-release gel injection
InactiveCN101336890APharmaceutical delivery mechanismPharmaceutical non-active ingredientsLestaurtinibSolvent
The invention relates to an anticancer slow-release gel injection which contains slow-release microspheres containing an angiogenesis inhibitor, an amphiphilic block polymer, a solvent and a slow-release agent, wherein the composition of the amphiphilic block polymer and the non-organic solvent exhibit the property of temperature-sensitive gelation. After the in vivo injection, the injection turns into a stagnant and biodegradable water-insoluble gel and the gel slowly releases the drug contained therein for a plurality of weeks to a plurality of months. After the intratumoral or peritumoral injection, the anticancer slow-release gel injection can significantly reduce the general drug reactions and is used for treating tumors of different stages. The angiogenesis inhibitor is selected from SU5416, SU6668, bosutinib, sprycel, erlotinib, vandetanib, gefitnib, canertinib, lapatinib, lestaurtinib, masitinib, vatalanib, mubritinib, tandutinib, nilotinib, marimastat, nilotinib, pelitinib, telatinib, sunitinib, sorafenib, zarnestra, sirolimus, imatinfb, lenalidomide and thalidomide.
Owner:济南基福医药科技有限公司
Method of treatment of philadelphia chromosome positive leukaemia
InactiveUS20120244116A1Organic active ingredientsPeptide/protein ingredientsBcr-Abl tyrosine-kinase inhibitorLestaurtinib
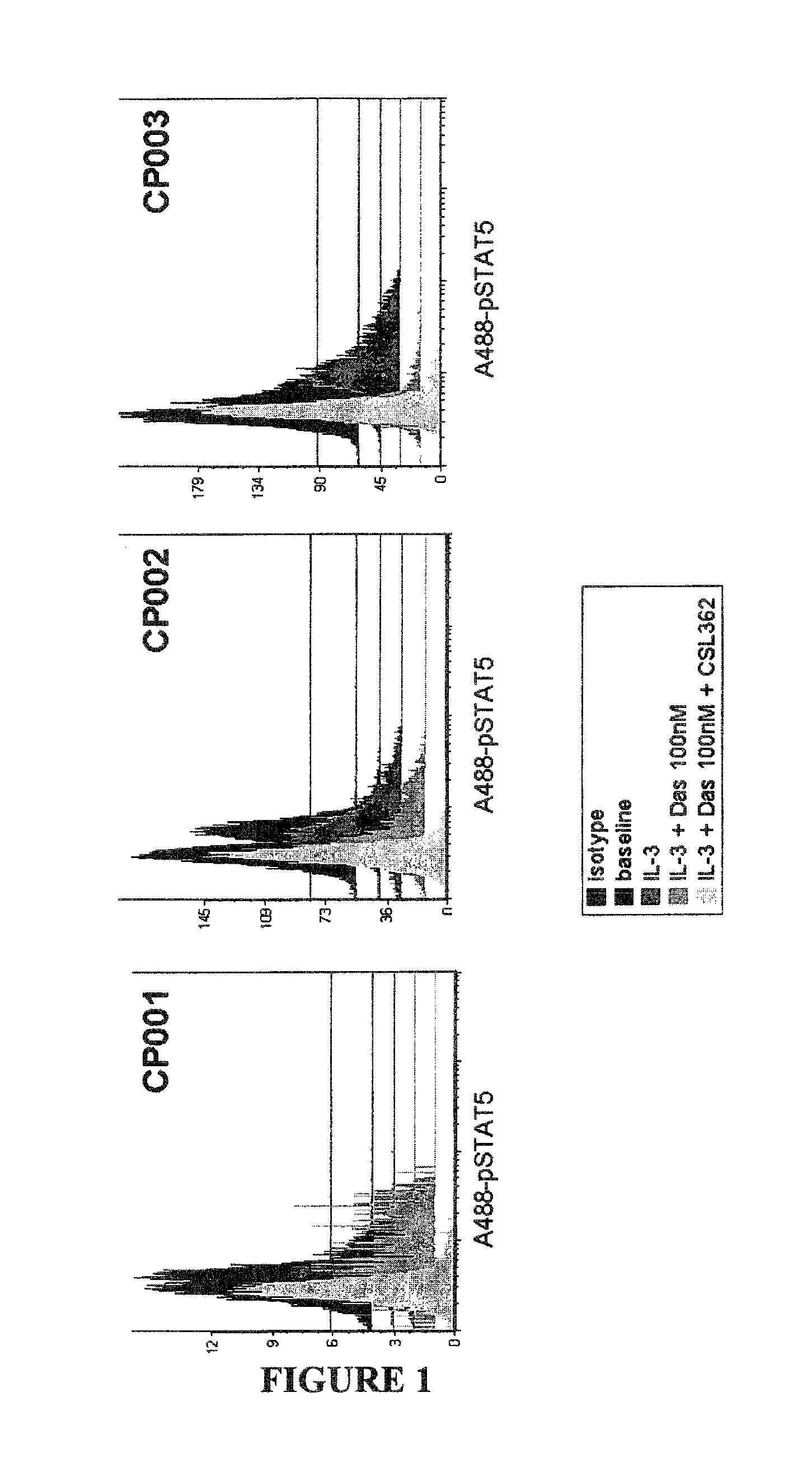
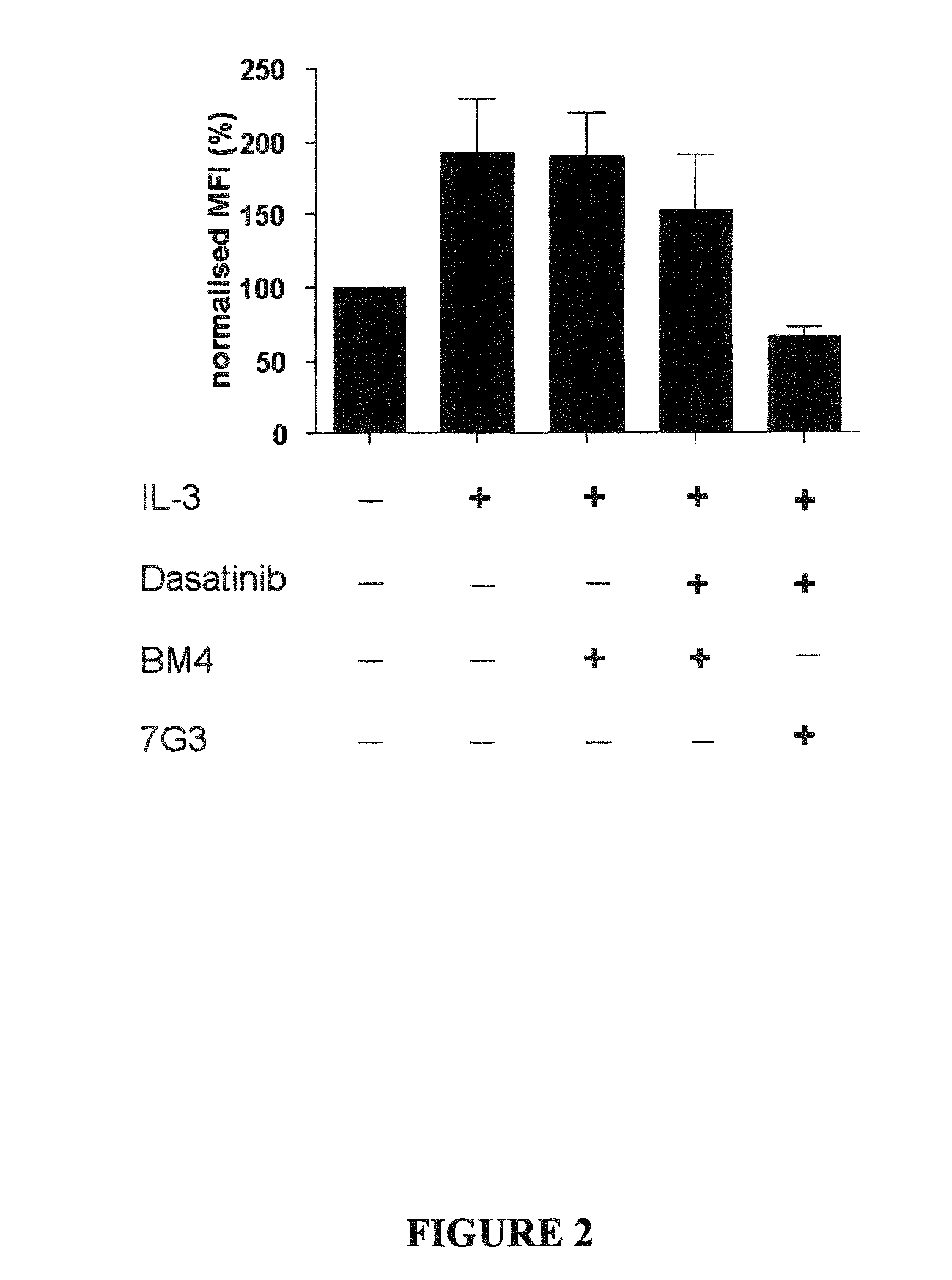
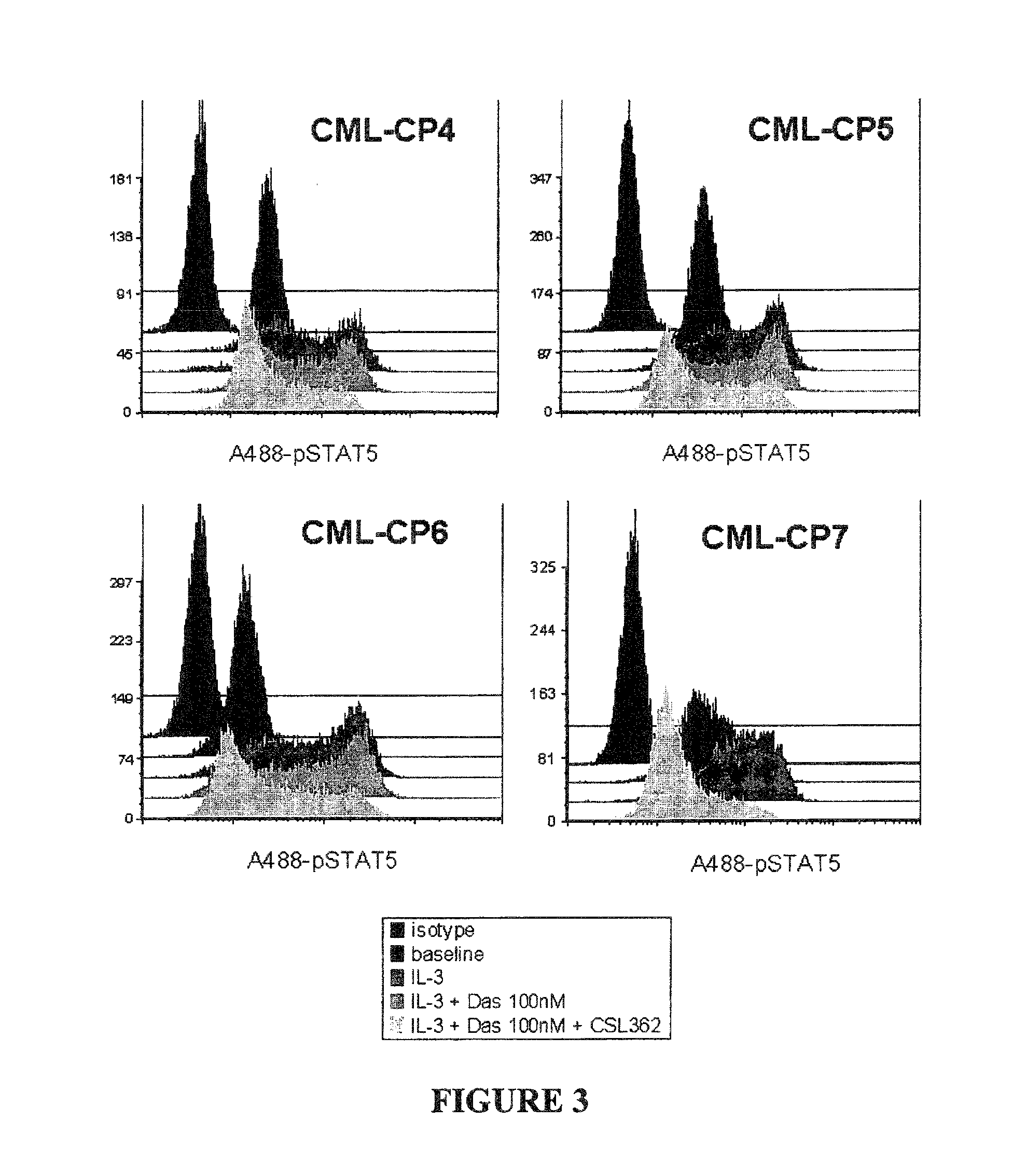
The invention provides a method for the treatment of Ph+ leukemia in a patient comprising administering to the patient (i) a BCR-ABL tyrosine kinase inhibitor, and (ii) an agent which selectively binds to a cell surface receptor expressed on Ph+ leukemic stem cells. The invention further provides for the use of (i) and (ii) in, or in the manufacture of a medicament for, the treatment of Ph+ leukemia in a patient; and a composition for the treatment of Ph+ leukemia in a patient comprising (i) and (ii); and kits comprising (i) and (ii). In some embodiments, the tyrosine kinase inhibitor is or is not imatinib; or is selected from the group consisting of dasatinib, nilotinib, bosutinib, axitinib, cediranib, crizotinib, damnacanthal, gefitinib, lapatinib, lestaurtinib, neratinib, semaxanib, sunitinib, toceranib, tyrphostins, vandetanib, vatalanib, INNO-406, AP24534, XL228, PHA-739358, MK-0457, SGX393 and DC2036; or is selected from the group consisting of dasatinib and nilotinib. In some embodiments, the agent binds to a receptor involved in signalling by at least one of IL-3, G-CSF and GM-CSF. In some embodiments, the agent is a mutein selected from the group consisting of IL-3 muteins, G-CSF muteins and GM-CSF muteins. In some embodiments, the mutein is an IL-3 mutein. In some embodiments, the agent is a soluble receptor which is capable of binding to IL-3.
Owner:CSL LTD
Preparation method of lapatinib intermediate and analogues thereof
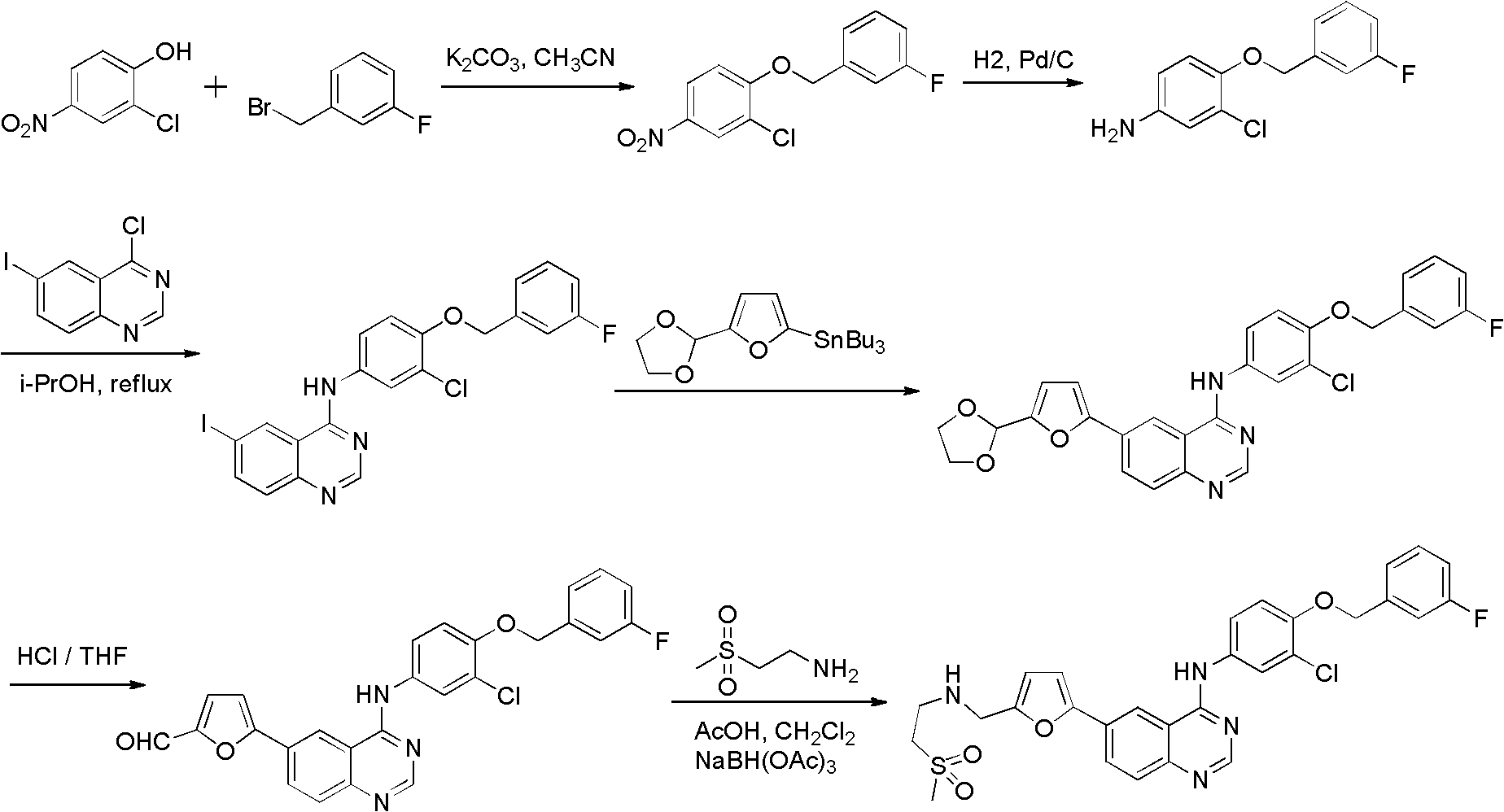
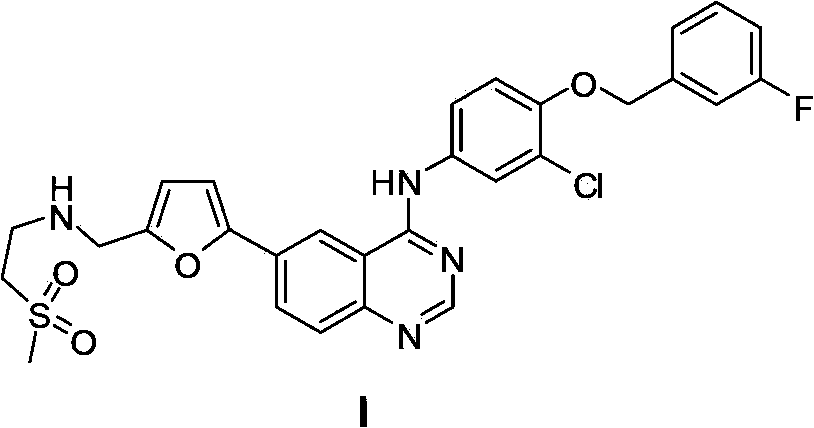
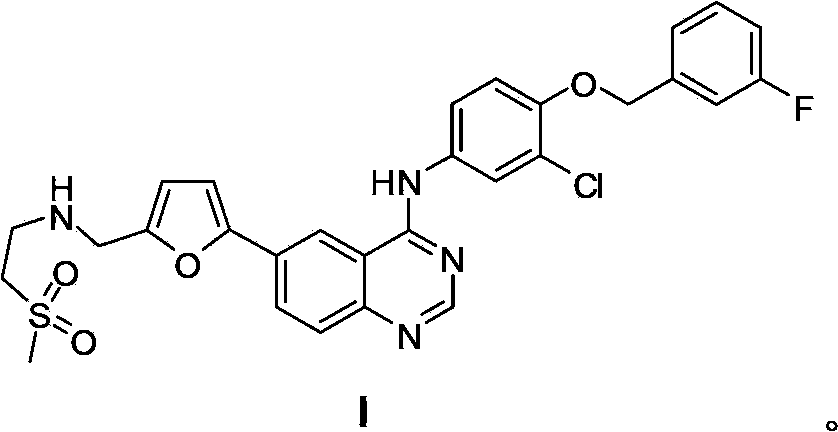
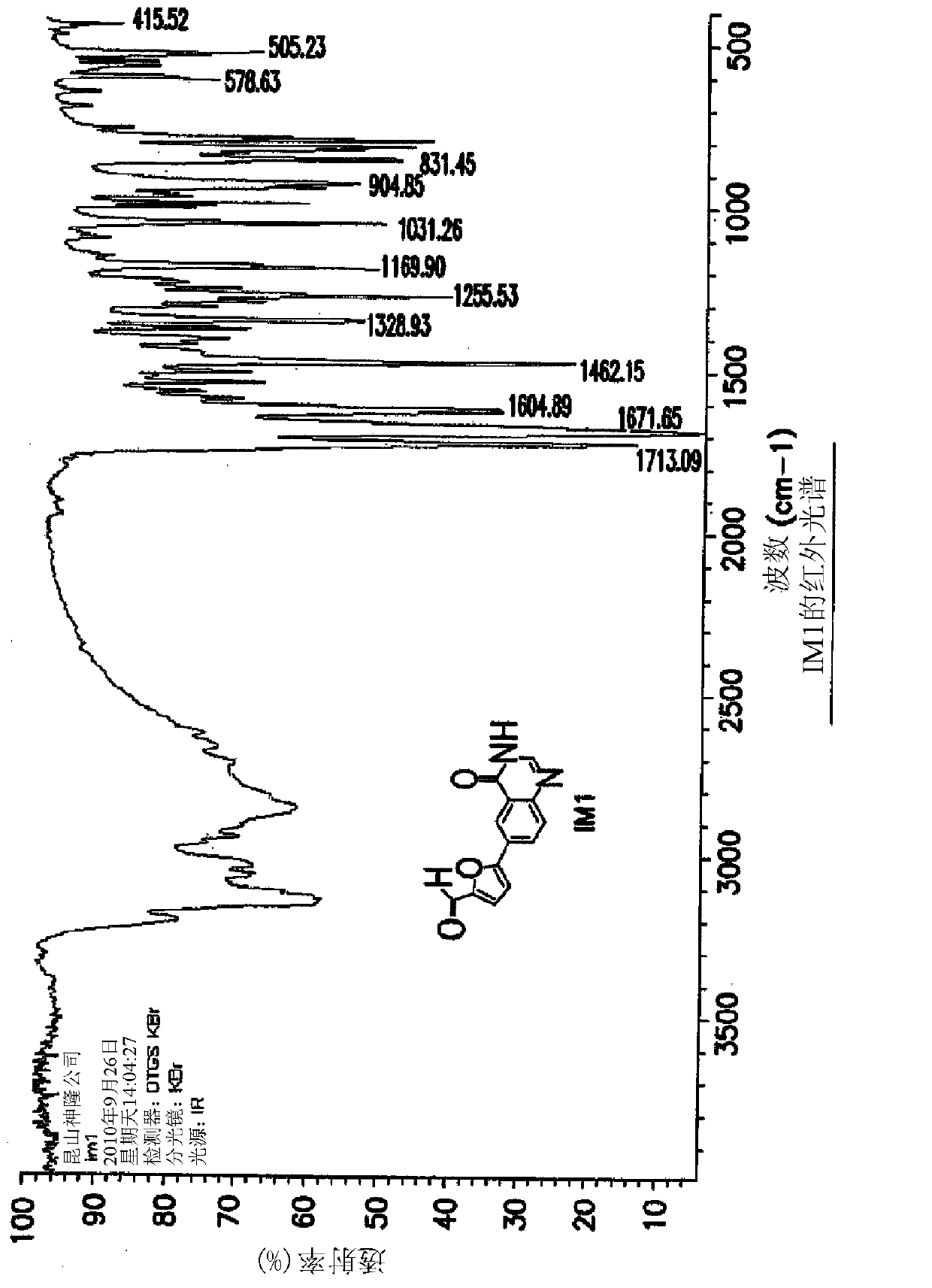
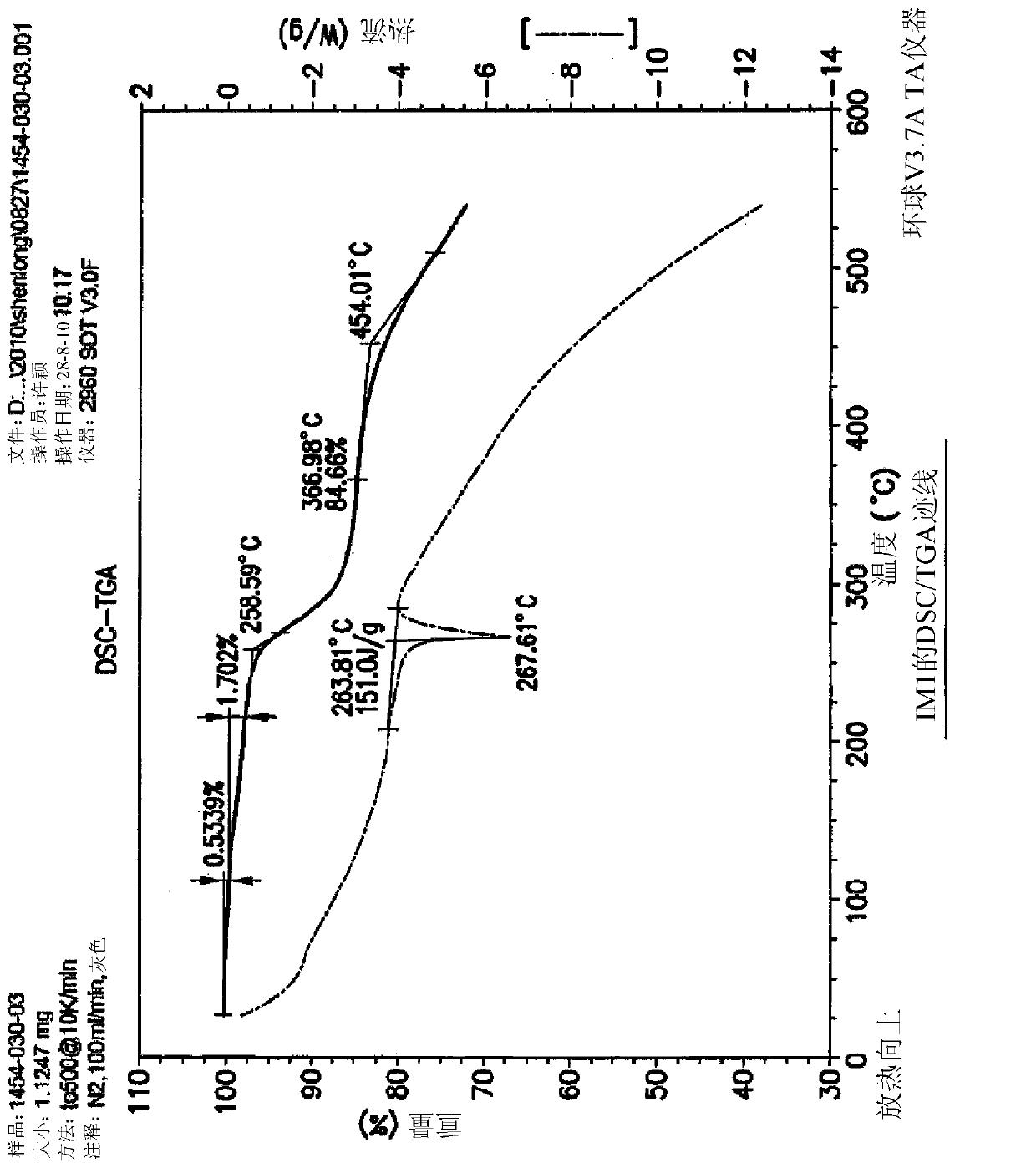
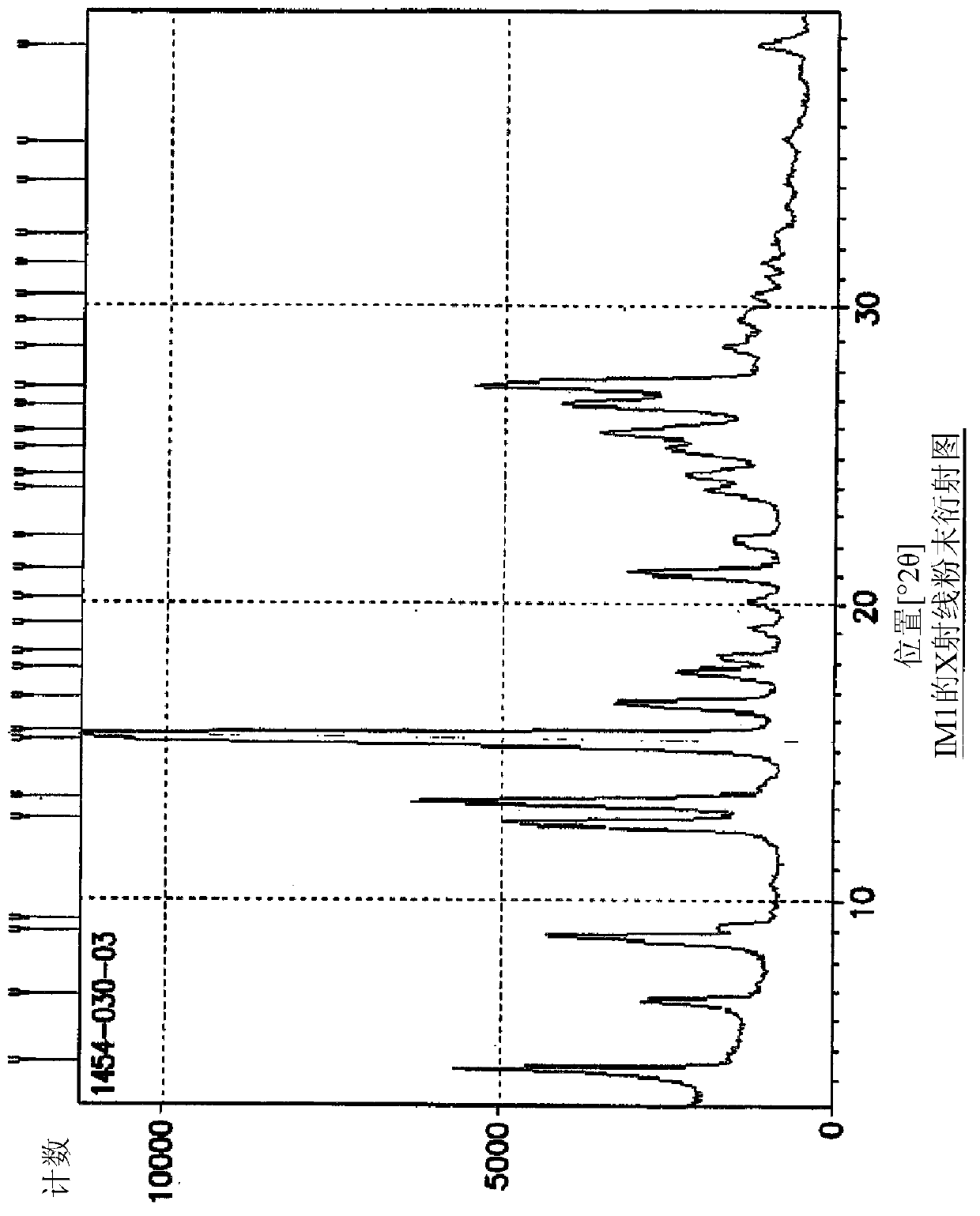
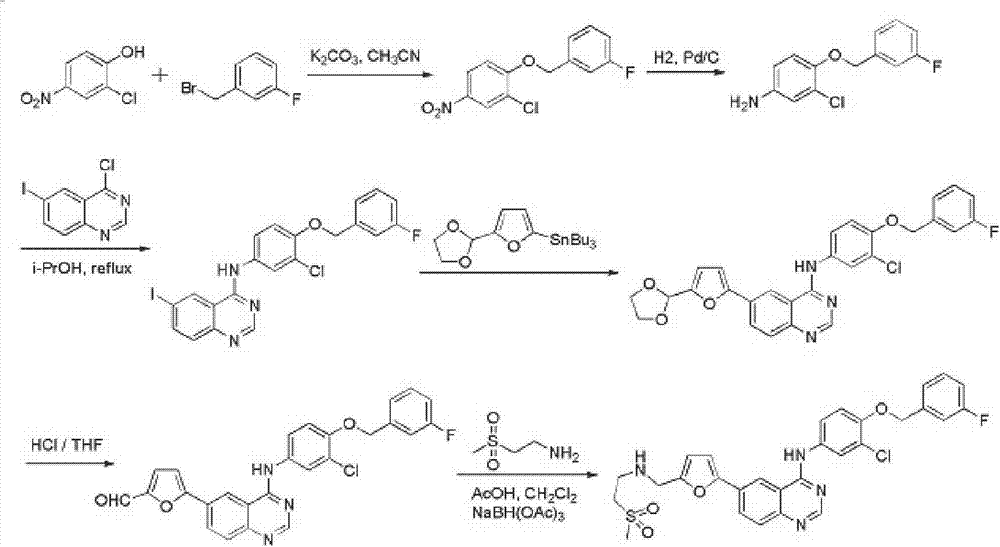
The invention discloses a compound preparation method, which comprises the following steps: a) a compound disclosed in formula I and 4-chlorine-6-iodine-quinazoline are in backflow reaction in isopropanol; b) kieselguhr, stannous chloride, trifluoroacetic acid, H2PdCl4 and polyvinylpyrrolidone are mixed in water and heated to 100-150 DEG C, and a Pd catalyst is obtained after reaction; c) the products obtained in step a), the Pd catalyst, 5-formyl furanboronic acid and K2CO3 are mixed to be reacted in a first organic solvent; and d) reaction products obtained in step c), organic base and 2-(methyl sulfone) ethylamine hydrochloride are mixed in a second organic solvent, and lapatinib basic groups or analogues thereof are obtained after reaction. The heterogeneous Pd catalyst is adopted, and because the heterogeneous Pd catalyst is easy to recycle, the preparation method provided by the invention protects the environment at the time of saving raw materials. Formula (I) is disclosed in the specification.
Owner:UNIV OF SCI & TECH OF CHINA
Serine protease molecules and therapies
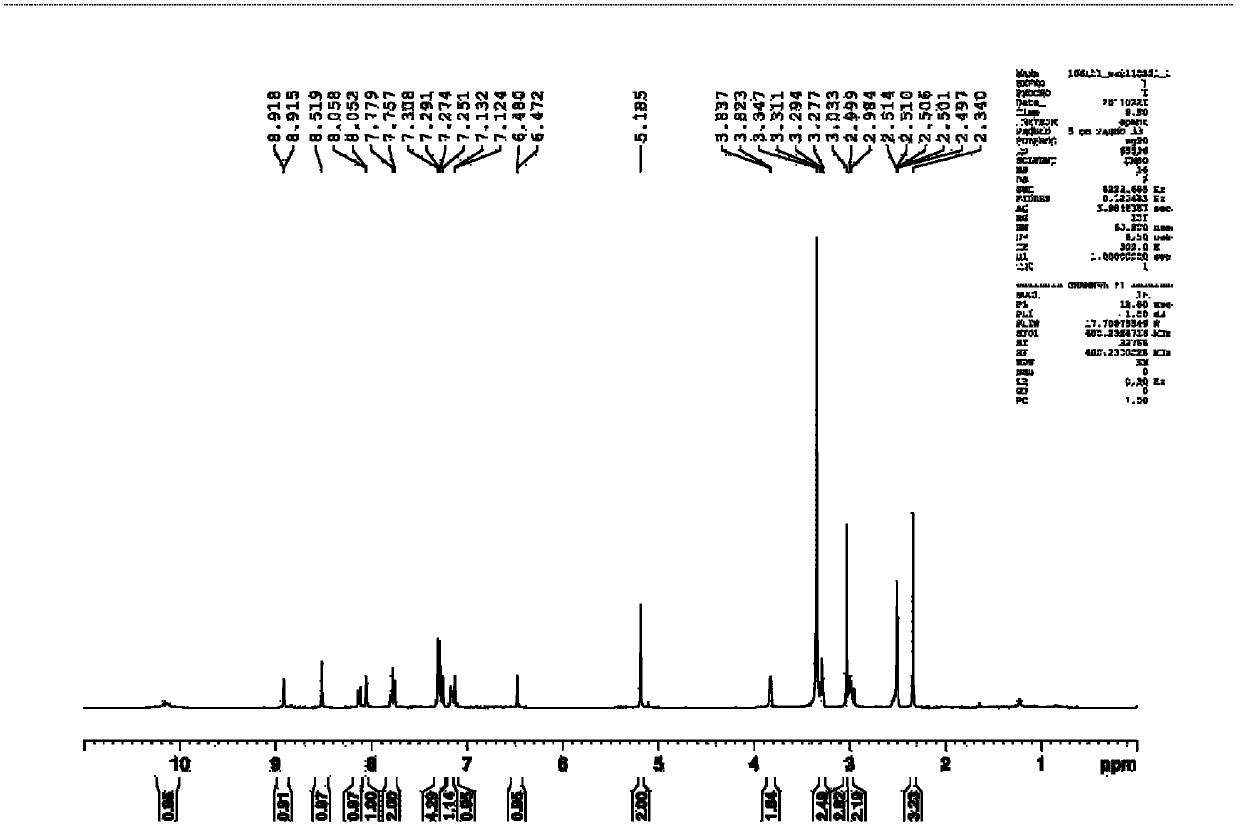
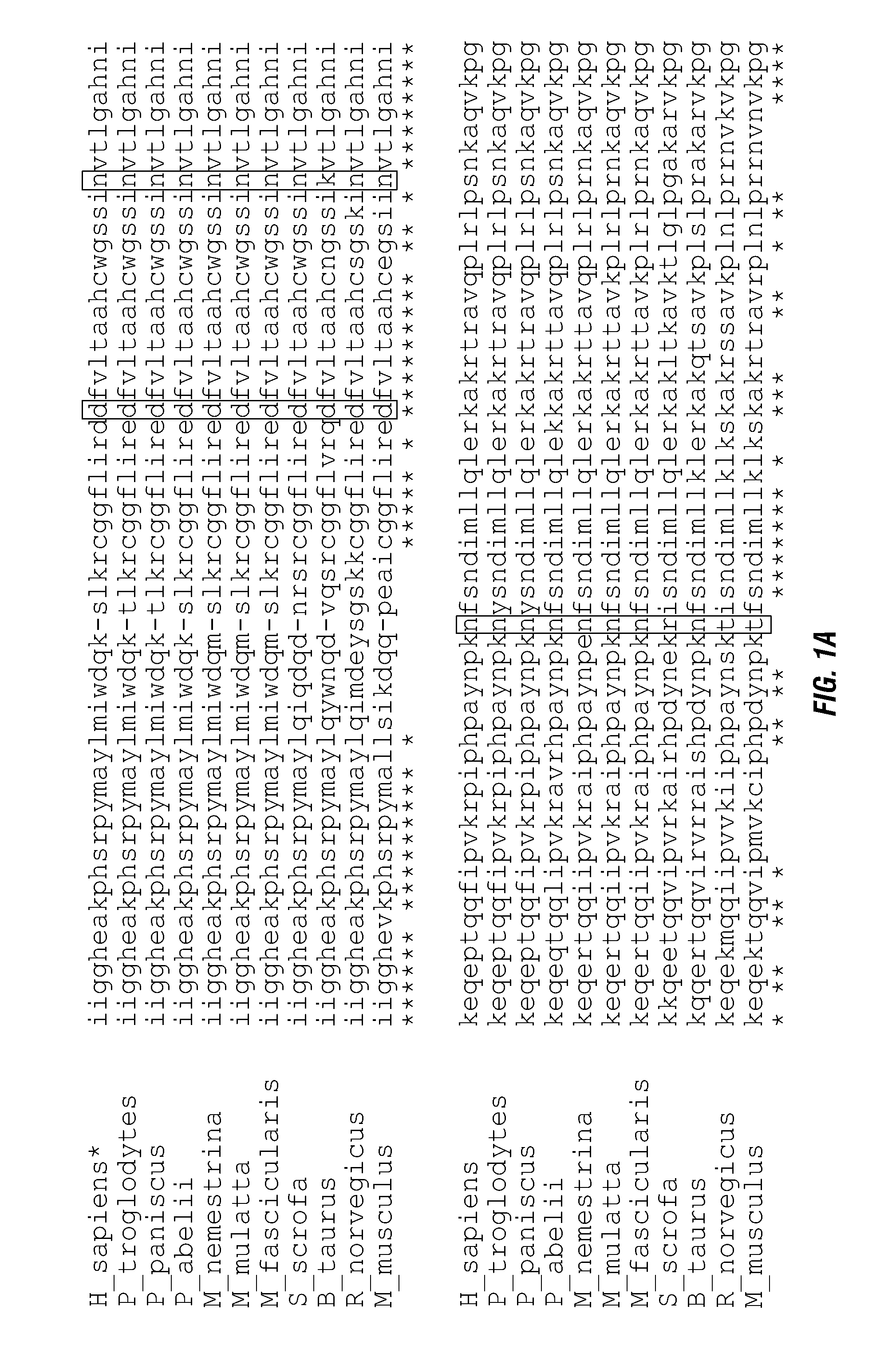
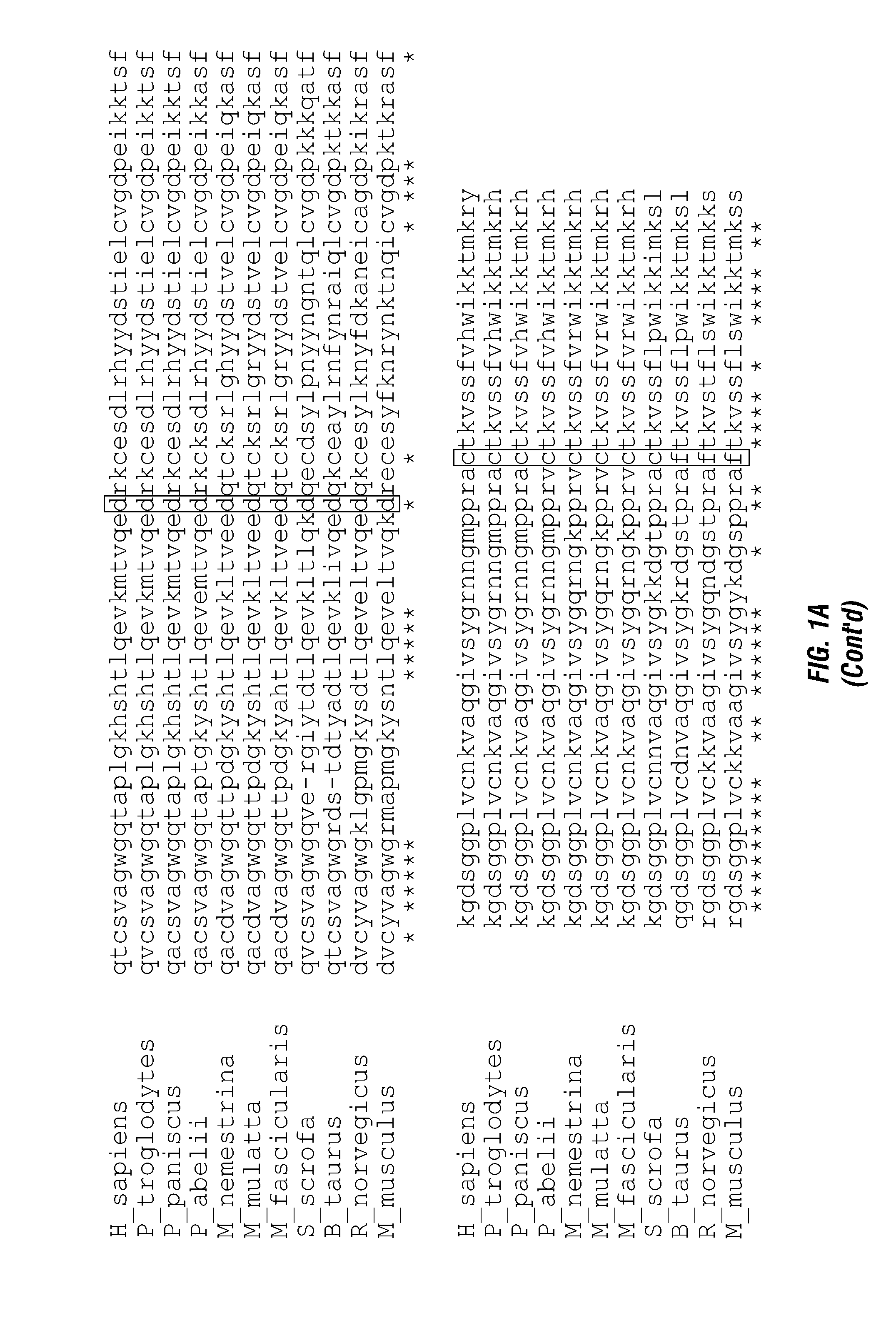
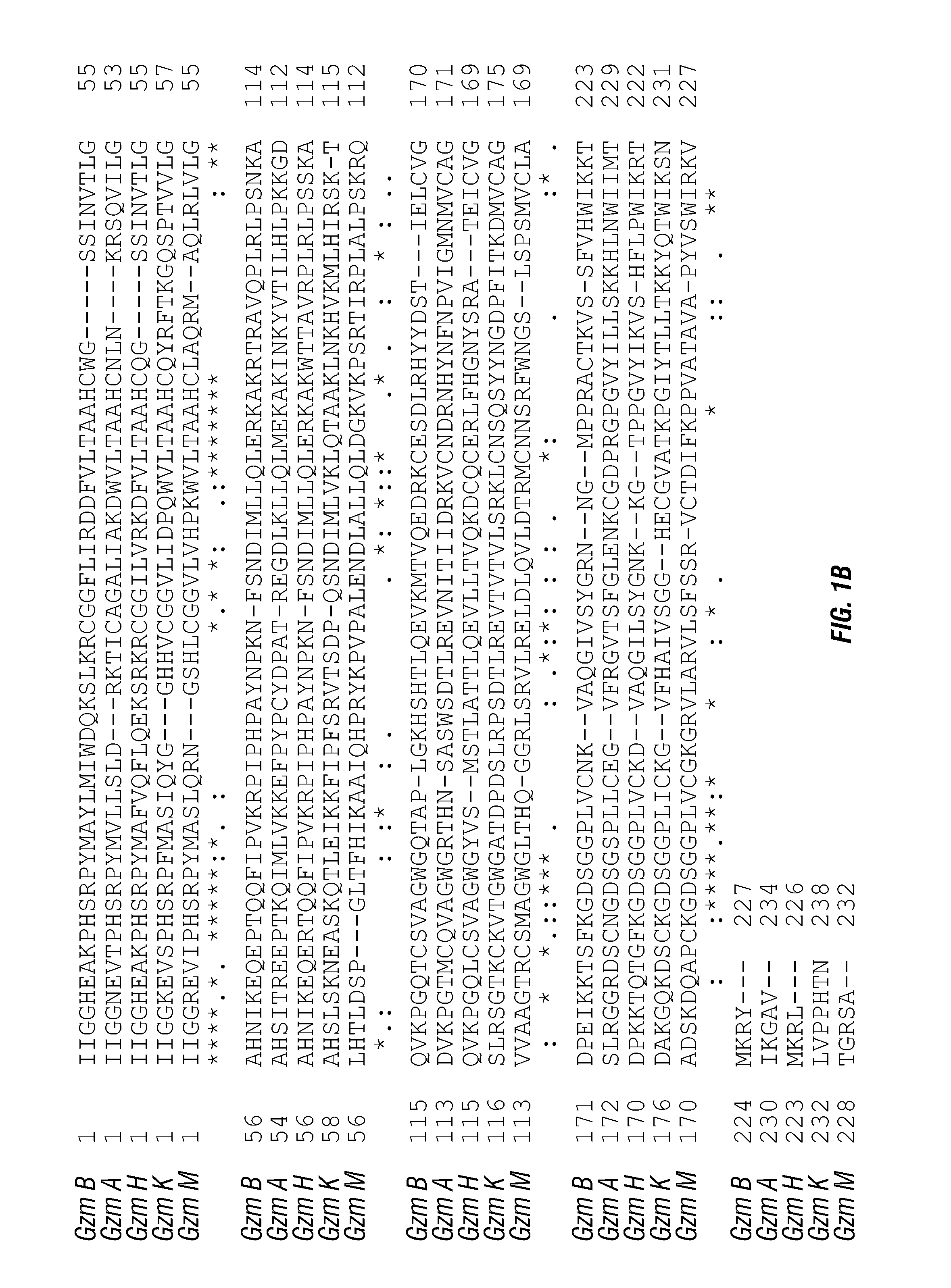
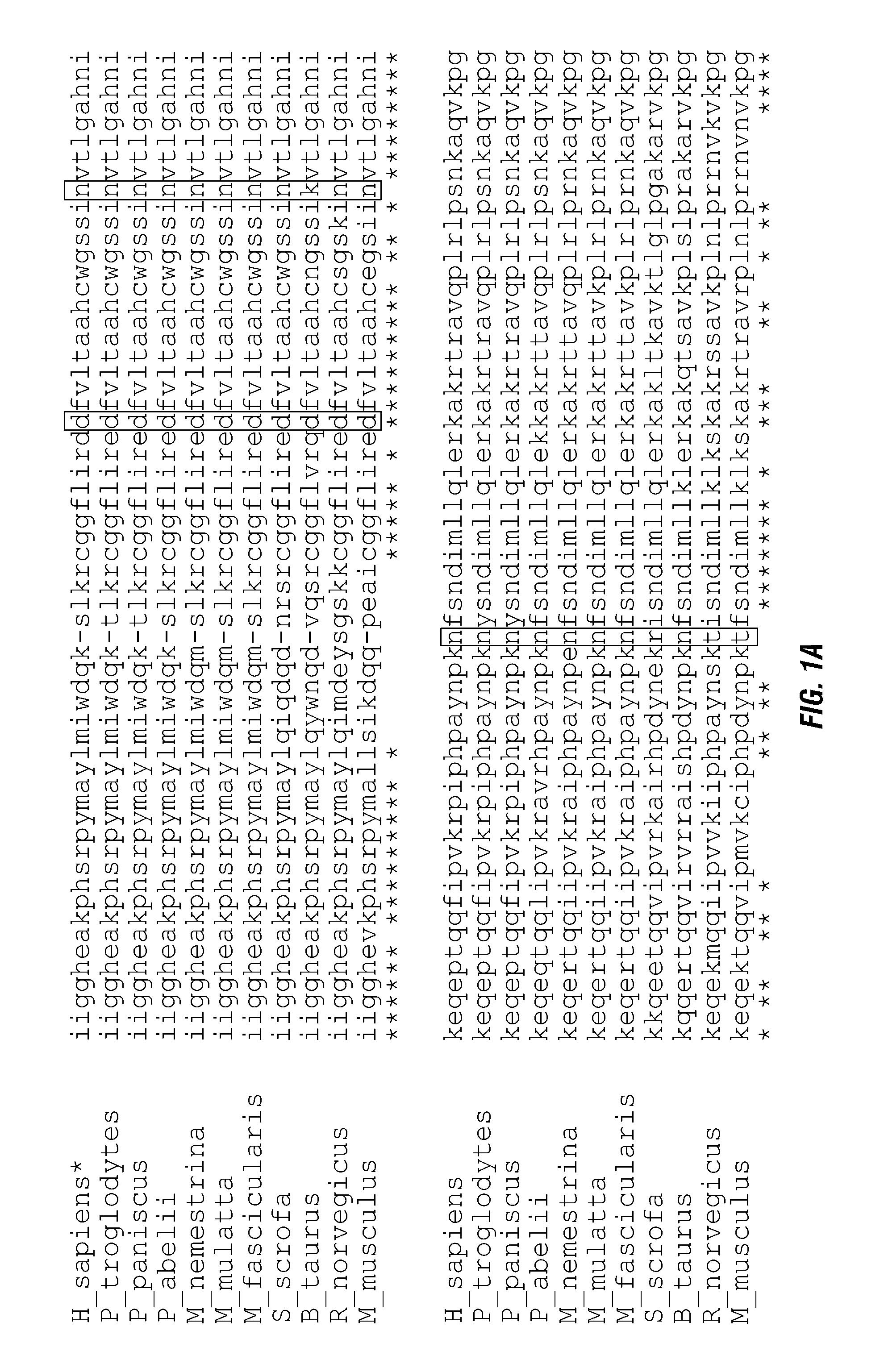
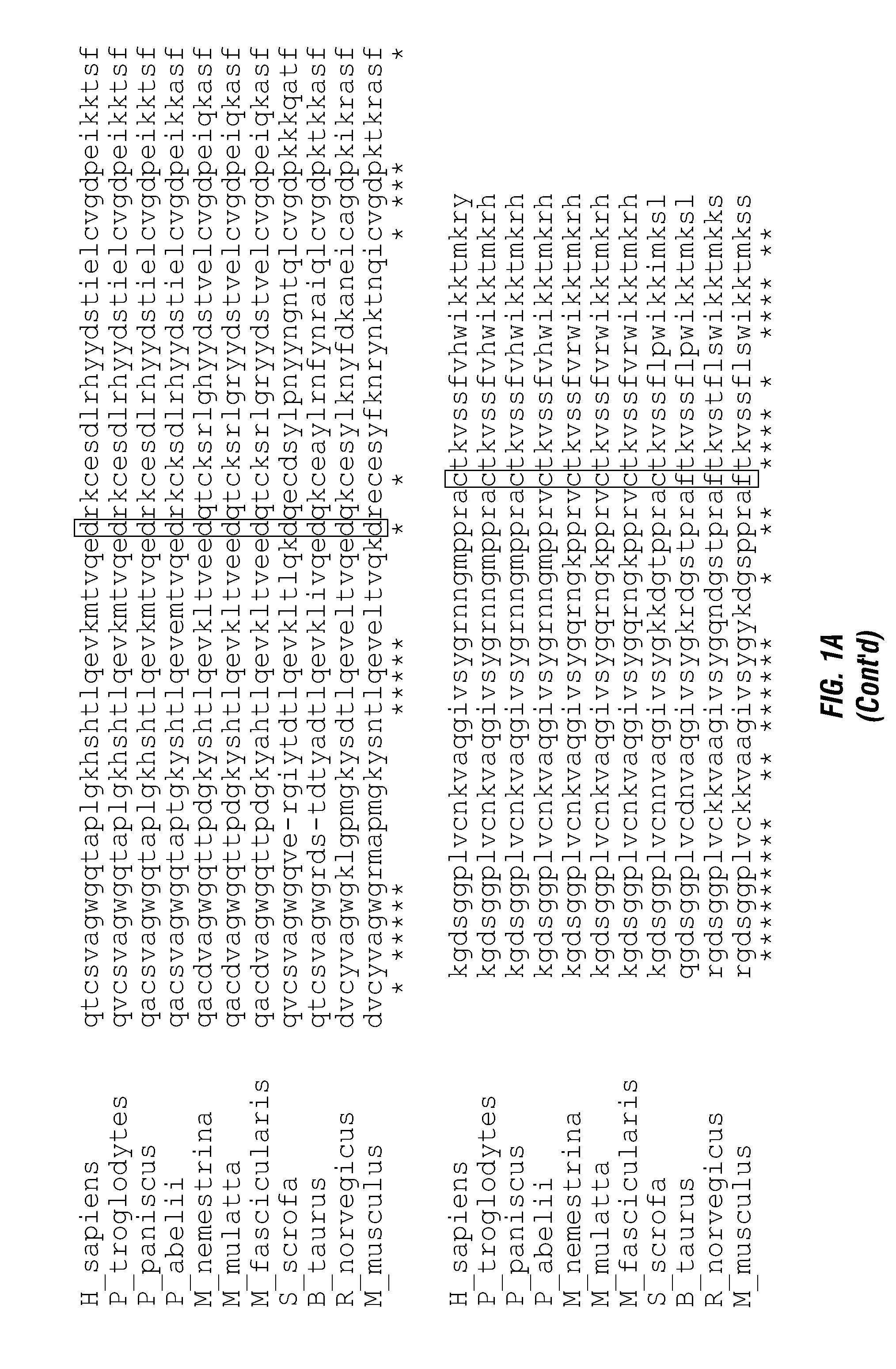
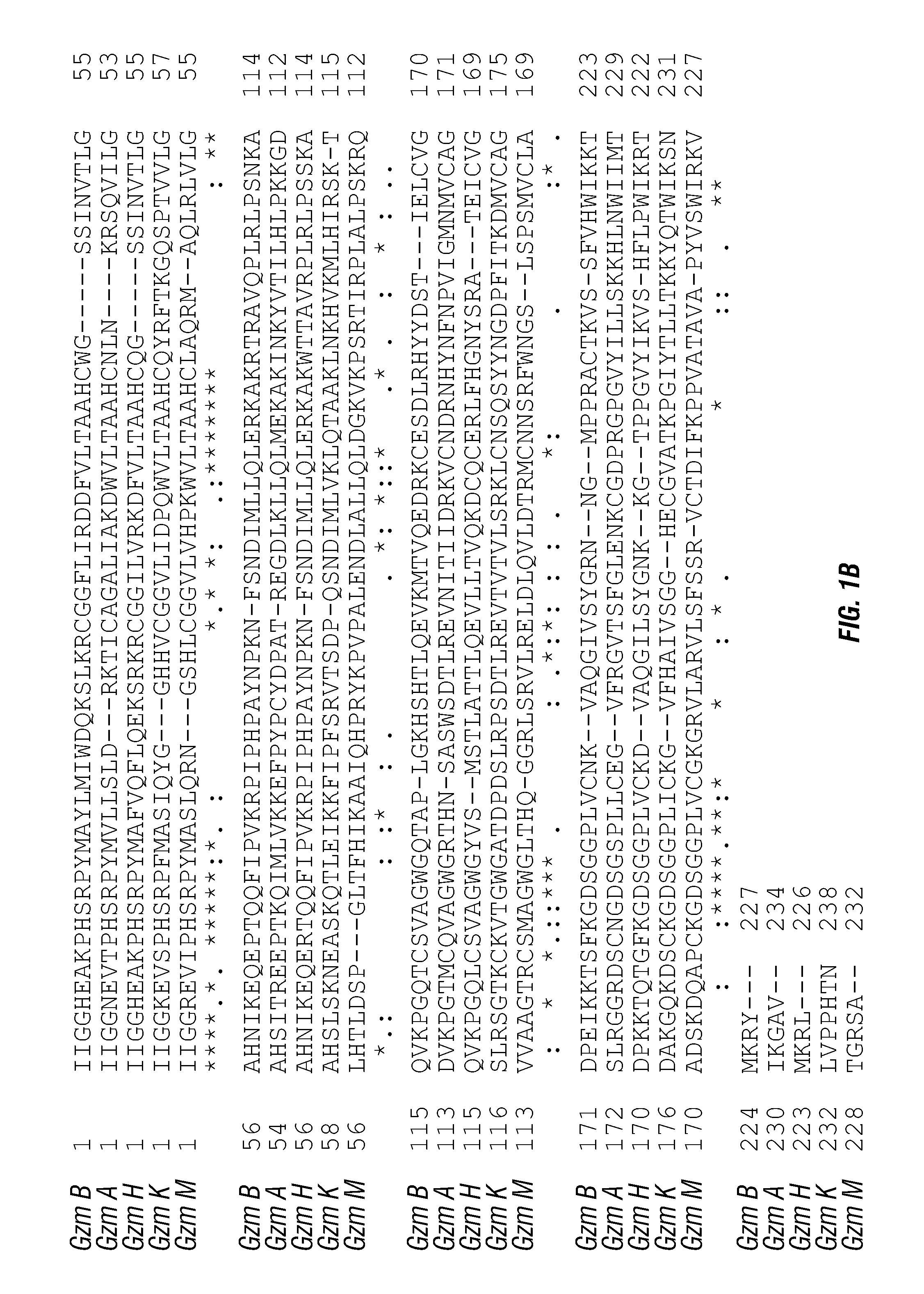
ActiveUS20140140976A1Enhanced intracellular activityReduce distractionsNervous disorderHydrolasesResistant cancerCancer research
Cell-targeted serine protease constructs are provided. Such constructs can be used in methods for targeted cell killing such as for treatment cell of proliferative diseases (e.g., cancer). In some aspects, recombinant serine proteases, such as Granzyme B polypeptides, are provided that exhibit improved stability and cell toxicity. Methods and compositions for treating lapatinib or trastuzumab-resistant cancers are also provided.
Owner:RES DEVMENT FOUND
Process for preparing lapatinib synthetic intermediate
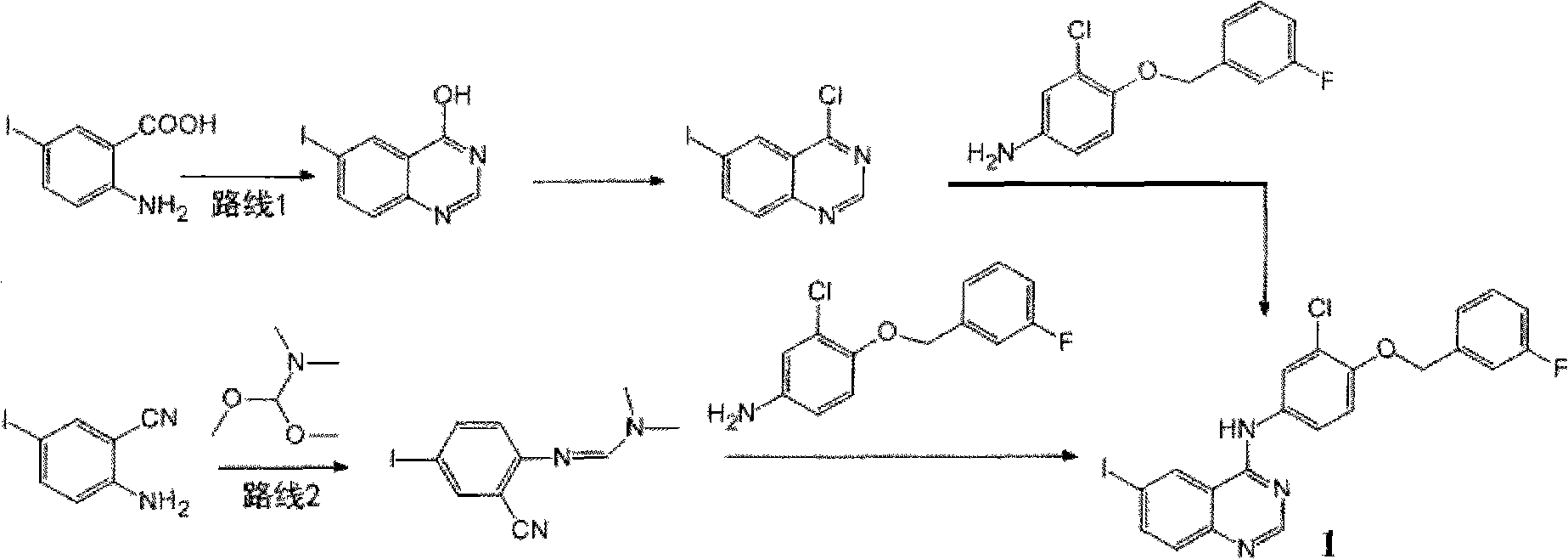
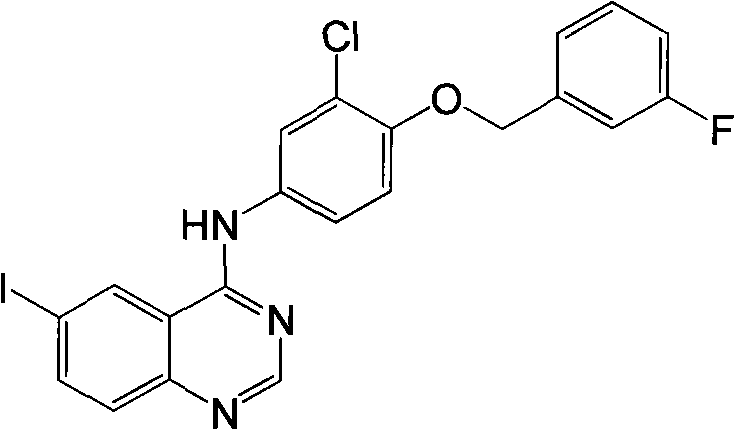
The invention relates to a process for preparing a lapatinib synthetic intermediate, which is characterized by comprising the following process steps: mixing 5-iodo-2-aminobenzonitrile and DMF-DMA; heating and refluxing the mixture at a temperature of between 90 and 100 DEG C for 1 to 2 hours, and performing reduced pressure distillation at a pressure of 0.1 MPa and at a temperature of between 70 and 80 DEG C for 10 to 30 minutes to remove excessive DMF-DMA; adding glacial acetic acid and 3-chloro-4-(3-fluorophenyl-methoxy) aniline; heating and refluxing the mixture at a temperature of between 90 and 100 DEG C for 1 hour, cooling the mixture to the room temperature, and pouring the mixture to ice water; performing vacuum filtration, and washing a filter cake with the ice water and then with methanol; and performing vacuum drying on the filter cake to obtain a faint yellow solid N-(3-chloro-4-(3-fluorophenylmethoxy)phenyl)-6-iodoquinazoline-4-amine. The route avoids the use of thionyl chloride or phosphorus oxychloride and reduces pollutions to the environment; besides, the process has high yield and simple operation and is suitable for industrial production.
Owner:NANJING MEDICAL UNIV
Preparation method of Lapatinib
ActiveCN102675297ASuitable for industrial productionSuitable for industrial scale-up productionOrganic chemistryFuranSuzuki reaction
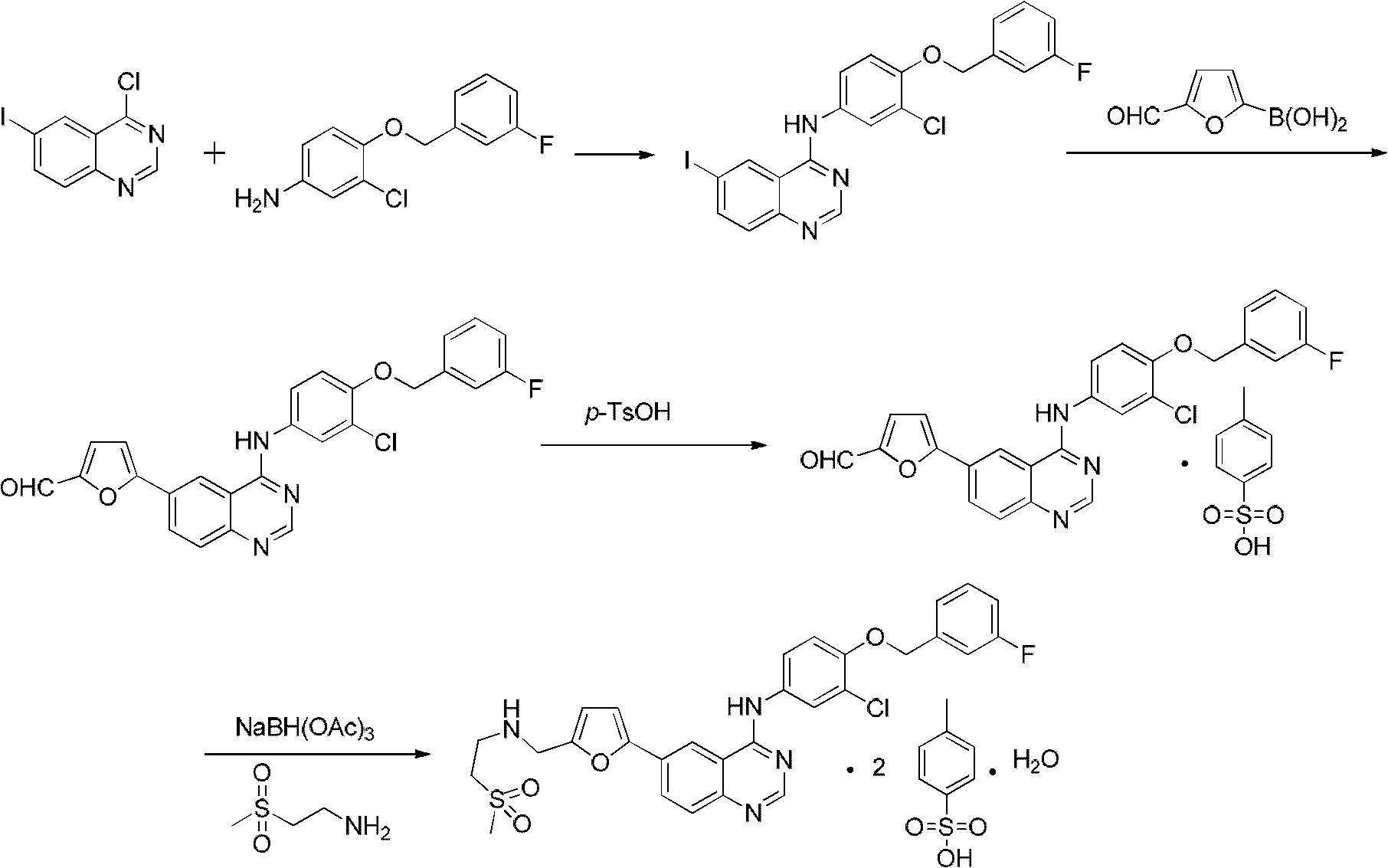
The invention relates to a preparation method of Lapatinib. The method comprises the steps of carrying out condensation, cyclization, reduction, addition, twice Suzuki reaction, deprotection, addition, condensation, Boc protection removal and salification reaction on 2-amino-5-bromobenzylcyanide and 5-bromo furan-2-carboxylic acid methyl ester as starting raw materials to obtain Lapatinib. The method is easily available in starting raw materials, simple in process operation and mild in reaction conditions and does not need specific reaction equipment; in the preparation process, no compounds difficult to separate exist, and the obtained intermediates and finished product can be obtained by recrystallization; and the method is suitable for industrialized amplification production.
Owner:HUBEI BIO PHARMA IND TECHCAL INST
Method of treatment of EGFR inhibitor toxicity
InactiveUS20110190244A1Reduce severityGood curative effectBiocideSenses disorderErlotinibTyrosine-kinase inhibitor
The invention provides a method of treating and / or preventing a toxicity associated with epidermal growth factor receptor (EGFR) inhibitor therapy in a subject, the method comprising administering to the subject an effective amount of a steroid sulfatase (STS) inhibitor. The toxicity may be ocular toxicity; or dermatologic toxicity, such as papulopustular rash. The EGFR inhibitor may be selected from the group consisting of: a small molecule; an antibody or derivative or fragment thereof; another agent that targets the extracellular or intracellular domain of the EGFR, such as a tyrosine kinase inhibitor selected from the group consisting of: erlotinib; gefitinib; lapatinib; and any combination thereof. The EGFR inhibitor may also be antibody selected from the group consisting of: cetuximab; panitumumab; and any combination thereof.Preferably the STS inhibitor is selected from the group consisting of: alternative STS substrates; reversible STS inhibitors; and irreversible STS inhibitors; and any combination thereof. A preferred STS inhibitor is the irreversible nonsteroidal STS inhibitor STX64.In some embodiments, the subject receiving EGFRI therapy has a cancer comprising cells that express wildtype k-ras and / or wildtype b-raf. In other embodiments, the cancer may be hormone-dependent. Cancers that may be treated with EGFRI therapy include colorectal cancer and non-small cell lung cancer.
Owner:PETER MACCALLUM CANCER INST
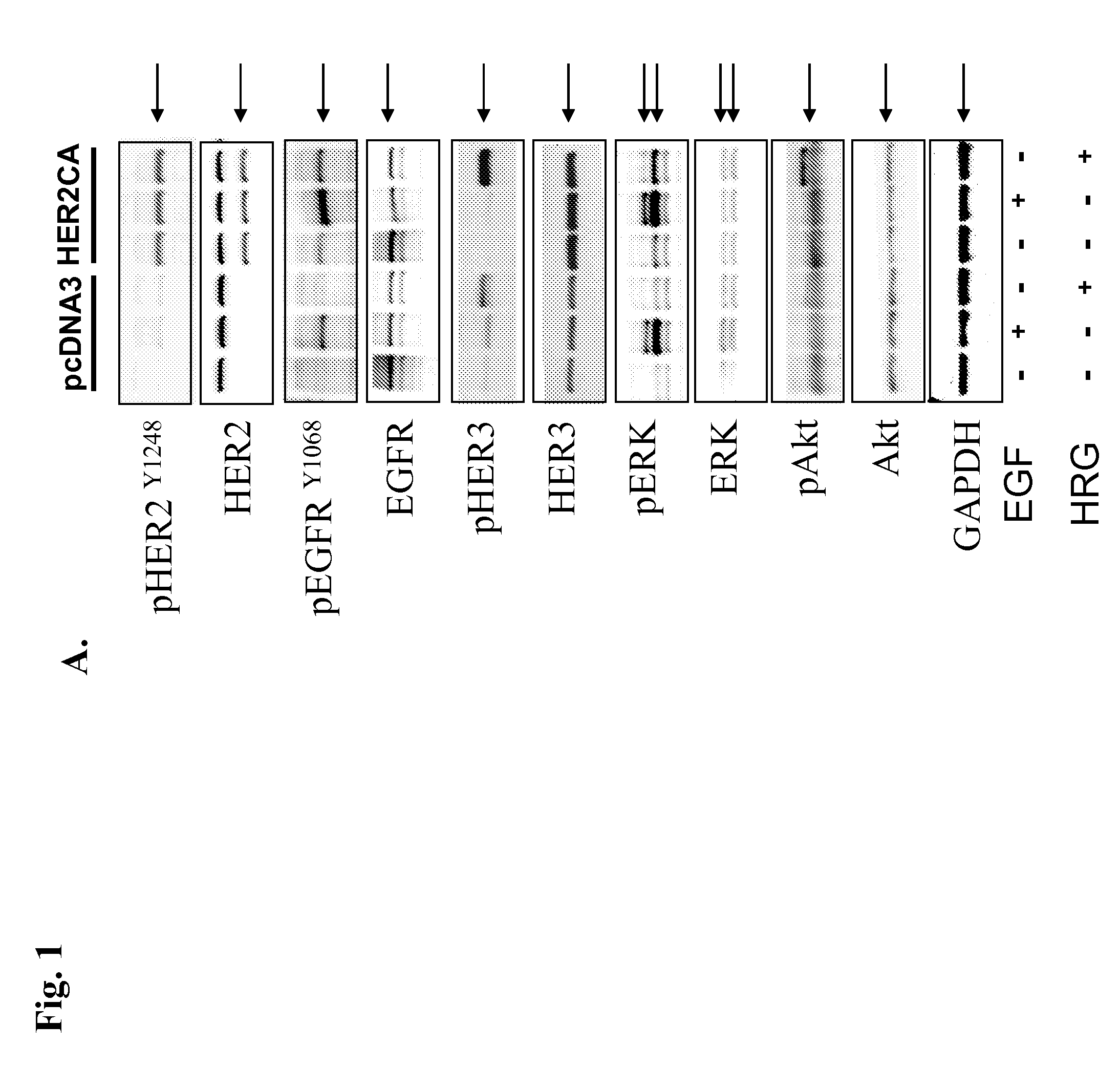
Detection of elevated levels of her-2/neu protein from non-isolated circulating cancer cells and treatment
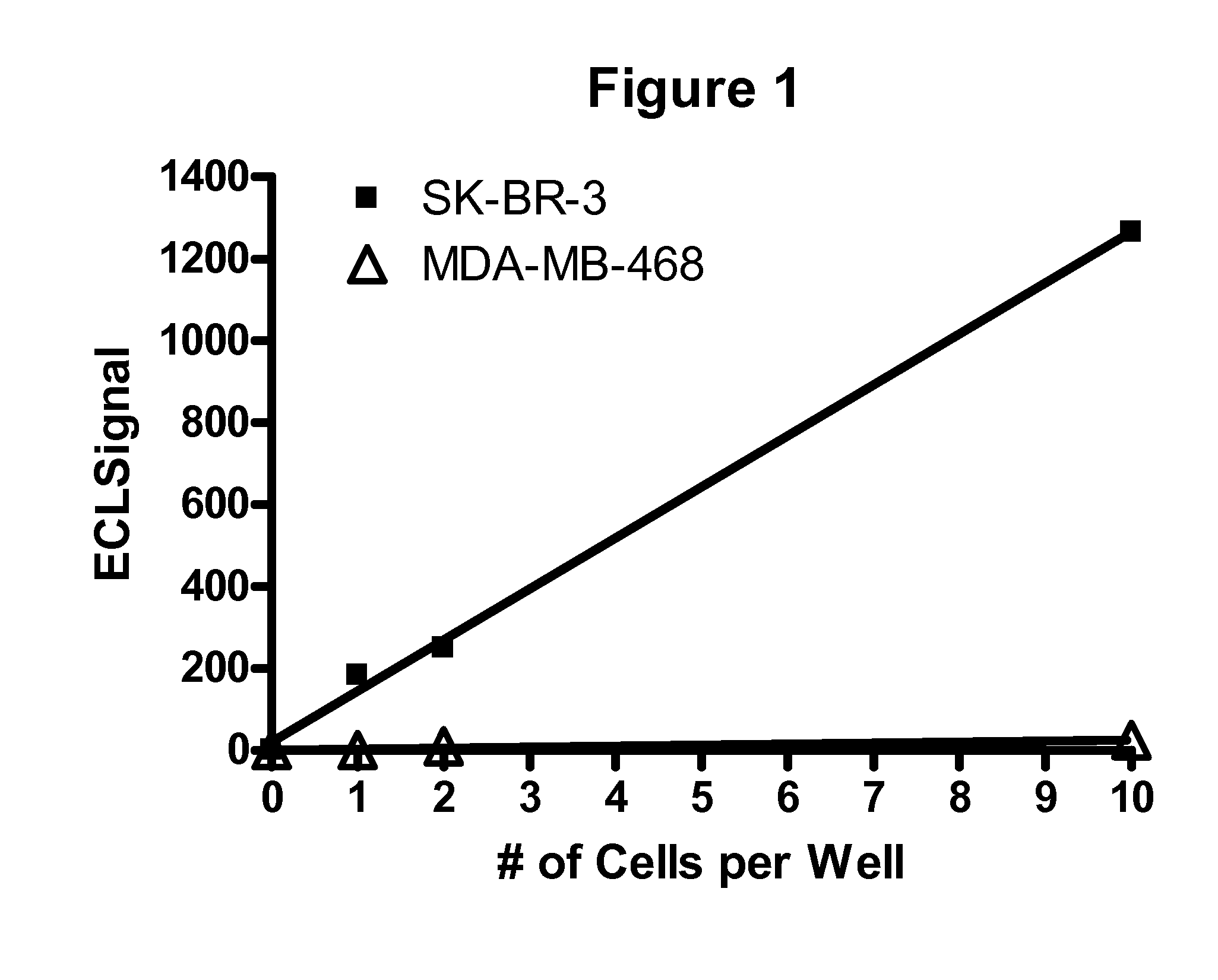
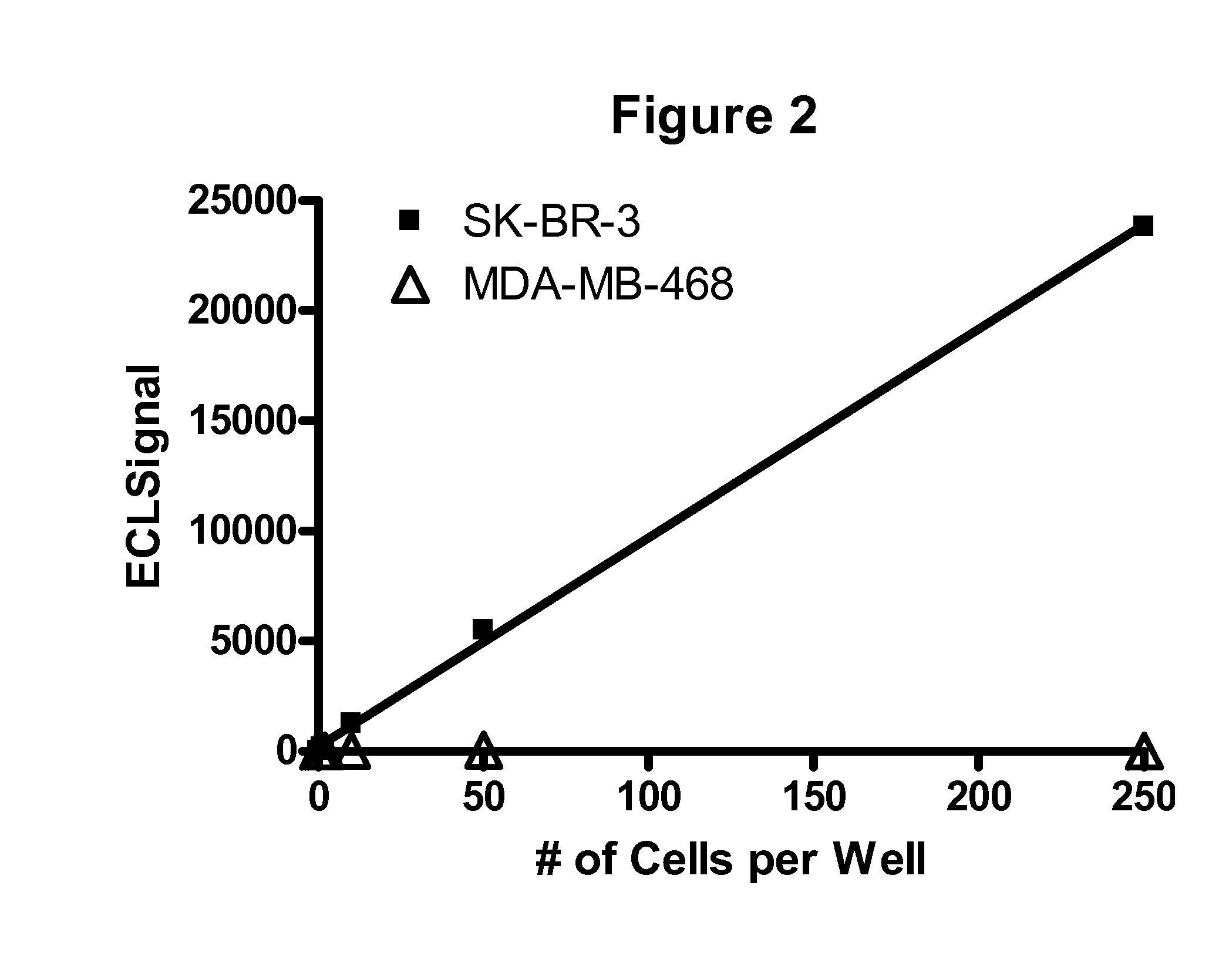
InactiveUS20100120072A1Delay in testingRapidity of assayDisease diagnosisAntineoplastic agentsAnticarcinogenCirculating cancer cell
The expression of Her-2 / neu protein on circulating cancer cells in a sample of blood or peripheral blood mononuclear cells (PBMCs) is detected by performing a sensitive Her-2 / neu immunoassay. There is no need to isolate the cancer cells before performing the immunoassay. A positive result indicates the expression of Her-2 / neu on cancer cells in the blood sample. This method can be used to identify cancer patients who are likely to benefit from treatment with an anticancer agent that targets Her-2 / neu, such as trastuzumab (HERCEPTIN), lapatinib, CP-724,714, NKI-272, and BMS-599626
Owner:WELLSTAT BIOLOGICS CORP
Salicyloyl anilines compound as well as preparation method and application thereof
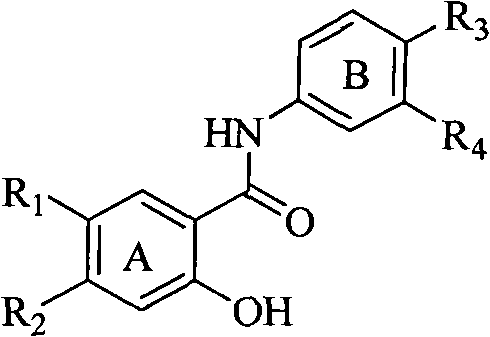
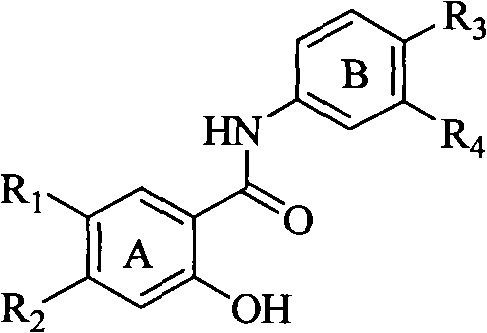
InactiveCN102249945AEasy to makeStrong inhibitory activityOrganic active ingredientsOrganic compound preparationIc50 valuesSide chain
The invention belongs to the field of medical compounds and relates to a salicyloyl anilines compound as well as a preparation method and application in preparing an anti-cancer medicine. According to the method disclosed by the invention, the salicyloyl aniline is taken as a parent and different side-chain substituent groups are introduced into a benzene ring of the parent to prepare the salicyloyl anilines compound of the following formulas, wherein in the formulas, R1, R2, R3 and R4 are defined as an specification. Compared with the Lapatinib, the prepared salicyloyl anilines compound represents that the IC50 value is obviously reduced or is at least equal to that of the Lapatinib. The experiment shows that the salicyloyl anilines compound provided by the invention has an obvious inhibition effect on tumor cells, especially ovarian cancer cells so that the salicyloyl anilines compound can be further used for preparing antitumor medicines. The chemical formula of the salicyloyl anilines compound is represented by the following formulas.
Owner:FUDAN UNIV
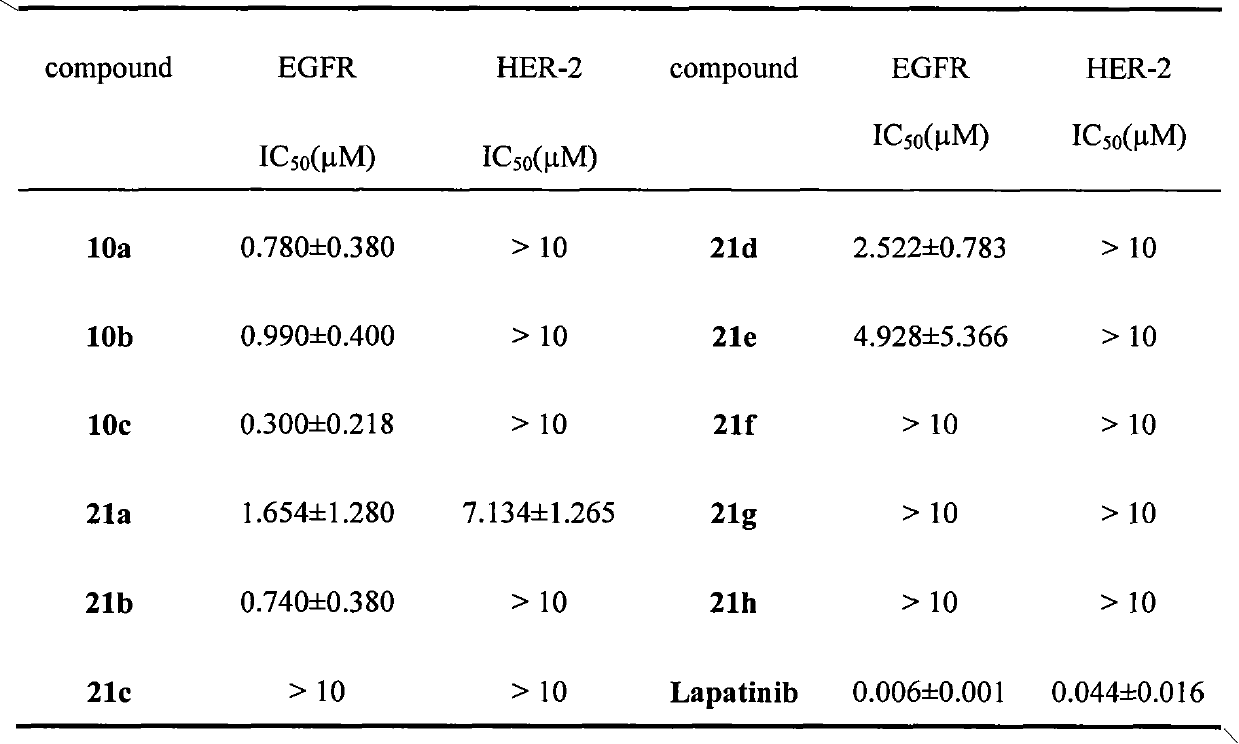
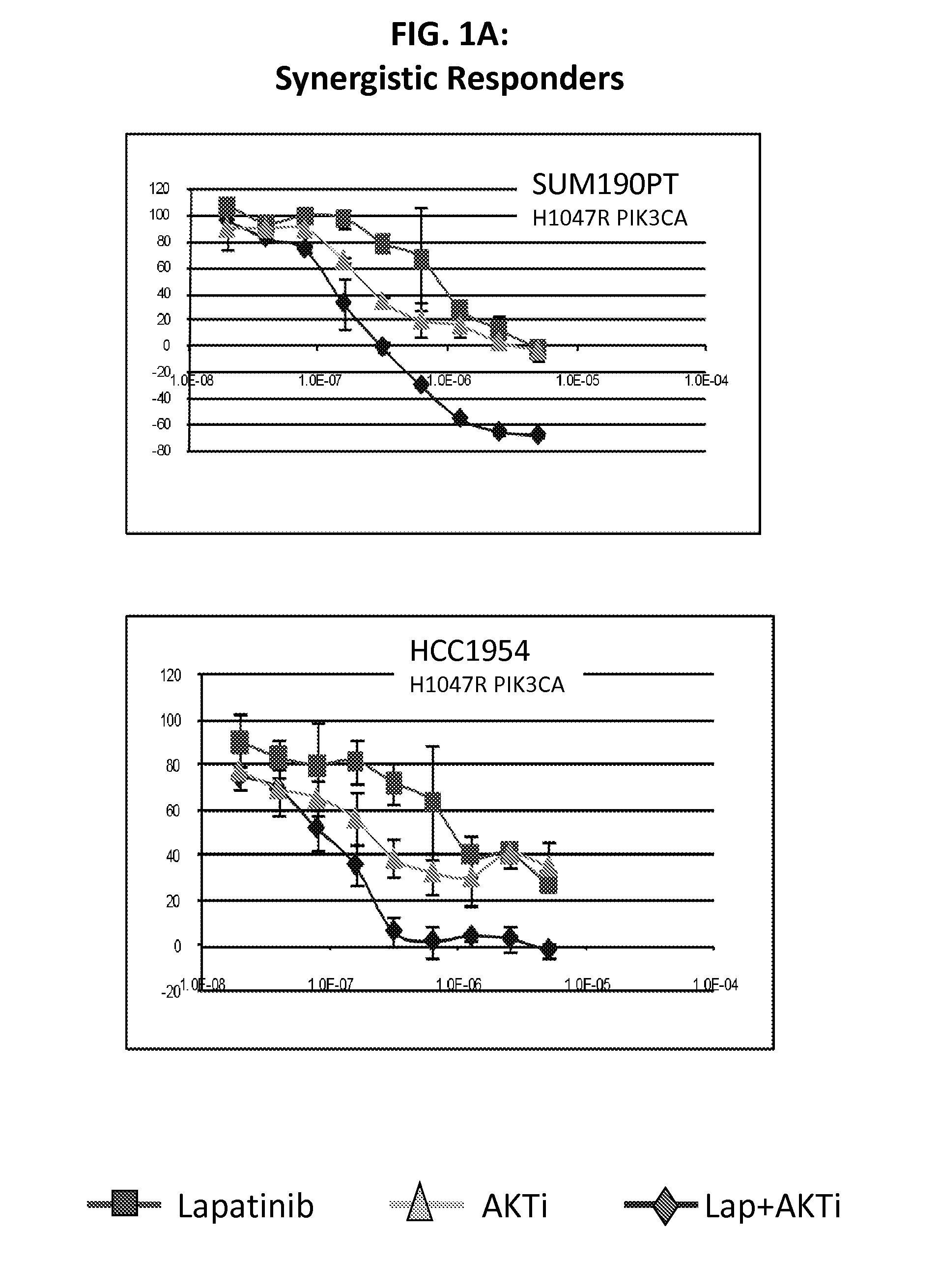
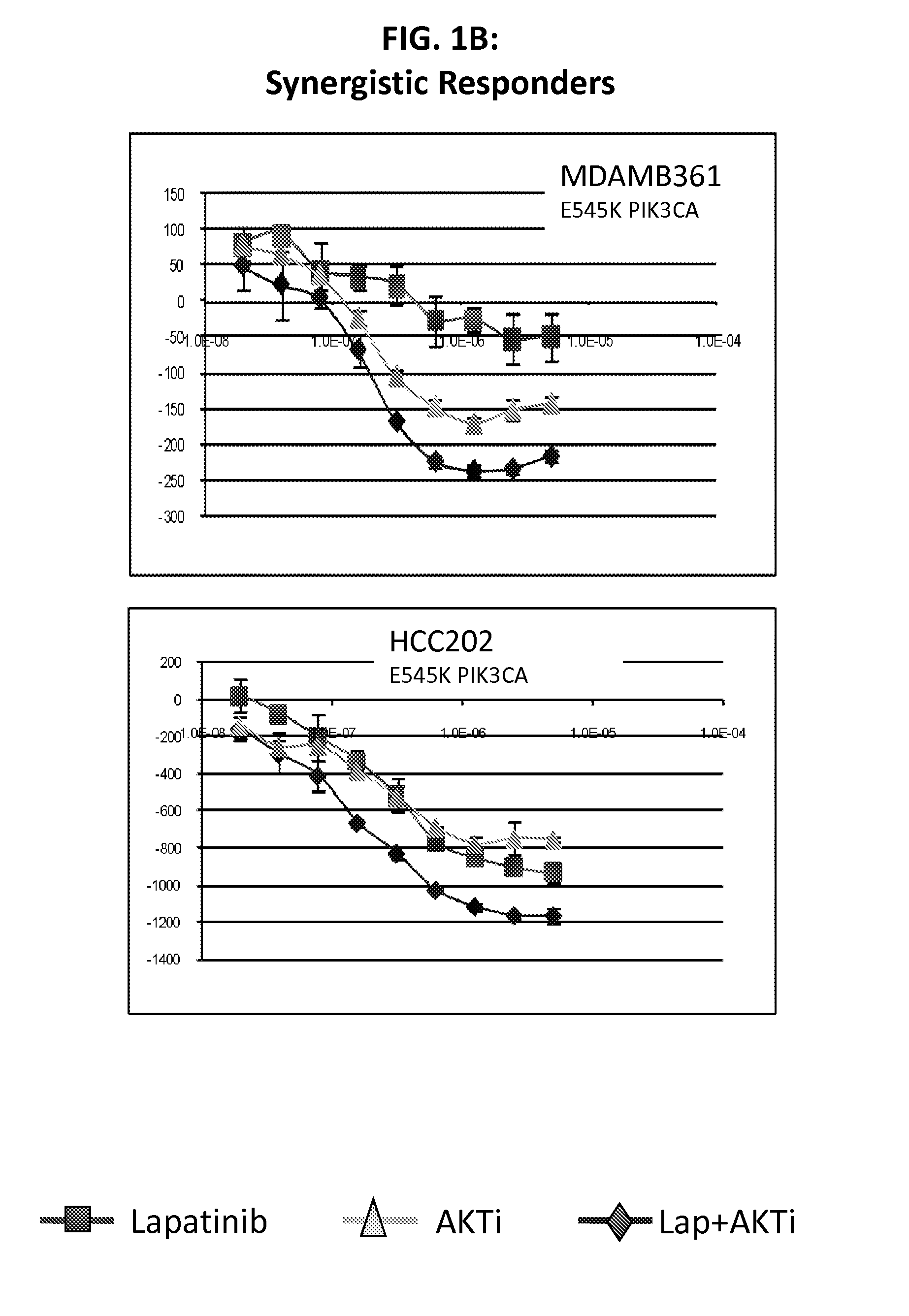
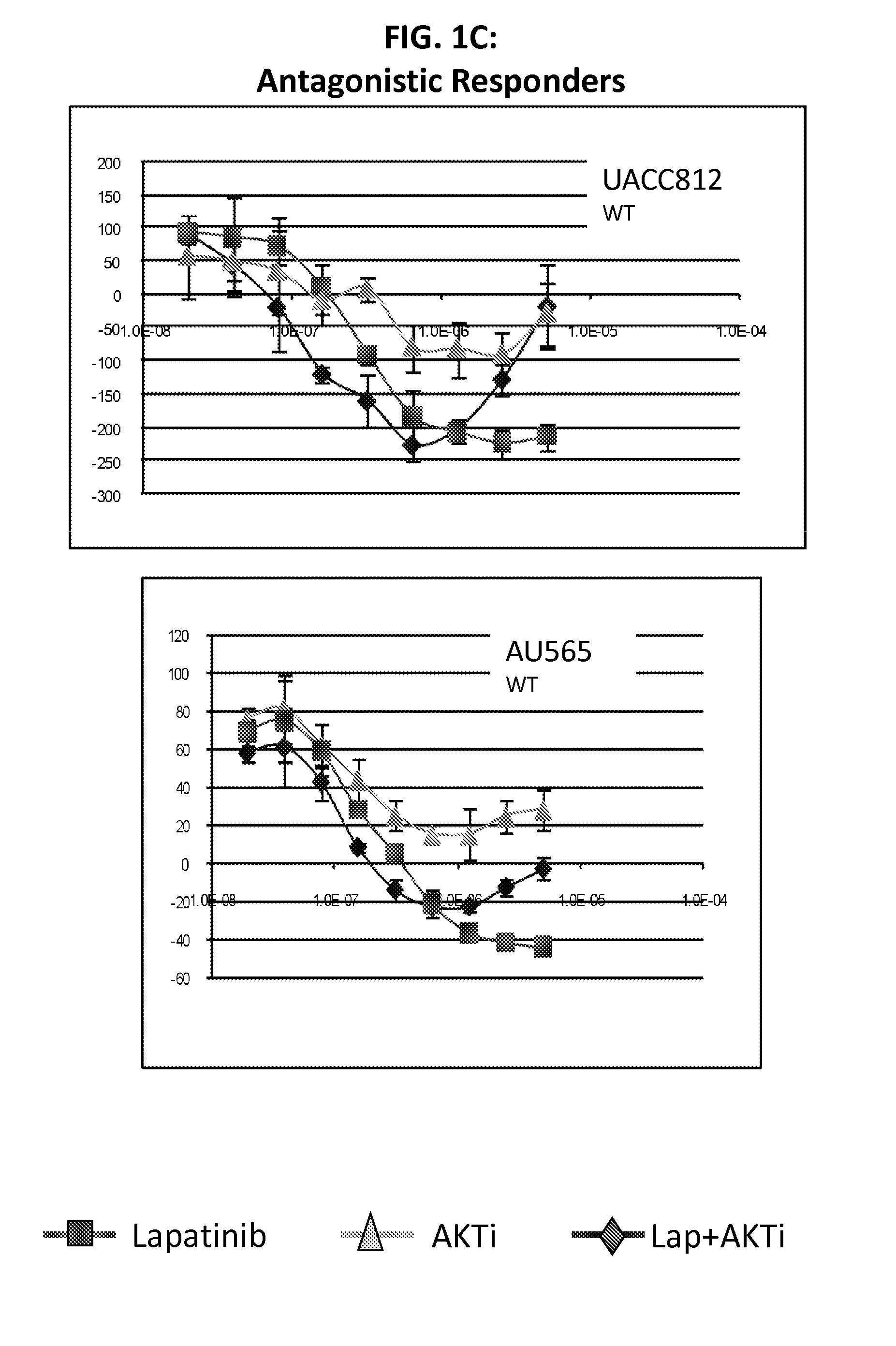
PIK3CA Mutation Status and SASH1 Expression Predicts Synergy Between Lapatinib and an AKT Inhibitor in HER2 Positive Breast Cancer
InactiveUS20130331405A1Improve expression levelOrganic active ingredientsBiocideProtein proteinHER2 Positive Breast Cancer
Methods for identifying a cancer patient, such as a breast cancer patient, suitable for treatment with a 4-anilinoquinazoline kinase inhibitor, such as lapatinib, and an AKT inhibitor, comprising detecting modulated expression of HER2 (ERBB2) and SASH1 or protein encoded thereof and detecting PIK3CA mutation status. High levels of expression in HER2 and high levels of SASH1 and / or positive PIK3CA mutation status indicate a patient that is suitable for treatment with a 4-anilinoquinazoline kinase inhibitor, such as lapatinib and an AKT inhibitor.
Owner:RGT UNIV OF CALIFORNIA
Anticancer composition containing Sirolimus
InactiveCN1973822AGrowth inhibitionInhibitionOrganic active ingredientsPeptide/protein ingredientsErlotinibMicrosphere
The anticancer composition containing sirolimus is one kind of slow releasing injection comprising slow released microsphere and special solvent containing suspending agent or slow releasing implant. The effective anticancer component is sirolimus, proteolytic enzyme selected from collagenase, hyaluornidase, etc and blood vessel inhibitor selected from gefitinib, thalidomide, etc. The slow releasing supplementary material is selected from polylactic acid, polyethylene glycol, polifeprosan, etc; the suspending agent is selected from sodium carboxymethyl cellulose, etc and has viscosity of 100cp-3000cp. The slow releasing injection and the slow releasing implant are used in treating tumor alone or in conjunction with chemotherapy.
Owner:SHANDONG LANJIN PHARMA +1
Serine protease molecules and therapies
Cell-targeted serine protease constructs are provided. Such constructs can be used in methods for targeted cell killing such as for treatment cell of proliferative diseases (e.g., cancer). In some aspects, recombinant serine proteases, such as Granzyme B polypeptides, are provided that exhibit improved stability and cell toxicity. Methods and compositions for treating lapatinib or trastuzumab-resistant cancers are also provided.
Owner:RES DEVMENT FOUND
Use of tyrosine kinase inhibitors for treatment of prolactinoma
InactiveUS20120308567A1Inhibiting and reducing prolactinomaPromoting prolactinoma prophylaxisOrganic active ingredientsBiocideTyrosine-kinase inhibitorProlactinoma
Owner:CEDARS SINAI MEDICAL CENT
New preparation method of lapatinib
ActiveCN103483324AEfficient manufacturingHigh purityOrganic chemistryN dimethylformamideDimethyl acetal
The present invention provides a preparation method of lapatinib. The method comprises contacting a compound shown as a formula 1 with a compound shown as a formula 2 to produce a compound shown as a formula 3; reducing the compound shown as the formula 3 to produce a compound shown as a formula 4; contacting a compound shown as a formula 5 with N,N-dimethylformamide dimethyl acetal to produce a compound shown as a formula 6; contacting the compound shown as the formula 6 with the compound shown as the formula 4 to produce a compound shown as a formula 7; in the presence of an acid, an alkali and NaNH(OAc)3, contacting a compound shown as a formula 8 with a compound shown as a formula 9 to produce a compound shown as a formula 10; in the presence of a catalyst and an alkali, contacting the compound shown as the formula 10 with a compound shown as a formula 11 to produce a transition intermediate, and contacting the transition intermediate with the compound shown as the formula 7 and p-toluenesulfonic acid to produce a compound shown as a formula I; through use of the method, the lapatinib can be effectively prepared.
Owner:HUBEI BIO PHARMA IND TECHCAL INST
Process and intermediates for preparing lapatinib
ActiveCN102812019AEnsure safetyReduce usageOrganic active ingredientsOrganic chemistry methodsMedicineBiology
Owner:SCINOPHARM TAIWAN LTD
Synthetic method of lapatinib and salt of lapatinib
ActiveCN102532109AAvoid makingReduce usageCarboxylic acid nitrile preparationOrganic compound preparationOxygenMethyl group
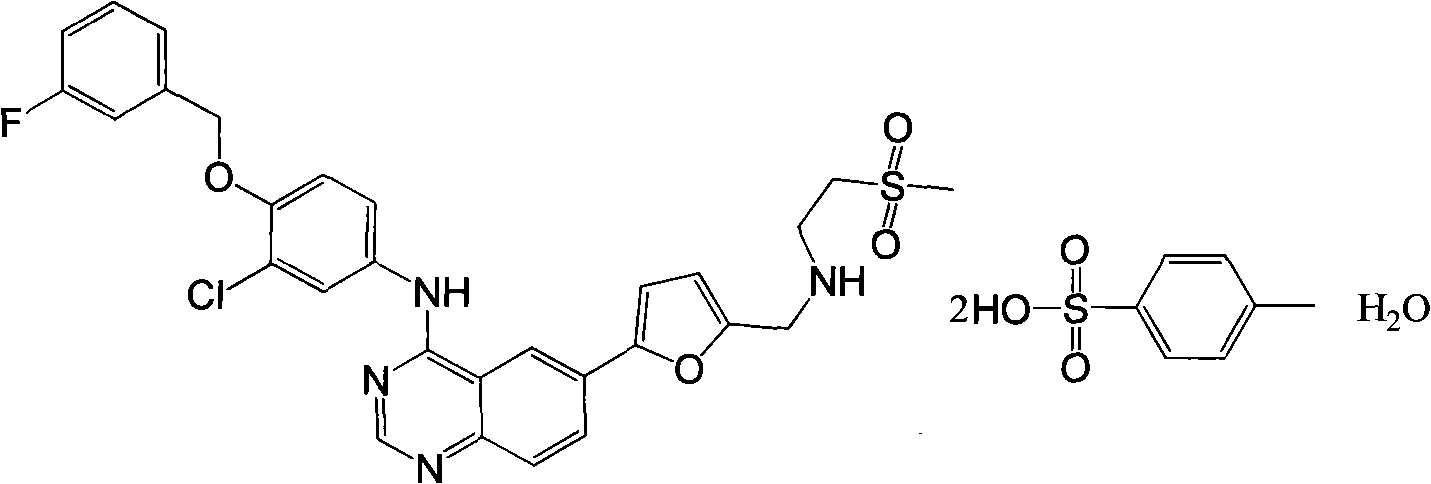
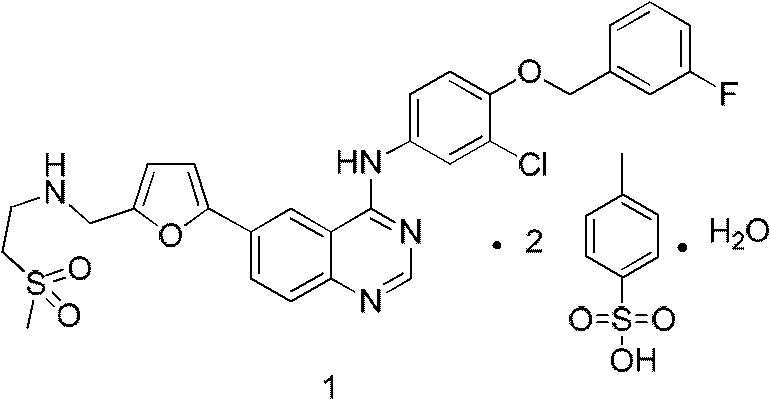
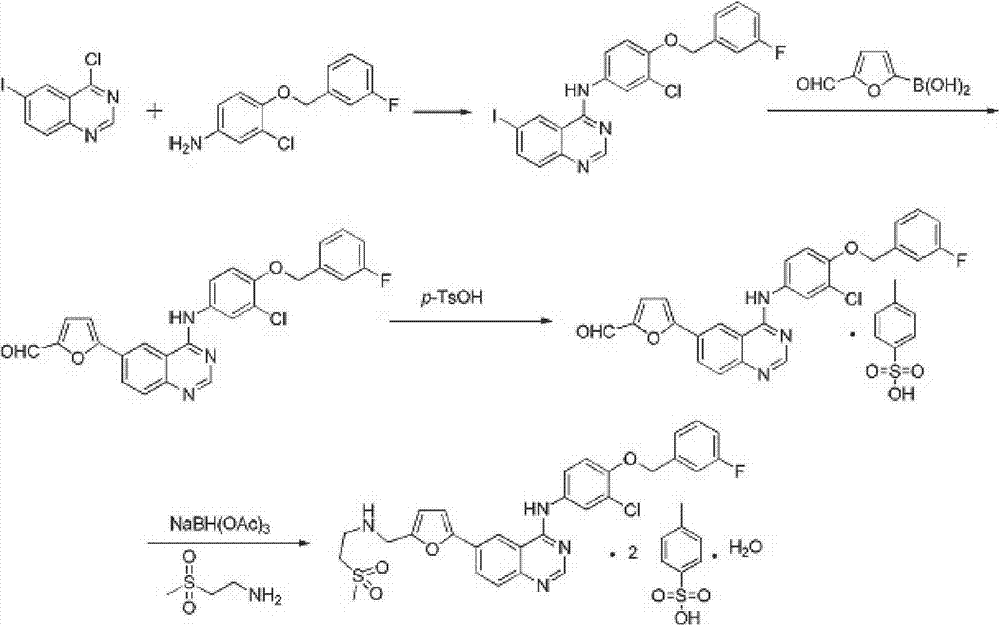
Provided is a novel preparation method of lapatinib and two pairs of tosylate monohydrate and compounding of relevant midbody N-[3-chlorine-4[(3-fluorine benzyl group) oxygen] phenyl group]-6-halogen-quinazoline-4-amine and 5-[(2'-methylsulfonyl ethyl amino) methyl-2-furanboric acid. The synthetic method comprises reaction steps of amidining, condensation, reduction amination, suzuki coupling, salify purification and the like. The method avoids preparation of an unstable midbody and using of chlorinated reagent with large pollution and danger, and has the advantages of being high in product purity, simple in operation, less in liquid and gas waste generated by reaction, convenient in aftertreatment and the like.
Owner:ZHEJIANG HISUN PHARMA CO LTD
Anti-cancer sustained-released agent
InactiveCN101396341AInhibition formationSpeed up entryPeptide/protein ingredientsSolution deliveryDepressantSuspending Agents
The invention relates to an anti-cancer sustained release injection, consisting of sustained release microspheres and menstruum, wherein, the sustained release microspheres comprise sustained release auxiliary material, angiogenesis inhibitor and / or proteolytic enzyme; and the menstruum contains suspending agent. The angiogenesis inhibitor is selected from gefitinib, erlotinib, lapatinib, vatalanib, pelitinib, endostatin, imatinib, semaxanib, dasatinib, avastin, sorafenib, sunitinib, telcyta or panitumumab; the proteolytic enzyme is selected from one or the combination of collagenase, hyaluronidase, relaxin and plasmase; the sustained release auxiliary material is selected from polifeprosan, decanedioic acid copolymer, EVAc, polylactic acid, the mixture or the copolymer thereof, and the like; and the suspending agent is selected from carboxymethyl cellulose and the like with the viscosity of 100cp to 3000cp (under the temperature of 25 DEG C to 30 DEG C). The injection can also be made into a sustained release implant. The sustained release injection is injected or arranged in or around the tumour and can improve the local drug concentration selectively, reduce the general reaction of the drug, inhibit the growth of tumour cell and blood vessel and enhance the treatment effect of non-operative treatments, such as radiotherapy, chemotherapy, etc.
Owner:SHANDONG LANJIN PHARMA +1
Synthetic method of lapatinib
The invention relates to a synthetic method of lapatinib. 2-furaldehyde diethyl acetal, N-[3-Cl-4-[(3- fluorobenzoic) methoxy] phenyl]-6-I-4-quinazoline amine and the like serve as raw material, are subjected to three steps of reaction to obtain the lapatinib. The method implements one-pot reaction and is easy to operate, the reaction conversion rate reaches up to 70%, and the purity reaches 98%. Involved solvents are small in toxicity, high in recycling rate and few in three wastes. The involved intermediates are good in crude product purity, can be directly used for latter reaction without the need for aftertreatment and purification, and are suitable for industrial magnification production.
Owner:CHANGZHOU HONGCHUANG POLYMER SCI & TECH LTD
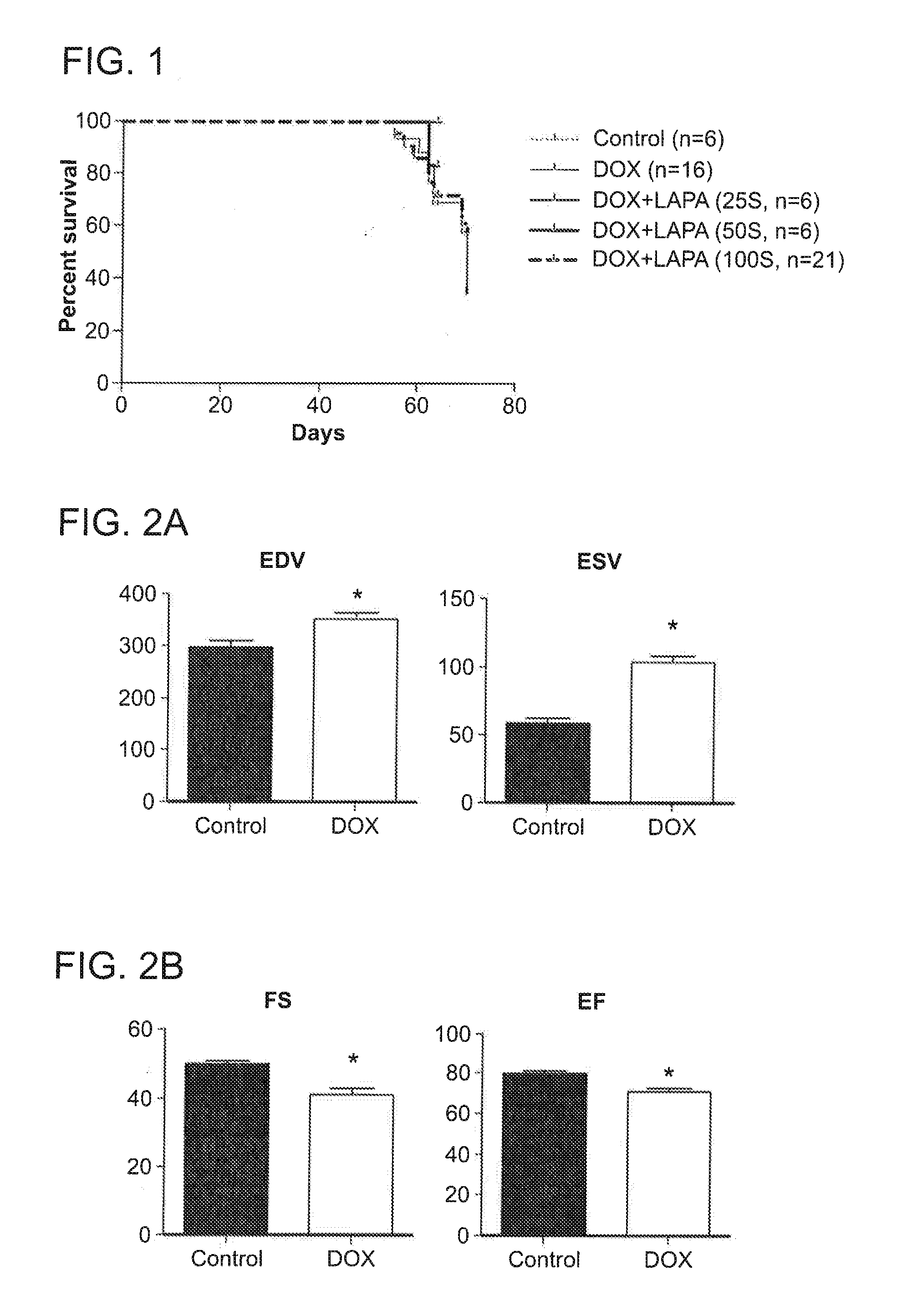
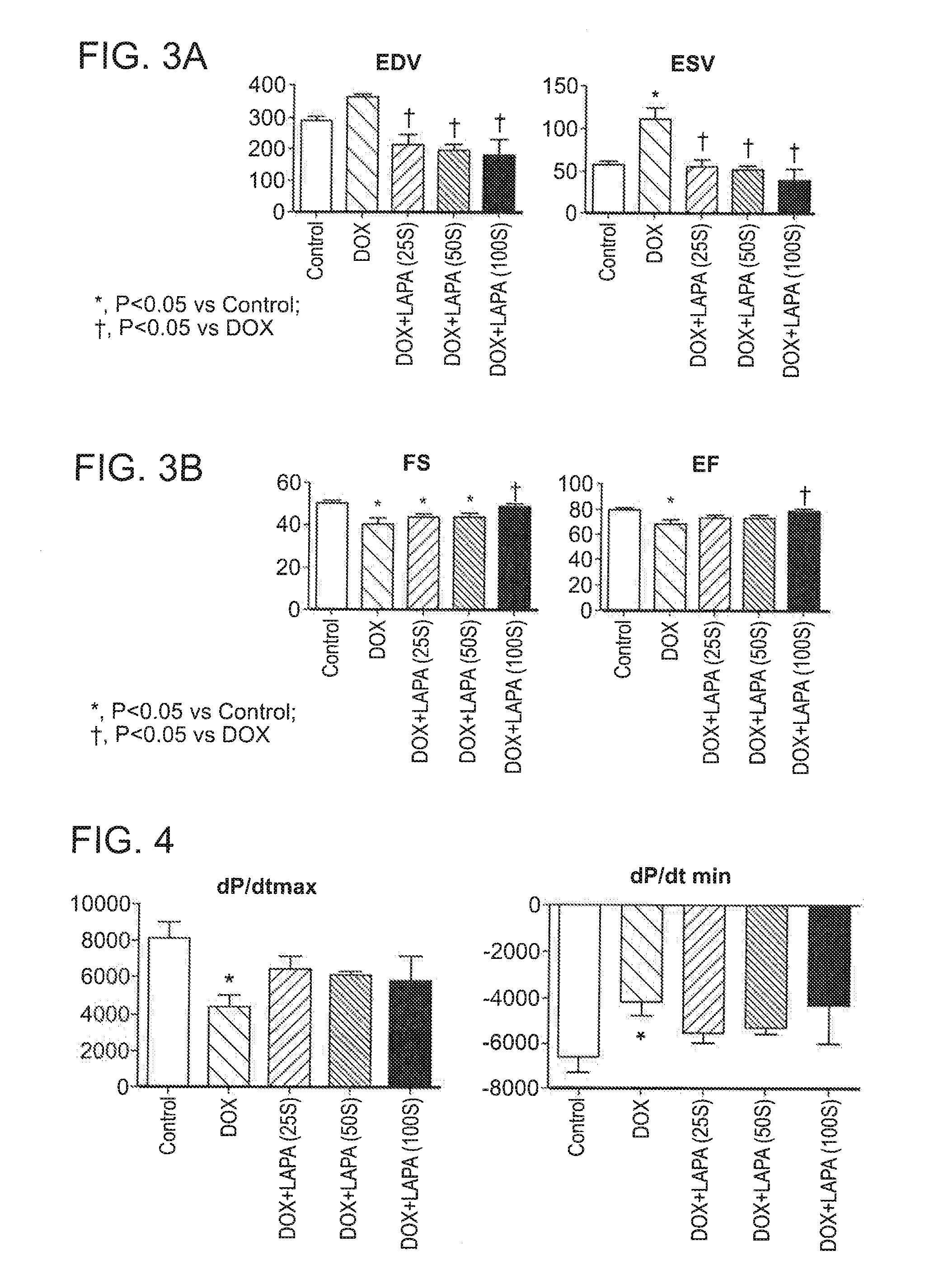
Compositions and methods for treating or preventing anthracycline induced cardiotoxicity
InactiveUS20140057861A1Treating and preventing cardiac toxicityTreat and prevent cardiotoxicityBiocideAntinoxious agentsAnthracycline induced cardiotoxicityHeart disease
The invention features methods and compositions feature lapatinib and / or rapamycin for treating or preventing a cardiac condition induced by anthracycline treatment.
Owner:GENESYS RES INST
Lapatinib injection and preparation method thereof
InactiveCN101444479AConvenient for clinical operationStable for clinical useOrganic active ingredientsPharmaceutical delivery mechanismIntramuscular injectionSolvent
The invention relates to the field of pharmaceutical technology and discloses a lapatinib injection which has convenient and stable clinical use, high bioavailability, and cheap price and can be used for treating solid tumors. The direct intravenous injection or the intramuscular injection can lead a drug to rapidly achieve the effective therapeutic concentration in vivo, reduce the first pass effect of the drug in liver and improve the bioavailability of the drug in vivo. In order to achieve the purposes, the lapatinib injection mainly contains lapatinib injection liquid or medicinal salt thereof and a solvent for injection; the injection liquid per1ml contains 10-500mg of the lapatinib injection liquid or the medicinal salt thereof, 200-300mg is preferential, and the pH of the injection liquid is 3.0-8.0.
Owner:李铁军
Synthetic method for lapatinib and lapatinib intermediates
InactiveCN104513231AReduce usageThe synthesis method is simpleOrganic chemistryOrganic solventDimethyl acetal
The invention provides a synthetic method for lapatinib. The method comprises the following steps: (a) in an organic solvent, reacting a compound (6) with a compound (7) in the presence of alkali to obtain a compound (8); (b) in the organic solvent, reacting the compound (8) with a compound (9) in the presence of alkali, and after the reaction is ended, adding a reducing agent for producing a reductive amination reaction to obtain a compound (10); (c) in the organic solvent, reacting the compound (10) with BOC anhydride in the presence of alkali to obtain a compound (11); (d) in the organic solvent, fully reacting the compound (11) with N,N-dimethylformamide dimethyl acetal under the backflow condition, and after the reaction is ended, adding a compound (4) and reacting under the backflow condition to obtain a compound (12); (e) in the organic solvent, producing a deprotection reaction of the compound (12) to obtain lapatinib. The invention further provides two new key intermediates. The method has the characteristics of few steps, high yield and suitability for industrialized production.
Owner:ARROMAX PHARMATECH
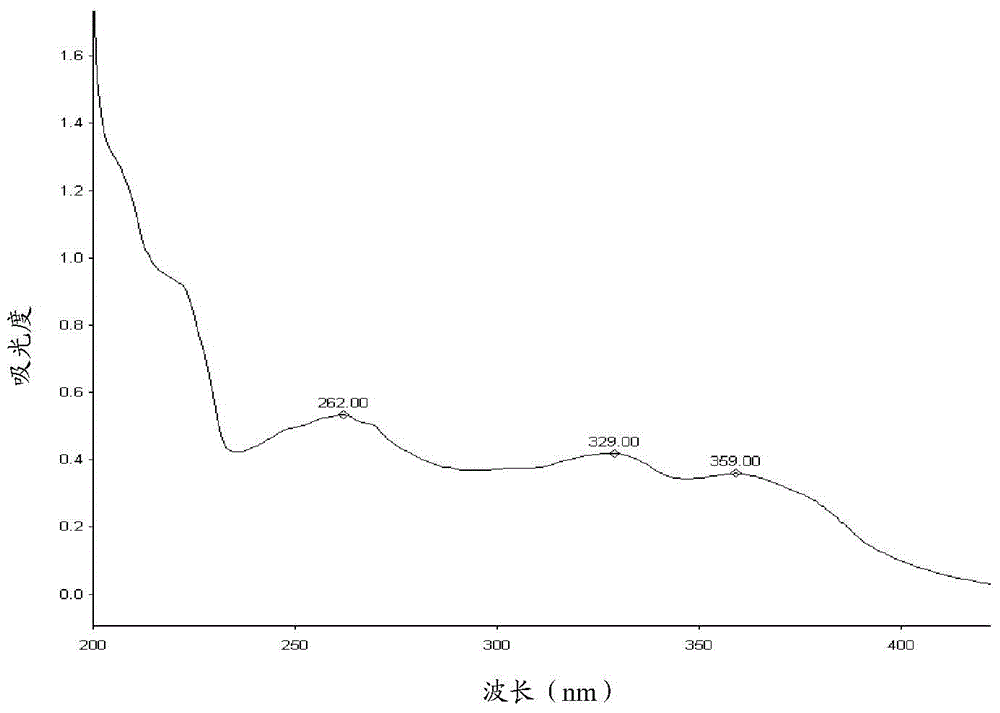
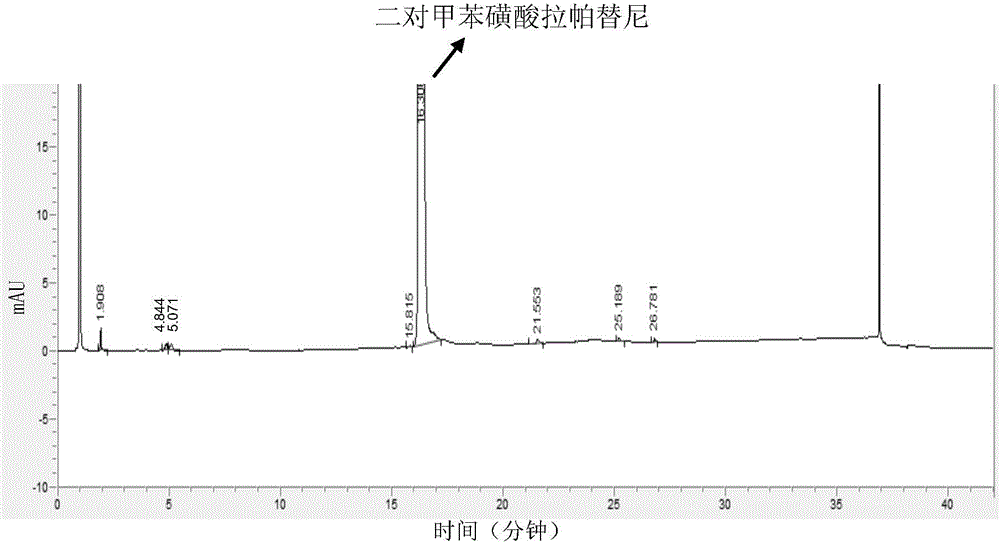
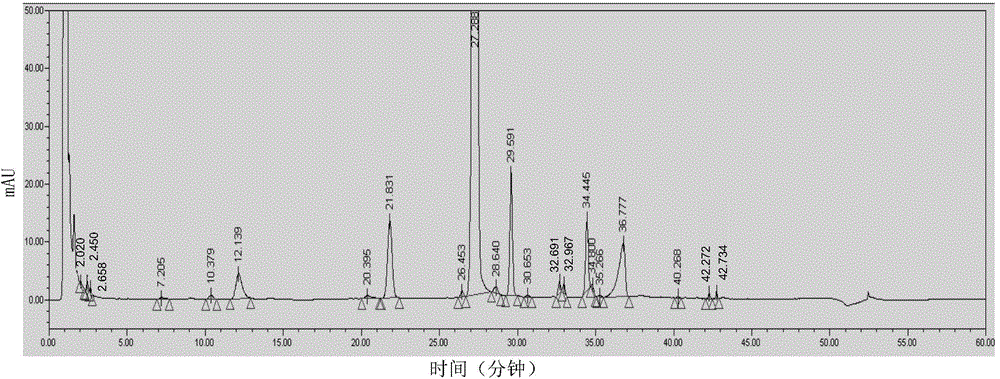
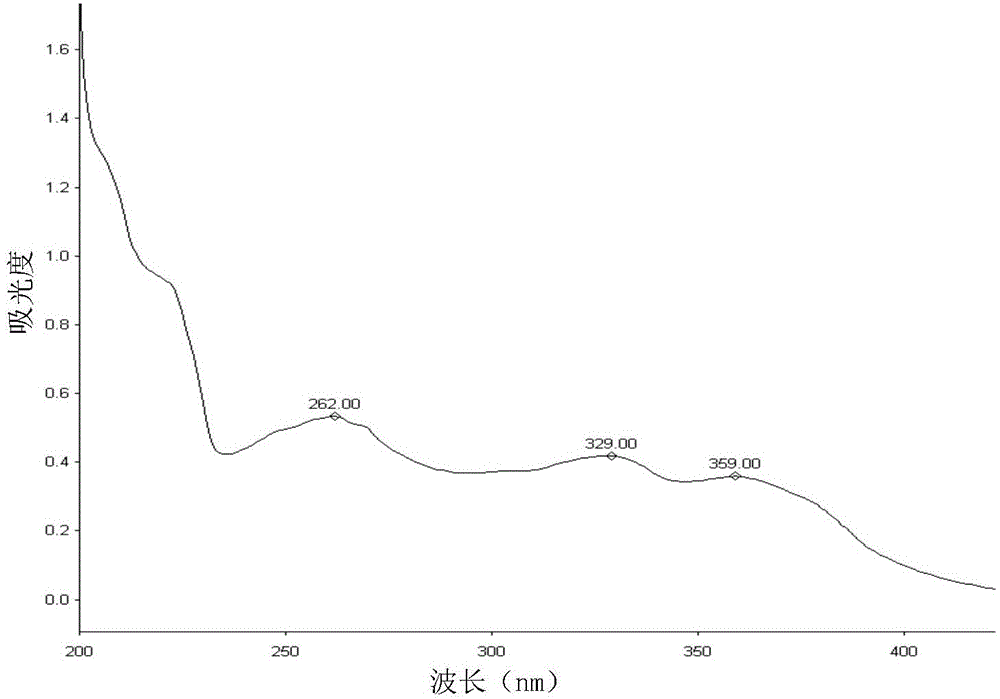
Method for analyzing lapatinib ditosylate isomer impurities
ActiveCN105738493AEfficient separationShort timeComponent separationColor/spectral properties measurementsUltraviolet detectorsIsocratic elution
The invention provides a method for analyzing lapatinib isomer impurities. The method is carried out by adoption of high performance liquid chromatography combined with an ultraviolet detector. In high performance liquid chromatography, a pentafluorophenyl column is adopted; a mobile phase comprises a mobile phase A and a mobile phase B, wherein an ammonium acetate buffer solution is taken as the mobile phase A, and methanol is taken as the mobile phase B; and an isocratic elution mode is adopted. According to the method, lapatinib ditosylate isomer impurities can be well separated. The method is high in sensitivity and good in specialization, and the separation degree can reach requirements.
Owner:HUBEI BIO PHARMA IND TECHCAL INST
An anticancer sustained releasing agent
InactiveCN1961861AInhibition formationSpeed up entryPeptide/protein ingredientsPharmaceutical delivery mechanismDepressantHyaluronidase
Disclosed is an anti-cancer slow release agent comprising slow release microspheres and dissolvent, wherein the slow release microspheres include slow release auxiliary materials, anti-angiogenesis agent and / or proteolytic enzyme, the dissolvent comprises suspension adjuvant. The anti-angiogenesis agent is selected from gefinitib, erlotinib, lapatinib, pelitinib, endostatin, imatinib, smasnib, avastin, sorafenib, sunitinib, oersted or panitoma, the proteolytic enzyme is selected from collagenase, hyaluronidase, relaxin and thrombase or the combination of them, the slow release auxiliary materials are selected from Polifeprosan, sebacylic acid copolymer, EVAc, polylactic acid and their mixture of copolymer, the viscosity of the suspension adjuvant is 100-3000cp (at 25-30 deg C), and is selected from sodium carboxymethylcellulose. The agent can also be prepared into implanting agent for injection or placement in or around tumor with the effects of selectively increasing local concentration, lowering general reaction of the drugs, suppressing growth of tumor cells and blood vessel, and improving the treatment effect of the non-operative treatment methods such as chemotherapy.
Owner:SHANDONG LANJIN PHARMA +1
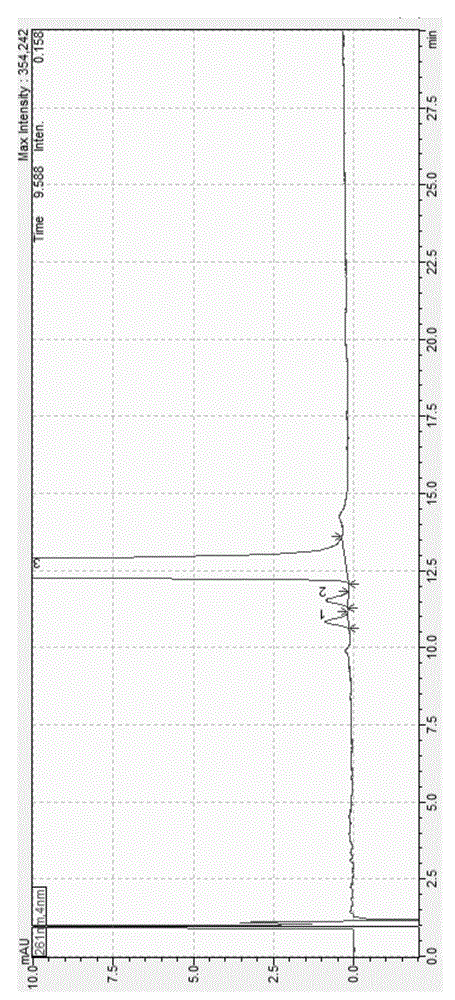
Analysis and detection method for impurity in lapatinib ditosylate bulk drug
The invention provides an analysis and detection method for impurity in a lapatinib ditosylate bulk drug. The method is carried out by combining high performance liquid chromatography of an ultraviolet detector, wherein, an octadecylsilane bonded silica gel column is employed; a mobile phase is composed of a mobile phase A and a mobile phase B; a buffer salting liquid is taken as the mobile phase A, the buffer salting liquid is a phosphate solution or an acetate solution; an organic solvent is employed as the mobile phase B, the organic solvent is selected from at least one of methanol, acetonitrile and ethanol; and an elution mode is linear elution. The method can separate lapatinib ditosylate and degrade impurity introduced in a synthesis process in a good mode, and has the advantages of high sensitivity and strong specialization.
Owner:HUMANWELL HEALTHCARE GRP
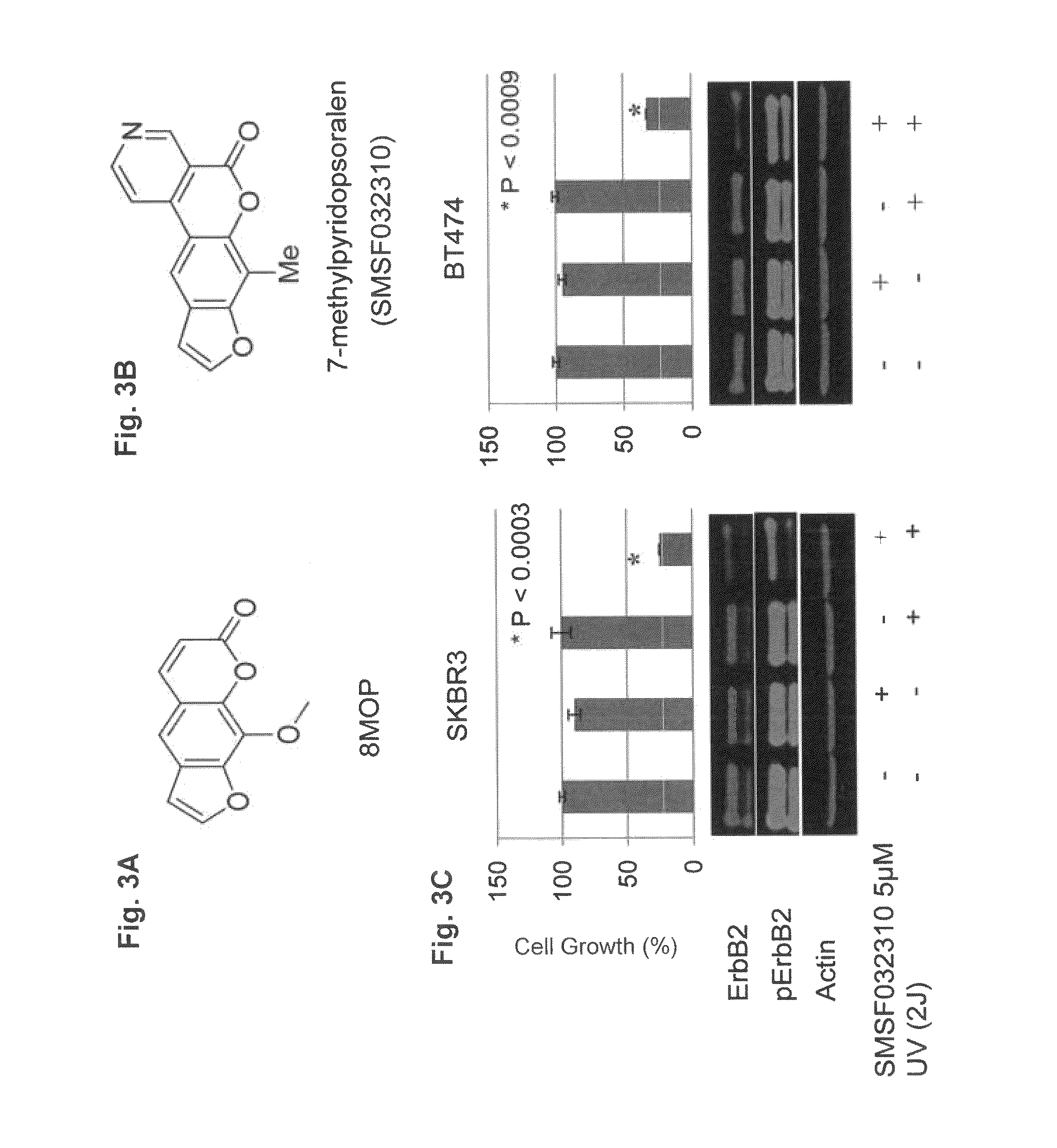
Use of psoralen derivatives and combination therapy for treatment of cell proliferation disorders
Methods for the treatment of a cell proliferation disease or disorder in a subject, involving applying a psoralen derivative lacking a DNA cross-linking motif to cancer cells, applying a psoralen or a derivative thereof and lapatinib, or applying a psoralen or derivative thereof and neratinib, to a subject and further applying initiation radiation energy form an energy source.
Owner:DUKE UNIV +1
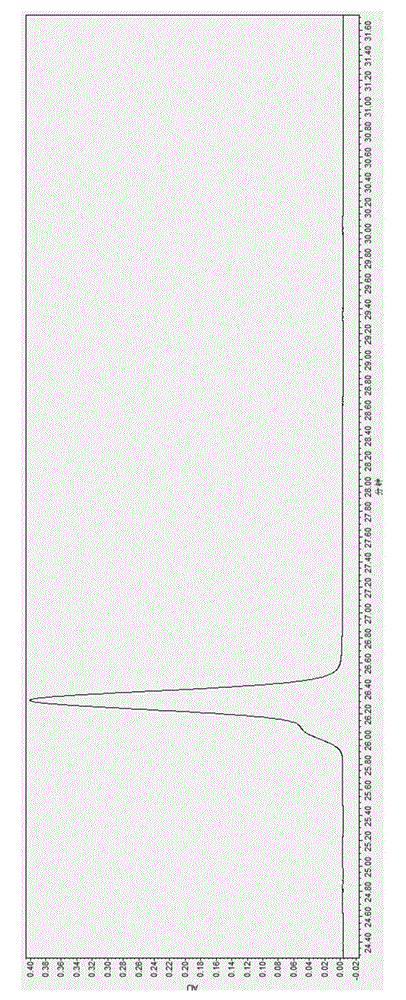
I-type crystal of dibenzenesulfonate of inhibitor of protein tyrosine kinase
InactiveCN104788435AImprove stabilityThe production process is stable, repeatable and controllableOrganic active ingredientsOrganic chemistry methodsProtein-Tyrosine KinasesX-ray
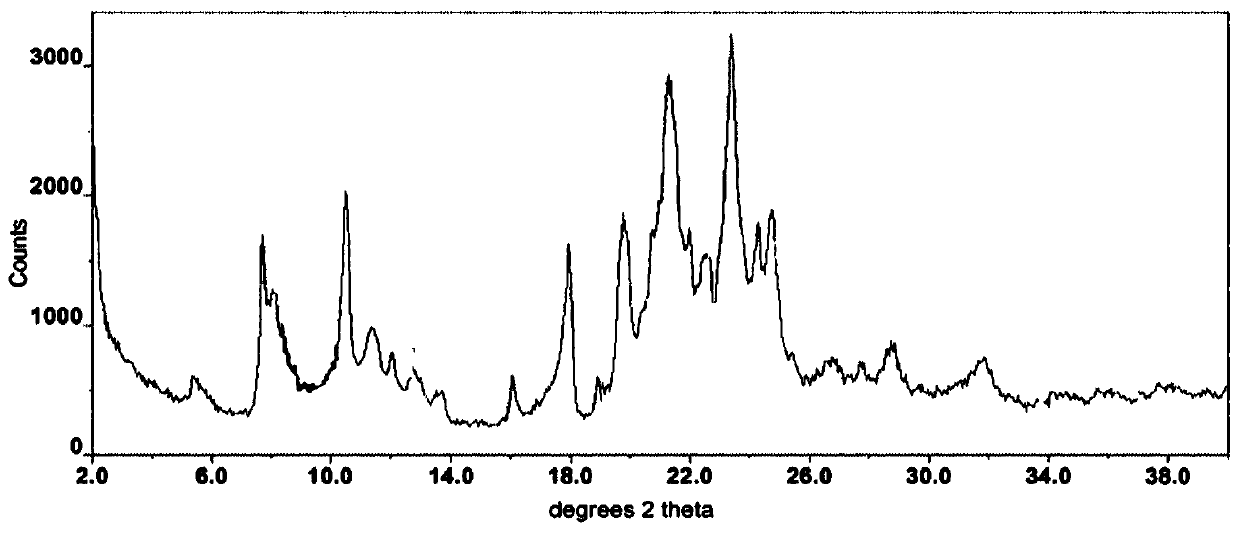
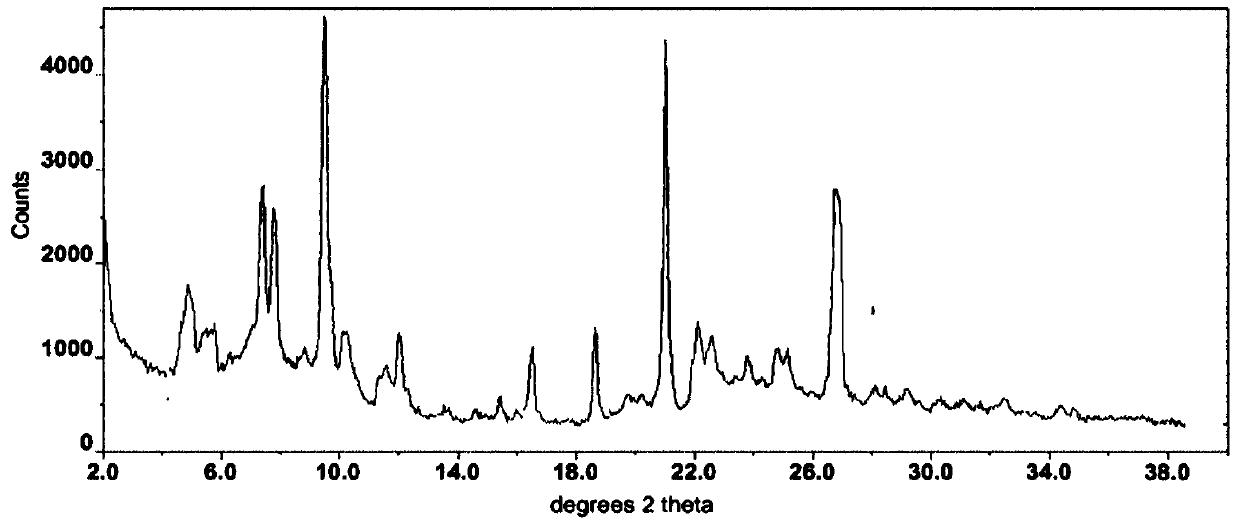
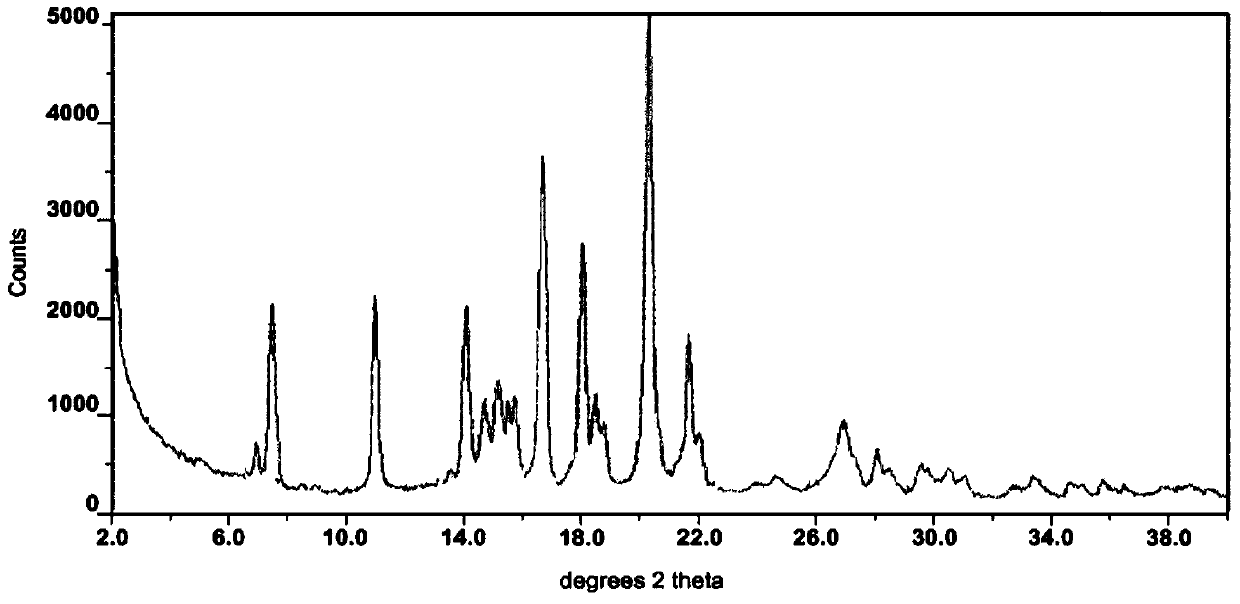
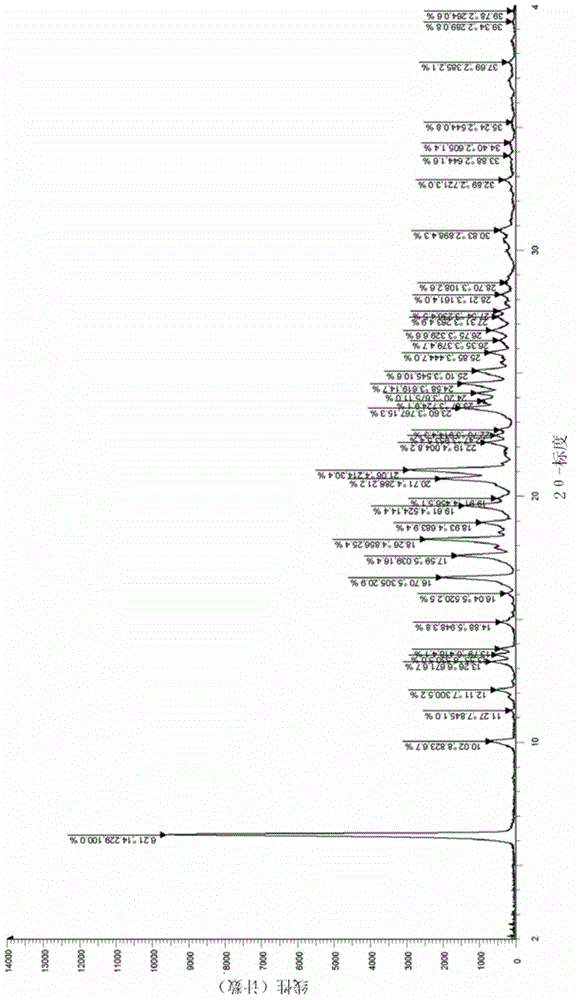
The invention relates to a I-type crystal of dibenzenesulfonate of an inhibitor of protein tyrosine kinase. Specifically, the invention relates to a I-type crystal of lapatinib dibenzenesulfonate. The crystal, radiated by Cu-K<alpha>, has the following characteristic peaks, represented by a 2theta angle and an inter-planar distance in an X-ray powder diffraction pattern: 6.21(14.23), 10.02(8.82), 12.11(7.30), 13.26(6.67), 13.55(6.53), 13.79(6.42), 14.88(5.95), 6.70(5.31), 17.59(5.04), 18.26(4.86), 18.93(4.68), 19.61(4.52), 20.71(4.29), 21.06(4.21), 22.19(4.00), 23.60(3.77), 24.20(3.68), 24.58(3.62), 25.10(3.55), 25.85(3.44), 26.75(3.33), 30.83(2.90), and 32.89(2.72). According to the invention, the I-type crystal of lapatinib dibenzenesulfonate has better stability, and can be preferably used for clinic treatment.
Owner:JIANGSU HENGRUI MEDICINE CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com