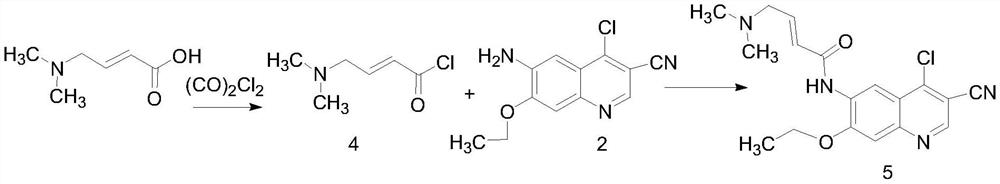
Patents
Literature
37 results about "Neratinib" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
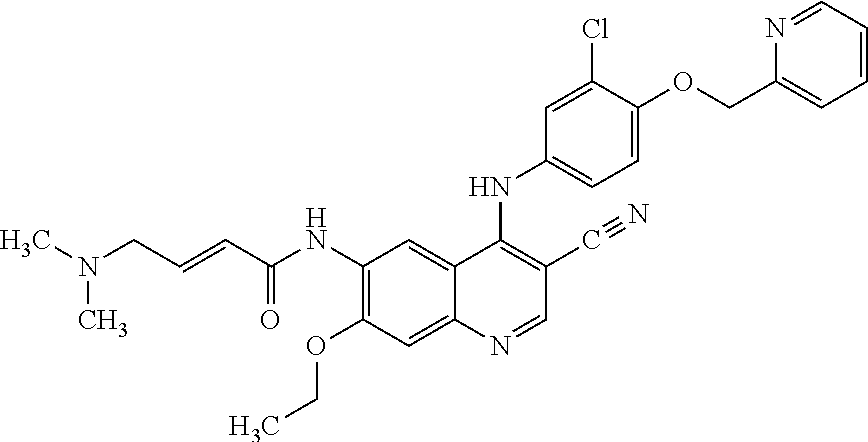
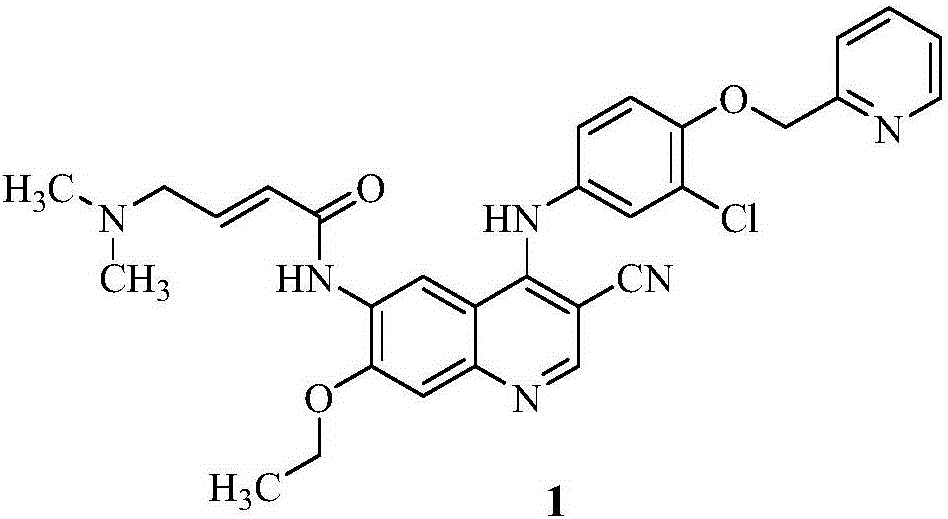
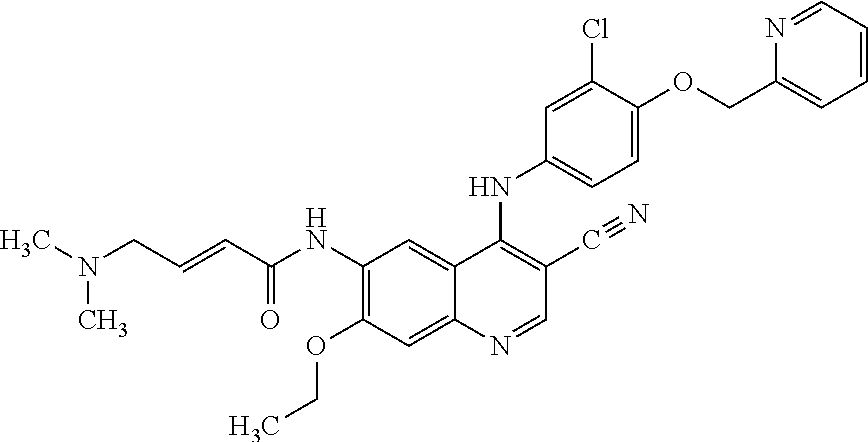
Neratinib is used to treat breast cancer after treatment with certain other medications (such as trastuzumab).
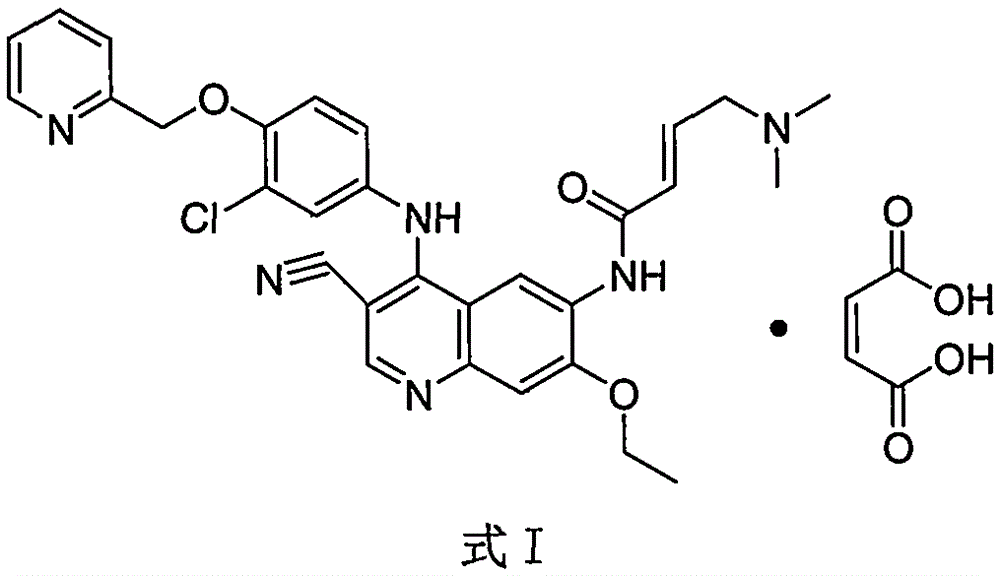
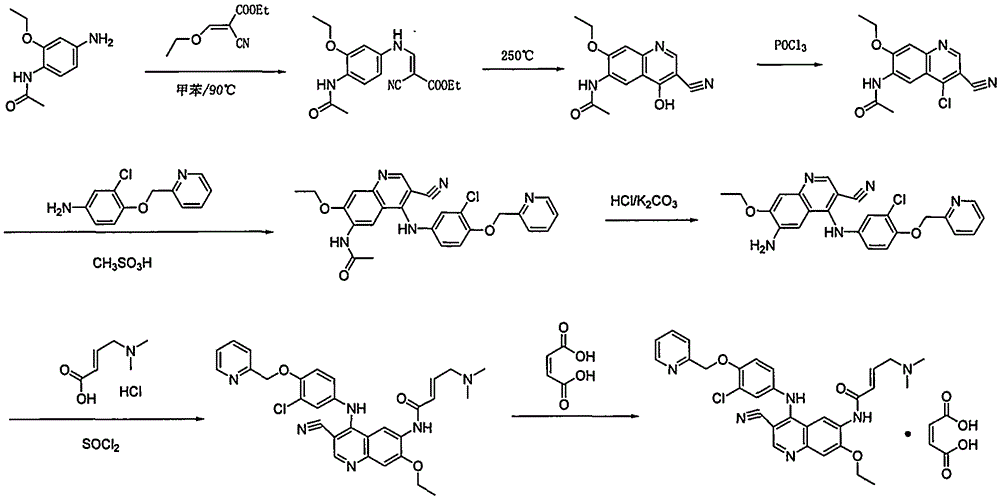
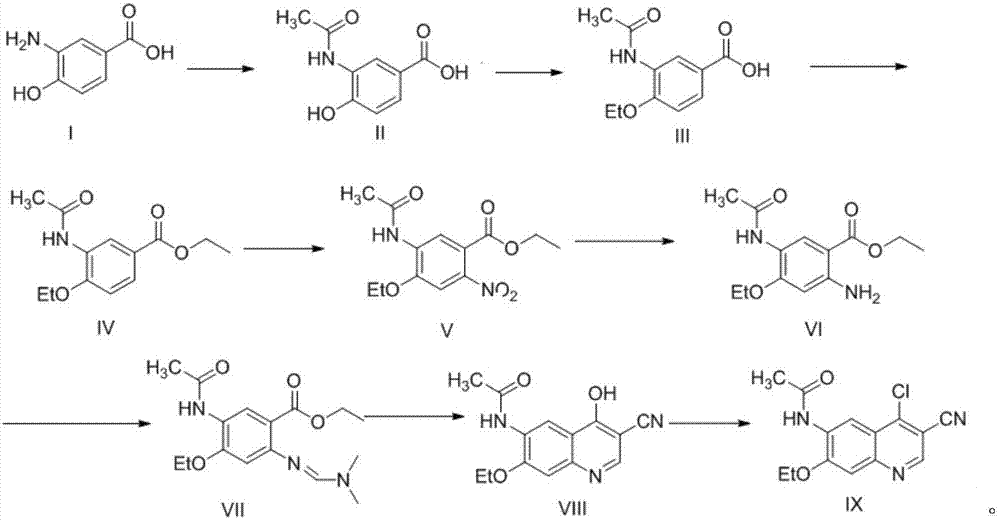
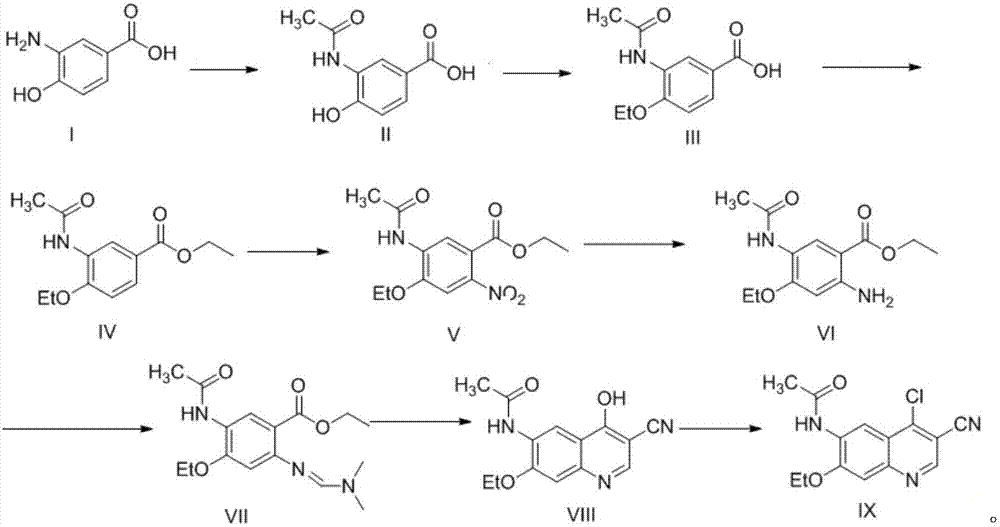
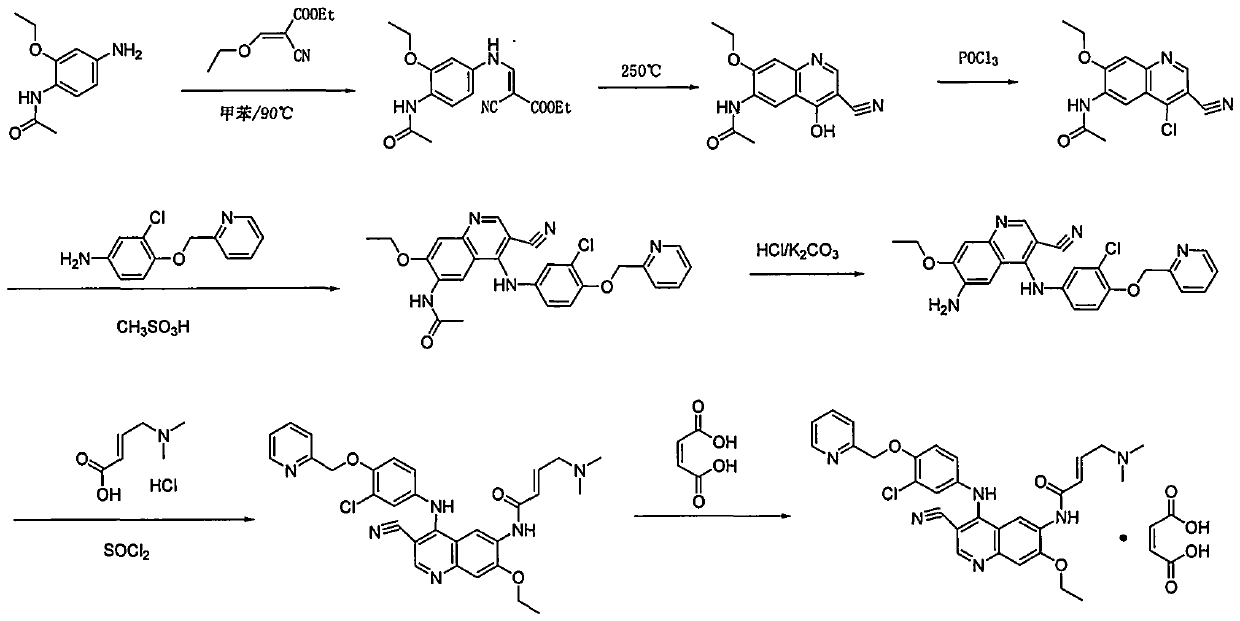
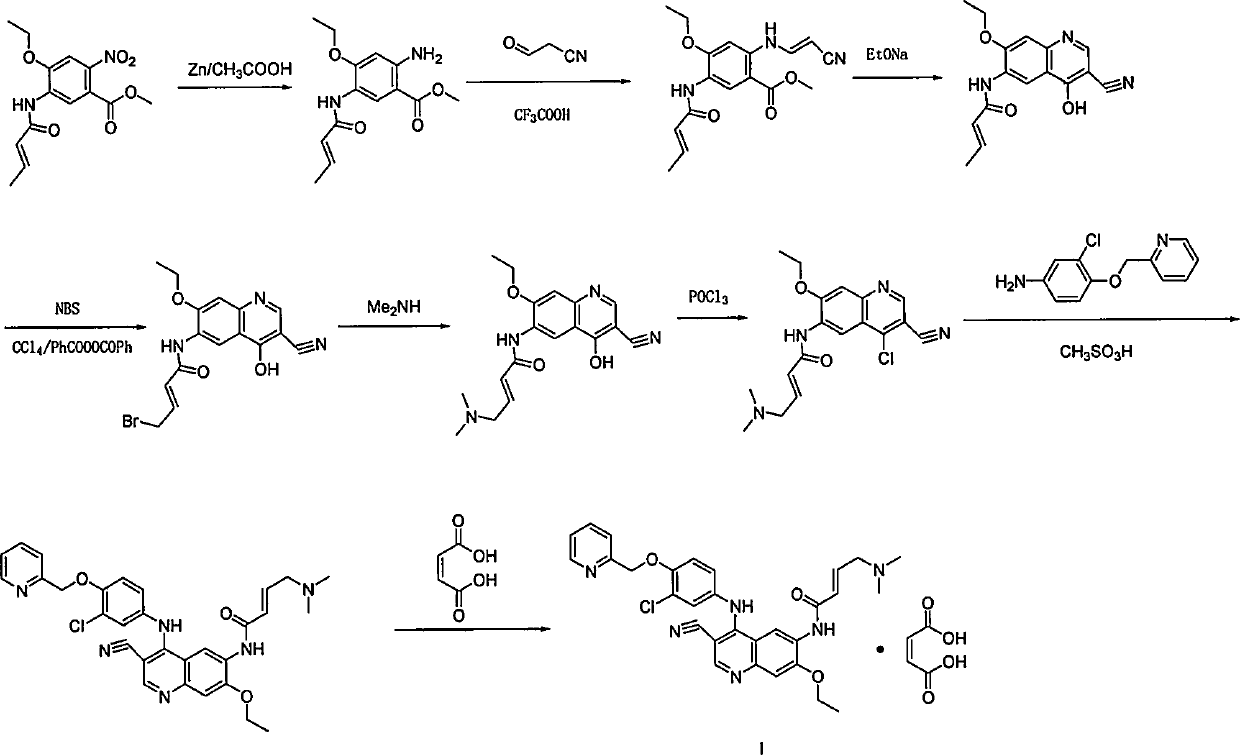
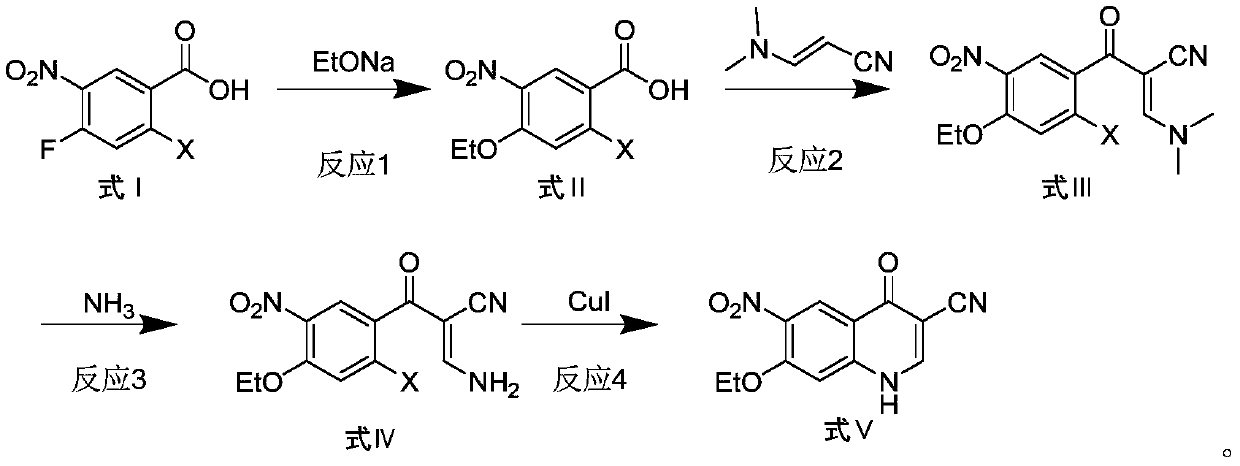
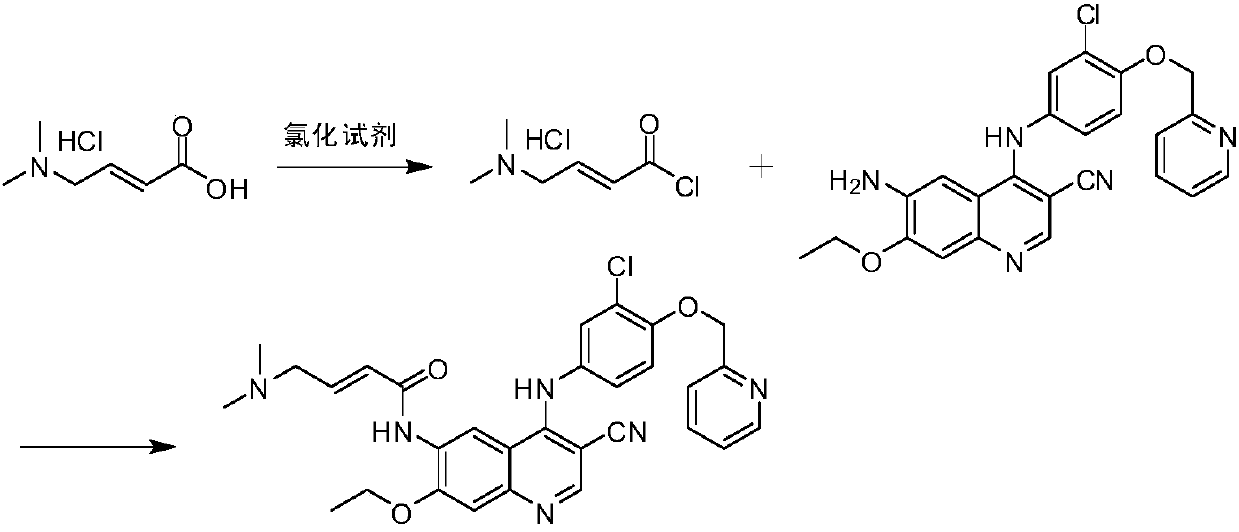
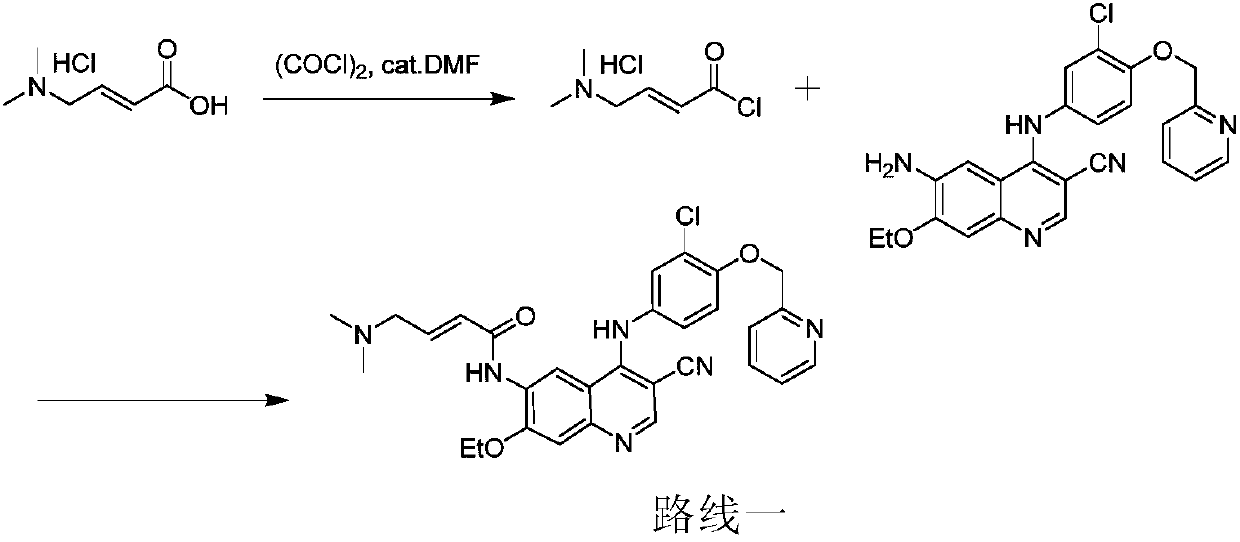
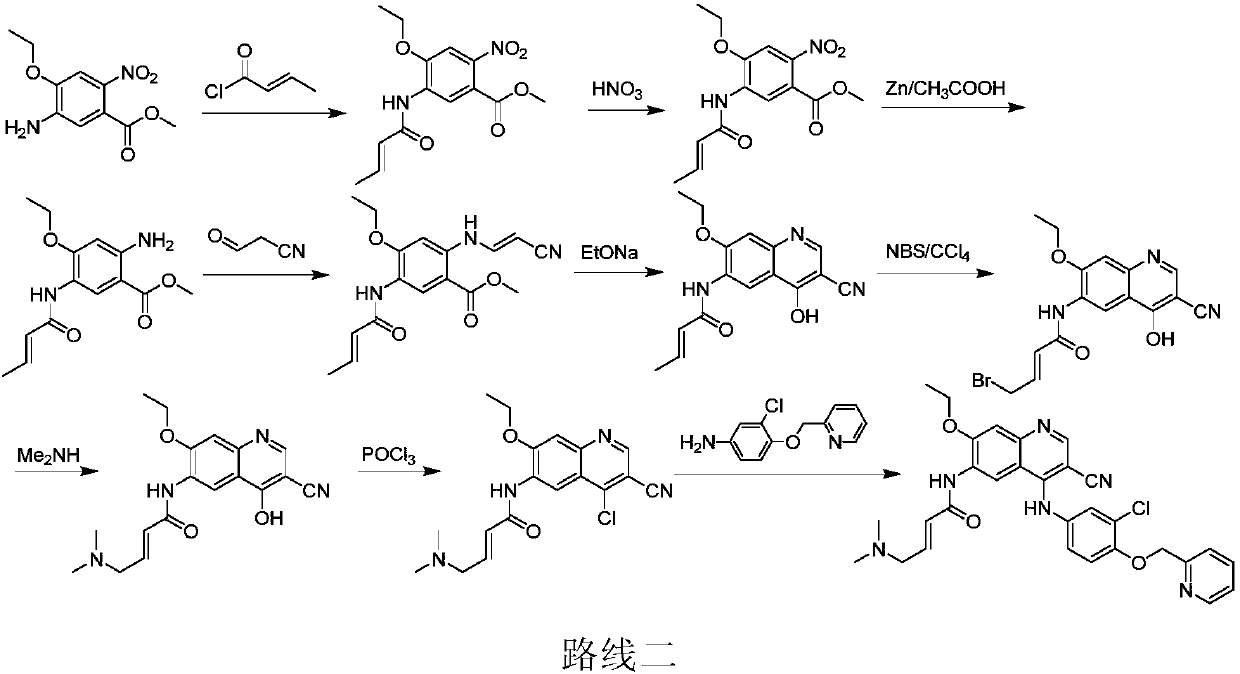
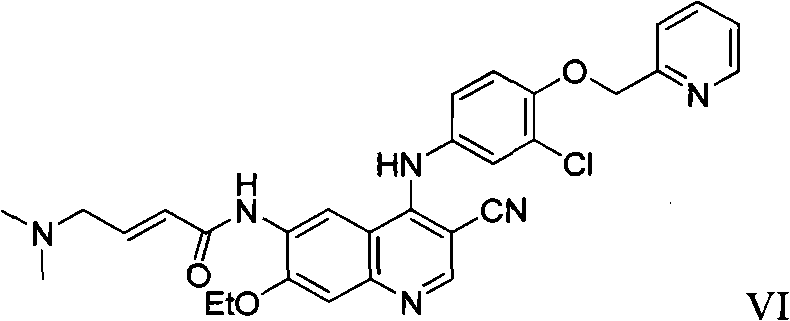
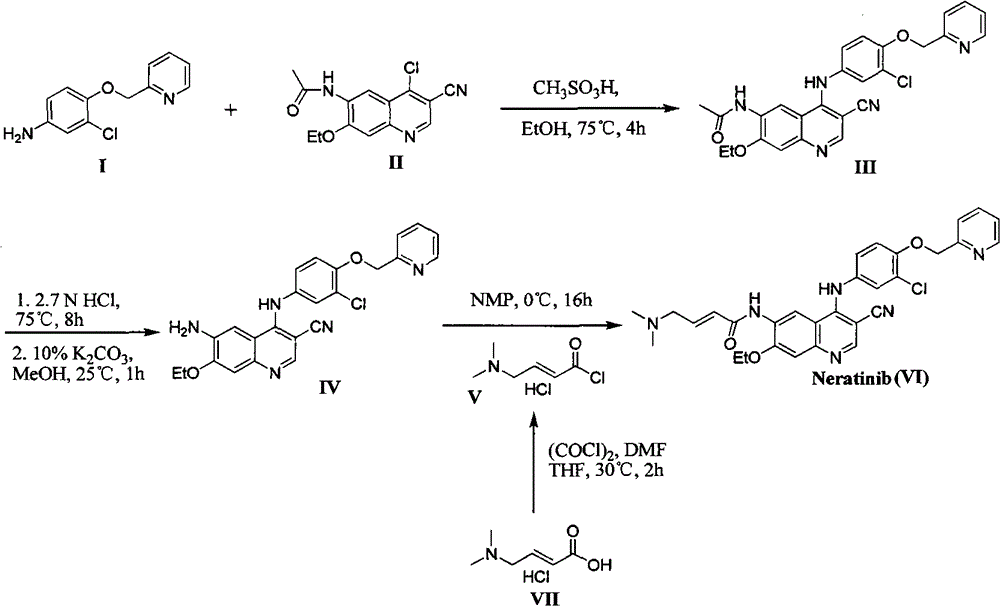
Preparation method of antineoplastic drug maleic acid neratinib
ActiveCN105330646AWide variety of sourcesShort synthetic routeCarboxylic acid salt preparationNitro compoundMedicinal chemistry
The invention provides a preparation method of antineoplastic drug maleic acid neratinib. The defects in the prior art are overcome. The preparation method comprises the steps that the formula II and the formula III are coupled to form the formula IV under the effect of a catalyst; a nitro-compound IV is reduced under the effect of a reduction system to form a formula V; an amino compound V and a formula VI are condensed to obtain neratinib VII, and then the neratinib VII and maleic acid form a salt to obtain the maleic acid neratinib I. By the adoption of the technical route, the preparation method has the advantages that the synthetic route is short, reaction conditions are mild, the yield is high, raw materials are wide in source, and environmental protection is achieved.
Owner:SHANGHAI XUNHE PHARMA TECH CO LTD
Method of treatment of philadelphia chromosome positive leukaemia
InactiveUS20120244116A1Organic active ingredientsPeptide/protein ingredientsBcr-Abl tyrosine-kinase inhibitorLestaurtinib
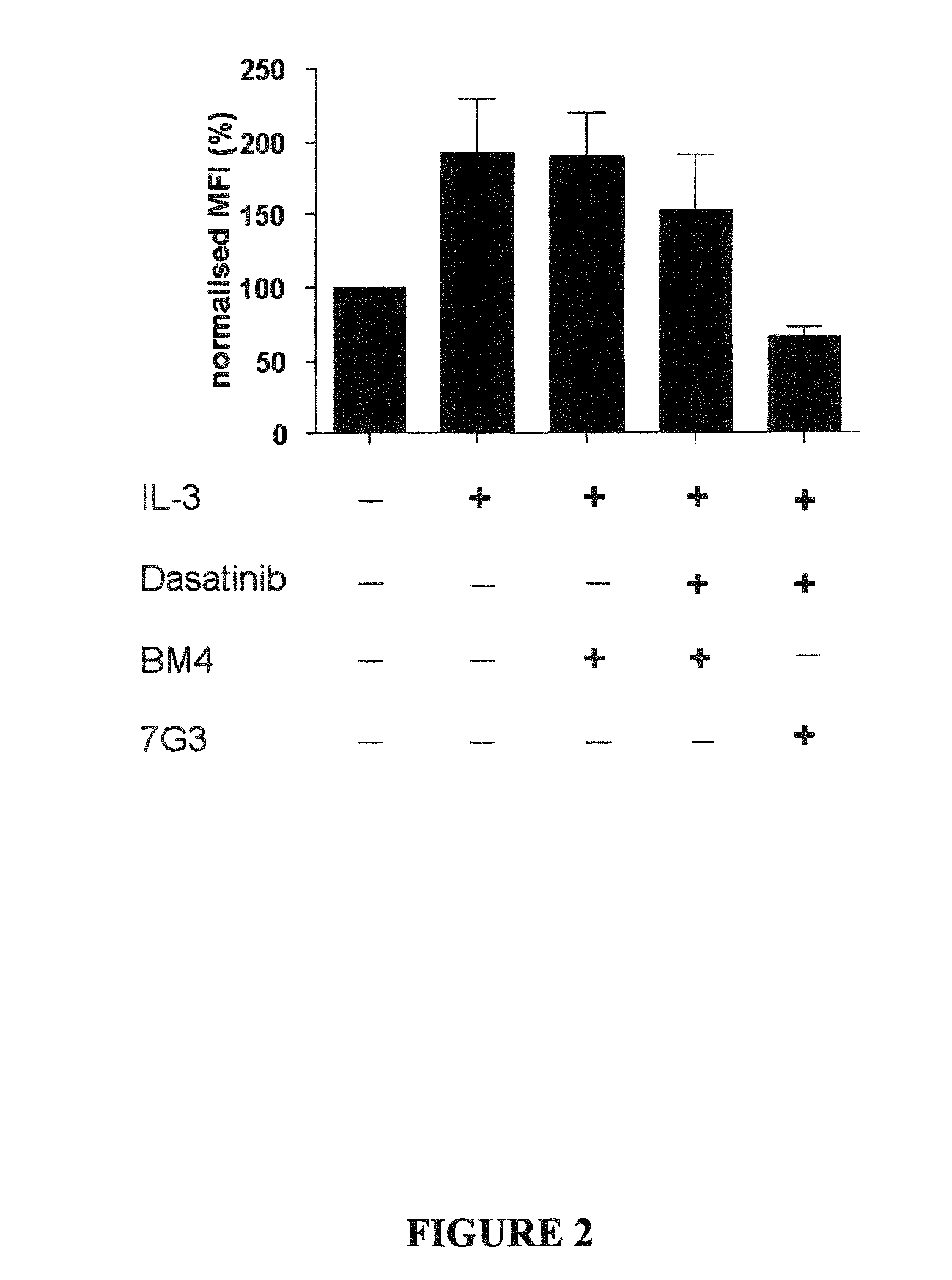
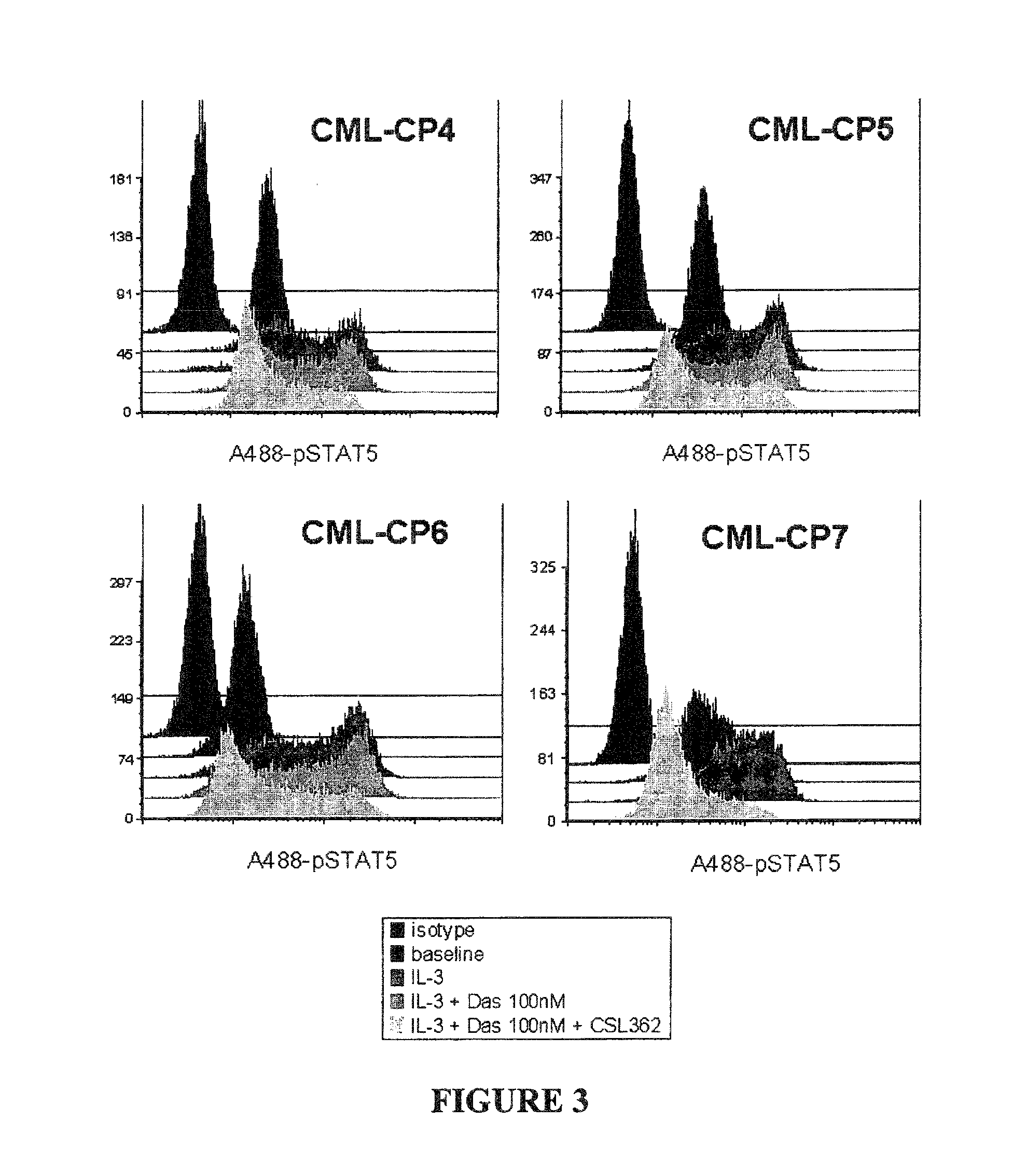
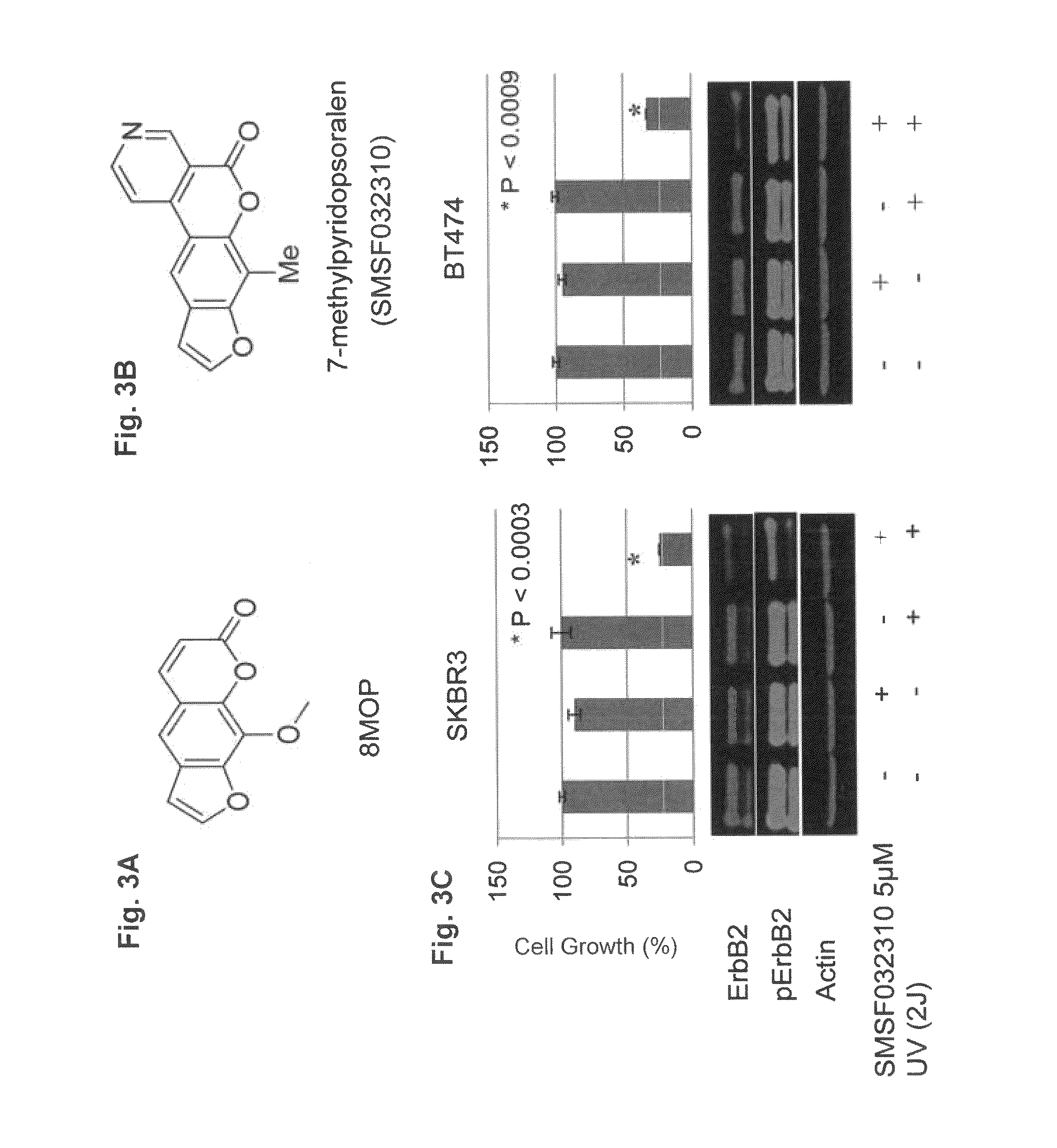
The invention provides a method for the treatment of Ph+ leukemia in a patient comprising administering to the patient (i) a BCR-ABL tyrosine kinase inhibitor, and (ii) an agent which selectively binds to a cell surface receptor expressed on Ph+ leukemic stem cells. The invention further provides for the use of (i) and (ii) in, or in the manufacture of a medicament for, the treatment of Ph+ leukemia in a patient; and a composition for the treatment of Ph+ leukemia in a patient comprising (i) and (ii); and kits comprising (i) and (ii). In some embodiments, the tyrosine kinase inhibitor is or is not imatinib; or is selected from the group consisting of dasatinib, nilotinib, bosutinib, axitinib, cediranib, crizotinib, damnacanthal, gefitinib, lapatinib, lestaurtinib, neratinib, semaxanib, sunitinib, toceranib, tyrphostins, vandetanib, vatalanib, INNO-406, AP24534, XL228, PHA-739358, MK-0457, SGX393 and DC2036; or is selected from the group consisting of dasatinib and nilotinib. In some embodiments, the agent binds to a receptor involved in signalling by at least one of IL-3, G-CSF and GM-CSF. In some embodiments, the agent is a mutein selected from the group consisting of IL-3 muteins, G-CSF muteins and GM-CSF muteins. In some embodiments, the mutein is an IL-3 mutein. In some embodiments, the agent is a soluble receptor which is capable of binding to IL-3.
Owner:CSL LTD
Neratinib sustained-release implant for treating solid tumor
InactiveCN101185633AOrganic active ingredientsPharmaceutical delivery mechanismProstate cancerTherapeutic effect
A sustained release implant includes 0.1%-50% (w / w) nilotinib, 50-99% sustained release excipients and 0-15% sustained release moderator. Sustained release excipients are mainly one or combination of poly (L-lactide-co-ethyl phosphate), poly (L-lactide-co- phosphoric acid propyl), polylactic acid, the copolymer of polylactic acid and hydroxyacetic acid and polifeprosan; sustained release moderator is one or combination of mannitol, sorbic alcohol and chondroitin; sustained release implant applied in local tumor can slowly release nilotinib onto local tumor, thus maintaining effective drug concentration of local tumor as well as significantly reducing overall toxic reaction; the invention not only reduces overall toxic reaction of nilotinib, but also selectively improves drug concentration in local tumor, enhancing the therapeutic effects of non-operative therapy such as chemotherapy drugs and radiotherapy. The implant can be used for treating solid tumors including lung cancer, esophageal carcinoma, gastric cancer, liver cancer, breast cancer, ovarian cancer, prostatic carcinoma, pancreatic cancer, bladder carcinoma, cerebroma, and colorectal cancer.
Owner:SHANDONG LANJIN PHARMA +1
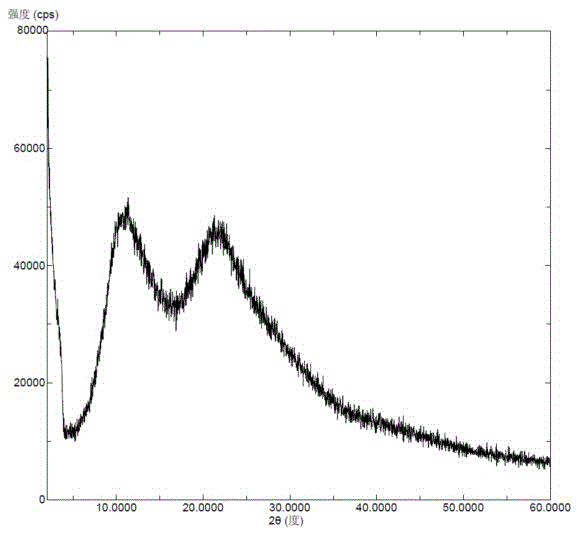
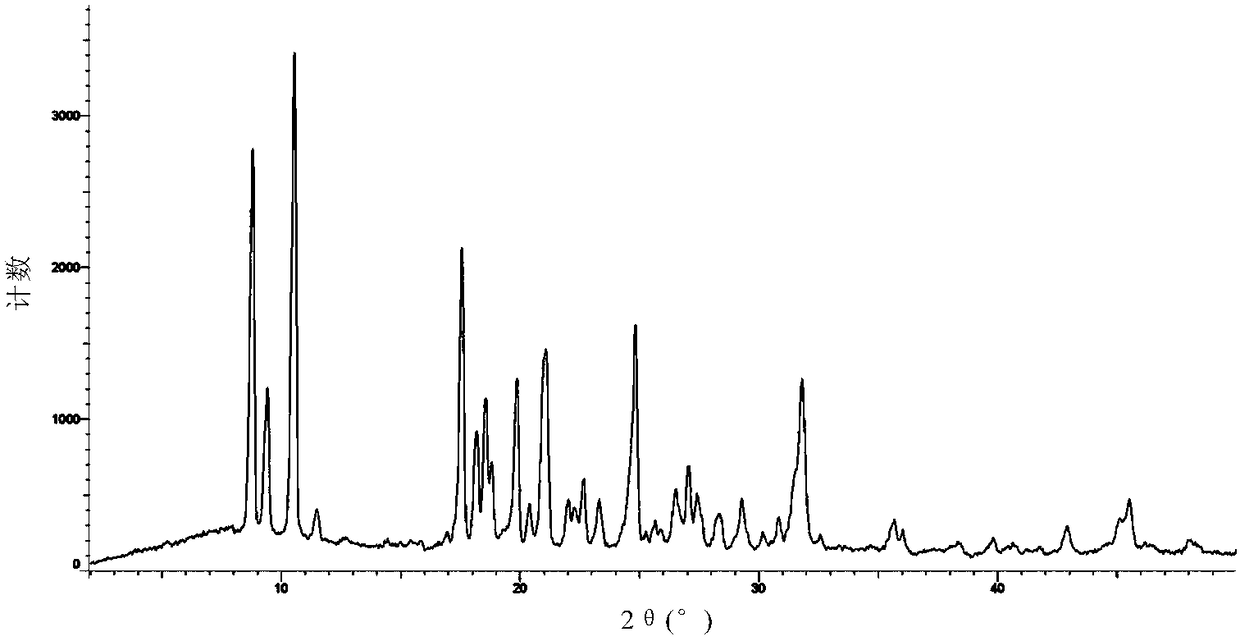
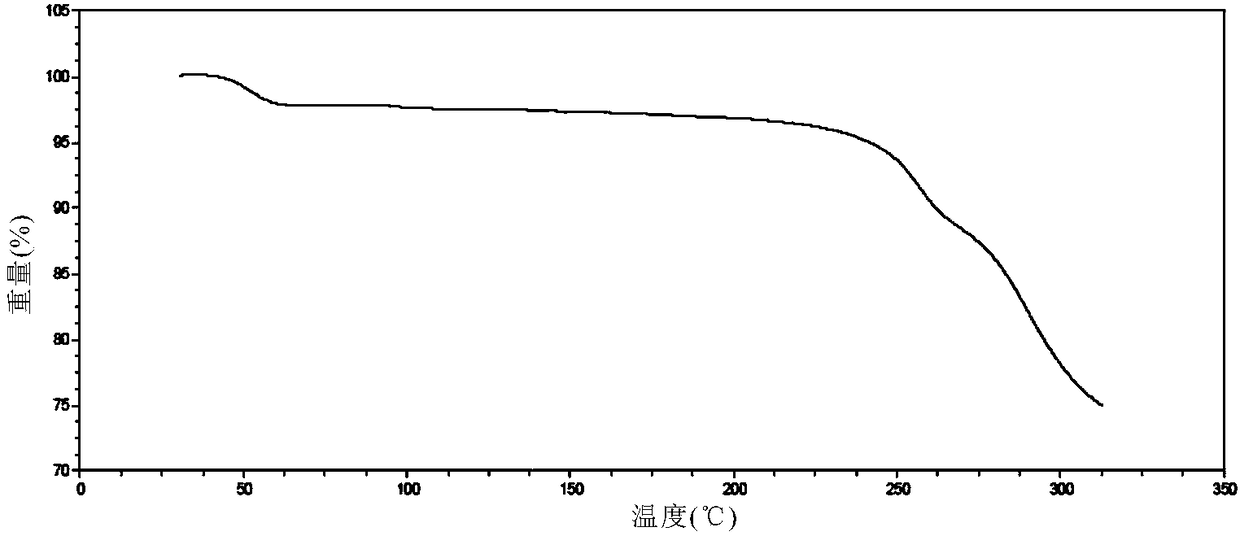
Solid dispersion prepared from amorphous neratinib or pharmaceutically acceptable salt thereof and medicinal auxiliary materials and preparation method thereof
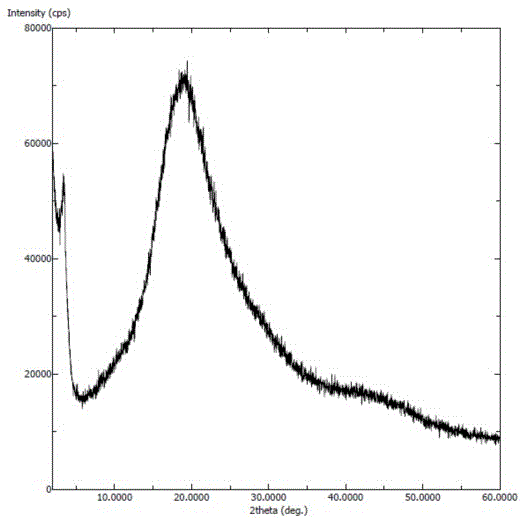
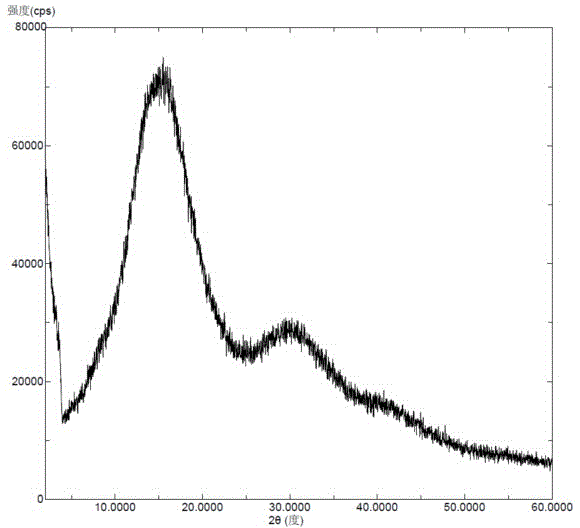
InactiveCN106831710AImprove stabilityLow pricePowder deliveryOrganic active ingredientsDissolutionBioavailability
Amorphous neratinib is characterized in that no characteristic peak of neratinib exists in an X-ray powder diffraction spectrum. Amorphous neratinib maleate is characterized in that no characteristic peak of neratinib maleate exists in an X-ray powder diffraction spectrum. The invention further discloses a preparation method of an amorphous state of the amorphous neratinib or pharmaceutically acceptable salt thereof, as well as a solid dispersion prepared from the amorphous neratinib or the pharmaceutically acceptable salt thereof and medicinal auxiliary materials and a preparation method thereof, wherein the neratinib or the pharmaceutically acceptable salt thereof are amorphous. The solid dispersion prepared from the neratinib or the pharmaceutically acceptable salt thereof and the medicinal auxiliary materials is good in stability and dispersion, so that the dissolution of the neratinib or the salt thereof is improved and thus the improvement on the bioavailability of a medicinal preparation and medicine absorption of a body is more facilitated; under an accelerated test condition, the solid dispersion keeps good physical and chemical stability. The preparation method of the amorphous solid dispersion is easy to operate, low in cost, good in reproducibility and easy to implement, and is suitable for industrial production.
Owner:CHANGZHOU AINUOXINRUI PHARMA LTD
Treatment regimen utilizing neratinib for breast cancer
ActiveUS20120071507A1Improving invasive disease free survivalImprove survivalBiocideSkeletal disorderRegimenExtended regimen
An extended regimen for treatment of HER-2 / neu overexpressed / amplified cancer is described, with involves delivering a course of neratinib therapy to HER-2 / neu overexpressed / amplified cancer patients following the completion of surgical and adjuvant therapy. The neratinib regimen may be continued for upwards of twelve months to five years. Also provided are pharmaceutical kits designed to facilitate compliance with the regimen.
Owner:WYETH LLC
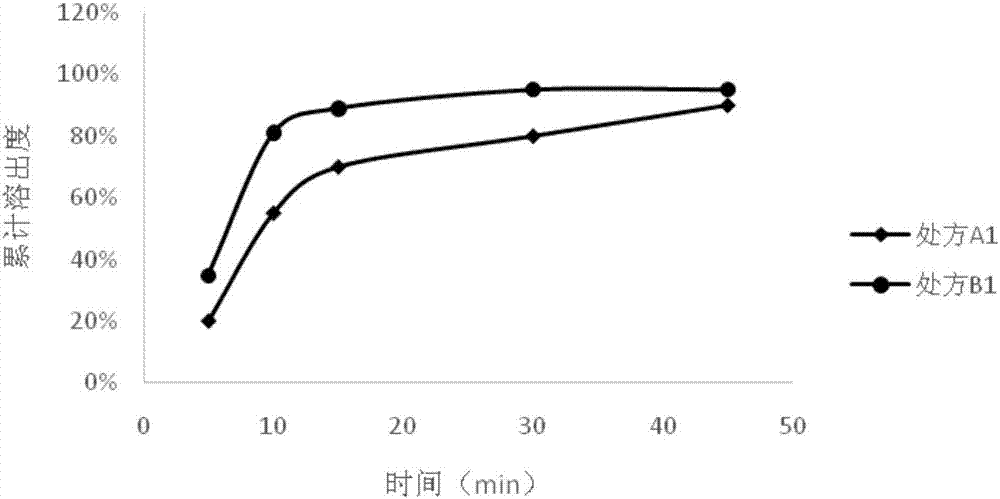
Preparation method of medicinal composition of neratinib or medicinal salt of neratinib
ActiveCN106913529AGood dissolution propertiesImprove stabilityOrganic active ingredientsPharmaceutical non-active ingredientsMedicinePharmaceutical formulation
The invention relates to a preparation method of a medicinal composition of neratinib or a medicinal salt of the neratinib. A medicinal preparation with good stability can be prepared through keeping the maximum water content of a granule in the granulation period to be 10% or less or / and controlling the water content of a final granule or the medicinal composition to be 2% or less in the preparation process of the neratinib granule, and the medicinal preparation has good dissolving property.
Owner:JIANGSU HENGRUI MEDICINE CO LTD +1
Use of psoralen derivatives and combination therapy for treatment of cell proliferation disorders
Methods for the treatment of a cell proliferation disease or disorder in a subject, involving applying a psoralen derivative lacking a DNA cross-linking motif to cancer cells, applying a psoralen or a derivative thereof and lapatinib, or applying a psoralen or derivative thereof and neratinib, to a subject and further applying initiation radiation energy form an energy source.
Owner:DUKE UNIV +1
Method of treatment of philadelphia chromosome positive leukemia
InactiveUS20150093355A1Organic active ingredientsPeptide/protein ingredientsBcr-Abl tyrosine-kinase inhibitorLestaurtinib
The invention provides a method for the treatment of Ph+ leukemia in a patient comprising administering to the patient (i) a BCR-ABL tyrosine kinase inhibitor, and (ii) an agent which selectively binds to a cell surface receptor expressed on Ph+ leukemic stem cells. The invention further provides for the use of (i) and (ii) in, or in the manufacture of a medicament for, the treatment of Ph+ leukemia in a patient; and a composition for the treatment of Ph+ leukemia in a patient comprising (i) and (ii); and kits comprising (i) and (ii). In some embodiments, the tyrosine kinase inhibitor is or is not imatinib; or is selected from the group consisting of dasatinib, nilotinib, bosutinib, axitinib, cediranib, crizotinib, damnacanthal, gefitinib, lapatinib, lestaurtinib, neratinib, semaxanib, sunitinib, toceranib, tyrphostins, vandetanib, vatalanib, INNO-406, AP24534, XL228, PHA-739358, MK-0457, SGX393 and DC2036; or is selected from the group consisting of dasatinib and nilotinib. In some embodiments, the agent binds to a receptor involved in signalling by at least one of IL-3, G-CSF and GM-CSF. In some embodiments, the agent is a mutein selected from the group consisting of IL-3 muteins, G-CSF muteins and GM-CSF muteins. In some embodiments, the mutein is an IL-3 mutein. In some embodiments, the agent is a soluble receptor which is capable of binding to IL-3.
Owner:CSL LTD
Method for purifying neratinib
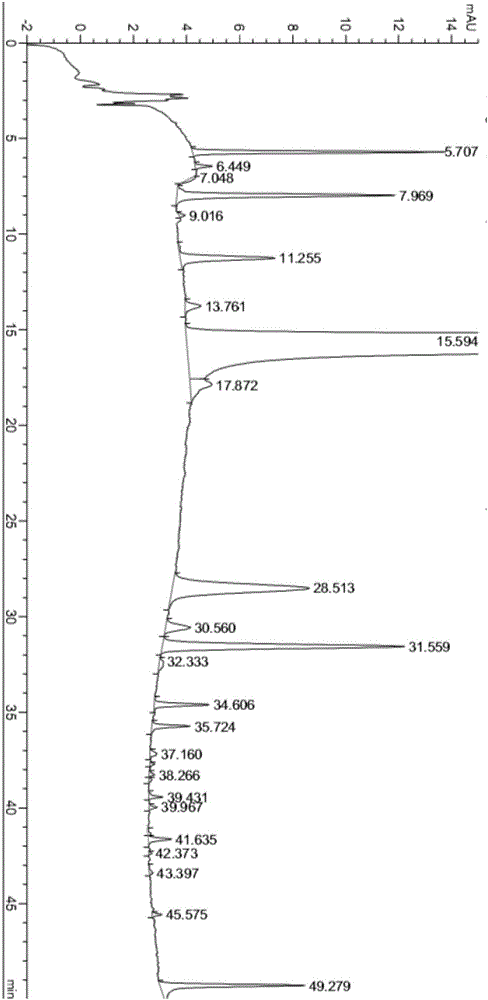
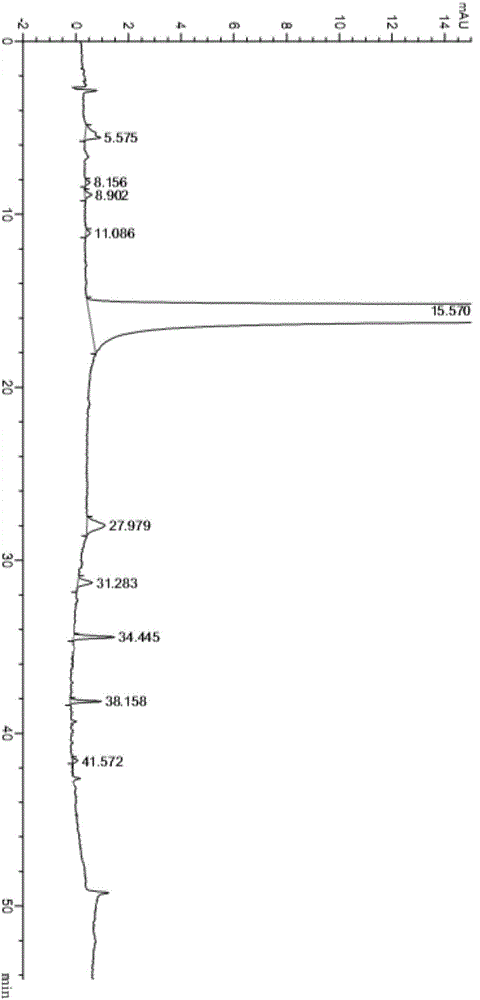
The invention relates to a method for purifying neratinib. The method provided by the invention is capable of effectively reducing the content of impurities in neratinib and preparing high-purity neratinib, and moreover is simple in operation, high in yield and very applicable to industrial production.
Owner:ZHEJIANG HISUN PHARMA CO LTD
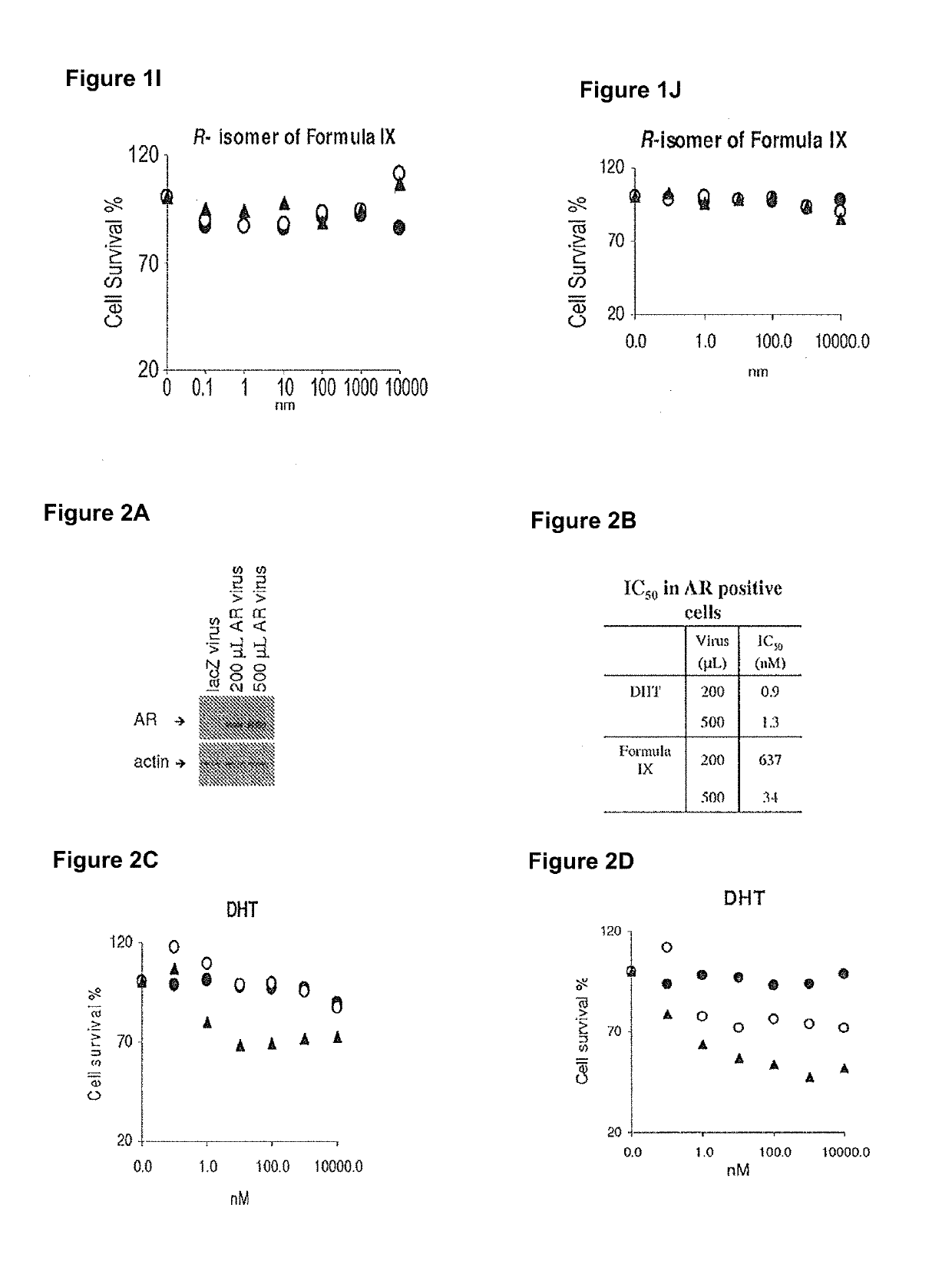
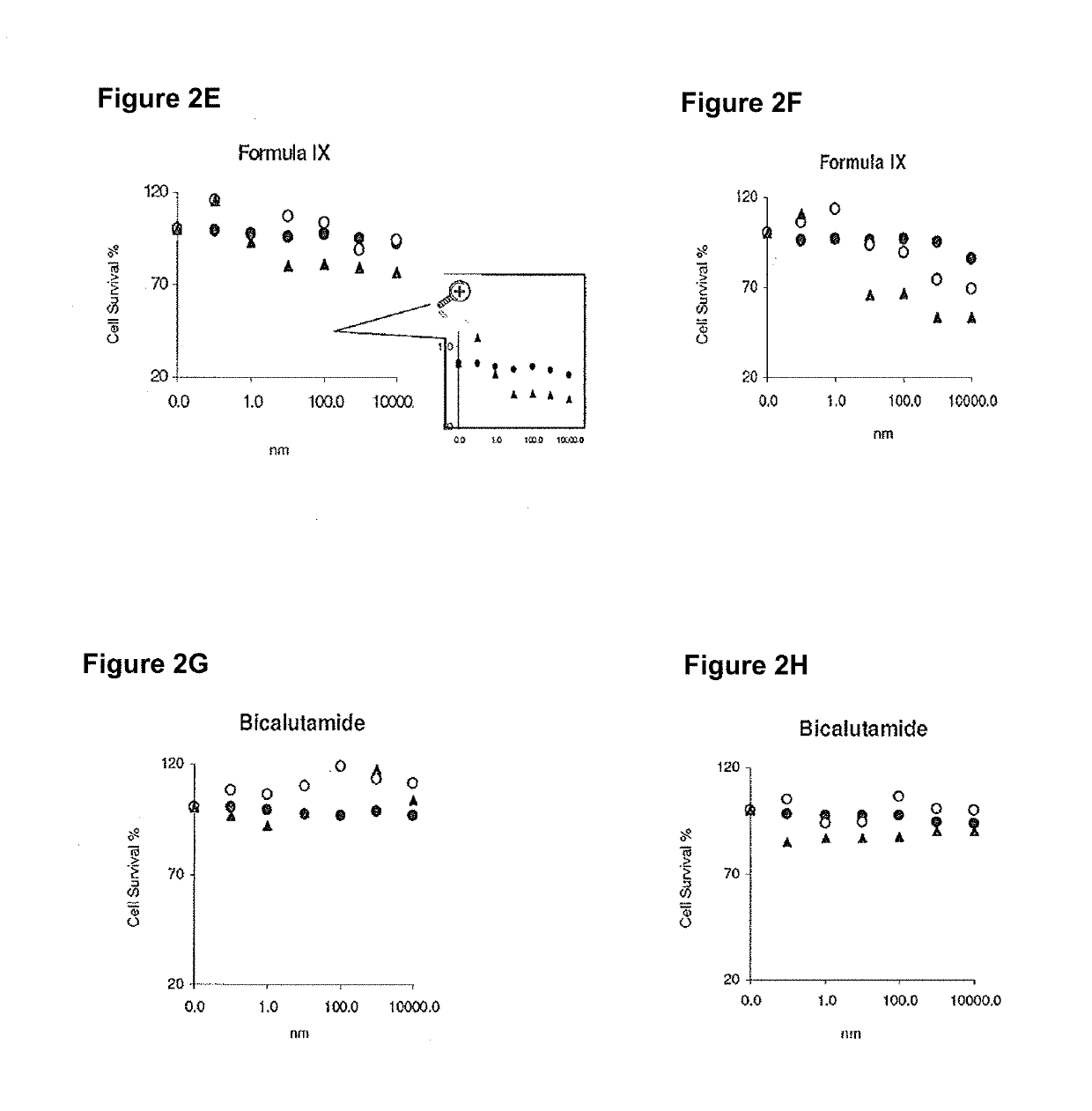
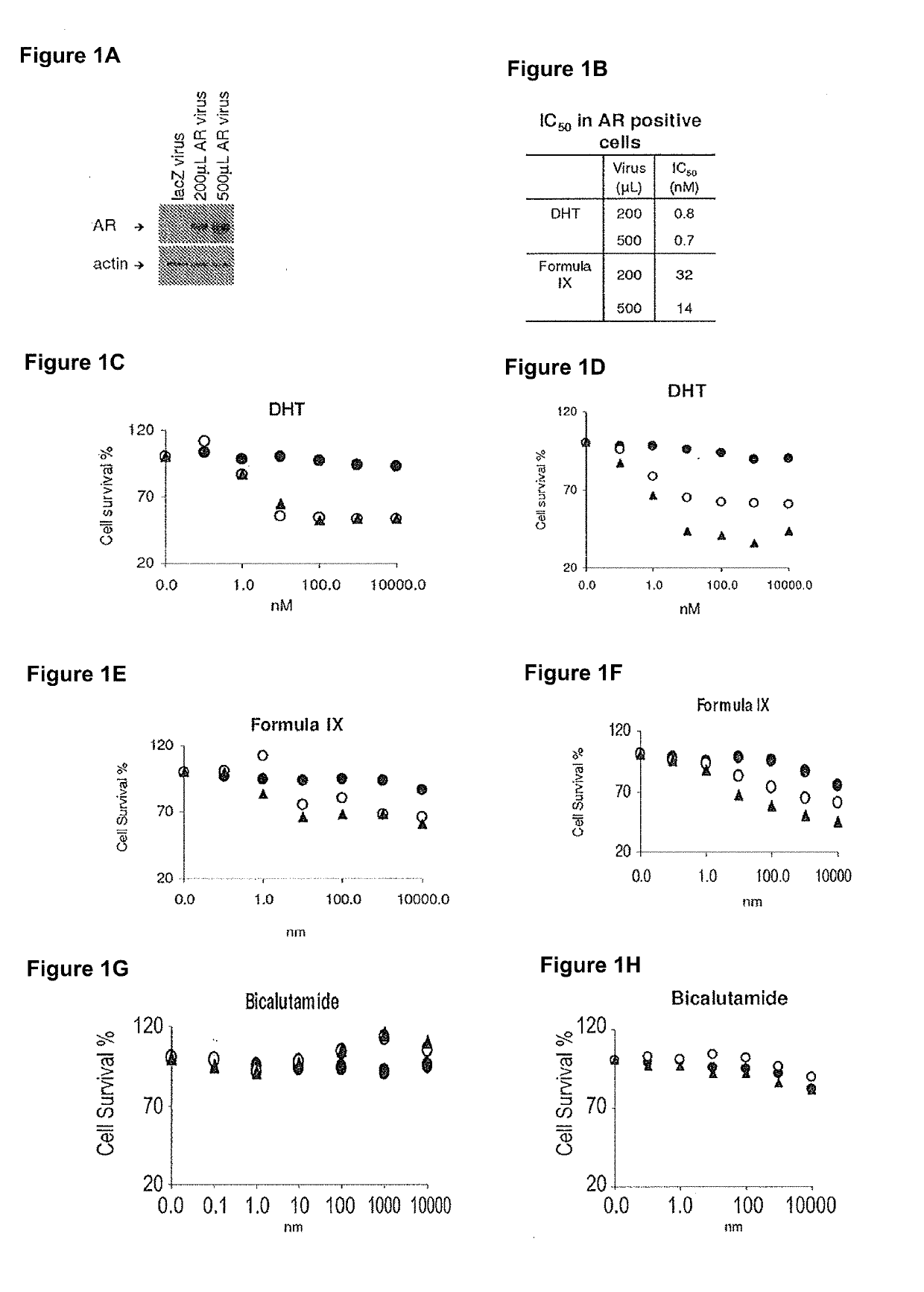
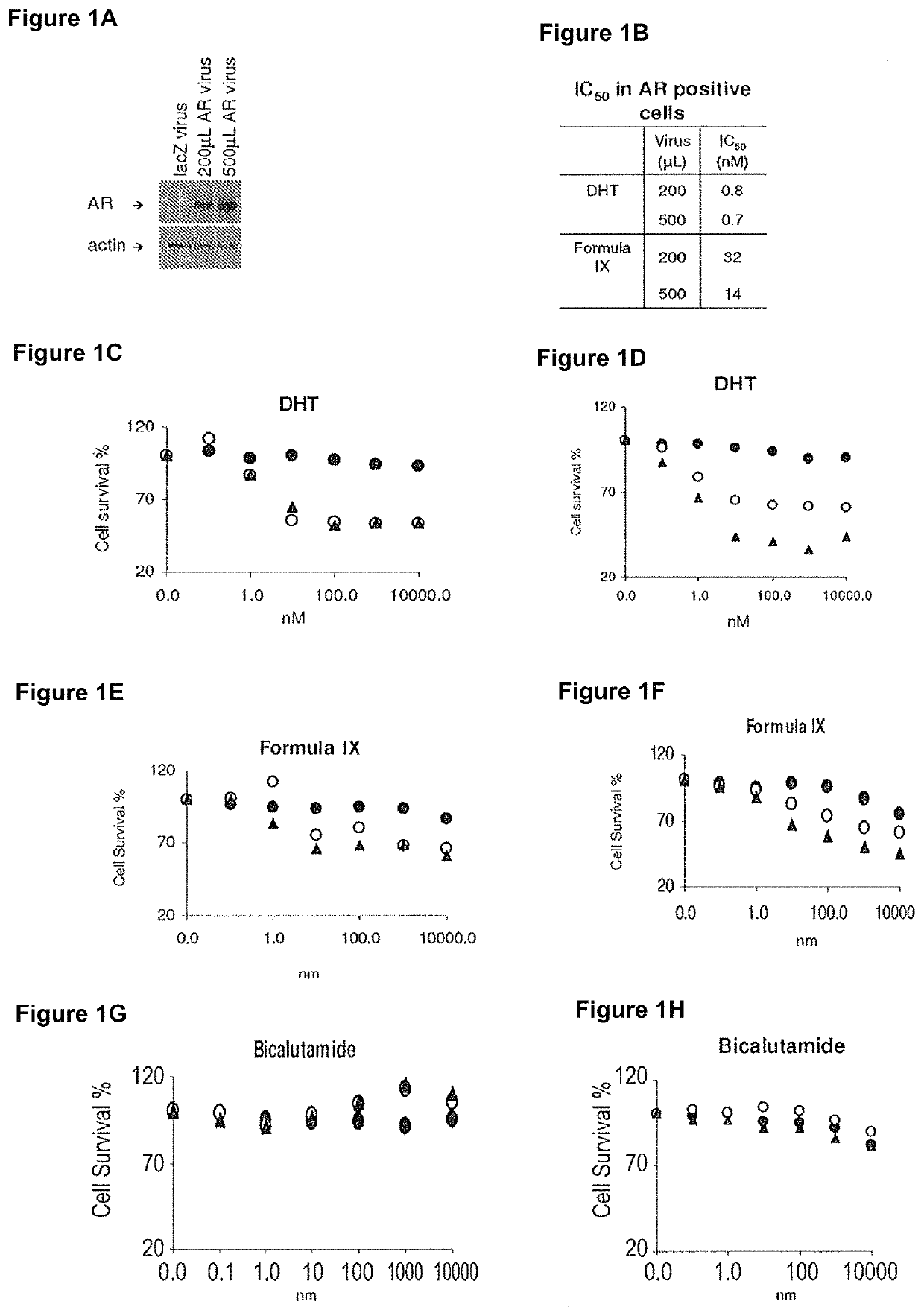
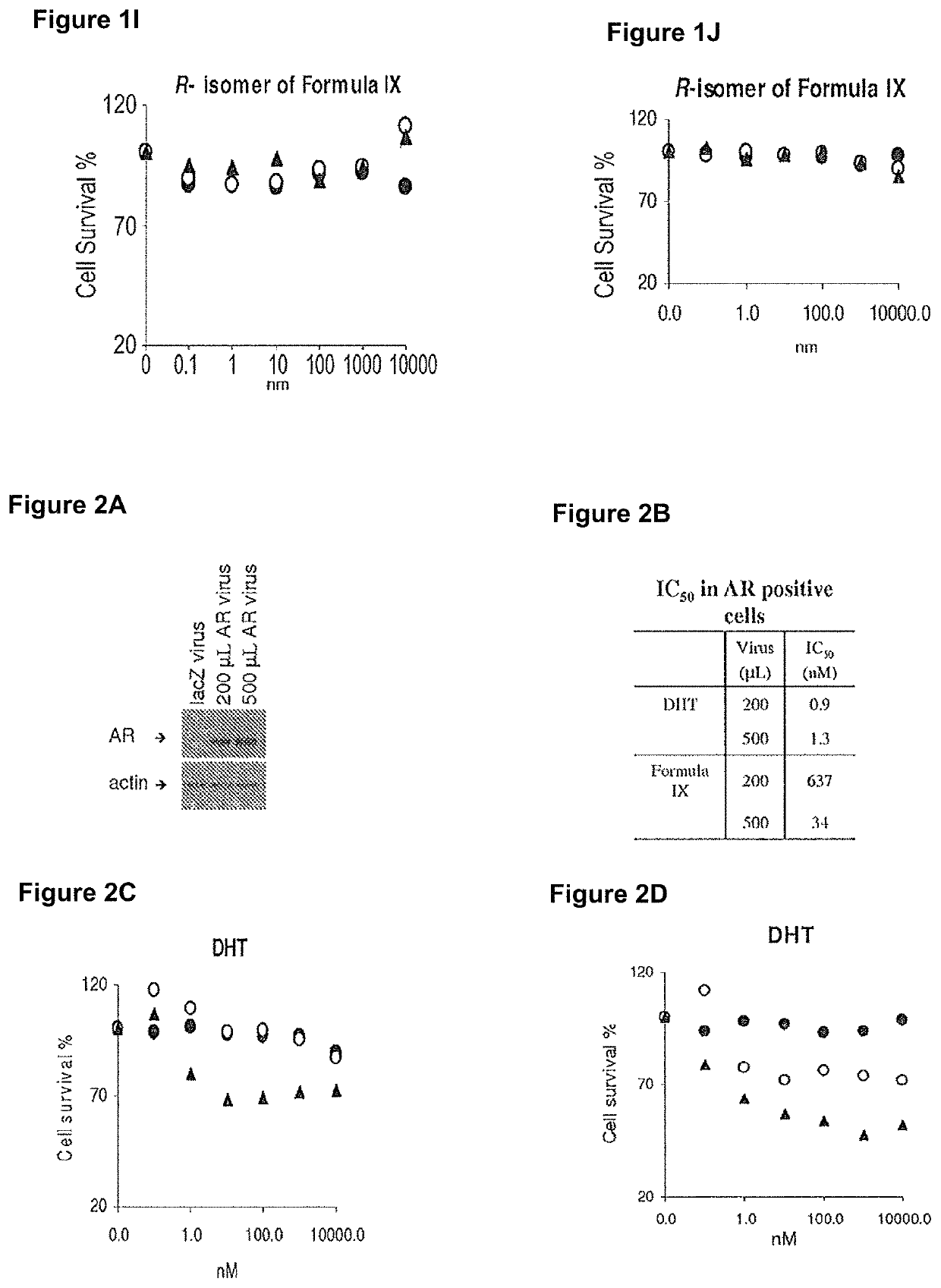
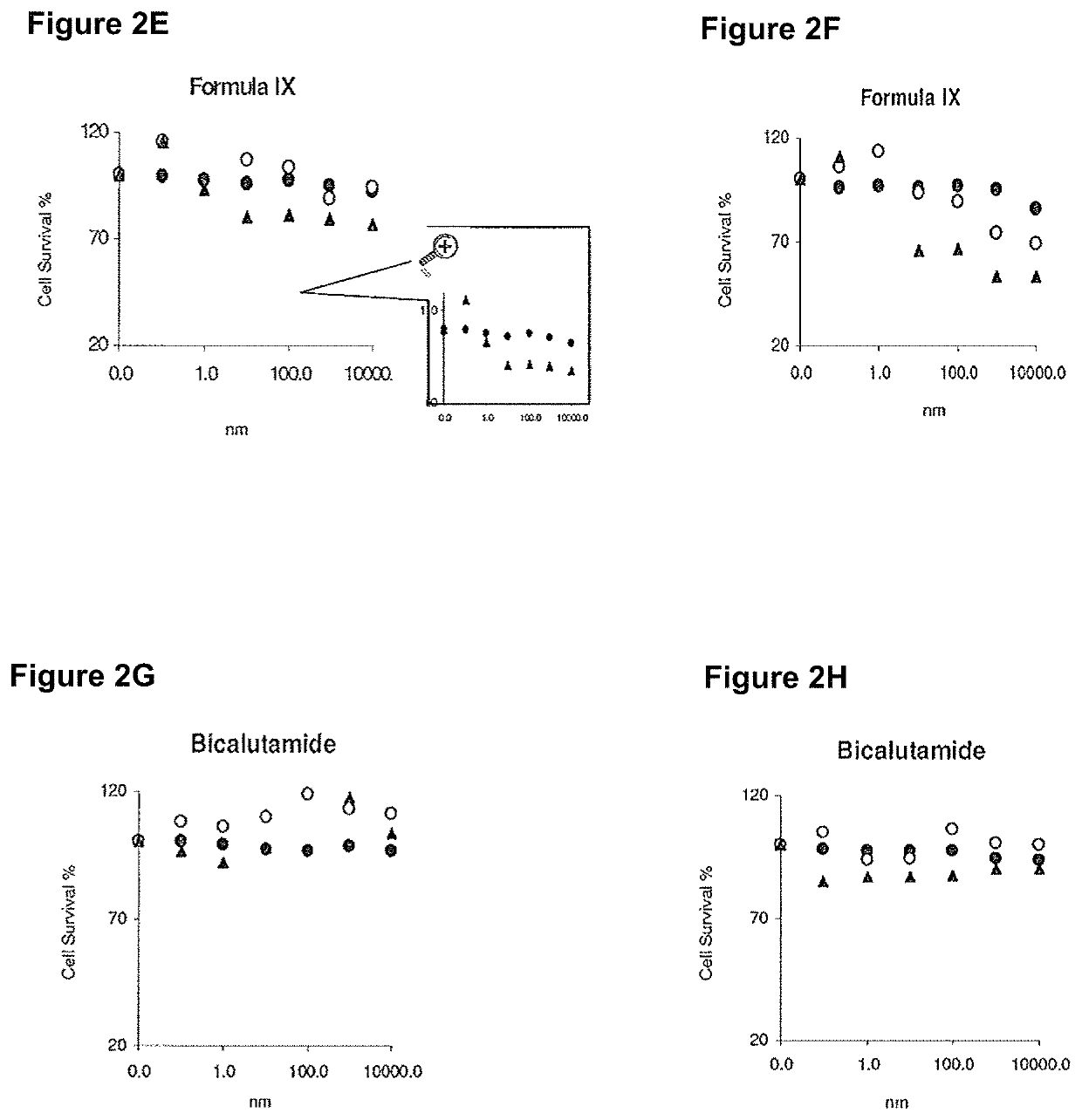
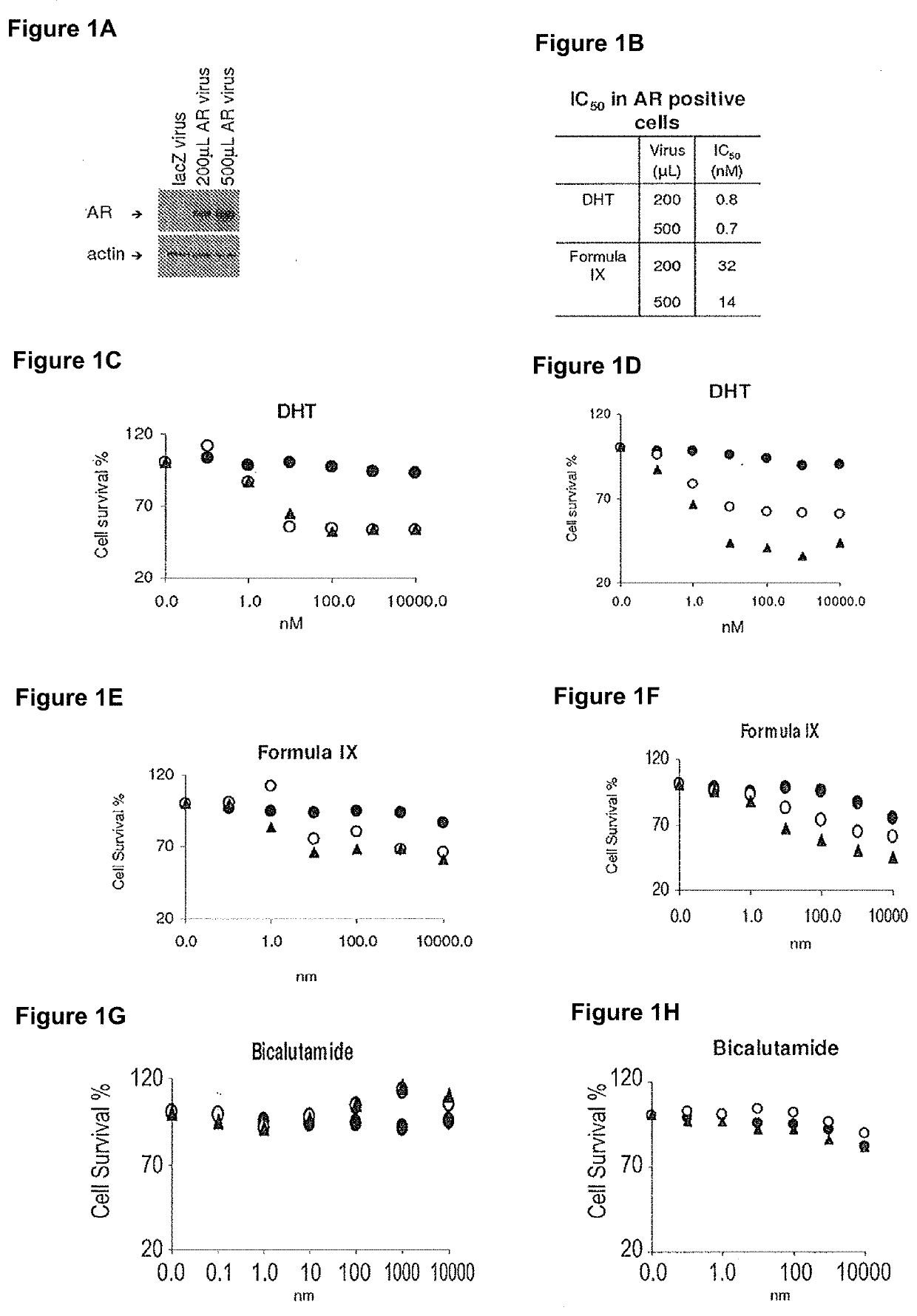
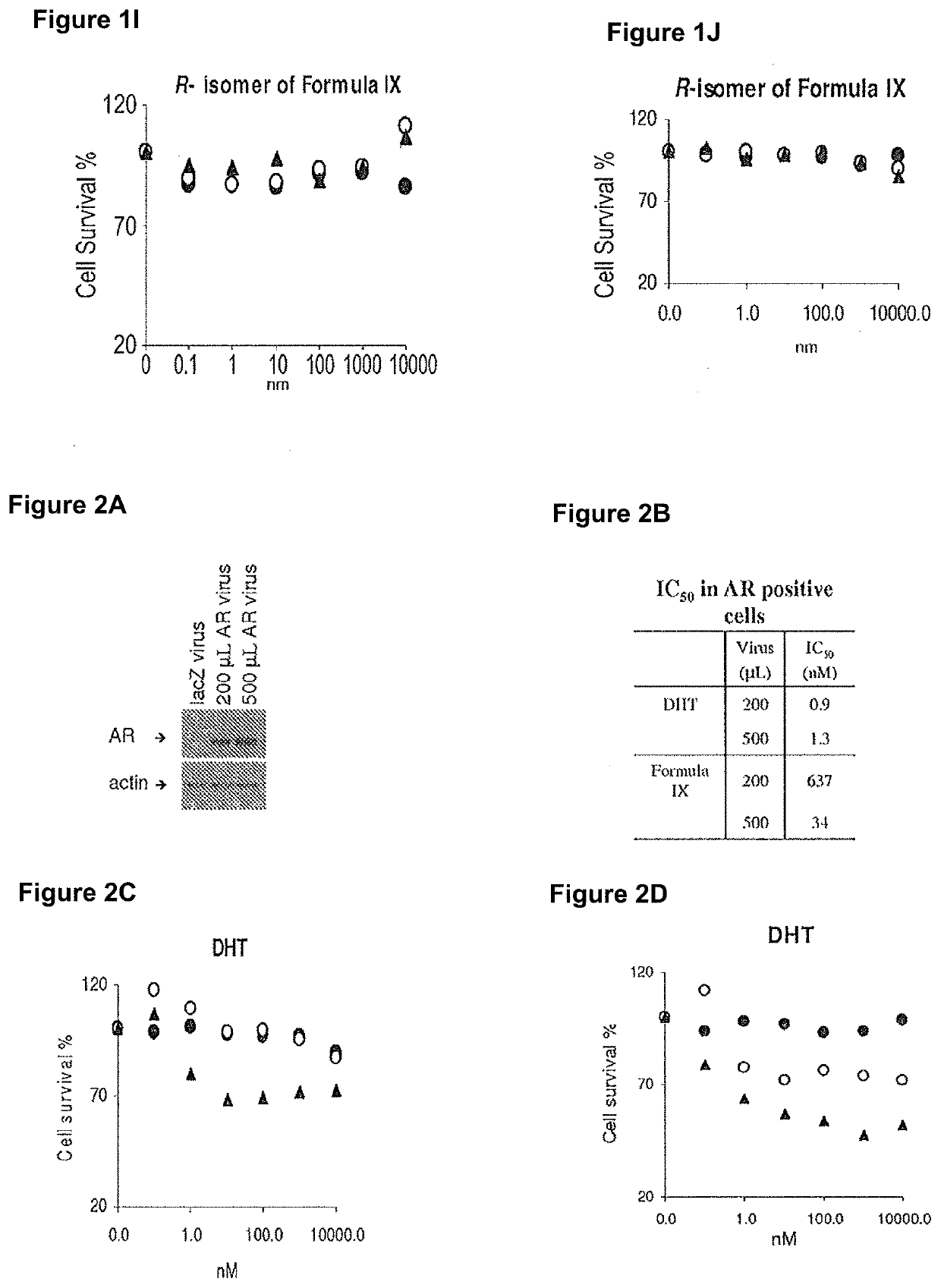
Method of treating er mutant expressing breast cancers with selective androgen receptor modulators (SARMS)
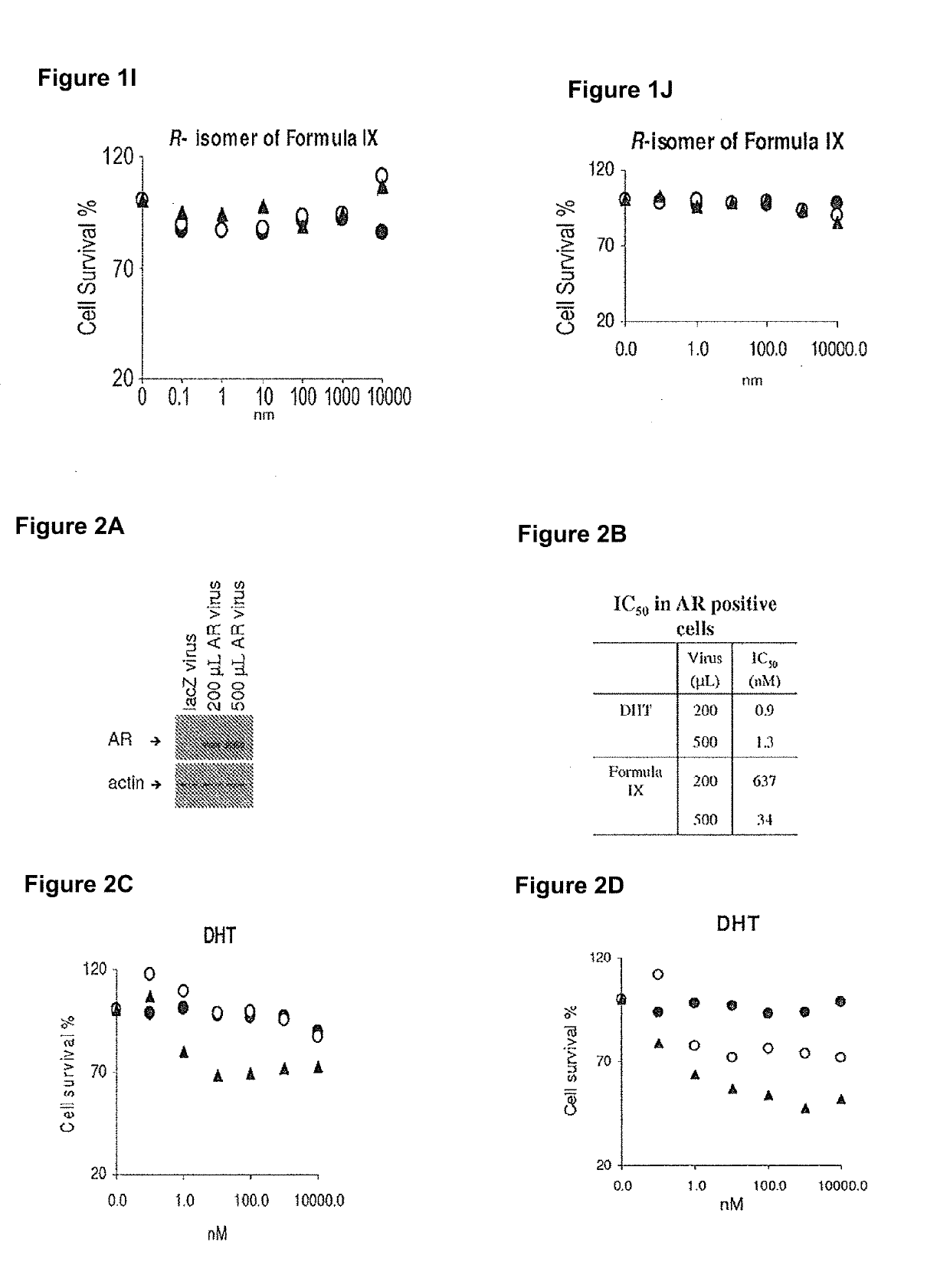
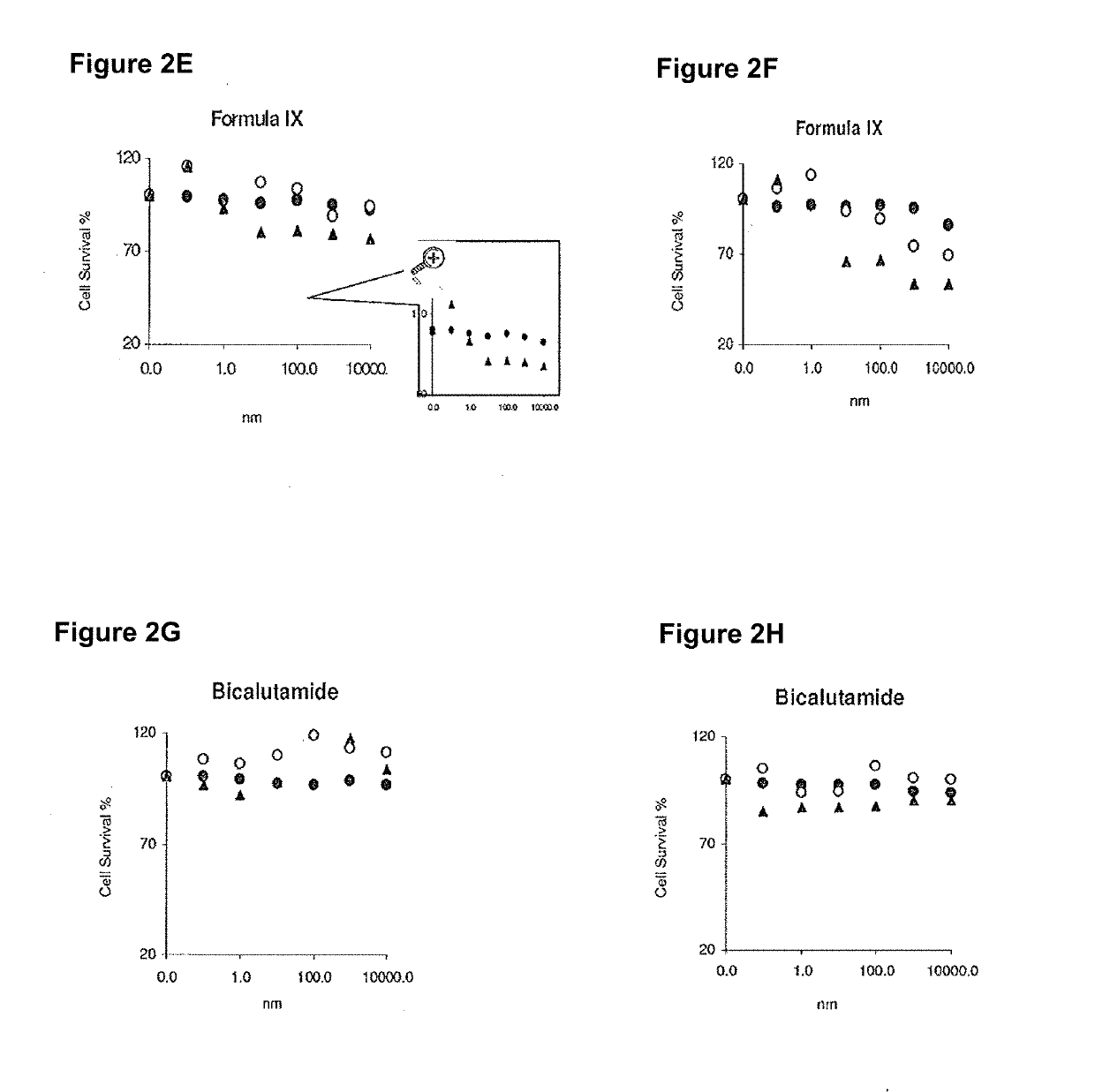
This invention relates to the treatment of breast cancer in a subject, for example a female subject. Including methods of: treating metastatic breast cancer; refractory breast cancer; AR-positive breast cancer; AR-positive refractory breast cancer; AR-positive metastatic breast cancer; AR-positive and ER-positive breast cancer; triple negative breast cancer; advanced breast cancer; breast cancer that has failed selective estrogen receptor modulator (SERM) (tamoxifen, toremifene, raloxifene), gonadotropin-releasing hormone (GnRH) agonist (goserelin), aromatase inhibitor (AI) (letrozole, anastrozole, exemestane), cyclin-dependent kinase 4 / 6 (CDK 4 / 6) inhibitor (palbociclib (Ibrance), ribociclib (Kisqali), abemaciclib (Vorzenio)), mTOR inhibitor (everolimus), trastuzumab (Herceptin, ado-trastuzumab emtansine), pertuzumab (Perjeta), lapatinib, neratinib (Nerlynx), olaparib (Lynparza) (an inhibitor of the enzyme poly ADP ribose polymerase (PARP)), bevacizumab (Avastin), and / or fulvestrant treatments; metastasis in a subject suffering from breast cancer; HER2-positive; and / or treating a subject suffering from ER mutant expressing breast cancer, comprising administering to the subject a therapeutically effective amount of a selective androgen receptor modulator (SARM) compound.
Owner:UNIV OF TENNESSEE RES FOUND
Neratinib compound
InactiveCN107778284AGood reproducibilityHigh purityOrganic active ingredientsOrganic chemistry methodsMedicinal chemistryPilot scale
The invention belongs to the technical field of medicine, and specifically relates to neratinib and a preparation method thereof. The new crystal form of neratinib obtained in the invention has the advantages of high purity, the largest impurity is less than 0.5‰, good stability, and heavy method. The reproducibility is good, and the purity and crystal form can be well reproduced when it is scaled up to the pilot scale.
Owner:TIANJIN HANKANG PHARMA BIOTECH
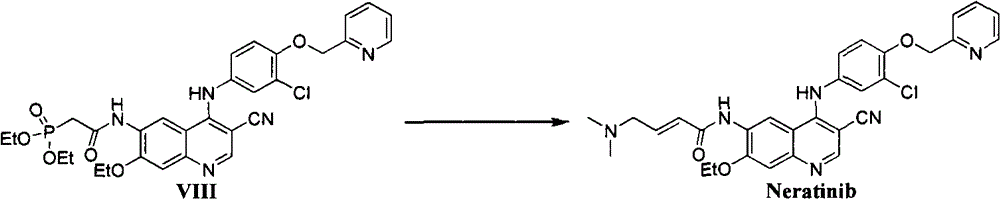
Preparation method of Neratinib intermediate
ActiveCN107325049AThe reaction process is simple to operateHigh yieldOrganic chemistryChemical synthesisMedicinal chemistry
The invention relates to the field of chemical synthesis, and specifically relates to a preparation method of a Neratinib intermediate. A compound (I) is taken as the raw material, and through a series of reactions, the intermediate compound (VIII) of a novel antitumor drug Neratinib is obtained. A high efficient synthesis route is provided and has the characteristics of low cost, few byproducts, high yield, and little environmental pollution.
Owner:江苏阿尔法集团盛基药业(宿迁)有限公司
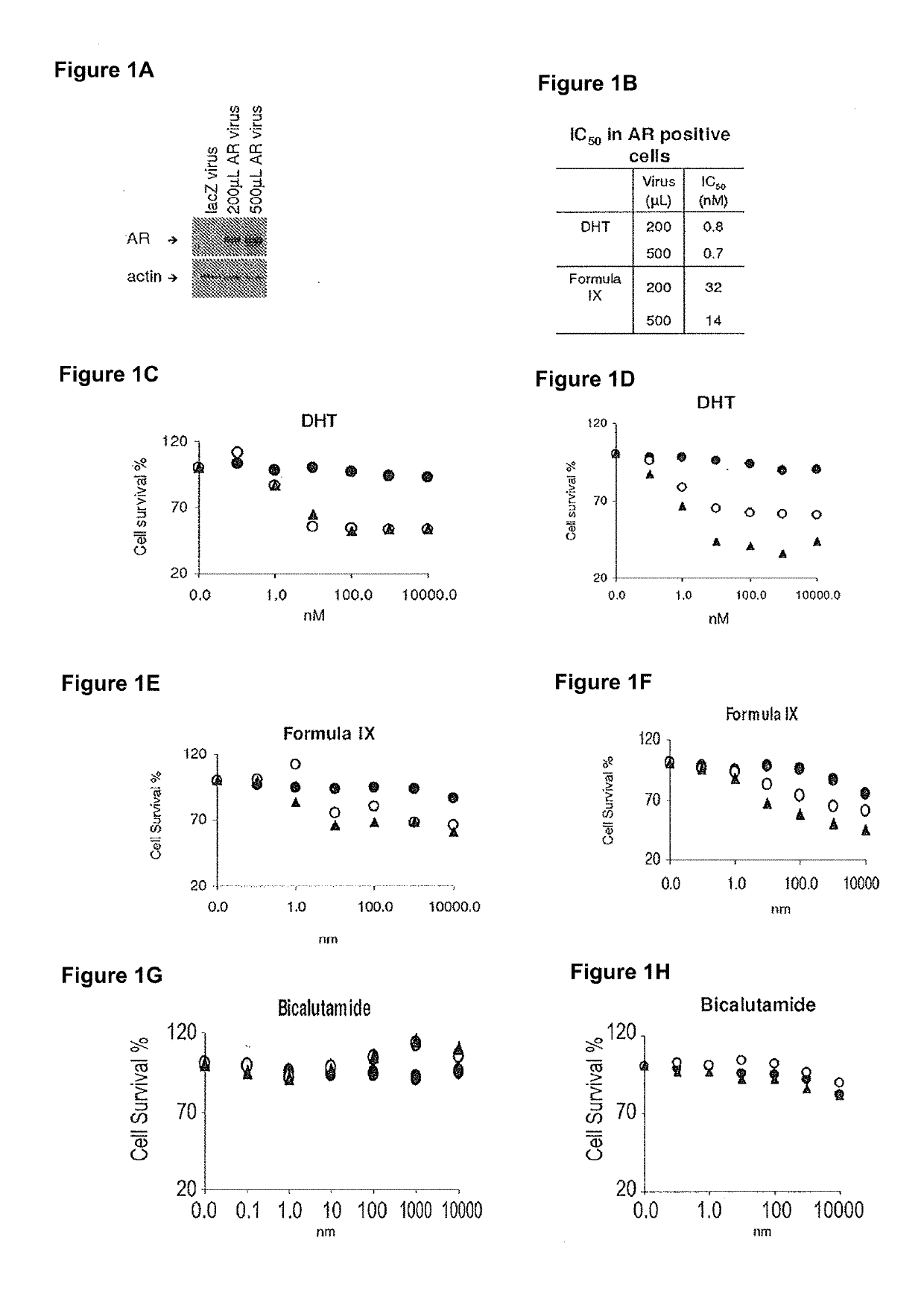
Non-invasive method of evaluating breast cancers for selective androgen receptor modulator (SARM) therapy
This invention relates to the treatment of breast cancer in a subject, for example a female subject. Including methods of: treating metastatic breast cancer; refractory breast cancer; AR-positive breast cancer; AR-positive refractory breast cancer; AR-positive metastatic breast cancer; AR-positive and ER-positive breast cancer; triple negative breast cancer; advanced breast cancer; breast cancer that has failed selective estrogen receptor modulator (SERM) (tamoxifen, toremifene, raloxifene), gonadotropin-releasing hormone (GnRH) agonist (goserelin), aromatase inhibitor (AI) (letrozole, anastrozole, exemestane), cyclin-dependent kinase 4 / 6 (CDK 4 / 6) inhibitor (palbociclib (Ibrance), ribociclib (Kisqali), abemaciclib (Vorzenio)), mTOR inhibitor (everolimus), trastuzumab (Herceptin, ado-trastuzumab emtansine), pertuzumab (Perjeta), lapatinib, neratinib (Nerlynx), olaparib (Lynparza) (an inhibitor of the enzyme poly ADP ribose polymerase (PARP)), bevacizumab (Avastin), and / or fulvestrant treatments; metastasis in a subject suffering from breast cancer; HER2-positive; treating a subject suffering from ER mutant expressing breast cancer and / or treating breast cancer in a subject, by first determining the 18F-16β-fluoro-5α-dihydrotestosterone (18F-DHT) tumor uptake and identifying said subject as having AR-positive breast cancer based on 18F-DHT tumor uptake, comprising administering to the subject a therapeutically effective amount of a selective androgen receptor modulator (SARM) compound.
Owner:UNIV OF TENNESSEE RES FOUND
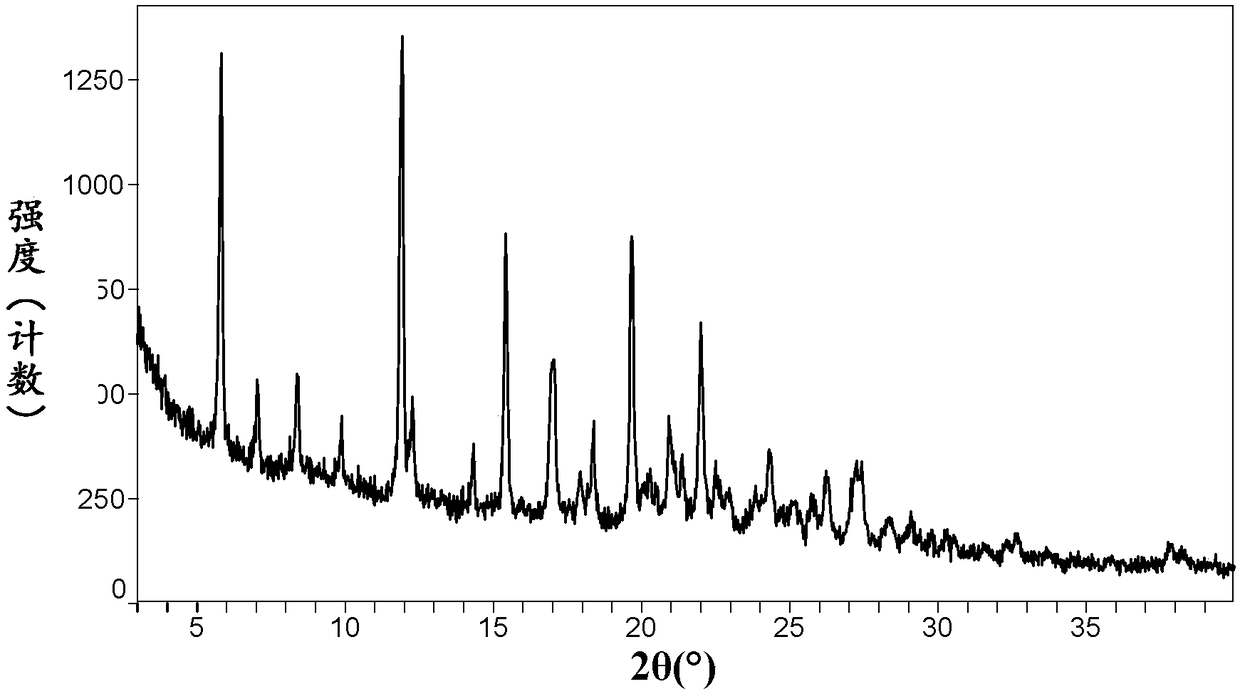
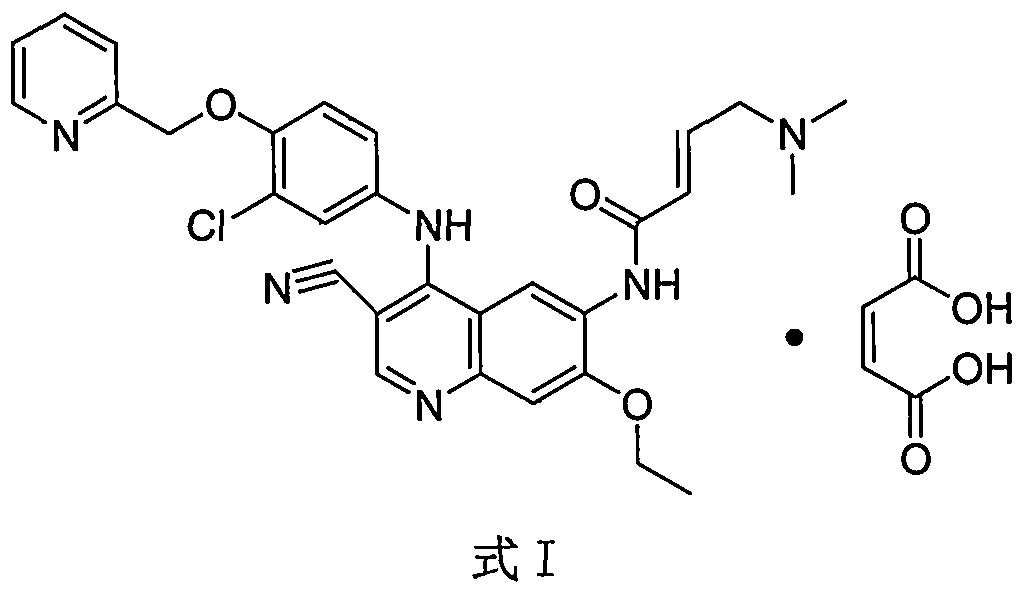
Crystal form of neratinib dimaleate, and preparation method and pharmaceutical composition thereof
InactiveCN108299391AGood solubility in waterImprove bioavailabilityOrganic active ingredientsOrganic chemistry methodsSolubilityMedicinal chemistry
The invention relates to a crystal form of neratinib dimaleate. Compared with the prior art, the crystal form of neratinib dimaleate in the invention has better solubility in water. The invention alsorelates to a preparation method for the crystal form of neratinib dimaleate, a pharmaceutical composition of neratinib dimaleate and application of neratinib dimaleate to preparation of drugs used for treating, inhibiting and / or preventing cancers.
Owner:SOLIPHARMA
Neratinib hydrochloride crystal form and preparation method thereof
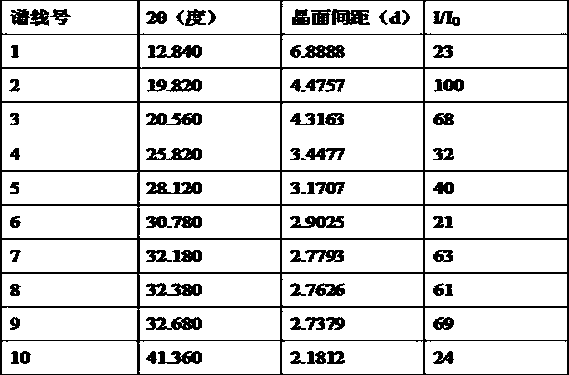
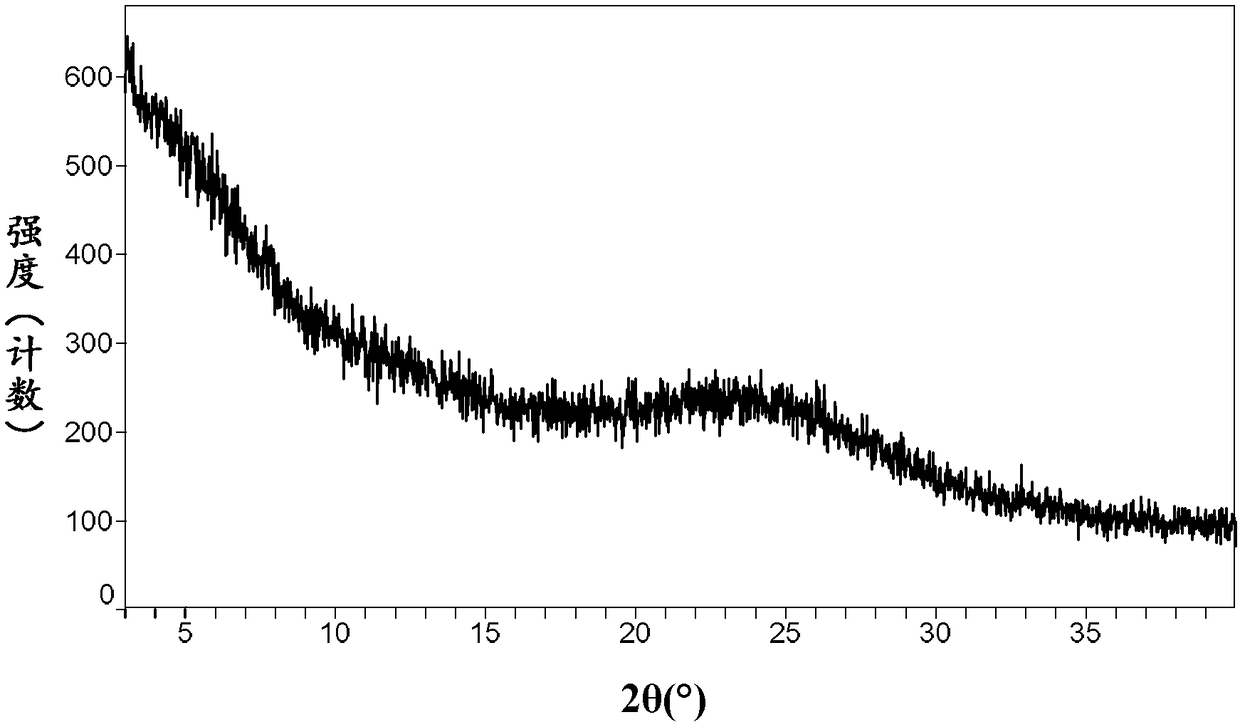
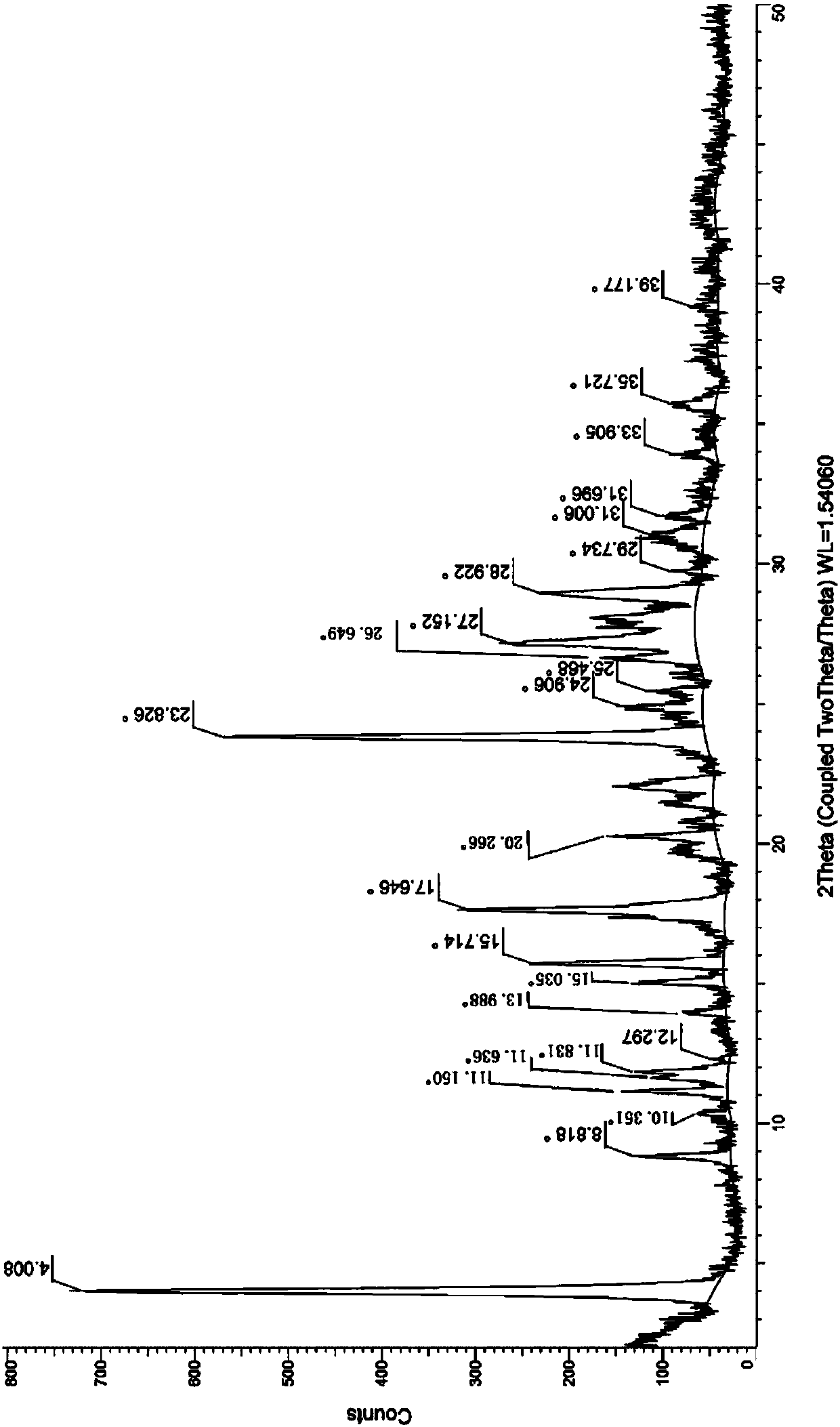
The invention relates to a neratinib hydrochloride new crystal form I and a preparation method thereof, and belongs to the technical field of medicines. An X-ray powder diffraction pattern of the neratinib hydrochloride crystal form I has characteristic absorption peaks when the reflection angle, namely 2*theta is 4.0+ / -0.2 degrees, 8.8+ / -0.2 degrees, 11.1+ / -0.2 degrees, 15.7+ / -0.2 degrees, 17.6+ / -0.2 degrees, 20.2+ / -0.2 degrees, 23.8+ / -0.2 degrees, 26.6+ / -0.2 degrees, 27.1+ / -0.2 degrees and 28.9+ / -0.2 degrees, and the crystal form I has the excellent stability and purity; and additionally, the preparation method of the crystal form I is simple, easy to operate, mild in reaction condition, small in type and using quantity of organic solvents and environmentally friendly, and facilitates industrialization innovation.
Owner:JIANGSU CHUANGUO PHARMA CO LTD +2
Method of treating ER mutant expressing breast cancers with selective androgen receptor modulators (SARMs)
This invention relates to the treatment of breast cancer in a subject, for example a female subject. Including methods of: treating metastatic breast cancer; refractory breast cancer; AR-positive breast cancer; AR-positive refractory breast cancer; AR-positive metastatic breast cancer; AR-positive and ER-positive breast cancer; triple negative breast cancer; advanced breast cancer; breast cancer that has failed selective estrogen receptor modulator (SERM) (tamoxifen, toremifene, raloxifene), gonadotropin-releasing hormone (GnRH) agonist (goserelin), aromatase inhibitor (AI) (letrozole, anastrozole, exemestane), cyclin-dependent kinase 4 / 6 (CDK 4 / 6) inhibitor (palbociclib (Ibrance), ribociclib (Kisqali), abemaciclib (Vorzenio)), mTOR inhibitor (everolimus), trastuzumab (Herceptin, ado-trastuzumab emtansine), pertuzumab (Perjeta), lapatinib, neratinib (Nerlynx), olaparib (Lynparza) (an inhibitor of the enzyme poly ADP ribose polymerase (PARP)), bevacizumab (Avastin), and / or fulvestrant treatments; metastasis in a subject suffering from breast cancer; HER2-positive; and / or treating a subject suffering from ER mutant expressing breast cancer, comprising administering to the subject a therapeutically effective amount of a selective androgen receptor modulator (SARM) compound.
Owner:UNIV OF TENNESSEE RES FOUND
A kind of preparation method of antineoplastic drug neratinib maleate
ActiveCN105330646BMild reaction conditionsRaw materials are easy to getCarboxylic acid salt preparationNitro compoundPtru catalyst
The invention provides a preparation method of antineoplastic drug maleic acid neratinib. The defects in the prior art are overcome. The preparation method comprises the steps that the formula II and the formula III are coupled to form the formula IV under the effect of a catalyst; a nitro-compound IV is reduced under the effect of a reduction system to form a formula V; an amino compound V and a formula VI are condensed to obtain neratinib VII, and then the neratinib VII and maleic acid form a salt to obtain the maleic acid neratinib I. By the adoption of the technical route, the preparation method has the advantages that the synthetic route is short, reaction conditions are mild, the yield is high, raw materials are wide in source, and environmental protection is achieved.
Owner:SHANGHAI XUNHE PHARMA TECH CO LTD
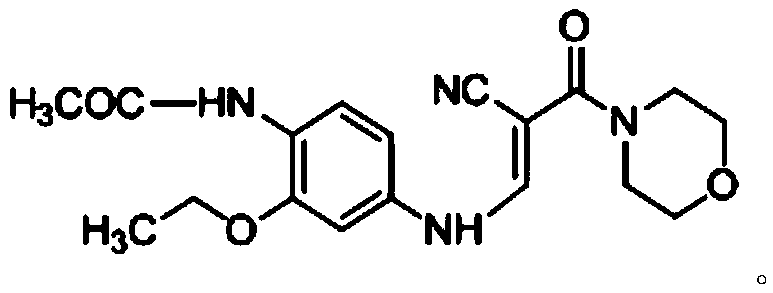
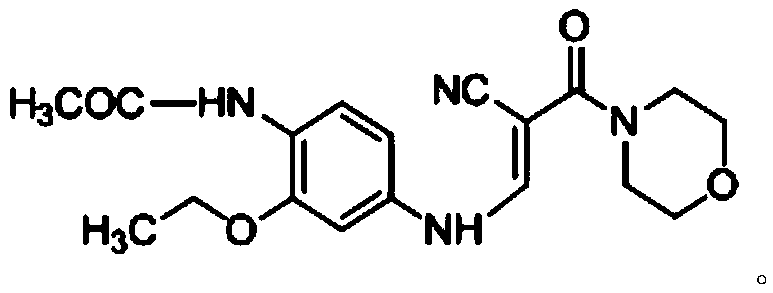
Preparation method of Neratinib intermediate
ActiveCN105503720ALow reaction temperatureAvoid high temperature reactionOrganic chemistryAcetamideNeratinib
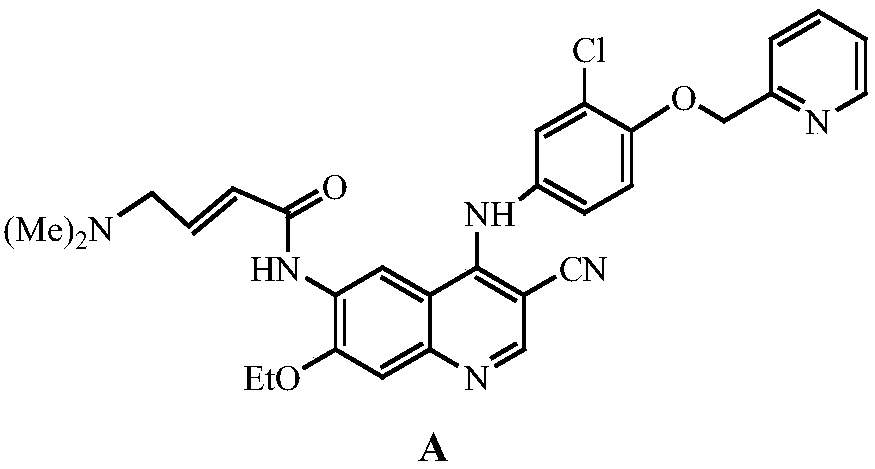
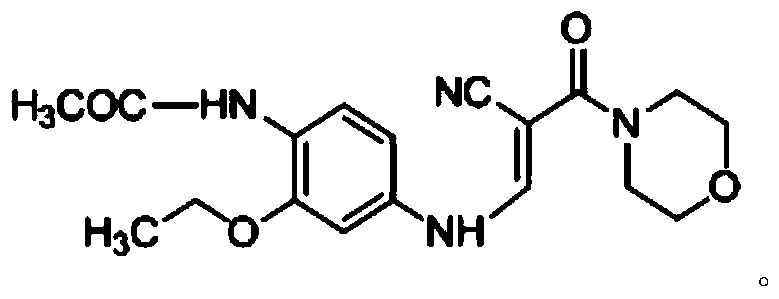
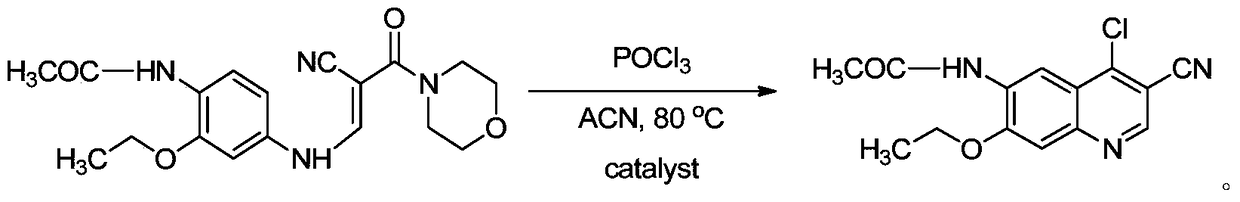
The invention discloses a preparation method of a Neratinib intermediate. The preparation method comprises the following steps: by taking a compound A and POCl3 as raw materials, and a nitrogenous compound as a catalyst, allowing the compound A and POCl3 to react with the nitrogenous compound to obtain the Neratinib intermediate, wherein the compound A is (E)-N-(4-((2-cyano-3-morpholino-3-oxopropionic-1-alky-1-base)amino)-2-ethoxy phenyl) acetamide. According to the method, the nitrogenous compound is used as the catalyst, high temperature is not needed in the reaction, the reaction can be performed at the temperature of 65 to 75 DEG C, compared with the traditional method requiring the high temperature of 200 to 250 DEG C, the reaction temperature is greatly reduced, the high-temperature reaction is avoided, operation safety is ensured, the reaction yield is also increased to 45 to 57 percent, and the yield is obviously increased.
Owner:CHONGQING WEIPENG PHARMA
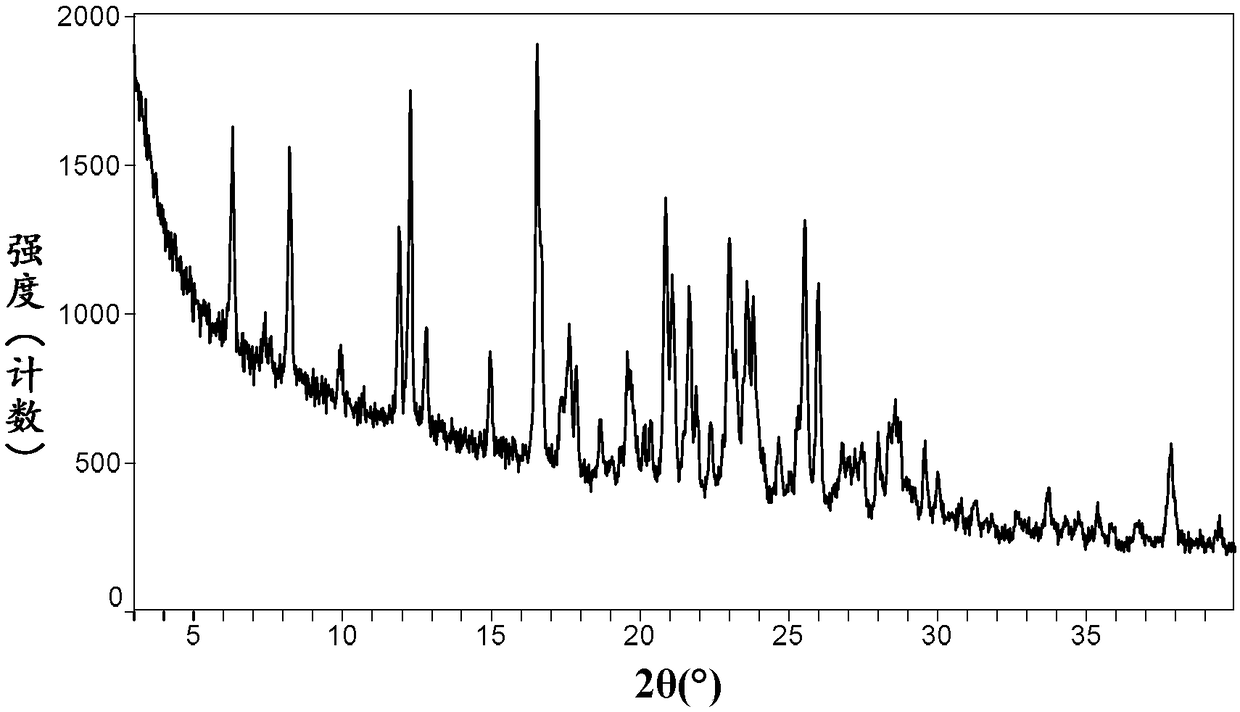
Crystal forms and preparation method of neratinib free alkali
InactiveCN108373467AOrganic active ingredientsOrganic chemistry methodsChemical qualityProcess efficiency
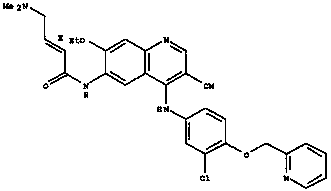
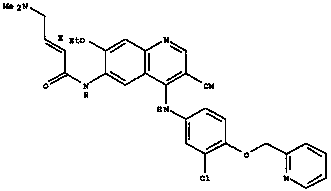
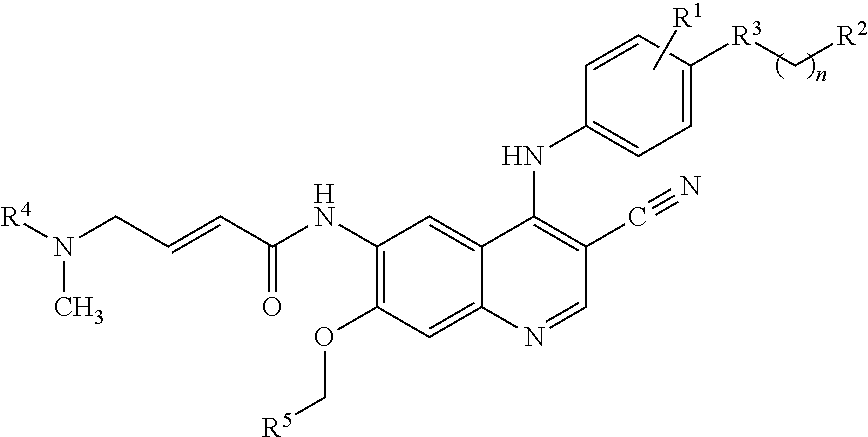
The invention relates to crystal forms and a preparation method of neratinib free alkali. Specifically, the invention discloses new crystal forms of a compound shown in a formula (I) or a solvate thereof, namely a crystal form III, a crystal form IV, a crystal form V and a crystal form VI respectively. The new crystal forms disclosed by the invention are beneficial to separation and purification of a compound free alkali shown in the formula (I), and process efficiency and chemical quality of a product are greatly improved. (The formula (I) is as shown in the description.).
Owner:JIANGSU CHUANGUO PHARMA CO LTD +2
Treatment regimen utilizing neratinib for breast cancer
An extended regimen for treatment of HER-2 / neu overexpressed / amplified cancer is described, with involves delivering a course of neratinib therapy to HER-2 / neu overexpressed / amplified cancer patients following the completion of surgical and adjuvant therapy. The neratinib regimen may be continued for upwards of twelve months to five years. Also provided are pharmaceutical kits designed to facilitate compliance with the regimen.
Owner:WYETH LLC
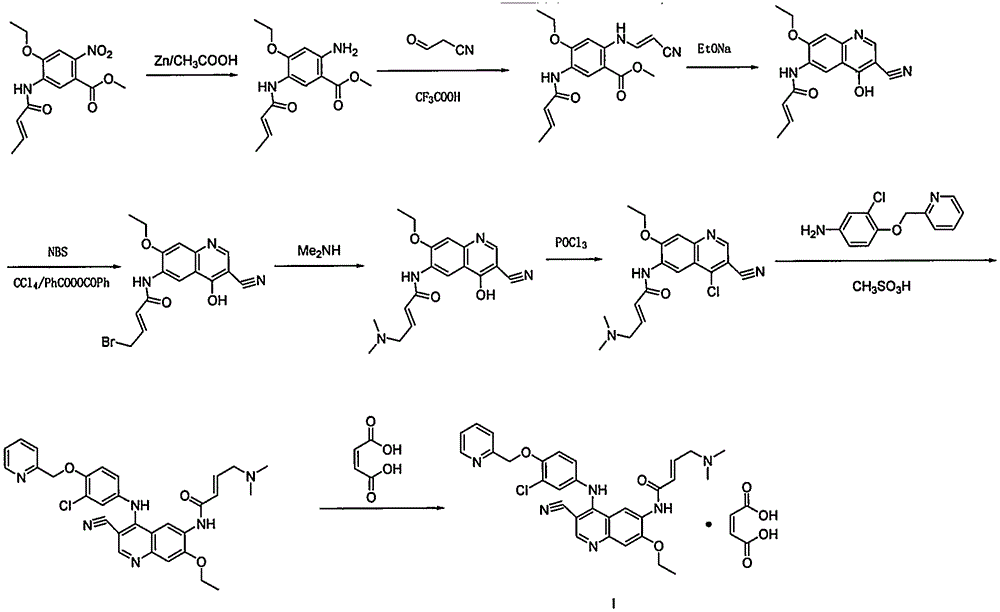
Method for synthesizing neratinib intermediate
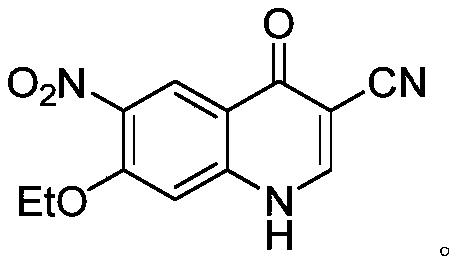
ActiveCN110845409AEase of commercializing bulk purchasesEasy to operateOrganic chemistryBenzoic acidQuinoline
The invention aims to provide a new route for preparing neratinib key intermediate 3-cyano-4-oxo-6-nitro-7-ethoxy-1, 4-dihydroquinoline, and the route takes 2-halo-4-fluoro-5-nitrobenzoic acid as a starting material, and the synthesis of the target intermediate is conveniently realized through four steps of reactions.
Owner:WISDOM PHARM CO LTD
A kind of preparation method of neratinib or its pharmaceutically acceptable salt pharmaceutical composition
ActiveCN106913529BImprove stabilityDegraded impurity reductionOrganic active ingredientsPharmaceutical non-active ingredientsPharmaceutical SubstancesPharmaceutical formulation
The invention relates to a preparation method of a medicinal composition of neratinib or a medicinal salt of the neratinib. A medicinal preparation with good stability can be prepared through keeping the maximum water content of a granule in the granulation period to be 10% or less or / and controlling the water content of a final granule or the medicinal composition to be 2% or less in the preparation process of the neratinib granule, and the medicinal preparation has good dissolving property.
Owner:JIANGSU HENGRUI MEDICINE CO LTD +1
A method for synthesizing neratinib intermediate 3-cyano-4-chloro-6-amino-7-ethoxyquinoline
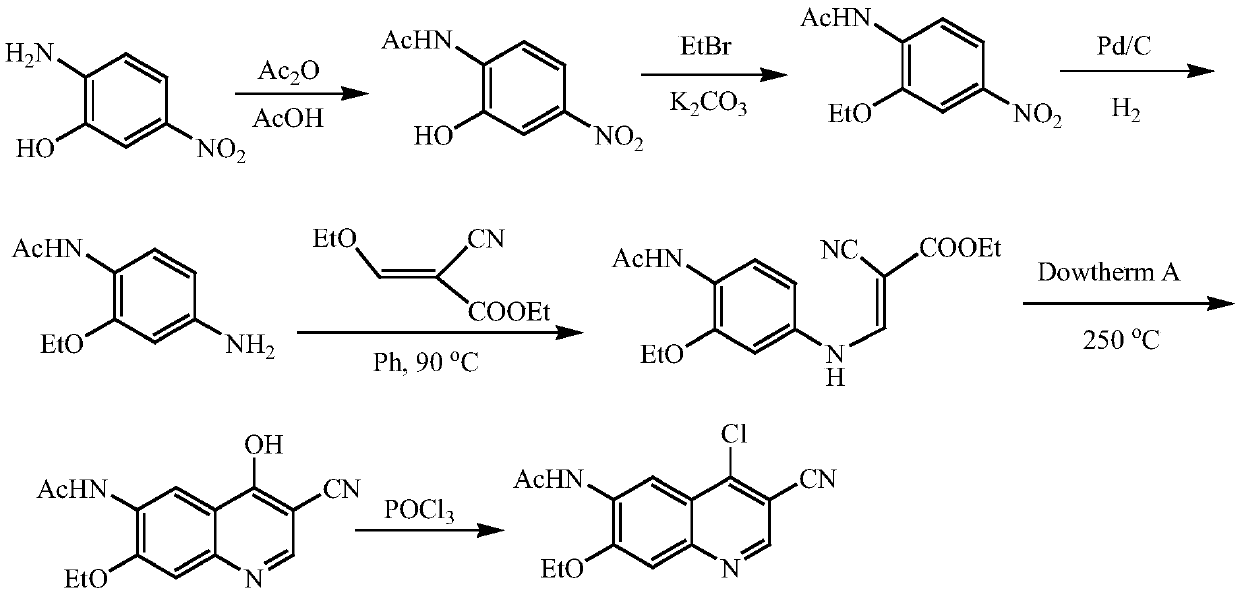
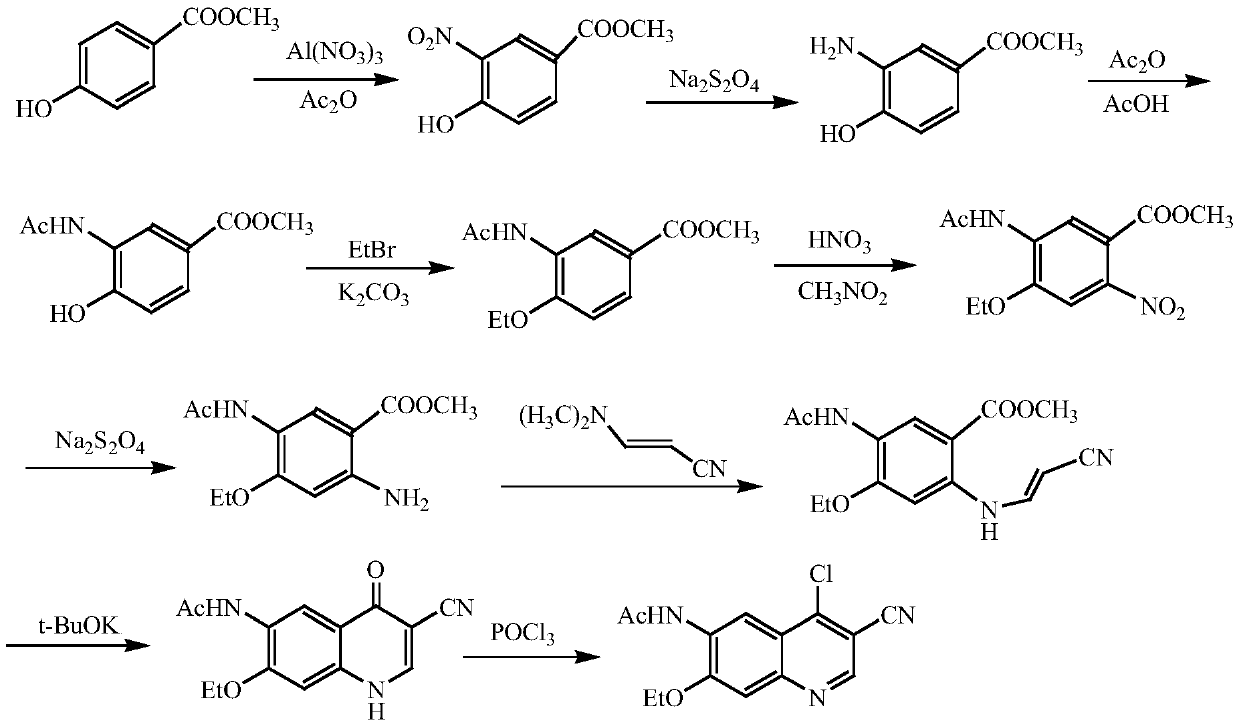
A method of synthesizing a neratinib intermediate that is 3-cyano-4-chloro-6-amino-7-ethoxyquinoline is disclosed. The method includes (1) subjecting methyl 4-ethoxy-2-chloro-5-nitrobenzoate and 3-amino acrylonitrile to a condensation reaction under the action of a catalyst 1 to obtain 2-(4-ethoxy-2-chloro-5-nitrobenzoyl)-3-amino acrylonitrile; (2) subjecting the 2-(4-ethoxy-2-chloro-5-nitrobenzoyl)-3-amino acrylonitrile to a cyclization reaction to obtain 3-cyano-4-oxo-6-nitro-7-ethoxy-1,4-dihydroquinoline; (3) subjecting the 3-cyano-4-oxo-6-nitro-7-ethoxy-1,4-dihydroquinoline and phosphorus oxychloride to a chlorination reaction to obtain 3-cyano-4-chloro-6-nitro-7-ethoxyquinoline; and (4) subjecting the 3-cyano-4-chloro-6-nitro-7-ethoxyquinoline and hydrazine hydrate to a reduction reaction under the action of a catalyst 2 to obtain a target product. According to the method, synthetic steps are few, reaction conditions are mild, agents are cheap and easily available, operation is simple and the total yield is high. The method provides a novel route for preparation of neratinib and the intermediate.
Owner:山东金吉利新材料有限公司
The preparation method of neratinib intermediate
ActiveCN105503720BLow reaction temperatureAvoid high temperature reactionOrganic chemistryCompound aReaction temperature
The invention discloses a preparation method of a Neratinib intermediate. The preparation method comprises the following steps: by taking a compound A and POCl3 as raw materials, and a nitrogenous compound as a catalyst, allowing the compound A and POCl3 to react with the nitrogenous compound to obtain the Neratinib intermediate, wherein the compound A is (E)-N-(4-((2-cyano-3-morpholino-3-oxopropionic-1-alky-1-base)amino)-2-ethoxy phenyl) acetamide. According to the method, the nitrogenous compound is used as the catalyst, high temperature is not needed in the reaction, the reaction can be performed at the temperature of 65 to 75 DEG C, compared with the traditional method requiring the high temperature of 200 to 250 DEG C, the reaction temperature is greatly reduced, the high-temperature reaction is avoided, operation safety is ensured, the reaction yield is also increased to 45 to 57 percent, and the yield is obviously increased.
Owner:CHONGQING WEIPENG PHARMA
A kind of purification method of Neratinib
The invention relates to a method for purifying neratinib. The method provided by the invention is capable of effectively reducing the content of impurities in neratinib and preparing high-purity neratinib, and moreover is simple in operation, high in yield and very applicable to industrial production.
Owner:ZHEJIANG HISUN PHARMA CO LTD
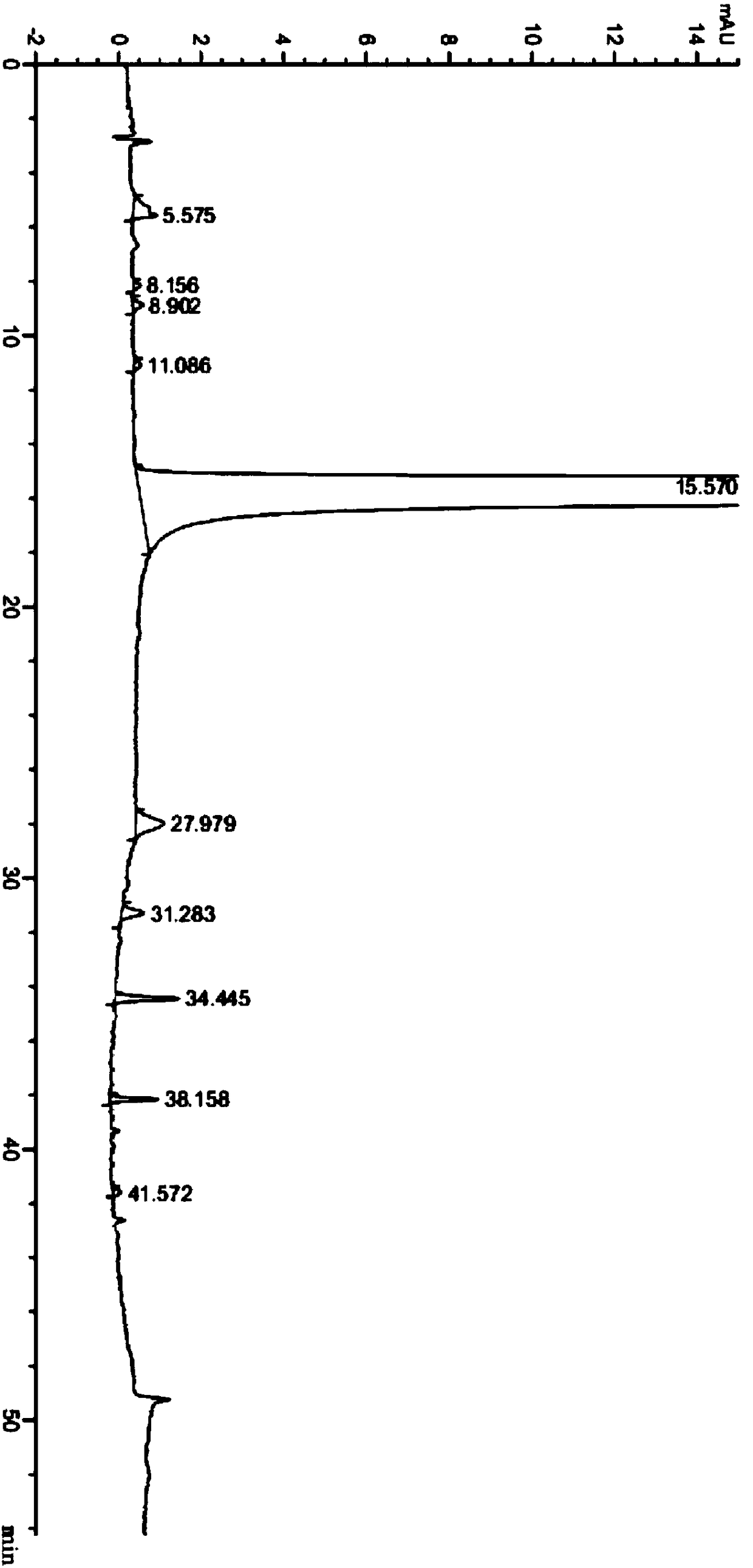
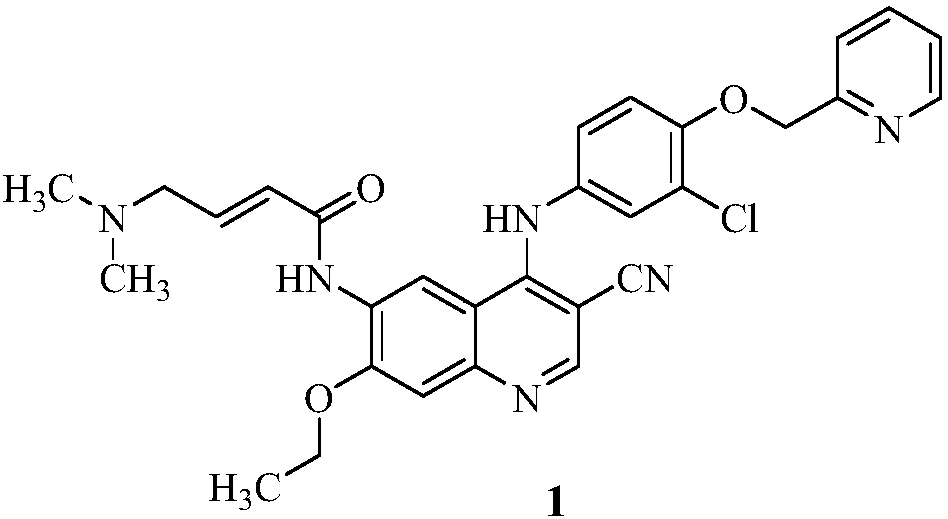
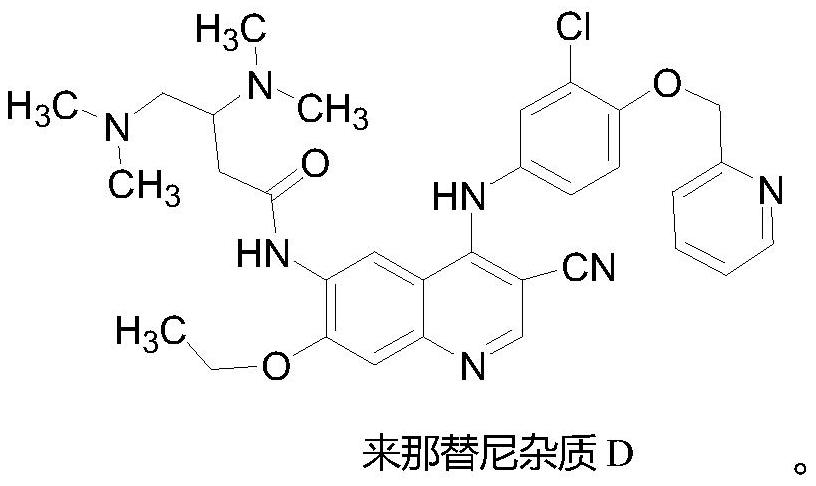
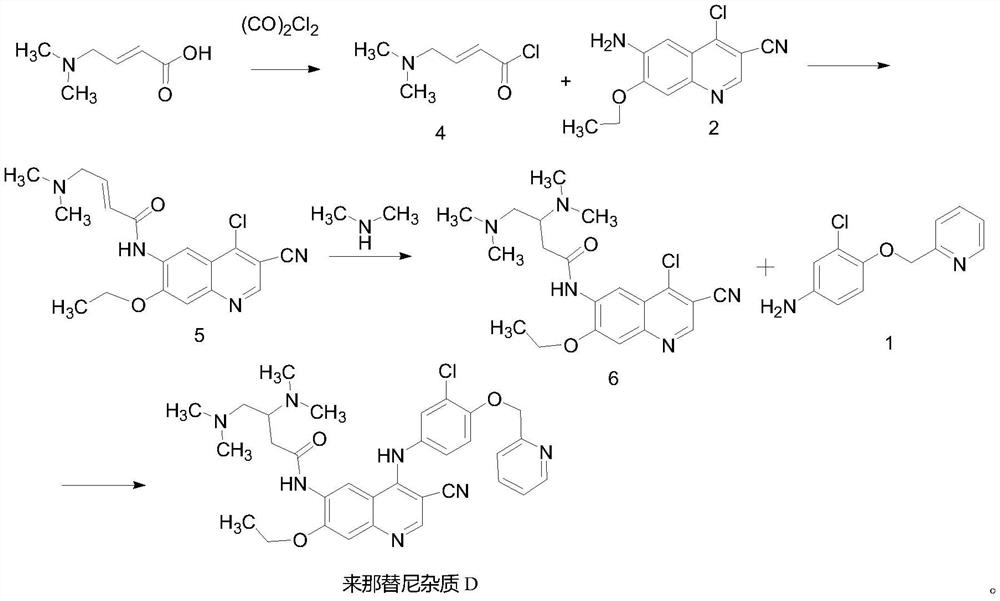
A kind of preparation method of neratinib impurity d
The invention provides a preparation method of neratinib impurity D, which uses N,N-dimethylaminotrans-crotonic acid as a raw material, and obtains the target product neratinib impurity D through 4-step reaction. The present invention uses zinc nitrate as a specific catalyst and acetonitrile as a solvent to prepare neratinib impurity D at a temperature of 60-85°C, and the yield can reach more than 48%, and the product can be realized without column treatment. The purity of more than 99.0% also solves the problems of ‑CN hydrolysis in the quinoline ring and side chain amide hydrolysis during the reaction process, ensuring the quality of the final product.
Owner:CHONGQING MEDICAL UNIVERSITY
Neratinib dimaleate crystal forms and preparation method thereof
InactiveCN108299394AImprove bioavailabilityEasy to convertOrganic active ingredientsOrganic chemistry methodsSolubilityAnalytical chemistry
The invention relates to neratinib dimaleate crystal forms and a preparation method thereof. Particularly, the invention discloses three crystal forms of neratinib dimaleate, i.e., crystal forms IV, Vand VI respectively, which are all anhydrous substances of the neratinib dimaleate, wherein the crystal forms IV and V have better solubility than a known crystal form II, the crystal form V has lower hygroscopicity, and the crystal forms cannot be transformed by a relative moisture change. The invention further discloses proper medicine research of the three crystal forms and the preparation method in industrial production.
Owner:JIANGSU CHUANGUO PHARMA CO LTD +2
Preparation method of neratinib
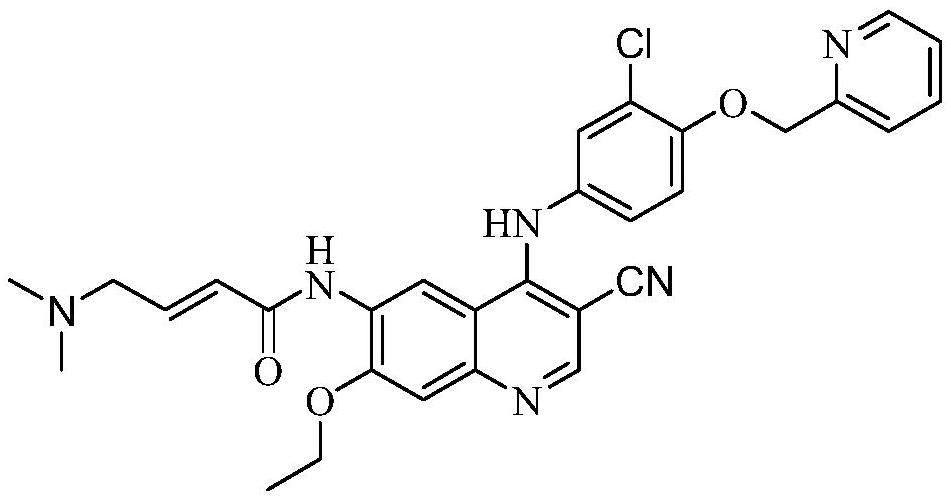
The invention relates to a preparation method of neratinib. The preparation method specifically comprises the steps: (1) in an organic solvent 1, trans-4-dimethylaminocrotonic acid hydrochloride and achloride agent react, and thus a solution containing (e)-4-(dimethylamino)but-2-enoyl chloride (hydrochloride) is obtained; (2) a solution of an organic solvent 2 containing 6-amino-4-[[3-chloro-4-[(pyridine-2-yl)methoxy]phenyl]amino]-3-cyano-7-ethoxyquinoline is added into the solution obtained in the step (1) to react, and then neratinib hydrochloride is obtained; and (3) the neratinib hydrochloride obtained in the step (2) is mixed with water and an organic solvent 3, a reaction is carried out after the pH value is regulated to be 7-10, and then the neratinib is obtained. The synthesis method has the advantages that the yield is high, the product purity is high, the production cost is low, operation is safe, easy and convenient, and large-scale industrial production is easy.
Owner:JIANGSU CHUANGUO PHARMA CO LTD +2
The preparation method of neratinib
The invention relates to a preparation method for neratinib. Specifically, N-[4-[[3-chloro-4-(2-pyridylmethoxy)phenyl]amino]-3-cyano-7-ethoxy-6-quinolyl]-2-diethyl phosphate-acetamide reacts with 2,2-diethoxy-N,N-dimethylethylamine or 2-(dimethylamino)acetaldehyde in the presence of a lithium salt and an alkali to produce neratinib. The method provided by the invention has the advantages of high yield, mild reaction conditions, usage of common and commercially available reagents, a low price, suitability for industrial production and good economic prospects.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD +1
Pharmaceutical compositions for treating breast cancers and methods of uses thereof
PendingUS20210228529A1Overcomes estrogen endocrine resistanceOvercome resistanceSuppositories deliveryOintment deliveryToremifeneEverolimus
This invention relates to the treatment of breast cancer in a subject, and the subject can be either a male or female subject. Including methods of: treating metastatic breast cancer; refractory breast cancer; AR-positive breast cancer; AR-positive refractory breast cancer; AR-positive metastatic breast cancer; AR-positive and ER-positive breast cancer; triple negative breast cancer; advanced breast cancer; breast cancer that has failed selective estrogen receptor modulator (SERM) (tamoxifen, toremifene, raloxifene), gonadotropin-releasing hormone (GnRH) agonist (goserelin), aromatase inhibitor (AI) (letrozole, anastrozole, exemestane), cyclin-dependent kinase 4 / 6 (CDK 4 / 6) inhibitor (palbociclib (Ibrance), ribociclib (Kisqali), lerociclib, abemaciclib (Vorzenio), trilaciclib, lerociclib), mTOR inhibitor (everolimus), trastuzumab (Herceptin, ado-trastuzumab emtansine), pertuzumab (Perjeta), alpelisib (Piqray) (an inhibitor of phosphatidylinositol-3-kinase subunit alpha (PI3Kα)), lapatinib, neratinib (Nerlynx), olaparib (Lynparza) (an inhibitor of the enzyme poly ADP ribose polymerase (PARP)), bevacizumab (Avastin), and / or fulvestrant treatments; metastasis in a subject suffering from breast cancer; HER2 -positive; treating a subject suffering from ER mutant expressing breast cancer and / or treating breast cancer in a subject, by first determining the 18F-16β-fluoro-5α-dihydrotestosterone (18F-DHT) tumor uptake and identifying said subject as having AR-positive breast cancer based on 18F-DHT tumor uptake, comprising administering to the subject a therapeutically effective amount of a selective androgen receptor modulator (SARM) compound and a cyclin-dependent kinase 4 / 6 (CDK 4 / 6) inhibitor.
Owner:UNIV OF TENNESSEE RES FOUND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com