Crystal form of neratinib dimaleate, and preparation method and pharmaceutical composition thereof
A technology of neratinib and maleic acid, applied in the field of medicinal chemistry crystallization, can solve the problems of poor efficacy, low bioavailability of solid pharmaceutical preparations, poor water solubility and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
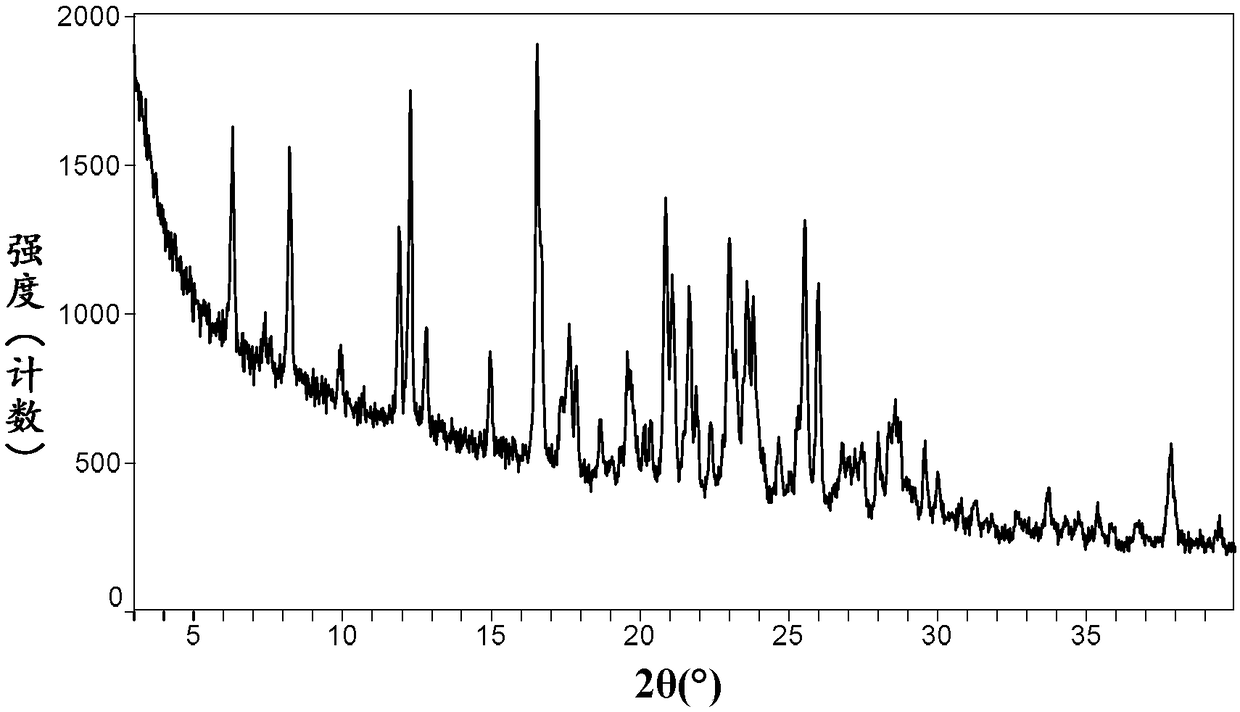
[0096] Preparation Example 1 Preparation of neratinib-maleate monohydrate crystal form II
[0097] Referring to the method of Example 1 in the patent document CN101918390B, neratinib monomaleate monohydrate crystal form II was prepared, and the specific operation was as follows: the crude product (E)-N-{4-[3-chloro-4- (2-pyridylmethoxy)anilino]-3-cyano-7-ethoxy-6-quinolyl}-4-(dimethylamino)-2-butenamide free base (0.100kg , 0.159 mol) was rinsed with a 10% solution of USP purified water in n-propanol (0.082 kg, 0.10 L), then a water:n-propanol solution (0.74 kg, 0.90 L) was added. Maleic acid (0.0191 kg, 0.164 mol) was added and the mixture was rinsed with 10% water: n-propanol (0.082 kg, 0.10 L). The mixture was heated rapidly to 50-60° C. for at least 15 minutes until a solution was obtained. The hot solution was clarified by passing it through a 0.2 Mm filter cartridge preheated to 50-60°C, and the filtrate was collected in a 2L multi-neck flask preheated to 45-55°C. T...
preparation example 2
[0099] Preparation example 2 Preparation of neratinib-maleate anhydrate crystal form I
[0100] Referring to the method of Example 2 in the patent document CN101918390B, neratinib-maleate anhydrate crystal form I was prepared, and the specific operation was as follows: the product (II type) obtained in Preparation Example 1 was dried (50°C, 10mmHg , 24h), to obtain 94.4g (88% yield) of crystals, namely anhydrous (E)-N-{4-[3-chloro-4-(2-pyridylmethoxy)anilino]-3- Cyano-7-ethoxy-6-quinolinyl}-4-(dimethylamino)-2-butenamide maleate (Type I).
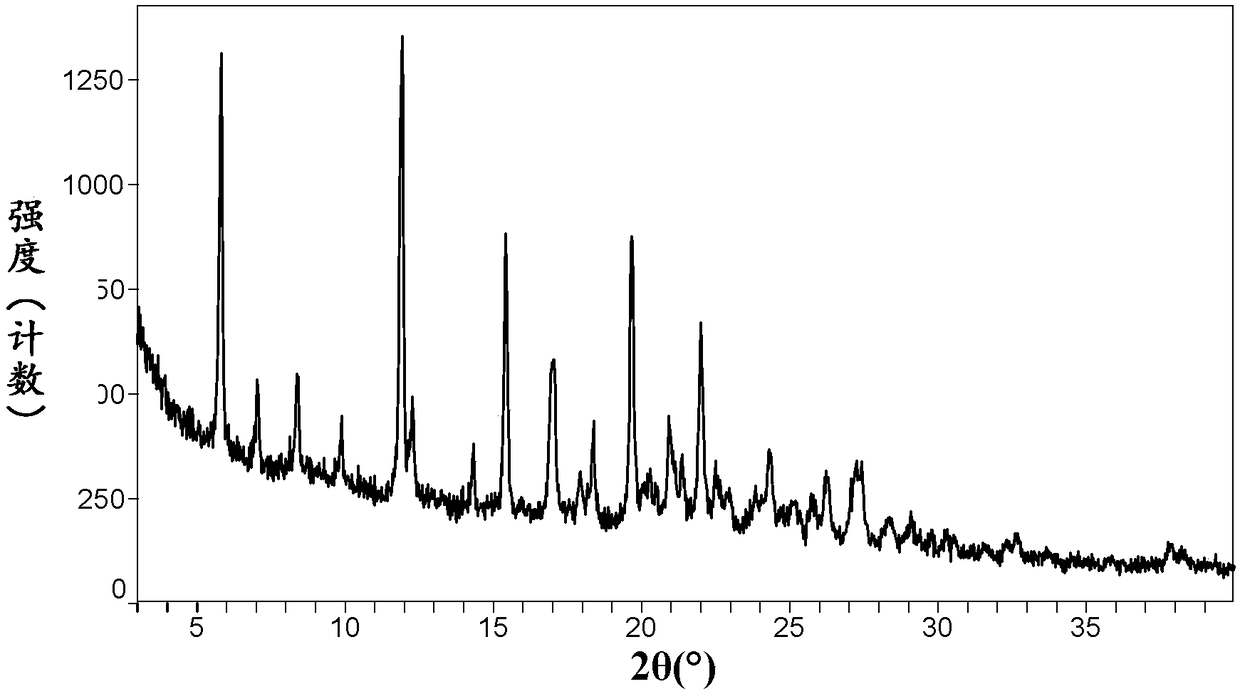
[0101] Its XRPD pattern is as follows figure 1 As shown, it is consistent with the XRPD spectrum of neratinib-maleate anhydrate crystal form I reported in the patent document CN101918390B.
[0102] It has been tested that it is easily transformed into monohydrate crystal form II at room temperature and in water.
Embodiment 1
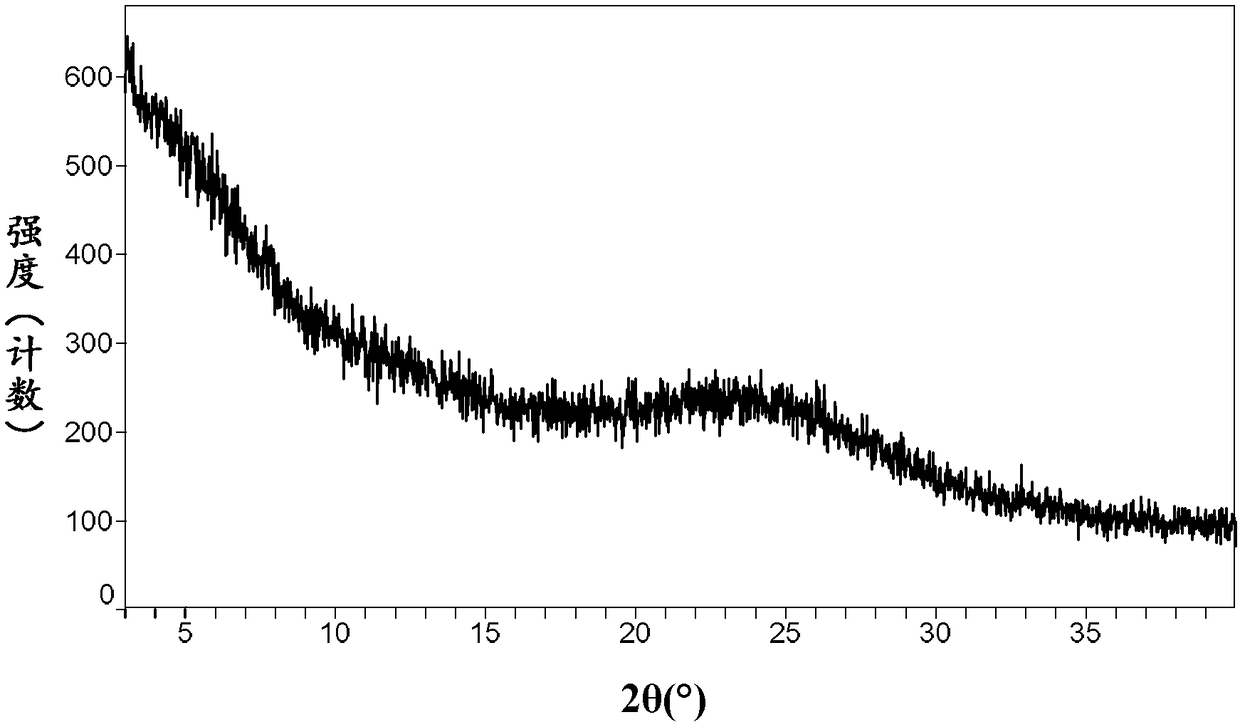
[0104] Take 20mg of neratinib-maleate anhydrous crystal form I, add 1.5mL of methanol, ultrasonically dissolve at 50°C, and concentrate to dryness under reduced pressure in a water bath at 30°C to obtain 18mg of neratinib-maleate of amorphous. XRPD patterns such as image 3 shown.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com