Antitumor carboxyalkyl achyranthis polysaccharide
The technology of Achyranthes polysaccharide and carboxyalkyl group is applied in the field of immunomodulatory Achyranthes polysaccharide derivatives and the preparation thereof, and can solve the problems of difficulty in preparing carboxyalkyl compounds, reduced antitumor activity, destruction of polysaccharide structure, etc. Anti-tumor immune function, simple synthesis method, and the effect of enhancing immune function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
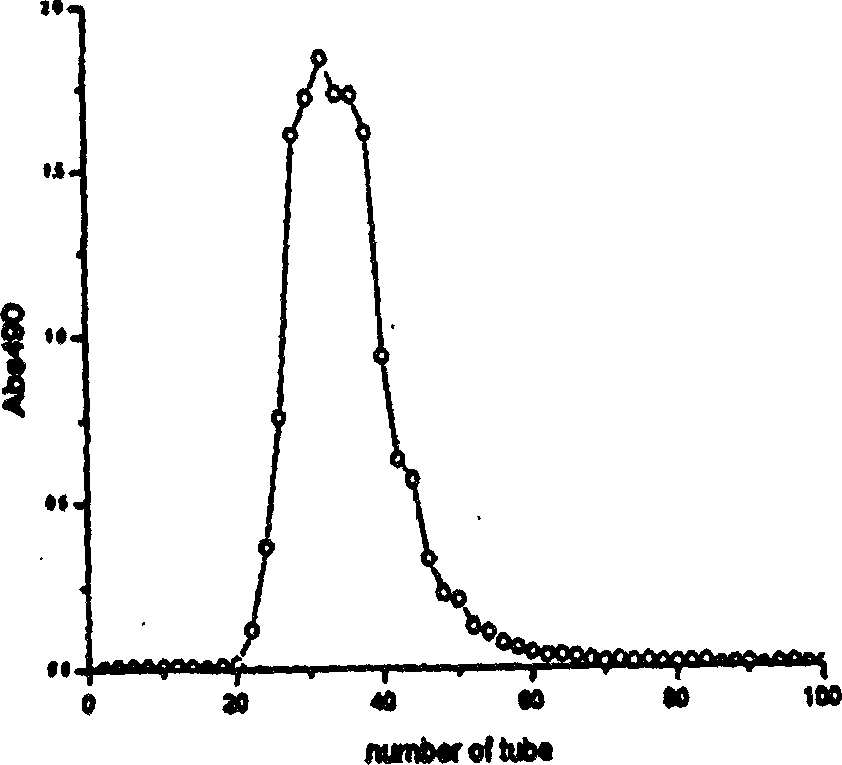
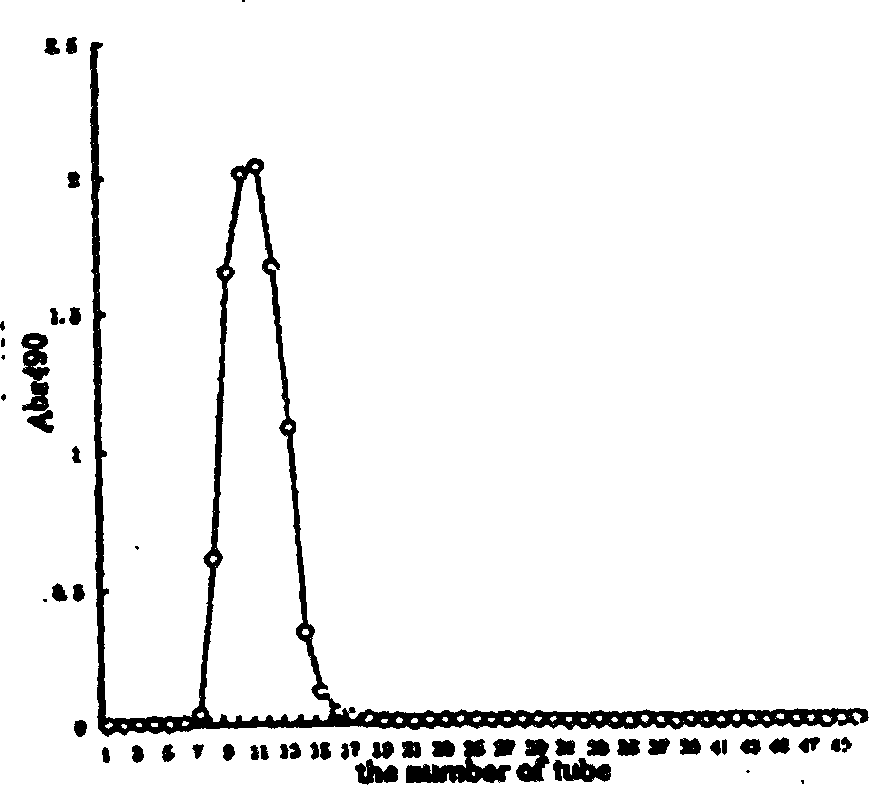
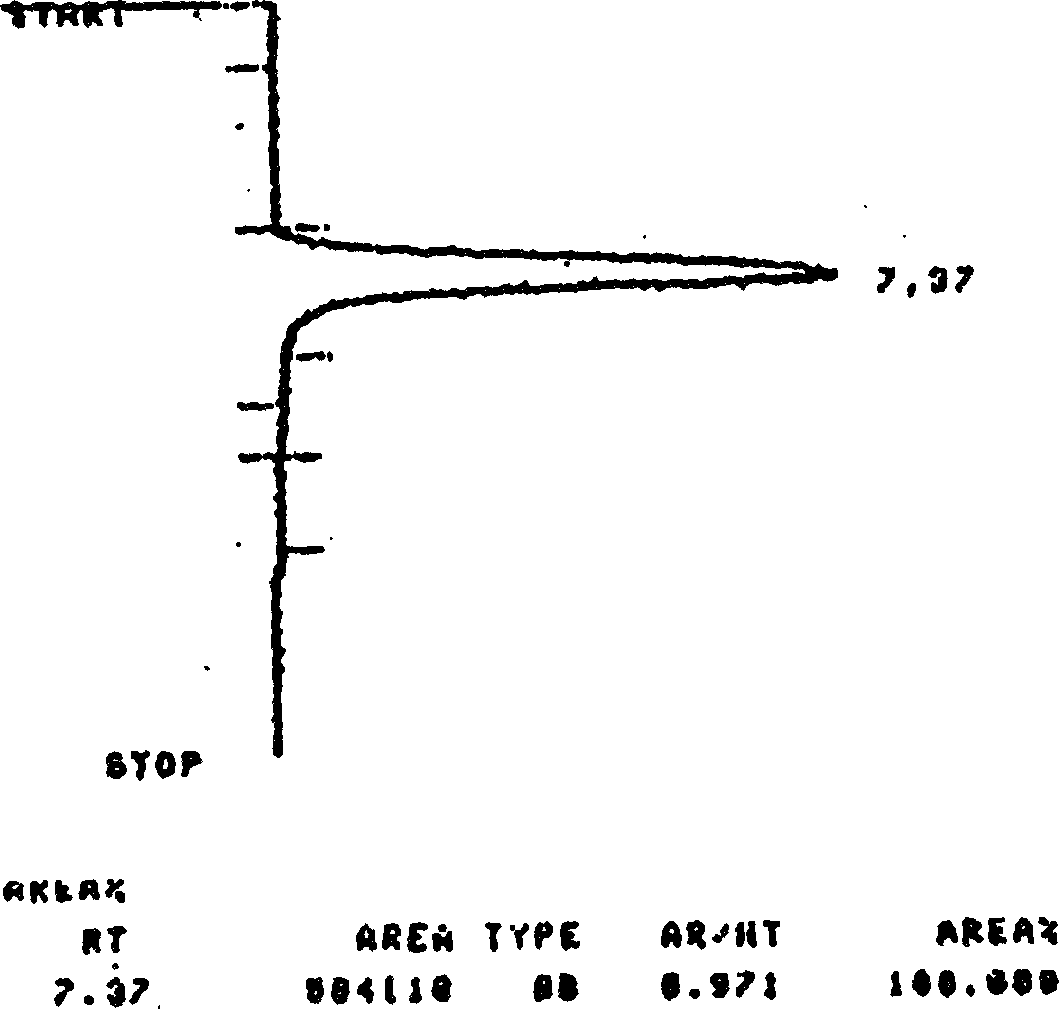
[0021] Take 250 mg of Achyranthes bidentata polysaccharide, dissolve it in 10 ml of double distilled water, add 247 mg of potassium hydroxide to dissolve, then add 286 mg of bromobutyric acid, and stir for reaction at 95°C for 1 hour. Then use 1mol / L hydrochloric acid to neutralize to pH=7, precipitate with 5 times the volume of acetone, centrifuge, dialyze, freeze-dry, and then purify through DEAE-Cellulose and SephadexG-25 column chromatography to obtain carboxypropyl achyranthes bidentata ( 241mg), the degree of carboxypropyl substitution in the carboxypropyl Achyranthes knuckle polysaccharide obtained by this method is 0.2, and it has anti-tumor and immune-enhancing activities.
Embodiment 2
[0023] Take 250 mg of Achyranthes bidentata polysaccharide and dissolve it in 5 ml of double distilled water, add 494 mg of sodium hydroxide to dissolve, then add 571 mg of chloroacetic acid, and stir and react at 95°C for 3 hours. Then use 1mol / L hydrochloric acid to neutralize to pH=7, precipitate with 5 times the volume of acetone, centrifuge, dialyze, freeze-dry, and then purify through DEAE-Cellulose and SephadexG-25 column chromatography to obtain carboxymethyl Achyranthes bidentata polysaccharide ( 286mg), the carboxymethyl substitution degree in the carboxymethyl Achyranthes knuckle polysaccharide obtained by this method is 0.7,
[0024] 13C-NMR: δ63.03, 62.97, 62.93, 62.88, 62.86, 62.79, 62.75, 62.71, 107.00, 106.87, 106.78, 106.71, 106.67, 106.51, 106.17, 106.13, 105.95, 105.838, 79.9 79.16,79.13,79.04,77.19,77.13,83.83,82.98,82.86,82.79,82.70,65.2,95.20,73.80,75.30,74.90,180.63,180.58,180.43,180.36,180.32,180.23,180.12,180.00,179.89,179.71, 179.62, 72.25, 72.22, 8...
Embodiment 3
[0026] Take 250 mg of Achyranthes bidentata polysaccharide, dissolve it with 2.5 ml of double distilled water, add 617 mg of potassium hydroxide to dissolve, then add 714 mg of chloroacetic acid, and stir for 10 hours at room temperature. Then use 1mol / L hydrochloric acid to neutralize to pH=7, precipitate with 5 times the volume of acetone, centrifuge, dialyze, freeze-dry, and then purify through DEAE-Cellulose and SephadexG-25 column chromatography to obtain carboxymethyl Achyranthes bidentata polysaccharide ( 340mg), the degree of carboxymethyl substitution in the carboxymethyl Achyranthes knuckle polysaccharide obtained by this method is 1.5, and has anti-tumor and immune-enhancing activities.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com