Neratinib hydrochloride crystal form and preparation method thereof
A technology of hydrochloride and dimethylaminocroton hydrochloride, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0054] The present invention also provides a preparation method of the aforementioned crystal form I, the preparation method comprising the following steps:
[0055] (i) Dissolve trans-4-dimethylaminocrotonyl chloride hydrochloride in the first inert solvent, react with chlorinating reagent to obtain trans-4-dimethylaminocrotonyl chloride hydrochloride The solution;
[0056] (ii) The solution obtained in step (1) is mixed with 6-amino-4-[[3-chloro-4-[(pyridin-2-yl)methoxy]phenyl]amino]-3-cyano-7- Ethoxyquinoline is reacted, the reaction is completed, and the crystal form I of the compound hydrochloride of formula (I) is obtained through post-processing.
[0057] Wherein, the molar ratio of trans-4-dimethylamino croton hydrochloride to the chlorination reagent is 1:1-3; the chlorination reagent is selected from oxalyl chloride, phosphorus oxychloride or thionyl chloride ; 6-amino-4-[[3-chloro-4-[(pyridin-2-yl)methoxy]phenyl]amino]-3-cyano-7-ethoxyquinoline and trans-4 - The ...
Embodiment 1
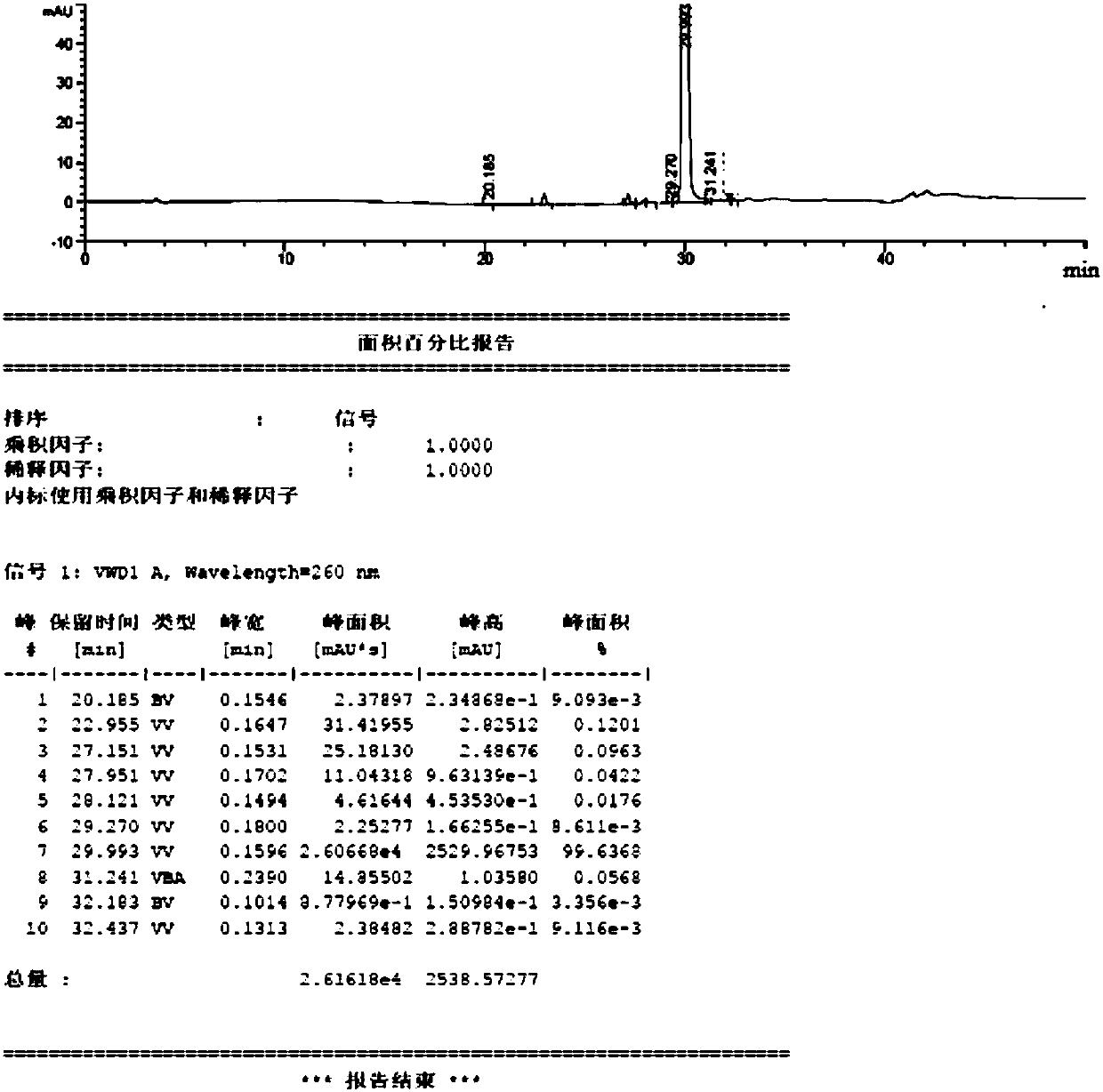
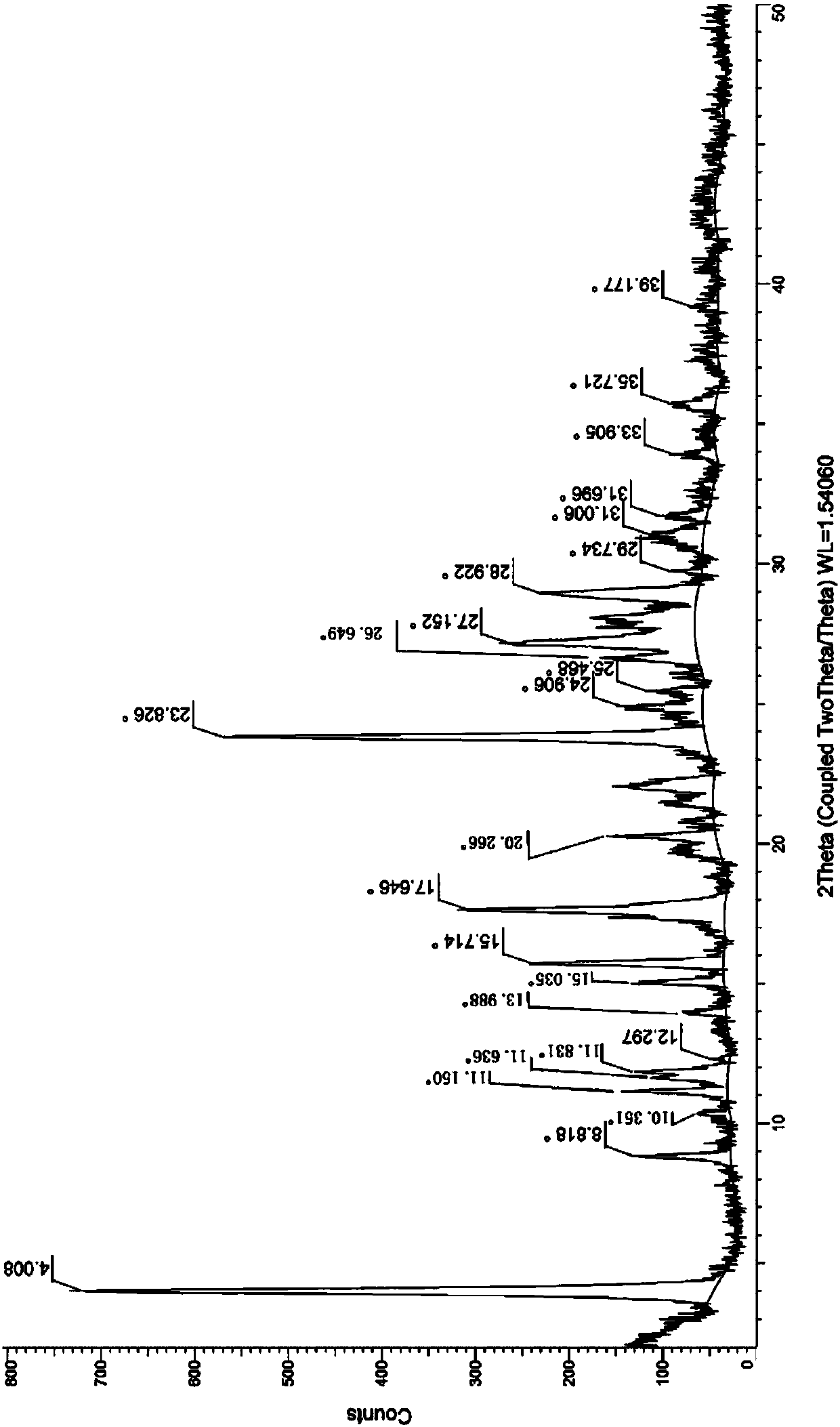
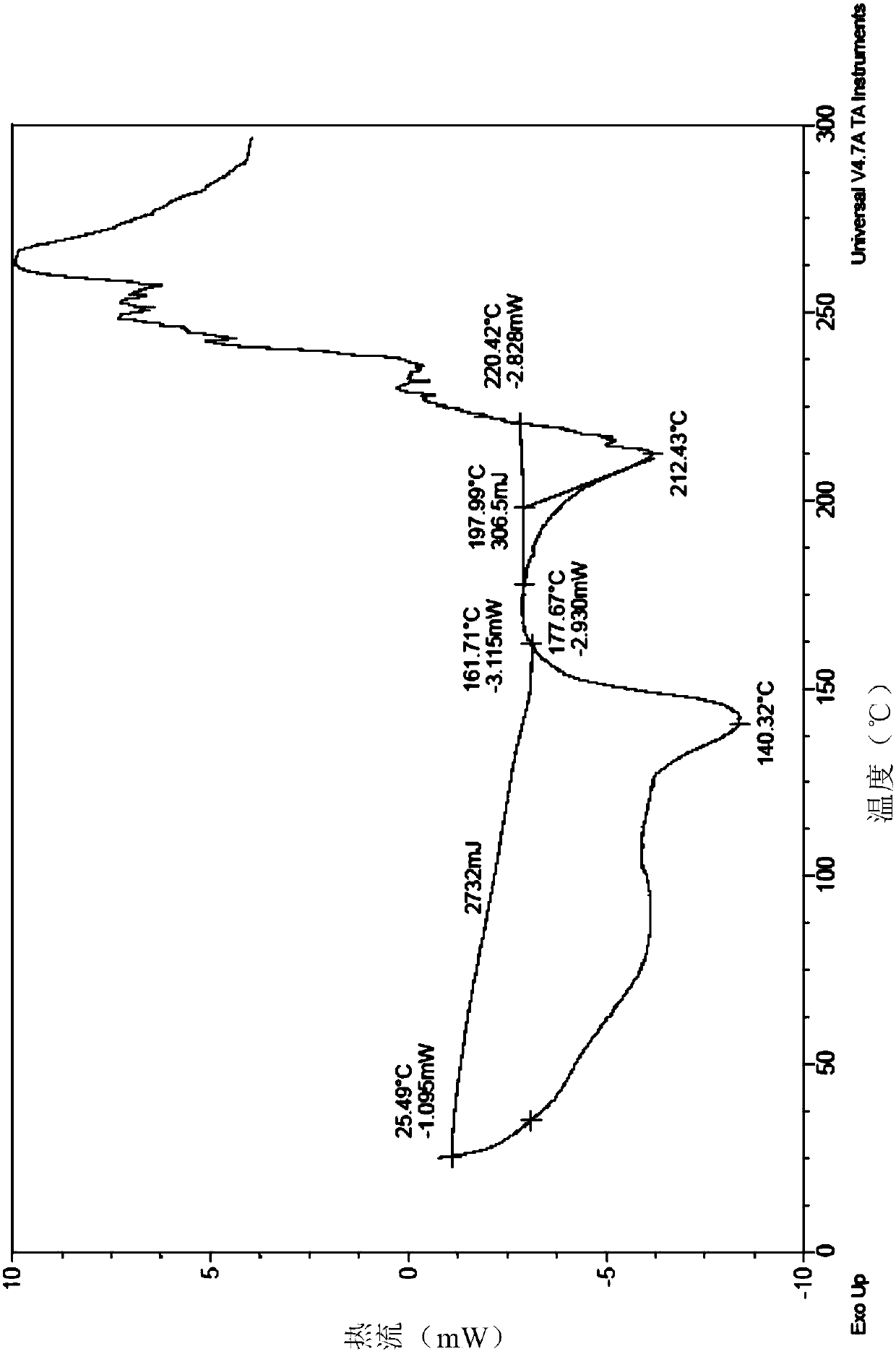
[0089] Example 1 Preparation of Neratinib Hydrochloride Form I
[0090] (1) Add 10g trans-4-dimethylamino croton hydrochloride, 50ml N-methylpyrrolidone in a 250ml there-necked flask and stir at room temperature, add 9.26g phosphorus oxychloride dropwise to a constant pressure dropping funnel, After 1 hour of dropwise addition, the reaction temperature was controlled at -15 to -10°C. During the reaction, the solution gradually changed from white to light brown, monitored by HPLC. After 2 hours, the reaction was stopped, and the resulting reaction solution was used for later use;
[0091] (2) Add 18 g of 6-amino-4-[[3-chloro-4-[(pyridine-2- base) methoxy] phenyl] amino] -3-cyano-7-ethoxyquinoline in 70ml N-methylpyrrolidone solution, after 1h dropwise addition, the reaction temperature was controlled at -15~-10°C, Insulated for 2 hours, 10ml of methanol was added dropwise, filtered with suction, rinsed twice with 10ml of dichloromethane, and vacuum-dried to obtain 24.2g of ner...
Embodiment 2
[0093] Example 2 Preparation of Neratinib Hydrochloride Form I
[0094] (1) Add 10g of trans-4-dimethylaminocroton hydrochloride and 80ml of N,N-dimethylformamide into a 250ml three-neck flask, stir at room temperature, add 7.66g of grass Acyl chloride, 1h dropwise, the reaction temperature is controlled at -10~-5°C, the solution gradually changes from white to light brown during the reaction, HPLC monitors, stop the reaction after 2h, and the obtained reaction solution is ready for use;
[0095](2) Add 18 g of 6-amino-4-[[3-chloro-4-[(pyridine-2- base)methoxy]phenyl]amino]-3-cyano-7-ethoxyquinoline in 70ml N,N-dimethylformamide solution, after 1h dropwise addition, the reaction temperature was controlled at -15~ -10°C, keep warm for 4 hours, add dropwise 10ml of methanol, filter with suction, rinse twice with 10ml of dichloromethane, and vacuum dry to obtain 23.1g of neratinib hydrochloride, with a yield of 90.84% and a purity of 98.26%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com