Crystal forms and preparation method of neratinib free alkali
A technology of crystal form and polymorph, applied in the field of new crystal form of neratinib free base and its preparation, can solve problems such as easy wrapping of mother liquor and difficulty in filtration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0147] Embodiment 1: the preparation of crystal form II
[0148] Weigh 1 g of the maleate salt of the compound of formula (I) and suspend it in 10 mL of methanol, add dropwise 2.5 ml of 5% NaOH, concentrate to about 5 times the amount, add 10 mL of isopropanol, concentrate to about 8 times the amount, and filter with suction , the filter cake was vacuum-dried at 25° C. to obtain 710 mg of solid, the yield was 88.7%, and the moisture content was 3.95%. The resulting solid is Form II of the compound of formula (I).
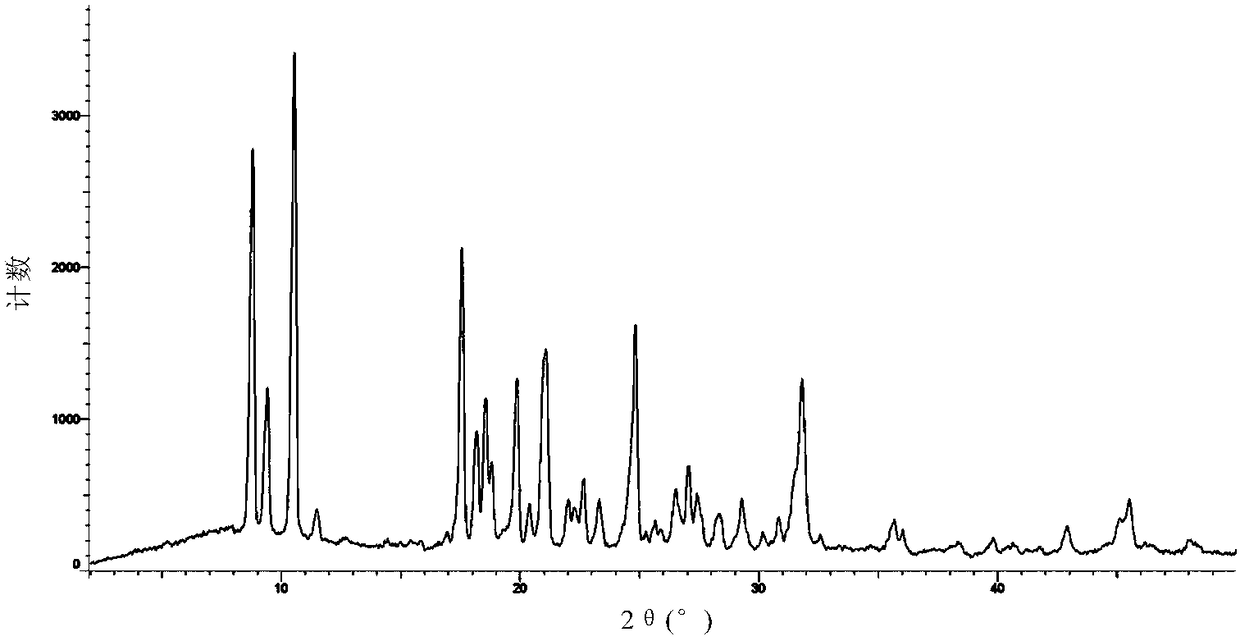
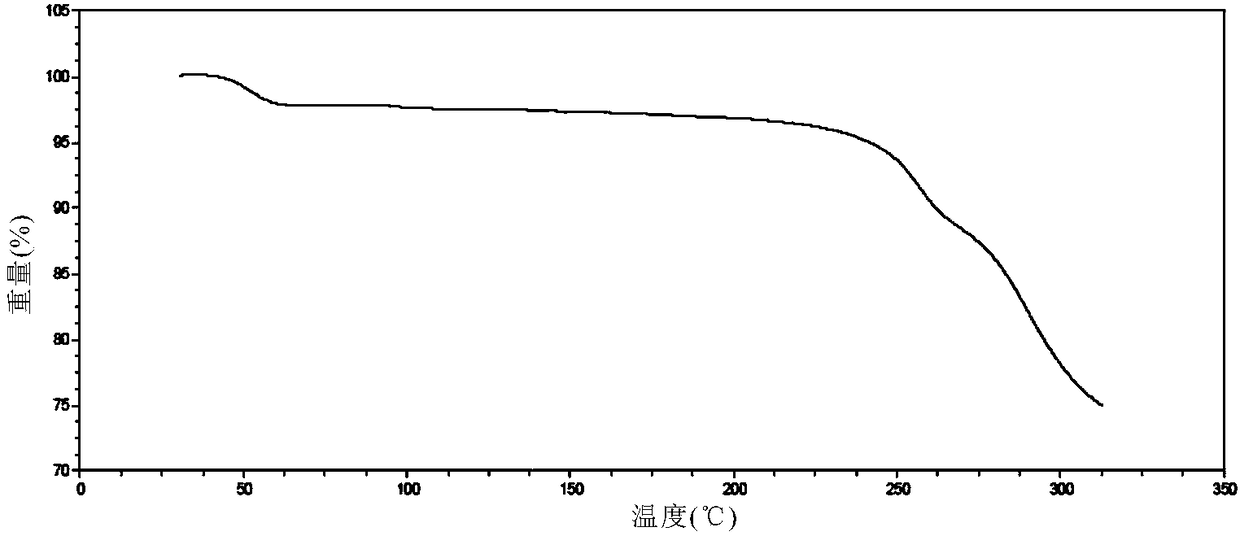
[0149] Carry out XRPD test to the obtained solid, its X-ray powder diffraction data are as shown in table 2, and its X-ray powder diffraction pattern is as follows Figure 4 Shown; Carry out TGA test to gained solid, its spectrogram is as Figure 5 As shown, there is about 2.9% weight loss when heated to 80°C, which is a hydrate; the spectra of the resulting solid under a 400-fold microscope are Image 6 shown.
[0150] Table 2
[0151]
[0152]
Embodiment 2
[0153] Embodiment 2: the preparation of crystal form III
[0154] Weigh 1g of the maleate salt of the compound of formula (I) and suspend it in 10mL of methanol, add dropwise 2.5ml of 5% NaOH, add the crystal form II in Example 1, concentrate to about 5 times the amount, add 10mL of isopropanol , concentrated to about 8 times the amount, suction filtered, and the filter cake was vacuum-dried at 40° C. to obtain 861.3 mg of solid with a yield of 91.7% and a moisture content of 5.45%. The resulting solid is Form III of the compound of formula (I).
[0155] Carry out XRPD test to the obtained solid, its X-ray powder diffraction data are as shown in table 3, and its X-ray powder diffraction pattern is as follows Figure 7 Shown; Carry out TGA test to gained solid, its spectrogram is as Figure 8 As shown, it has about 5.0% weight loss when heated to 140°C, which is a hydrate; the spectrum of the resulting solid under a microscope at 400 times is as Figure 9 shown.
[0156] ta...
Embodiment 3
[0158] Embodiment 3: the preparation of crystal form III
[0159] Weigh 1g of the maleate salt of the compound of formula (I) and suspend it in 10mL of methanol, add dropwise 2.5ml of 5% NaOH, add the crystal form III in Example 2, concentrate to about 5 times the amount, add dropwise 10mL of water, pump Filter, rinse with 2 times the amount of water, and dry the filter cake under vacuum at 40°C to obtain 867.9 mg of solid with a yield of 92.4%. The resulting solid is Form III of the compound of formula (I).
[0160] Carry out XRPD test to the obtained solid, its X-ray powder diffraction pattern is basically as Figure 7 Shown; Carry out TGA test to gained solid, its spectrogram is basically as Figure 8 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com