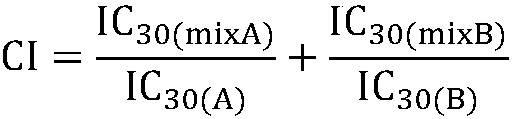Patents
Literature
73 results about "Axitinib" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
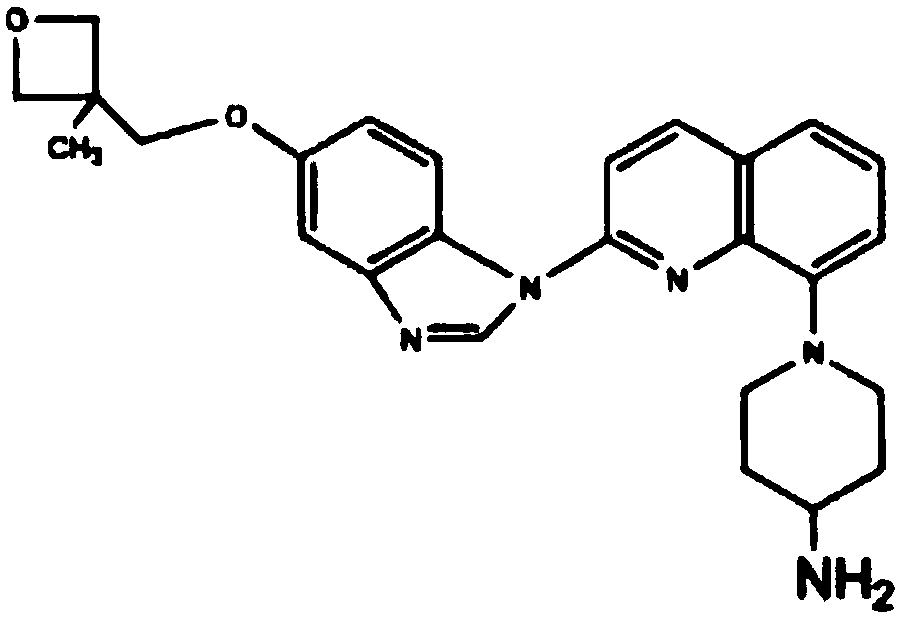
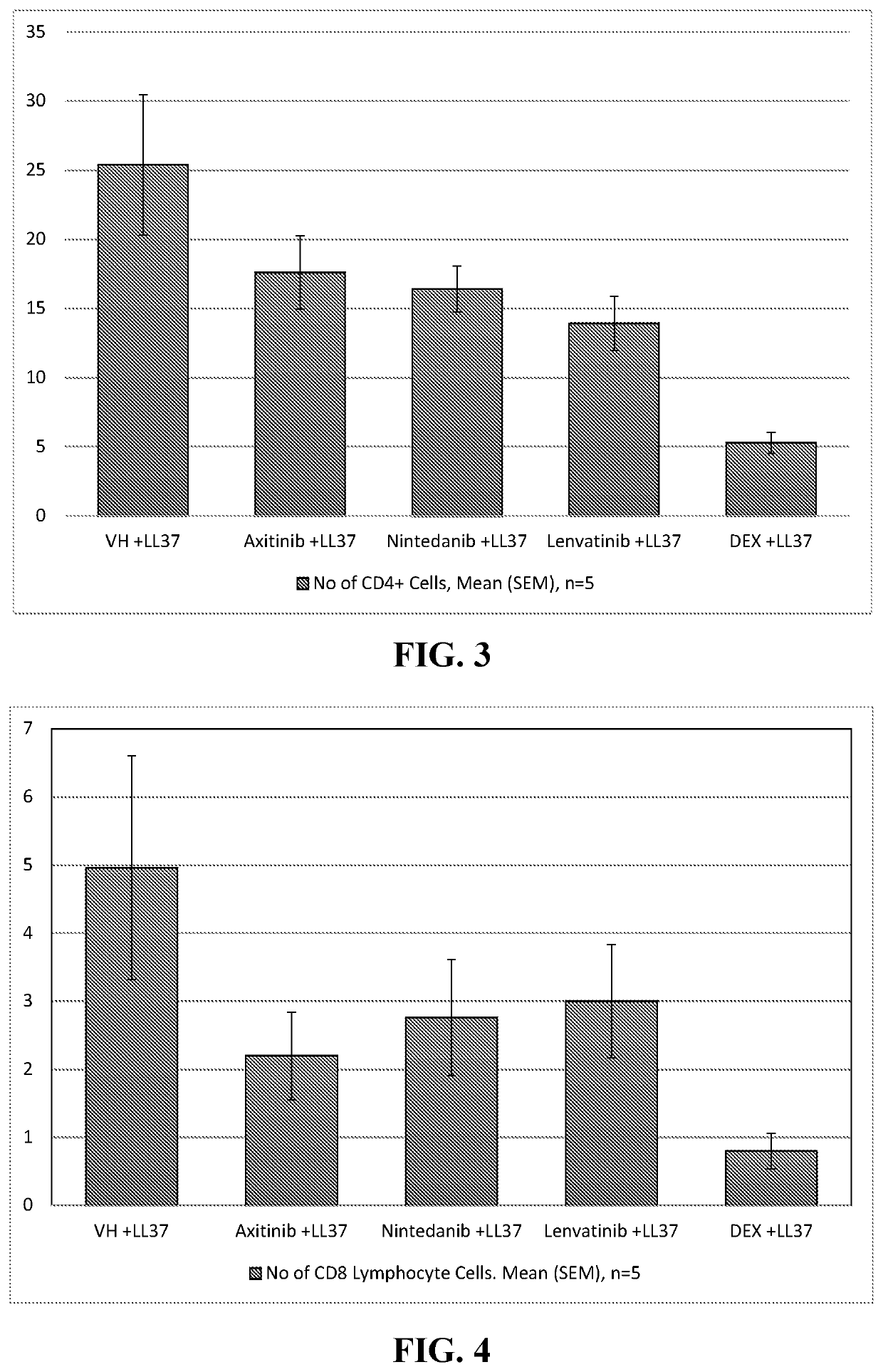
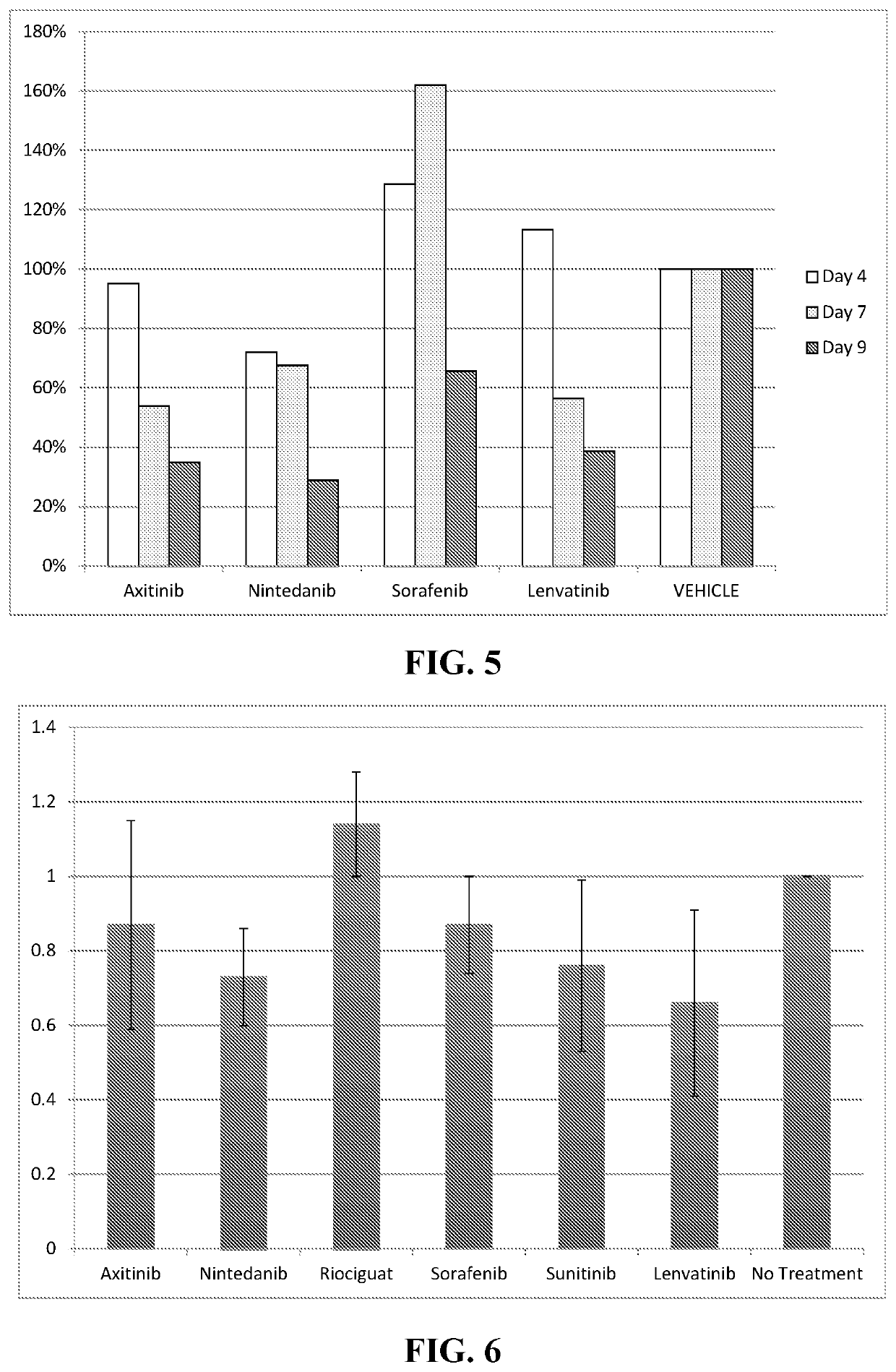
This medication is used to treat kidney cancer after previous treatment has not been effective.
Eutectic complex composed of resveratrol and protein kinase inhibitor, and composition comprising eutectic complex
InactiveCN110283052AHydroxy compound active ingredientsOrganic chemistry methodsLenvatinibPTK Inhibitors
The invention provides a eutectic complex composed of resveratrol and a protein kinase inhibitor, and a composition comprising the eutectic complex. The eutectic complex is characterized in that the protein kinase inhibitor is selected from one of imatinib, gefitinib, erlotinib, sunitinib, sorafenib, dasatinib, lapatinib, nilotinib, pazopanib, afatinib, crizotinib, axitinib, regorafenib, ibrutinib, lenvatinib, palbociclib, osimertinib and alectinib or pharmaceutically acceptable salt thereof. The eutectic complex provided by the invention can produce synergistic effects of inhibiting histamine release.
Owner:黄泳华
Method of treatment of philadelphia chromosome positive leukaemia
InactiveUS20120244116A1Organic active ingredientsPeptide/protein ingredientsBcr-Abl tyrosine-kinase inhibitorLestaurtinib
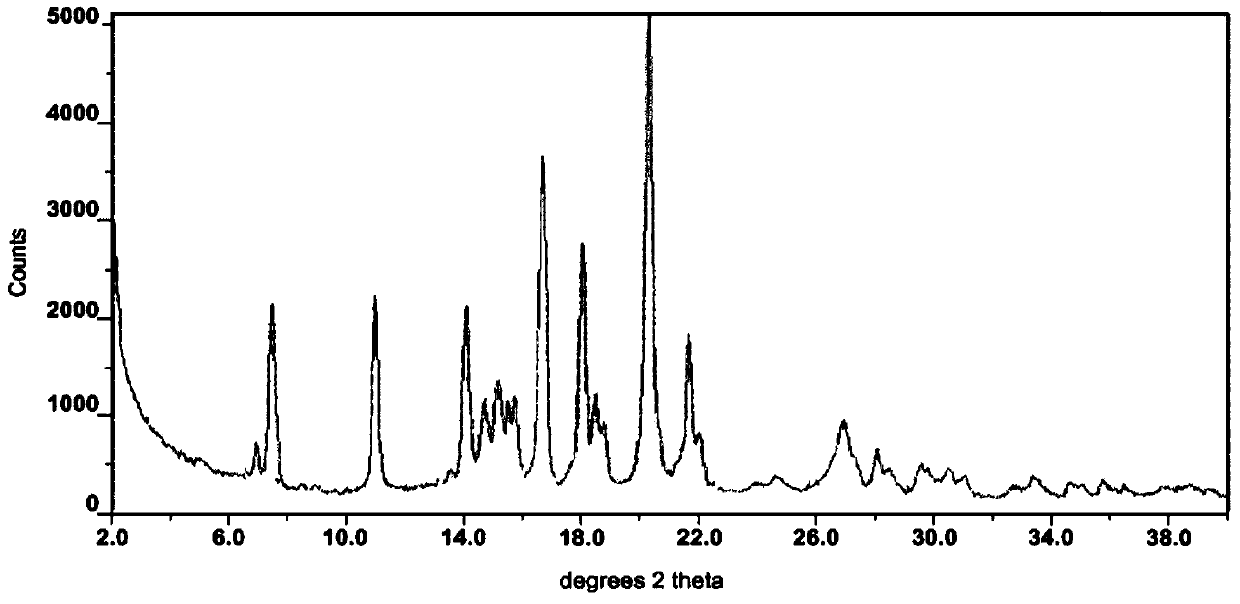
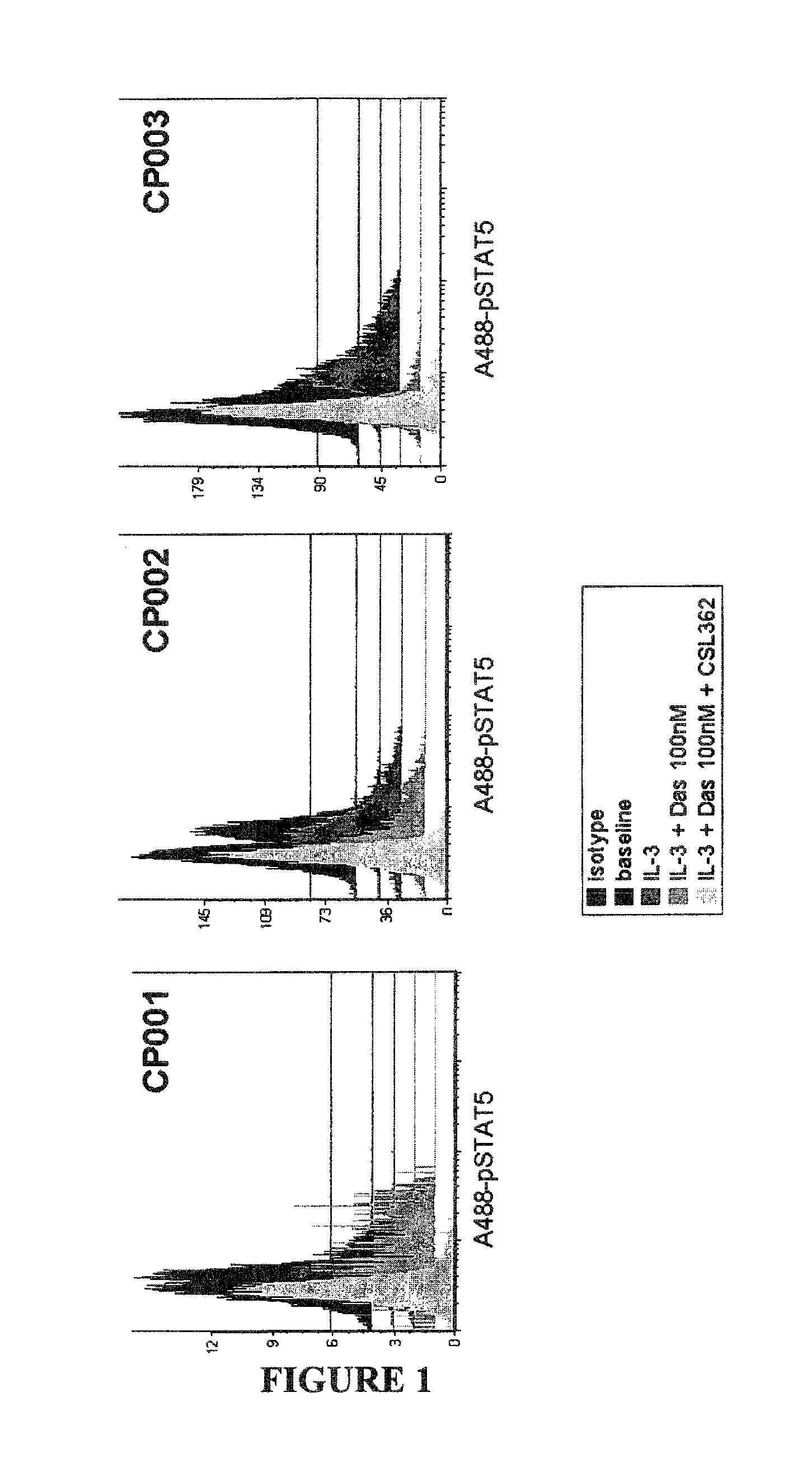
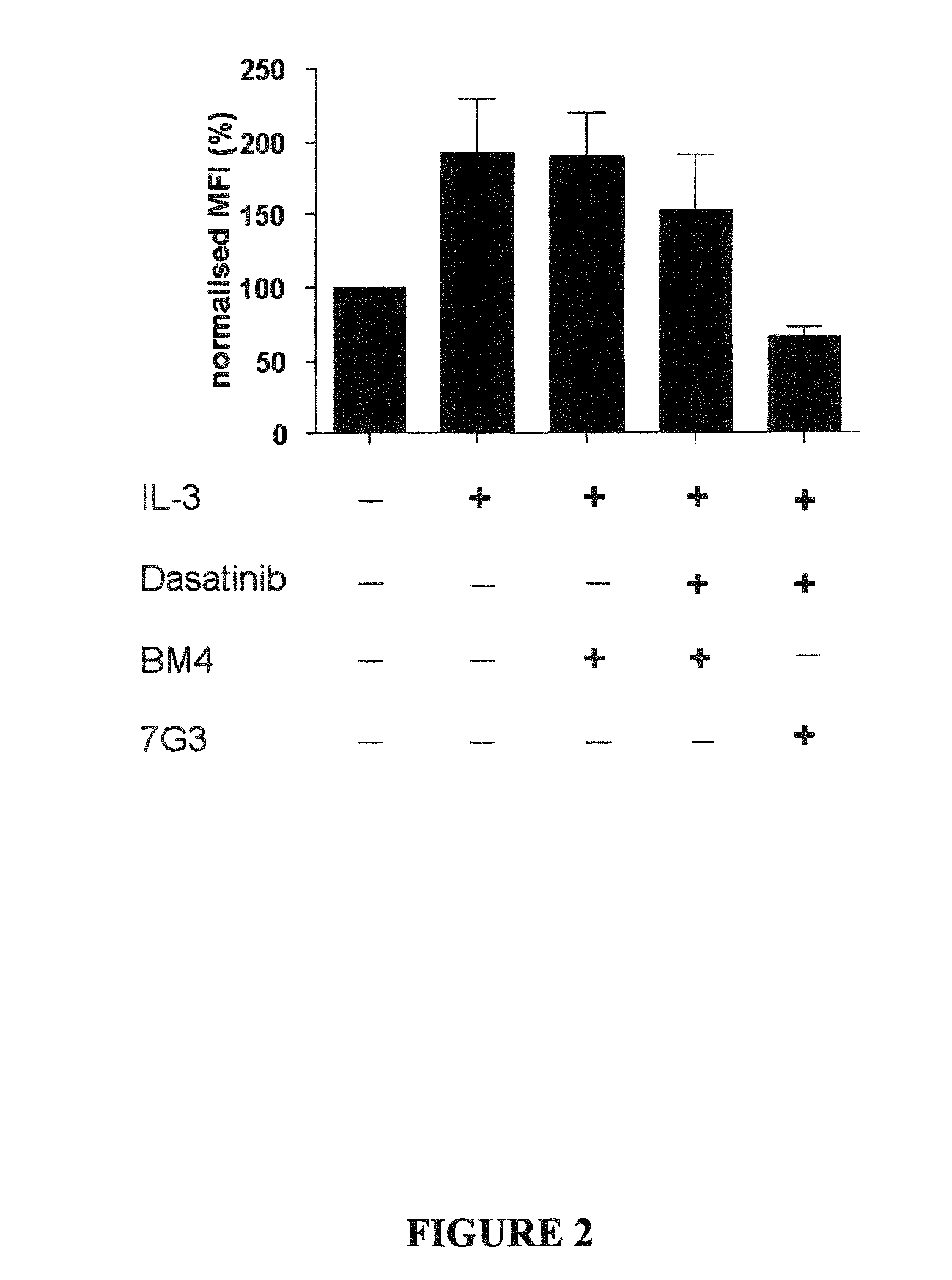
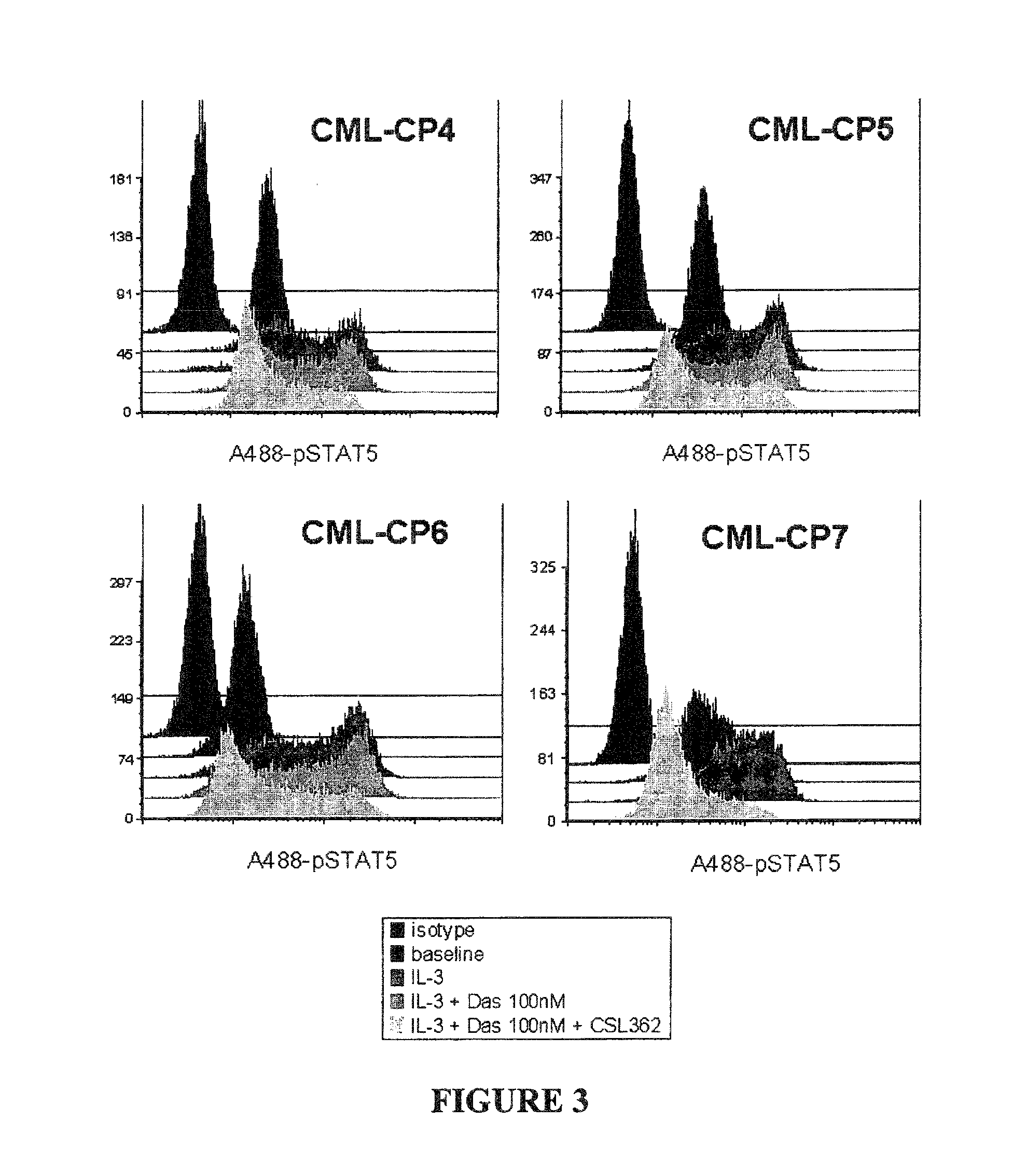
The invention provides a method for the treatment of Ph+ leukemia in a patient comprising administering to the patient (i) a BCR-ABL tyrosine kinase inhibitor, and (ii) an agent which selectively binds to a cell surface receptor expressed on Ph+ leukemic stem cells. The invention further provides for the use of (i) and (ii) in, or in the manufacture of a medicament for, the treatment of Ph+ leukemia in a patient; and a composition for the treatment of Ph+ leukemia in a patient comprising (i) and (ii); and kits comprising (i) and (ii). In some embodiments, the tyrosine kinase inhibitor is or is not imatinib; or is selected from the group consisting of dasatinib, nilotinib, bosutinib, axitinib, cediranib, crizotinib, damnacanthal, gefitinib, lapatinib, lestaurtinib, neratinib, semaxanib, sunitinib, toceranib, tyrphostins, vandetanib, vatalanib, INNO-406, AP24534, XL228, PHA-739358, MK-0457, SGX393 and DC2036; or is selected from the group consisting of dasatinib and nilotinib. In some embodiments, the agent binds to a receptor involved in signalling by at least one of IL-3, G-CSF and GM-CSF. In some embodiments, the agent is a mutein selected from the group consisting of IL-3 muteins, G-CSF muteins and GM-CSF muteins. In some embodiments, the mutein is an IL-3 mutein. In some embodiments, the agent is a soluble receptor which is capable of binding to IL-3.
Owner:CSL LTD
Pharmaceutical compositions of n-methyl-2-[3-((e)-2-pyridin-2-yl-vinyl)-1h-indazol-6-ylsulfanyl]-benzamide
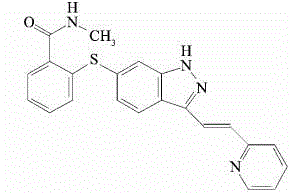
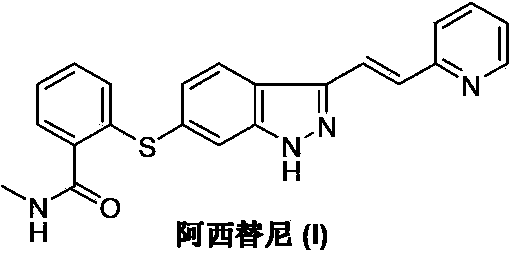
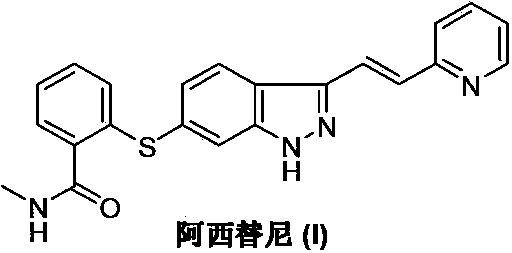
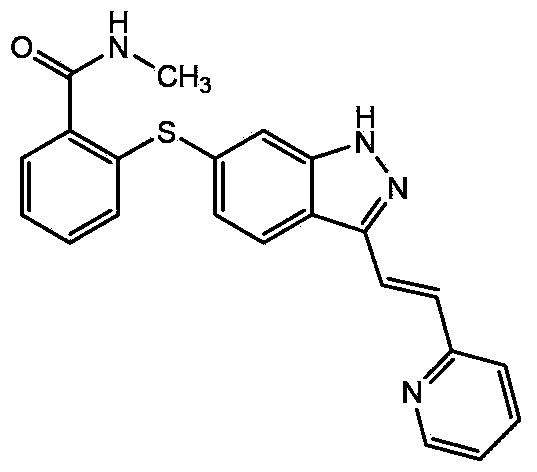
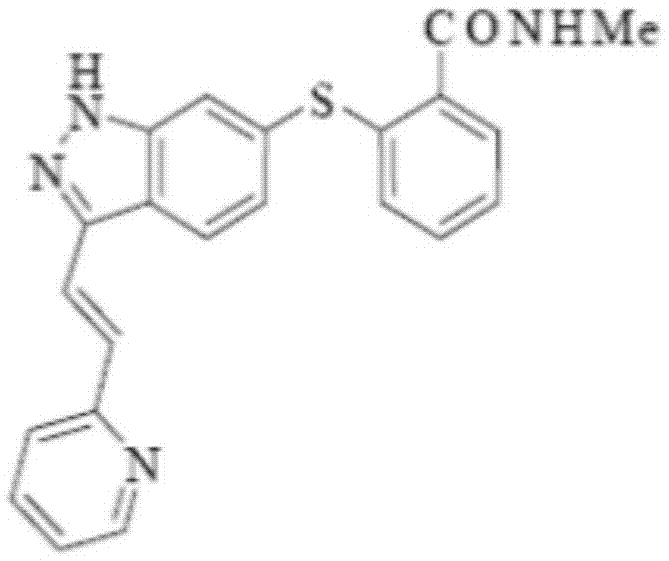
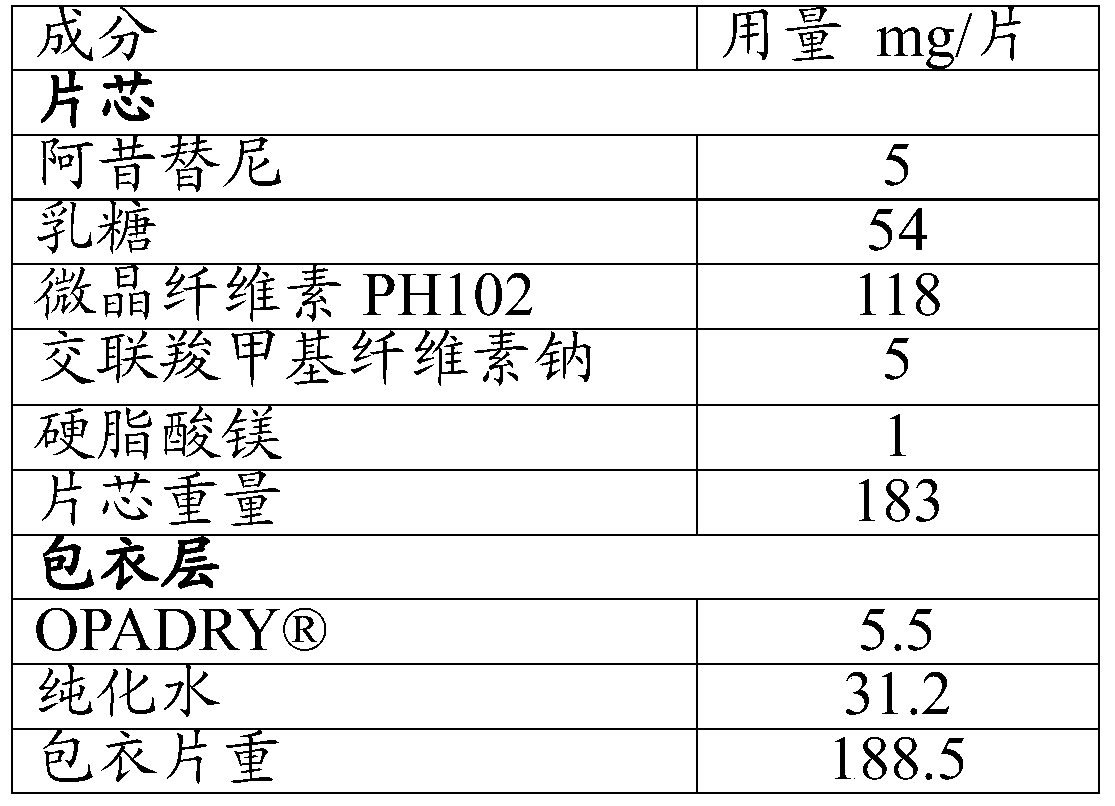
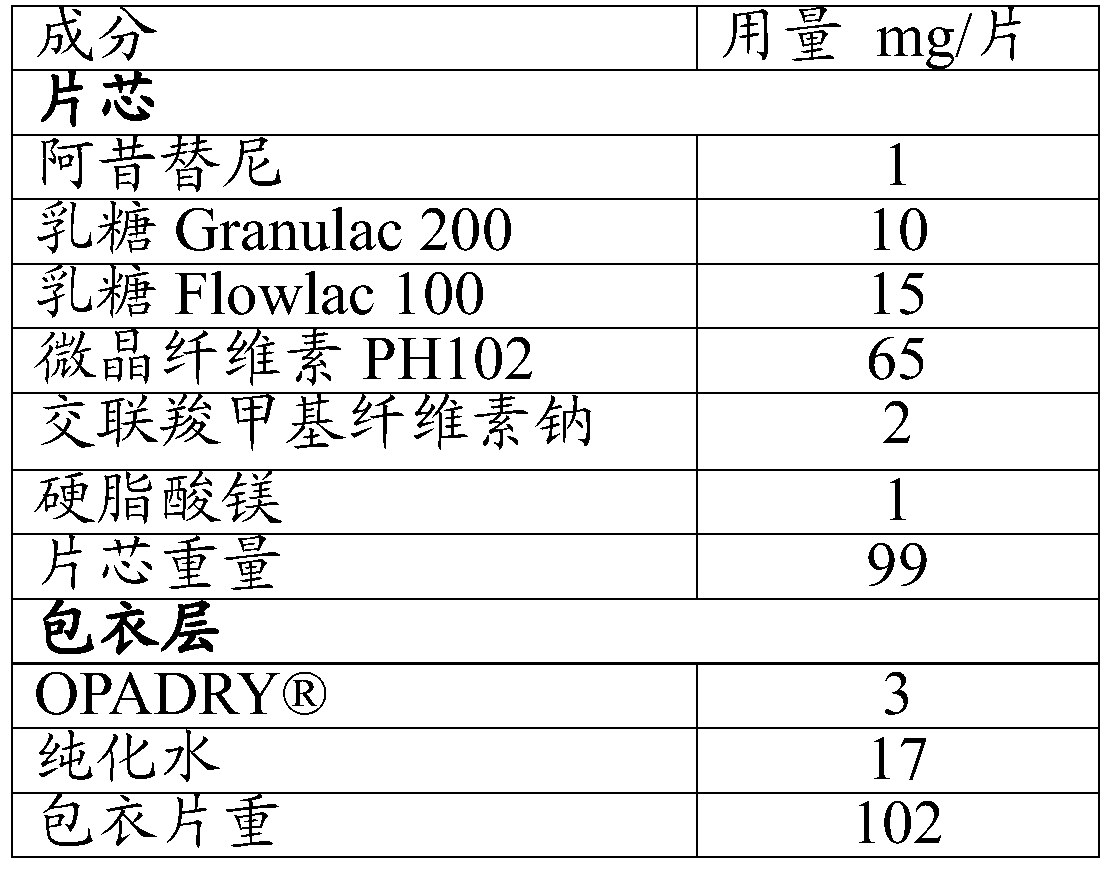
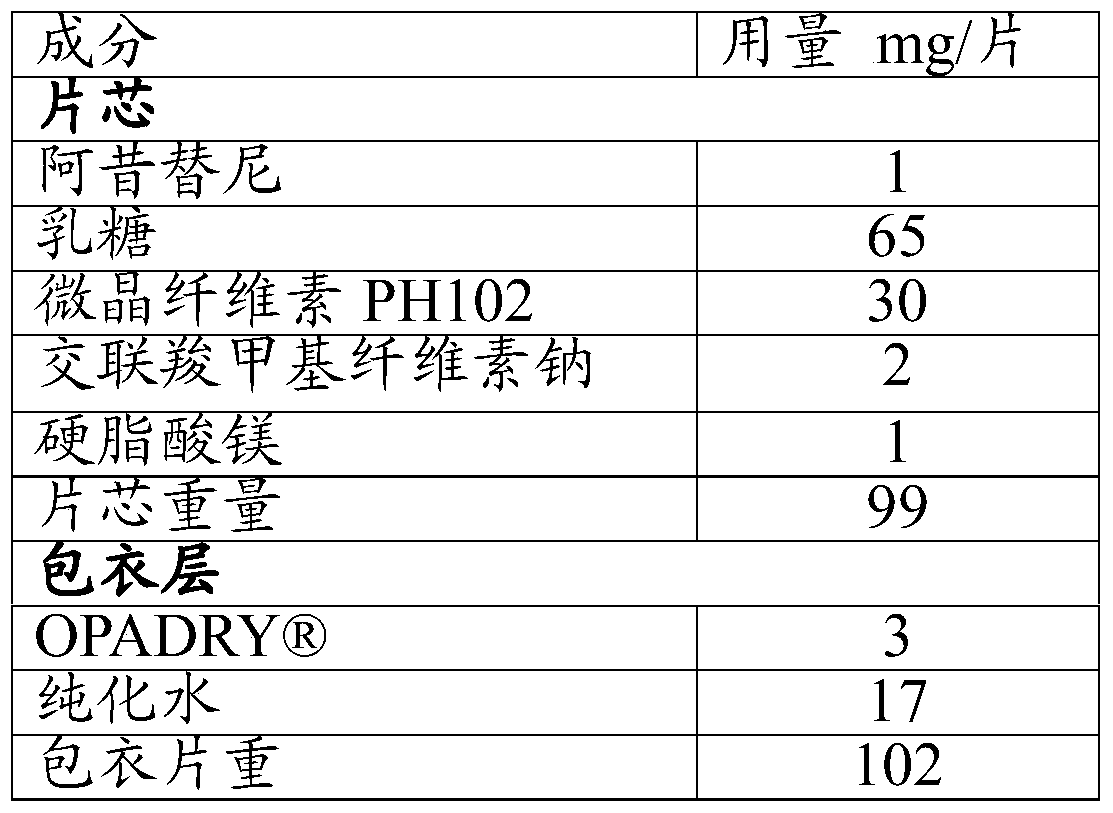
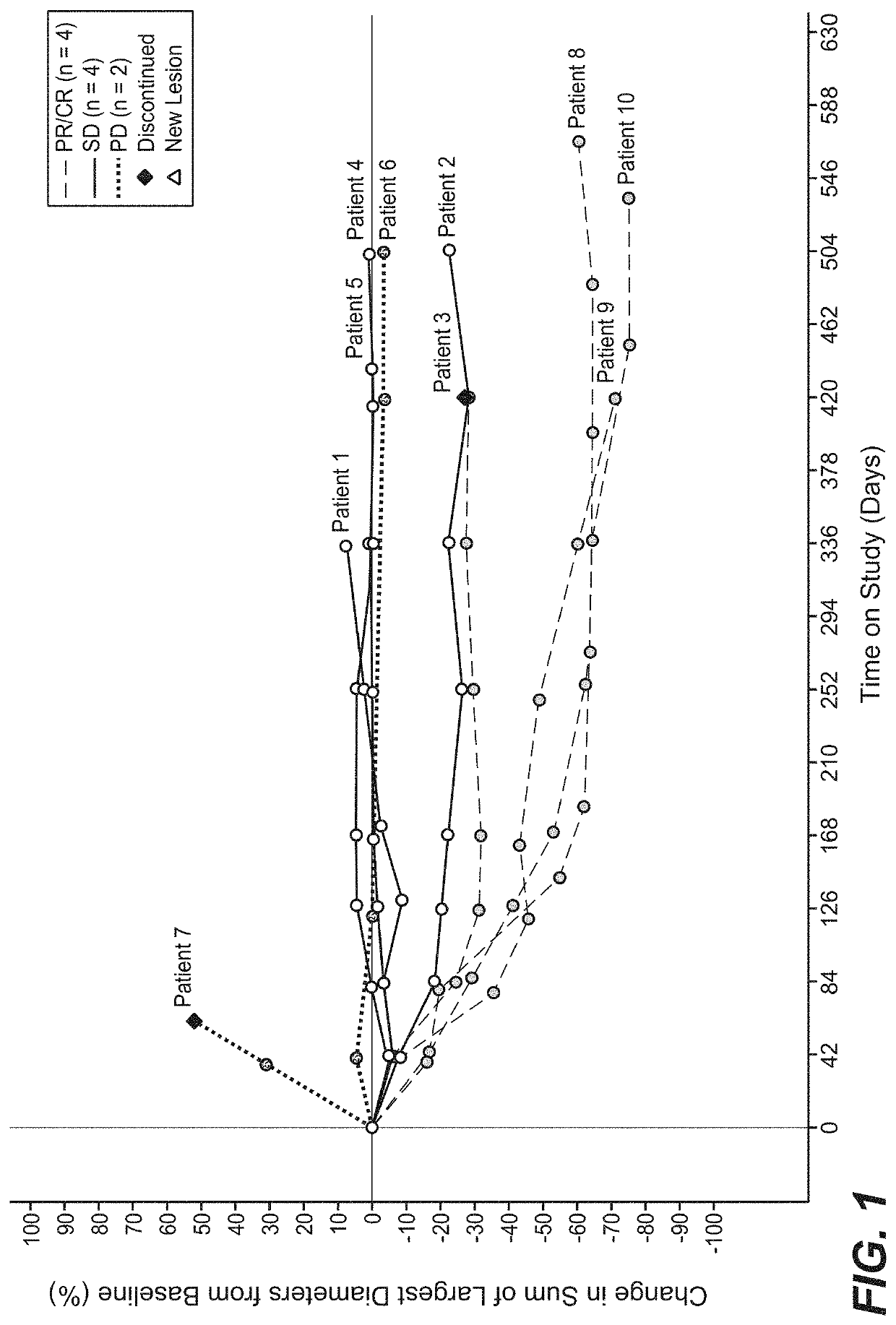
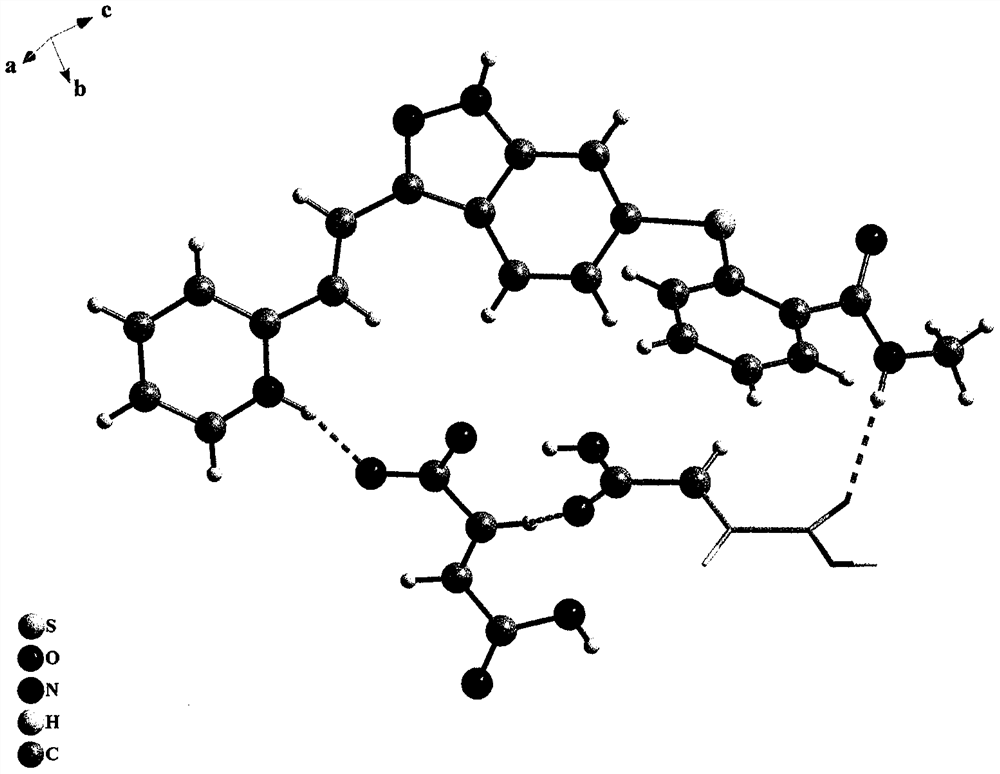
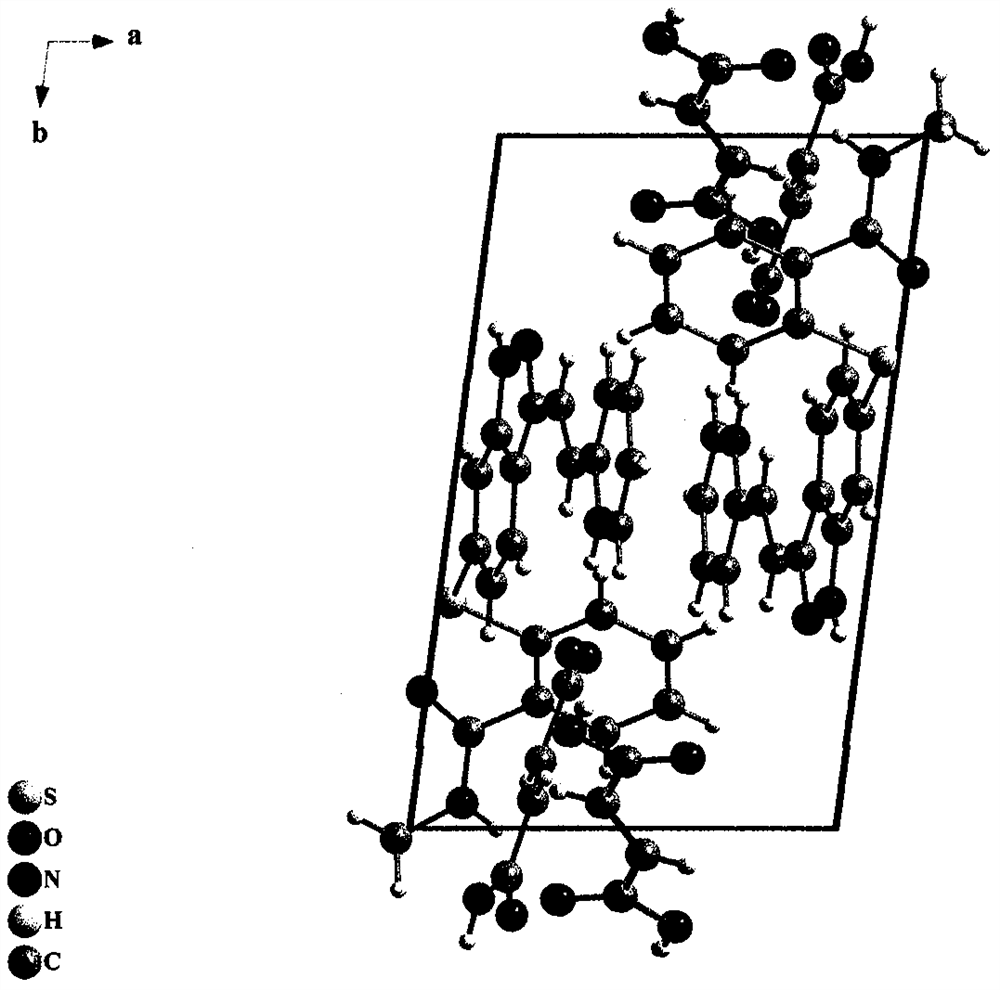
The present invention relates to pharmaceutical compositions containing axitinib, which is known as N-methyl-2-[3-((E)-2-pyridin-2-yl-vinyl)-1H-indazol-6-ylsulfanyl]-benzamide or 6-[2-(methylcarbamoyl)phenylsulfanyl]-3-E-[2-(pyridin-2-yl)ethenyl]indazole, or crystalline forms thereof, that protect axitinib from degradation, including photodegradation, as well as the therapeutic use of such compositions. The present invention also relates to novel photodegradants of axitinib.
Owner:PFIZER INC
Method for preparing intermediate of axitinib and application of intermediate in preparation of axitinib
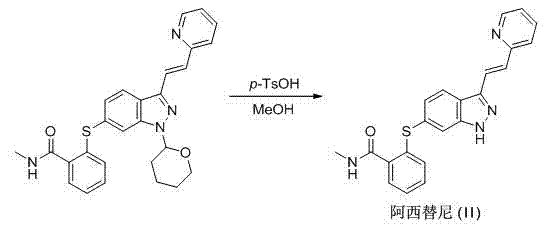
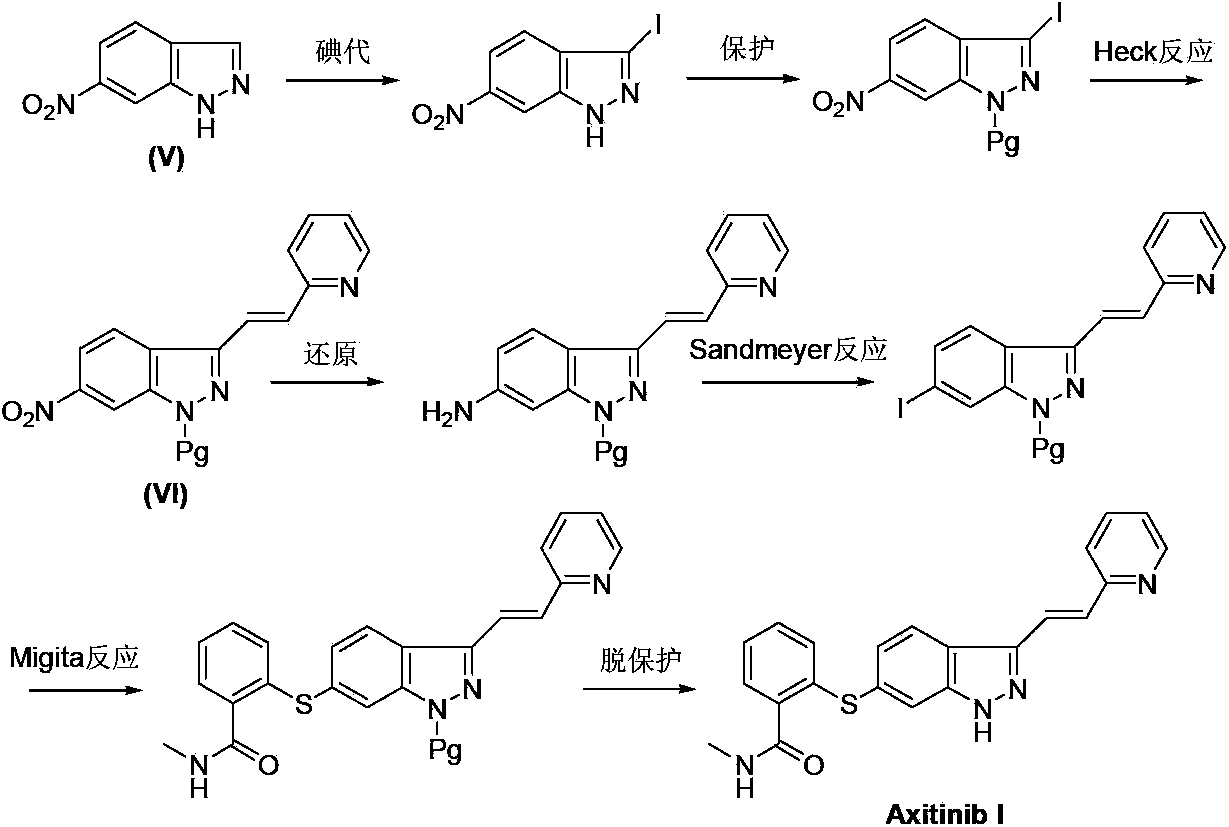
The invention relates to a method for preparing an intermediate of axitinib and application of the intermediate in preparation of axitinib. The preparation method for the intermediate of axitinib 3-iodine-6-nitro-1-(teralin-2H-pyran-2-base)-1H-indazole comprises the following steps that 6-nitroindazole and 3,4-dihydro-2H-pyran react under the action of catalyst so as to protect perssad tetralin-2H-pyran-2-base at N-H site, and the key intermediate with high yield is obtained through iodination at site 3. The application of the intermediate in preparation of axitinib is as follows: Heck coupled reaction is carried out on the intermediate and 2-vinyl pyridine, then nitro reduction and diazo-reaction for iodination are sequentially carried out, finally, the axitinib is obtained after docking of 2-sulfydryl-N-methyl benzamide and deprotection. The related main initial raw materials are easy to purchase in markets, and the method has high yield and high molecule economic efficiency, is efficient and environment-friendly, and is suitable for industrial mass production.
Owner:湖南欧亚药业有限公司
Stable axitinib compound
InactiveCN104650034AHigh purityImprove stabilityOrganic active ingredientsOrganic chemistryRenal Cell CancersHigh humidity
The present invention belongs to the technical field of medicine, and particularly relates to an axitinib crystal and a preparation method thereof. The obtained axitinib crystal of the present invention has advantages of high purity and good stability, wherein the moisture absorption weight increase of the axitinib crystal is not significant even under the high humidity condition. The present invention further relates to applications of a composition using the axitinib crystal in treatment of advanced renal cell carcinoma.
Owner:TIANJIN HANKANG PHARMA BIOTECH
Preparation method of Axitinib
ActiveCN103387565AEase of industrial productionRaw materials are easy to getOrganic chemistryHalogenSulfur
The invention discloses a preparation method of Axitinib(I). The preparation method comprises the following steps of: with 6-halo-1H-indole (II) as a raw material, carrying out an oxidation rearrangement reaction to obtain 6-halo-3-formyl-1H-indole (III), carrying out a Migita reaction on the 6-halo-3-formyl-1H-indole (III) serving as an intermediate and N-methyl-2-mercaptobenzamide to obtain N-methyl-2-[(3-formyl-1H-6-yl)sulfur]benzamide (IV), and carrying out a Witting reaction on a ylide reagent (V) and the N-methyl-2-[(3-formyl-1H-6-yl)sulfur]benzamide(IV) serving as an intermediate to prepare Axitinib (I). The preparation method has the advantages that the raw materials are easily available and the process is concise, economicAL and environment-friendly; therefore, the preparation method is suitable for industrial production.
Owner:临泉县联正电子商务有限公司
New application of axitinib
ActiveCN105287552APromote browningResistance insensitivityOrganic active ingredientsMetabolism disorderFatty liverCell marker
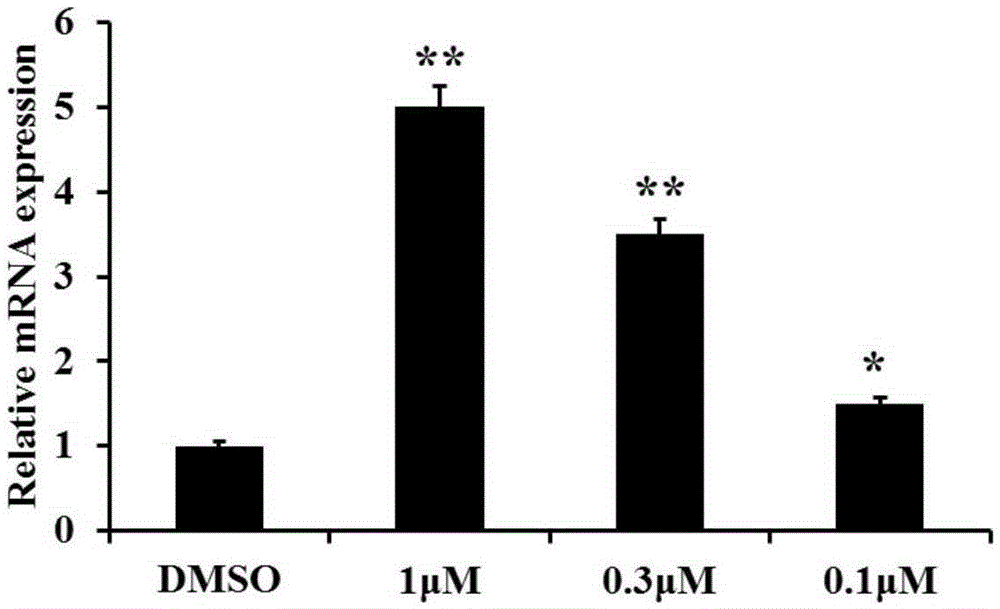
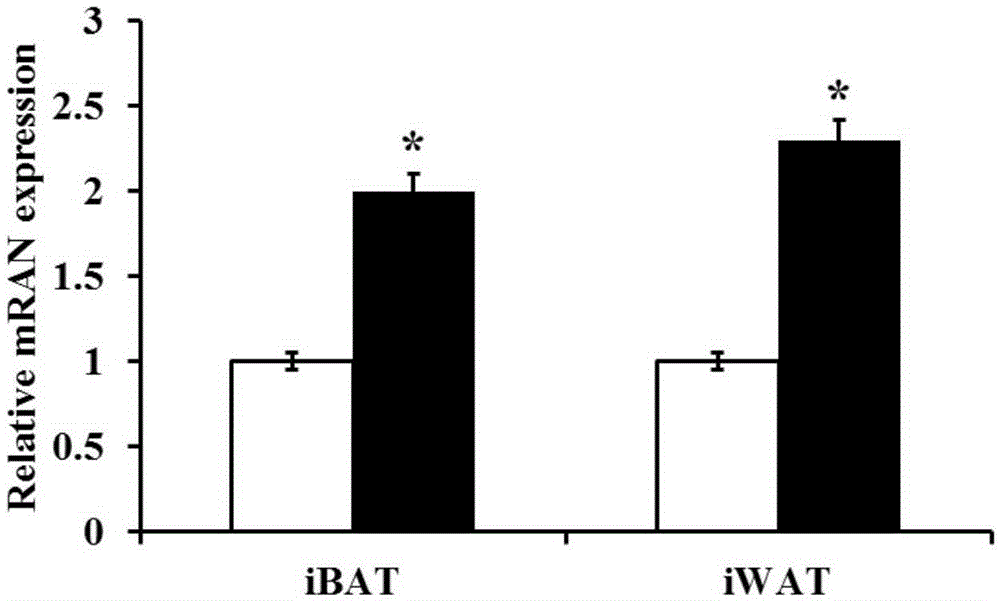
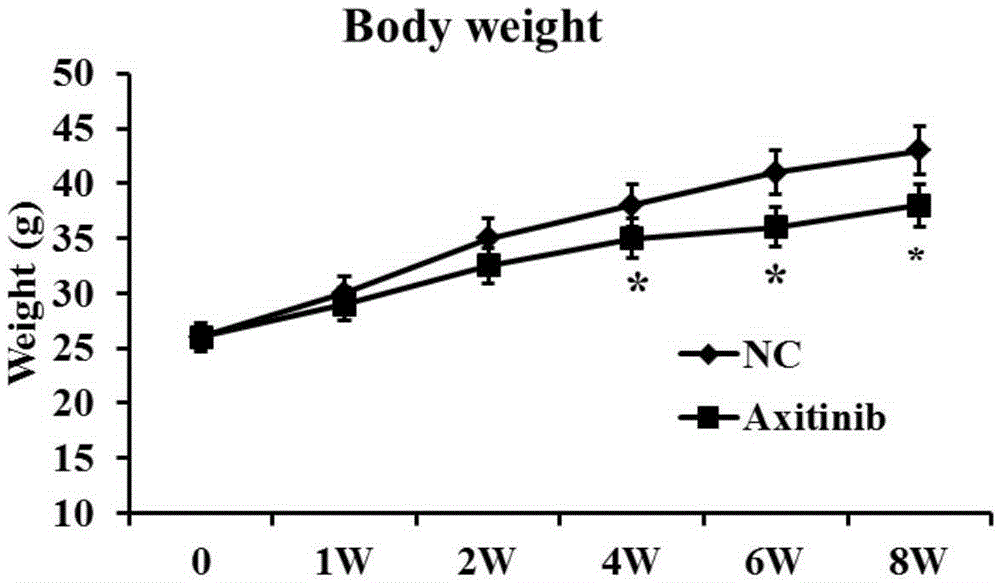
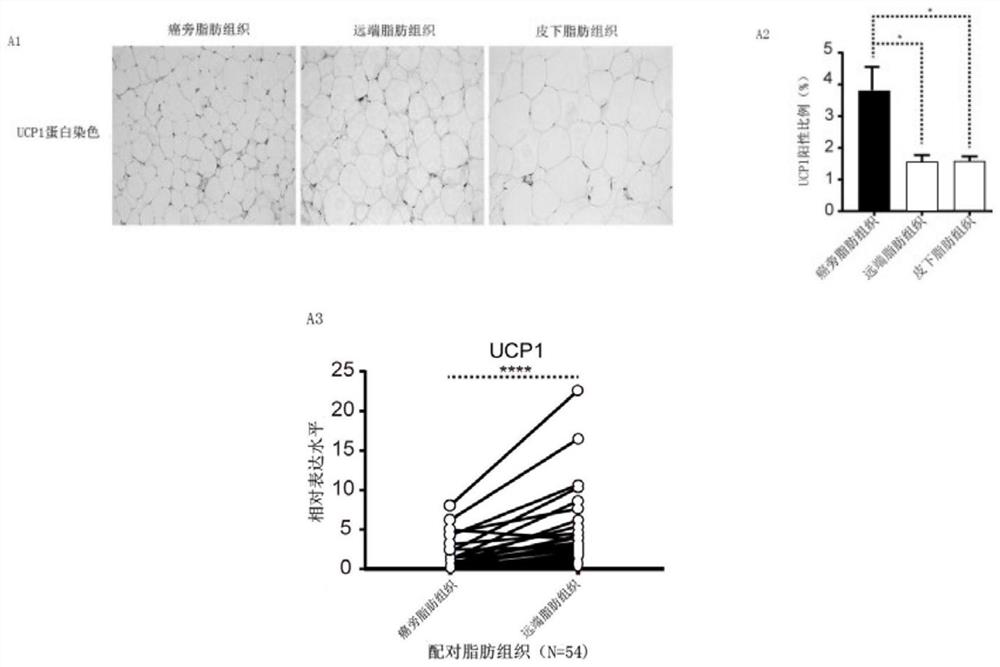
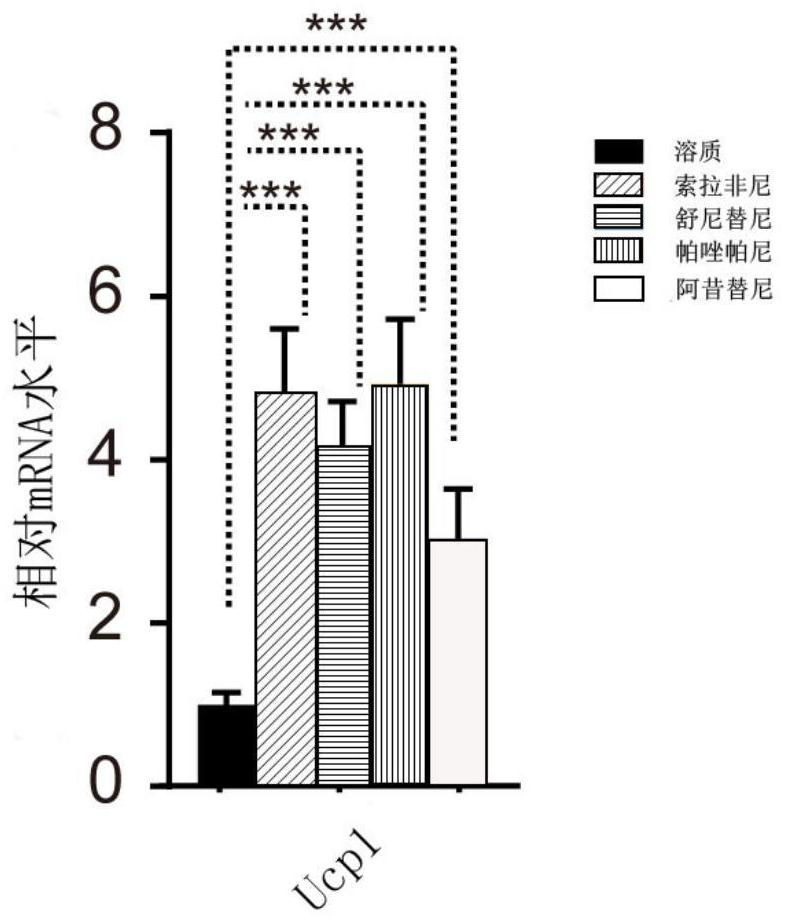
The invention discloses new application of axitinib. Axitinib can be used for preparing medicine for promoting white fat cell browning and used for preventing and treating obesity and diseases related to obesity. The medicine can promote primary white fat cell browning in vitro, thereby enhancing expression of brown fat cell marker gene uncoupling protein 1 (UCP1), and can promote the increase of beige fat cells in white fat tissue of groin and the increase of expression of UCP1; the medicine can resist obesity and insulin insensitivity caused by high-fat diet and has an effect on treating fatty livers.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
Axitinib crystal form preparation method
ActiveCN104140414AReduce pollutionLow requirements for production equipmentOrganic chemistry methodsX-raySolvent
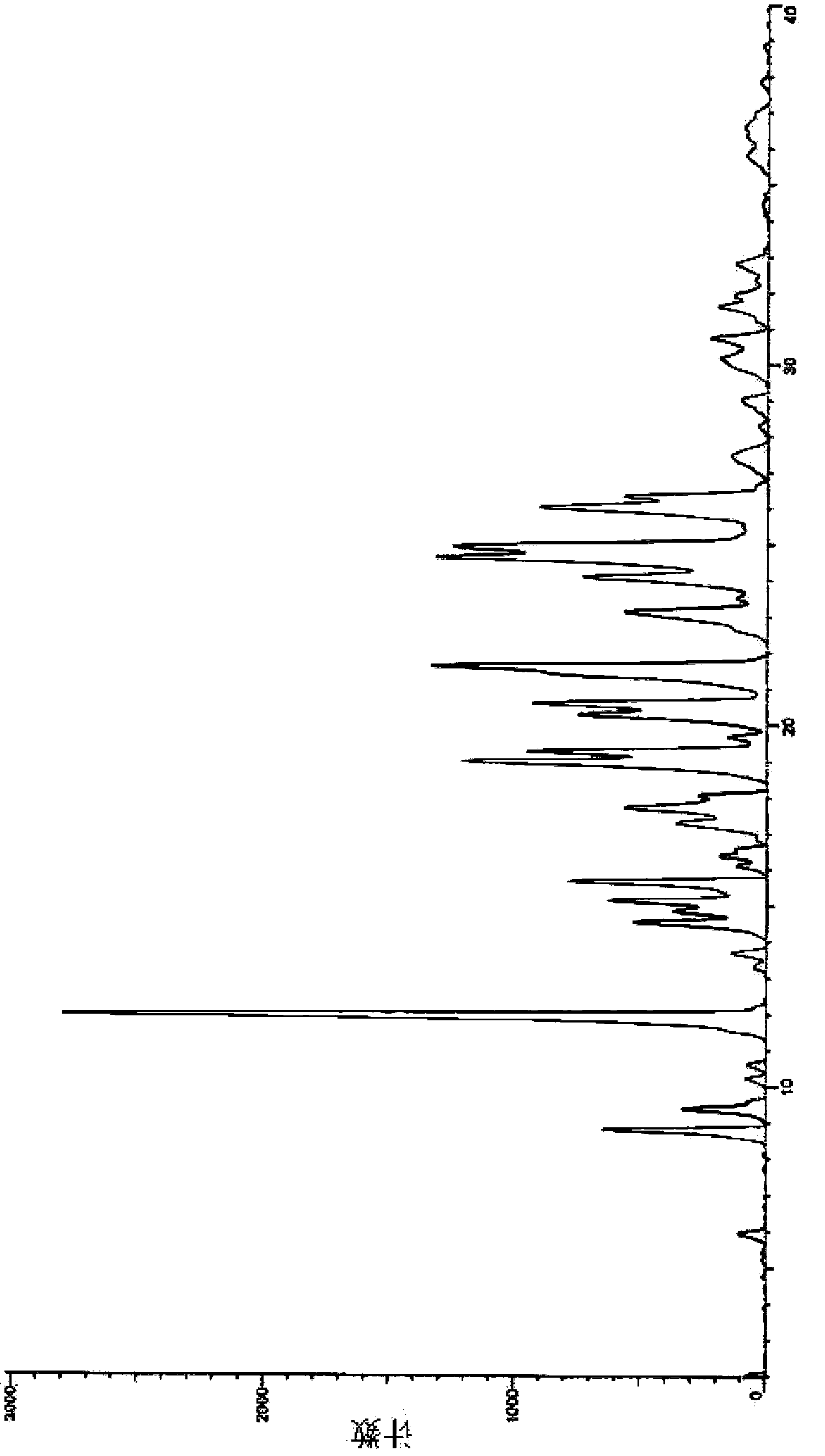
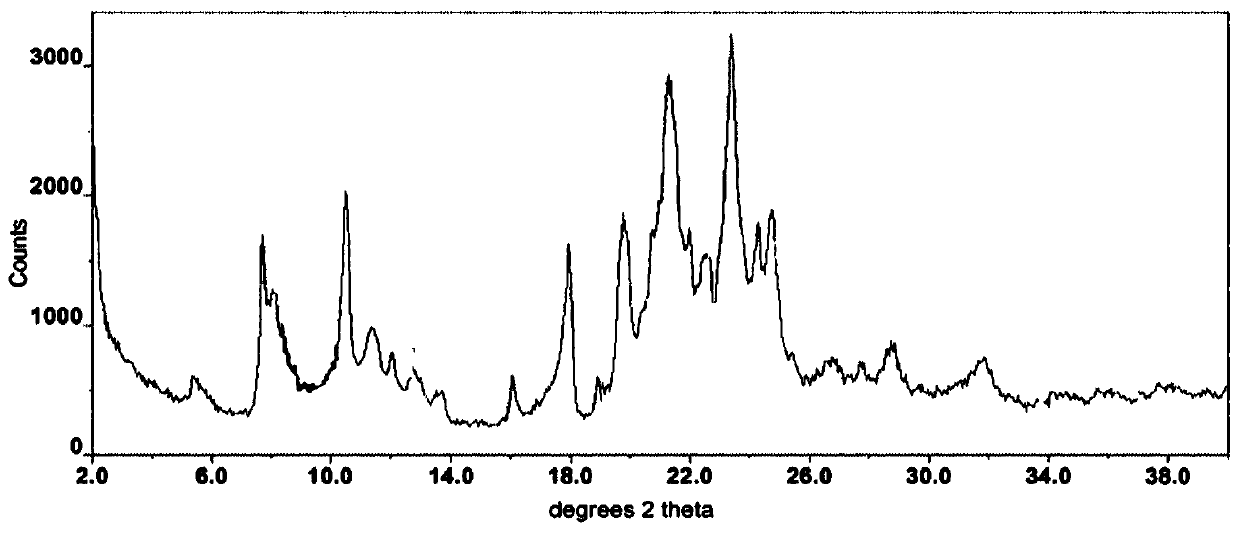
The invention relates to an axitinib crystal form preparation method. Specifically, XRD (X-ray diffraction) spectrum characteristic peaks of axitinib crystal form are shown as 2 theta angle (+ / -0.1), and located in the 8.8, 9.4, 11.9, 14.5, 15.1, 15.6, 19.0, 19.3, 20.3, 20.6, 21.6, 23.1, 24.1, 24.6, 24.9 and 26.0. The preparation method is as follows: putting axitinib or an axitinib solvate into a solvent, heating for slurrying, performing heat preservation, stirring, cooling to ambient temperature for granulation, filtering, and drying to obtain the target crystal form.
Owner:JIANGSU HANSOH PHARMA CO LTD
Axitinib tablet and preparation method thereof
ActiveCN106913547APromote dissolutionSimple preparation processOrganic active ingredientsPharmaceutical non-active ingredientsMesoporous silicaSilicon dioxide
The invention discloses an axitinib tablet and a preparation method thereof. The preparation method comprises the following steps: controlling crystallization process so as to obtain an axitinib-mesoporous silica compound, then mixing the axitinib-mesoporous silica compound with a filler and a disintegrating agent, carrying out granulation and drying, adding a lubricant into dry particles and then carrying out tableting. Compared with the prior art, the axitinib tablet prepared in the invention can be rapidly dissolved out in water, and the preparation method is simple in process and suitable for industrial production.
Owner:SHANDONG NEWTIME PHARMA
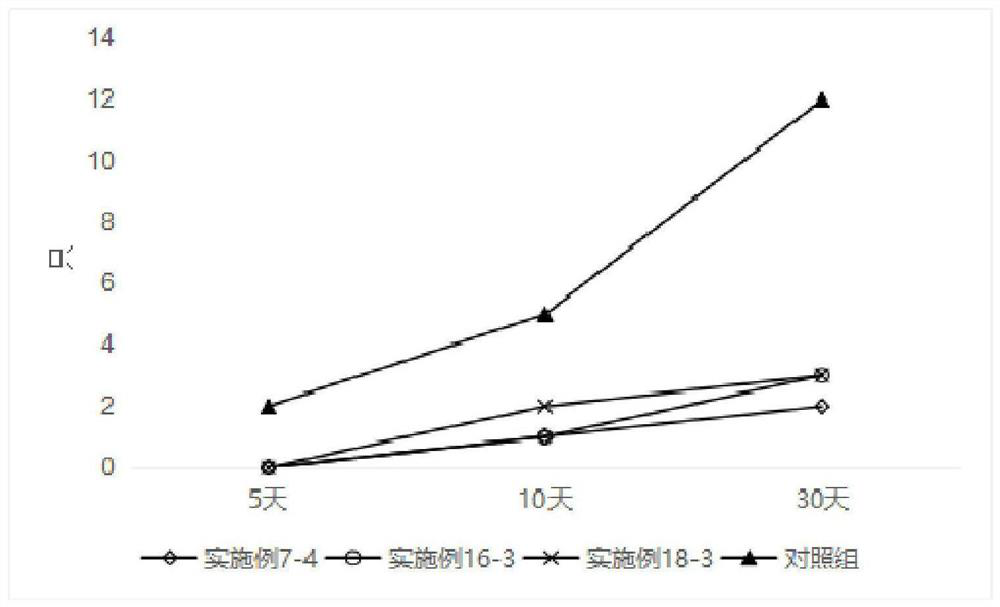
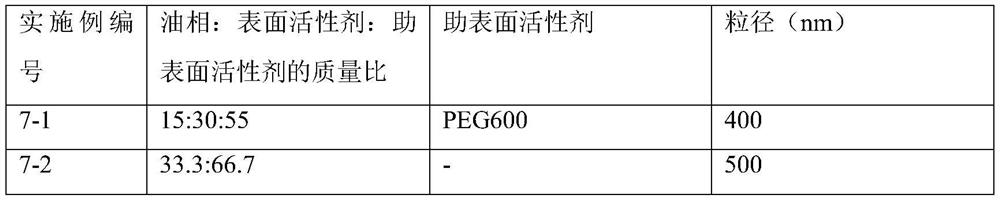
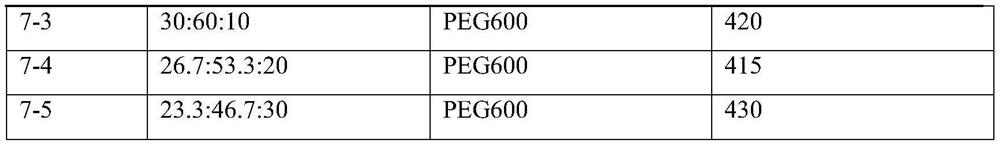
Self-microemulsion preparation of axitinib
ActiveCN112618488AReduce stimulationLittle side effectsOrganic active ingredientsEmulsion deliveryPotocytosisActive agent
The invention belongs to the technical field of medicines, and particularly discloses a self-microemulsion preparation of axitinib. The prepared preparation is a self-microemulsion composition and comprises the following components in percentage by mass: 0.1-10% of axitinib, 5-70% of oil phase, 10-90% of a surfactant and 0-50% of a cosurfactant. The dissolution rate and the mixing uniformity of the prepared axitinib preparation meet the requirements, the axitinib preparation is spontaneously dispersed in gastrointestinal fluid under gastrointestinal peristalsis after being orally taken to form O / W type nanoemulsion, and drug molecules are wrapped in a carrier, so that the particle size of the drug molecules is correspondingly increased, the membrane permeation mode is changed after the nanoemulsion is in contact with small intestine epidermal cells, original passive diffusion transport is changed into endocytosis transport, and stimulation of the nanoemulsion to gastrointestinal tracts is reduced through active cytosis or endocytosis absorption, so that stimulation caused by too high local concentration of the medicines and long-time contact with the gastrointestinal walls is reduced, and side effects of the gastrointestinal tracts of the medicines can be reduced.
Owner:HUNAN HUIZE BIO PHARMA CO LTD
Axitinib sustained-release implplant treating for solid tumor
InactiveCN101204367AOrganic active ingredientsPharmaceutical delivery mechanismWhole bodyProstate cancer
The invention relates to an Axitinib sustained-release implant for treating a solid tumor, which is characterized in that: the sustained-release implant contains an effective anticancer amount of Axitinib and sustained-release excipients and a certain amount of sustained-release regulator. The solid tumors include the liver cancer, the lung cancer, the esophageal canner, the gastric canner, the breast canner, the ovarian canner, the prostate canner, the bladder canner and the rectum canner. The sustained-release excipients are mainly a copolymer of polylactic acid, glycollic acid and hydroxyacetic acid and one of the three materials, polifeprosan, poly-(L-lactide-co-ethyl phosphonate) and Poly(L-lactide-co-propyl phosphonate) or a combination of the three materials, in the degradation and absorption process of which the Axitinib is sustainedly released to part of the tumor, thus the entire toxicity of the Axitinib is significantly reduced while an effective medicine consistency is maintained on part of the tumor. That the sustained-release implant is implanted inside part of the tumor can not only reduce the entire toxicity of the Axitinib, but also enhance the medicine consistency on part of the tumor, thereby increasing the curing effect of non-operative therapeutics such as chemotherapeutic drugs and radiotherapy.
Owner:JINAN SHUAIHUA PHARMA TECH
A kind of preparation method of Axitinib tablet
ActiveCN105769785BSimple preparation processShort production hoursOrganic active ingredientsPharmaceutical non-active ingredientsPhysical chemistryTableting
The invention provides a method for preparing axitinib tablets through a dry powder tabletting method.The method comprises the steps that 1, raw and auxiliary material components comprising 0.5 wt%-3 wt% of axitinib, 85 wt%-97 wt%of a diluent, 1 wt%-10 wt% of a disintegrating agent and 0.25 wt%-2 wt% of a lubricating agent are weighed; 2, the axitinib and the auxiliary materials are mixed in batches; 3, a mixture obtained in the step 2 is tabletted and coated to obtain the axitinib tablets, wherein the particle size D90 of the axitinib is smaller than or equal to 60 micrometers, the particle size of at least 90% of the diluent is smaller than 240 micrometers, the particle size of at least 30% of the diluent ranges from 50 micrometers to 140 micrometers, and the particle size of 2-20 wt% of the diluent is smaller than 32 micrometers.The method for preparing the axitinib tablets through the dry powder tabletting method is simple and reliable; the axitinib tablets prepared through the method are uniform in content, small in tablet weight difference, low in tablet cracking rate and qualified in dissolution curve, and the related substance content meets the specification; meanwhile, the method has the advantages that the technology is simple, the production period is short, the production efficiency is improved, and the cost is reduced.
Owner:SICHUAN KELUN PHARMA RES INST CO LTD
Diagnostic and therapeutic methods for cancer
ActiveUS20190369098A1Organic active ingredientsMicrobiological testing/measurementTyrosine kinaseKidney cancer
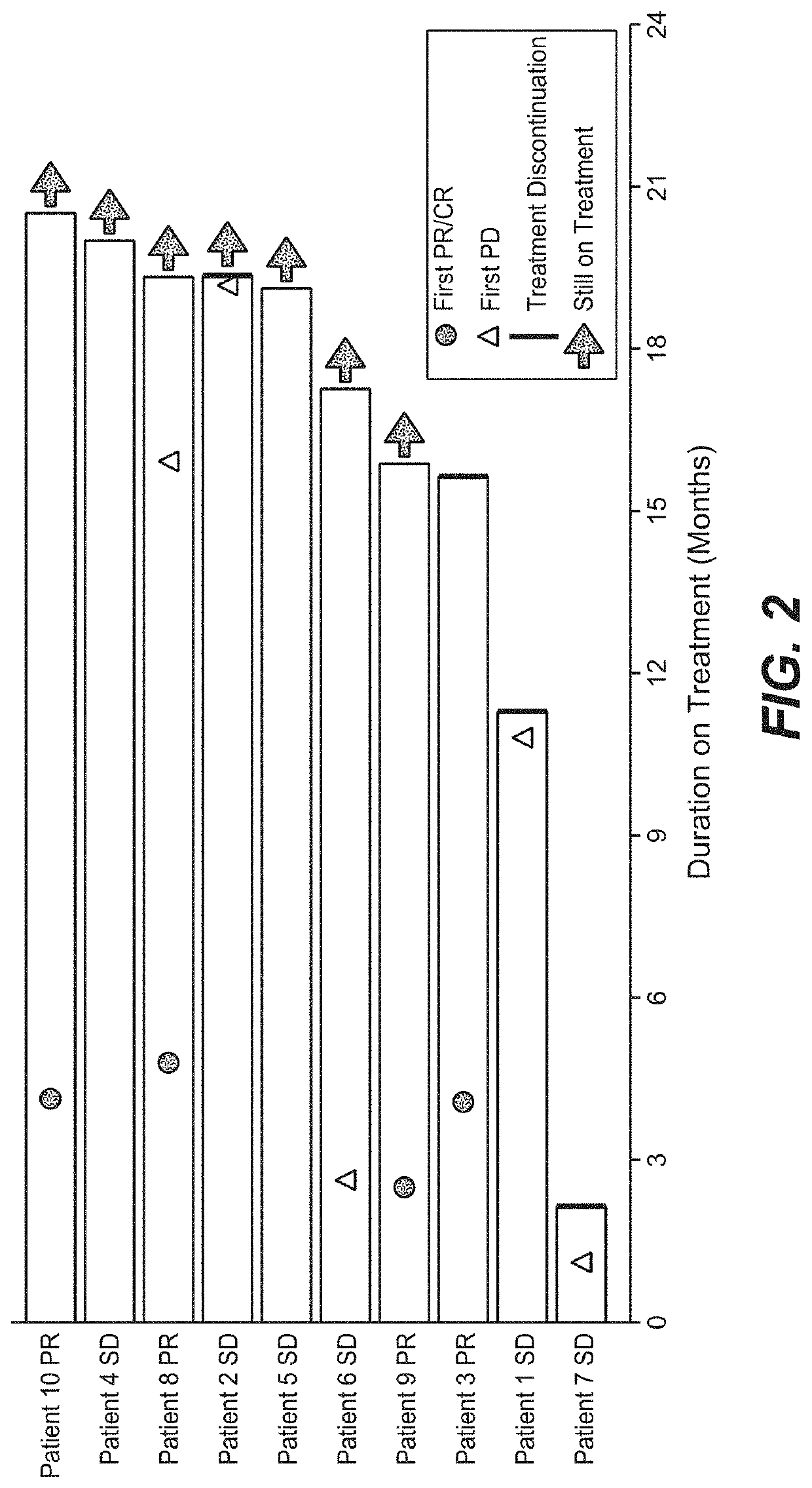
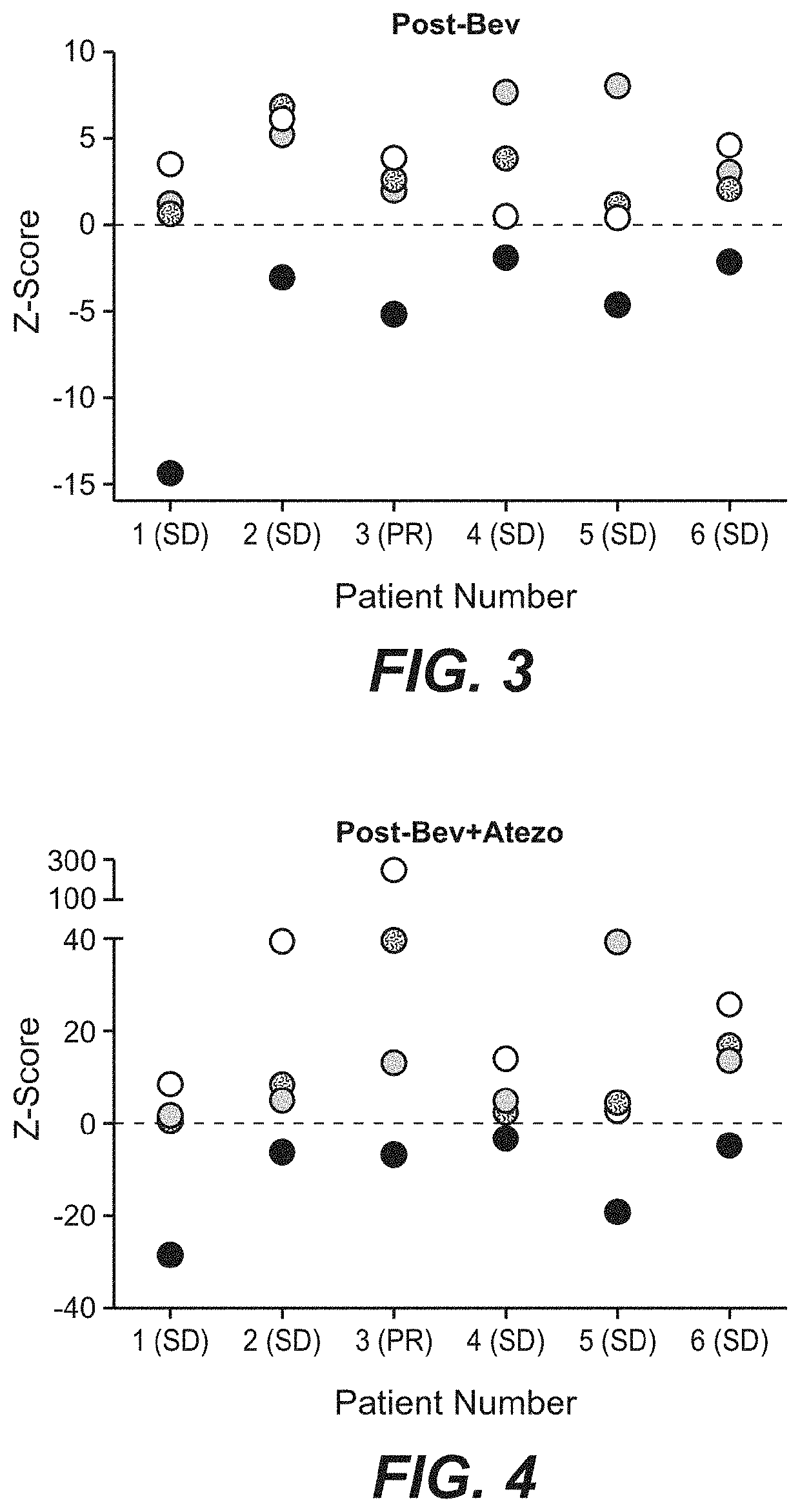
The present invention provides diagnostic methods, therapeutic methods, and compositions for the treatment of cancer (e.g., kidney cancer (e.g., renal cell carcinoma (RCC)), lung cancer (e.g., non-small cell lung cancer (NSCLC)), bladder cancer (e.g., urothelial bladder cancer (UBC)), liver cancer (e.g., hepatocellular carcinoma (HCC)), ovarian cancer, or breast cancer (e.g., triple-negative breast cancer (TNBC))). The invention is based, at least in part, on the discovery that expression levels of one or more biomarkers described herein in a sample from an individual having cancer can be used in methods of predicting the therapeutic efficacy of treatment with a VEGF antagonist (e.g., an anti-VEGF antibody, (e.g., bevacizumab) or a VEGFR inhibitor (e.g., a multi-targeted tyrosine kinase inhibitor (e.g., sunitinib, axitinib, pazopanib, or cabozantinib))) and a PD-L1 axis binding antagonist (e.g., a PD-L1 binding antagonist (e.g., anti-PD-L1 antibody, e.g., atezolizumab (MPDL3280A)) or a PD-1 binding antagonist (e.g., anti-PD-1 antibody)), or with an angiogenesis inhibitor (e.g., a VEGF antagonist (e.g., a VEGFR inhibitor, (e.g., a multi-targeted tyrosine kinase inhibitor (e.g., sunitinib, axitinib, pazopanib, or cabozantinib)))).
Owner:GENENTECH INC
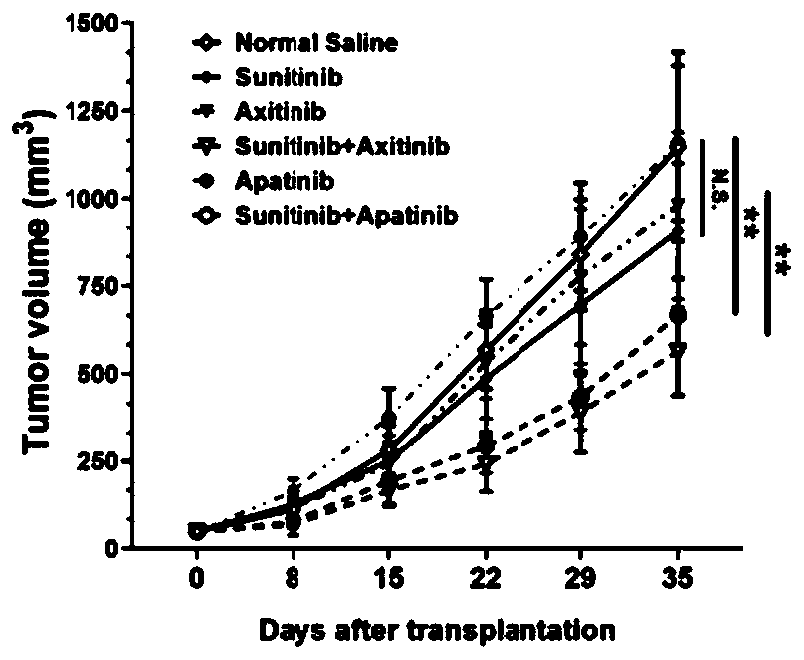
Pharmaceutical composition containing sunitinib as well as preparation and application of pharmaceutical composition
ActiveCN111558044ADelay drug resistancePrevent proliferationOrganic active ingredientsAntineoplastic agentsCancer cellSide effect
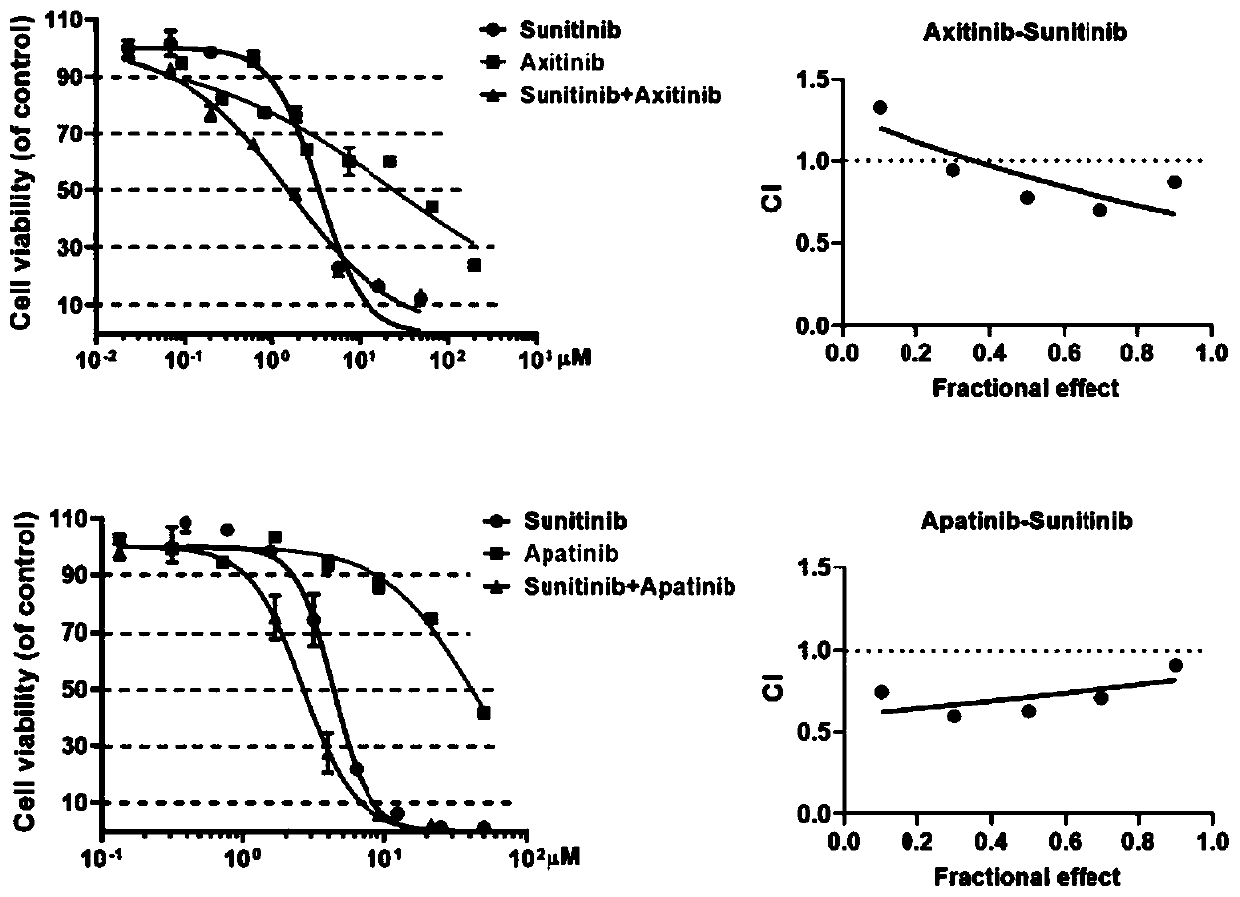
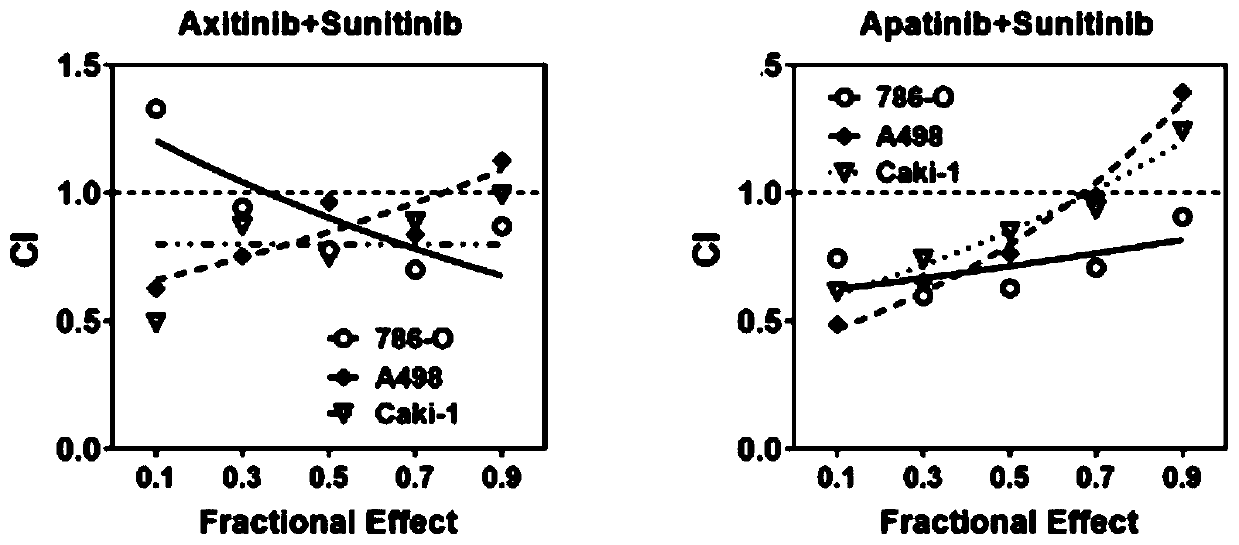
The invention provides a pharmaceutical composition containing sunitinib as well as a preparation and an application of the pharmaceutical composition. On the basis of the original sunitinib, at leastone tyrosine kinase receptor inhibitor, especially apatinib and / or axitinib, is further added to generate a very significant synergistic effect with the sunitinib, so that the proliferation of cancercells is obviously inhibited, and the sunitinib has very significant killing efficiency on the cancer cells. The pharmaceutical composition provided by the invention can obviously improve the problemof serious drug resistance caused by singly adopting sunitinib in the prior art and the problem of obviously reduced treatment effect when the conventional use amount of sunitinib is reduced; clinical treatment efficiency is greatly improved, treatment toxic and side effects of a patient are reduced, a new scheme is provided for clinical treatment, and the traditional Chinese medicine compositionhas very wide market prospects and extremely important social significance.
Owner:SUN YAT SEN UNIV CANCER CENT
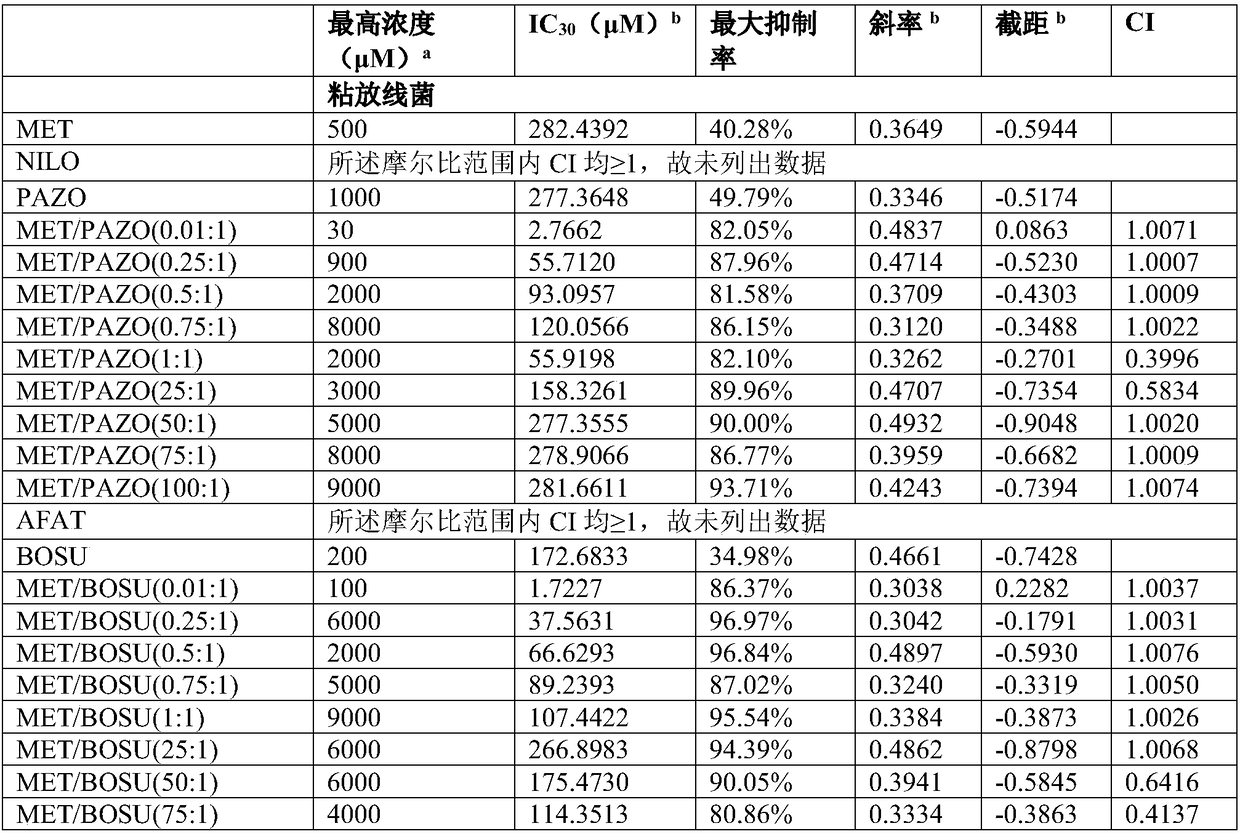
Composition containing protein kinase inhibitor and metformin
The invention firstly provides a composition containing a protein kinase inhibitor and metformin or pharmaceutically acceptable salts thereof. The composition is characterized in that the protein kinase inhibitor is one selected from nilotinib, pazopanib, afatinib, bosutinib, crizotinib, axitinib and regorafenib or pharmaceutically acceptable salts or solvates thereof or solvates of the pharmaceutically acceptable salts thereof, and the molar ratio of the metformin to the protein kinase inhibitor is (0.01-100):1. In-vitro bacteriostatic tests find that the composition containing the metforminand the protein kinase inhibitor can achieve a synergistic bacteriostatic effect on various bacteria such as staphylococcus aureus in the molar ratio of (0.01-100):1 (at an inhibition rate of 30%, thecombined medication index CI is smaller than 1).
Owner:黄泳华
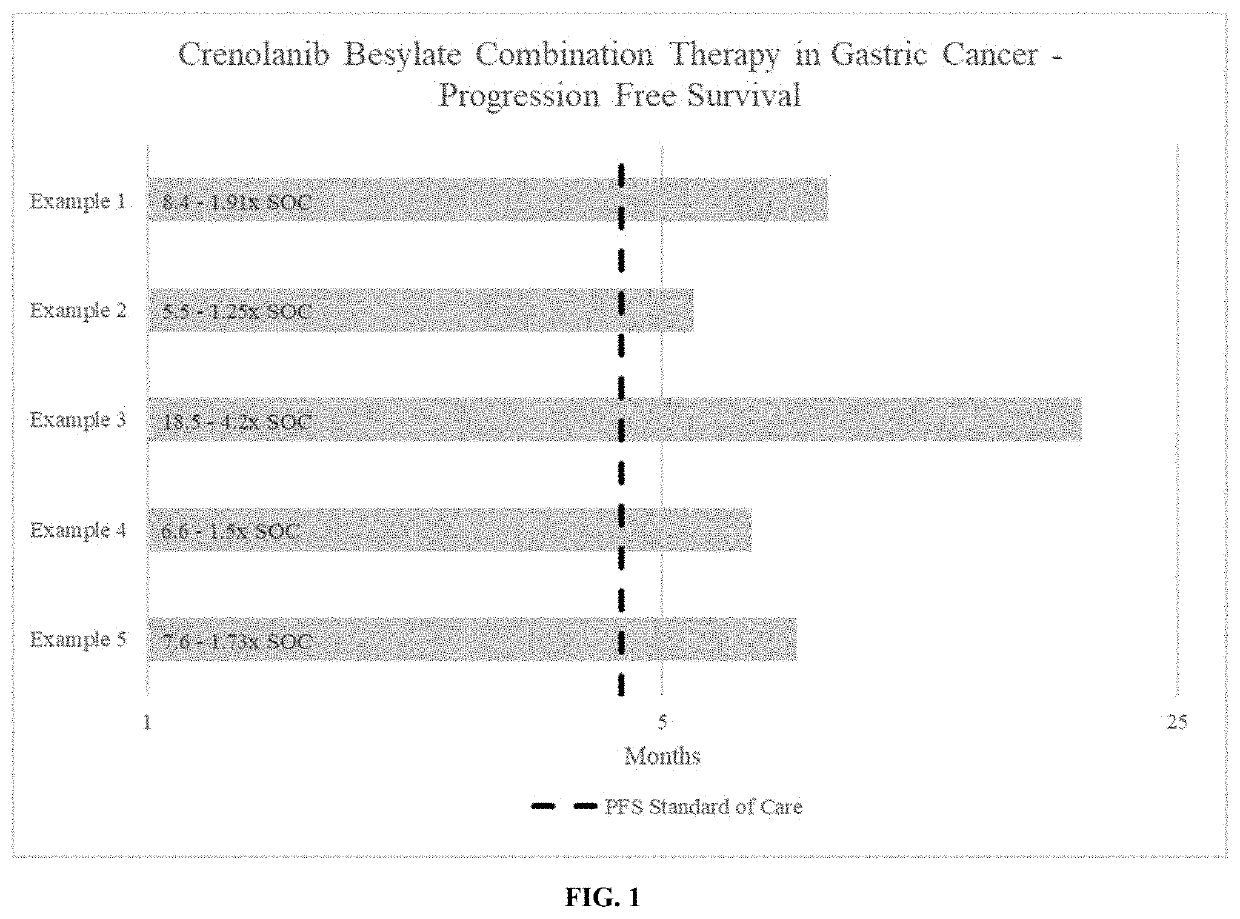
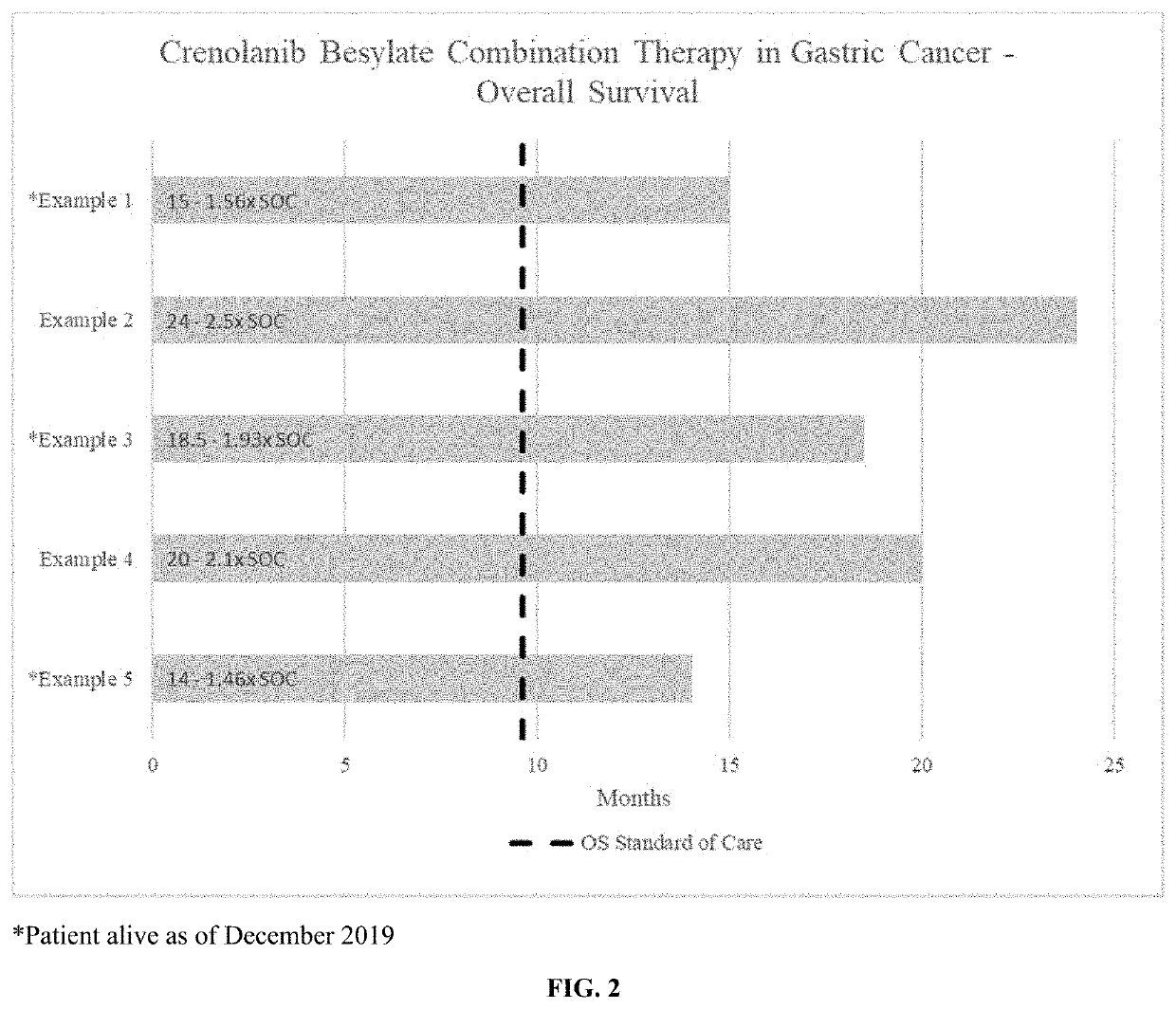
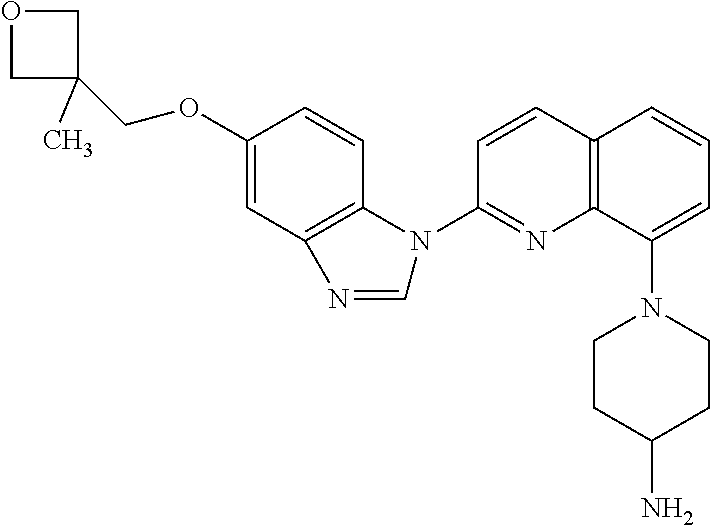
Crenolanib combination therapy
The present invention includes methods for treating a proliferative disorder by blocking both PDGFR and VEGFR signaling comprising a therapeutically effective amount of crenolanib or salt in combination with a VEGF / VEGFR inhibitor that is not axitinib wherein the crenolanib, VEGF / VEGFR inhibitor that is not axitinib are provided at least one of sequentially or concomitantly, in a subject for use in the treatment of the proliferative disorder, wherein the subject is a human subject.
Owner:AROG PHARMA
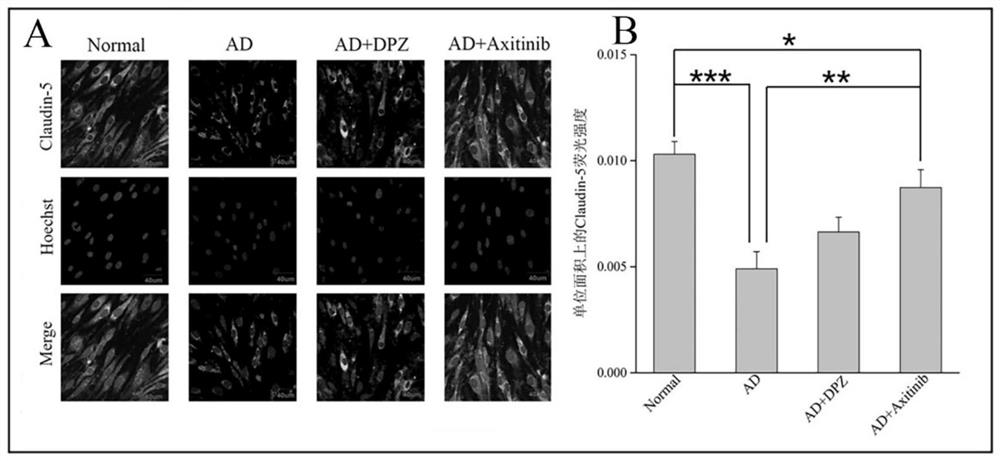
Application of VEGFR inhibitor in preparation of anti-Alzheimer's disease medicine
PendingCN113750236AReduce penetrationImprove securityOrganic active ingredientsNervous disorderDiseaseDonepezil
The invention discloses an application of a VEGFR inhibitor in preparation of an anti-Alzheimer's disease medicine. The VEGFR inhibitor is axitinib and an analogue thereof. It is found that axitinib has an unexpected curative effect on Alzheimer's disease, and Alzheimer's disease model animal in-vivo experiments prove that after administration of axitinib, beta-amyloid protein deposition in the brain of a model animal is significantly reduced, the content of acetylcholin esterase is reduced, the oxidative stress level and inflammatory response of the lesion part of the model animal are relieved, the learning and memory ability and learning and memory behaviors of the model animal on space and direction are improved, and the curative effect is obviously higher than that of a clinical first-line treatment drug donepezil. The axitinib and the analogue thereof provide a new means for clinical treatment of the Alzheimer's disease, and the axitinib and the analogue thereof are high in human body safety, low in price and easy to prepare. According to the invention, non-intracranial administration mode is adopted, safety is high, administration is convenient and fast, and patient compliance is high. The application of a VEGFR inhibitor in preparation of an anti-Alzheimer's disease medicine has wide market prospects and great social significance.
Owner:ZHEJIANG UNIV
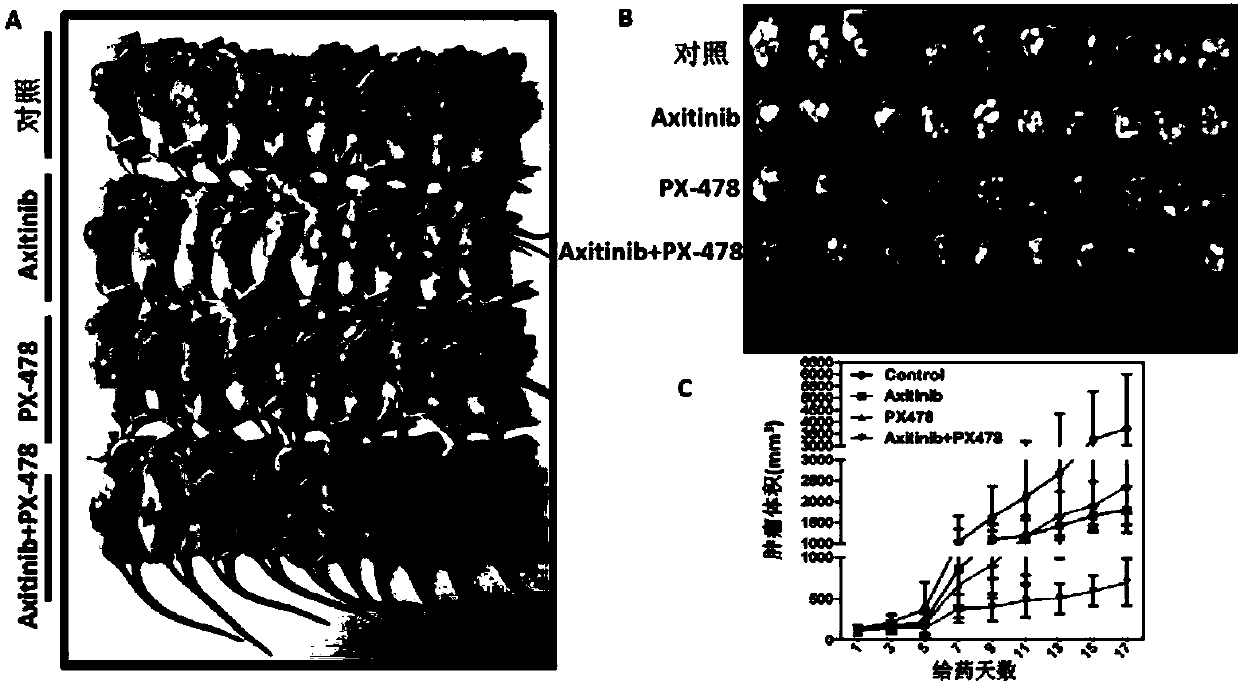
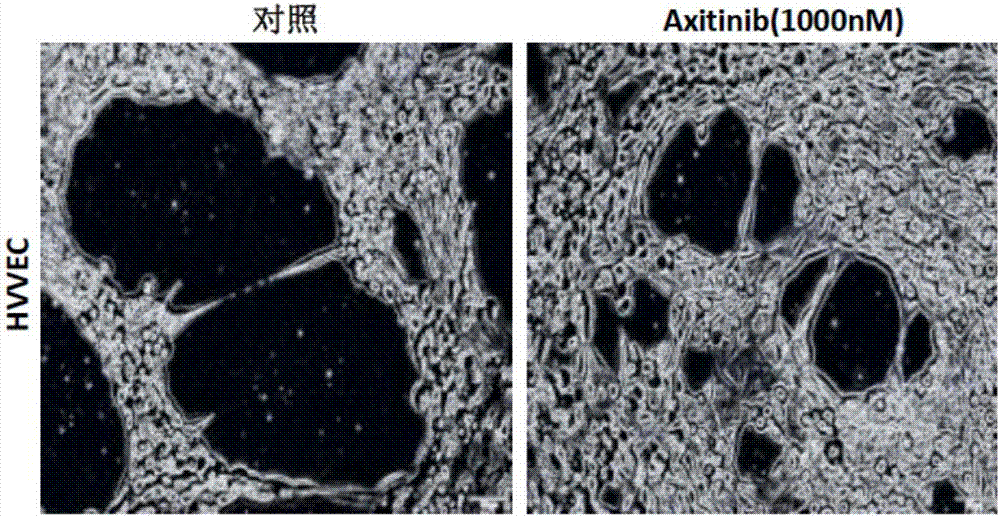
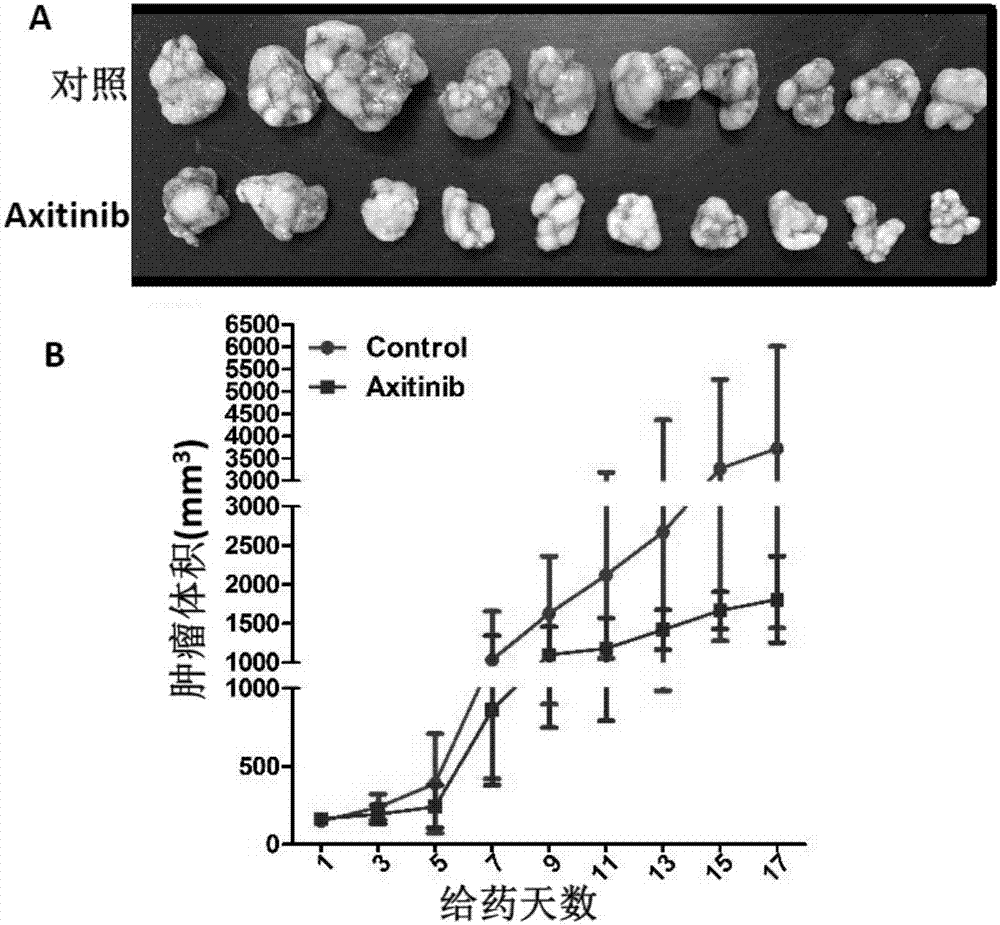
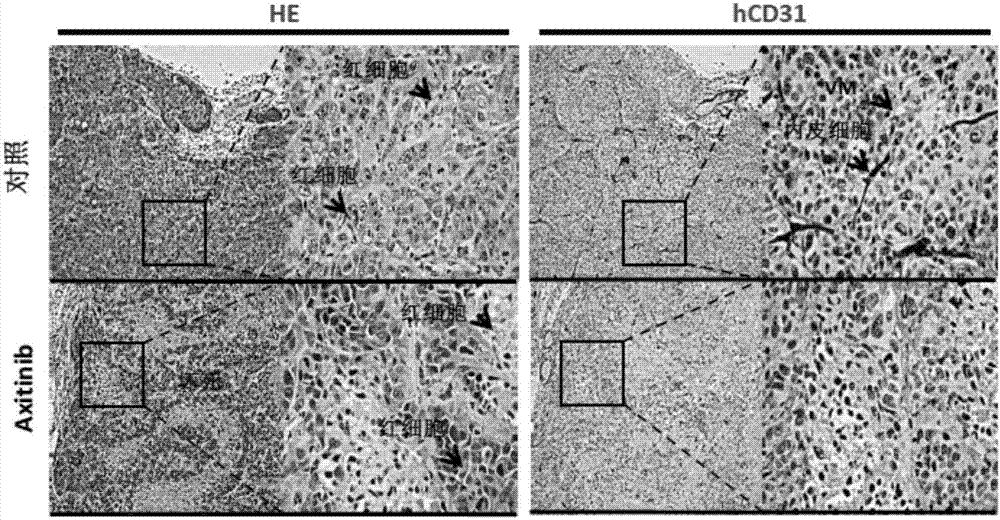
Application of axitinib and PX-478 in treatment of nasopharyngeal carcinoma
ActiveCN107693517ASignificant effectGrowth inhibitionOrganic active ingredientsPeptide/protein ingredientsRegimenCurative effect
The invention discloses PX-478, and an application of combined use of axitinib and PX-478 in treatment of nasopharyngeal carcinoma. The PX-478 is discovered to significantly inhibit formation of VM ofnasopharyngeal carcinoma cells, inhibit the growth of transplantation tumor and have a significant curative effect on nasopharyngeal carcinoma for a first time. At the same time, a research discoversthat the axitinib and PX-478 combined medication achieves synergistic effect and has a more significant curative effect on nasopharyngeal carcinoma. The invention not only provides the new application of axitinib and PX-478, but also provides a new treatment medication regimen for treatment of nasopharyngeal carcinoma, and has a good application prospect in prevention and treatment of nasopharyngeal carcinoma.
Owner:SUN YAT SEN UNIV +1
Pharmaceutical composition containing crenolanib and application of pharmaceutical composition
Owner:AROG PHARMA
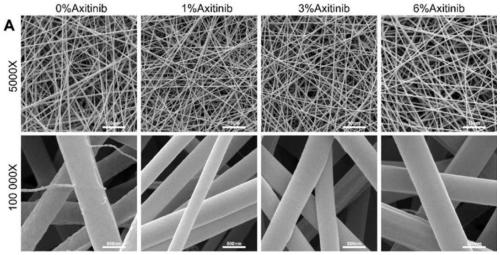
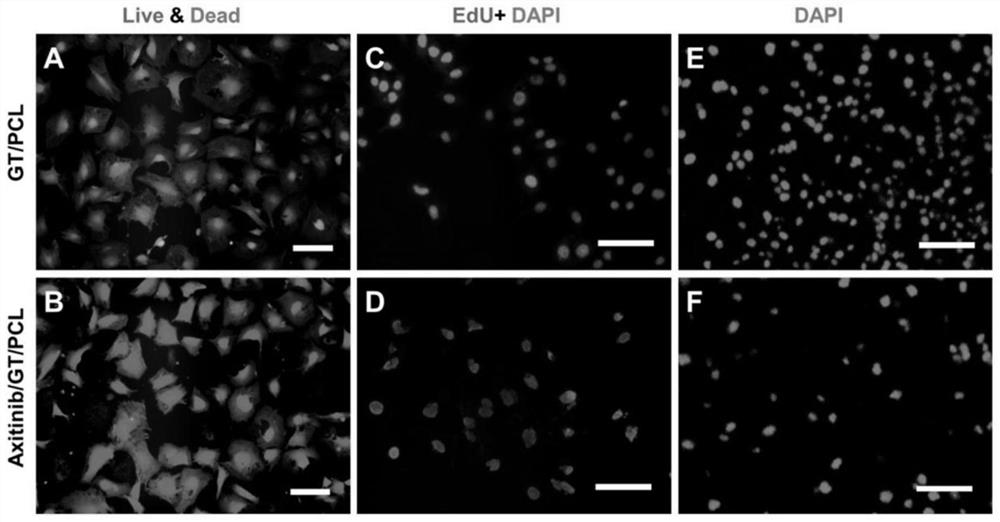
Axitinib-contained nanofiber electrospun membrane and preparation method and application thereof
The invention provides an axitinib-contained nanofiber electrospun membrane. The nanofiber electrospun membrane consists of axitinib, collagen and polycaprolactone. The invention further provides a preparation method and application of the nanofiber electrospun membrane. The nanofiber electrospun membrane has good biocompatibility, can be degraded and absorbed and can remarkably inhibit blood vessel generation, and therefore the problem that cartilages are prone to ossification when regenerated in a subcutaneous environment is solved.
Owner:SHANGHAI CHILDRENS MEDICAL CENT AFFILIATED TO SHANGHAI JIAOTONG UNIV SCHOOL OF MEDICINE
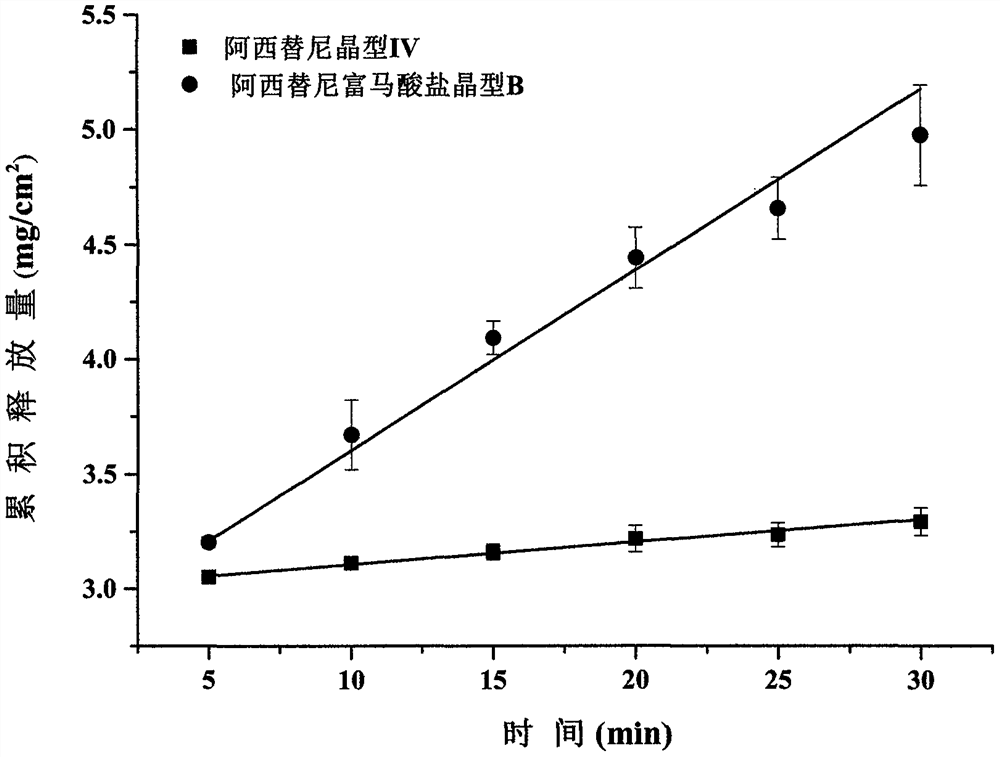
New crystal form of axitinib fumarate and preparation method thereof
InactiveCN112174933AImprove securityImprove oral bioavailabilityOrganic active ingredientsOrganic chemistry methodsBioavailabilityPowder diffraction
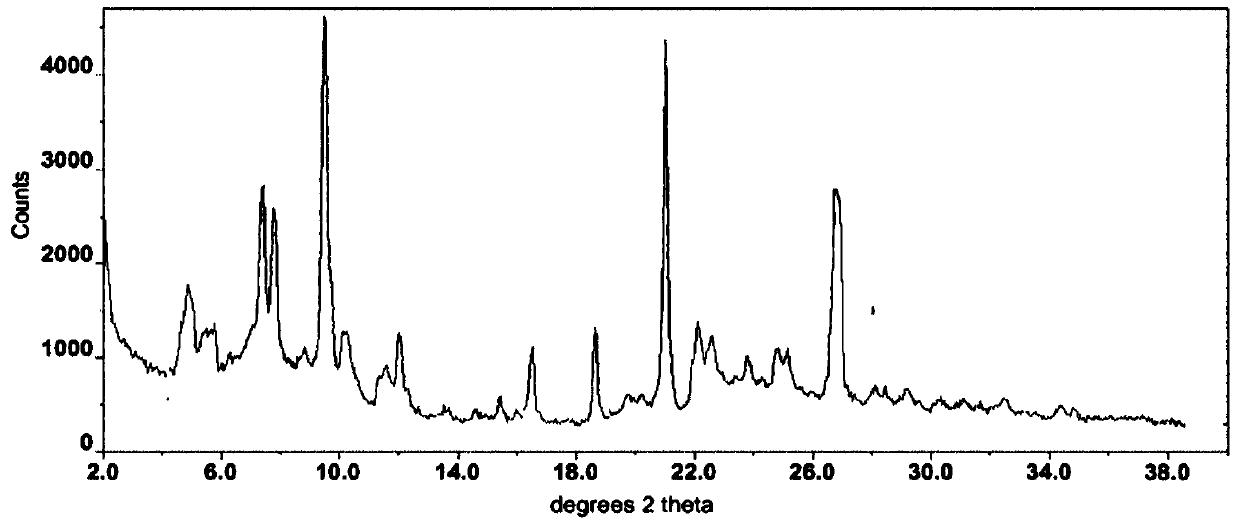
The invention discloses a new crystal form of axitinib fumarate and a preparation method thereof. In the new crystal form, the molar ratio of axitinib to fumaric acid is 1: 1.5, and the X-ray powder diffraction pattern of the crystal form has characteristic peaks when the 2 theta value is 7.1 + / -0.2 degrees, 12.5 + / -0.2 degrees, 15.1 + / -0.2 degrees, 17.4 + / -0.2 degrees and 23.4 + / -0.2 degrees. Thecrystal form preparation method provided by the invention is simple in process, easy in crystallization process control, good in reproducibility and suitable for industrial production. The new crystal form of the axitinib fumarate is remarkably improved in the aspects of light stability and dissolution property, and is beneficial to improving the safety and oral bioavailability of axitinib.
Owner:TIANJIN UNIVERSITY OF TECHNOLOGY
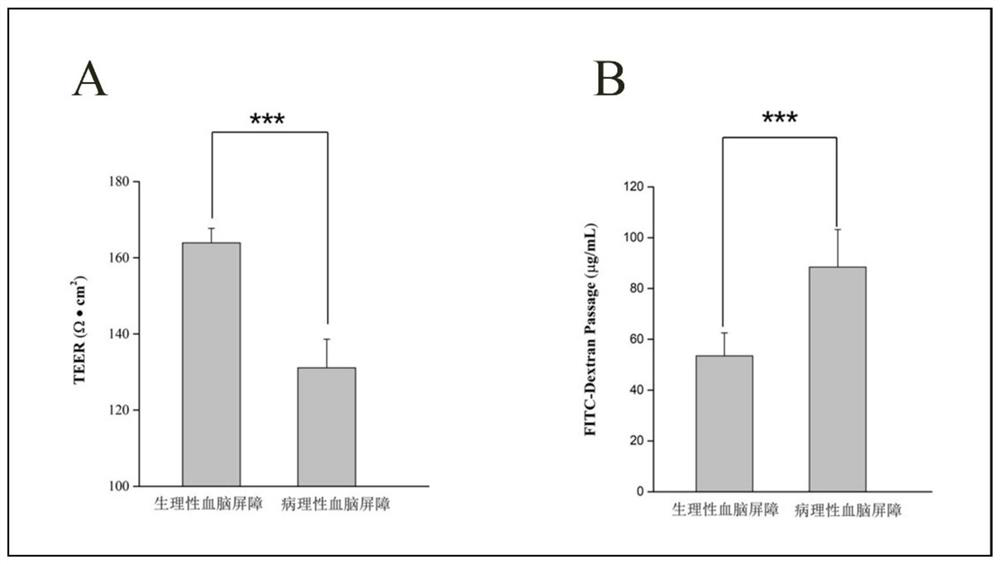
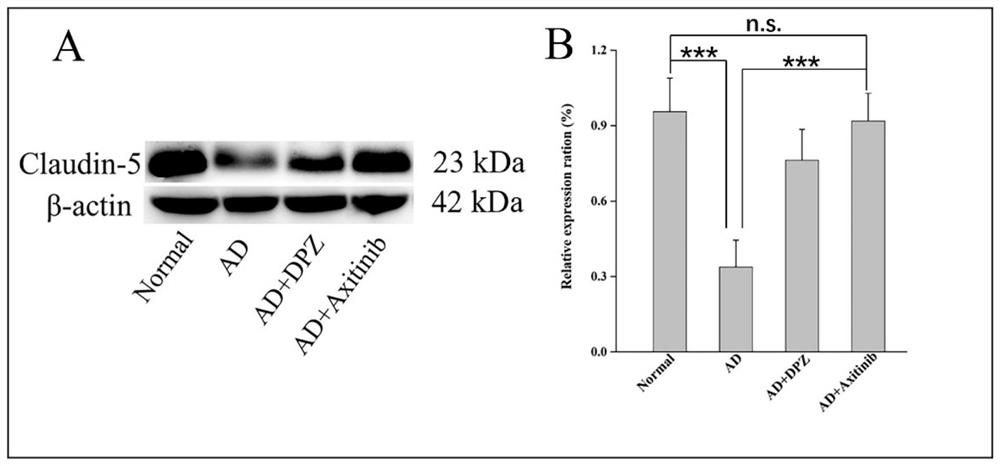
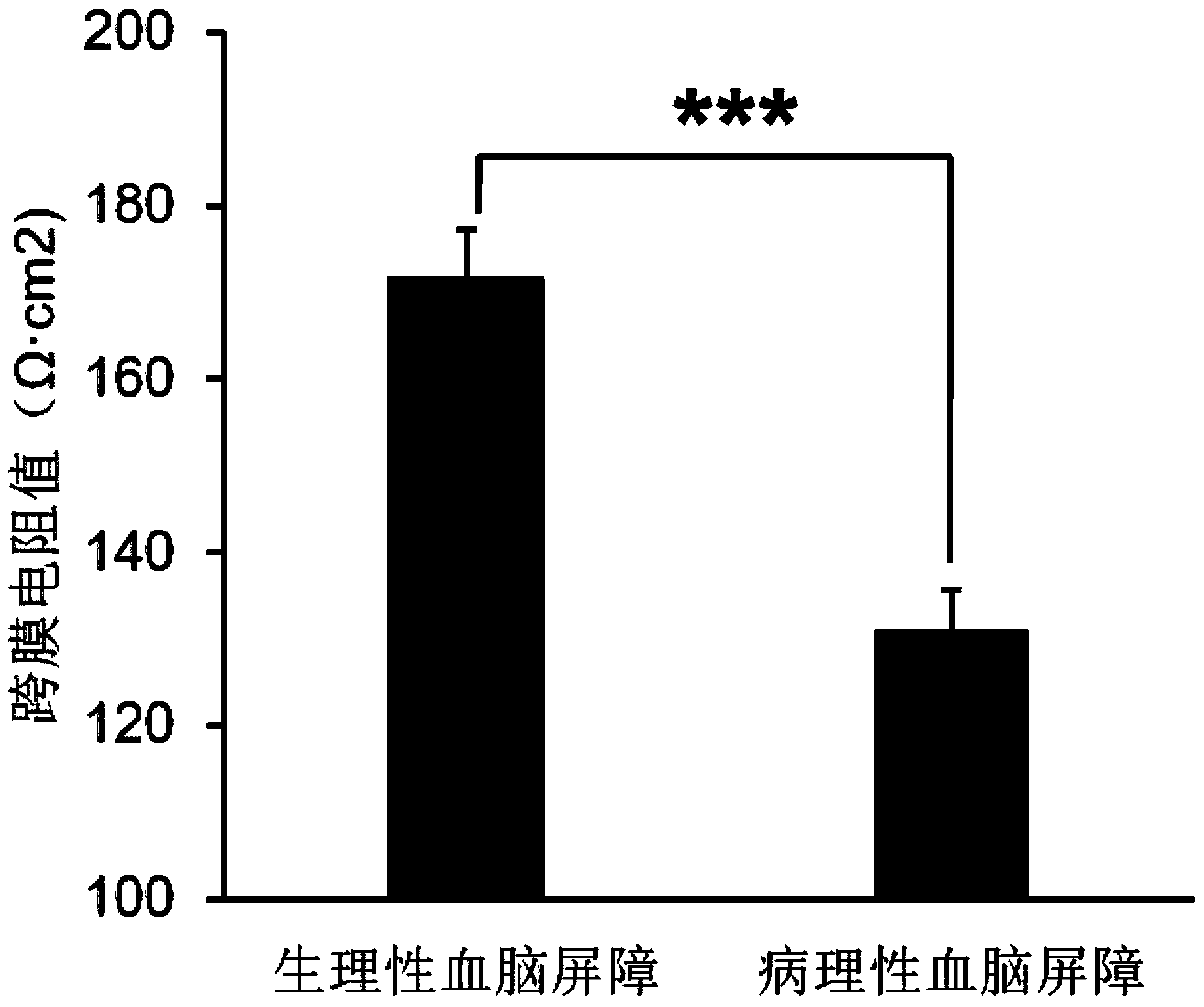
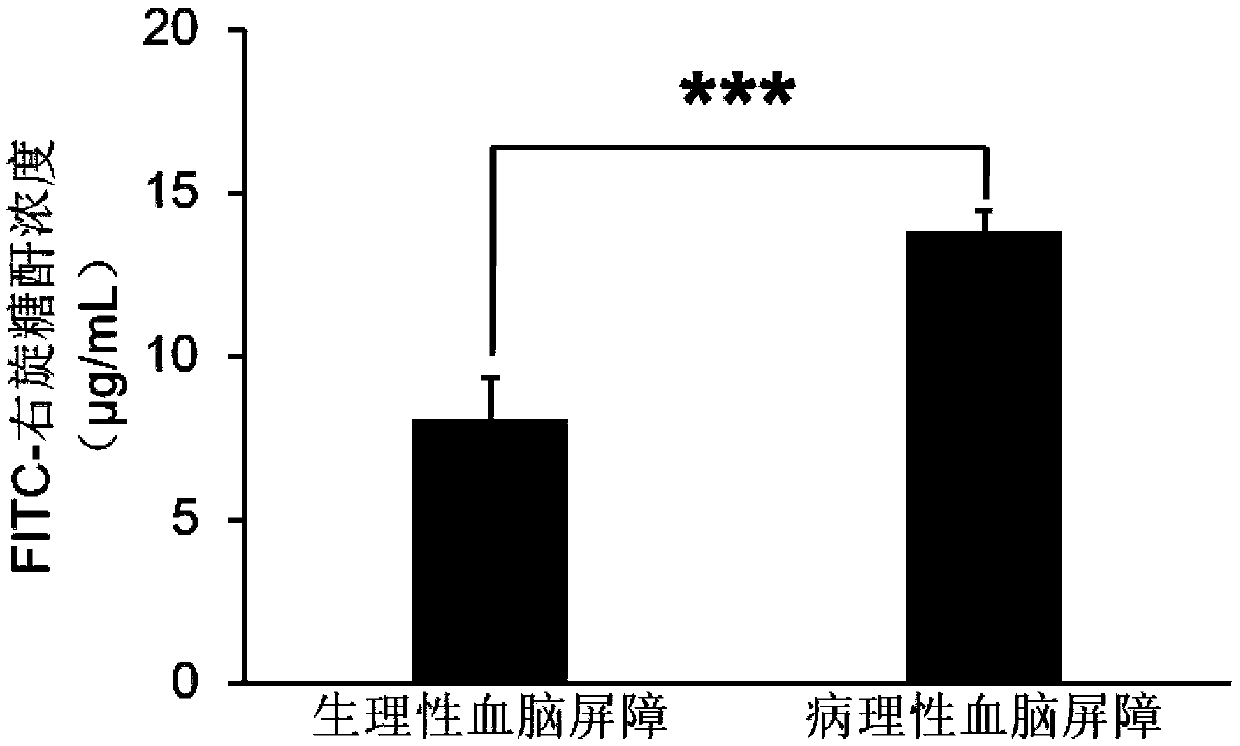
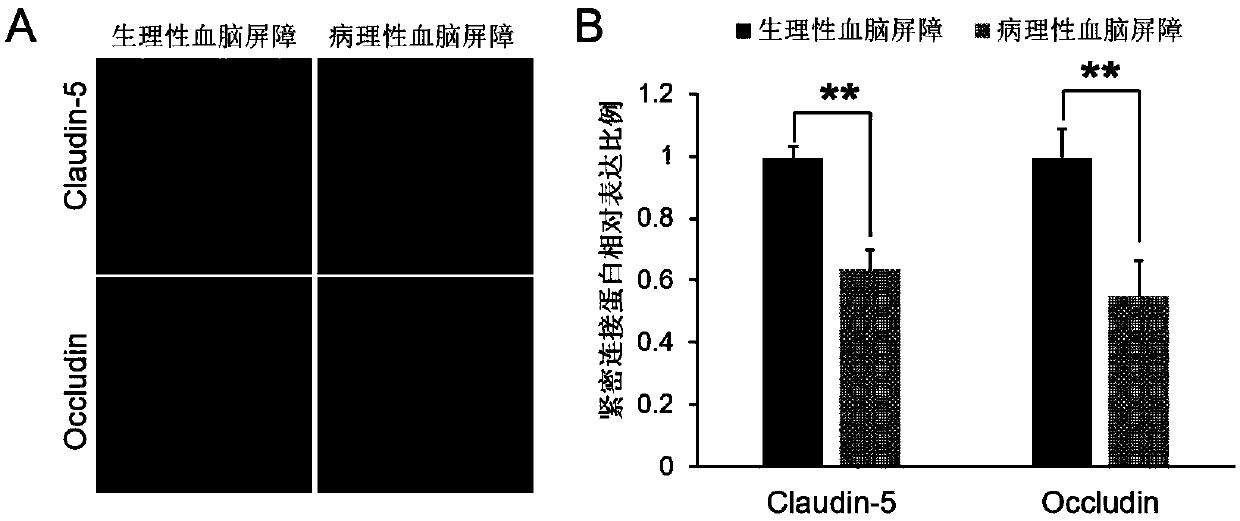
Use of axitinib and analogue thereof in preparation of blood-brain barrier permeability regulating agent
ActiveCN111374977AReduce penetrationAchieve therapeutic effectOrganic active ingredientsNervous disorderDiseaseBlood brain barrier penetration
The invention relates to use of axitinib and an analogue thereof in preparation of a blood-brain barrier permeability regulating agent. The blood-brain barrier permeability regulating agent can reducethe blood-brain barrier permeability and promote the recovery of a blood-brain barrier function from a pathological damage state to an approximate physiological barrier state. The axitinib and the analogue thereof, by suppressing vascular endothelial cell growth factor-phosphatidylinositol kinase-protein kinase B signaling pathway, can reduce the down-regulating degree of pathological blood brainbarrier tightly attached protein Claudin-5 / Occludin and reduce the blood-brain barrier permeability. The blood-brain barrier permeability regulating agent can be used for treatment of related diseases that cause changes in the blood-brain barrier permeability.
Owner:ZHEJIANG UNIV
Axitinib tablet
ActiveCN112999176ASimple processQuality improvementOrganic active ingredientsInorganic non-active ingredientsAlcoholTableting
The invention belongs to the technical field of pharmaceutical preparations, and particularly relates to axitinib tablets. The preparation process of the axitinib tablet provided by the invention comprises the following steps: adding axitinib into a volatile hydrochloric acid alcohol solution, uniformly stirring, adding into a filling agent and a disintegrating agent, stirring, granulating, drying, adding a lubricating agent, mixing and tabletting to obtain the axitinib tablet. The prepared axitinib tablet is high in dissolution speed, high in stability, simple in preparation process and suitable for large-scale production.
Owner:LUNAN PHARMA GROUP CORPORATION
Axitinib-loaded nanofiber membrane as well as preparation method and application thereof to surgical postoperative adhesion prevention
ActiveCN112210891AGood biocompatibilityImprove mechanical propertiesOrganic active ingredientsSurgical drugsSurgical operationBiocompatibility
The invention discloses an application of a nanofiber membrane loaded with a small molecule drug for inhibiting a vascular endothelial growth factor and / or inhibiting a vascular endothelial growth factor receptor to preparation of a surgical postoperative anti-adhesion medical instrument. The nanofiber membrane has the advantages that the nanofiber membrane provided in the invention is good in biocompatibility, excellent in mechanical property, flexible and smooth after encountering water, good in air permeability and capable of effectively preventing the heart from adhering to surrounding tissues. In addition, the nanofiber membrane can also be applied to adhesion prevention in other surgical operations for abdominal cavities, pelvic cavities, tendons and the like.
Owner:SHANGHAI CHILDRENS MEDICAL CENT AFFILIATED TO SHANGHAI JIAOTONG UNIV SCHOOL OF MEDICINE
Anticancer sustained-release gel injection and preparation method thereof
InactiveCN101283974APharmaceutical delivery mechanismPharmaceutical non-active ingredientsTherapeutic effectSolvent
A sustained-release gel injection comprises angiogenesis inhibitor and / or carmustine or nimustine, amphiphilic block copolymer and solvent, wherein the angiogenesis inhibitor is selected from SU5416, SU6668, alemtuzumab, axitinib, ibritumomab, bevacizumab, sutent, dasatinib, tarceva, vandetanib, gefitinib, lapatinib, lapatinib, sunitinib, sorafenib, endostatain, engiostatin, tositumomab, tipifarnib, sirolimus, cetuximab, ematinib, lenalidomide and thalidomide. The mixture of amphiphilic block copolymer and solvent has temperature sensitive gelatinization characteristic, is fluid liquid at room temperature, and automatically becomes non-flowing biodegradable water insoluble gel in warm-blooded animals. The preparation can locally slowly release at tumor foci and maintain effective drug concentration for several weeks to several months; and has effects on killing tumor cells, effectively inhibiting tumor angiogenesis, reducing systemic toxicity of drug, and enhancing the treatment effect of chemotherapy and radiotherapy (particularly radioactive particles).
Owner:济南基福医药科技有限公司
Preparation method of axitinib
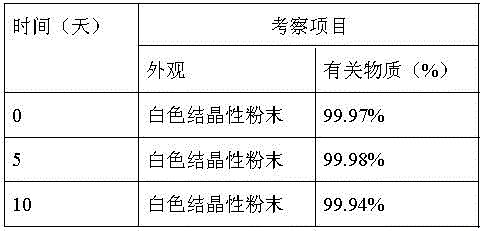
The invention relates to a method for refining axitinib, which belongs to the technical field of preparation of raw materials. The preparation method of axitinib of the present invention comprises the following steps: the crude product of axitinib is dissolved in a mixed solvent of acetone and ethanol, adding 5% gac of axitinib weight, stirring, and filtering; Slowly add 95% ethanol which is 0.6-1.2 times the volume of the solution in the first step to the filtrate of the first step, after the addition is completed, quickly cool the system to -8°C to -3°C, and continue stirring; in the third step, filter to obtain the present invention product. The invention provides a high-purity axitinib bulk drug with a D90 of 100-120 microns. The process of the invention is simple and easy to operate, and is suitable for industrial production. The purity of the product of the invention can reach more than 99.95%.
Owner:WEIHAI GUANBIAO INFORMATION TECH
Application of axitinib in treating nasopharynx cancer
InactiveCN107468691ASignificant effectGrowth inhibitionOrganic active ingredientsAntineoplastic agentsCurative effectNasopharyngeal carcinoma
The invention discloses application of axitinib in treating nasopharynx cancer. The invention reports that axitinib is capable of remarkably inhibiting growth of nasopharynx cancer transplantation tumor and has a remarkable treatment effect on nasopharynx cancer for a first time. The invention provides novel application of axitinib, also provides a novel medicine for treating nasopharynx cancer, and has very good application prospects in preventing and treating nasopharynx cancer. In addition, axitinib is already a clinical medicine and has a solid clinical application foundation.
Owner:SUN YAT SEN UNIV +1
Preparation method of axitinib
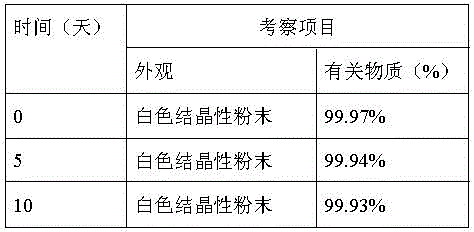
The invention relates to a method for refining axitinib, which belongs to the technical field of preparation of raw materials. The preparation method of axitinib of the present invention comprises the following steps: the crude product of axitinib is dissolved in a mixed solvent of acetone and ethanol, adding 5% gac of axitinib weight, stirring, and filtering; Slowly add 95% ethanol that is 0.6-1.2 times the volume of the first-step solution into the first-step filtrate dropwise, after the dropwise addition, quickly cool the system to -8°C to -3°C, and continue stirring; the third step is filtered to obtain the present invention product. The invention provides a high-purity axitinib bulk drug with a D90 of 100-120 microns. The process of the invention is simple and easy to operate, and is suitable for industrial production. The product purity of the invention can reach more than 99.95%.
Owner:WEIHAI GUANBIAO INFORMATION TECH
Pharmaceutical composition for treating renal clear cell carcinoma and application thereof
ActiveCN111789952AImprove anti-cancer effectAddressing issues that impair anticancer efficacy and lead to drug resistanceUrinary disorderAntineoplastic agentsSide effectWhite Adipocytes
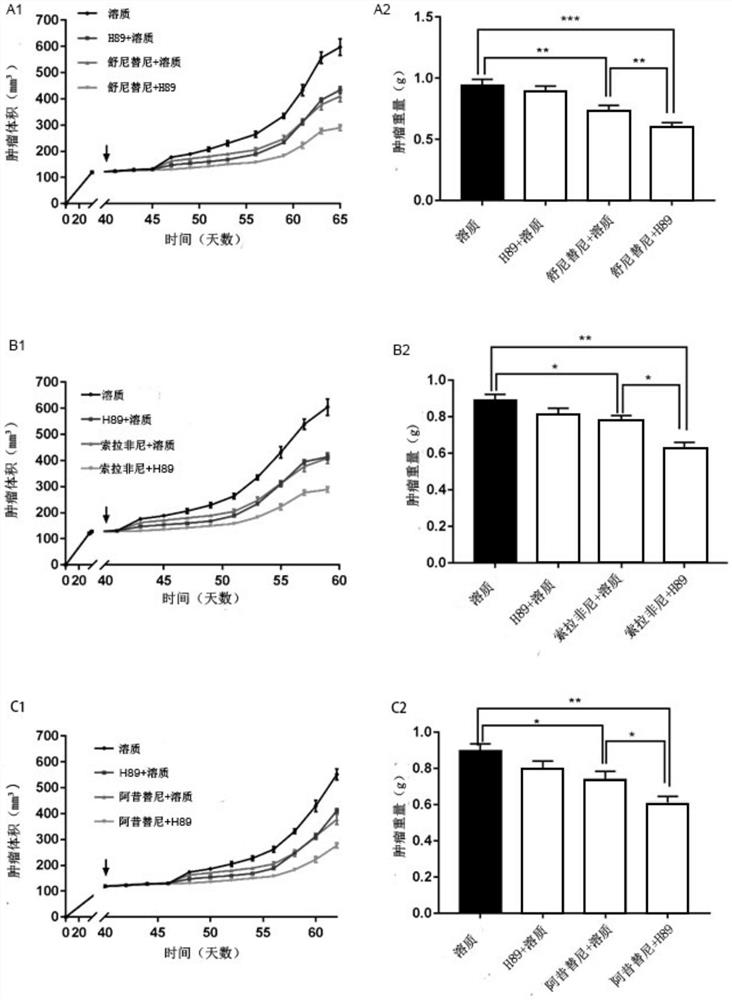
The invention provides a pharmaceutical composition for treating the renal clear cell carcinoma and an application thereof. The pharmaceutical composition comprises a fat browning inhibitor and a first-line renal cancer treatment drug, wherein the first-line renal cancer treatment drug is selected from sunitinib, sorafenib, axitinib and pazopanib to form four combinations. The fat browning inhibitor in the pharmaceutical composition can improve the anti-cancer effects of the four kinds of first-line renal cancer treatment drugs, solve the problem that the four kinds of first-line renal cancertreatment drugs can cause white fat cell browning, further possibly damage the anti-cancer curative effect and cause drug resistance, reduce the treatment side effects of the drugs, and provide a newstrategy for treatment of the renal clear cell carcinoma.
Owner:SHANGHAI SIXTH PEOPLES HOSPITAL
Multikinase inhibitors of VEGF and tfg beta and uses thereof
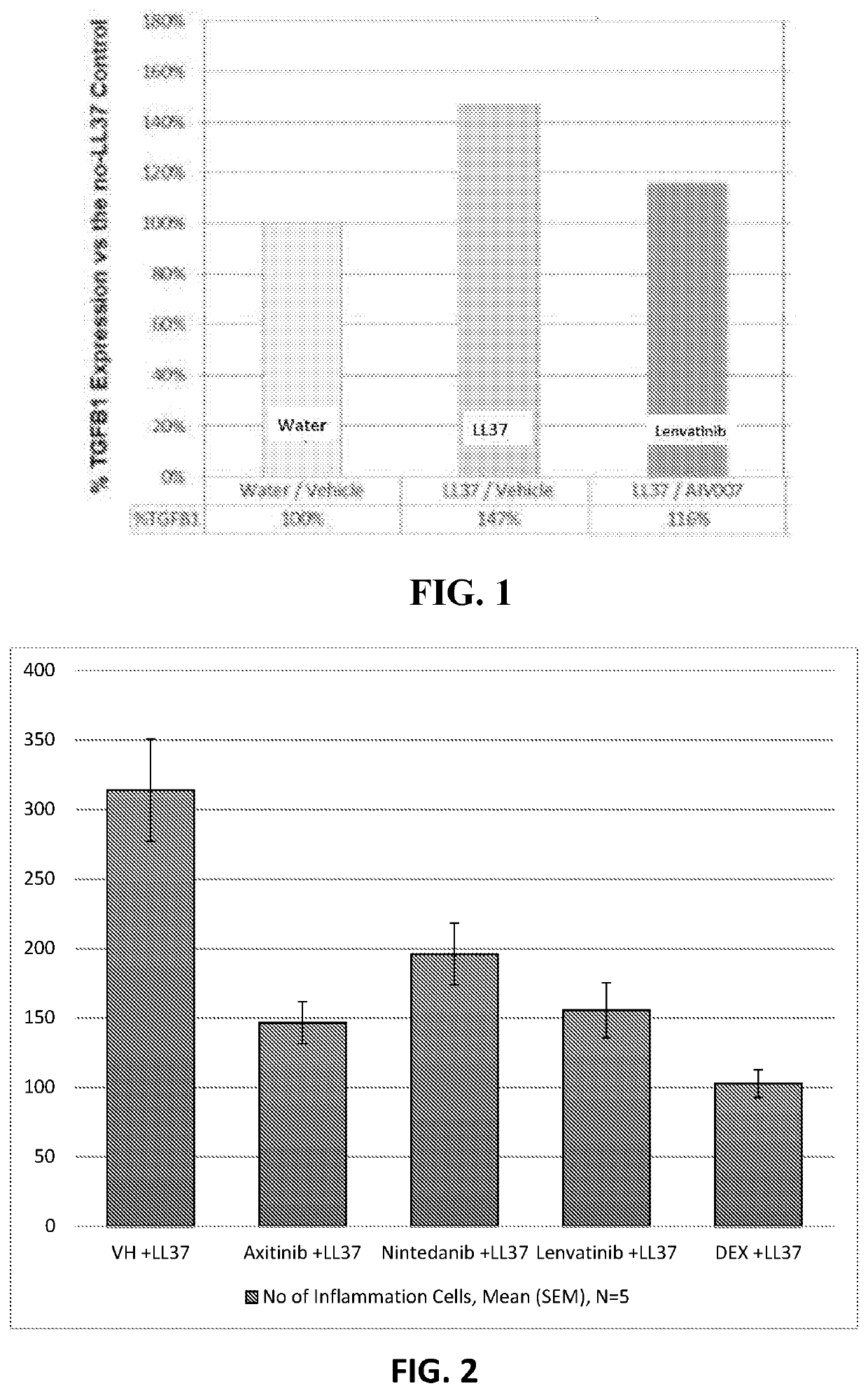
A pharmaceutical composition for prevention or treatment of a disease or disorder characterized by chronic inflammation, with associated angiogenesis and fibrosis, wherein the disease or disorder is selected from the group consisting of rosacea, psoriasis, erythema multiforme, bullous pemphigoid, hereditary hemorrhagic telangiectasia, rheumatoid arthritis, atopic dermatitis, and dermal wound healing. The pharmaceutical composition includes at least one multi-kinase inhibitor selected from the group consisting of axitinib, nintedanib, and lenvatinib.
Owner:AIVIVA BIOPHARMA INC
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Pharmaceutical compositions of n-methyl-2-[3-((e)-2-pyridin-2-yl-vinyl)-1h-indazol-6-ylsulfanyl]-benzamide Pharmaceutical compositions of n-methyl-2-[3-((e)-2-pyridin-2-yl-vinyl)-1h-indazol-6-ylsulfanyl]-benzamide](https://images-eureka.patsnap.com/patent_img/0f92072d-341c-4606-84fb-0ef5edd7f488/US20140248347A1-20140904-D00001.png)
![Pharmaceutical compositions of n-methyl-2-[3-((e)-2-pyridin-2-yl-vinyl)-1h-indazol-6-ylsulfanyl]-benzamide Pharmaceutical compositions of n-methyl-2-[3-((e)-2-pyridin-2-yl-vinyl)-1h-indazol-6-ylsulfanyl]-benzamide](https://images-eureka.patsnap.com/patent_img/0f92072d-341c-4606-84fb-0ef5edd7f488/US20140248347A1-20140904-D00002.png)
![Pharmaceutical compositions of n-methyl-2-[3-((e)-2-pyridin-2-yl-vinyl)-1h-indazol-6-ylsulfanyl]-benzamide Pharmaceutical compositions of n-methyl-2-[3-((e)-2-pyridin-2-yl-vinyl)-1h-indazol-6-ylsulfanyl]-benzamide](https://images-eureka.patsnap.com/patent_img/0f92072d-341c-4606-84fb-0ef5edd7f488/US20140248347A1-20140904-D00003.png)