A kind of preparation method of Axitinib tablet
A technology for axitinib tablets and diluents, which is applied in the field of preparation of axitinib tablets, can solve problems such as poor compressibility, poor material fluidity, splits, etc., to shorten the production cycle, improve production efficiency, and prepare The effect of simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
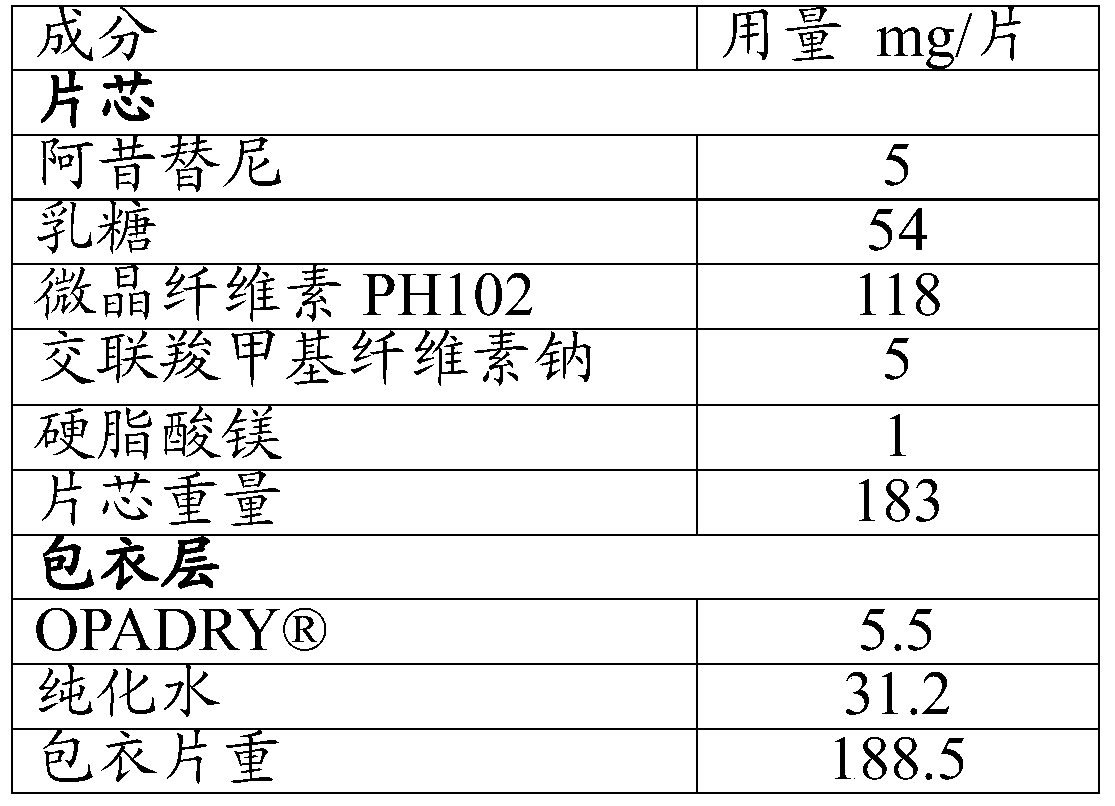
[0052] Prescription (specification 5mg / tablet):
[0053]
[0054] Preparation Process:
[0055] 1) Weigh the components of raw and auxiliary materials according to the prescription quantity, of which
[0056] The particle size of axitinib is D90≤60 μm; 54g lactose Flowlac 100 and 118g microcrystalline cellulose PH102 are respectively passed through a 60-mesh sieve, and mixed for 10 minutes at 30 rpm in a three-dimensional mixer to obtain a diluent, the 90% of the particle size of the diluent is less than 240 μm, 50% of the particle size is between 50-140 μm, and 7 wt% of the particle size is less than 32 μm;
[0057] Cross-linked sodium carboxymethyl cellulose through an 80-mesh sieve;
[0058] 2) Mix axitinib and croscarmellose sodium uniformly in a three-dimensional mixer to obtain the mixture ①;
[0059] The mixture ① is mixed with about 10% diluent in a three-dimensional mixer at 30 rpm for 5 minutes; the resulting mixture is mixed with about 30% diluent in a three-d...
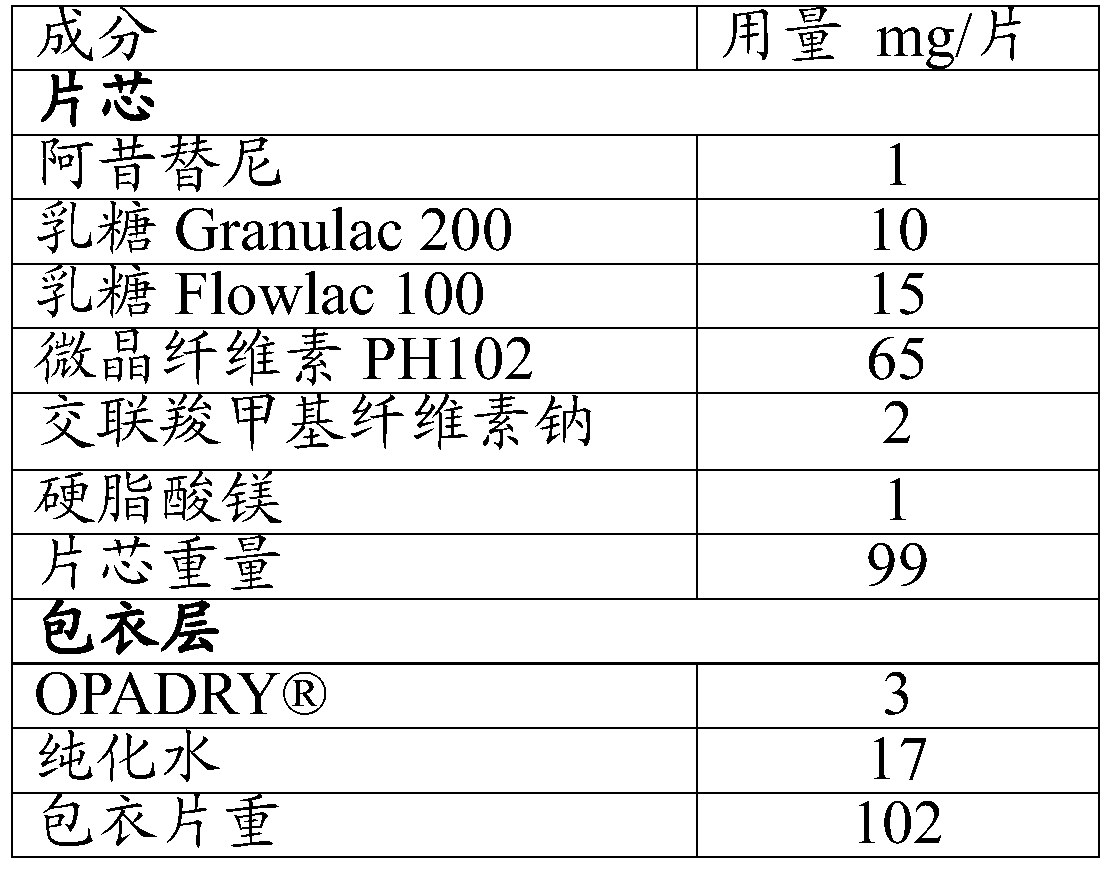
Embodiment 2
[0063] Except that the particle size of axitinib in step 1) is D90≤36 μm, other operations are basically the same as in Example 1 to prepare axitinib tablets.
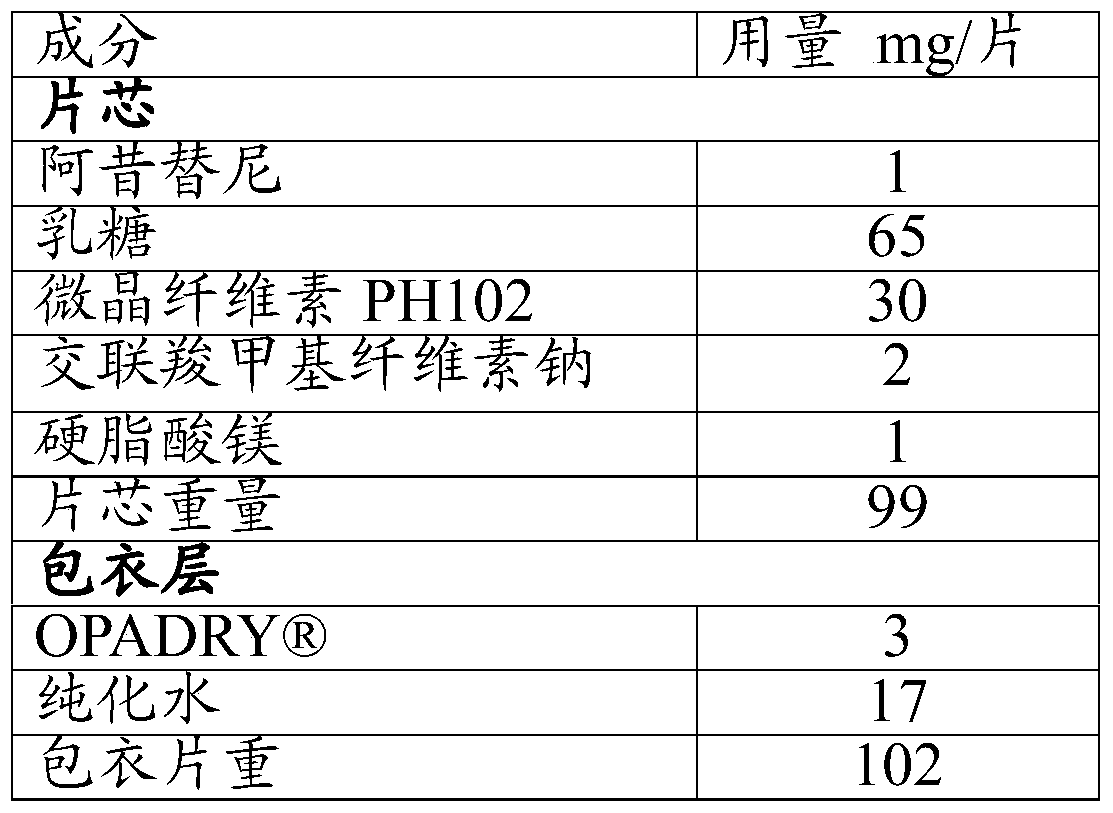
Embodiment 3
[0065] Except that the particle size of axitinib in step 1) is D90≤12 μm, other operations are basically the same as in Example 1 to prepare axitinib tablets.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com