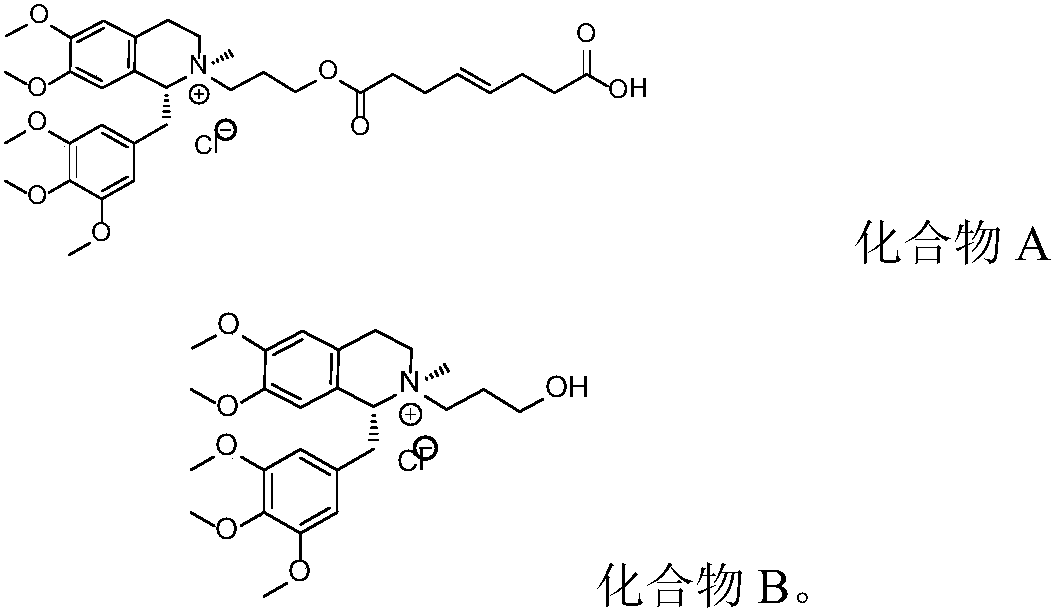
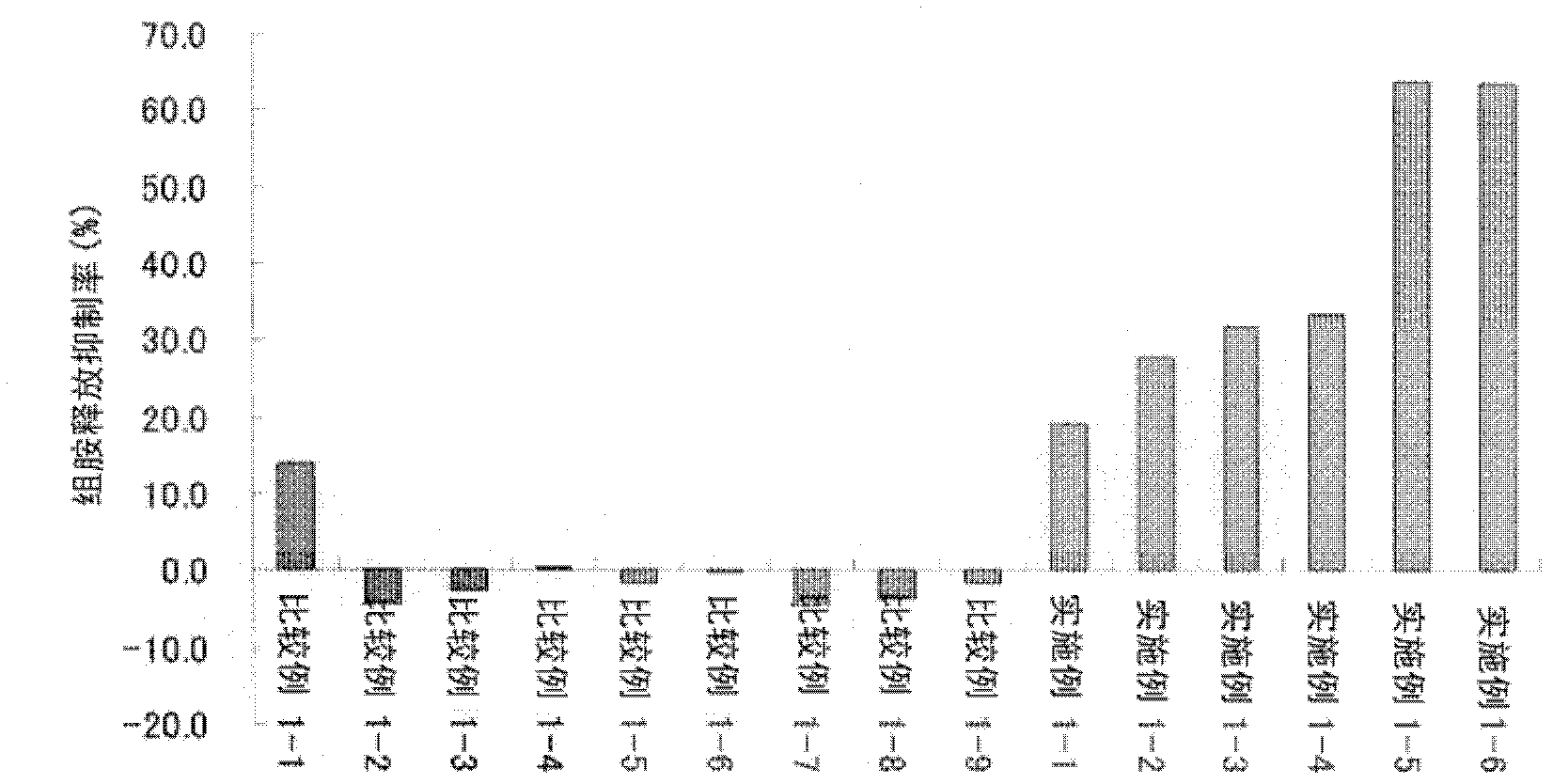
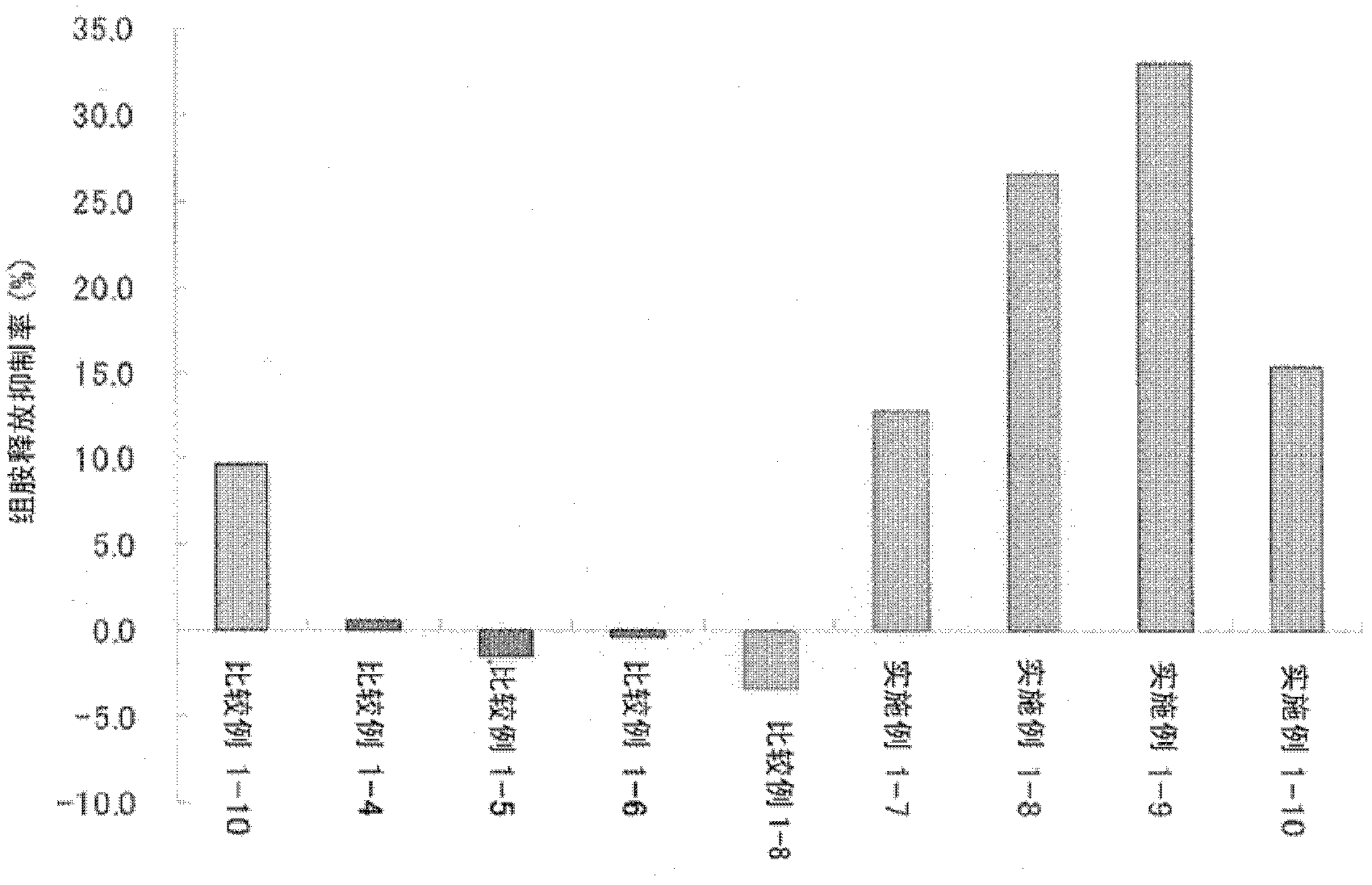
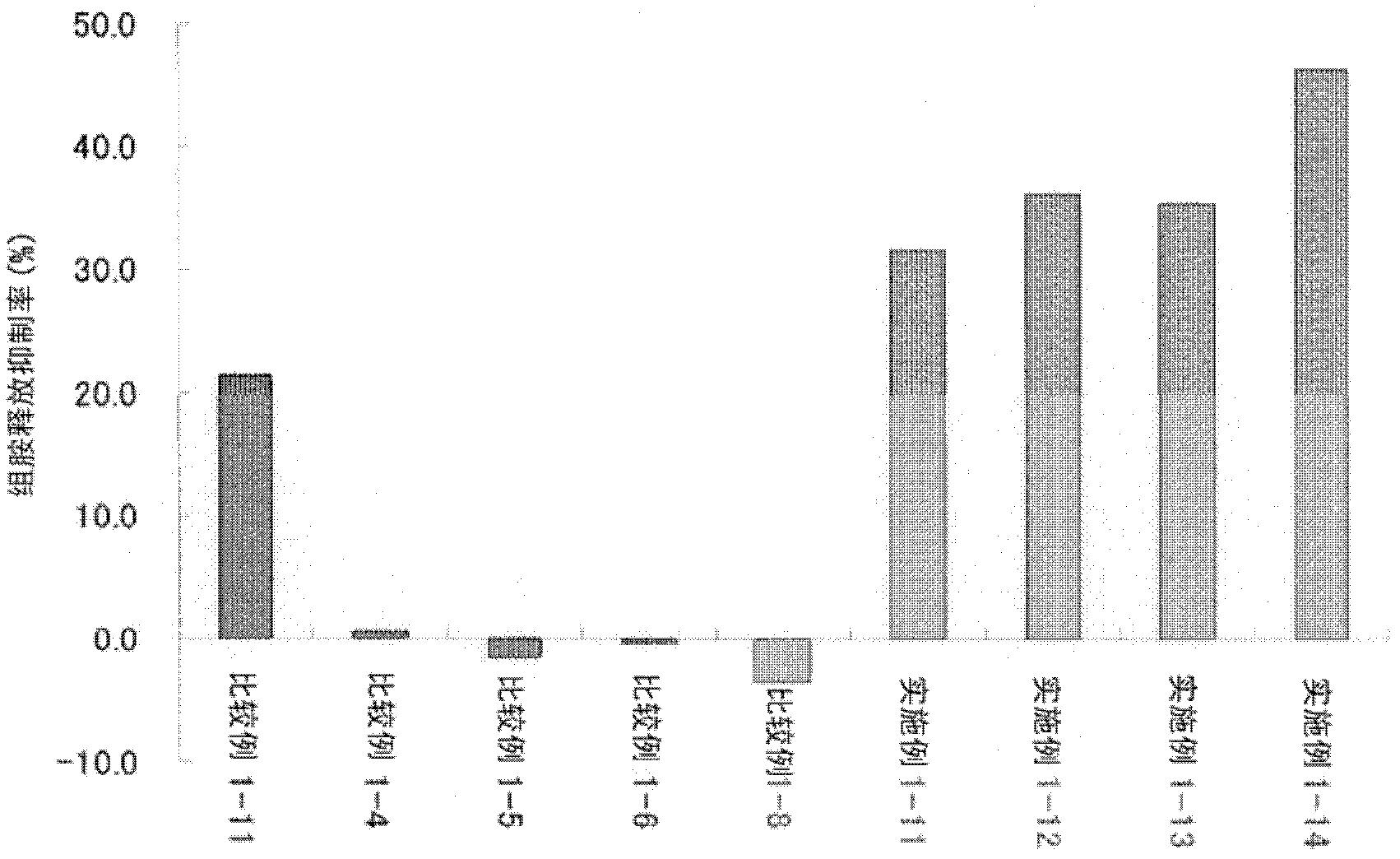
Patents
Literature
91 results about "Histamine Releases" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Histamine release is a biological response mediated by the immune system in response to allergens and certain other triggers from the outside environment or the body itself.
Composition comprising the extract of Actinidia arguta and related species for the prevention and treatment of allergic disease and non-allergic inflammatory disease
ActiveUS9131722B2Reduce actionTreat and prevent allergic diseaseBiocideSenses disorderFood additiveDisease
The present invention provides a pharmaceutical composition comprising the extract of hardy kiwifruit as an active ingredient in an effective amount to treat and prevent allergic disease and non-allergic inflammatory disease by reducing inflammation action, by inhibiting histamine release from mast cell, and by increasing the level of Th1 cytokines, IgG2a in serum and reducing the level of Th2 cytokines and IgE in serum. The present invention also provides a use of above extract for the preparation of pharmaceutical composition. The present invention also provides a health food or food additives, a cosmetic composition, a feed or feed additives comprising above extract for prevention or alleviation of allergic disease and non-allergic inflammatory disease by reducing inflammation action, by inhibiting histamine release from mast cell, and by increasing the level of Th1 cytokines, IgG2a in serum, and reducing the level of Th2 cytokines and IgE in serum.
Owner:VIROMED CO LTD
Co-Administration of an Agent Linked to an Internalization Peptide with an Anti-Inflammatory
ActiveUS20090176713A1Reduce capacityInhibit the inflammatory responseOrganic active ingredientsNervous disorderCo administrationBiotin
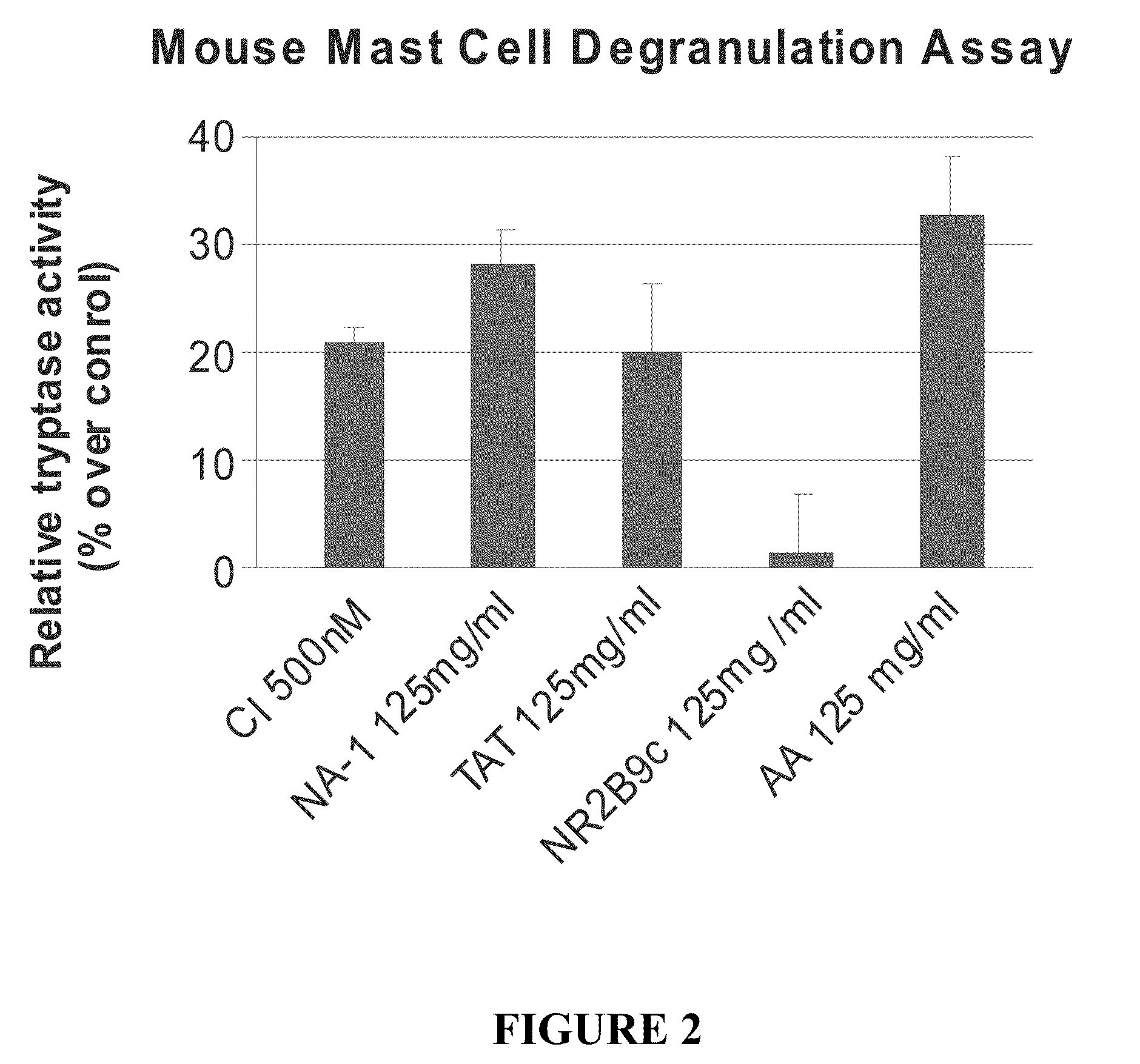
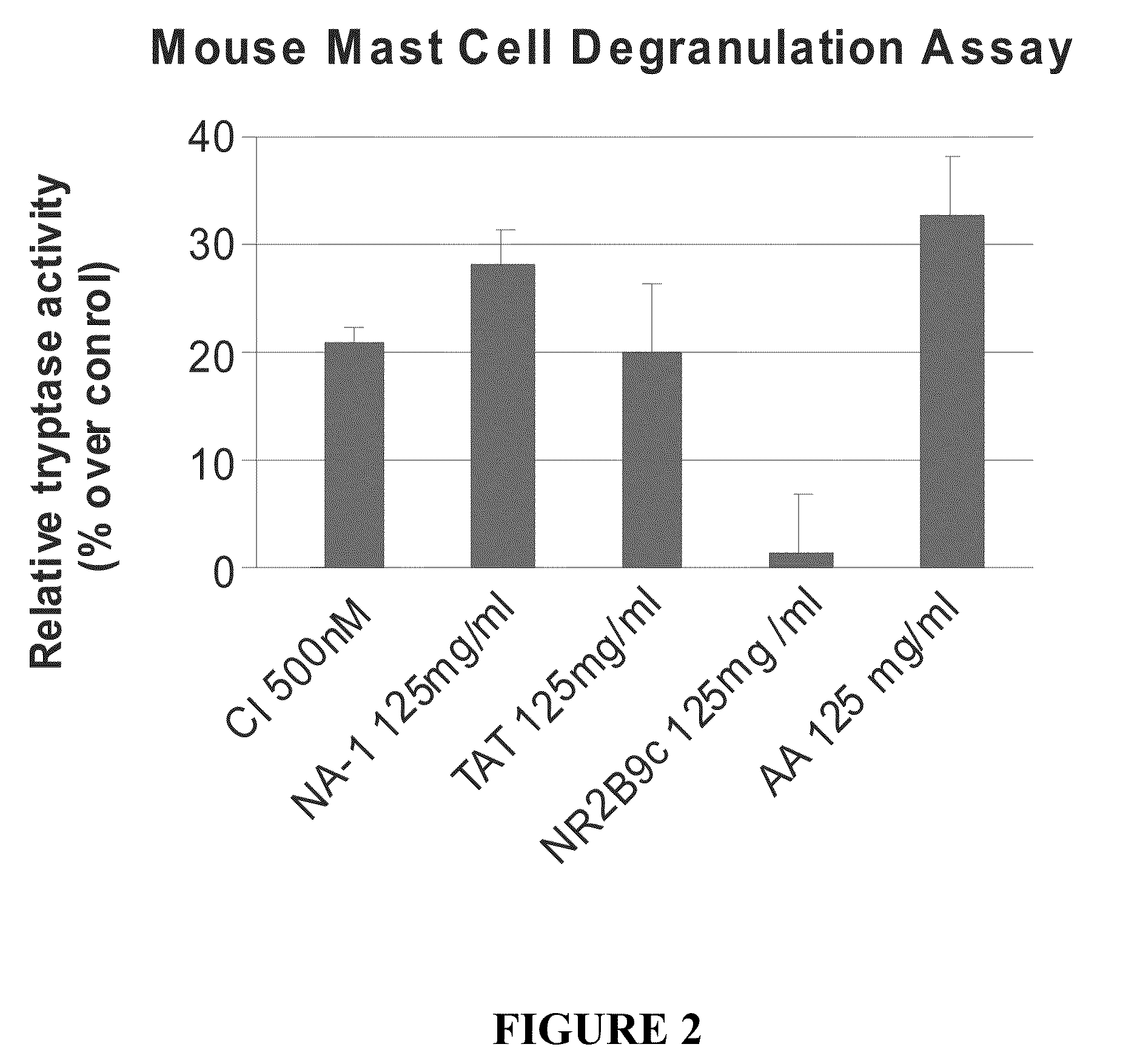
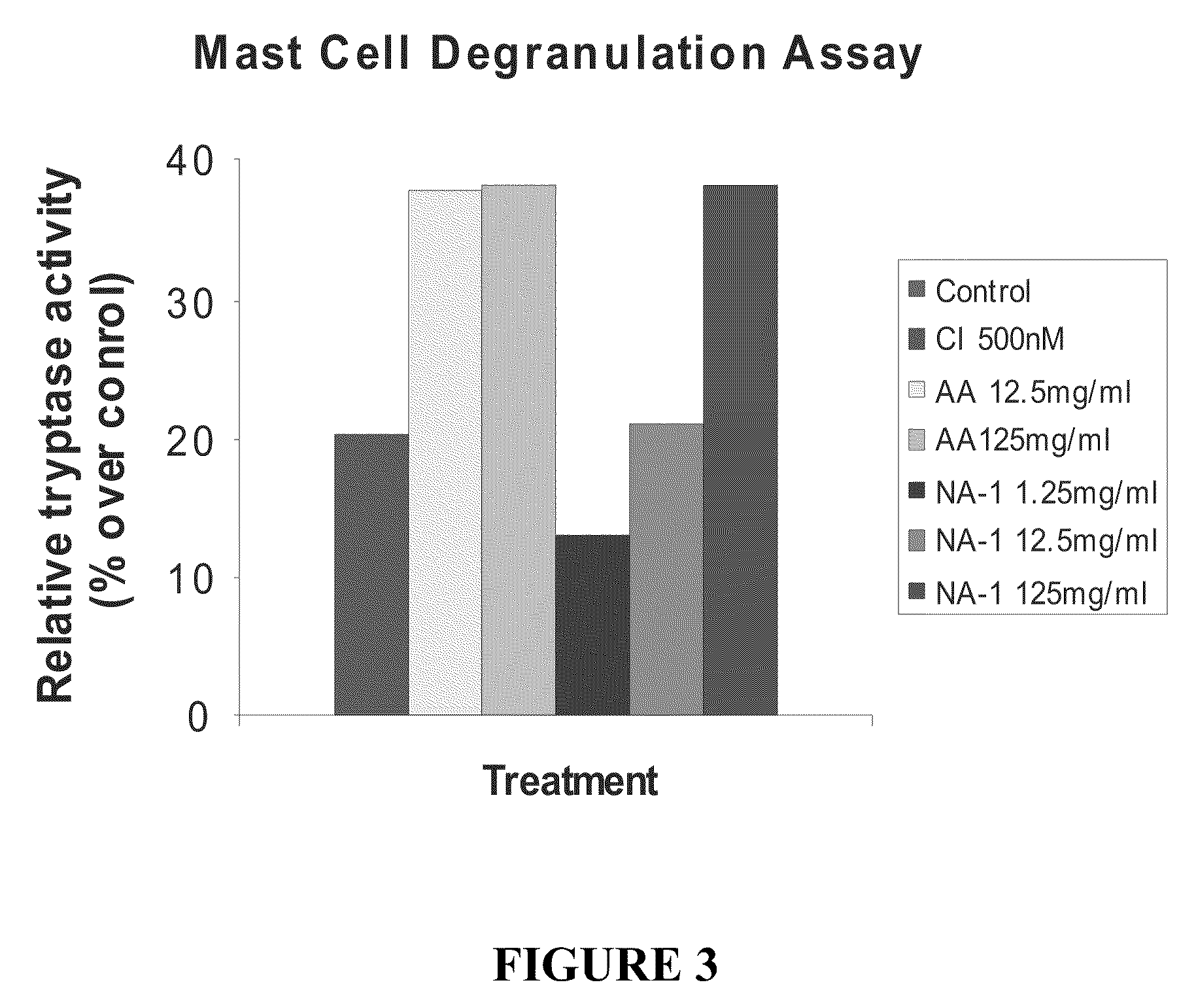
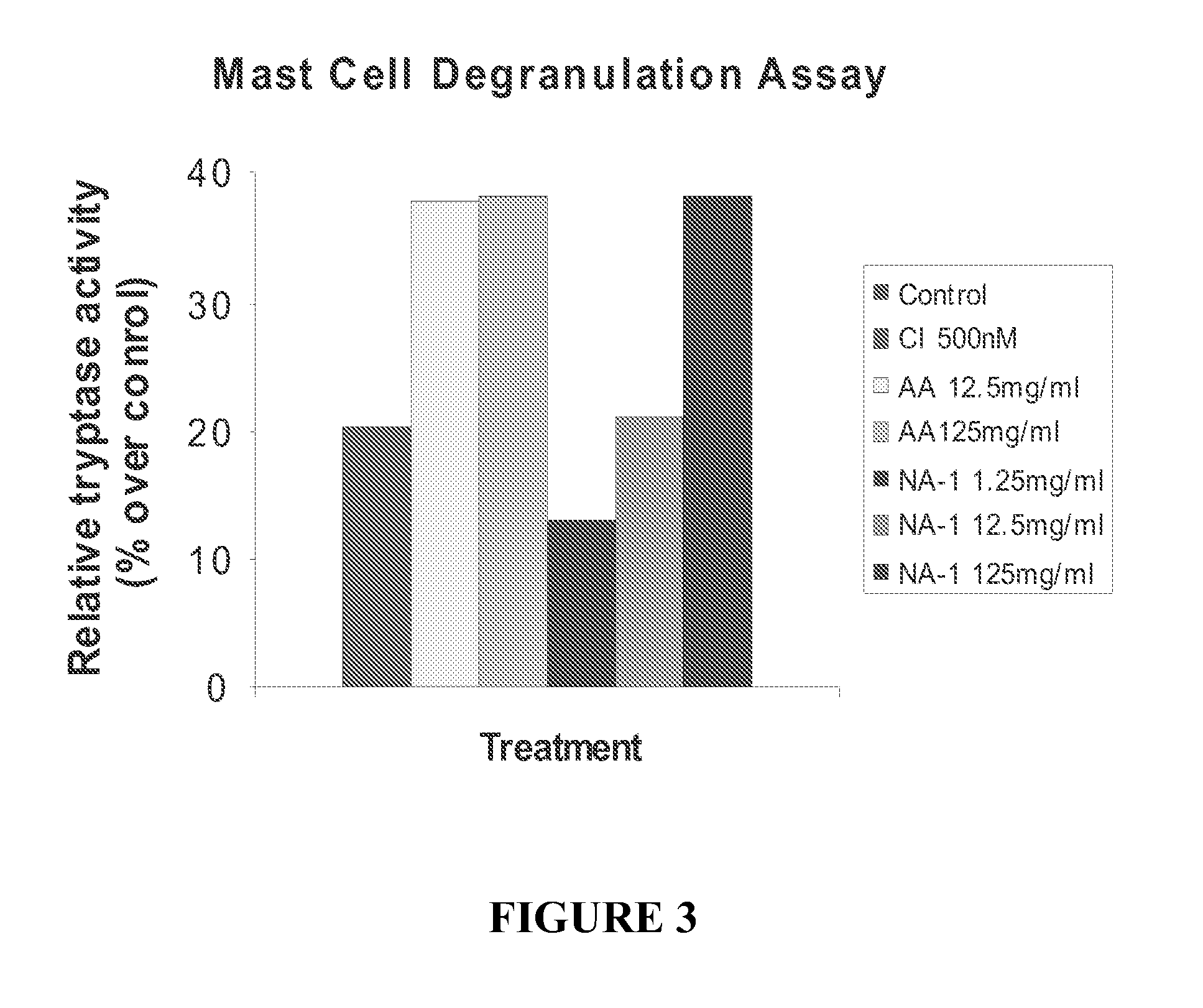
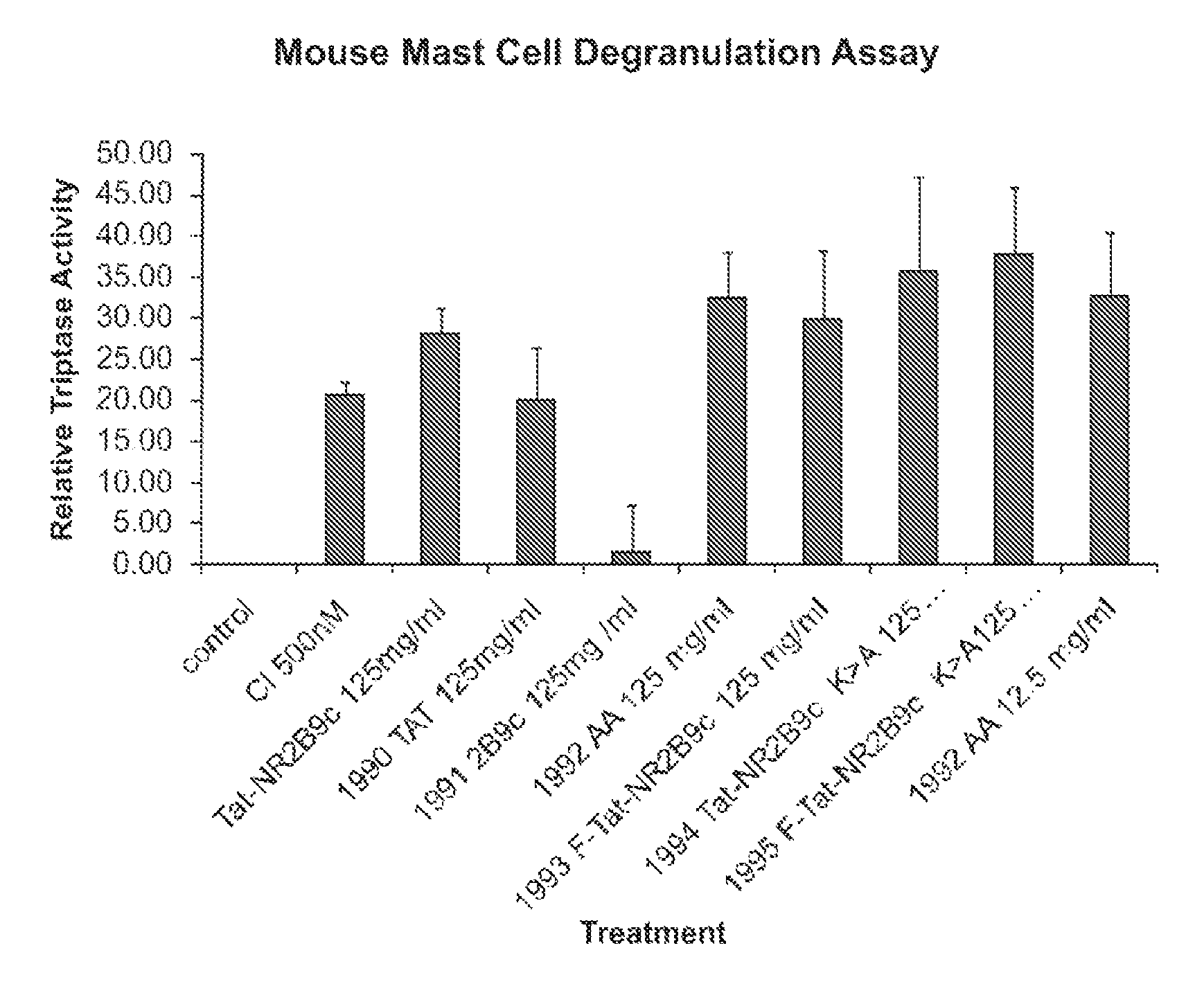
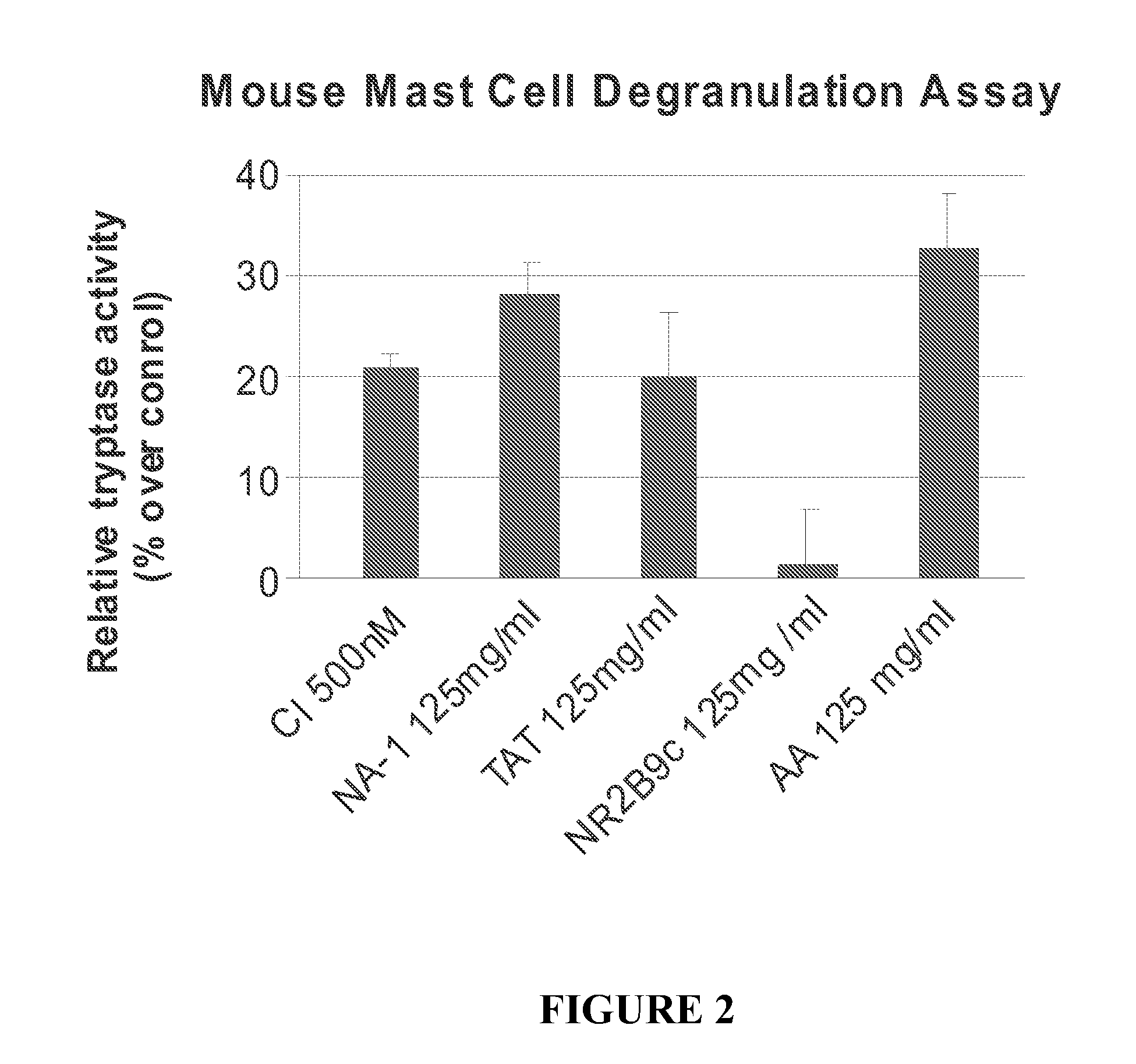
The invention provides methods of delivering pharmacologic agents linked to an internalization peptide, in which an inflammatory response inducible by the internalization peptide is inhibited by co-administration of an anti-inflammatory or by linking the internalization peptide to biotin or similar molecule. Such methods are premised in part on the results described in the examples whereby administration of a pharmacological agent linked to tat high dosages is closely followed by an inflammatory response, which includes mast cell degranulation, histamine release and the typical sequelae of histamine release, such as redness, heat, swelling, and hypotension.
Owner:NONO INC
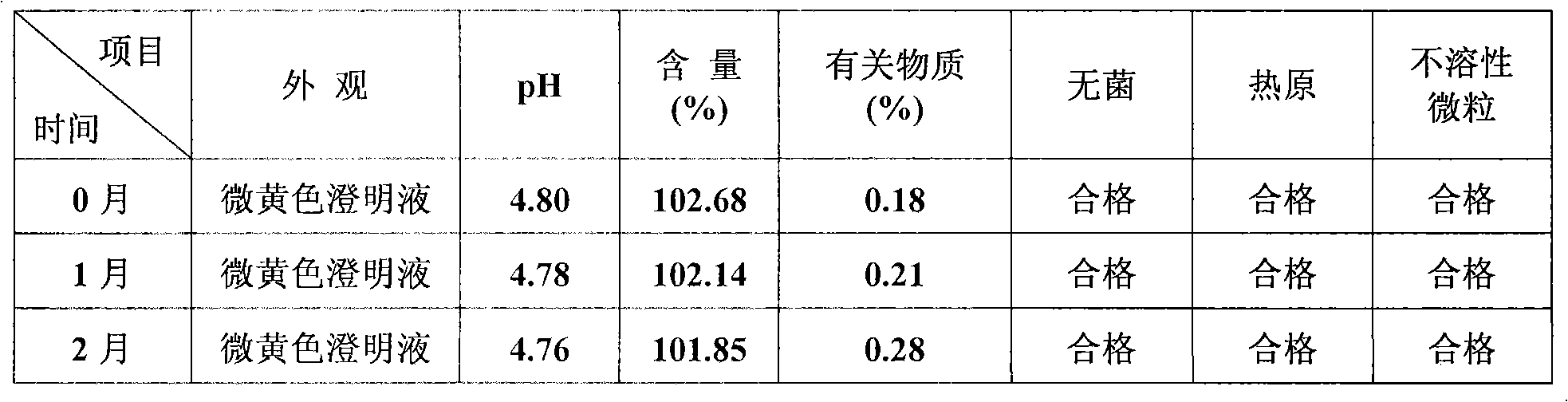
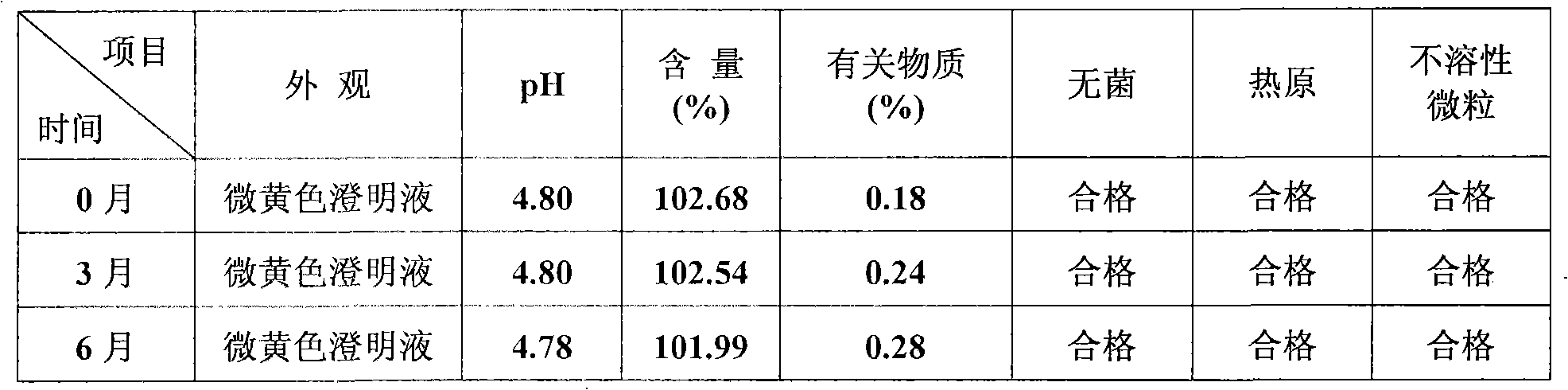
Coenzyme Q10 injection
InactiveCN101278907AStrong solubilizing abilityLow toxicityOrganic active ingredientsPharmaceutical delivery mechanismParenteral nutritionHydroxystearic Acid
The invention discloses the novel combined coenzyme Q10 parenteral solution, mainly containing the coenzyme Q10 as active component, polyethylene glycol 15-hydroxyl stearate as solubilizing agent and water for injection as solvent. One or more solvents of cosolvent, acidity regulator, osmoregulator and stabilizing agents can be also added. Low hemolyzation, extreme low histamine release and higher physiological tolerance of a novel solubilizing agent Solutol HS 15 adopted by the parenteral solution remarkably improves clinical medication security and the compliance of a patient; and the parenteral solution has the advantages of good stability, longer period of validity, higher medication convenience for a clinician, better storage and stable transportation, higher security for the clinical medication and better compliance of the patient. The parenteral solution also has the advantages of simple preparation technology, simple and convenient quality control and lower production cost, thereby being beneficial to industrialization production.
Owner:郑微
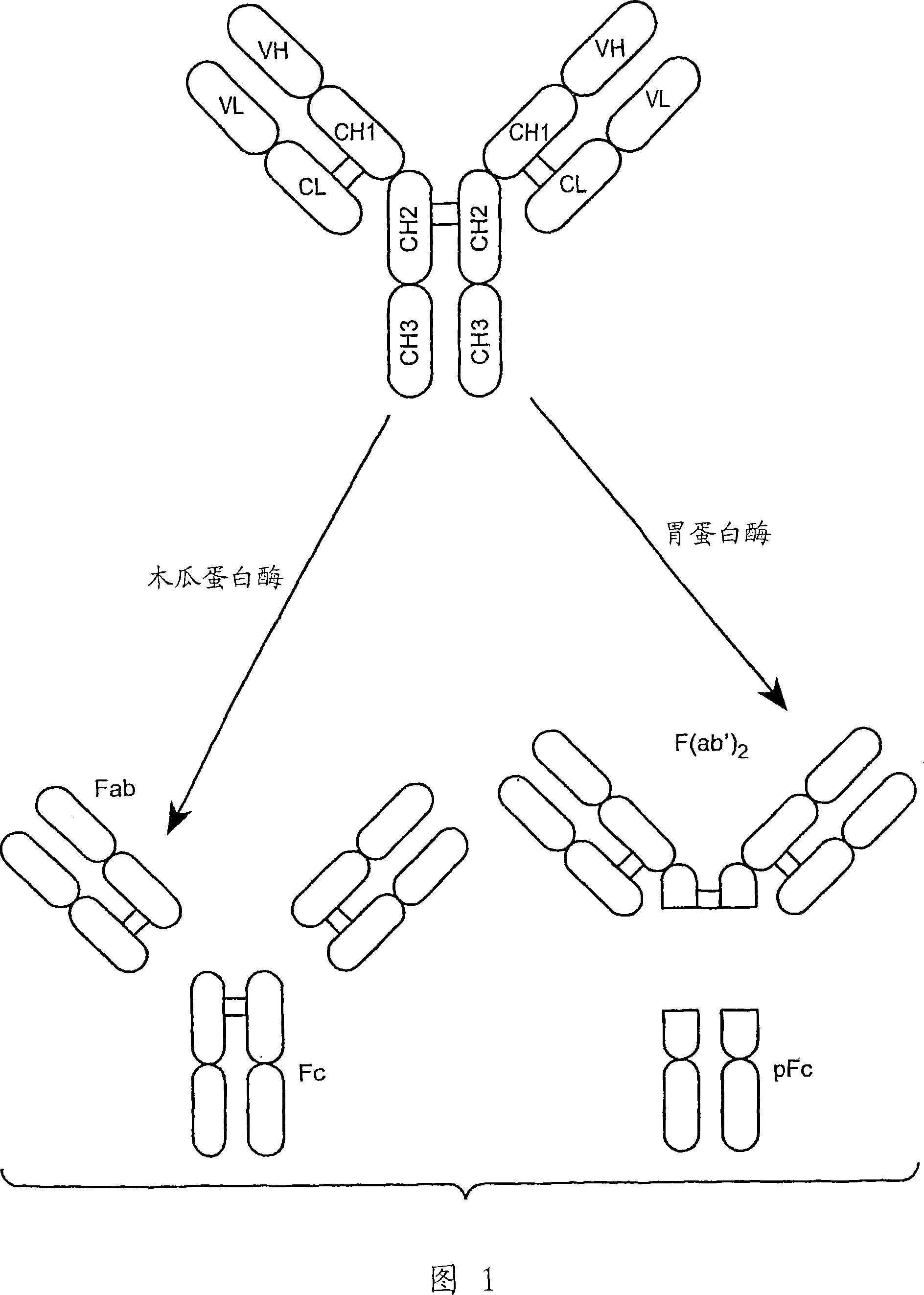
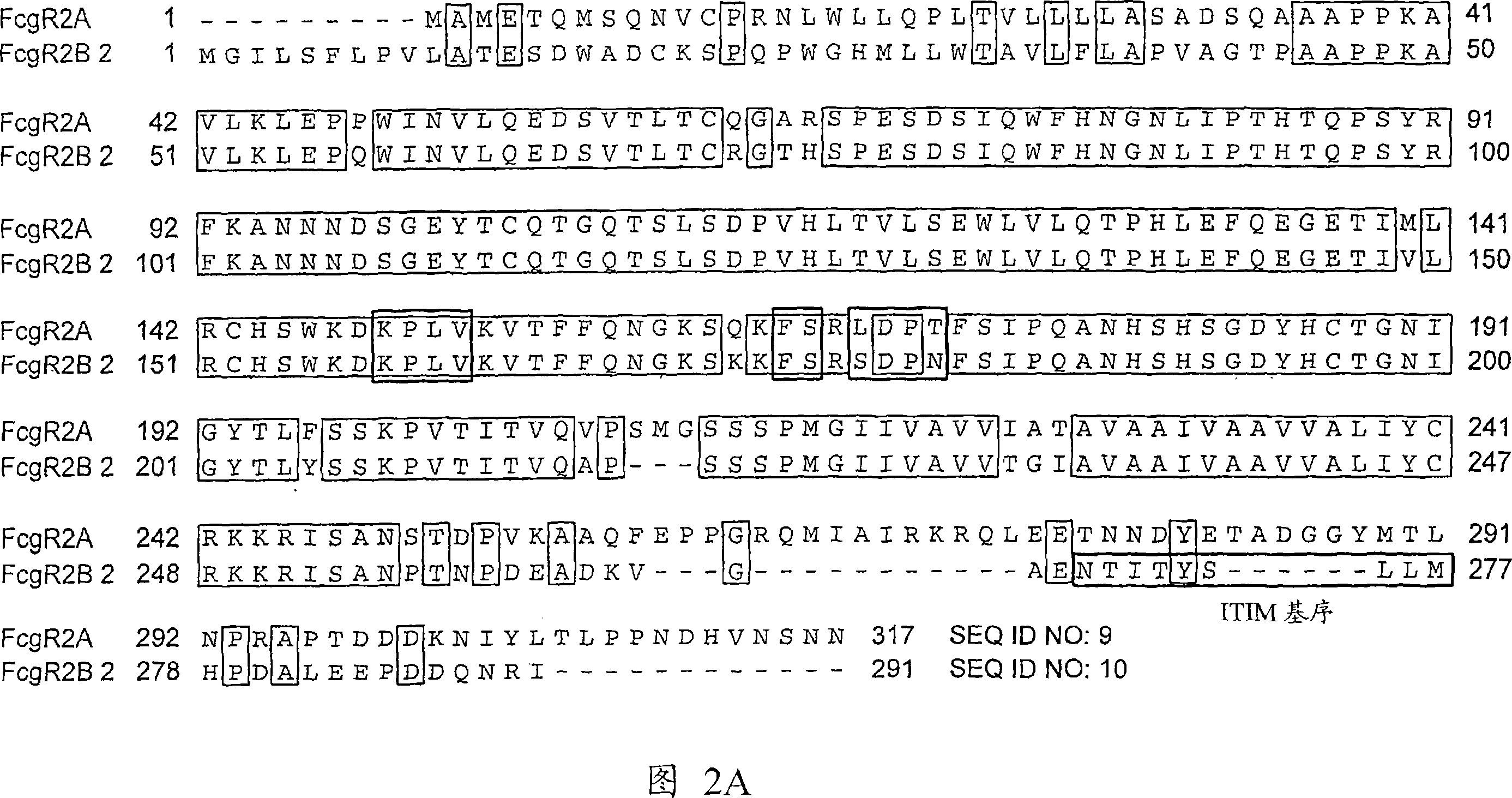
Anti-Fc-gamma RIIB receptor antibody and uses therefor
The present application describes antibodies that selectively bind human FcyRIIB, with little or no binding to other human FcgammaRs, e.g., human FcgammaRIIA. The invention also provides isolated bispecific antibodies comprising an antibody that selectively binds FcgammaRIIB, and a second antibody that specifically binds an activating receptor. Various uses, including therapeutic uses, for those antibodies are also described, including administration with anti-tumor antibodies and methods of inhibiting immune responses and suppressing histamine release.
Owner:GENENTECH INC
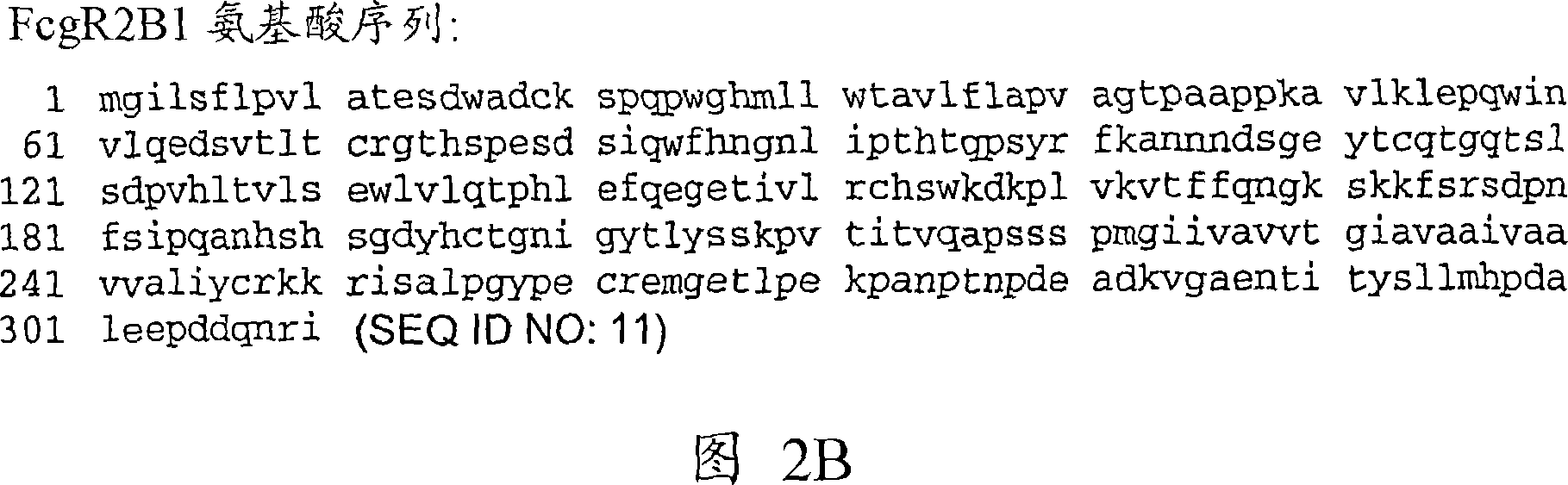
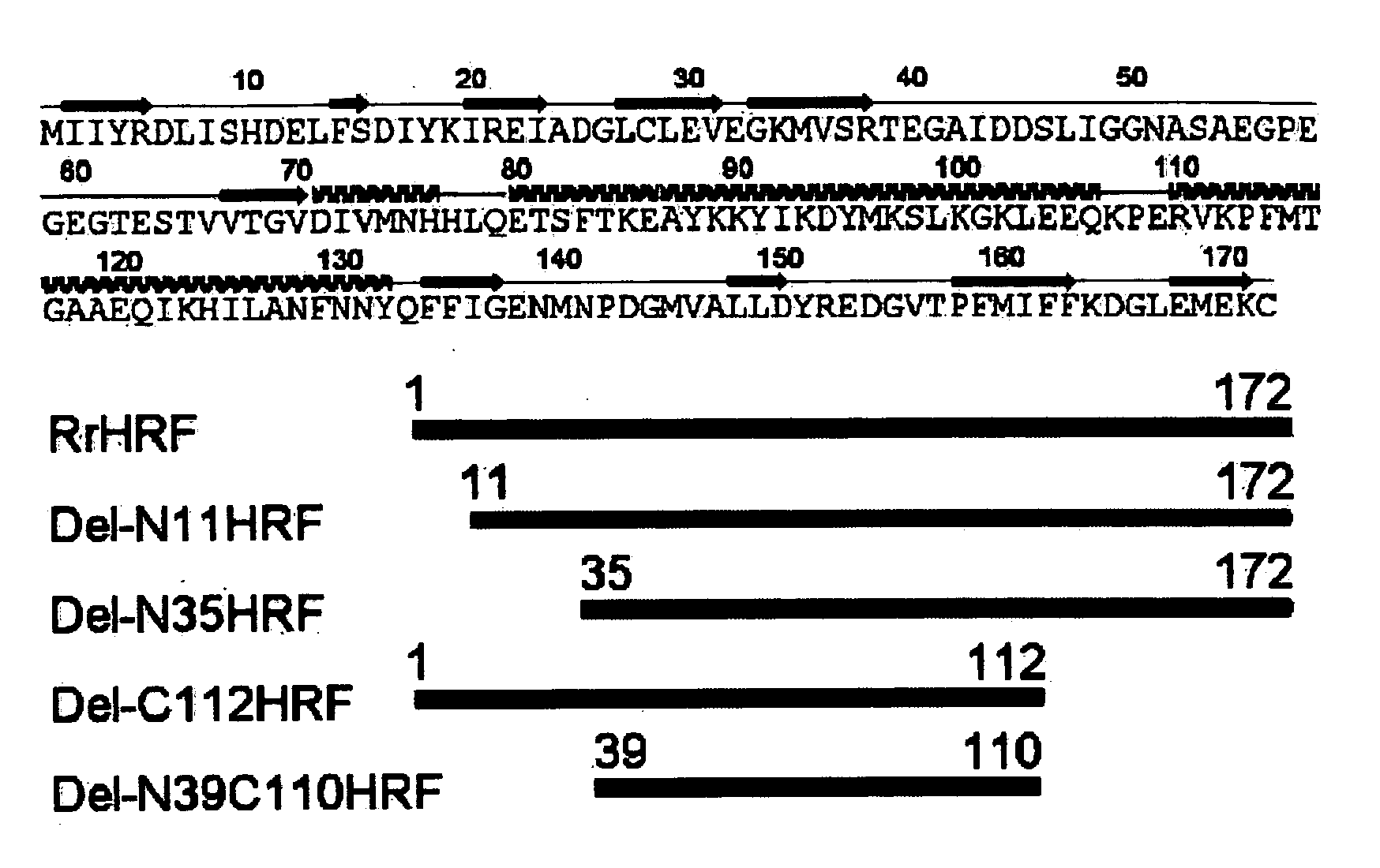
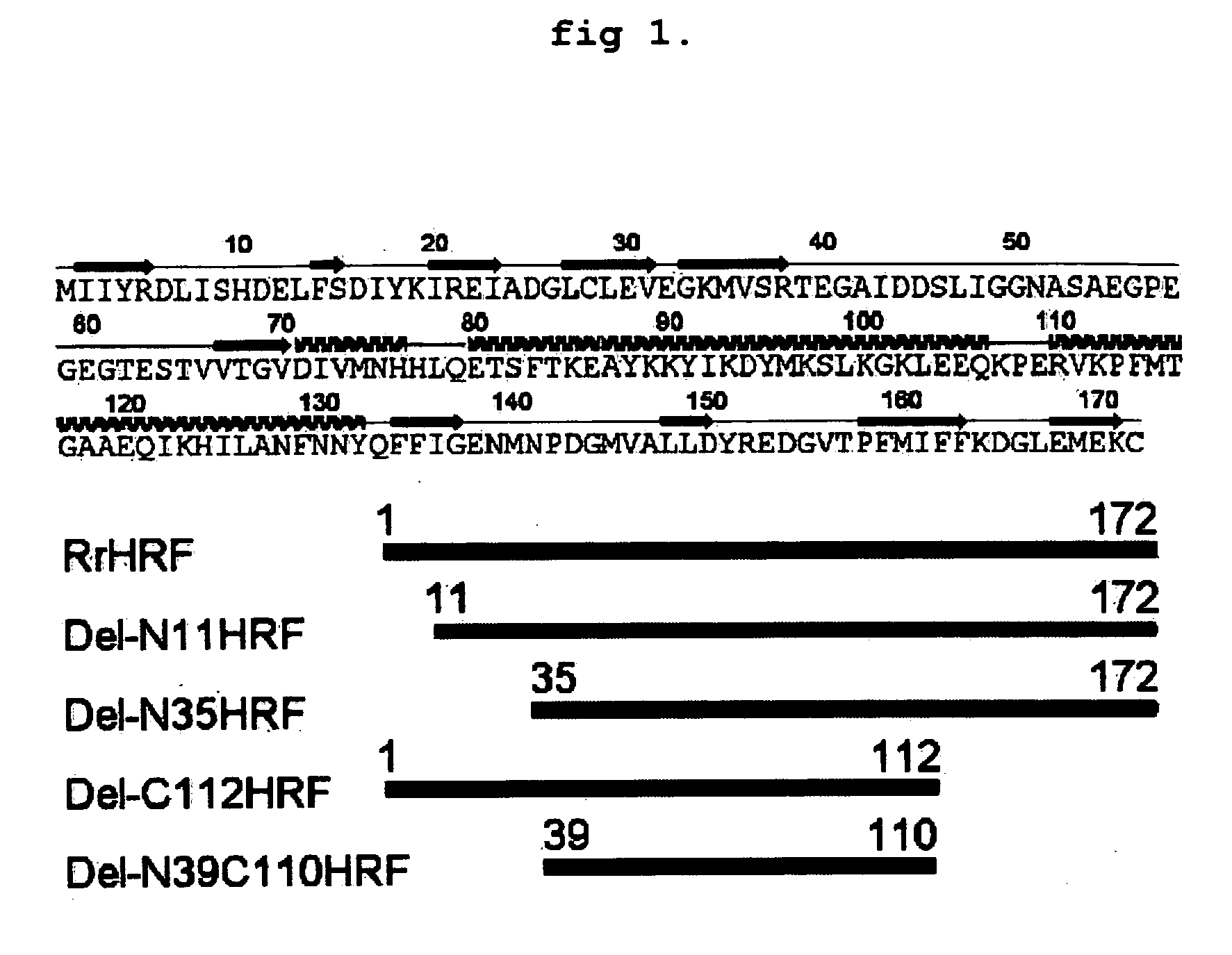
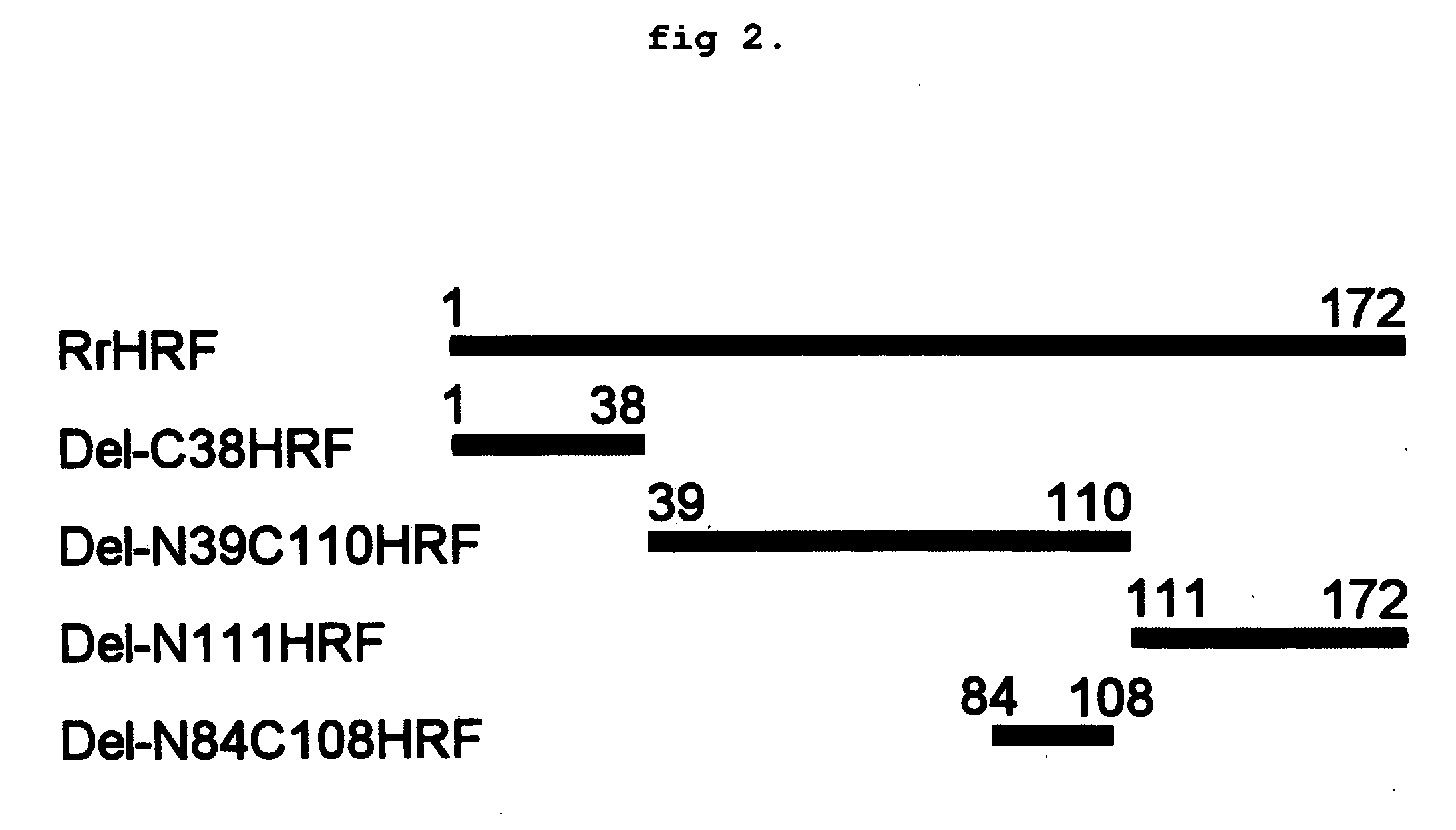
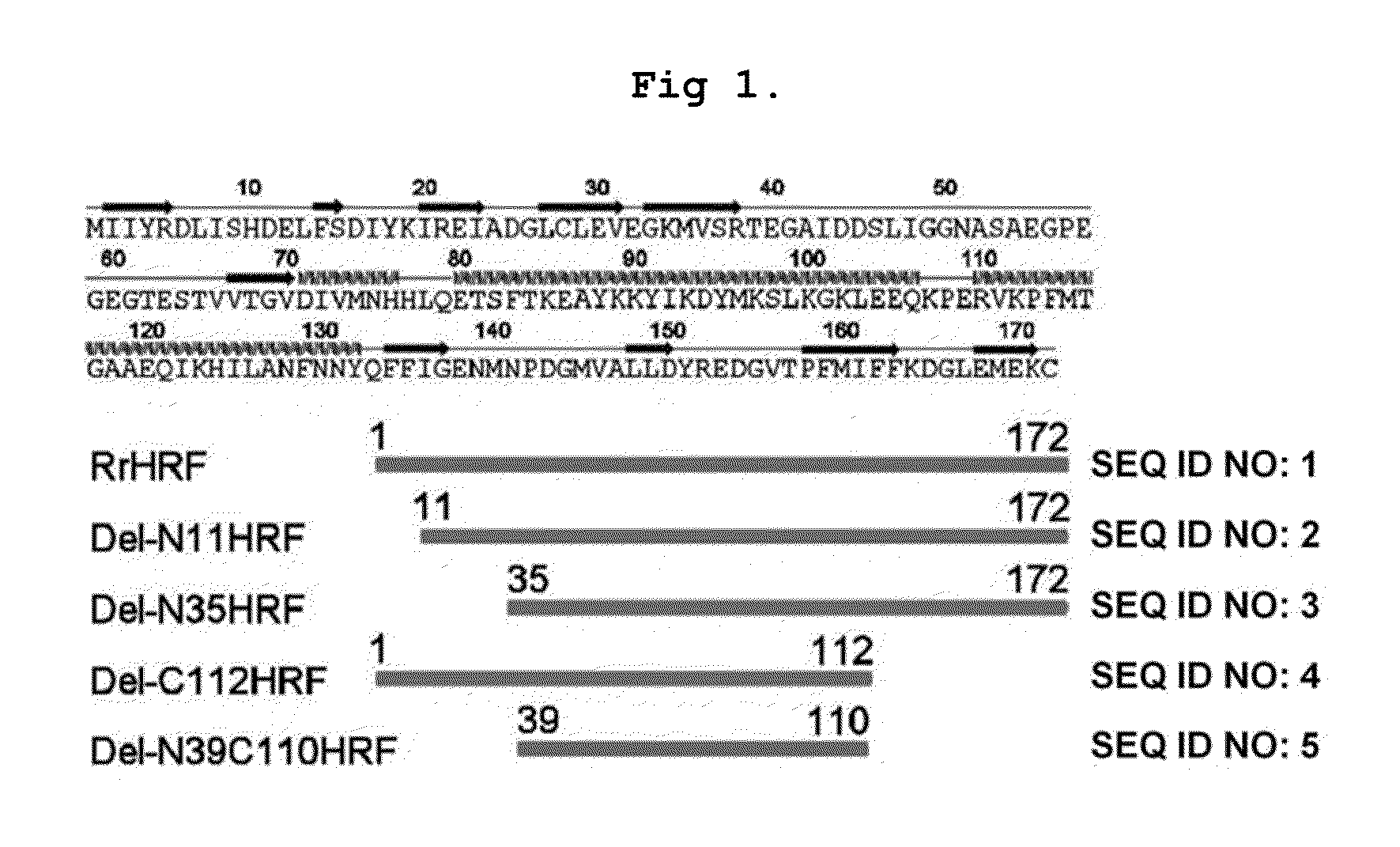
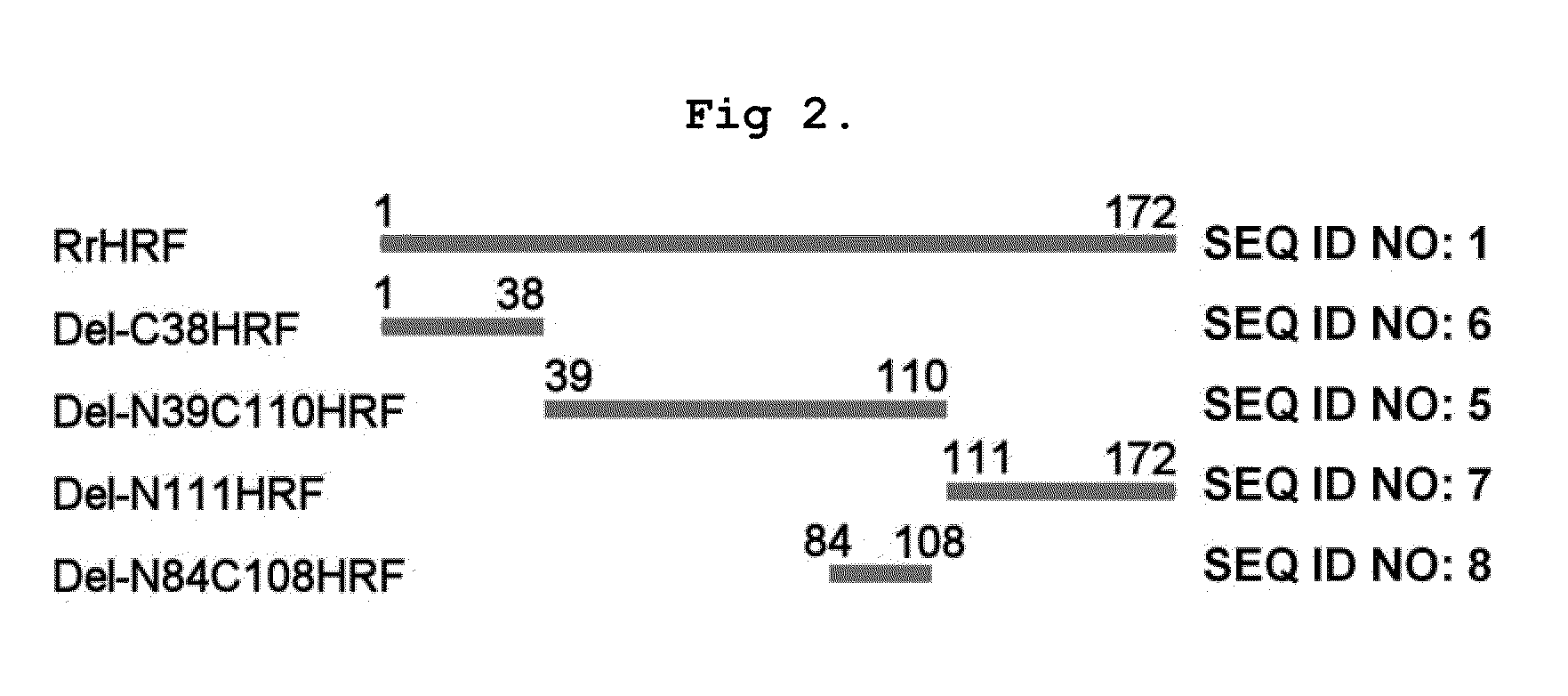
DELETION FORMS OF IgE-DEPENDENT HISTAMINE RELEASING FACTOR HAVING HISTAMINE RELEASING ACTIVITY, HRF-BINDING PEPTIDES AND THE USES THEREOF
ActiveUS20060165677A1Easy to synthesizePeptide/protein ingredientsImmunoglobulins against growth factorsBinding peptideHypersensitive response
The present invention relates to IgE-dependent histamine releasing factor (HRF) and HRF-binding peptides, more precisely, deletion forms of HRF which are able to be formed as dimers containing amino acid sequence represented by SEQ ID NO:3, genes encoding thereof and novel HRF-binding peptides having an activity of inhibiting HRF. The deletion forms of HRF which are able to be formed as dimers of the present invention induces intracellular secretion of histamine and IL-8, making an excellent candidate for a drug for inhibiting allergic reaction triggered by HRF and a kit for detecting HRF in serum of an allergy patient. In addition, novel HFR-binding peptides of the present invention bind to HRF to inhibit the actions of HFR, so they can be effectively used for the prevention and the treatment of allergic diseases of animals including asthma and rhinitis or malaria.
Owner:EWHA UNIV IND COLLABORATION FOUND
Application method of natural plant pepper extract
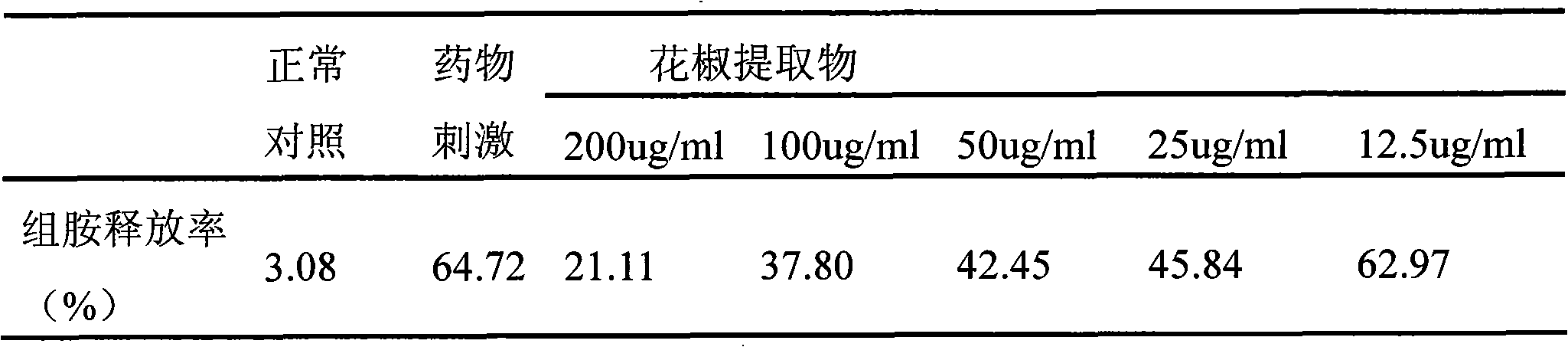
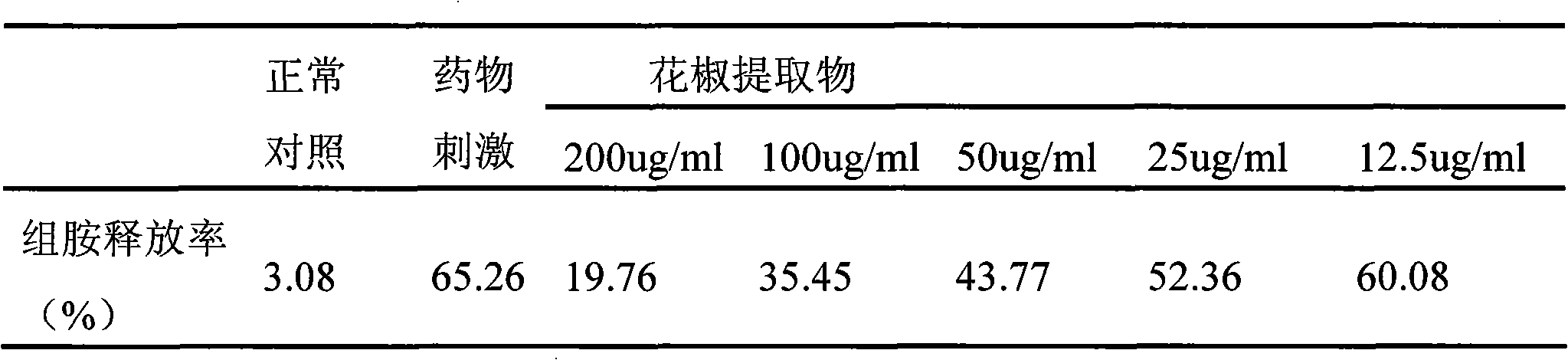
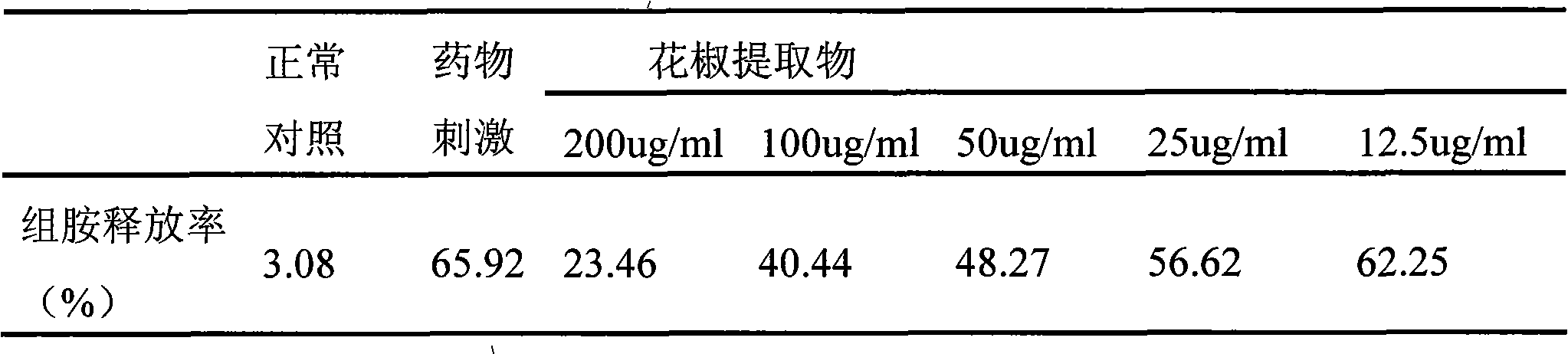
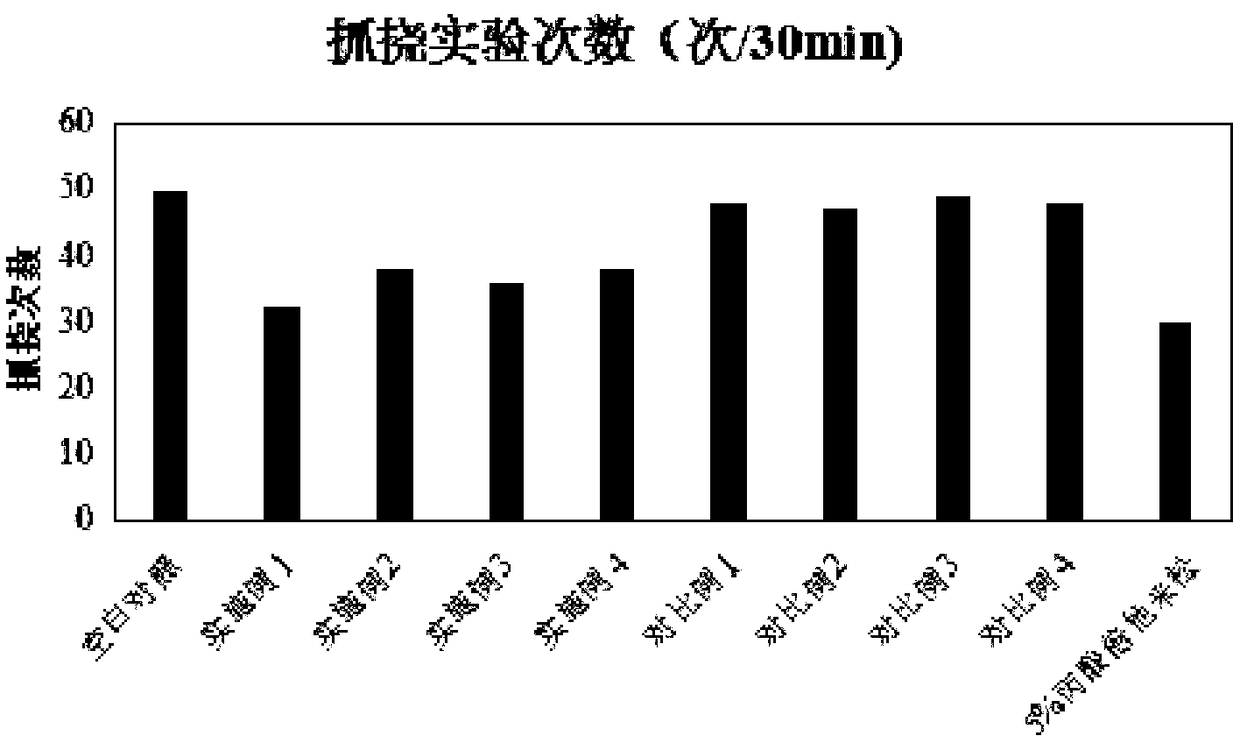
ActiveCN101612243AInhibition releaseAnti-allergicCosmetic preparationsToilet preparationsIrritabilityHistamine Releases
Owner:晟薇药业(上海)有限公司
Antioxidant-Containing Food Composition For Use In Inhibiting Histamine Pathways In Companion Animals
InactiveUS20090156658A1Inhibition capacityInhibiting the deterioration of the mental capacity of an agedBiocideOrganic active ingredientsInterstitial cystitisHistamine Releases
The invention encompasses compositions for inhibiting histamine release pathways in a companion animal, for example, felines and in treating or preventing idiopathic cystitis or interstitial cystitis. The compositions of the invention include an amount of lipoic acid that is effective in inhibiting histamine release pathways in a companion animal, for example, felines and in treating or preventing idiopathic cystitis or interstitial cystitis.
Owner:HILLS PET NUTRITION INC
Histamine release inhibitor
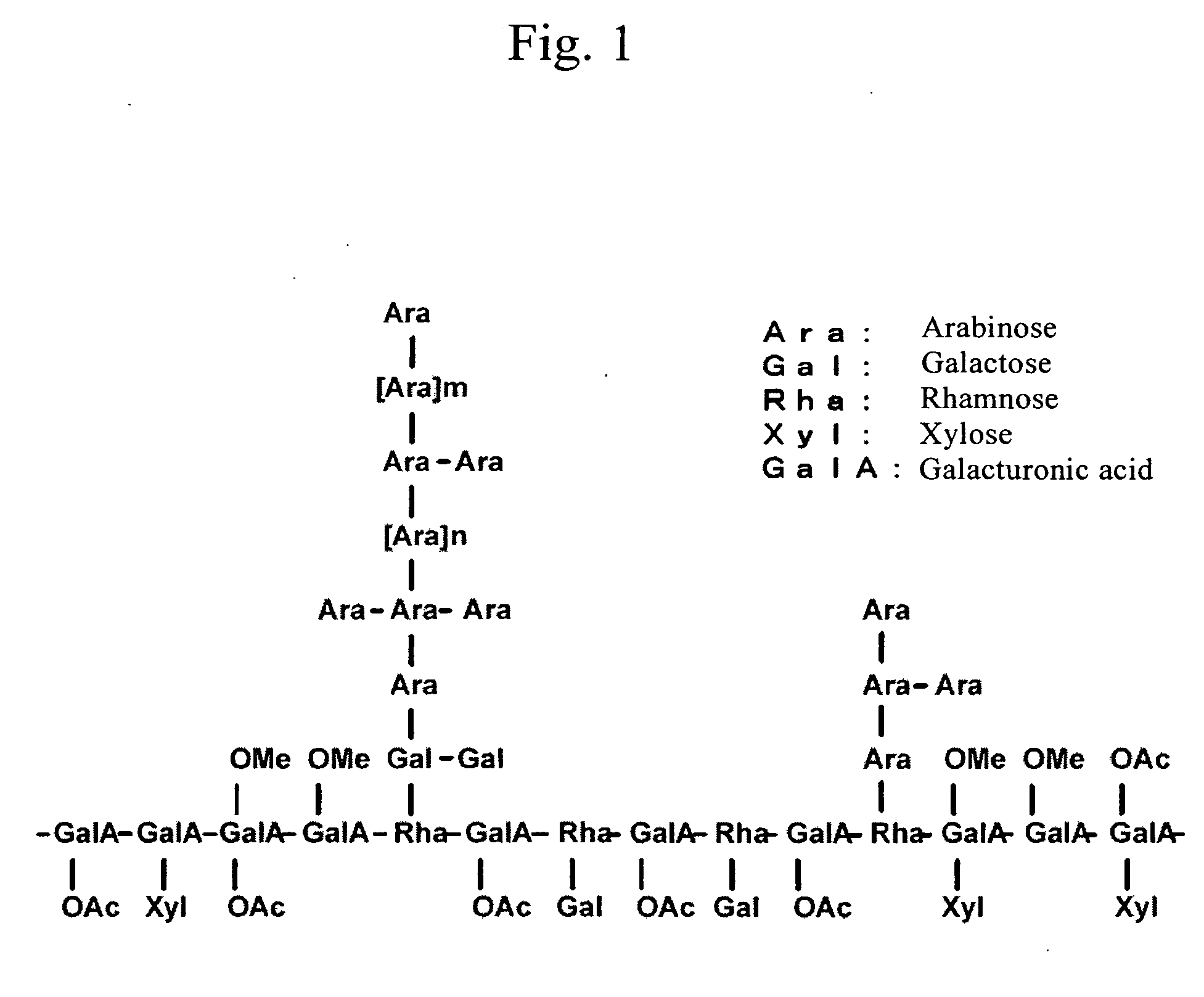
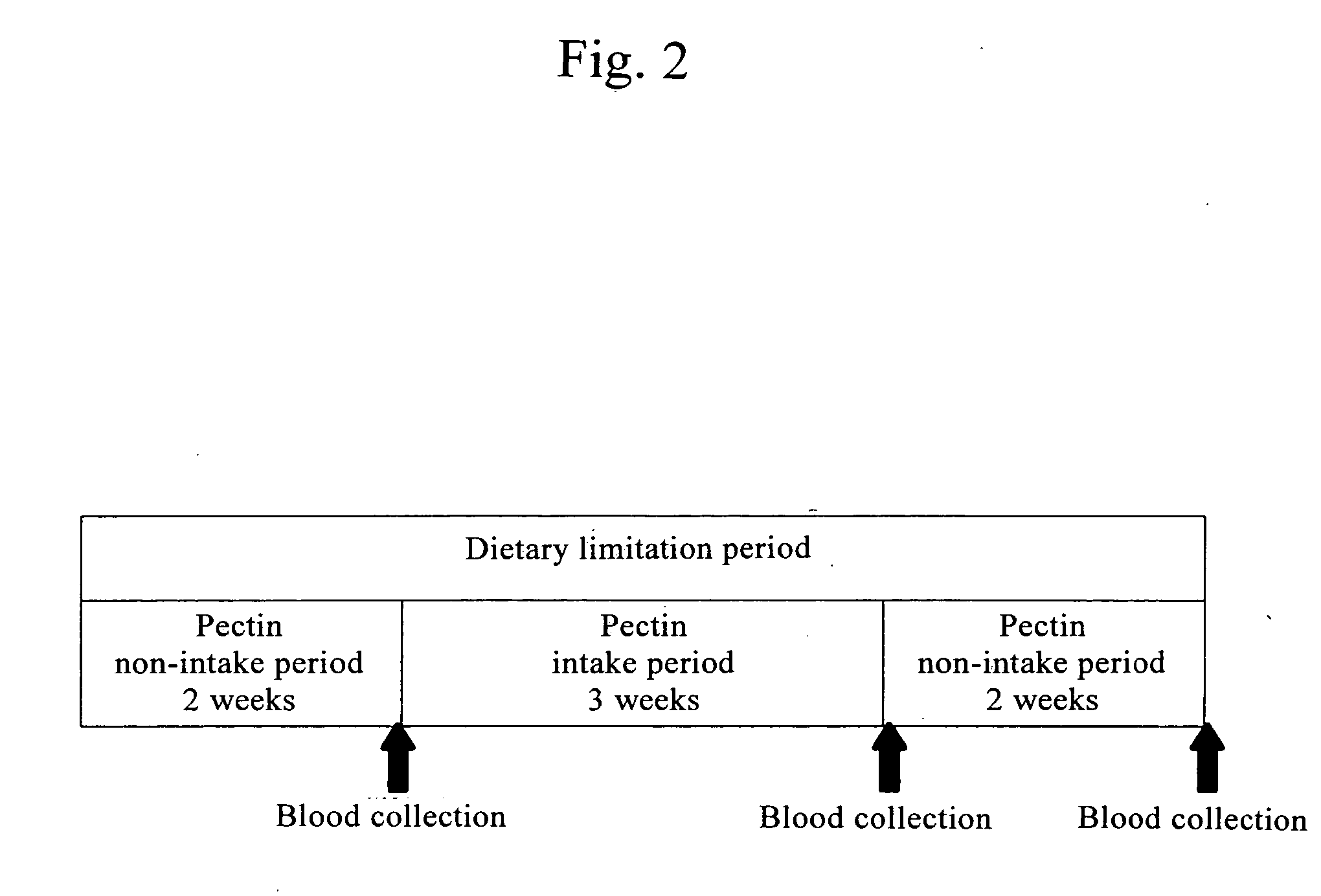
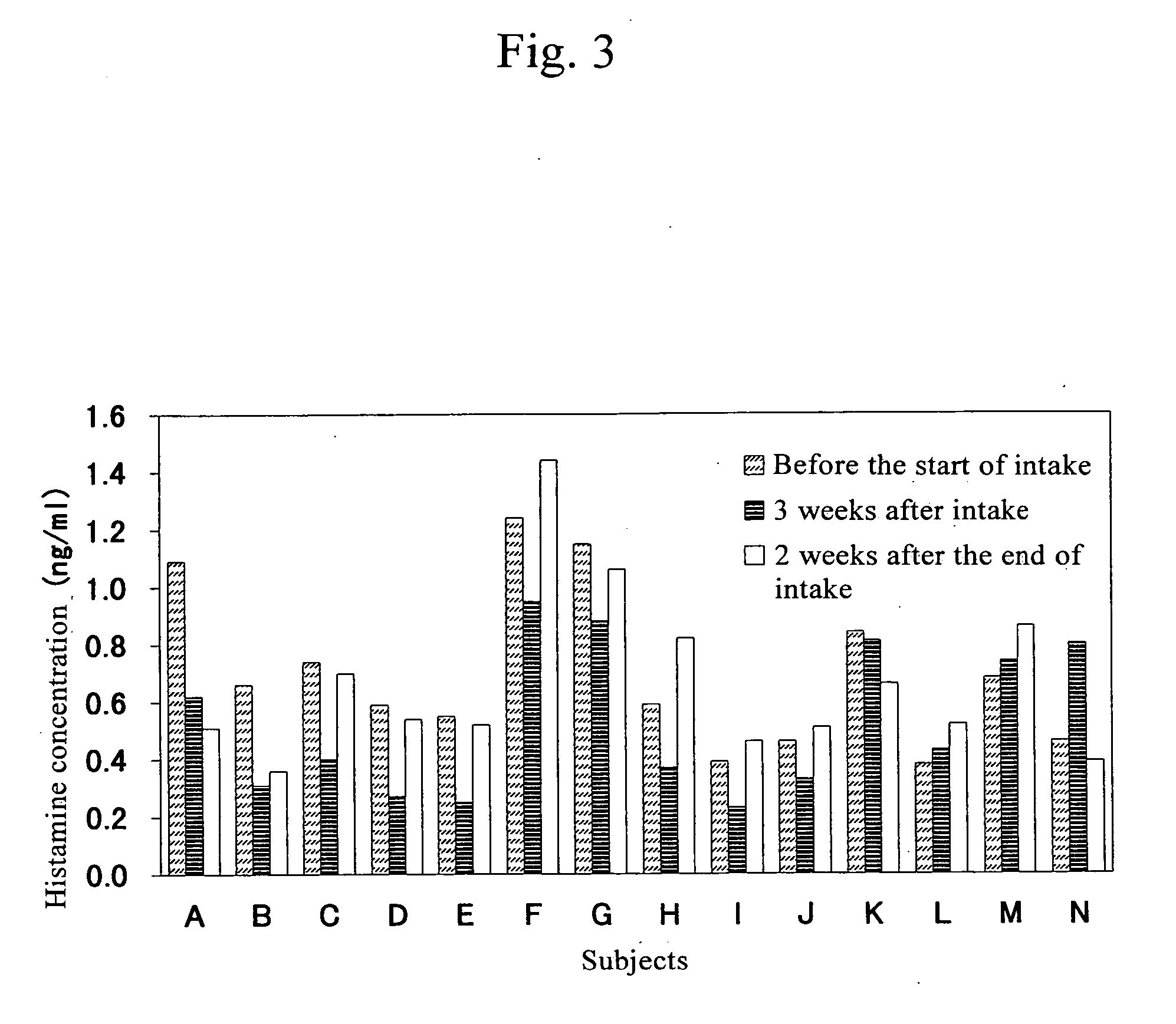
The present invention provides a histamine release inhibitor comprising a pectin or a salt thereof or a pectin hydrolysate as an active ingredient, and a pharmaceutical composition, a cosmetic, and food and drink comprising the inhibitor.
Owner:INC ADMINISTRATIVE AGENCY NAT AGRI & BIO ORIENTED RES ORG
Eutectic complex composed of resveratrol and protein kinase inhibitor, and composition comprising eutectic complex
InactiveCN110283052AHydroxy compound active ingredientsOrganic chemistry methodsLenvatinibPTK Inhibitors
The invention provides a eutectic complex composed of resveratrol and a protein kinase inhibitor, and a composition comprising the eutectic complex. The eutectic complex is characterized in that the protein kinase inhibitor is selected from one of imatinib, gefitinib, erlotinib, sunitinib, sorafenib, dasatinib, lapatinib, nilotinib, pazopanib, afatinib, crizotinib, axitinib, regorafenib, ibrutinib, lenvatinib, palbociclib, osimertinib and alectinib or pharmaceutically acceptable salt thereof. The eutectic complex provided by the invention can produce synergistic effects of inhibiting histamine release.
Owner:黄泳华
Composition with anti-allergy and relieving effects and preparation method and application thereof
ActiveCN105902447AFull coordinationReduce releaseCosmetic preparationsToilet preparationsMirabilis jalapaIrritation
The invention discloses a composition with anti-allergy and relieving effects and a preparation method and application thereof. The composition with anti-allergy and relieving effects has the functions of reducing allergic source irritation, inhibiting histamine release and hyaluronidase activity and repairing sensitive skin injured barriers. The composite is characterized by being mainly prepared from, by weight, 10-20% of plant extracts, 5-10% of trehalose, 20-30% of 1,3-propylene glycol and the balance water. The plant extracts mainly comprise, by weight, 10-15 parts of mirabilis jalapa, 10-15 parts of radix ophiopogonis, 10-15 parts of sophora flavescens and 5-10 parts of cactus. The composition belongs to the technical field of cosmetics.
Owner:GUANGDONG BAWEI BIOLOGICAL TECH CO LTD
Co-administration of an agent linked to an internalization peptide with an anti-inflammatory
ActiveUS8080518B2Reduce capacityTreating or effecting prophylaxis of a diseaseOrganic active ingredientsNervous disorderPharmacometricsBiotin
Owner:NONO INC
Anti-cancer drug preparation taking Solutol HS 15 as solubilizer
The invention discloses an injectable preparation of an anti-cancer drug, belonging to the technical field of medicines. The anti-cancer active ingredient in the preparation is paclitaxel or Docetaxol, and the dosage thereof is 0.01-10 percent (w / v); a solubilizer in the preparation is novel auxiliary polyethylene glycol-12-hydroxystearic acid (Solutol HS 15) with the characteristics of low hemolysis and low histamine release, and the dosage thereof is 5-50 percent (w / v). The preparation does not contain other surface active agent except Solutol HS 15. The preparation can eliminate or reduce anaphylactic reaction or haemocylolysis caused by Cremophor EL and Tween-80, has the characteristics of toxicity reduction, higher safety, certain slow release and targeting action, simple prescription constitution, simple and convenient process and low cost and has wide application prospect in the practice of preparing the anti-cancer drug.
Owner:BEIJING SL PHARMA
Composition and method for modulating inflammatory molecules with amylase
InactiveUS20130273026A1Reduce inflammationCause ‘sickness behavior’Peptide/protein ingredientsMetabolism disorderAmylaseMast cell
A method and composition for treating in a mammalian subject a condition accompanied or caused by IgE mediated histamine release from mast cells comprising administering to a subject in need of such treatment a therapeutically effective amount of the pharmaceutical composition an amylase peptide or derivative thereof.
Owner:GAVINI MADHAVI +1
Nitrofurazone washing fluid, preparation method and application thereof
InactiveCN102210651AEasy to useThe scope of clinical application is smallAntibacterial agentsOrganic active ingredientsSolventPatient compliance
The invention discloses a novel nitrofurazone washing fluid, which mainly comprises an active component nitrofurazone, a solubilizing agent polyethyleneglycol 15-hydroxyl stearate and a solvent injection water. Furthermore, one or more of cosolvent, pH regulator, osmotic pressure regulator and stabilizing agent can be added into the washing fluid. In the washing fluid, a novel solubilizer Solutol HS 15 has low haemolysis, extremely low histamine liberation and higher physiological tolerance, so that safety of clinical administration and compliance of patients are remarkably improved; the washing fluid has excellent stability and longer validity, and higher administration convenience is given to clinical doctors; and the washing fluid has better storage and transportation stability, higher clinical administration safety and patient compliance. The washing fluid has a simple preparation process, convenience in quality control, lower production cost and industrial production.
Owner:85 HOSPITAL OF PEOPLES LIBERATION ARMY
Co-Administration of An Agent Linked to an Internalization Peptide With an Anti-Inflammatory
ActiveUS20120252731A1Reduce capacityInhibit the inflammatory responseNervous disorderPeptide/protein ingredientsCo administrationBiotin
The invention provides methods of delivering pharmacologic agents linked to an internalization peptide, in which an inflammatory response inducible by the internalization peptide is inhibited by co-administration of an anti-inflammatory or by linking the internalization peptide to biotin or similar molecule. Such methods are premised in part on the results described in the examples whereby administration of a pharmacological agent linked to tat at high dosages is closely followed by an inflammatory response, which includes mast cell degranulation, histamine release and the typical sequelae of histamine release, such as redness, heat, swelling, and hypotension.
Owner:NONO INC
Cabazitaxel composition for injection and preparation method thereof
ActiveCN108066774AGood stabilization timeImprove stabilityPowder deliveryOrganic active ingredientsSolubilitySide effect
The invention relates to a cabazitaxel composition for injection. The cabazitaxel composition comprises, by weight, 1 part of cabazitaxel, 1-100 parts of cyclodextrin, 10-200 parts of cosolvent, 1-60parts of povidone (PVP) and 0.02-0.1 part of additive. The composition does not contain polysorbate and alcohol and is low in histamine release; using of antihistamine, corticosteroid and H2 antagonist are not needed before administration; the composition is a single small bottle ready to use, and the composition does not need to be diluted in two steps. The composition has the advantages of highcabazitaxel solubility, high stability, long redissolving stable time and convenience for clinical use. The composition does not contain polysorbate and alcohol, so that side effects like allergy, irritation and addiction are lowered. In addition, the invention further relates to preparation method of the cabazitaxel composition.
Owner:BIKA BIOTECH GUANGZHOU CO LTD
Non-depolarizing muscle relaxant composition as well as preparation method and application thereof
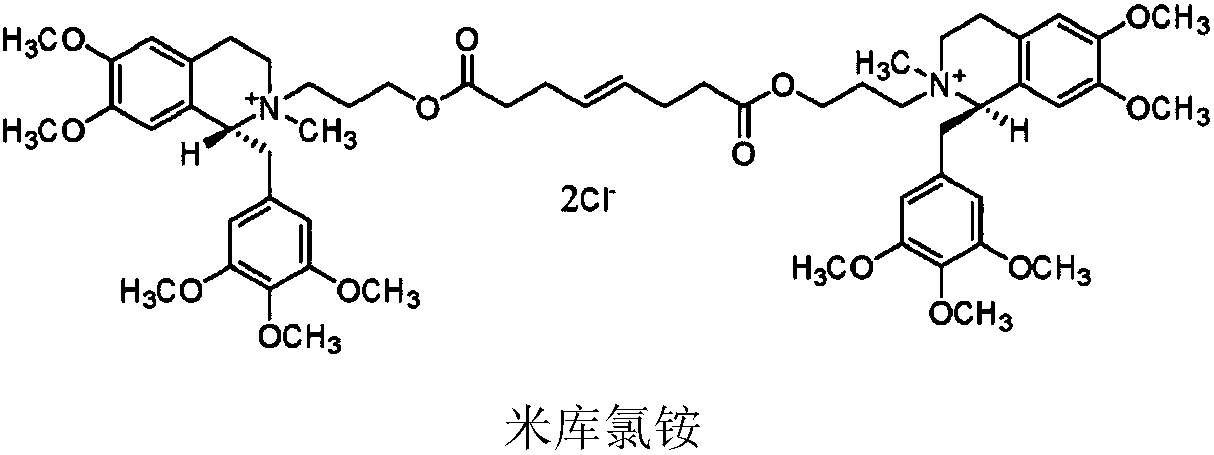
PendingCN108926564AGood storage stabilityPromote recoveryOrganic active ingredientsMuscular disorderSide effectCompound c
The invention discloses a mivacurium chloride composition as well as a preparation method and an application thereof. The mivacurium chloride composition consists of mivacurium chloride, a compound A,a compound B as well as an optional compound C and an optional compound D, wherein in terms of total weight of the mivacurium chloride composition, the compound A accounts for 0.001-0.15wt%, preferably 0.001-0.13wt%, and more preferably 0.001-0.1wt%; and the compound B accounts for 0.001-0.15wt%, preferably 0.006-0.15wt%, and more preferably 0.01-0.15wt%. The mivacurium chloride composition provided by the invention is good in stability in long-term preservation, relatively low in histamine release action and low in toxic and side effects.
Owner:SICHUAN CREDIT PHARMA
Ophthalmic composition
ActiveCN102548562AExcellent histamine release inhibitory effectSuppress discomfortAntibacterial agentsSenses disorderMentholSide chain
Disclosed are: an ophthalmic composition according to a non-conventional novel preparation, which has histamine release inhibitory action; and an ophthalmic composition for a silicone hydrogel contact lens, which is capable of suppressing adsorption of terpenoid to a silicone hydrogel contact lens. Specifically, the ophthalmic composition is prepared using (A) 0.001-2 w / v% of a polymer that has a phosphorylcholine analogous group in a side chain in combination with (B-1) 0.001-0.02 w / v% of menthol. Meanwhile, the ophthalmic composition for a silicone hydrogel contact lens is prepared using (A) a polymer that has a phosphorylcholine analogous group in a side chain in combination with (B-2) terpenoid.
Owner:ROHTO PHARM CO LTD
Atopic dermatitis inducer
InactiveUS20060134706A1Microbiological testing/measurementImmunoglobulins against animals/humansAntigenMast cell
An atopic dermatitis inducer binding to a human own IgE antibody and activating mast cells and basophiles, which includes a purified human secretion fraction, or an antigenic molecule or an antigenic determinant in the purified fraction, and obtained through the following steps of: filtering a human secretion, removing insoluble matters and collecting the filtrate; mixing the filtrate with a ConA-affinity carrier and collecting the supernatant; and separating a component having a histamine-releasing activity from the supernatant by column chromatography. This inducer is effective in diagnosing and treating human atopic dermatitis.
Owner:SHIONOGI & CO LTD
Antipyrotic
InactiveCN101198341AInhibition releaseInhibit aggregationOrganic active ingredientsCosmetic preparationsOrganic solventMedicine
An anti-inflammatory agent which comprises an oil-soluble licorice extract obtained by extraction treatment of either a leguminous plant of the genus Glycyrrhiza or a water extract of a leguminous plant of the genus Glycyrrhiza with an organic solvent, and has at least one effect selected from an inhibitory effect on hyaluronidase activity, an inhibitory effect on hexosaminidase release (i.e., an inhibitory effect on histamine release), an inhibitory effect on platelet aggregation and an inhibitory effect on phospholipase A2 activity.
Owner:MARUZEN PHARMA
Application method of natural plant peony extract
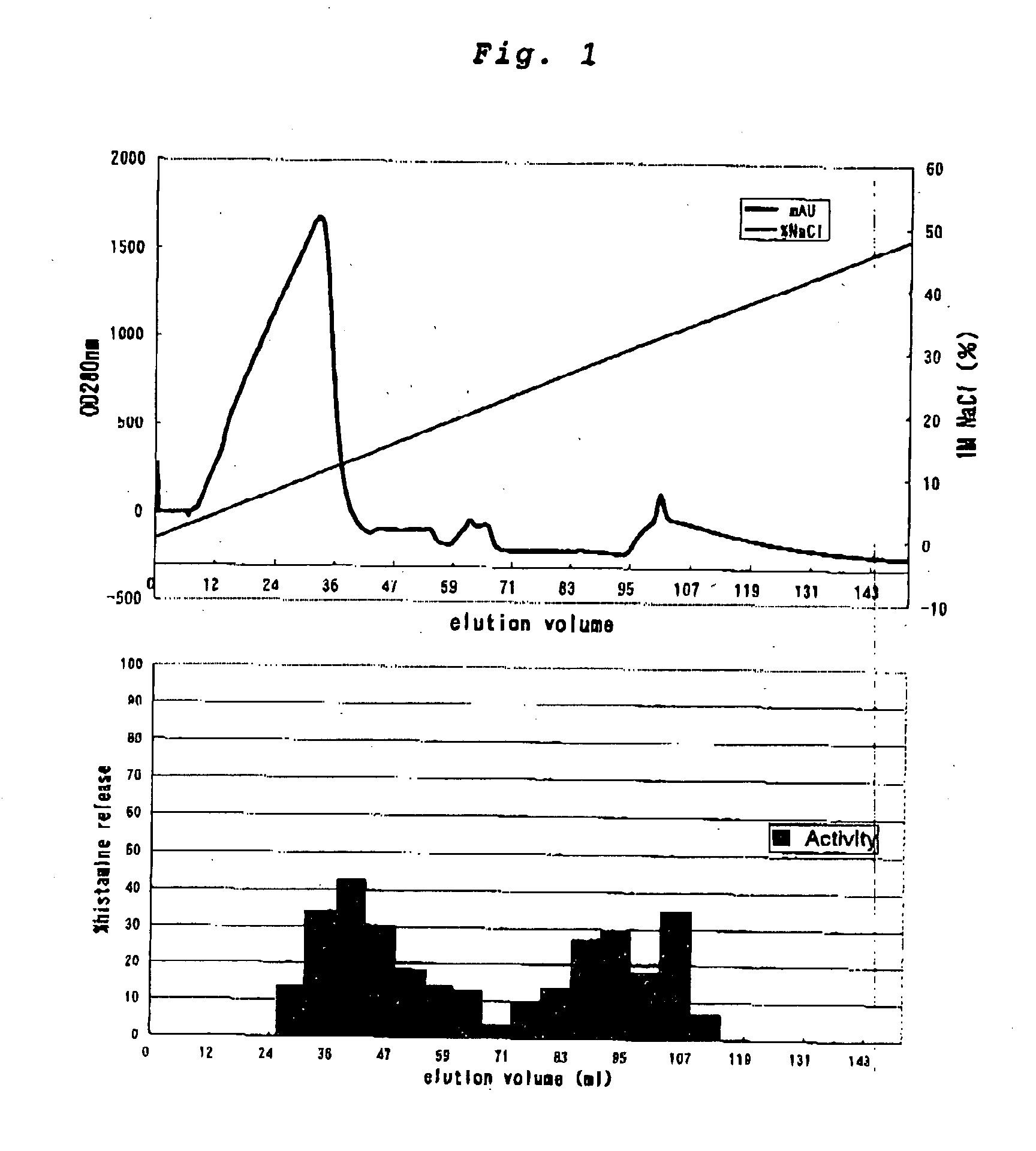
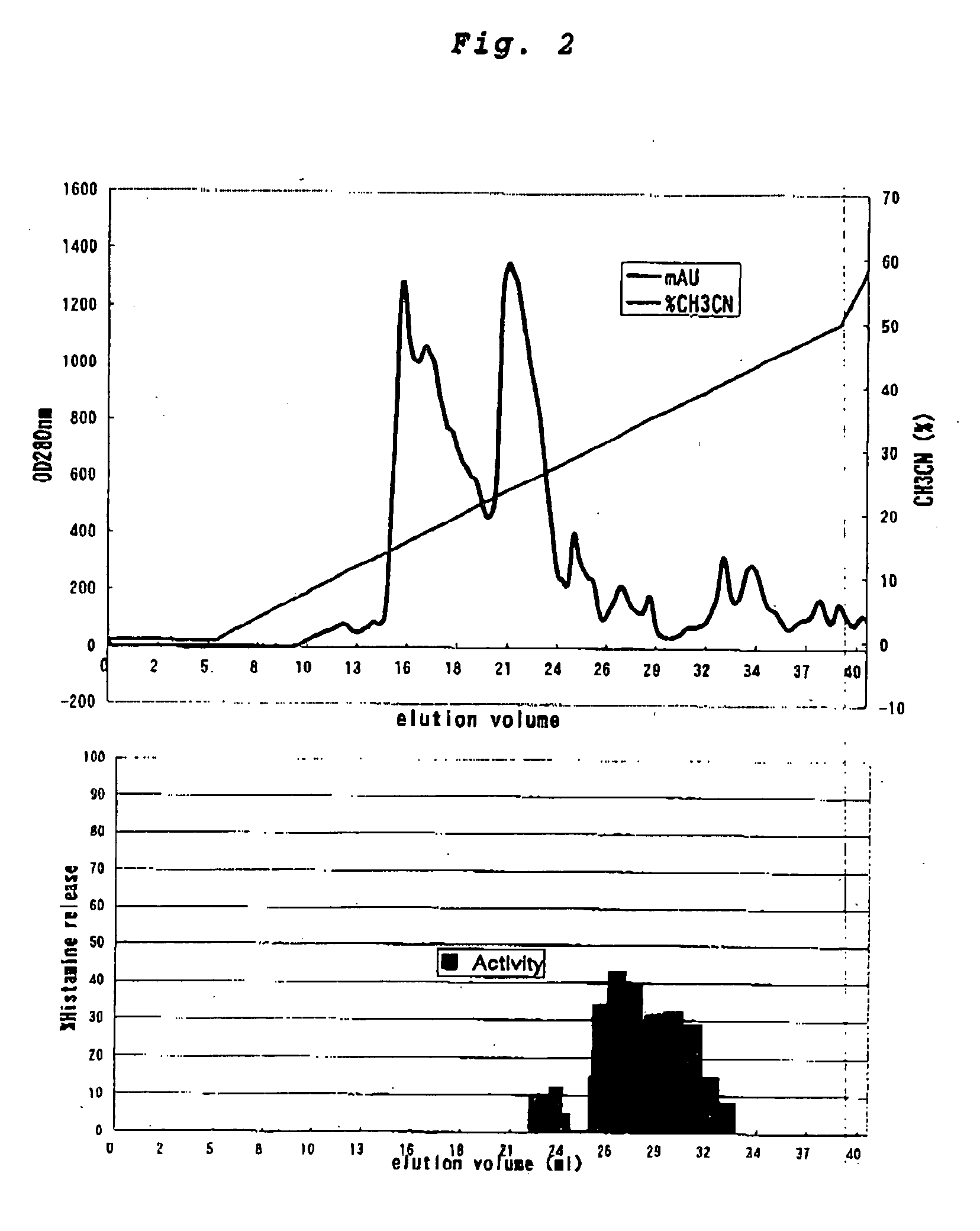
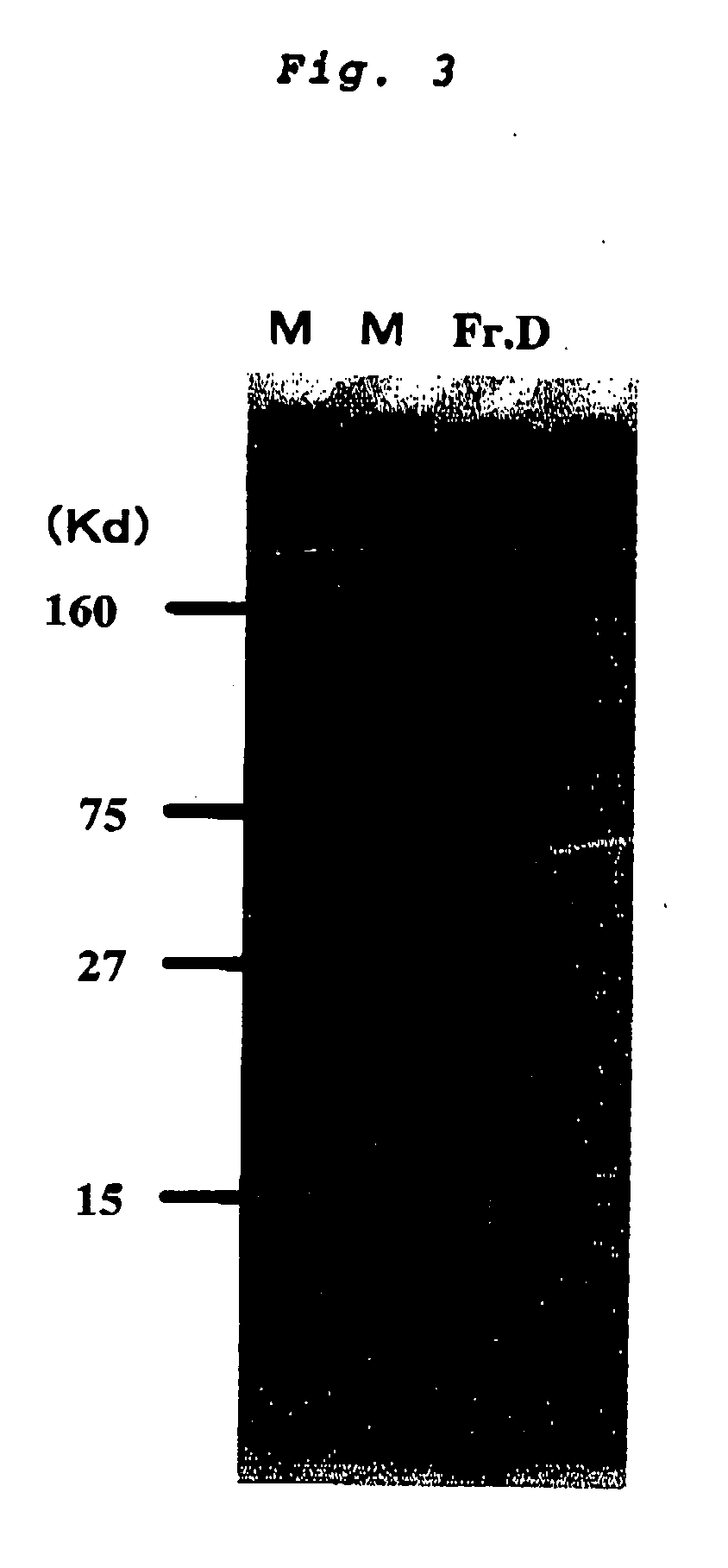
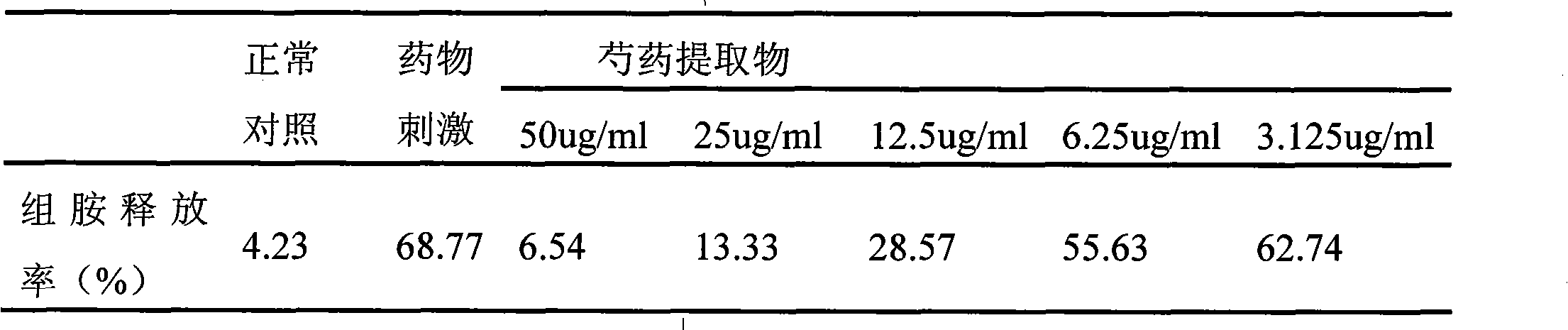
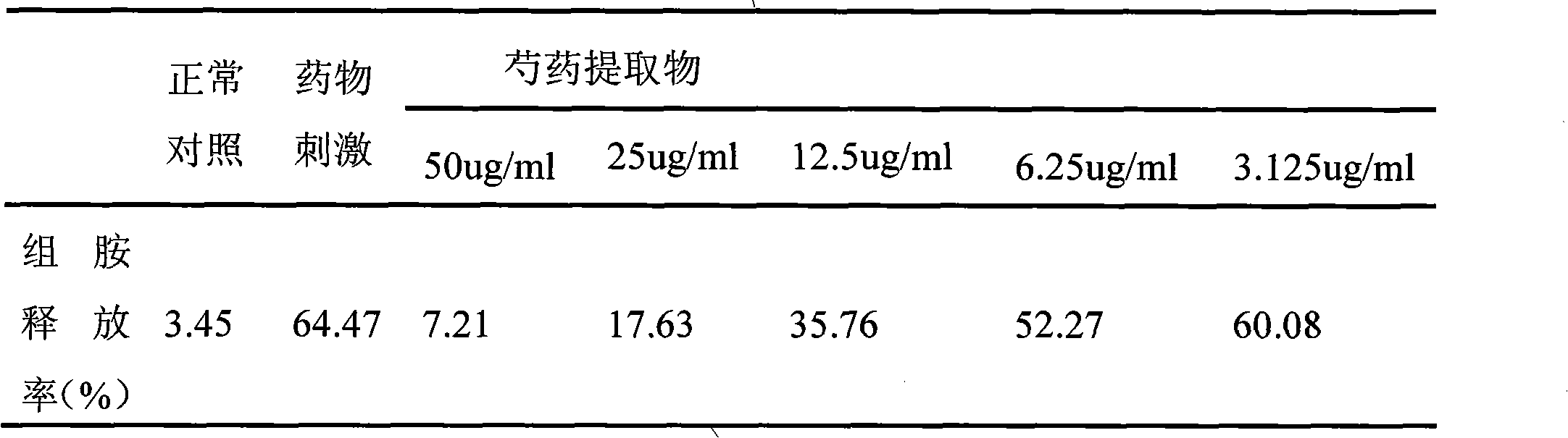
InactiveCN101612212AInhibition releaseTo achieve anti-allergic effectCosmetic preparationsToilet preparationsHistamine ReleasesIrritability
The invention relates to an application method of a natural plant peony extract. In the application method, the natural plant peony extract is applied to medicines, cosmetics and health-care foods for suppressing histamine release. The invention adopts a test model suppressing the histamine release of mass cells and validates the irritability-resisting and itching-relieving effects of the natural plant peony extract.
Owner:泊恩诗(上海)化妆品有限公司 +4
Methods for preparing pharmaceutical formulations
InactiveUS6911455B2Reduce aggregationInhibitionBiocidePeptide/protein ingredientsPharmaceutical formulationExcipient
The invention relates to pharmaceutical formulations and methods for preparing pharmaceutical formulations of histamine releasers. The present invention provides methods for determining the concentration of physiologically acceptable excipients for use in the formulations of the invention. The present invention also provides methods for suppressing pharmaceutically-induced histamine release by administering to an animal, the formulations of the present invention. A kit useful for preparing pharmaceutical formulations of histamine releasers is also provided.
Owner:SMITHKLINE BECKMAN CORP
Luteinizing hormone-releasing hormone (LHRH) antagonist derivative, preparation method of LHRH antagonist derivative and application of LHRH antagonist derivative
InactiveCN102675418AGood LHRH antagonist activityLow histamine releasing activityPeptide/protein ingredientsLuteinising hormone-releasing hormoneDiseaseSexual hormones
The invention belongs to the field of medicine and chemistry and particularly relates to a luteinizing hormone-releasing hormone (LHRH) antagonist derivative which is represented as formula (I), a preparation method of the LHRH antagonist derivative and application for preparation of drugs curing related sex hormone diseases or contraceptives. The invention also relates to a stereoisomeride of the LHRH antagonist derivative which is represented as the formula (I), a solvate of the LHRH antagonist derivative which is represented as the formula (I), or physio-toxicity-free salt of the LHRH antagonist derivative which is represented as the formula (I) and a pharmaceutical composition which contains compounds such as the LHRH antagonist derivative, the stereoisomeride, the solvate or the salt. An LHRH antagonist is modified with a water soluble group and a water soluble vitamin structure, the obtained LHRH antagonist derivative can maintain the activity of original antagonists, has low histamine-releasing activity and is beneficial to the clinic application and the water solubility is increased. The formula (I) is R-D-NAI-D-Cpa-D-Aaa3-Ser-Aaa5-Aaa6-Leu-Aaa8-Pro-D-Ala-B.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
Composition comprising the extract of actinidia arguta and related species for the prevention and treatment of allergic disease and non-allergic inflammatory disease
InactiveUS20070122508A1Confirm inhibitory effectReduce actionBiocideSenses disorderFood additiveActinidia
The present invention provides a pharmaceutical composition comprising the extract of hardy kiwifruit as an active ingredient in an effective amount to treat and prevent allergic disease and non-allergic inflammatory disease by reducing inflammation action, by inhibiting histamine release from mast cell, and by increasing the level of Th1 cytokines, IgG2a in serum and reducing the level of Th2 cytokines and IgE in serum. The present invention also provides a use of above extract for the preparation of pharmaceutical composition. The present invention also provides a health food or food additives, a cosmetic composition, a feed or feed additives comprising above extract for prevention or alleviation of allergic disease and non-allergic inflammatory disease by reducing inflammation action, by inhibiting histamine release from mast cell, and by increasing the level of Th1 cytokines, IgG2a in serum, and reducing the level of Th2 cytokines and IgE in serum.
Owner:HELIXIR
Deletion forms of IGE-dependent histamine releasing factor having histamine releasing activity, HRF-binding peptides and the uses thereof
The present invention relates to IgE-dependent histamine releasing factor (HRF) and HRF-binding peptides, more precisely, deletion forms of HRF which are able to be formed as dimers containing amino acid sequence represented by SEQ ID NO:3, genes encoding thereof and novel HRF-binding peptides having an activity of inhibiting HRF. The deletion forms of HRF which are able to be formed as dimers of the present invention induces intracellular secretion of histamine and IL-8, making an excellent candidate for a drug for inhibiting allergic reaction triggered by HRF and a kit for detecting HRF in serum of an allergy patient. In addition, novel HFR-binding peptides of the present invention bind to HRF to inhibit the actions of HFR, so they can be effectively used for the prevention and the treatment of allergic diseases of animals including asthma and rhinitis or malaria.
Owner:EWHA UNIV IND COLLABORATION FOUND
Viable cell analysis system for allergen screening
InactiveCN104232466ABioreactor/fermenter combinationsBiological substance pretreatmentsIrritationDimethyl siloxane
The invention discloses a method for fast detecting allergens, belonging to the technical field of detection. The integrated type allergen viable cell detection system integrates cell fixation, allergen irritation and histamine release and determination. The system comprises three cavities consisting of four layers of dimethyl siloxane microchips, wherein the cavities are connected respectively through a silane capillary tube, the cavity at the first layer can be connected with a flow injector or a syringe through a plurality of joints, the cavity at the second layer is an effect cell cavity, and the cavity at the third layer is a histamine reaction basin cavity. After liquid to be detected continuously and fully reacts with effect cells, a solution reaches a histamine reaction basin through a filter membrane and fully reacts with fluorescent derivatization liquid input in advance through reaction liquid injection holes, the generation condition of histamine can be judged under a fluorescent microscope according to fluorescence intensity, and further whether the reaction liquid contains allergens can be judged. The method has excellent sensitivity, fast response and direct visibility, the detection time can be remarkably shortened, the stability is high, and the method has great application potential in detection of allergens in cosmetics, food safety and clinical medicines.
Owner:TIANJIN MOSBIO SCI & TECH CO LTD
Anti-sweat antigen monoclonal antibody
ActiveUS20110117103A1High sensitivity detectionEasy to optimizeCompound screeningSenses disorderAntigen stimulationMonoclonal antibody
[Object of the Invention] To provide an antibody that inhibits histamine releasing activity induced by an antigenic substance contained in sweat.[Means for Attaining the Object] An antibody which can react with a sweat antigen composition and inhibit the histamine releasing activity of the composition on a sweat antigen stimulation-responsive cell.
Owner:HIROSHIMA UNIVERSITY
Co-administration of an agent linked to an internalization peptide with an anti-inflammatory
ActiveUS8933013B2Prevent slippingAvoid developmentPolypeptide with localisation/targeting motifNervous disorderPharmacometricsBiotin
The invention provides methods of delivering pharmacologic agents linked to an internalization peptide, in which an inflammatory response inducible by the internalization peptide is inhibited by co-administration of an anti-inflammatory or by linking the internalization peptide to biotin or similar molecule. Such methods are premised in part on the results described in the examples whereby administration of a pharmacological agent linked to tat at high dosages is closely followed by an inflammatory response, which includes mast cell degranulation, histamine release and the typical sequelae of histamine release, such as redness, heat, swelling, and hypotension.
Owner:NONO INC
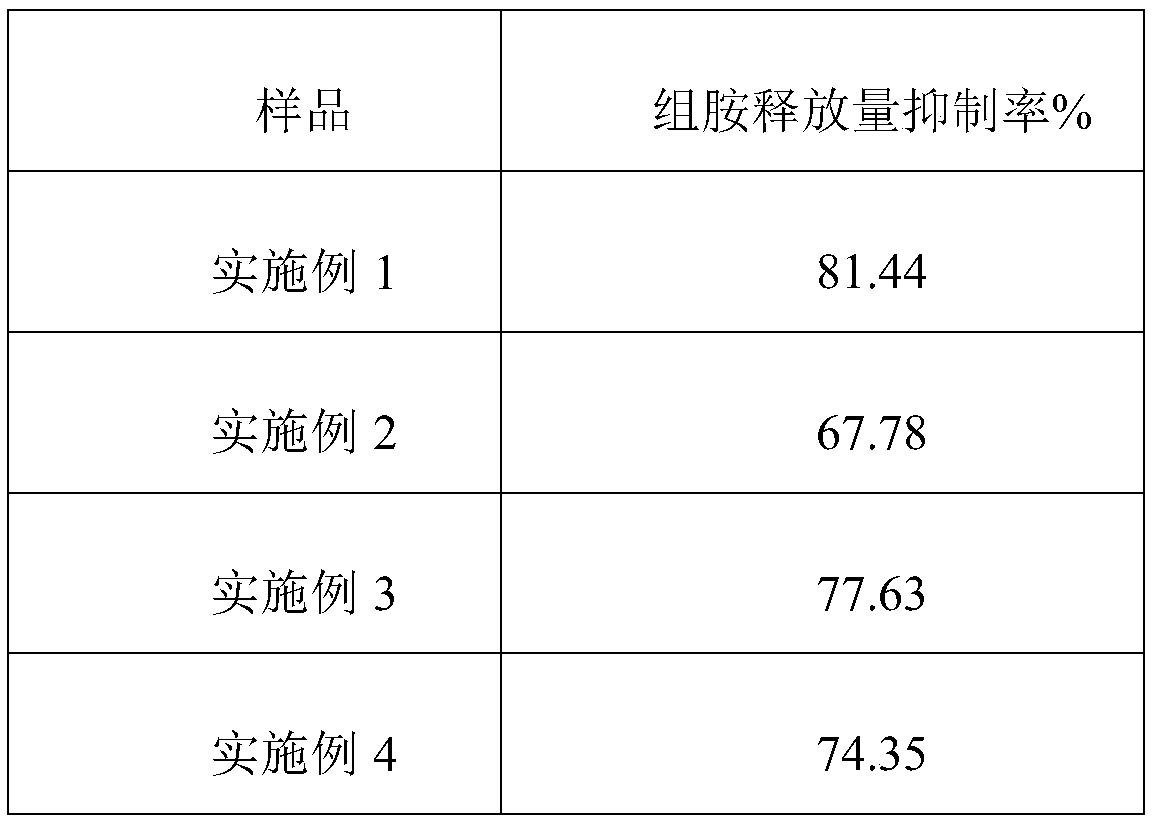
Anti-allergy repair composition with histamine release inhibition effect as well as preparation method and application thereof
ActiveCN108938498AInhibitionImprove immunityCosmetic preparationsToilet preparationsCentella asiatica extractAlcohol
The invention belongs to the field of cosmetics and in particular relates to an anti-allergy repair composition with a histamine release inhibition effect. The anti-allergy repair composition comprises the following components in percentage by weight: 5-15% of fructus momordicae extract, 0.5-5% of glycerine phosphoinositol choline salt, 0.2-2% of licorice extract, 0.1-2% of hydrocotyle asiatica extract, 0.1-5% of polypeptides, 0.5-3% of agastache rugosa extract, 1-5% of Chinese gentian root, 30-50% of polyhydric alcohols and 30-40% of water. The composition can achieve the effects of effectively inhibiting histamine release, reducing allergic reactions and eliminating skin itch brought by histamine. Meanwhile, the invention further discloses a preparation method and application of the composition.
Owner:广州天然国度生物科技有限公司
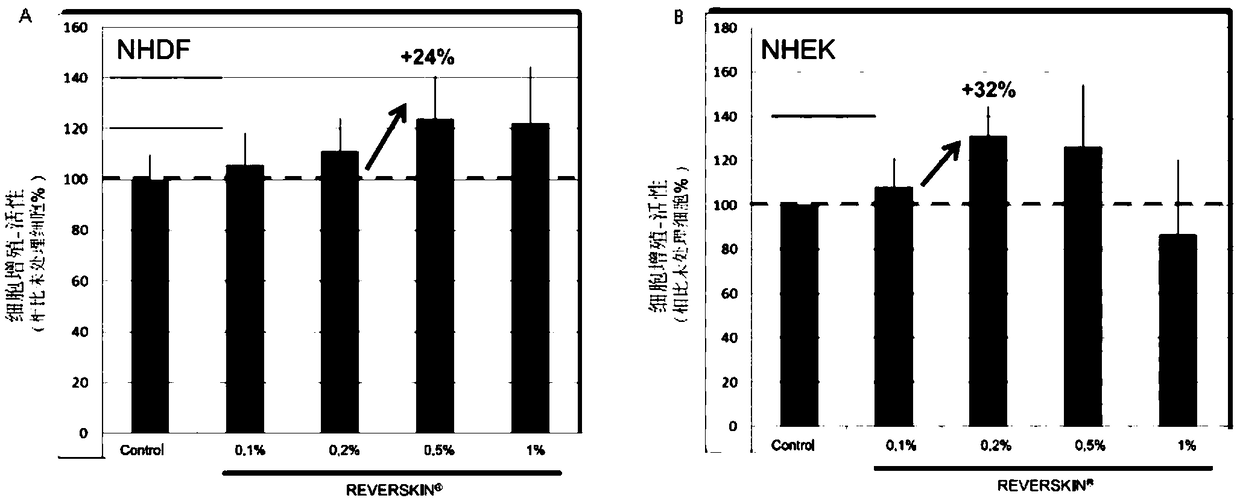
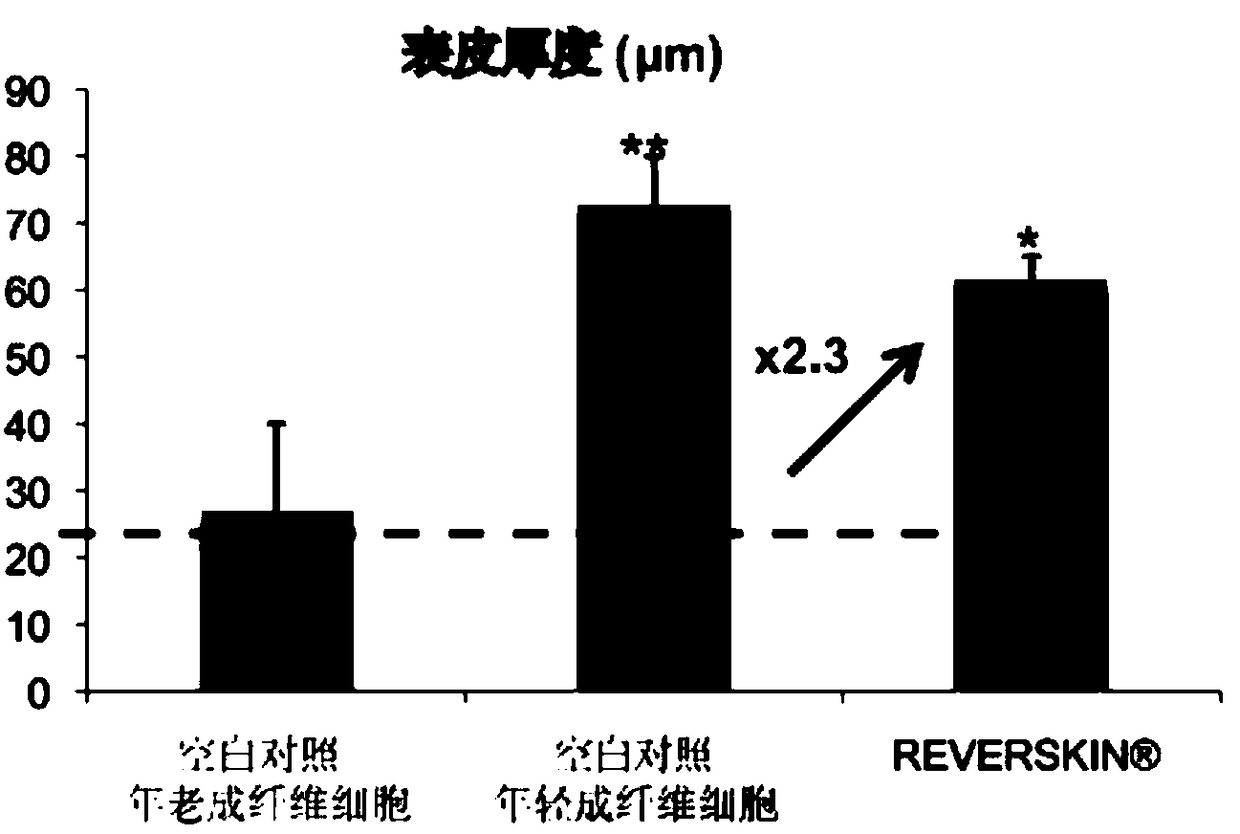
Skin barrier repairing composite and application thereof
ActiveCN109431910ARecovery functionIncrease vitalityCosmetic preparationsHair cosmeticsAllergyGluconates
The invention relates to a skin barrier repairing composite and application thereof. The skin barrier repairing composite comprises sodium pyrrolidonecarboxylate, ecdysterone, an itching relieving anti-allergy composite and a metabolic balance composite, wherein the itching relieving anti-allergy composite comprises a radix stephaniae tetrandrae extractive, potassium lactate, dipotassium glycyrrhizinate and phenoxyethanol; the metabolic balance composite comprises magnesium aspartate, zinc gluconate and copper gluconate. According to the skin barrier repairing composite, the sodium pyrrolidonecarboxylate and the ecdysterone are adopted to repair the skin barrier, the metabolic balance composite (M3) is adopted to improve cell activity and accelerate metabolic balance, the itching relievinganti-allergy composite (Comthing tetra) is adopted to lower itching and scratching caused by inflammation or histamine release, the skin barrier is protected from mechanical injury, the scalp barrierfunction is effectively recovered, and the susceptibility of the scalp is lowered.
Owner:HUNAN YUJIA COSMETICS MFG CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com