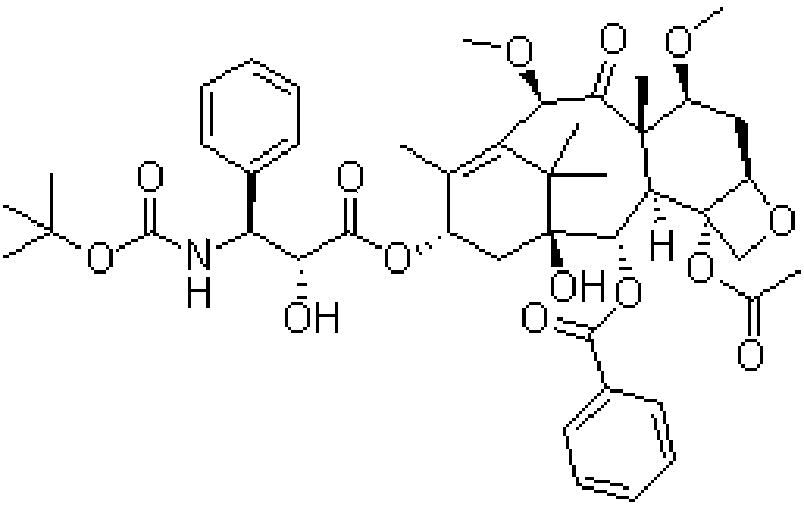
Cabazitaxel composition for injection and preparation method thereof
A technology of cabazitaxel and composition, which is applied in the field of cabazitaxel composition for injection and its preparation, can solve the problems such as the inability to improve the solubility of cabazitaxel, and achieves a cabazitaxel with long reconstitution stability time, convenient clinical use and high solubility. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041]
[0042]
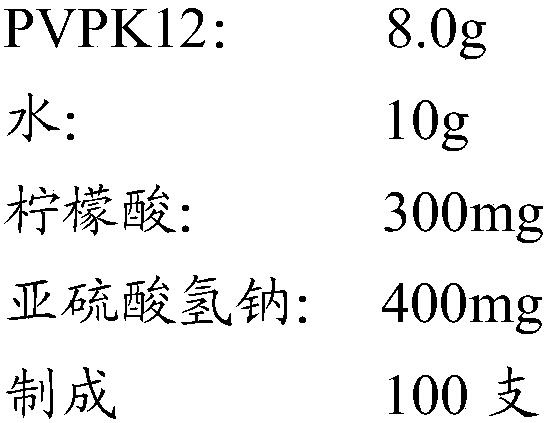
[0043] Weigh the SBE-β-CD of the recipe quantity, add the PEG300 and water of the recipe quantity, stir and dissolve at 23~25 ℃, add the PVPK12 and citric acid of the recipe quantity, add the sodium bisulfite and cabazita of the recipe quantity after stirring and dissolving Continue to stir for 2 h after stirring to dissolve the solution to make the solution uniform. Take samples to measure the pH value and content. After passing the test, filter them with a 0.2 μm polytetrafluoroethylene (PTFE) membrane, sub-pack vials, fill with nitrogen, press stoppers, roll caps, and label them.
Embodiment 2
[0045]
[0046] Weigh the prescribed amount of SBE-β-CD, add the prescribed amount of PEG300 and water, stir and dissolve at 22 to 25°C, add the prescribed amount of PVPK12 and citric acid, add the prescribed amount of cabazitaxel after stirring and dissolving, and stir and dissolve. Continue stirring for 2h to make the solution homogeneous. Take samples to measure the pH value and content. After passing the test, filter them with a 0.2 μm polytetrafluoroethylene (PTFE) membrane, sub-pack vials, fill with nitrogen, press stoppers, roll caps, and label them.
Embodiment 3
[0048]
[0049]
[0050] Weigh the prescribed amount of SBE-β-CD, add the prescribed amount of PEG300 and water, stir and dissolve at 18 to 20 ° C, add the prescribed amount of PVPK12 and citric acid, add the prescribed amount of cabazitaxel after stirring and dissolving, and stir and dissolve. Continue stirring for 100 min to make the solution homogeneous. Take samples to measure the pH value and content. After passing the test, filter them with a 0.2 μm polytetrafluoroethylene (PTFE) membrane, sub-pack vials, fill with nitrogen, press stoppers, roll caps, and label them.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com