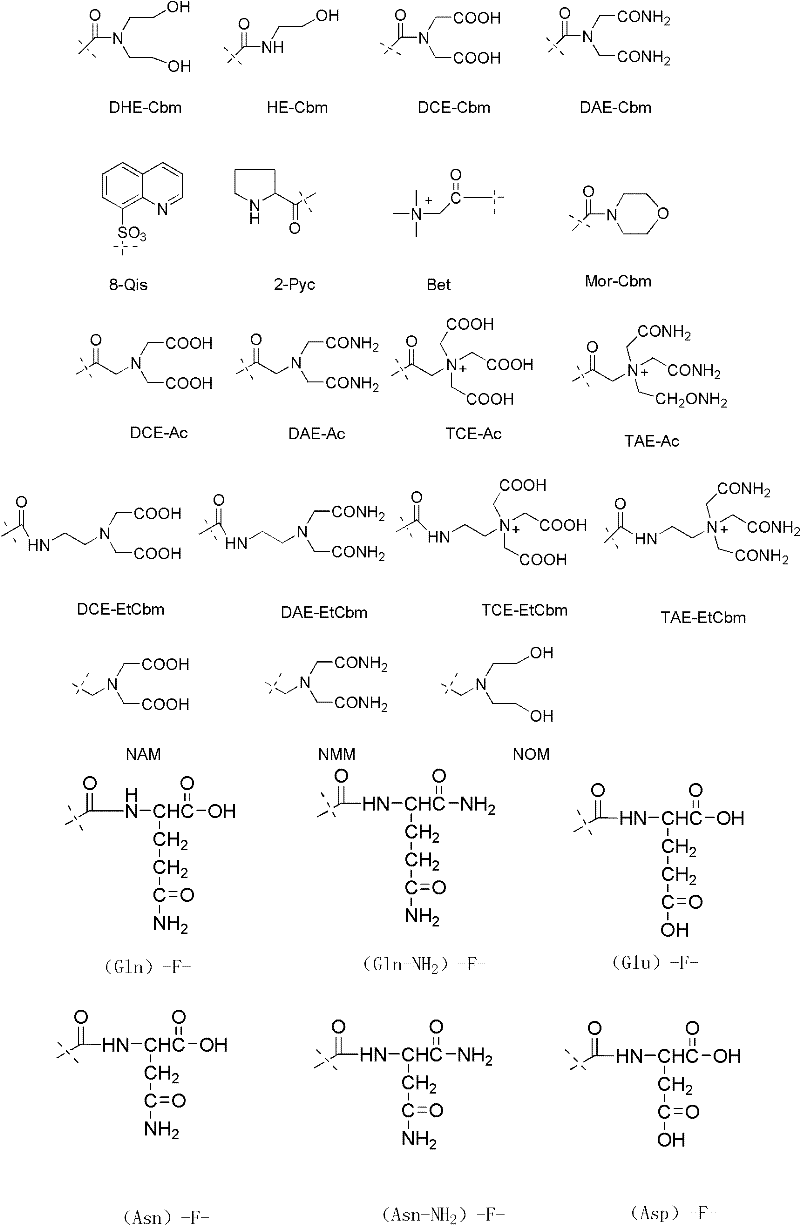
Luteinizing hormone-releasing hormone (LHRH) antagonist derivative, preparation method of LHRH antagonist derivative and application of LHRH antagonist derivative
A solvate and physiological technology, applied in the fields of chemistry and medicine, can solve problems such as easy gel formation, short half-life, and limited application of LHRH antagonists
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0168] Embodiment 1: the synthesis of Boc-Aph(Bet)-OH:
[0169] Reference method (Gao Yongqing, Zhou Ning, Lu Yujian, Shi Weiguo, Cheng Maosheng, Liu Keliang, Indirect introduction of aromatic nitro-p-aminophenylalanine in ammonium formate catalytic transfer hydrogenation reduction peptide chain, Chemical Journal of Chinese Universities, 2010, 31 (4), 718-722.) Synthesis of N α -tert-butoxycarbonyl-4-(phenoxycarbonyl)aminophenylalanine methyl ester [Boc-Aph-OMe]
[0170] 5.88g (20mmol) of Boc-Aph-OMe was dissolved in 50ml of DCM, 6.9ml (40mmol) of DIEA was added, and 3.44g (20mmol) of freshly prepared betaine acid chloride hydrochloride was added in batches. After the disappearance of the raw material was detected by TLC, a small amount of water was added, extracted with ethyl acetate, washed with saturated sodium chloride, and dried over anhydrous sodium sulfate. filter. Concentrate and perform silica gel column chromatography. 4.26 g of [Boc-Aph(Bet)-OMe] was obtained as...
Embodiment 2
[0172] Embodiment 2: the synthesis of Boc-Aph(DHE-Cbm)-OH:
[0173] Reference method ( Zhou Ning , Fu Huijun, Rong Yi, Cheng Maosheng, Liu Keliang, Design, Synthesis and Application of Unnatural Amino Acids Containing Complexing Functional Groups in Bioactive Peptides, Chemical Journal of Chinese Universities, 2007, 28(4): 668-671. ) to synthesize N α - tert-butoxycarbonyl-4-(phenoxycarbonyl)aminophenylalanine [Boc-Aph(PhOCO)-OH] and N,N-diethylbenzyl ether-imine hydrochloride (NDOB)
[0174] Add 4.00g (10mmol) Boc-Aph(PhOCO)-OH, 1.4ml (200mmol) pyridine, and 10ml methanol into a 50ml round bottom flask. After the raw materials are dissolved, add 32.2g (100mmol) NDOB. After TLC detects that the raw materials disappear, depressurize Evaporate to dryness and adjust pH to 3 with dilute hydrochloric acid, extract with ethyl acetate, wash with saturated sodium chloride, and dry over anhydrous sodium sulfate. Filter and evaporate to dryness. Silica gel column chromatography gav...
Embodiment 3
[0175] Embodiment 3: the synthesis of decapeptide derivatives
[0176] With 1g of MBHA resin (0.96mmol) as the solid phase carrier, DCC / HOBt as the condensing agent, according to the amino acid sequence of the compound, according to the standard Boc solid-phase peptide synthesis method (references: Huang Weide, Chen Changqing, Polypeptide Synthesis, Science Press , 1985), first synthesized Leu 7 -Ilys(Cbz)[or Arg(Tos)] 8 -Pro 9 -D-Ala 10 - MBHA resin.
[0177] A: Synthesis of compound 69: Connect the remaining amino acids in sequence according to the standard Boc strategy, put the above peptide resin into the reactor of the HF cutter, add 1.0mL of anisole, and vacuum the system of the HF cutter after installation , cooled the reactor with liquid nitrogen, transferred to about 10 mL of liquid HF, and reacted at 0°C for 40 minutes. Use an oil pump to pump out HF, remove the reactor, add frozen anhydrous ether to precipitate a solid, and then transfer the suspension to a fun...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com