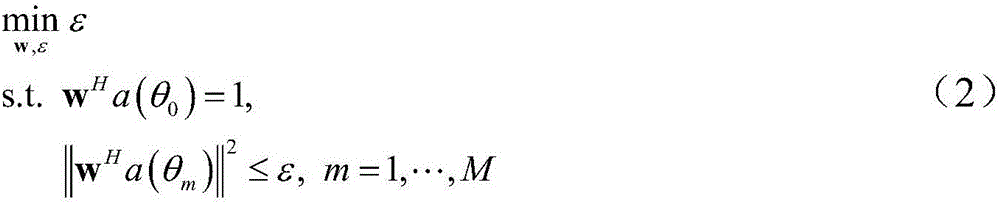Patents
Literature
604results about How to "Improve side effects" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
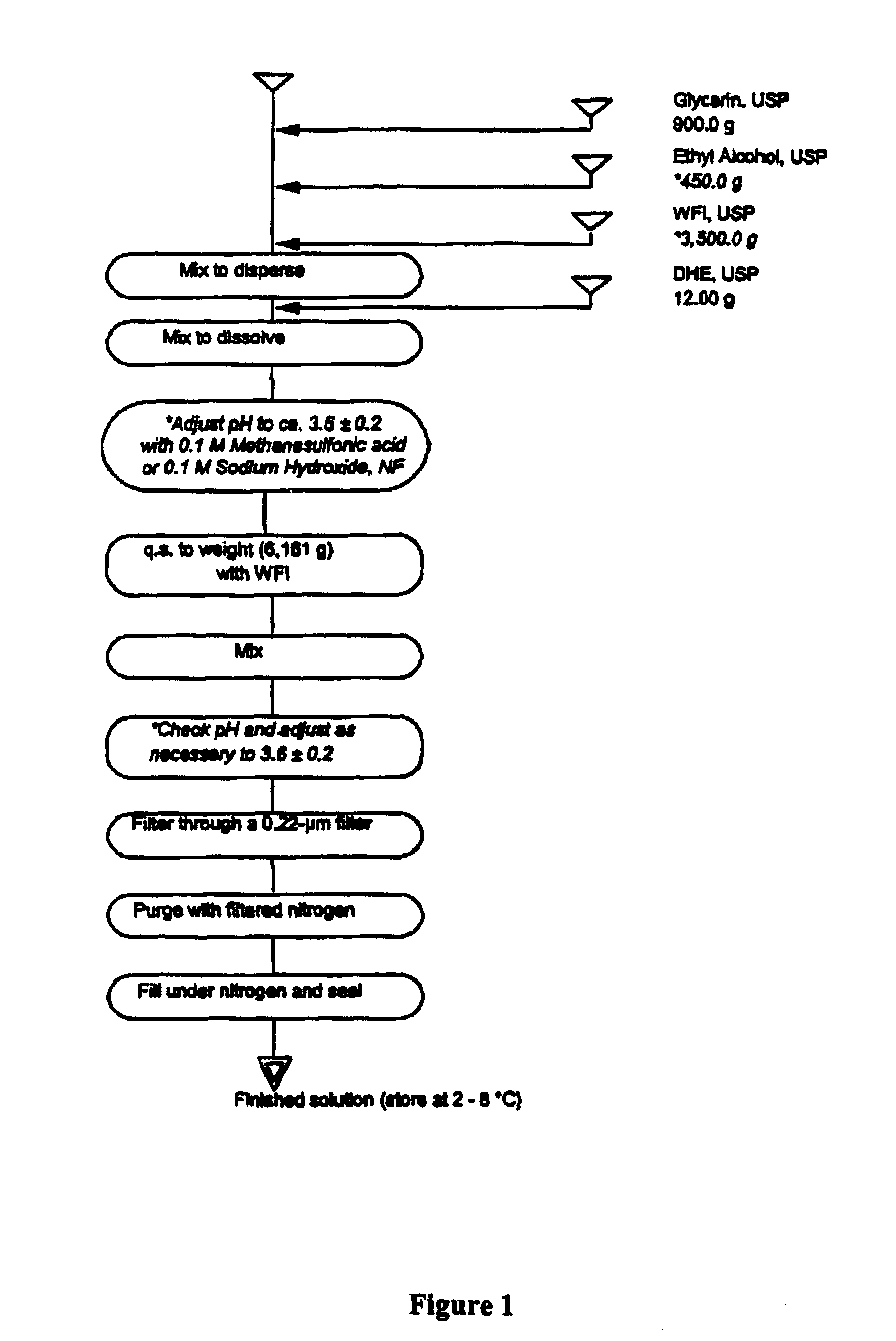
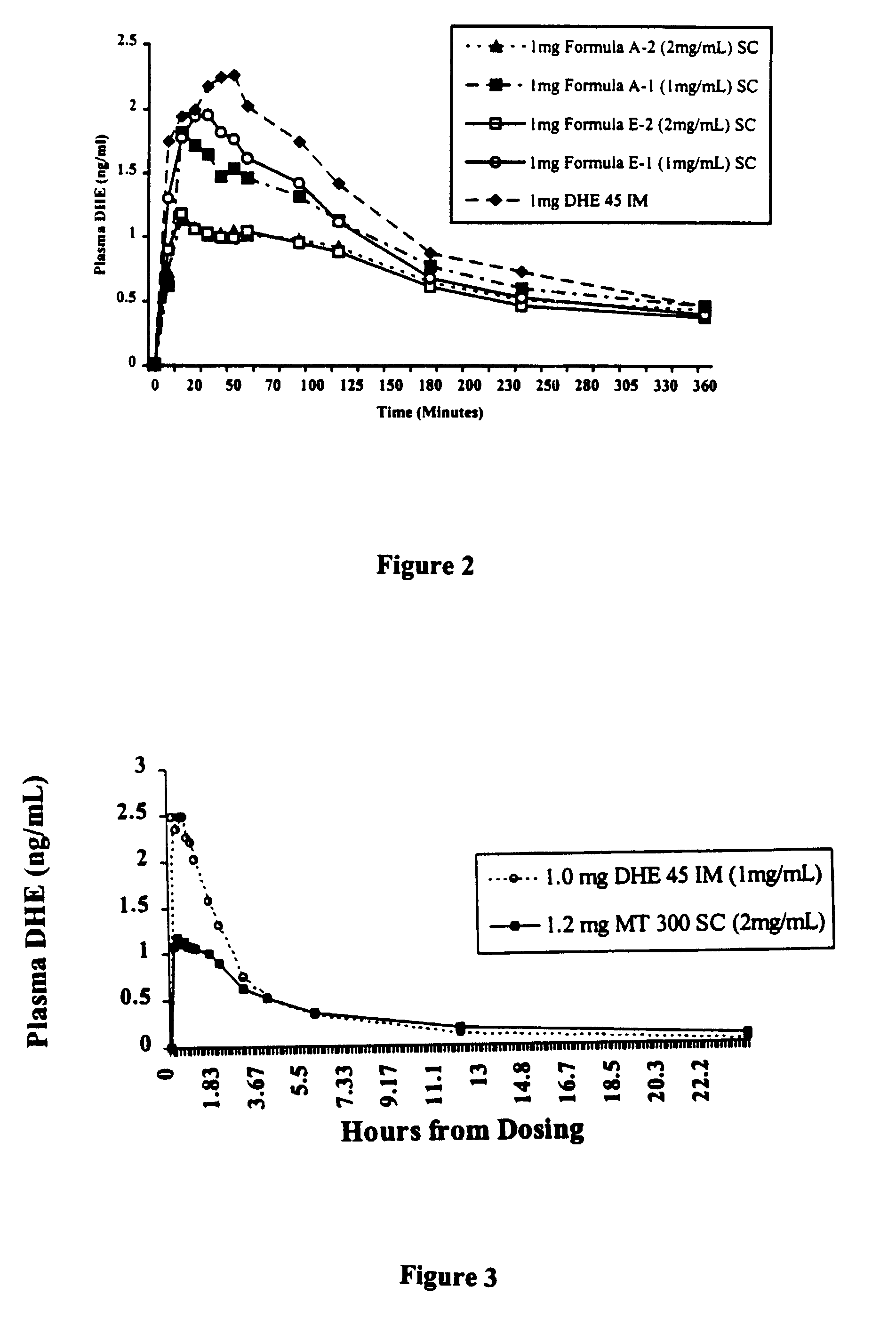
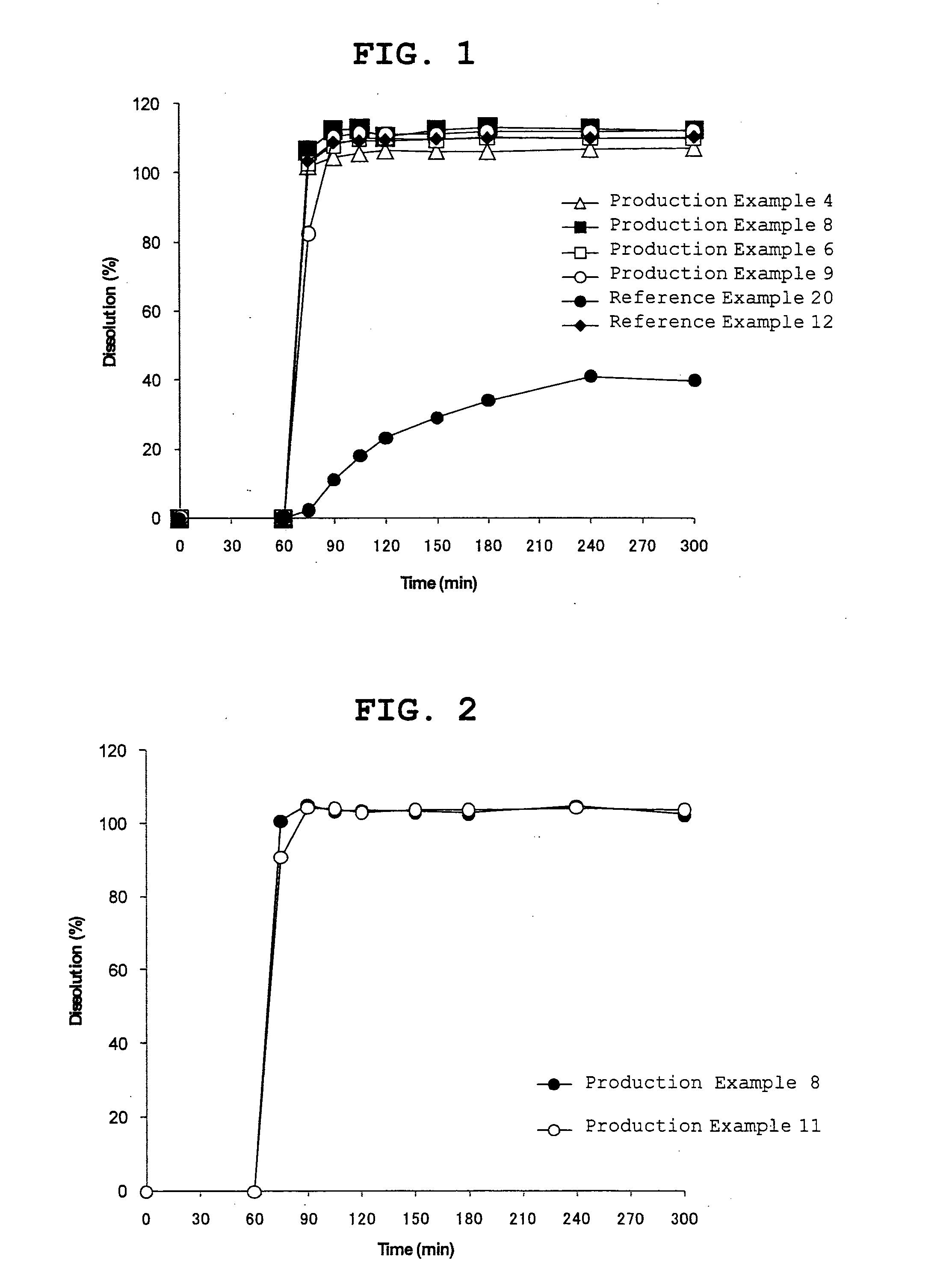
High potency dihydroergotamine compositions
InactiveUS7060694B2Improvement of side effectsEliminate side effectsBiocideNervous disorderDihydroergotamineHeadache severe
The present invention is directed to improved formulations for dihydroergotamine in which the drug is present at a concentration of at least 2.9 mM. The invention encompasses methods for using these formulations in treating patients for migraine headaches and the packaging of formulation into prefilled syringes for self-administration by patients.
Owner:POZEN INC
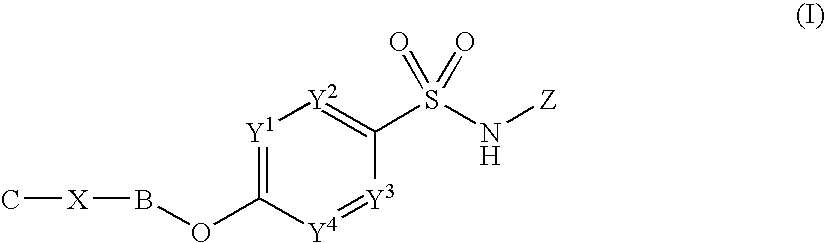
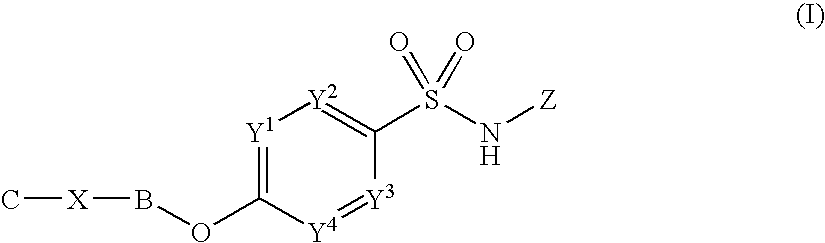
Sulfonamide derivatives
InactiveUS20100197655A1Good potencyLow affinityBiocideNervous disorderCombinatorial chemistryPerylene derivatives
Owner:PFIZER LTD +1
Methods and systems for ablating tissue
InactiveUS20080045890A1Improve side effectsLess damage to the heart's electrical functionsBalloon catheterMedical devicesAbnormal tissue growthHypertrophic cardiomyopathy
Methods and systems for treating patients requiring tissue ablation for volumetric tissue reduction rely on the injection of ethanol and other tissue-ablating agents into the perivascular space surrounding body lumens, particularly blood vessels or vessels of the alimentary canal, reproductive system and urinary tract. Injection of tissue-ablating agents is intended treat conditions such as hypertrophic cardiomyopathy, benign and malignant tumors, benign prostatic hyperplasia, and uterine fibroids, for example. Injection may be achieved using intravascular catheters which advance needles radially outward from a body vessel lumen or by transmyocardial injection from an epicardial or endocardial surface of the heart.
Owner:MERCATOR MEDSYST
Oral pharmaceutical products containing 17 beta-estradiol-3-lower alkanoate, method of administering the same and process of preparation
InactiveUS6962908B2Improve bioavailabilityIncrease intakePowder deliveryBiocideAcetic acidOral medication
A pharmaceutical dosage unit for oral administration to a human female comprising a therapeutically effective amount of 17β-estradiol-3-lower alkanoate, most preferably 17β-estradiol-3-acetate, and a pharmaceutically acceptable carrier is disclosed. Also disclosed is a method for treating a human female in need of 17β-estradiol and a contraceptive method by oral administration of the pharmaceutical dosage unit and a method of preparing a pharmaceutical composition that may be used to form the pharmaceutical dosage unit of the invention.
Owner:ALLERGAN THERAPEUTICS LLC
Compositions and dosage forms for gastric delivery of irinotecan and methods of treatment that use it to inhibit cancer cell proliferation
InactiveUS6881420B2Improve oral bioavailabilityReduced bioavailabilityBiocideCapsule deliveryWhole bodyCancer cell proliferation
The present invention provides oral dosage forms and compositions for administering antineoplastic agents, such as irinotecan, etoposide, paclitaxel, doxorubicin and vincristine, whose oral effectiveness is limited by pre-systemic and systemic deactivation in the GI tract. Gelling of the gastric retention vehicle composition, and in the case of solid forms concomitant expansion of the composition, retains the antineoplastic drug in the patient's stomach, minimizing pre-systemic and / or systemic deactivation of the drug.
Owner:TEVA PHARM USA INC
Polycyclic guanine phosphodiesterase V inhibitors
InactiveUS6969719B2High selectivityImprove side effectsOrganic active ingredientsBiocideMedicineSexual dysfunction
Owner:MERCK SHARP & DOHME CORP
Combination therapy for effecting weight loss and treating obesity
InactiveUS7056890B2Efficient and effective treatmentReduce effectBiocideCarbohydrate active ingredientsSYMPATHOMIMETIC AGENTSDrug
The present invention features a novel therapy for effecting weight loss which involves treating a subject with a sympathomimetic agent (e.g., phentermine or a phentermine-like drug) in combination with an anticonvulsant sulfamate derivative (e.g., topiramate) such that the subject experiences weight loss.The combination methods of the present invention also are effective against symptoms associated with Syndrome X. The invention also features pharmaceutical compositions and kits for use in the practice of these novel therapies.
Owner:VIVUS
Plasma device for selective treatment of electropored cells
InactiveUS20110112528A1Increased cell absorptionMinimal scarringBioreactor/fermenter combinationsElectrotherapyCancer cellMedicine
A method and device for treatment of living cells with cold atmospheric pressure plasma while simultaneously applying selective electroporation of the cells are provided. The method is useful for the local selective killing of cancer cells, improvement of wound treatment and sterilization or decontamination of objects.
Owner:LEIBNIZ INST FUR PLASMAFORSCHUNG & TECH
Three-dimensional cartoon face texture generation method and device
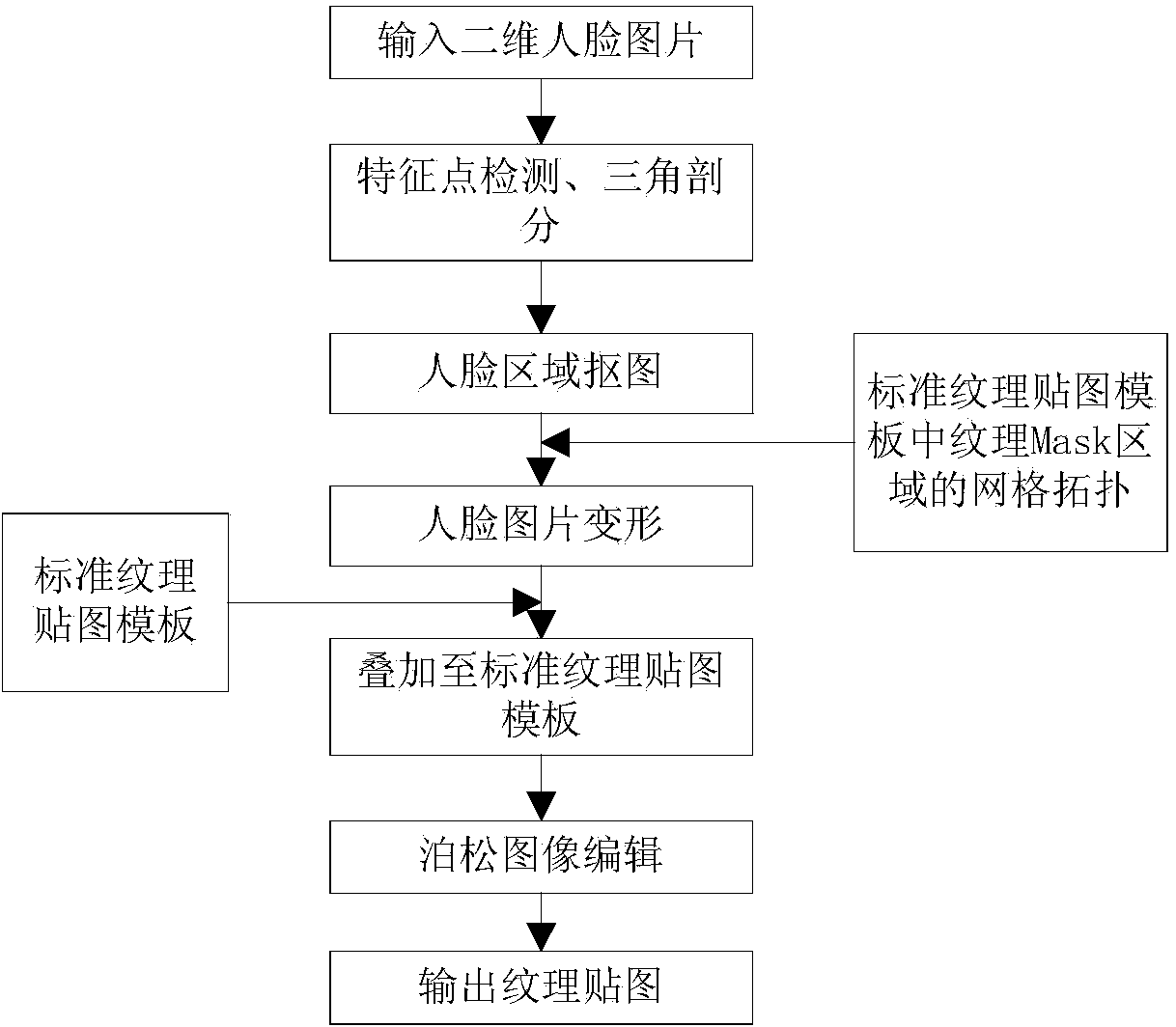
InactiveCN103646416AImprove side effectsHigh similarity3D-image renderingComputer graphics (images)Face model
The invention provides a three-dimensional cartoon face texture generation method and device. The three-dimensional cartoon face texture generation method comprises obtaining a standard texture mapping template of a three-dimensional cartoon face model and defining a texture Mask area in the standard texture mapping template; triangulating feature points inside a two-dimensional picture on the basis of a triangular mesh topological structure of the texture Mask area and obtaining a triangular mesh inside part of the two-dimensional picture corresponding to the triangular meshes inside the texture Mask area. The three-dimensional cartoon face texture generation method further comprises deforming the part of the two-dimensional picture and superposing the deformed part of the two-dimensional picture on the standard texture mapping template. The three-dimensional cartoon face texture generation method and device can generate a three-dimensional cartoon face texture mapping of relatively good effects simply by utilizing a single front face picture, and can enhance the similarity between a two-dimensional picture model and a picture to some extent.
Owner:INST OF COMPUTING TECH CHINESE ACAD OF SCI
Site-specific intestinal delivery of adsorbents, alone or in combination with degrading molecules
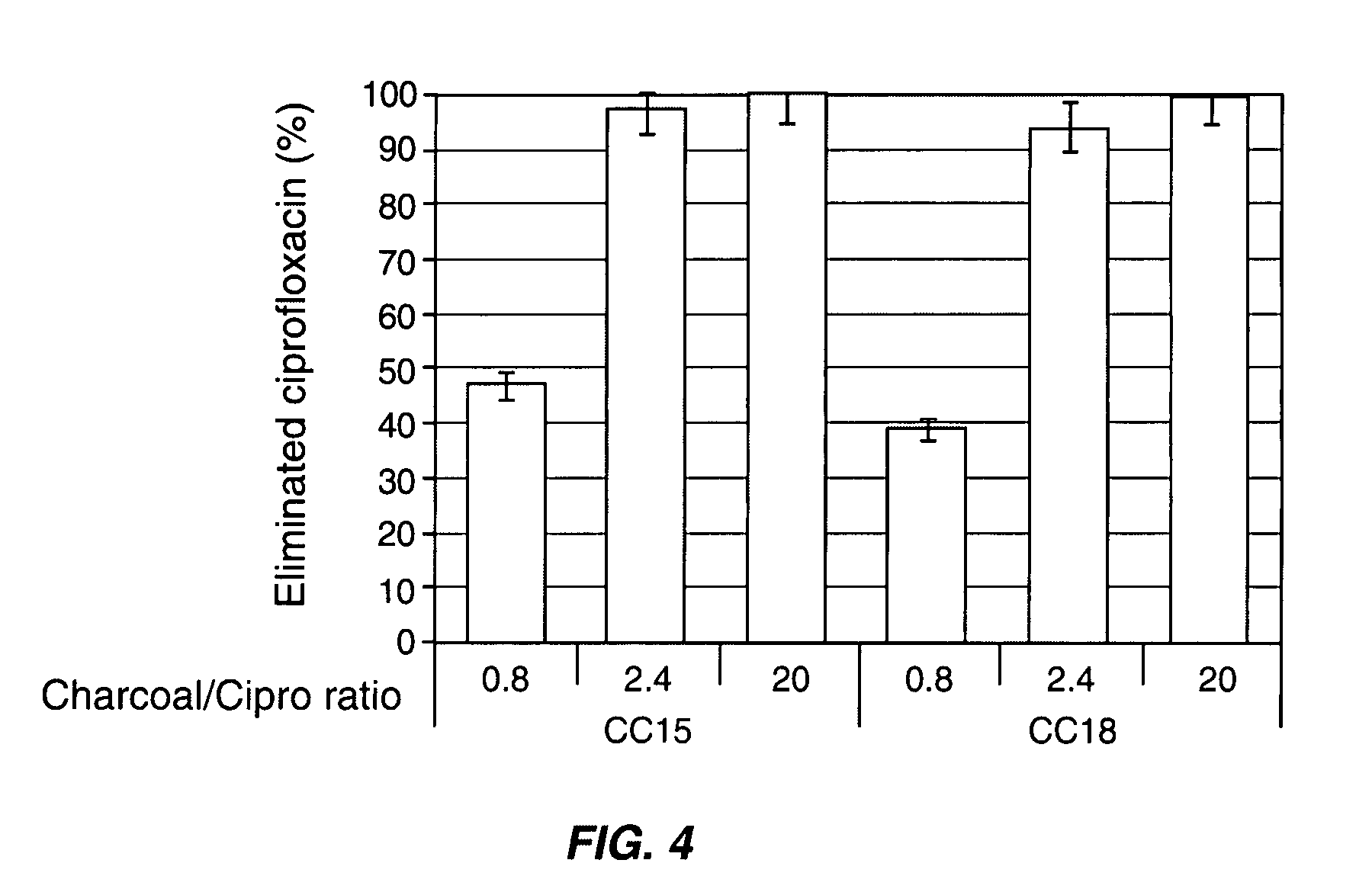
InactiveUS8048413B2Reduce concentrationImprove side effectsAntibacterial agentsBiocideDrug adsorptionMetabolite
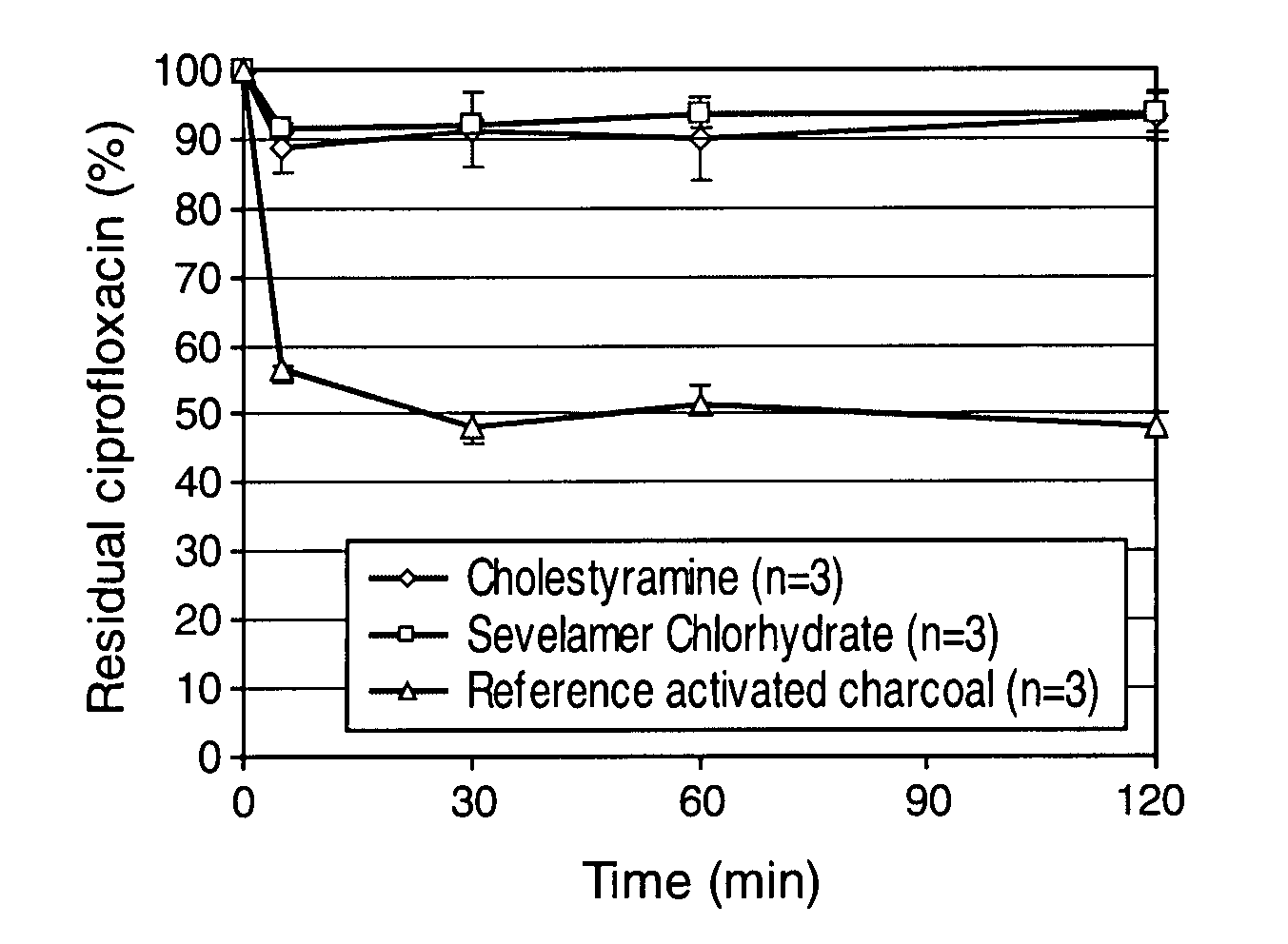
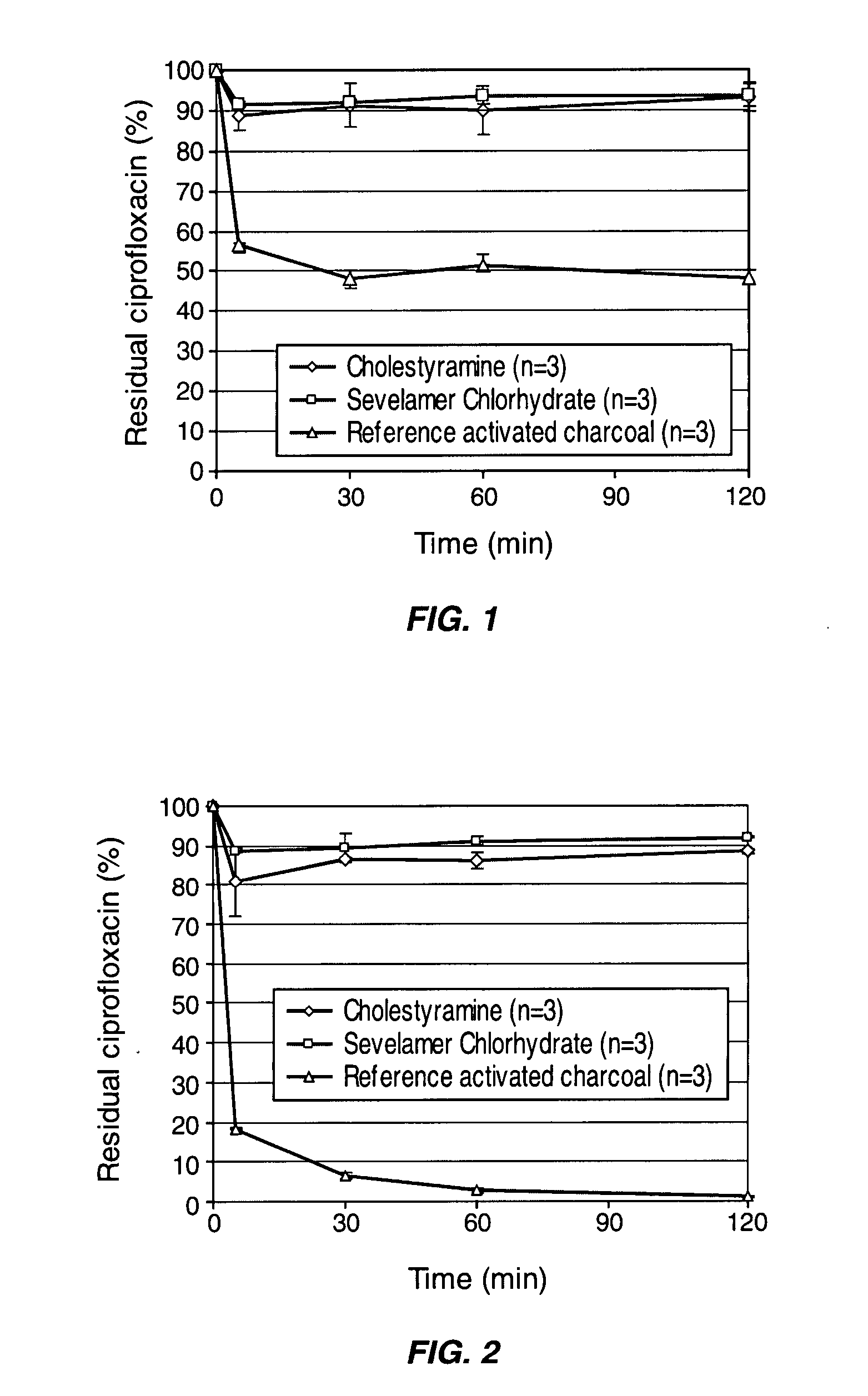
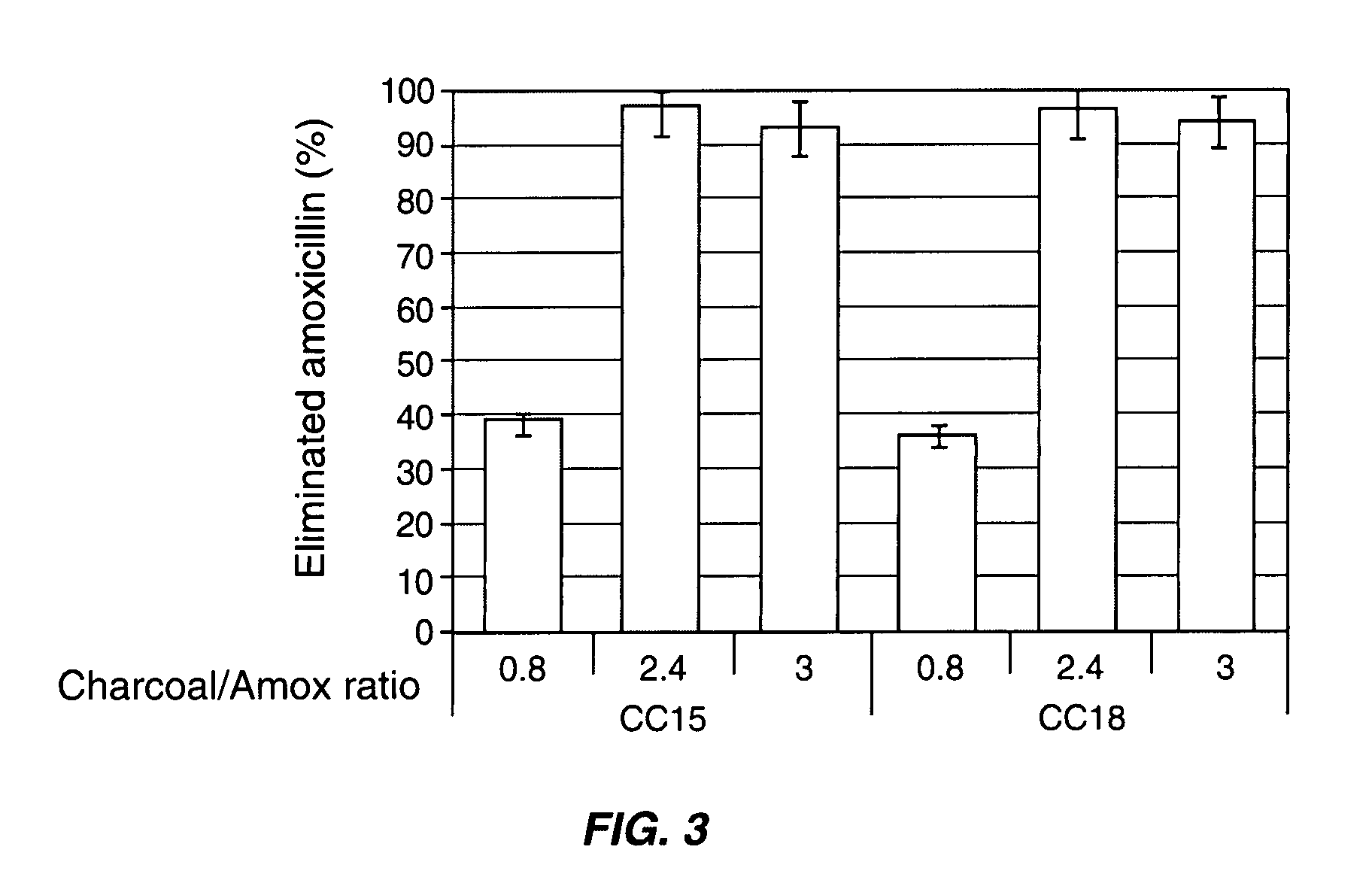
Compositions which deliver adsorbents, alone or in combination with active drug “degrading molecules,” in a site-specific manner to the intestine, and which eliminate or at least lower the concentration of residual unwanted material within the intestine, are disclosed. Methods of treatment using the compositions are also disclosed. The material to be eliminated can include residual active antibiotics, metabolites, bacterial or other toxins, and drugs which cause side effects in the gastrointestinal tract. The adsorbents can be formulated in capsules, tablets or any acceptable pharmaceutical composition, and are ideally designed to specifically release the adsorbents in a programmed manner at a specific site of the intestinal tract. The programmed delivery prevents adsorbents from interfering with the normal absorption process of a given molecule after oral absorption, until it reaches the lower part of the small intestine. The compositions can be used to adsorb, and therefore remove, any residual drug, metabolite thereof, or bacterial toxin after oral or parenteral administration which would otherwise cause adverse effects in the lower intestine and / or colon.
Owner:CENT NAT DE LA RECHERCHE SCI +3
Methods of providing sustained treatment with opioids
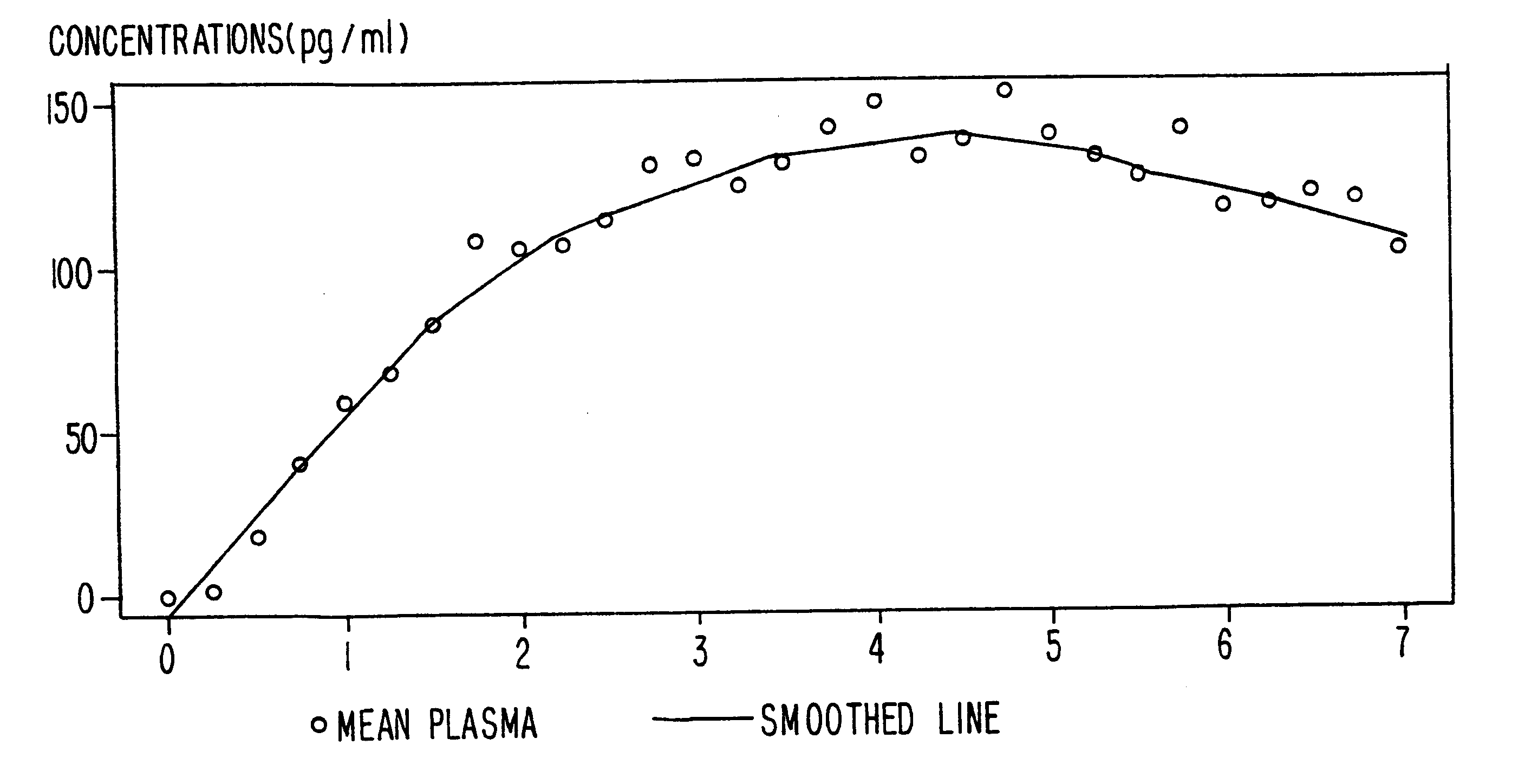
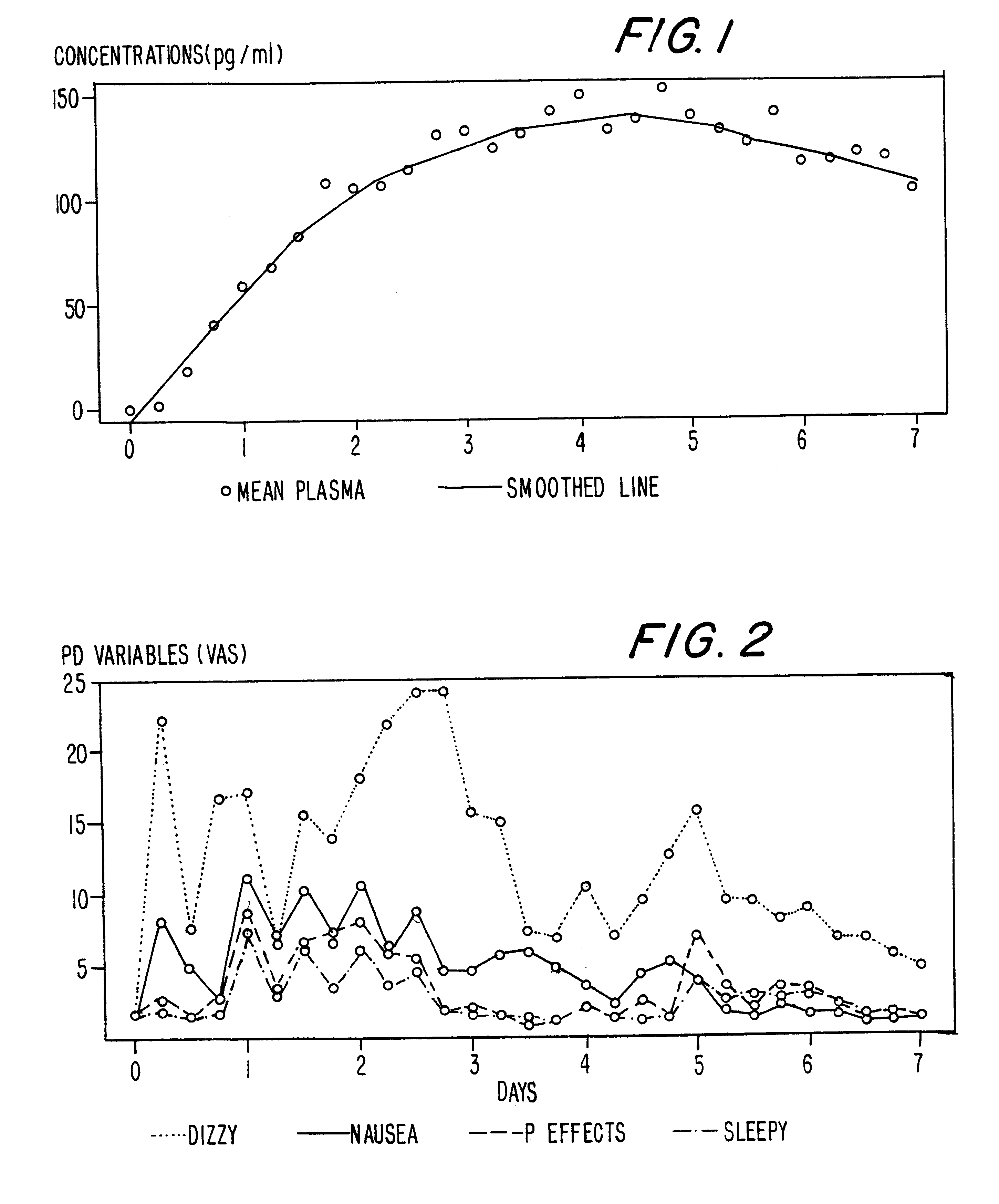
InactiveUS6231886B1Reduced plasma concentrationEfficient managementOrganic active ingredientsNervous disorderPlasma concentrationPlasma glucose
A method of effectively treating pain in humans is achieved by administering buprenorphine in accordance with first order kinetics over an initial three-day dosing interval, such that a maximum plasma concentration from about 20 pg / ml to about 1052 pg / ml is attained, and thereafter maintaining the administration of buprenorphine for at least an addition two-day dosing interval in accordance with substantially zero order kinetics, such that the patients experience analgesia throughout the at least two-day additional dosing interval.
Owner:PURDUE PHARMA LP
Quaternary opioid carboxamides
ActiveUS20090197905A1Good to excellent peripheral opioid antagonism activityLess susceptibleBiocideNervous disorderSide effectMedicine
Owner:RENESSELAER POLYTECHNIC INST
Blend oil and preparation method thereof
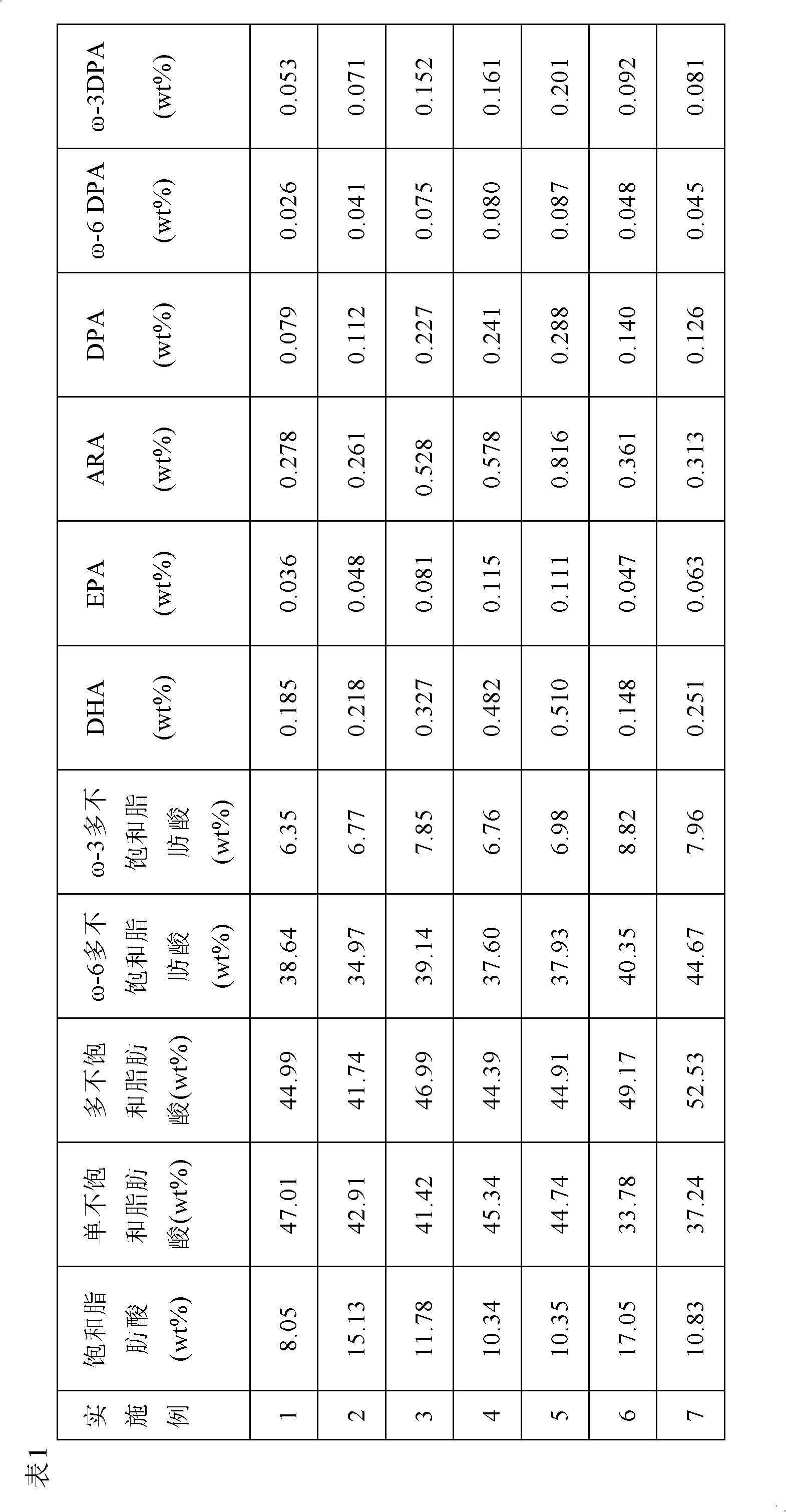
ActiveCN103156003AMake up for nutritional deficienciesReduce lossesEdible oils/fatsChemistryDocosapentaenoic acid
The invention provides blend oil. The blend oil comprises docosahexaenoic acid (DHA), eicosapentaenoic acid (EPA), eicosapentaenoic (ARA), and docosapentaenoic acid (DPA), wherein weight ratio of the ARA to the DHA equals to 1-3:1, preference ratio is 1.2-2.4:1, more preferable ratio is 1.2-1.6:1, and optimization ratio is 1.5:1. The blend oil and a preparation method of the blend oil are healthy and balanced in the DHA, the EPA, the DPA, and the ARA.
Owner:WILMAR SHANGHAI BIOTECH RES & DEV CENT
Sulfonamide derivatives
InactiveUS8153814B2Good potencyLow affinityBiocideNervous disorderSulfanilamideCombinatorial chemistry
Owner:PFIZER LTD +1
Highly stable lithium nickel cobalt aluminate positive electrode material and its preparation method
ActiveCN104218243AFacilitate conductionImprove side effectsCell electrodesSecondary cellsBattery chargeHigh energy
The invention provides a highly stable lithium nickel cobalt aluminate positive electrode material and its preparation method. The characteristic chemical formula of the lithium nickel cobalt aluminate material is LinNi1-x-yCoxAlyO2.mLiMaOb, wherein n is not less than 0.95 and not more than 1.15, x is more than 0.00 and less than 0.30, y is not less than 0.01 and not more than 0.10, m is more than 0.00 and less than 0.05, a is more than 0.0 and less than 3.0, b is a valence matching coefficient, and b is (M valence * a +1) / 2. The morphology of the material is a spheroidic secondary particle structure formed by a primary particle, the average particle size of the primary particle is 0.10-2.5mum, and the average particle size of the secondary particle is 3.0-20.0mum. LiMaOb is a composite oxide lithium ion conductor coating and is uniformly distributed on the surface of the primary and secondary particle, so the removal and embedding of lithium ions in the battery charge and discharge process are promoted, and the side reactions of the lithium nickel cobalt aluminate material and an electrolyte can be inhibited. The above lithium ion battery positive electrode material has the advantages of high energy, high safety and long cycle life.
Owner:HUBEI RONGBAI LITHIUM BATTERY MATERIAL CO LTD
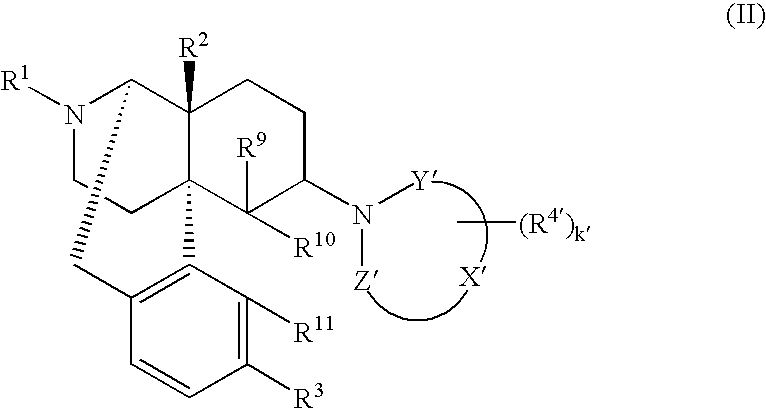
Remedies or preventives for urinary frequency or urinary incontinence and morphinan derivatives having nitrogen-containing heterocyclic group
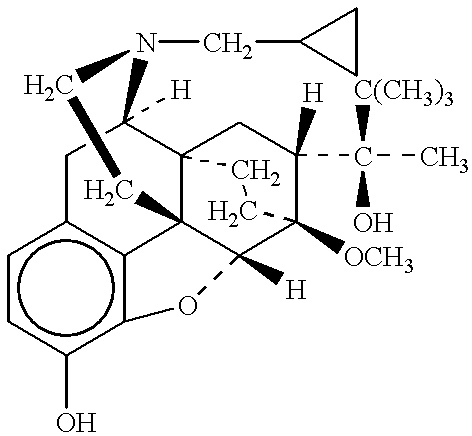
InactiveUS20060040970A1Efficient processImprovement of side effectsBiocideOrganic chemistryAdditive ingredientMorphine
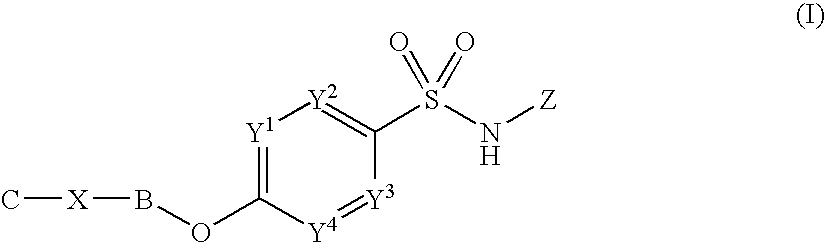
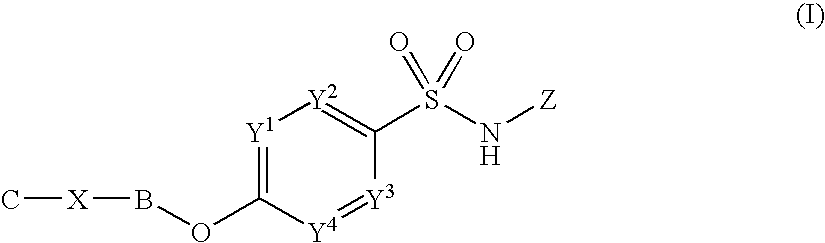
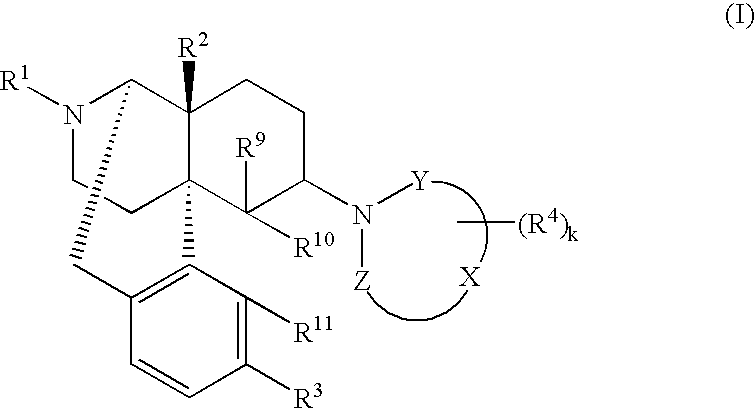
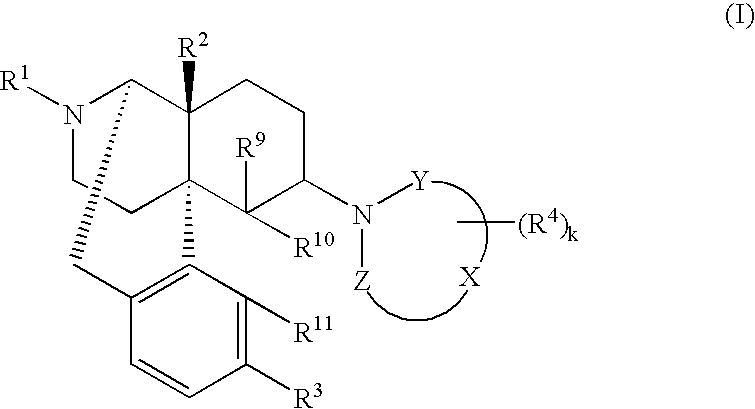
The invention provides a morphinan derivative of the Formula (I): (wherein R1 is methyl, cyclopropylmethyl or the like; R2 and R3 are hydroxy, methoxy, acetoxy or the like; both Y and Z are valence bonds, —C(═O)— or the like; X is C2-C5 carbon chain (one of the carbon atoms may be substituted by oxygen, sulfur or nitrogen) constituting a part of the ring structure, or the like; (R4)k is substituted or non-substituted benzene fused ring, carbonyl group or the like; R9 is hydrogen or the like; R10 and R11 are bound to represent —O—, or the like, and R6 is hydrogen or the like) or a pharmaceutically acceptable acid addition salt thereof. The invention also provides a therapeutic or prophylactic agent for urinary frequency or urinary incontinence, comprising as an effective ingredient the morphinan derivative or the pharmaceutically acceptable acid addition salt thereof; a method for therapy or prophylaxis of the diseases.
Owner:TORAY IND INC
Cancer pain-relieving plaster
InactiveCN103705897ARelief the painRelieve painInorganic boron active ingredientsHydroxy compound active ingredientsCentipedeSteeping
The invention discloses a cancer pain-relieving plaster prepared by a preparation A and a preparation B, wherein the preparation A is prepared by five branches, five skins, one brick, one stone, five cores, and the like; the preparation B is prepared by poria cocos, pinellia ternate, jackinthepulpit tuber rhizome, trichosanthes kirilowii maxim, centipede, zaocys dhumnade, and the like. When the cancer pain-relieving plaster is prepared, the preparation B is added into the preparation A to be decocted for 4.5-5.5 hours, red lead is added to be mixed to pasty fluid, then cold water is added for steeping to releasing fire-toxin, water is changed once one day, and the plaster is obtained after steeping for 7 days. When the plaster is used, the plaster is coated on cotton cloth or plaster paster. The cancer pain-relieving plaster mainly aims at tumour cancers of lung cancer, gastric cancer, liver cancer, pancreatic cancer, colon caner, rectal caner, kidney cancer, bladder caner, breast cancer, cervical cancer, ovarian cancer, esophagus cancer, laryngeal cancer, and the like and is obvious in pain-relieving effect.
Owner:陈延玲 +1
Combination therapy for effecting weight loss and treating obesity
InactiveUS7674776B2Improve side effectsAmeliorating sleep apneaBiocideMetabolism disorderSYMPATHOMIMETIC AGENTSCombined Modality Therapy
The present invention features a novel therapy for effecting weight loss which involves treating a subject with a sympathomimetic agent (e.g., phentermine or a phentermine-like drug) in combination with an anticonvulsant sulfamate derivative (e.g., topiramate) such that the subject experiences weight loss.The combination methods of the present invention also are effective against symptoms associated with Syndrome X. The invention also features pharmaceutical compositions and kits for use in the practice of these novel therapies.
Owner:VIVUS
Orally disintegrating tablet
InactiveUS20130273157A1Avoid breakingControl releaseBiocideDigestive systemLansoprazoleControlled release
A orally disintegrating tablet is obtained by tableting fine granules showing controlled release of lansoprazole and an additive, which is capable of suppressing breakage of the fine granules during tableting, and can control the release of lansoprazole for a long time, and can maintain a therapeutically effective concentration for a prolonged time, and shows superior disintegration property in the oral cavity.
Owner:TAKEDA PHARMA CO LTD
Treatment of chronic obstructive pulmonary disease with nebulized beta 2-agonist or combined nebulized beta 2-agonist and anticholinergic administration
InactiveUS20110132355A1Good curative effectExtended durationDispersion deliverySolution deliveryMuscarinic antagonistAnticholinergic agents
Inhalation solutions for administration of beta 2-agonists or combinations of muscarinic antagonists and beta 2-agonists for the treatment of breathing disorders, such as COPD, are provided. The inhalation solutions are administered by nebulization, particularly with a high efficiency nebulizer.
Owner:SUNOVION RESPIRATORY DEV
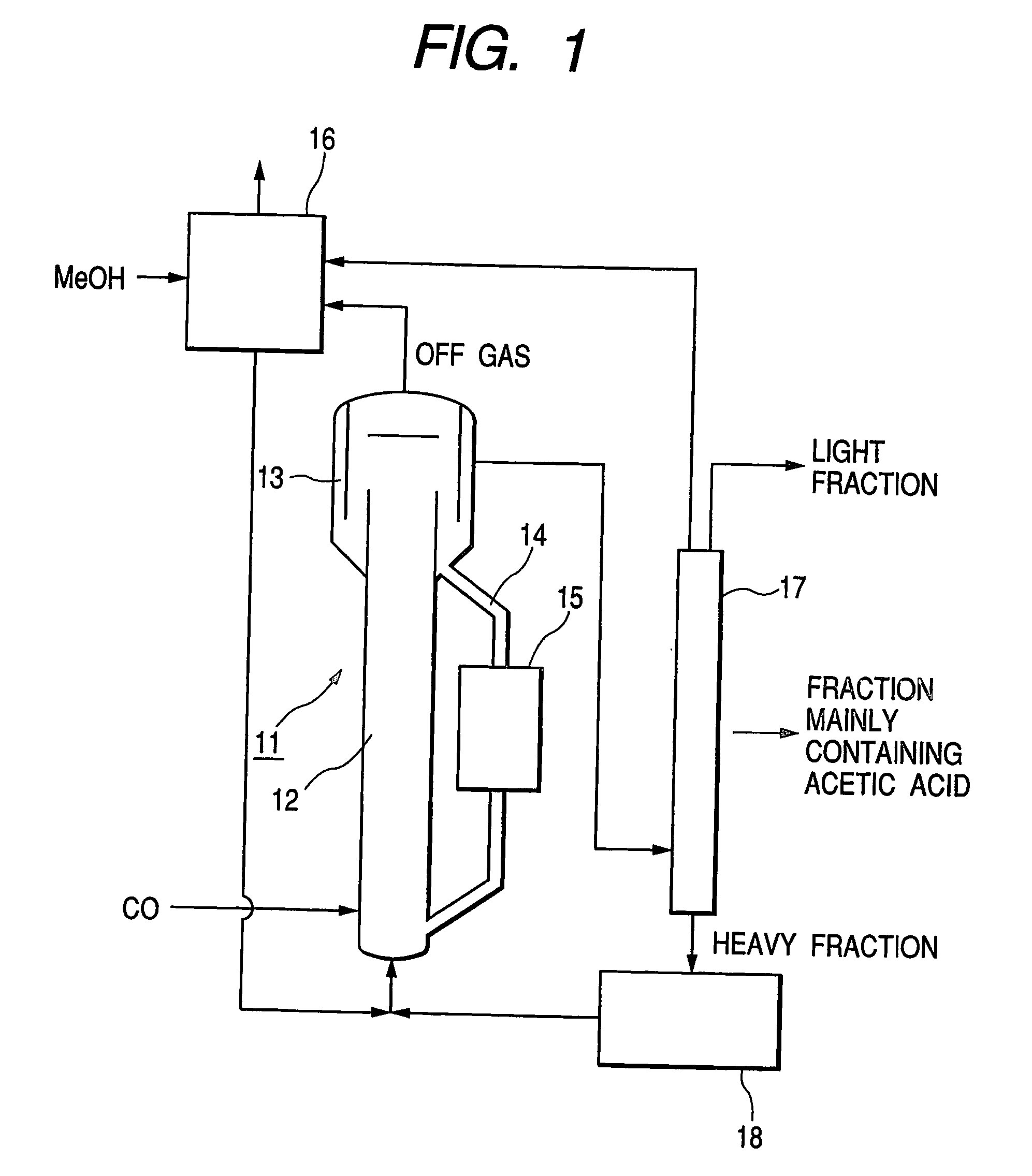
Method of manufacturing acetic acid
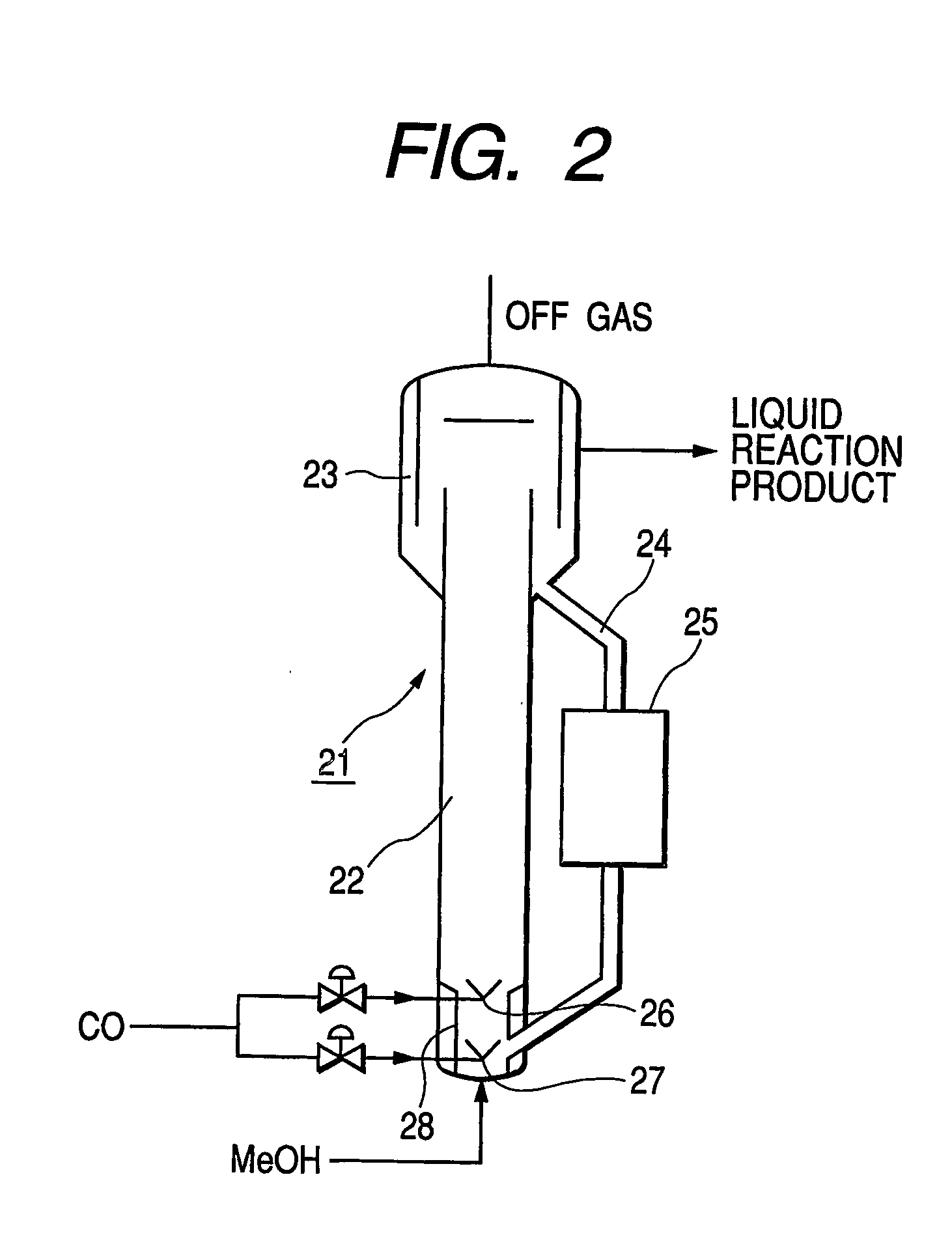
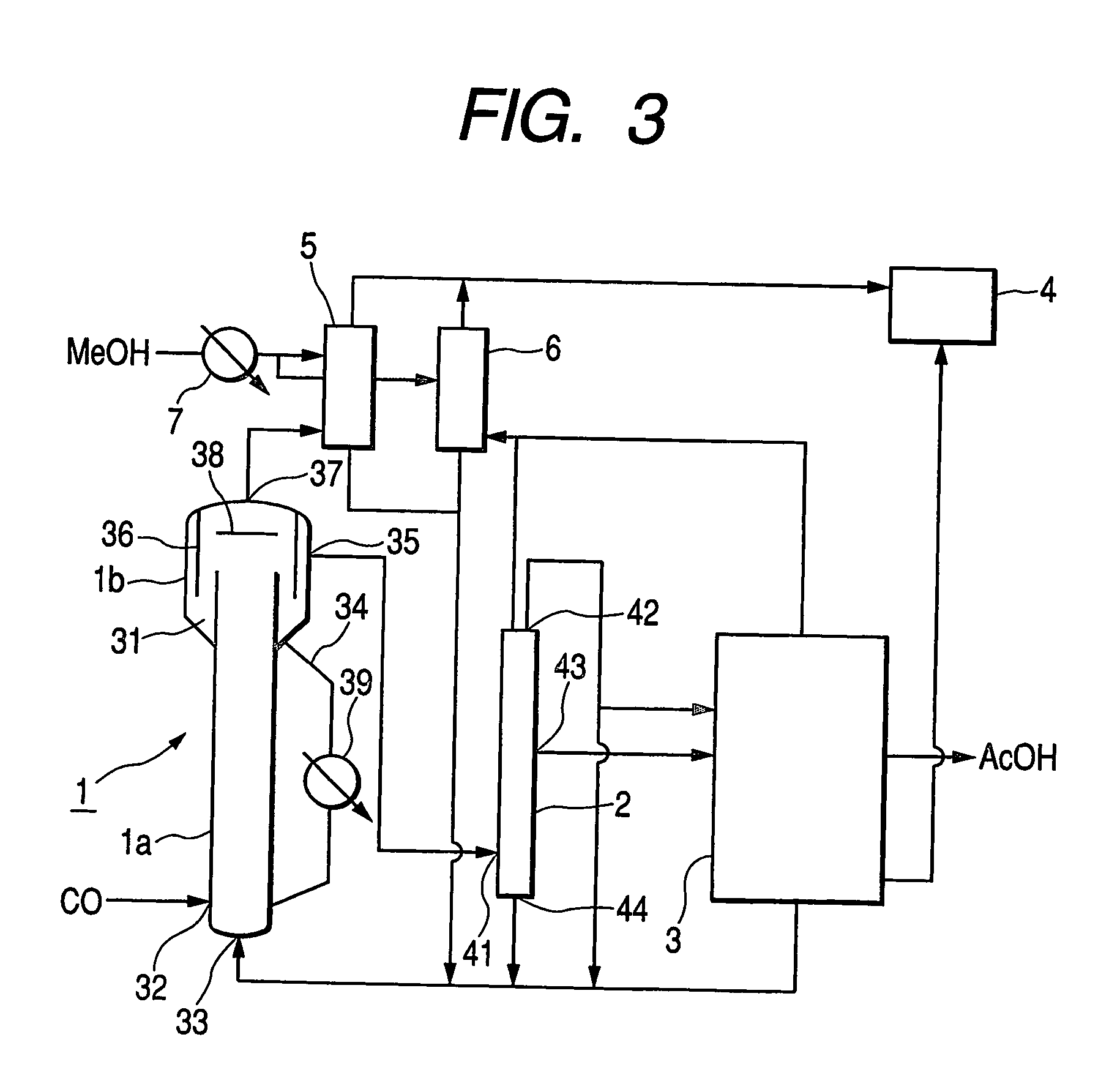
ActiveUS20060281944A1Decrease productivityImprove side effectsOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsAcetic acidCarbonylation
Acetic acid is manufactured by carbonylating methanol with carbon monoxide by way of a heterogeneous catalytic reaction in a bubble column reactor. The carbonylating reaction is conducted with a solid catalyst concentration of not less than 100 kg / m3 in terms of the reaction volume. For the reaction, the partial pressure of carbon monoxide in the reactor is confined to a range between 1.0 and 2.5 MPa while the exhaustion ratio of carbon monoxide is confined to a range between 3 and 15% of the theoretical reaction volume of carbon monoxide and the liquid superficial velocity is made to be found in a range between 0.2 and 1.0 m / sec.
Owner:KELLOGG BROWN & ROOT LLC
Combination therapy for effecting weight loss and treating obesity
InactiveUS7553818B2Improve side effectsAmeliorating sleep apneaBiocideCarbohydrate active ingredientsSYMPATHOMIMETIC AGENTSCombined Modality Therapy
The present invention features a novel therapy for effecting weight loss which involves treating a subject with a sympathomimetic agent (e.g., phentermine or a phentermine-like drug) in combination with an anticonvulsant sulfamate derivative (e.g., topiramate) such that the subject experiences weight loss.The combination methods of the present invention also are effective against symptoms associated with Syndrome X. The invention also features pharmaceutical compositions and kits for use in the practice of these novel therapies.
Owner:VIVUS
Combination therapy for effecting weight loss and treating obesity
InactiveUS7659256B2Improve side effectsAmeliorating sleep apneaBiocideCarbohydrate active ingredientsSYMPATHOMIMETIC AGENTSSympatholytic Drugs
Owner:VIVUS
Preparation method of positive pole piece containing positive electrode material coated by electricity and lithium conducting composite material and lithium-ion battery
ActiveCN110148709AEasy transferImprove performanceSecondary cellsPositive electrodesCross-linkPole piece
The invention provides a preparation method of a positive pole piece containing a positive electrode material coated by an electricity and lithium conducting composite material, and belongs to the technical field of lithium-ion batteries. The preparation method comprises the steps of uniformly stirring a vinyl sulfonic acid monomer, a cross-linking agent, an initiator and a solvent, then adding apositive electrode material, removing the solvent after impregnation, reacting for 10h at a temperature of 90 DEG C to obtain a positive electrode material coated by a cross-linked polymer; preparinga mixed solution by lithium salt and a low-boiling-point solvent, adding the positive electrode material coated by the cross-linked polymer, removing the low-boiling-point solvent until sulfonate or sulfonic acid cations in a coating layer of the positive electrode material is / are converted into lithium sulfonate structure to obtain a positive electrode material coated by a lithium sulfonate cross-linked polymer electrolyte; and uniformly mixing the positive electrode material coated by the lithium sulfonate cross-linked polymer electrolyte, a binder, a lithium conducting material and a conductive agent, and then forming a positive pole piece after coating and drying. The positive electrode material has lithium ion and electricity conducting properties, and improves the performances of thelithium-ion batteries.
Owner:ZHUHAI COSMX BATTERY CO LTD
Low-side-lobe beam pattern integrated design method based on convex optimization
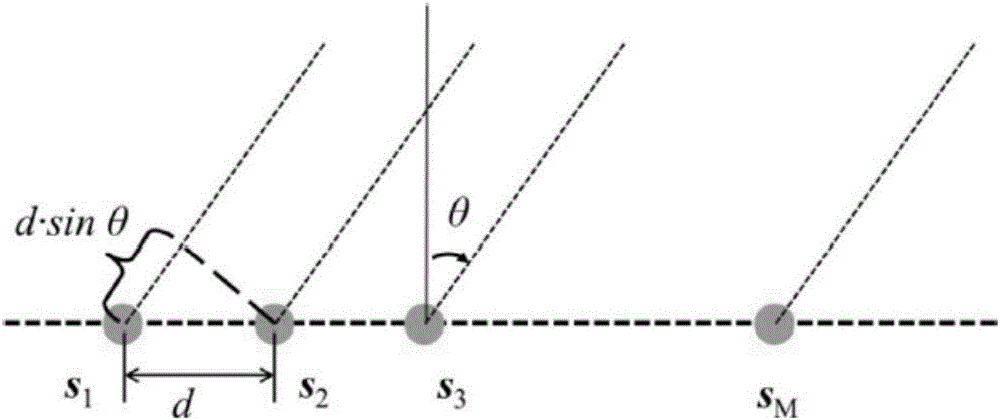
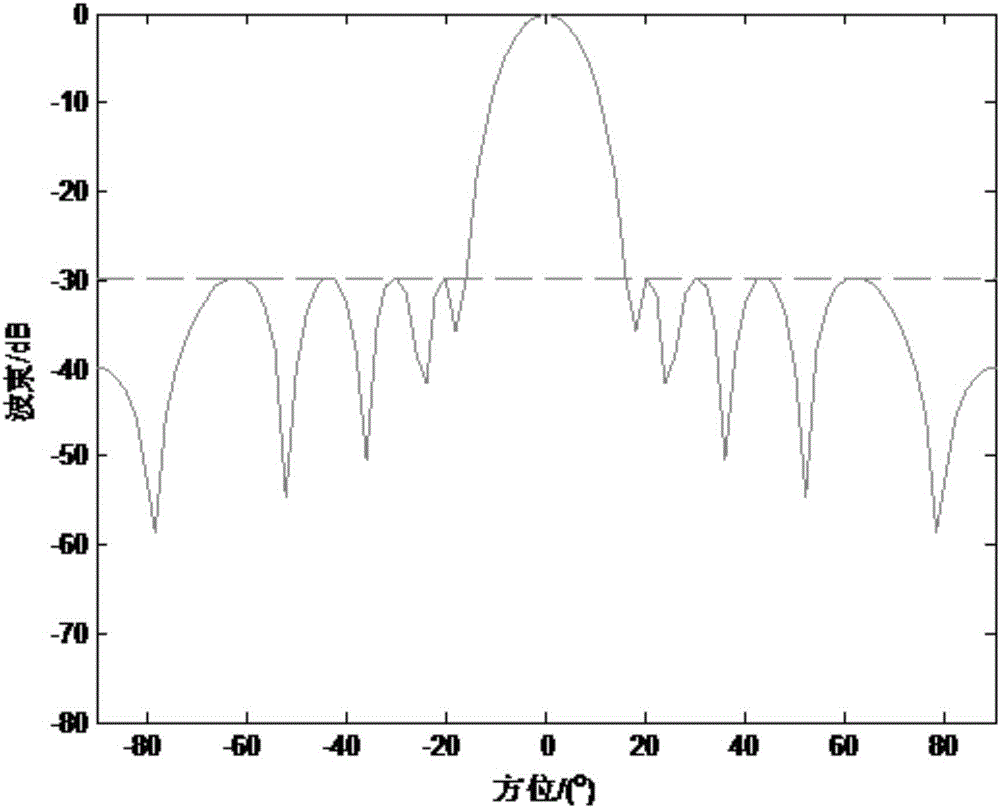
ActiveCN106682405AImprove robustnessReduce sidelobeSpecial data processing applicationsSide effectBeam pattern
The invention discloses a low-side-lobe beam pattern integrated design method based on convex optimization. Auxiliary variables are introduced to achieve variable separation by adopting an alternative direction multiplier method (ADMM), a large amount of original inequality constraint limits are converted into solvable problems, iteration solution is performed by applying an ADMM thought, and accordingly parameters are determined to obtain an ideal wave beam, namely side-lobe values are reduced. The robustness of a wave beam forming device is good in the process by optimizing wave beam side lobes, the side effect of the side lobes on array gains is low, the obtained side lobe values are low, and the low-side-lobe beam pattern integrated design method is simple and simple in operation and has a very good practical value.
Owner:NORTHWESTERN POLYTECHNICAL UNIV
Use of lotus procyanidin as advanced glycosylation end product formation inhibitor
InactiveCN103070400AHigh inhibition rateEnhanced inhibitory effectMilk preparationFood preparationAdditive ingredientAdduct
The present invention discloses a use of lotus procyanidin as an advanced glycosylation end product formation inhibitor. The drug composition adopting the lotus procyanidin as a main active component comprises the following nature active component extracts, by weight, 70-80% of lotus procyanidin, 10-20% of a synergist such as VE, VC, epigallocatechin gallate (EGCG), cysteine and other ??complexes, and 5-10% of other nature extracts such as a lotus leaf extract, a ginkgo leaf extract and the like. According to the present invention, a high performance liquid chromatography method is adopted to detect a methylglyoxal removing effect of the lotus procyanidin and the main structure unit thereof (catechin), wherein methylglyoxal is an important intermediate substance during an advanced glycosylation end product formation process; a high performance liquid chromatography / multi-stage mass spectrometry method is adopted to identify adducts of four catechin and methylglyoxal and adducts of nine lotus procyanidin and methylglyoxal; and results show that lotus procyanidin provides good inhibition effects for advanced glycosylation end product formation in simulated physiological environments and simulated food systems.
Owner:HUAZHONG AGRI UNIV
Rose enzyme and preparation technology thereof
The invention discloses a rose enzyme and a preparation technology of the rose enzyme. The rose enzyme comprises the following components by weight ratio: 80-100kg of water, 25-30kg of rose, 8-10kg of brown sugar or honey and 5-8kg of traditional Chinese medicine auxiliary material; the preparation technology of the rose enzyme utilizes pure natural rose petals and the traditional Chinese medicine auxiliary material to produce the enzyme by natural fermentation; the preparation method of the rose enzyme is simple, easy to operate and short in fermentation period; the rose enzyme is good in mouth feel, can be easily absorbed by a human body, is free from toxin, harm and side effects for the human body, does not contain any additive, preservative and hormone, and enables a user not to worry about getting fat and having dependence on the product; the food is high in safety, the nutrient content of the rose enzyme is not reduced, and the rose enzyme also has good functions of health care and treatment for gynecological diseases; the rose enzyme is better in health care effect, can be eaten for a long time and is an excellent health care product for women; the rose enzyme not only can be produced on a large scale, but also can be widely used in families.
Owner:QUJING DEV ZONE ZHUOCHENG BIOLOGICAL TECH DEV
Medicinal composition for treating diabetes mellitus and application thereof
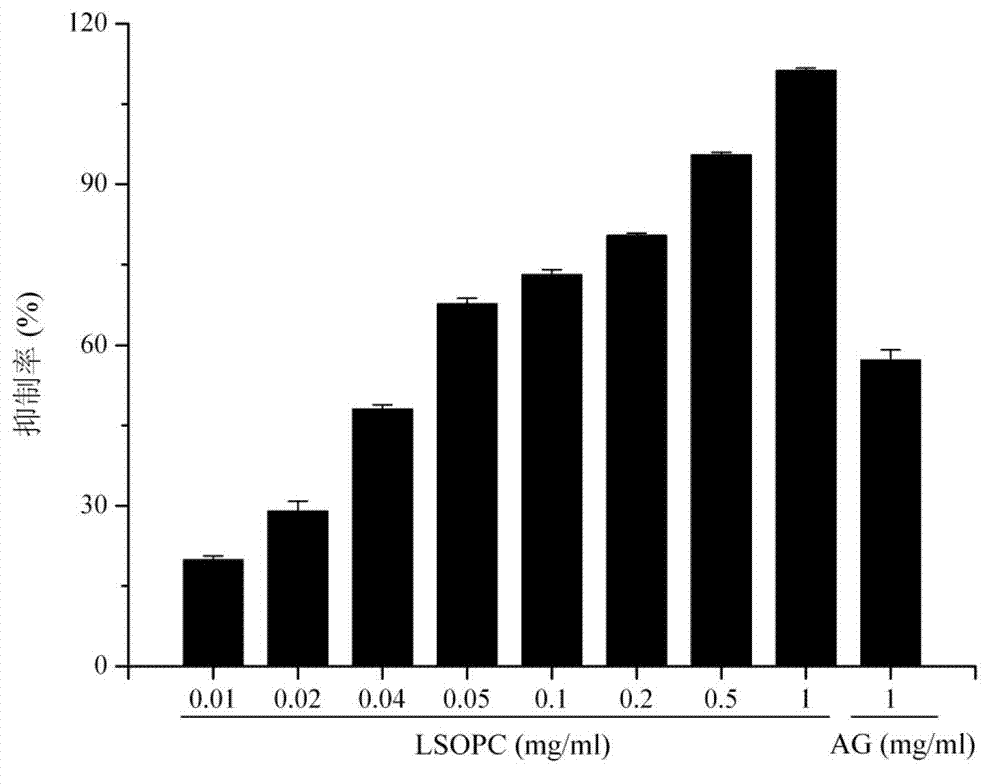
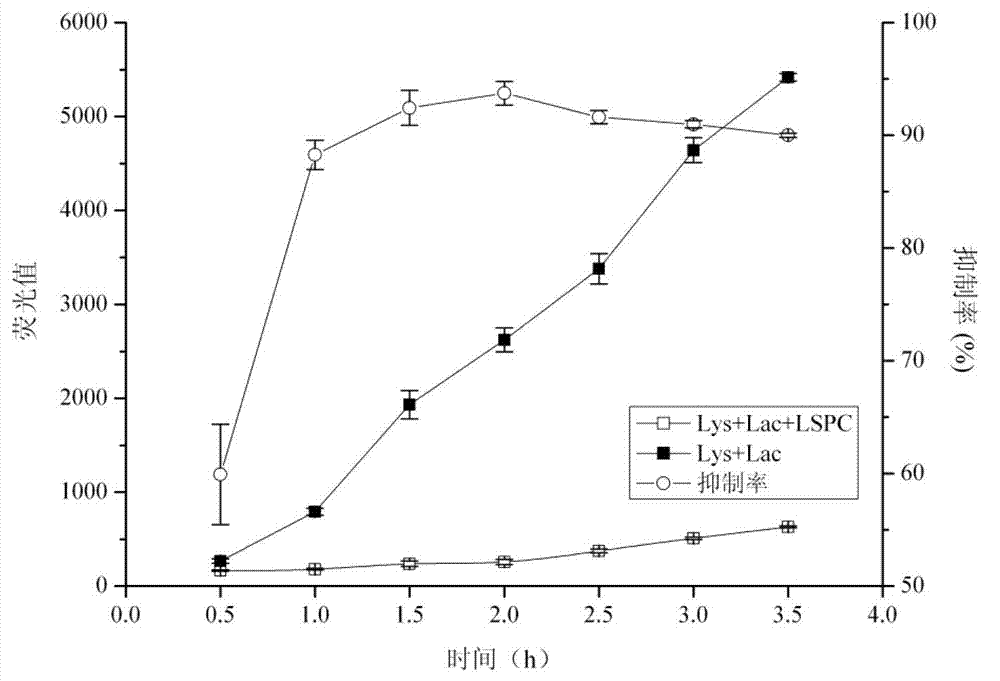
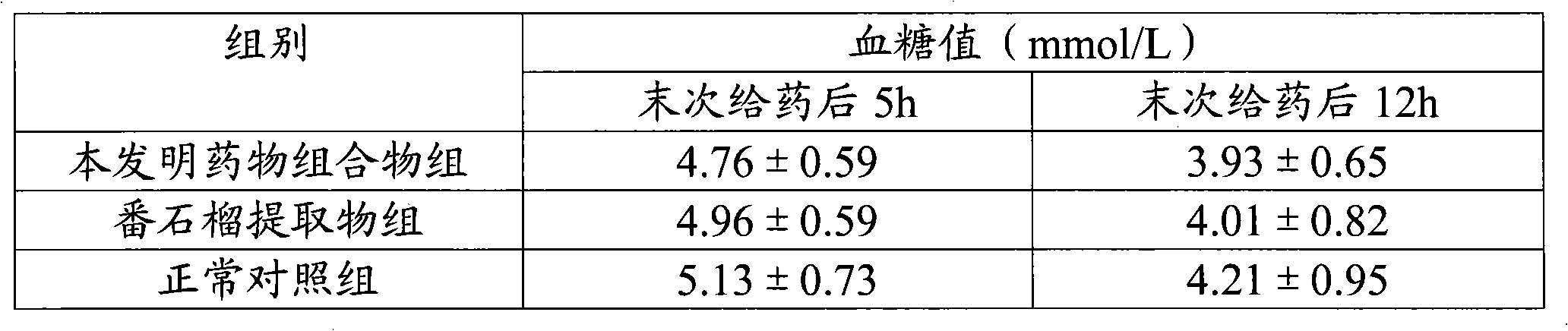
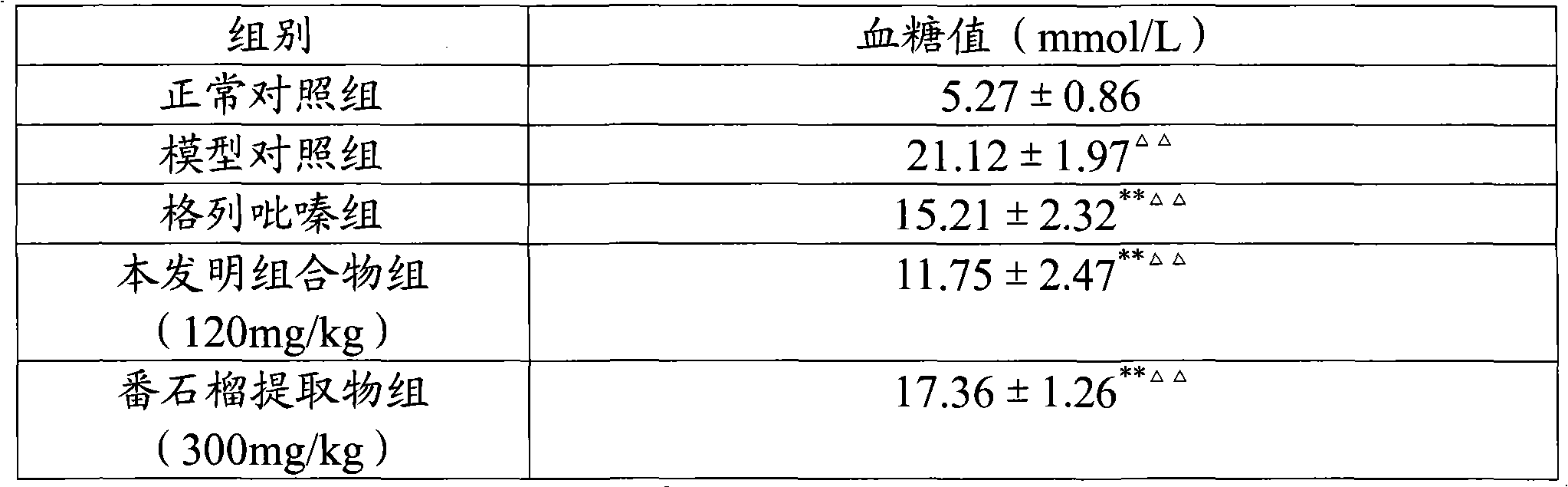
ActiveCN101940620AGood curative effectReduced responseHeavy metal active ingredientsOrganic active ingredientsDiabetes mellitusWestern medicine
The invention relates to a medicinal composition for treating diabetes mellitus and application thereof. The medicinal composition contains 20 to 200 parts of guava by weight, 0.01 to 0.2 part (converted to the weight of chromium) of medicinal compound containing chromium ions by weight, and 0.1 to 2 parts of evening primrose oil weight. The medicinal composition is a compound preparation combined by traditional Chinese herb and west medicine chromium picolinate to ensure that the two medicines can play the effect of synergy, so the medicinal composition not only has the advantages of accurate curative effect and quick response of the west medicine, but also can play the effects of harmonized medicines, compatibility between monarch and ministerial medicines, and abnormal metabolism balancing in human bodies in the traditional Chinese medicinal composition, and has the effect of treating both principal and secondary aspect of diabetes mellitus, hyperlipemia, in particular II-type diabetes mellitus with the hyperlipemia.
Owner:广州市和藤医药研究开发有限公司
Site-specific intestinal delivery of adsorbents, alone or in combination with degrading molecules
ActiveUS20080031867A1Quick releaseQuick effectAntibacterial agentsBiocideIntestinal structureMetabolite
Compositions which deliver adsorbents, alone or in combination with active drug “degrading molecules,” in a site-specific manner to the intestine, and which eliminate or at least lower the concentration of residual unwanted material within the intestine, are disclosed. Methods of treatment using the compositions are also disclosed. The material to be eliminated can include residual active antibiotics, metabolites, bacterial or other toxins, and drugs which cause side effects in the gastrointestinal tract. The adsorbents can be formulated in capsules, tablets or any acceptable pharmaceutical composition, and are ideally designed to specifically release the adsorbents in a programmed manner at a specific site of the intestinal tract. The programmed delivery prevents adsorbents from interfering with the normal absorption process of a given molecule after oral absorption, until it reaches the lower part of the small intestine. The compositions can be used to adsorb, and therefore remove, any residual drug, metabolite thereof, or bacterial toxin after oral or parenteral administration which would otherwise cause adverse effects in the lower intestine and / or colon.
Owner:CENT NAT DE LA RECHERCHE SCI +3
Traditional Chinese medicine composition with estrogen-like action and use thereof
InactiveCN101396421AIt has two-way regulationImprove side effectsSexual disorderPlant ingredientsVasomotor symptomDisease
The invention relates to a composition with the plant estrogen-like effects, and the composition is prepared by the following pharmaceutical raw materials by weight percentage: 12-64 percent eucommia bark and 6-38 percent malaytea scurfpea fruit. The traditional Chinese medicine composition has the plant estrogen-like effects and can bi-directionally regulate the estrogen level of human body, delay the arrival of menopause and be applicable to improving the perimenopausal syndrome of women: menstrual disorders, vasomotor symptoms (flushes and hot flushes), mental and neurological disorders (depression, anxiety, excitability and insomnia), cardiovascular and cerebrovascular symptoms (hypertension, hyperlipidemia, heart palpitations, atherosclerosis, coronary heart disease, senile dementia, and the like), bone and joint symptoms (osteoporosis, arthralgia, and the like), symptoms of urogenital tract and other diseases.
Owner:SHANGHAI UNIV OF T C M
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com