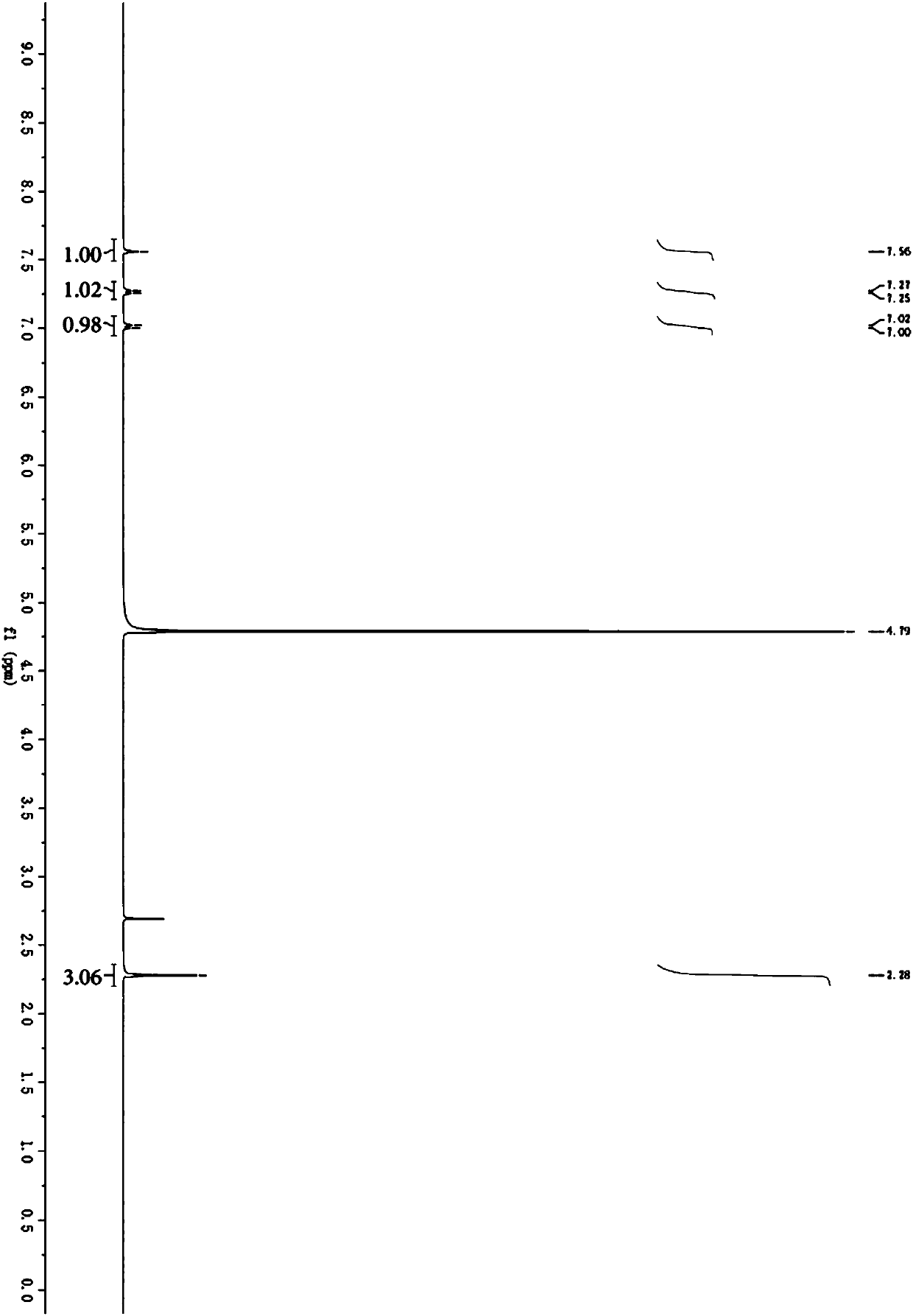
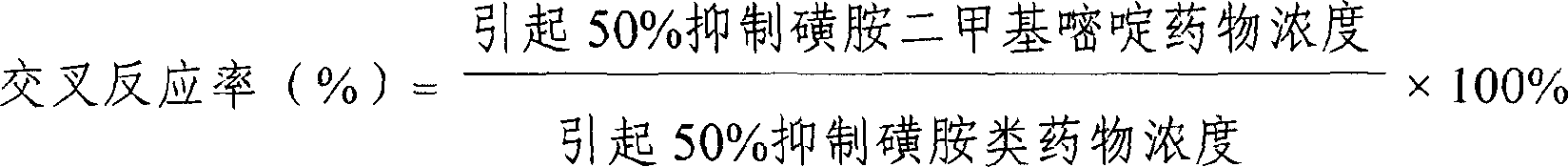Patents
Literature
915 results about "Sulfanilamide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This medication is used to treat vaginal yeast infections.
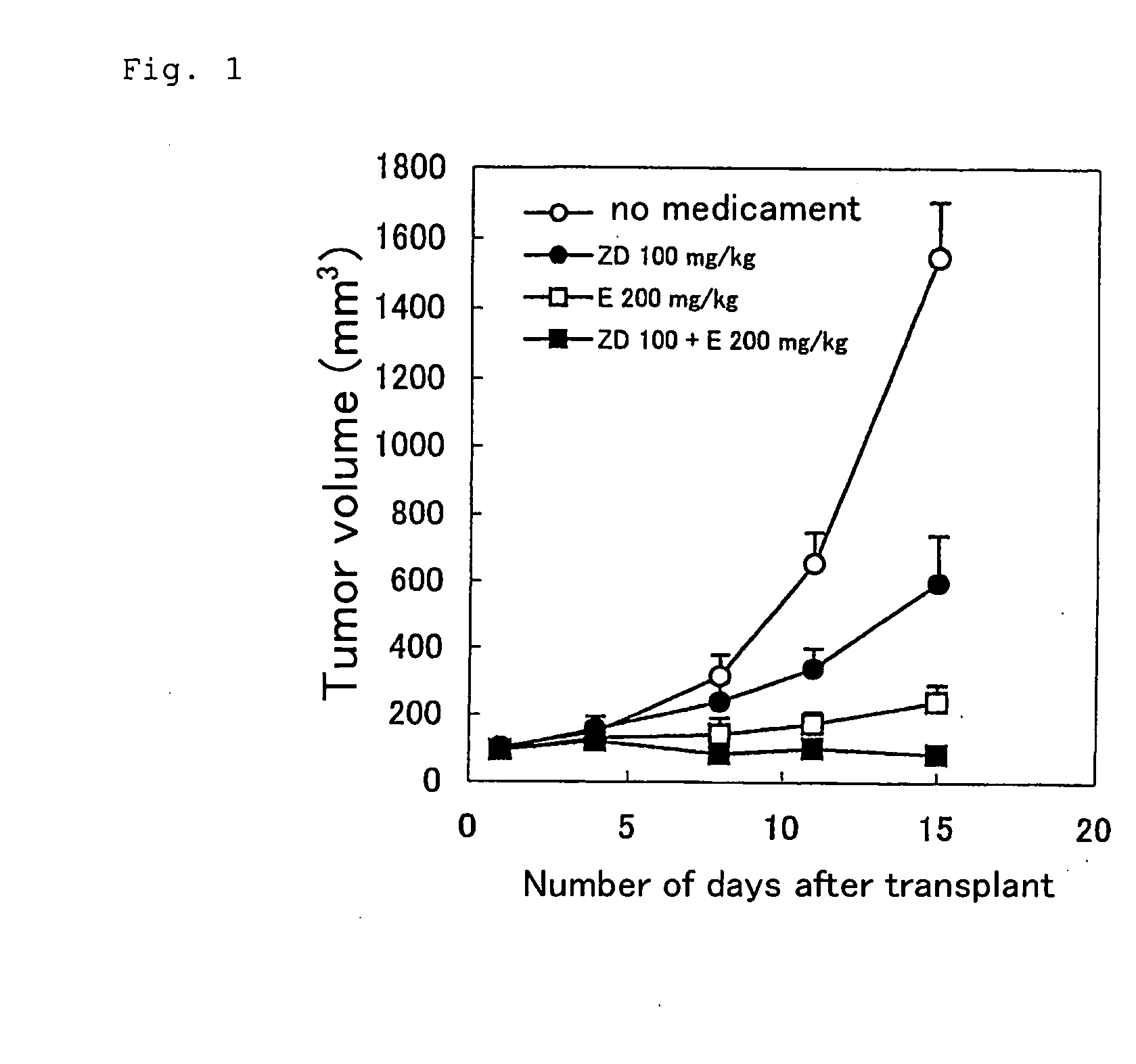
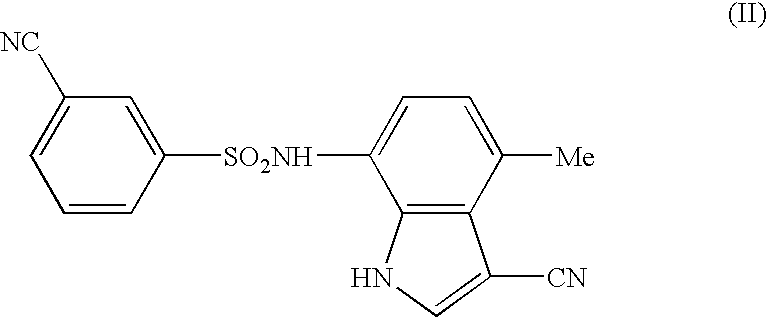
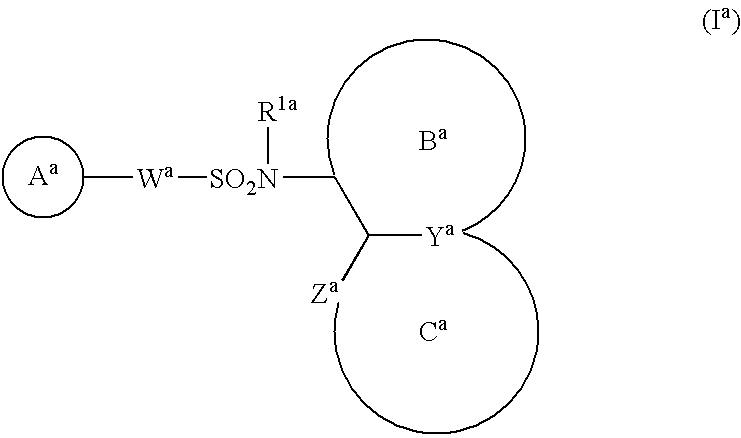
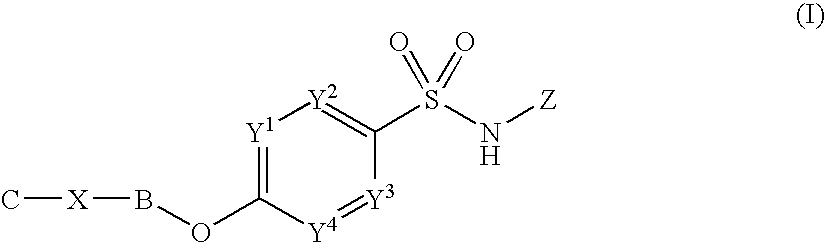
Antitumor agent comprising combination of sulfonamide-containing heterocyclic compound with an angiogenesis inhibitor
InactiveUS20050119303A1Efficient combinationBiocideAnimal repellantsAbnormal tissue growthAngiogenesis growth factor
The present invention provides a composition and a kit for treating tumors, which permits a sulfonamide-containing heterocyclic compound to exhibit its angiogenesis inhibitory activity and antitumor activity more effectively. According to the present invention, the sulfonamide-containing heterocyclic compound can be used in treating cancers more effectively by combination with a VEGF inhibitor / FGF inhibitor.
Owner:EISIA R&D MANAGEMENT CO LTD
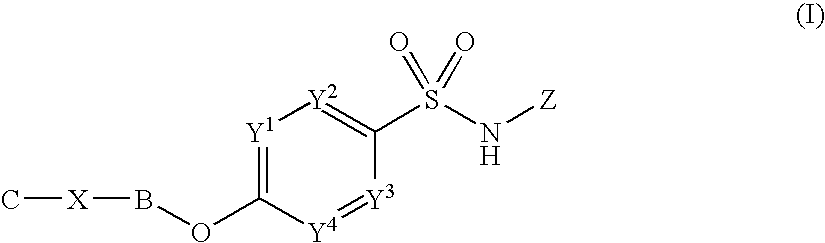
Sulfonamide derivatives
InactiveUS8153814B2Good potencyLow affinityBiocideNervous disorderSulfanilamideCombinatorial chemistry
Owner:PFIZER LTD +1
Method for simultaneously analyzing and detecting residual veterinary drug compositions in animal tissue
InactiveCN103713056AImprove linearitySimple processing and analysisComponent separationSulfur drugSulfanilamide
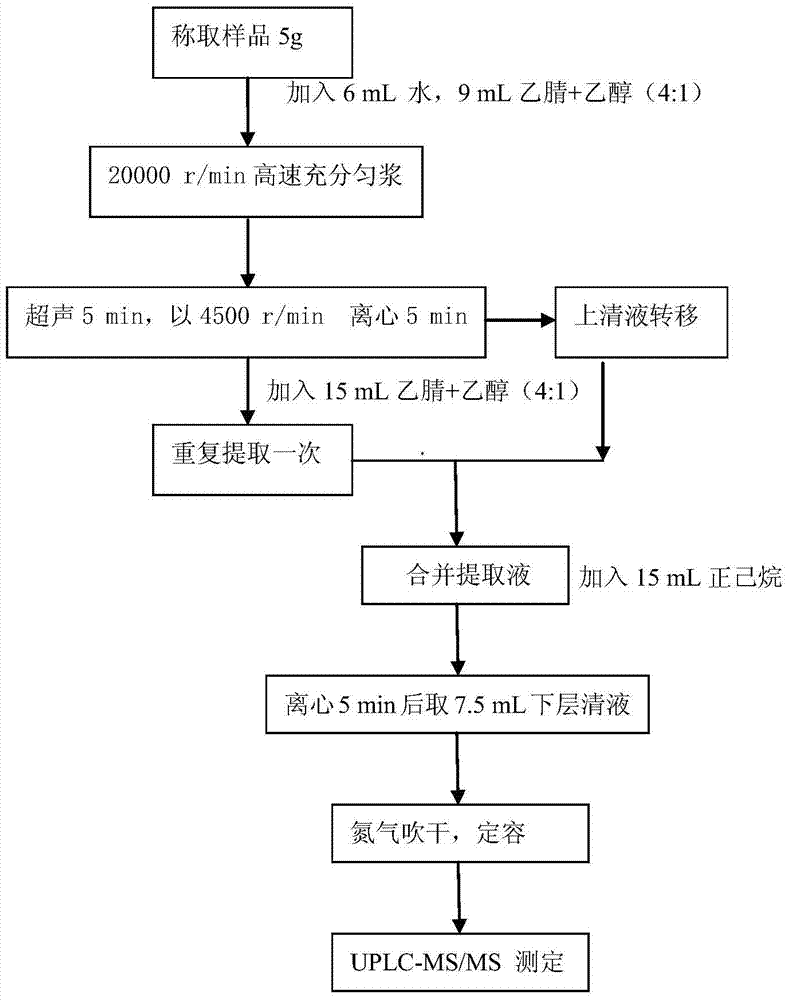
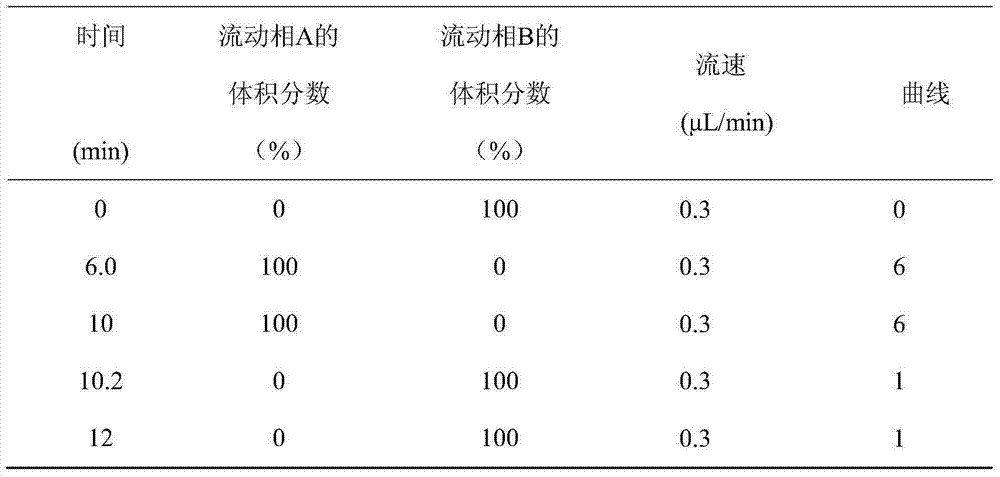
A disclosed method for simultaneously analyzing and detecting residual veterinary drug compositions in animal tissue comprises: performing homogenate on an animal tissue sample by 50% acetonitrile and ethanol, performing ultrasonic extraction and hexane purifying, and performing further precipitation by acetonitrile and ethanol, concentrating, utilizing UPLC-MS / MS to perform multi-reaction monitoring (MRM) determination under the negative ion mode, and quantifying according to a standard curve and an external standard method, detecting and calculating to obtain the content of 46 residual veterinary drug compositions in animal tissue. The method is applicable to present commonly-used veterinary drugs such as 18 kinds of quinolone drugs, 22 kinds of sulfanilamide drugs, dapsone, phenylethanolamine A, amoxicillin, adamantanamine, rimantadine, ethoxyquin and the like, and is capable of performing one-step simultaneous rapid accurate detection, and the application scope of the method is enlarged.
Owner:INSPECTION & QUARANTINE TECH CENT OF NINGBO ENTRY EXIT INSPECTION & QUARANTINE BUREAU
Preparation method of bismuth oxychloride/graphene composite visible light catalyst
InactiveCN104001495AEfficient degradationWater/sewage treatment by irradiationWater contaminantsWater bathsHydrazine compound
The invention discloses a preparation method of a bismuth oxychloride / graphene composite visible light catalyst. The preparation method comprises the following steps of (1) preparing a silvery white pure bismuth oxychloride photocatalyst; (2) preparing 100 ml of graphene oxide solution with the mass concentration of 10 to 60 mg / l, performing ultrasonic dispersion for 1 h, adding 0.2 g of bismuth oxychloride photocatalyst prepared in the step (1), mixing and adsorbing for 2 hours, then adding hydrazine hydrate, uniformly mixing, and then reducing in a water bath at 80 DEG C until the solution uniformly becomes black; (3) naturally cooling the solution to room temperature after reaction, filtering, washing with water and ethanol respectively for three times, and then drying in a constant-temperature drying box at 60 DEG C for 8 hours, so as to prepare the bismuth oxychloride / graphene composite visible light catalyst. The bismuth oxychloride / graphene composite visible light catalyst prepared with the preparation method responds to sunlight, natural sunlight can be utilized to effectively degrade sulfanilamide waste water, and the bismuth oxychloride / graphene composite visible light catalyst has the advantages of stable performance and no toxicity and has a strong market application prospect.
Owner:HENAN NORMAL UNIV
Method for simultaneously measuring various drug residues in honey by utilizing liquid chromatogram tandem mass spectrum isotope dilution method
The invention relates to a method for detecting various drug residues in honey, in particular relating to a method for simultaneously measuring various drug residues in honey by utilizing a liquid chromatogram tandem mass spectrum isotope dilution method. The method provided by the invention comprises the following steps: directly diluting by virtue of a phosphate buffer solution the pH value of which is equal to 8; carrying out HLB (Hydrophile Lipophile Balance) extraction and purification; carrying out measurement by utilizing the liquid chromatogram tandem mass spectrum isotope dilution method (LC-MS / MS); and quantifying by utilizing the internal standard method and external standard method of isotope internal standard dilution; and measuring low-limit sulfanilamide drugs to be 1.0 mu g / kg, nitromidazoles drugs to be 1.0 mu g / kg, carbostyril drugs to be 2.0 mu g / kg, macrolide drugs to be 3.0 mu g / kg, lincosamides drugs to be 2.0 mu g / kg and praziquantel to be 0.3 mu g / kg. The method provided by the invention is simple, convenient and rapid and has the advantages of small resource consumption and low detection cost; the front processing procedure, drug variety and instruments for measurement can be better complementary with the existing method; and the method provided by the invention is suitable for the simultaneous measurement requirements of various drugs in the honey and can provide a powerful technical guarantee for maintaining the food safety and guaranteeing that Chinese honey is successfully exported.
Owner:THE INSPECTION & QUARANTINE TECH CENT ZHEJIANG ENTRY EXIT INSPECTION & QUARANTINE BUREAU
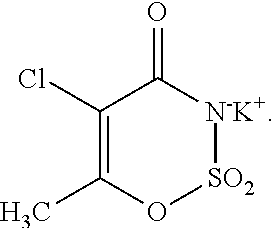
Method for determining N-methylamino ammate in production of acesulfame
ActiveCN103018368AAccurate measurementRapid determinationComponent separationOrganic solventColumn temperature
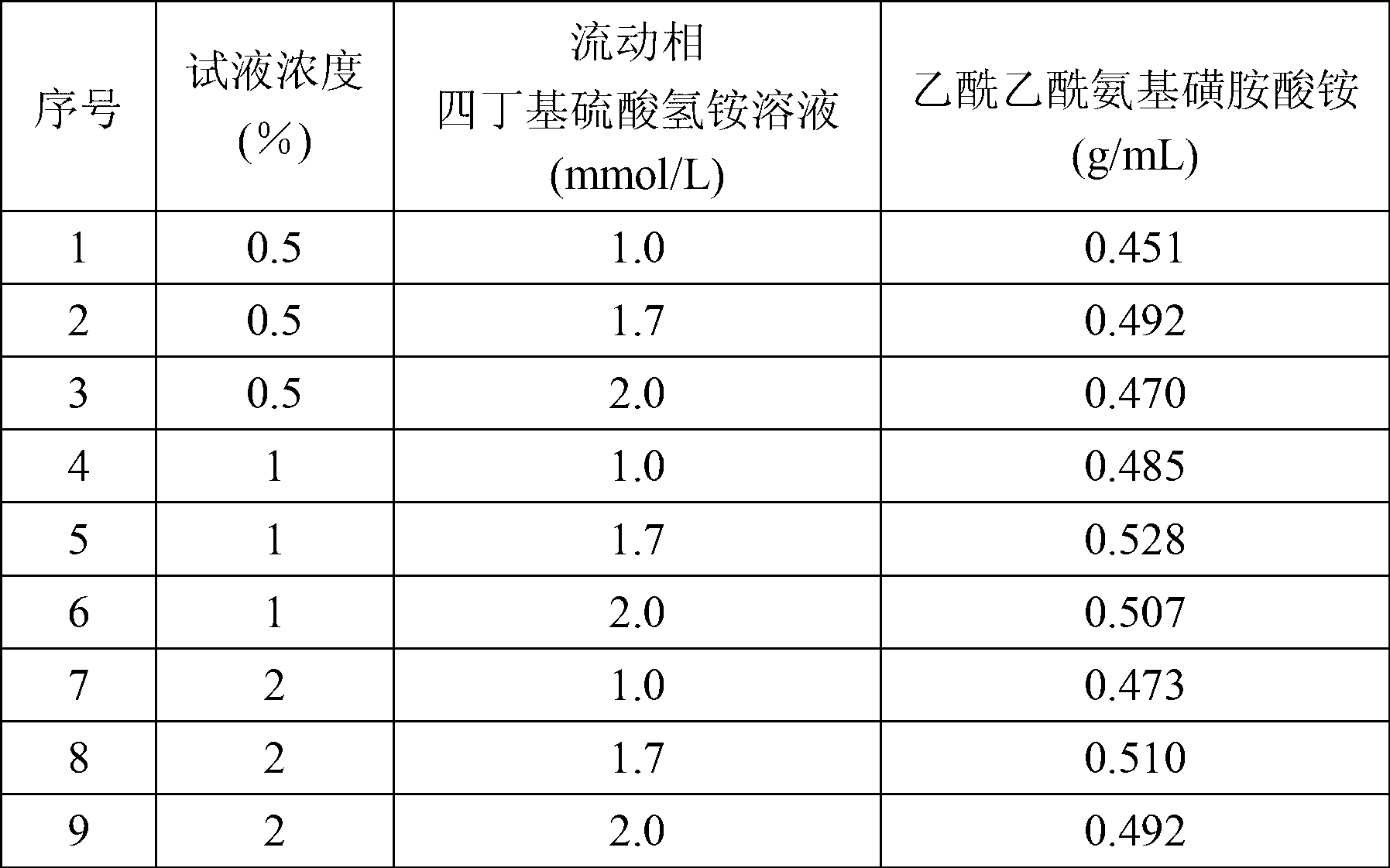
The invention discloses a method for determining N-methylamino ammate in production of acesulfame. The method mainly comprises the steps of: (1) selecting an analytical chromatographic condition, an ODS column (4.6mm*25cm) or other equivalent chromatographic columns, wherein the wavelength is 250nm, the flow velocity is 1ml / min, the column temperature is 25 DEG C, and the sample amount is 20mu l; (2) preparing tetrabutylammonium hydrogen sulfate solution from deionized water until the molar concentration is 1.0-2.0mmol / L, and preparing a mobile phase from the prepared tetrabutylammonium hydrogen sulfate solution and chromatographic grade methanol according to the volume ratio of 60 to 40; (3) diluting the sample to be tested by an organic solvent according to the volume ratio until the concentration is 0.5-2.0%, so as to prepare a test solution; (4) absorbing the test solution by a microinjector, injecting into an efficient liquid chromatograph, and analyzing by an area external standard method; and (5) recording the content of the N-methylamino ammate in the sample to be tested. By adopting the method disclosed by the invention, the content of the N-methylamino ammate can be accurately and rapidly determined.
Owner:南通宏信化工有限公司
Fluorizated cellulose acetate film and preparation method
ActiveCN101716471AEasy to synthesizeEasy to makeSemi-permeable membranesWater/sewage treatment bu osmosis/dialysisCeriumSulfanilamide
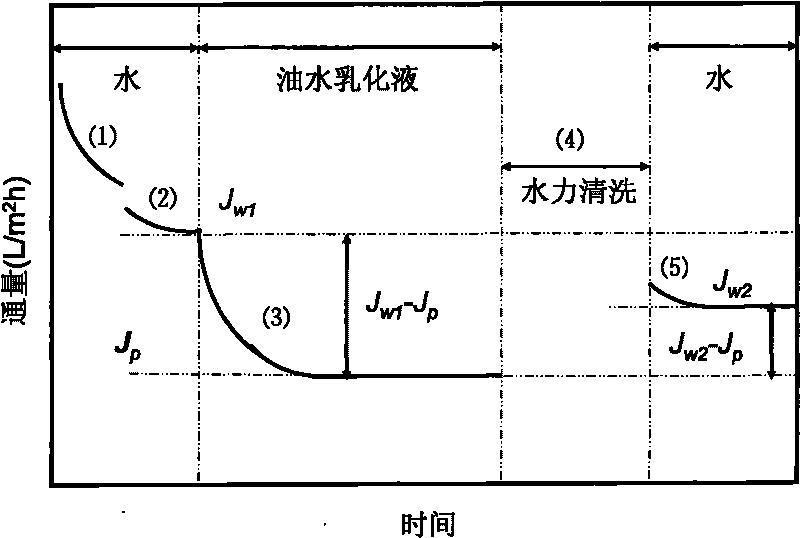
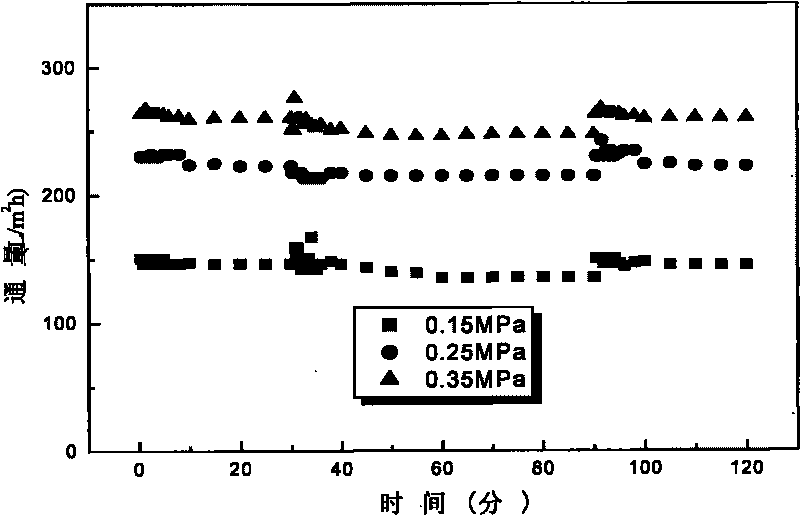
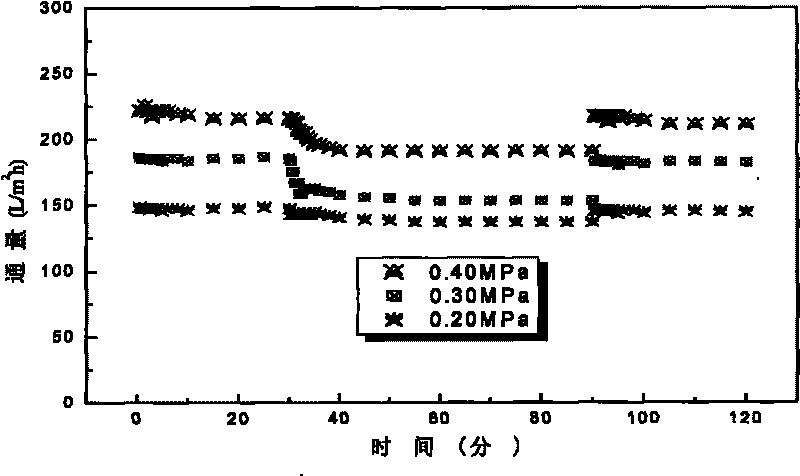
The invention relates to a fluorizated cellulose acetate film and a preparation method. The fluorizated cellulose acetate film is prepared by taking cerium salt as an initiator and grafting hydrophobic and oleophobic fluoroester methacrylic acid (FMA, such as methacrylic acid-12-fluorine heptyl ester G04, methacrylic acid hexafluoro-butyl ester G02 and the like) and hydrophilic polyglycol ester methacrylic acid (PEGMA) or methacrylic acid MAA and sulfanilamide MPDSAH sequentially onto cellulose acetate by a water-phase free-radical interfacial polymerization method; and then, taking the synthesized fluorizated cellulose acetate as a film material and preparing an asymmetrical oil-water separation film with pollution resistance and ultra-low flux depression by a non-solvent initiating phase conversion method. When the fluorizated cellulose acetate film is used for processing oil-water emulsion, the detention rate is as high as 99.8%, the rate of flux depression can be lowered to 3.4%, the water flux can be kept at 247.1L / m2h, and the rate of flux recovery is as high as 100%. The fluorizated cellulose acetate oil-water separation film has superior anti-pollution performance and strong recycle property.
Owner:南通诚恩机械有限公司
Bacteriostatic porous polyelectrolyte material and its prepn process
InactiveCN1810298AStrong initial bactericidal abilityReduce usageAbsorbent padsBandagesPvp iodineFreeze-drying
One kind of porous bacteriostatic polyelectrolyte material suitable for stopping bleeding and dressing wound is disclosed. The material features that it is prepared with chitosan solution in 1-5 wt% concentration and sodium alginate solution 1-5 wt% concentration in the weight ratio of 0.25-4 as material and through mixing and freeze drying. The chitosan solution is prepared through dissolving chitosan in water solution of acetic acid. The material includes also sulfadizine silver or PVP-iodine bacteriostatic agent in the amount of 3-10 wt% of chitosan and sodium alginate. The porous material has no toxicity, relatively great pores, high water absorption rate and short bleeding stopping period.
Owner:WUHAN UNIV OF TECH
Method for detecting residue of seven synthetic antibacterial agents in aquatic products
InactiveCN101625339ARapid determinationSensitive assayComponent separationPreparing sample for investigationSolid phase extractionPyrimethamine / Sulfadiazine
A detection method simultaneously detects residue of multiple synthetic antibacterial agents in aquatic products by a high performance liquid chromatography. The synthetic antibacterial agents mainly comprise sulfadiazine SD, sulfamerazine SMR, sulfadimidine SDD, sulfadimethoxine SDM, furazolidone FZD, oxolinic acid OXA, nalidixic acid NAA and the like. The multiple synthetic antibacterial agents in an aquatic product sample are extracted by acetonitrile, degreased by normal hexane, concentrated, purified by a solid phase extraction column (alumina neutral) or a C18 solid phase extraction column, analyzed by a liquid chromatograph, detected by an ultraviolet detector, and quantified by an external standard method. The method can quickly, sensitively and accurately detect multiple target compounds to greatly reduce detection cost and shorten detection time.
Owner:吴光红 +1
Method for purifying sulfanilamide drug by using molecularly imprinted polymer
InactiveCN102344527AImprove featuresHigh selectivityOrganic chemistryOther chemical processesSulfur drugCross-link
The present invention belongs to the technical field of bioengineering, and relates to a method for purifying sulfanilamide by using a molecularly imprinted polymer of a sulfanilamide drug. The method comprises the following steps: (1) mixing and dissolving template molecules of a sulfanilamide drug mixture, a functional monomer of methacrylic acid and a cross-linking agent of ethylene glycol dimethacrylate in a pore forming agent (a ratio of methanol to acetonitrile is 2:3); adding an initiator, and sealing; (2) crushing the synthesized polymer, removing fine particles, removing the template molecules through extraction; (3) drying the template molecule-removed polymer overnight at a temperature of 60 DEG C to obtain the molecularly imprinted polymer of the sulfanilamide; (4) filling the molecularly imprinted polymer into a solid phase extraction column tube; (5) activating the solid phase extraction column, adding an animal tissue extraction solution or a water sample, wherein the animal tissue extraction solution is dissolved in 40% methanol; then leaching the solid phase extraction column to elute and purify the sulfanilamide. The method provided by the present invention has advantages of high selectivity, high specificity and adverse environment resistance, and has broad application potential.
Owner:SHANGHAI ACAD OF AGRI SCI
Method for detecting sulfamethazine in animal-derived food
InactiveCN103105387AShort detection timeHigh detection sensitivityPreparing sample for investigationRaman scatteringEvaporationSulfanilamide
The invention provides a method for detecting sulfamethazine in animal-derived food. The method comprises the following steps of: synthesizing a molecularly imprinted material; preparing a practical sample; extracting the sulfamethazine from an aquatic product by ethyl acetate; carrying out rotary evaporation on an extracting solution; dissolving the product in a methanol aqueous solution again; performing purification and enrichment by a molecularly imprinted solid phase extraction column; and detecting the sulfamethazine by a Raman enhanced spectrum with combination of shell isolated nanoparticles. The method has the positive effects that a synthesized molecularly imprinted material is high in specificity and can be taken as a filler of the solid phase extraction column and used for separating and gathering target molecules to be detected from a practical sample; the method can be used for detecting the sulfamethazine by Raman enhancement with combination of the shell isolated nanoparticles and is high in detection sensitivity, high in speed, less in sample comsuption and convenient to operate; and the method can be used for rapidly detecting the sulfamethazine in the animal-derived food and can reach the detection sensitivity of 50ppb.
Owner:FUZHOU UNIV
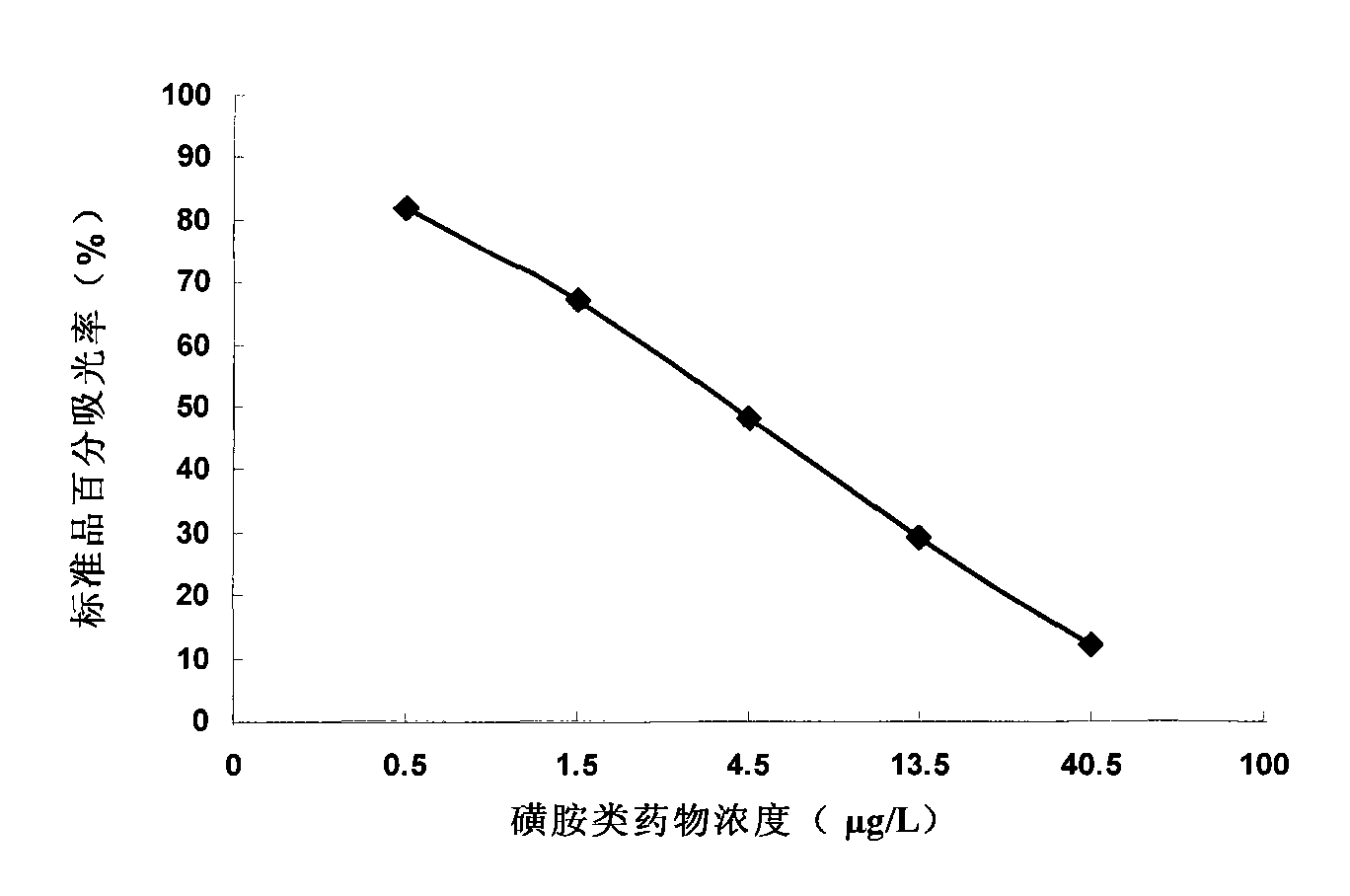
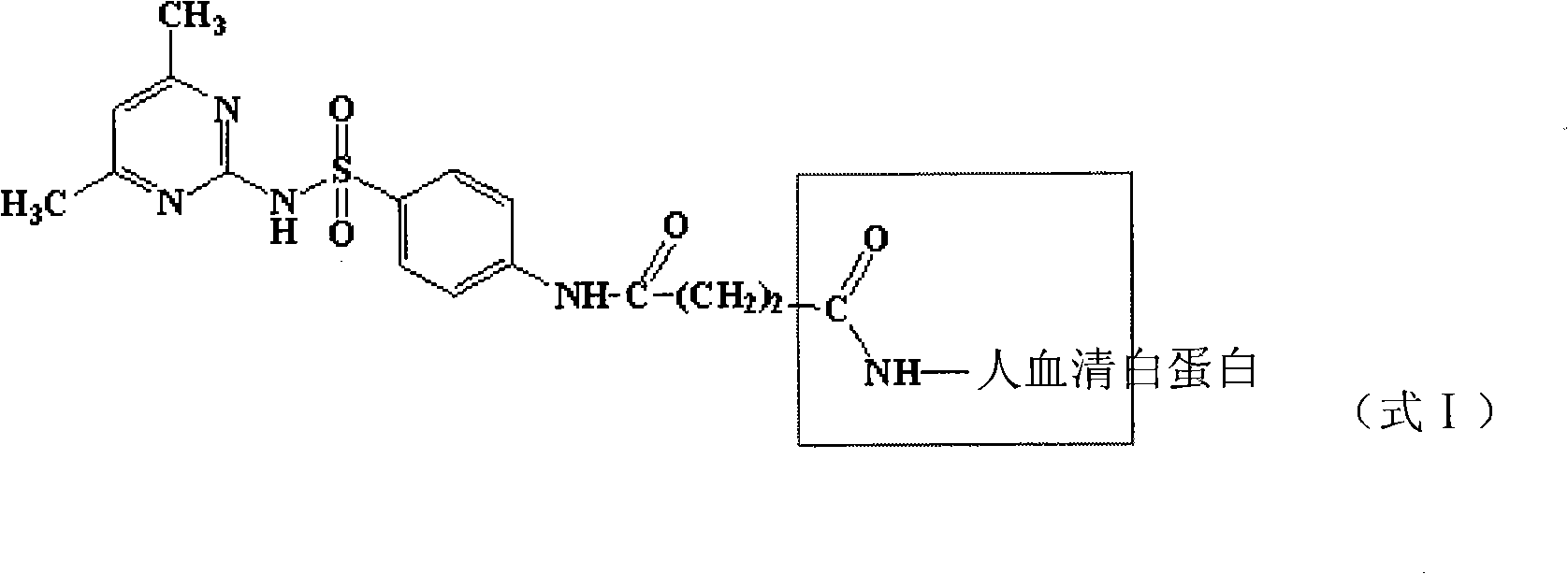
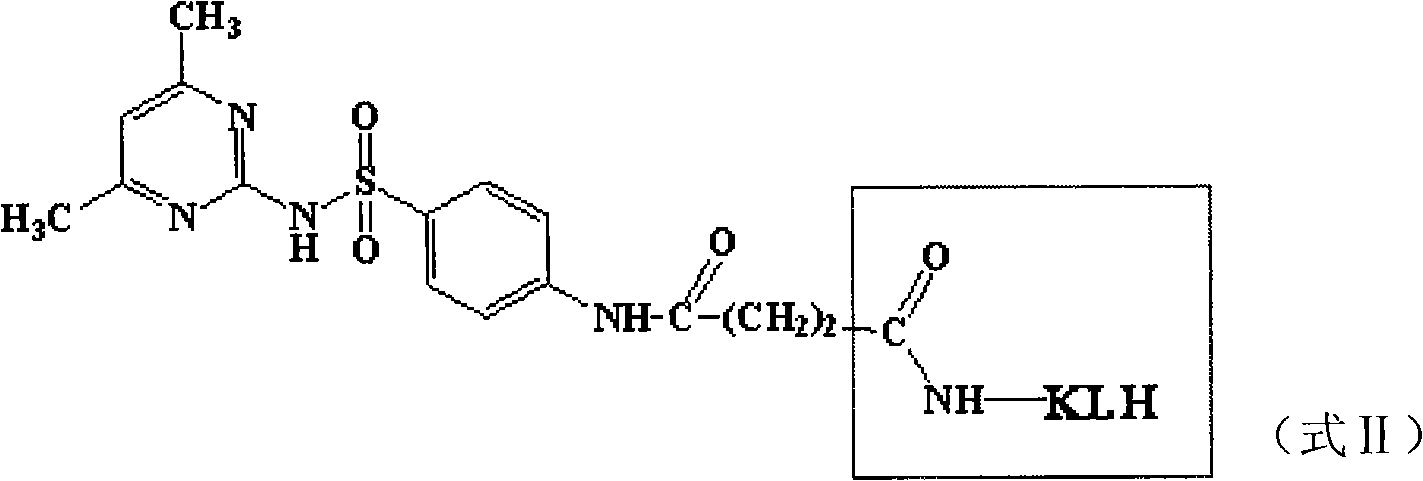
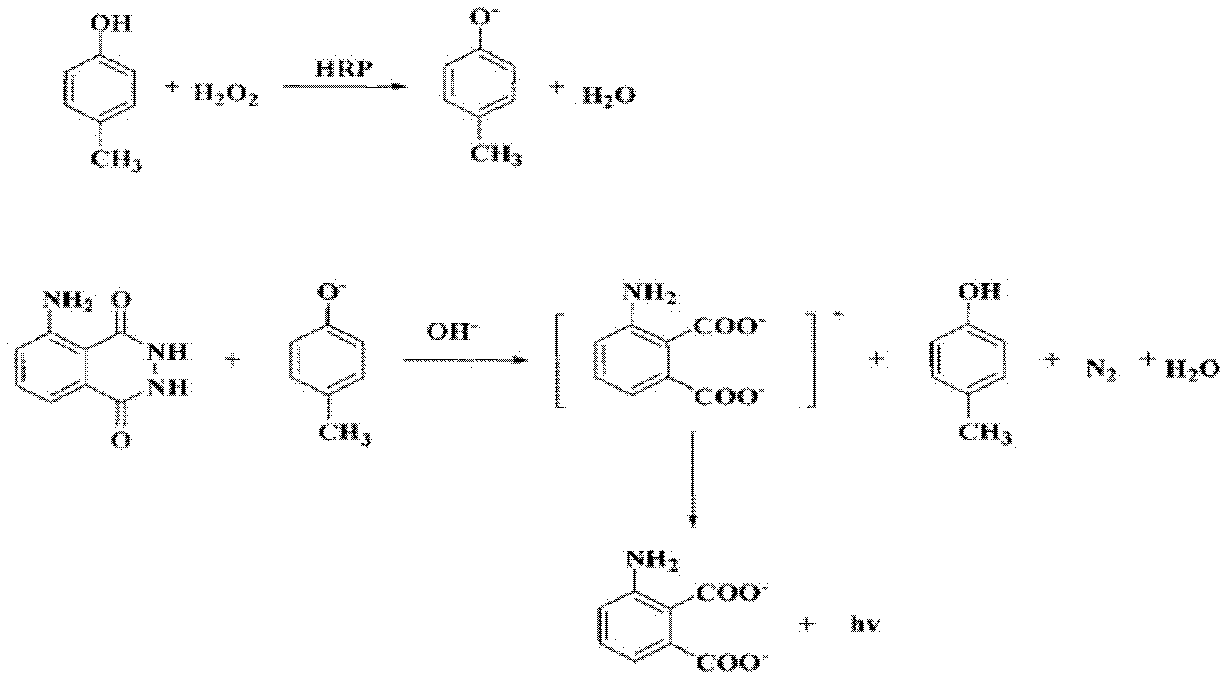
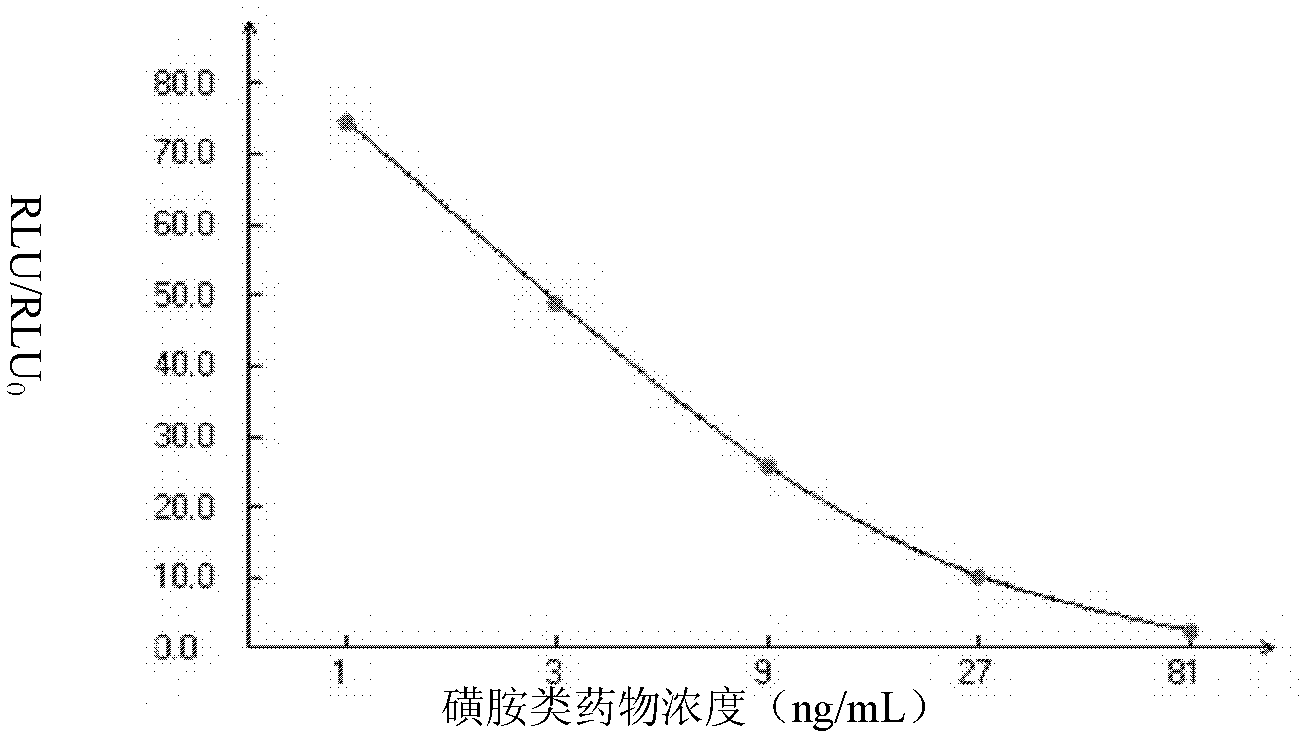
Method for detecting sulfanilamide medicine and special enzyme-linked immunoassay reagent kit thereof
The invention discloses a method for detecting a sulfanilamide medicine and a special enzyme-linked immunoassay reagent kit thereof. The enzyme-linked immunoassay reagent kit comprises a sulfanilamide medicine monoclonal antibody and the sulfanilamide medicine. The sulfanilamide medicine monoclonal antibody is secreted by the hybridoma cell line SAs of a sulfanilamide monoclonal antibody, whose preservation number is CGMCC No. 3393. In the enzyme-linked immunoassay reagent kit of the invention, an indirect competition ELISA (Enzyme-Linked Immuno Sorbent Assembly) method is mainly used for qualitatively or quantitatively detecting the content of the sulfanilamide medicine in products (especially milk, pork, chicken, eggs, honey, fish, shrimp and the like) eaten by animals or people. The reagent kit and the detection method of the invention have low requirements on the pretreatment of samples and simple pretreatment process of the samples and can detect mass samples quickly at the same time. By using the sulfanilamide medicine monoclonal antibody with high specificity, the detection method is convenient and simple, and the invention has the characteristics of high specificity, high sensibility, high precision, high accuracy and the like.
Owner:北京维德维康生物技术有限公司
Topical spray for burn treatment and anti-infection
InactiveUS6987133B2Function increaseReduce disadvantagesBiocideCosmetic preparationsCross-linkAntimicrobial drug
This invention relates to a topical spray preparation for burn treatment and microbial infections on human being or animals. This non-aerosol preparation contains an antimicrobial drug, i.e., silver sulfadiazine, as is dispersed or solubilized in a cream or lotion base matrix which can be sprayed directly from a common trigger spray device. The key component of the matrix can be characterized by it having a suitable molecular weight polymer of cross-linked acrylic acid, such as Carbomers or non-ionic surfactants such as polyoxyethylene alkyl ethers, or any combination of the above materials.
Owner:SAGE PHARMA
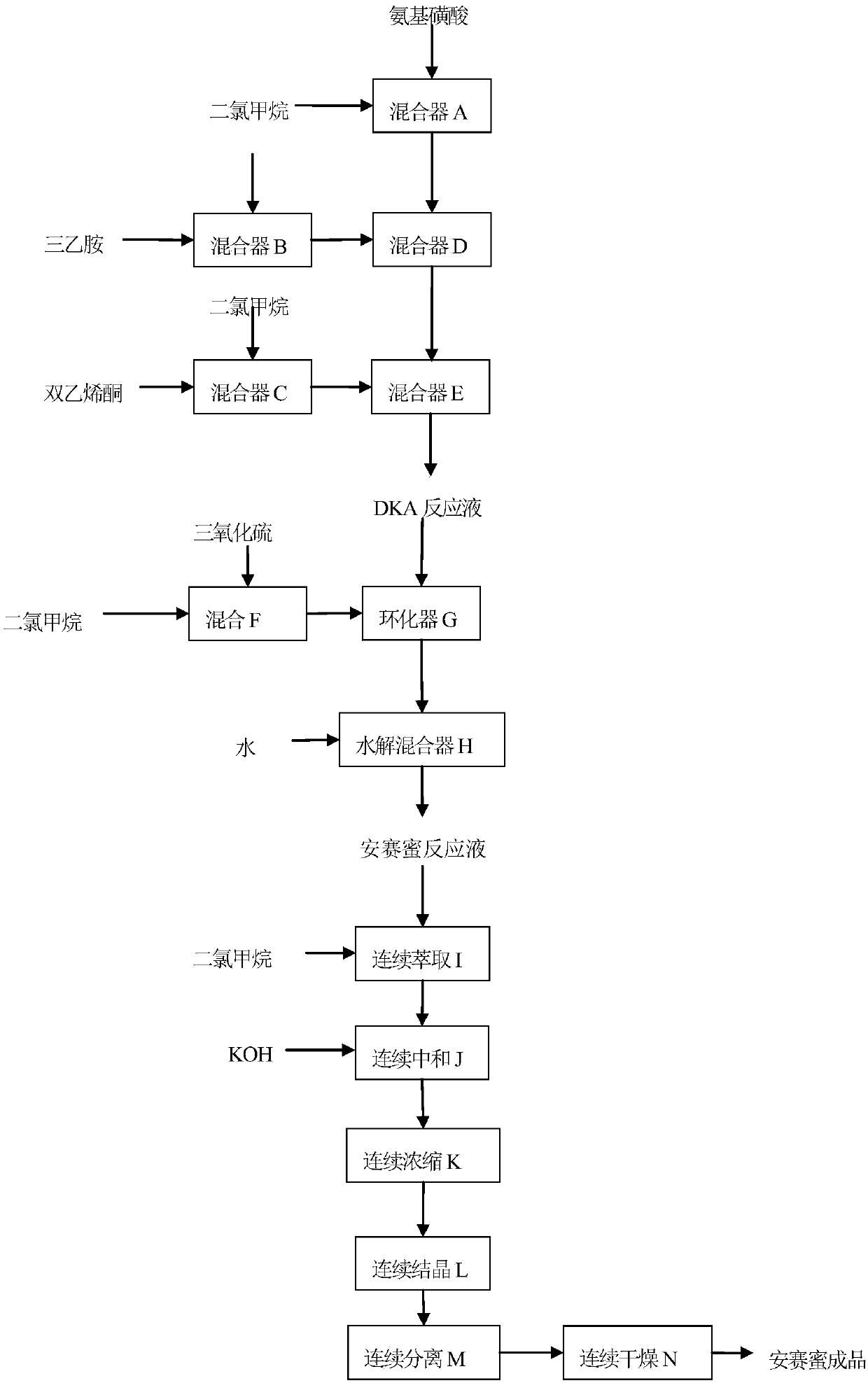
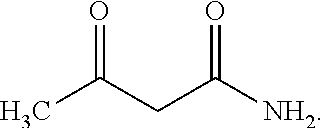
Method for continuously producing acesulfame potassium
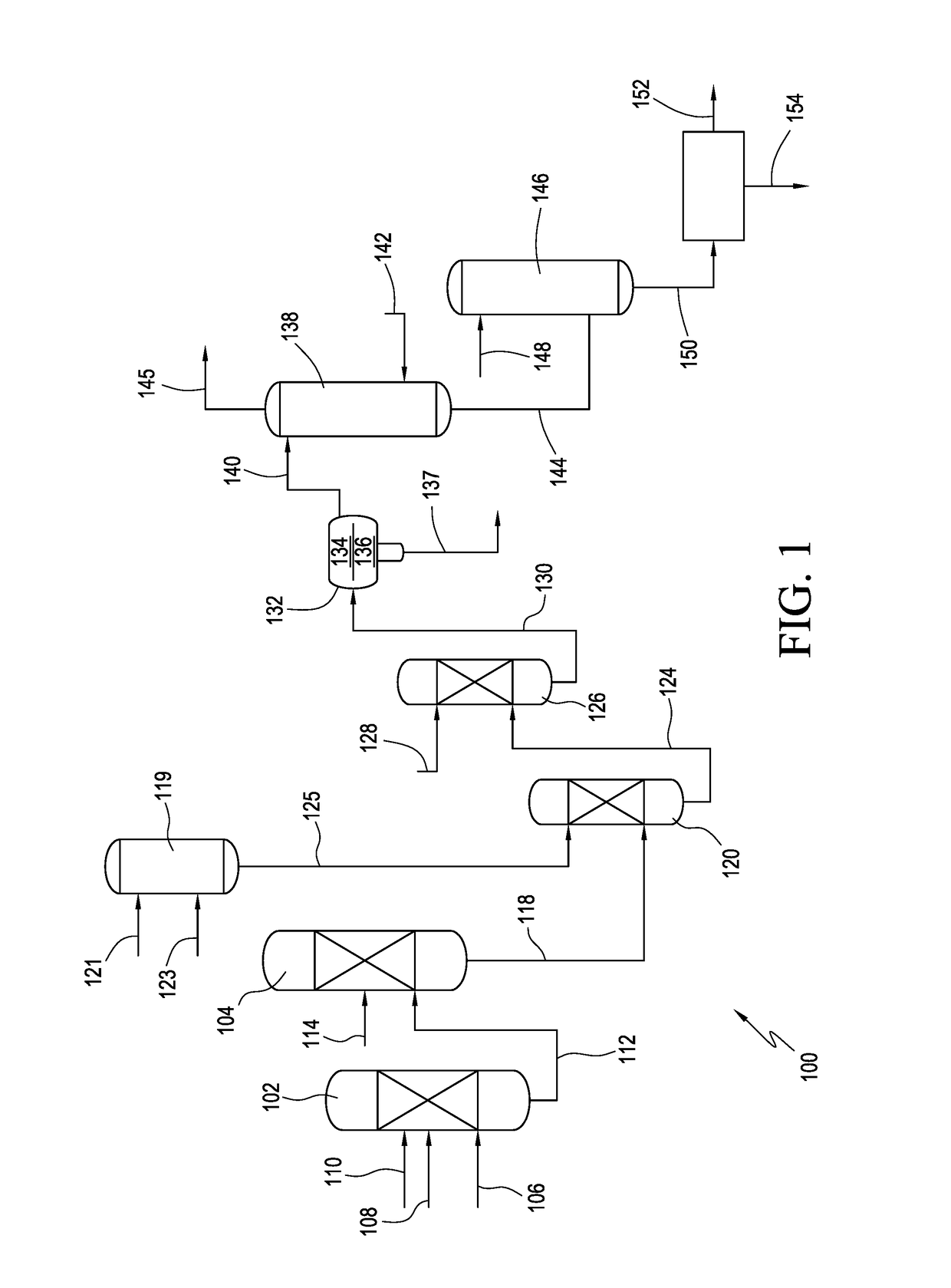
The invention belongs to the field of chemical production, and provides a method for continuously producing acesulfame potassium, which comprises the following steps: continuously mixing and dissolving sulfamic acid and dichloromethane, continuously neutralizing with a triethylamine solution, introducing the neutralized reaction solution and ketene dimer into a continuous reactor, and carrying outaddition acylation reaction to obtain a DKA reaction solution; sulfur trioxide and solvent micro-mixing: S03, enabling dichloromethane to enter a micro-mixer, so as to prepare a cyclizing agent; cyclization and hydrolysis: continuously feeding the DKA reaction solution and a cyclizing agent into a cyclization microreactor to generate a cyclization reaction solution, and continuously feeding the cyclization reaction solution into a hydrolysis microreactor to obtain an acesulfamic acid reaction solution; enabling the acesulfame acid reaction liquid and dichloromethane to enter continuous extraction equipment, enabling an extracted organic phase and a potassium hydroxide aqueous solution to enter a continuous neutralization reactor to obtain acesulfame acid potassium reaction liquid, and subjecting the acesulfame acid potassium reaction liquid to continuous concentration, continuous crystallization, continuous separation and continuous drying to obtain the acesulfame acid potassium finished product. The process has the characteristics of simple process, low cost, good product quality, continuous whole process and the like.
Owner:NANTONG ACETIC ACID CHEM +1
Preparation of zero-valent iron supported MC (mesoporous carbon) composite and method for degrading sulfachloropyridazine by persulfate activation based on zero-valent iron supported MC (mesoporous carbon) composite
InactiveCN108355610AEasy to operateStrong repeatabilityOther chemical processesWater contaminantsSulfate radicalsBiological activation
The invention discloses a preparation method of a zero-valent iron supported MC (mesoporous carbon) composite and a method for degrading sulfachloropyridazine by sulfate radicals produced by persulfate activation. The zero-valent iron supported MC composite is prepared with a liquid-phase reduction method, and zero-valent iron is efficiently and uniformly supported on the basis of the larger specific surface area and more pore structures of MC. According to the method, sodium persulfate is activated efficiently and continuously by using the synergistic effect of adsorption and catalysis of theprepared composite, sulfate radicals are produced for degrading sulfachloropyridazine, and degradation effect of sulfachloropyridazine is improved. The composite has higher stability and can still maintain higher activity after repeated recycling. With adoption of the method for treating non-biodegradable sulfanilamide antibiotic wastewater, especially sulfamethazine-containing antibiotic wastewater, biodegradability of the antibiotic wastewater can be improved, and the composite has the advantages of being environmentally friendly, simple and convenient to operate, high in catalytic activity, good in recyclability and the like, and has a wide application prospect.
Owner:HENAN UNIVERSITY OF TECHNOLOGY
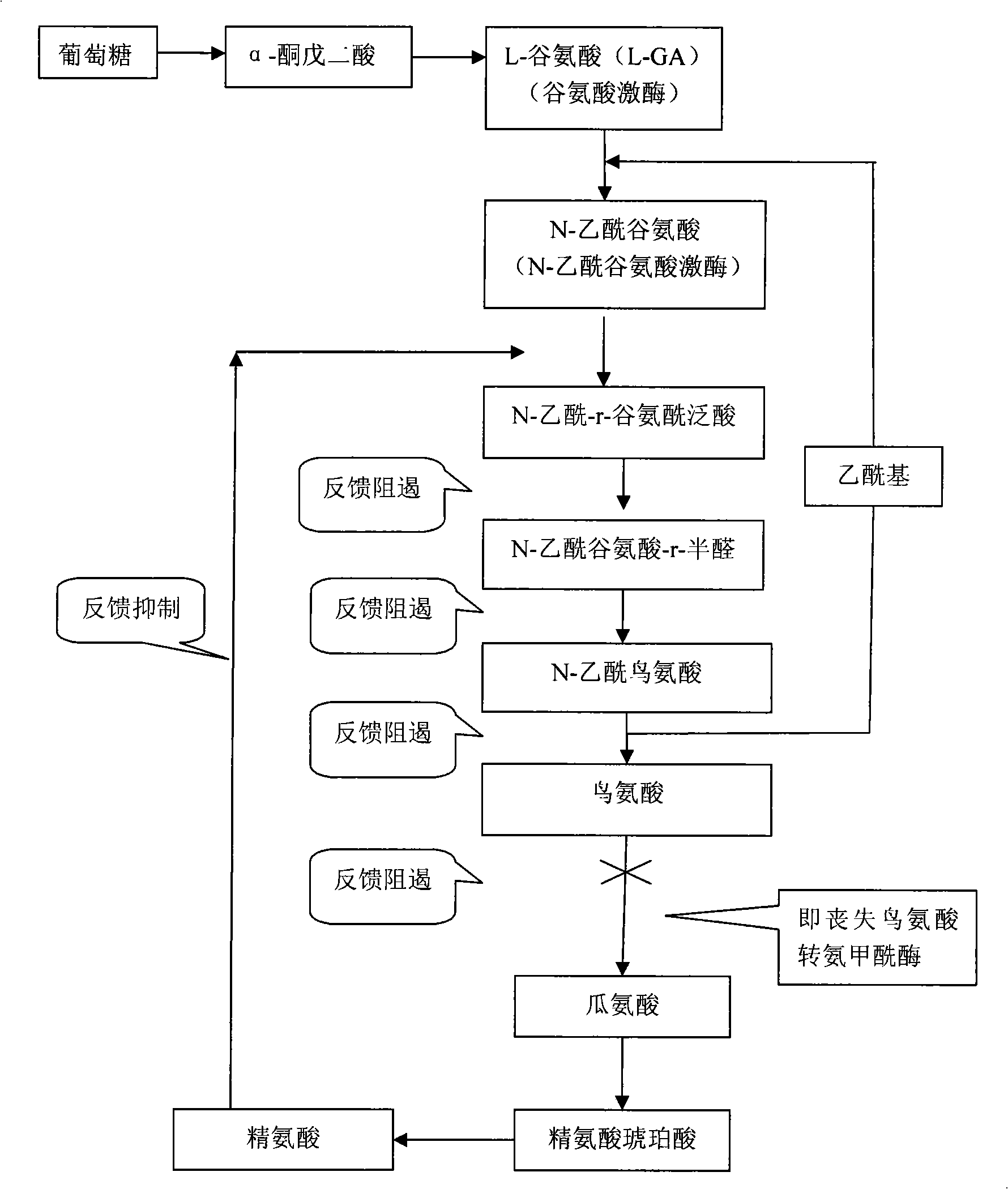
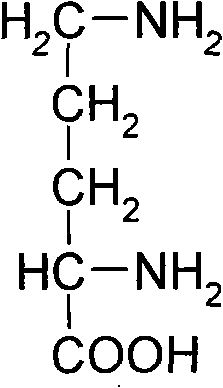
Method for producing L-ornithine by microorganism fermentation
InactiveCN101323866AFast acid productionImprove sugar conversion rateBacteriaMicroorganism based processesSolubilityChemical treatment
The invention provides a method for producing L-ornithine by microbial fermentation, namely, using Corynebacterium glutamate to obtain the strains of CS-189(cit<(-)>+SG<r>) by chemical treatment, and obtain the L-ornithine hydrochloride by fermentation, culture solution micro-filtration by a ceramic membrane, ion exchange resin and extraction by a hydrothermal crystallization method with decompression concentration. The strains used in the method are auxotrophy resistant to sulfaguanidine, which significantly enhances acid yield by fermentation. Meanwhile, aiming at the problem that the solubility of the L-ornithine hydrochloride in water is extremely difficult, the hydrothermal crystallization method is adopted to obtain better extraction rate under the condition that flammable and explosive alcohol is not used. The method simplifies processes and reduces extraction cost.
Owner:上海聚瑞生物技术有限公司
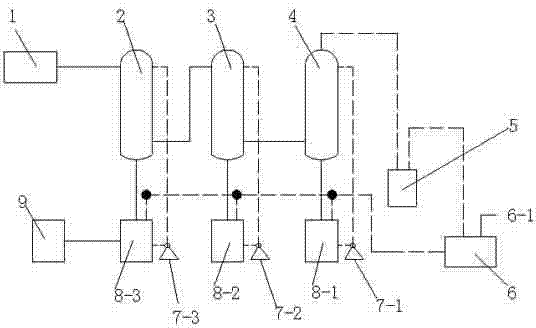
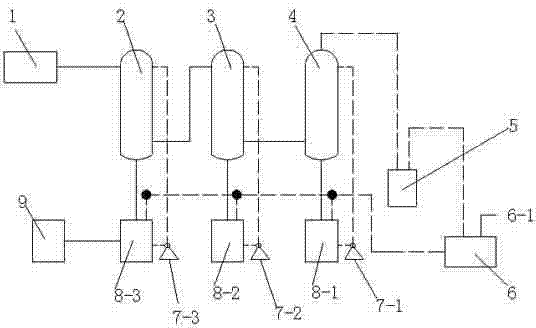
Hydrogen chloride gas circulating recovery system
InactiveCN102302890AHigh recovery rateQuality improvementChlorine/hydrogen-chlorideDispersed particle separationSulfanilamideProcess engineering
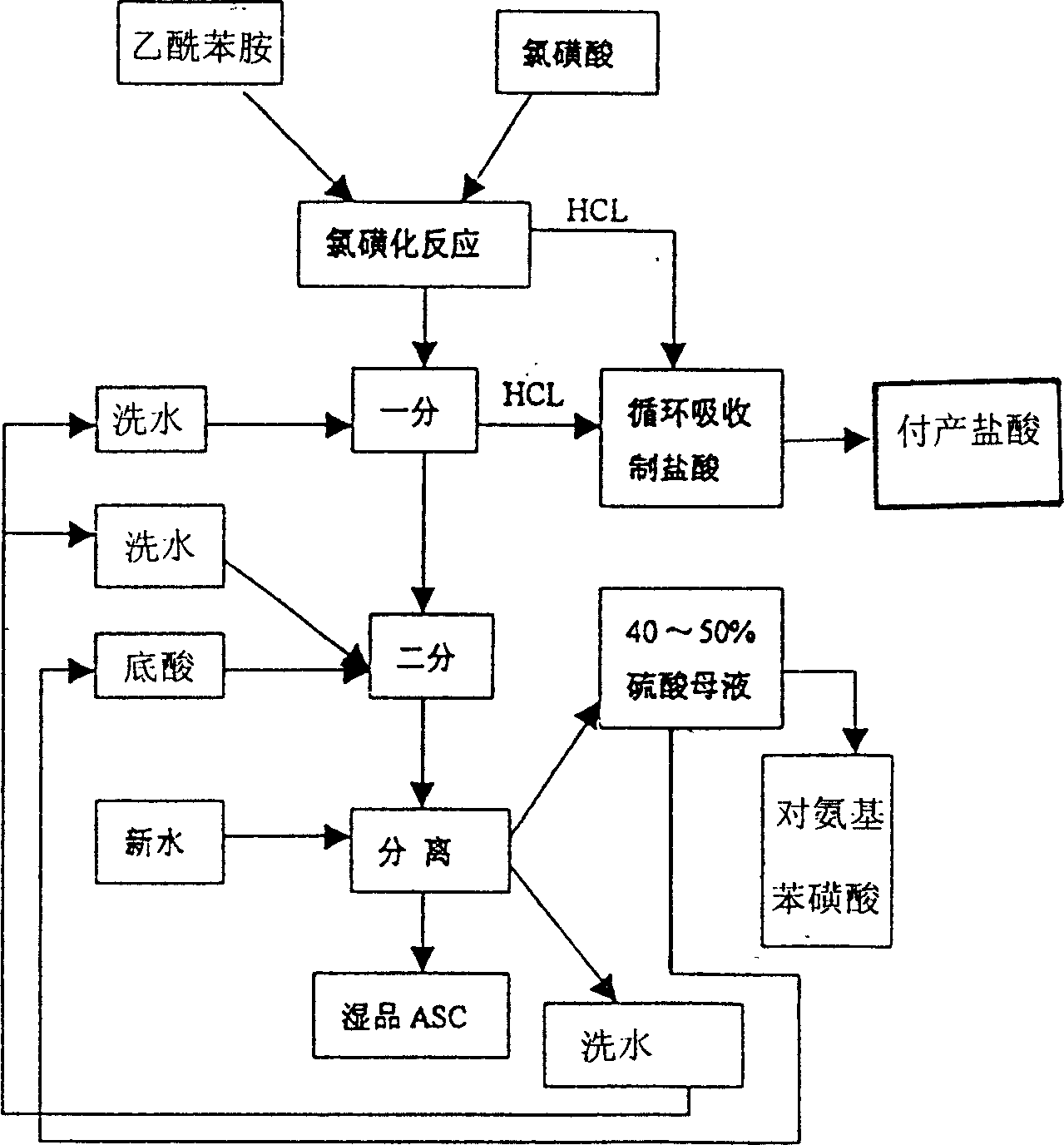
The invention relates to a hydrogen chloride gas circulating recovery system, which is characterized in that: a main body comprises a hydrogen chloride tail gas tank, a first-stage falling film tower, a second-stage falling film tower and a third-stage packing tower which are parallelly arranged, and a vacuum buffer tank which participates in forming a circulating system, wherein a gas outlet of the vacuum buffer tank is connected with a gas inlet of a water ring vacuum unit; a discharge hole of the water ring vacuum unit is connected to feeding holes of a circulating tank 1, a circulating tank 2 and a circulating tank 3 respectively; and a discharge hole on the left side of the circulating tank 3 is connected with a circulating finished product tank. Hydrogen chloride tail gas generated in the process of producing industrial sulfanilamide is subjected to circulating absorption through the structure, and the obtained hydrochloric acid can be recycled, so that the resource utilization rate is fully improved, pollution-free production is promoted, production cost is reduced, and the zero emission index of hydrogen chloride gas is met.
Owner:SUZHOU WUGAN PHARMA
Sulfanilamide drug chemiluminescence enzyme-linked immunodetection kit
The present invention discloses a sulfanilamide drug chemiluminescence enzyme-linked immunodetection kit, which comprises a kit body, an enzyme label plate placed inside the kit body, and reagents placed inside the kit body, and is characterized in that every hole of the enzyme label plate is coated with coating antigen, the coating antigen is a sulfanilamide mother nucleus and carrier protein conjugate, and the reagents comprise sulfanilamide monoclonal antibody, horseradish peroxidase-labeled goat anti-mouse antibody, a series of sulfanilamide standard solutions, a concentrated phosphate buffer, a concentrated washing solution and a chemiluminescence solution. The sulfanilamide drug chemiluminescence enzyme-linked immunodetection kit has characteristics of high sensitivity, simple and rapid detection, high accuracy, and more drug detection types, provides a substantially reduced operation time compared to the conventional colorimetric ELISA method, and can be used for detection of residues of the 17 sulfanilamide drugs in animal tissues (pork, chicken, pork liver and chicken liver), aquatic products (fish and shrimp), eggs, milk and milk powder.
Owner:BEIJING KWINBON BIOTECH
Preparation method of sulfadoxine
A preparation method of sulfadoxine belongs to the field of sulfanilamide antimicrobial drug preparation. Cyclization reaction comprises the following steps of: firstly pouring a sodium methoxide solution into a reactive pan, then successively adding methanamide and methyl ethyl methoxymalonate, keeping warm, recovering methanol, cooling for crystallization, drying by centrifugation, discharging,and drying to obtain 5-methoxy-4,6-disodium dihydroxypyrimidine; Chlorination reaction comprises the following steps of: firstly putting phosphorus oxychloride into a reaction vessel for heating, adding 5-methoxy-4,6-disodium dihydroxypyrimidine into the reaction vessel to react, decompressing and recovering phosphorus oxychloride until the material is dry, cooling, adding trichloro ethylene withuniformly stirring, putting into a hydrolysis pan for hydrolyzation, collecting a trichloro ethylene layer after standing and delaminating, followed by a neutralization reaction, controlling pH value, washing, removing a water layer, recovering trichloro ethylene, and releasing crystals to obtain 5-methoxy-4,6-dichloropyrimidine. The preparation method provided by the invention can be used to guarantee the product purity, prolong the service life of equipment, avoid the damage to the environment and human body, reduce emission, and save energy, and accords with foreign pharmacopoeia standard requirements.
Owner:CHANGSHU JINSHEN MEDICAL PROD CO LTD
Synthetic method of deuterium-marked sulfanilamide
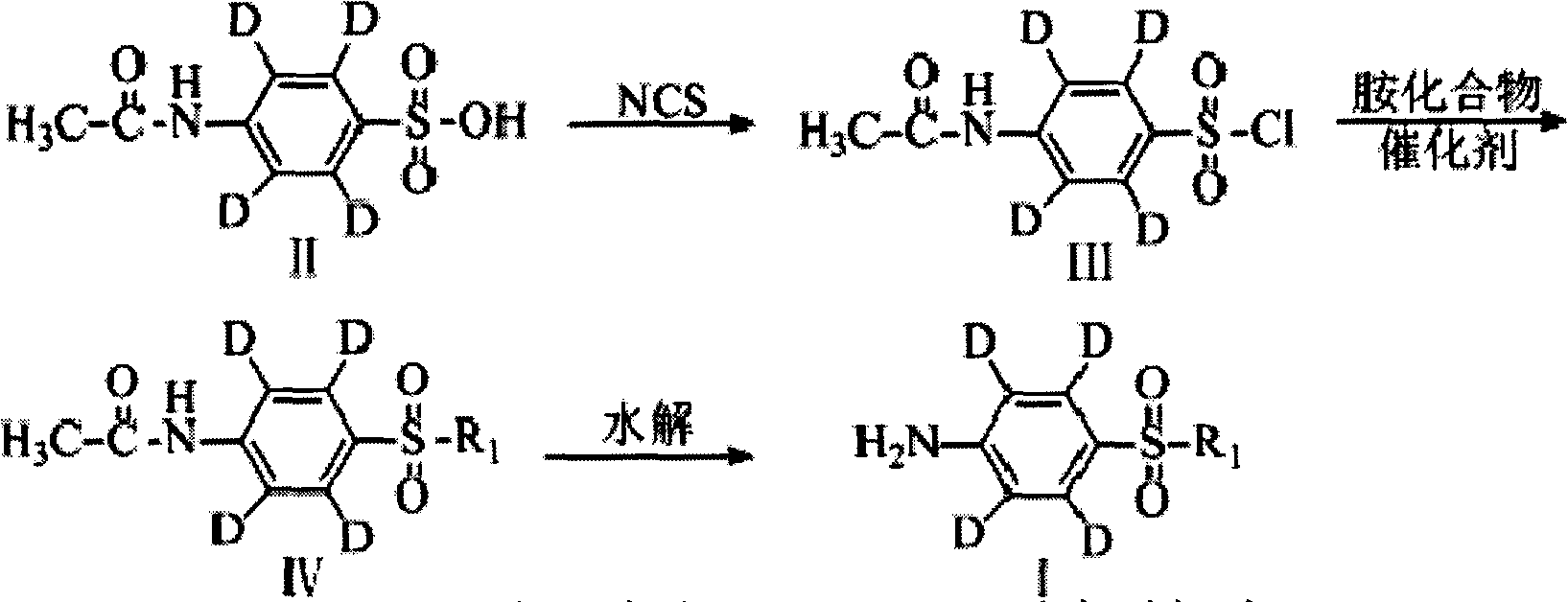
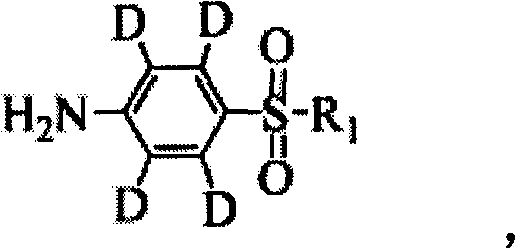
ActiveCN102603655ARaw materials are easy to getMild reaction conditionsOrganic chemistrySulfonyl chlorideN-Chlorosuccinimide
The invention relates to a synthetic method of deuterium-marked sulfanilamide. The method comprises the following steps of: reacting deuterated sulfonic acid with N-chlorosuccinimide (NCS) to obtain deuterated sulfonyl chloride which is not required to be separated; and adding amine for undergoing an amination reaction under the action of a metal catalyst to obtain deuterium-marked sulfanilamide. Compared with the prior art, the method has the advantages that: acyl chloride synthesis and the amination reaction are performed with a 'one pot process', operation is simple and convenient, the yield is high, and a synthesized product comprises sulfapyridine-benzene ring-D4, sulfadimidine-benzene ring-D4, sulfameter-benzene ring-D4, sulfaquinoxaline-benzene ring-D4, sulfisoxazole-benzene ring-D4 and sulfamethoxazole-benzene ring-D4.
Owner:SHANGHAI RES INST OF CHEM IND
Chinese medicine lotion preparation for curing acute conjunctivitis
InactiveCN101279027AEasy to makeLow toxicitySenses disorderAnthropod material medical ingredientsWhite blood cellSulfanilamide
The invention relates to a preparation method of a traditional Chinese medicine lotion for curing acute conjunctivitis, which pertains to the technical field of traditional Chinese medicine preparation methods. At present, acute conjunctivitis is cured by adopting a sulfa drug which is easy to cause gastrointestinal tract reaction or crystalluria, hematuria or tetter, antipyretic, leukocyte reduction, etc. The technical proposal of the invention is that: chrysanthemum, mint, cicada slough, mulberry leaf, fructus viticis, taraxacum mongolicum, viola yedoensis makino, herba patriniae, blackberrylily, ampelopsis japonica mak, cyrtomium fortunei, smilax glabra, bistortae, polygonum bistatum, herba lobeliae radicantis, potentilla discolor bunge, barberry root, cayratia japonica, tupan root, prunella vulgaris, clearson, seed of feather cockscomb, pale butter-fly bush bud, pipewort, equisetum hyemale, bat dung, princesplume ladysthumb fruit, and unripe licorice are taken as raw materials, the raw materials are soaked by using 1600ml water for 30mintues and boiled by small fire, residues are filtered by using gauze, thus preparing 1000ml of traditional Chinese medicine liquid which is the traditional Chinese medicine lotion for curing acute conjunctivitis. The lotion of the invention has the advantages of simple preparation method, low toxicity, fewer side effects of the prepared traditional Chinese medicine lotion and being able to directly achieve to the focus.
Owner:高秀真
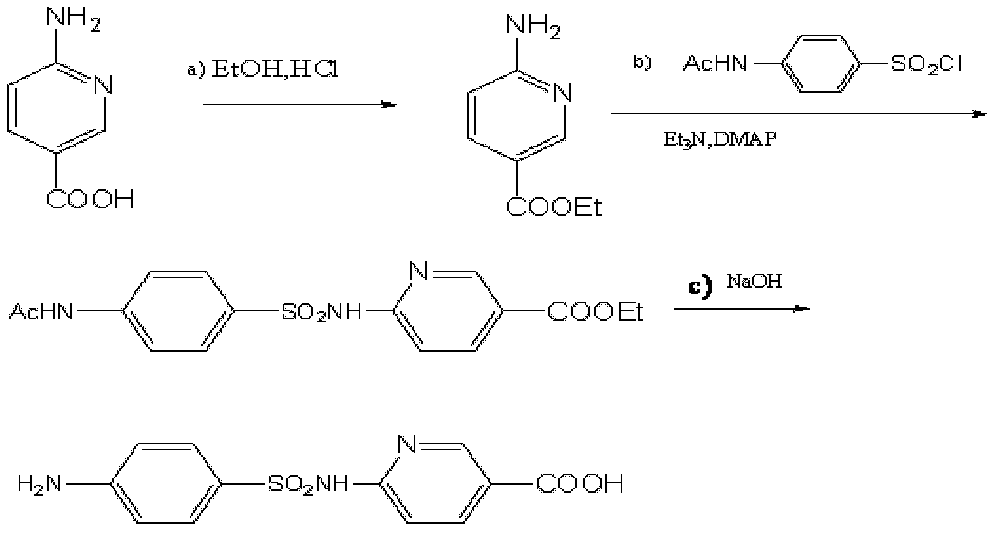
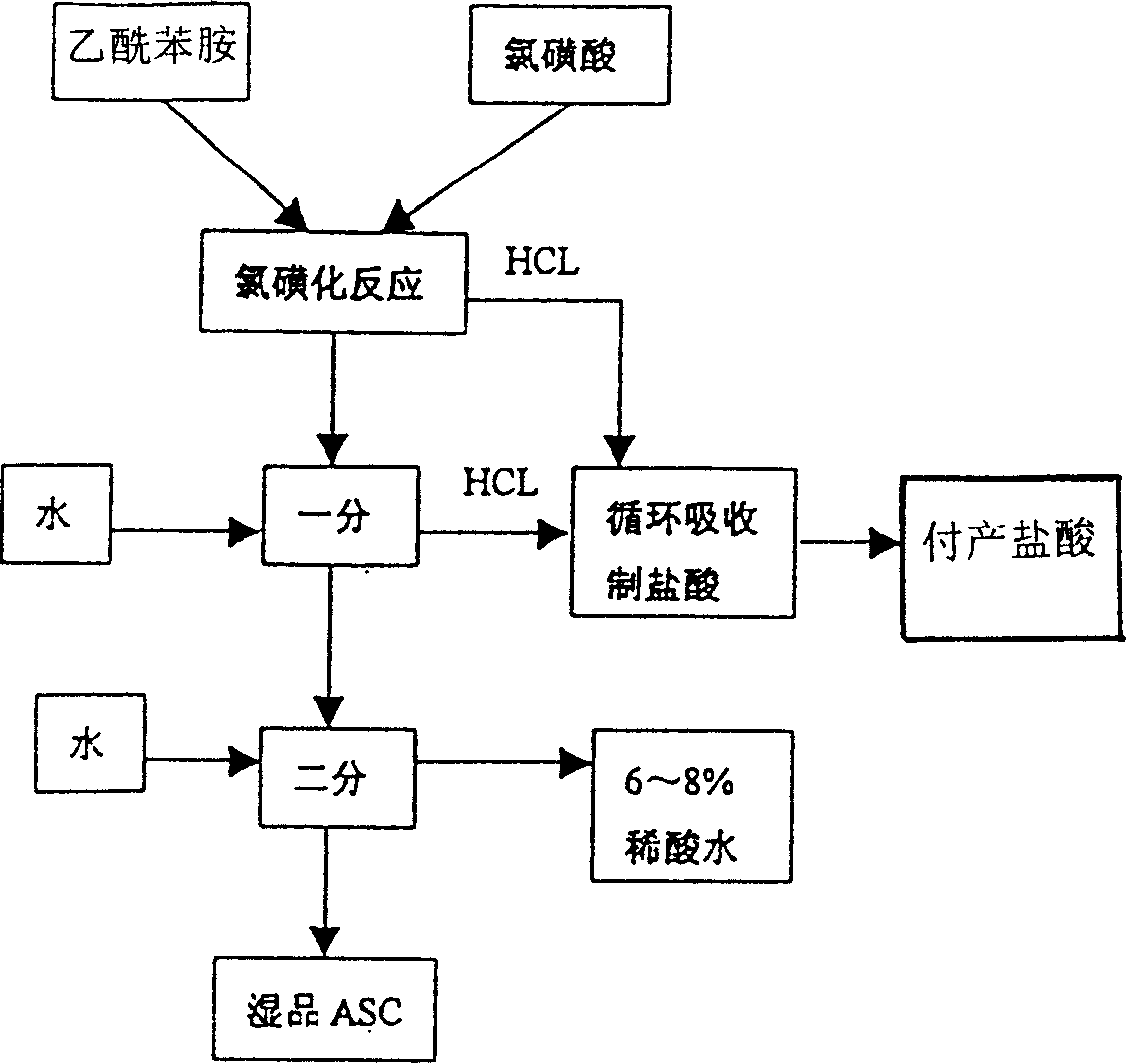
Process for producing sulfanilic amide medicine mother substance p-acetamido benzene sulfonyl chloride
InactiveCN1683331ATo achieve the goal of zero emissionsConserve waterSulfonic acid preparationSulfonyl chlorideChlorosulfuric acid
The production process of p-acetamido benzene sulfonyl chloride as intermediate for sulfanilamide medicine includes the following steps: chlorosulfonating acetylaminobenzene as main material with chlorosulfonic acid to produce sulfonated oil and absorbing hydrogen chloride gas to prepare hydrochloric acid; separating sulfonated oil, adding water and decomposing chlorosulfonic acid to obtain separated oil while absorbing produced hydrogen chloride gas; separating the separated oil and adding water to deposit out p-acetamido benzene sulfonyl chloride; further separating, eliminating p-acetamido benzene sulfonyl chloride mother liquid, water washing the crystal while side producing sulfuric acid; using the side produced sulfuric acid in recovering p-amido benzene sulfonic acid; and reusing crystal eliminating water in the separation. The present invention realizes the comprehensive utilization, protects environment and lowers the cost.
Owner:黄升
Acesulfame potassium compositions and processes for producing same
Improved processes for producing high purity acesulfame potassium. In one embodiment, the process comprises the steps of contacting a solvent, e.g., dichloromethane, and a cyclizing agent, e.g., sulfur trioxide, to form a cyclizing agent composition and reacting an acetoacetamide salt with the cyclizing agent in the composition to form a cyclic sulfur trioxide adduct. The contact time is less than 60 minutes. The process also comprises forming from the cyclic sulfur trioxide adduct composition a finished acesulfame potassium composition comprising non-chlorinated, e.g., non-chlorinated, acesulfame potassium and less than 35 wppm 5-halo acesulfame potassium, preferably less than 5 wppm.
Owner:CELANESE INT CORP
Soluble powder for treating livestock and poultry bacterial infection and parasitic disease
InactiveCN101450055AThe curative effect is sureGood curative effectOrganic active ingredientsPharmaceutical delivery mechanismCoccidiosisOral medication
The invention relates to a soluble powder for treating livestock and poultry bacterial infection and parasitosis, comprising nortloxacin lactate, trimethoprim and auxiliary materials, the medicament of the invention is taken as antibiotic medicament, is mainly used for treating various diseases and parasitosis caused by sensitive bacterias, has better curative effects for pig toxoplasmosis, pig hydropsy, poultry, rabbit coccidiosis, the nortloxacin lactate and the trimethoprim are compatible with each other for using and generating cooperating action, the medicament of the invention has excellent oral administration absorption effect and high safety.
Owner:TIANJIN RINGPU BIO TECH
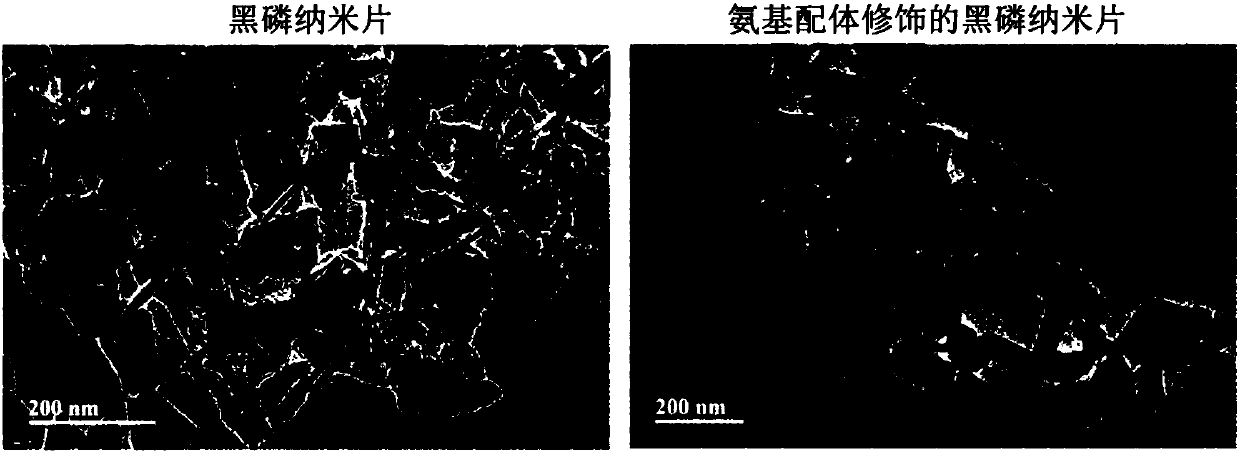
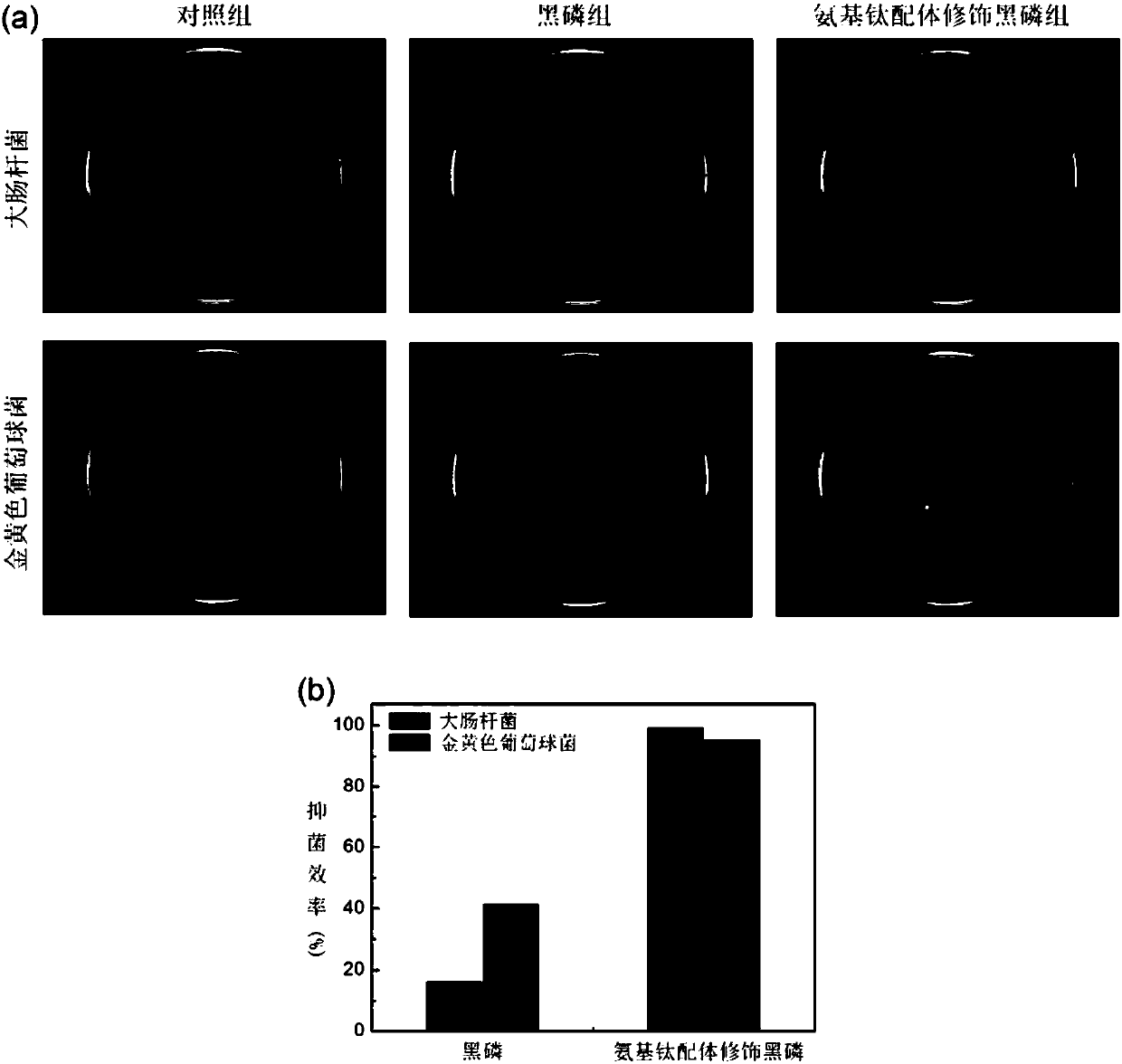
Antibacterial black phosphorus nanometer material and preparation method thereof
ActiveCN108042565AGood antibacterial effectGood inhibitory effectAntibacterial agentsInorganic phosphorous active ingredientsSulfanilamideBlack phosphorus
The invention discloses an amino ligand surface modified black phosphorus nanometer material and a preparation method thereof, and applications of the amino ligand surface modified black phosphorus nanometer material in antibacterial field. According to the present invention, the antibacterial amino group in the sulfanilamide-based group is introduced into the surface of the nanometer material soas to provide the antibacterial performances of wide antibacterial spectrum, high sterilization efficiency and less drug resistance for the nanometer material; the preparation method has characteristics of simple and easy operation, high yield, good reproducibility, low cost and large-scale production; the use of the black phosphorus nanometer material does not cause secondary pollution to the environment; and the modified two-dimensional black phosphorus nanometer material can significantly inhibit the growth of gram-negative bacteria and gram-positive bacteria, and has excellent antibacterial effect so as to provide great application potential in the field of medical materials.
Owner:SHENZHEN INST OF ADVANCED TECH
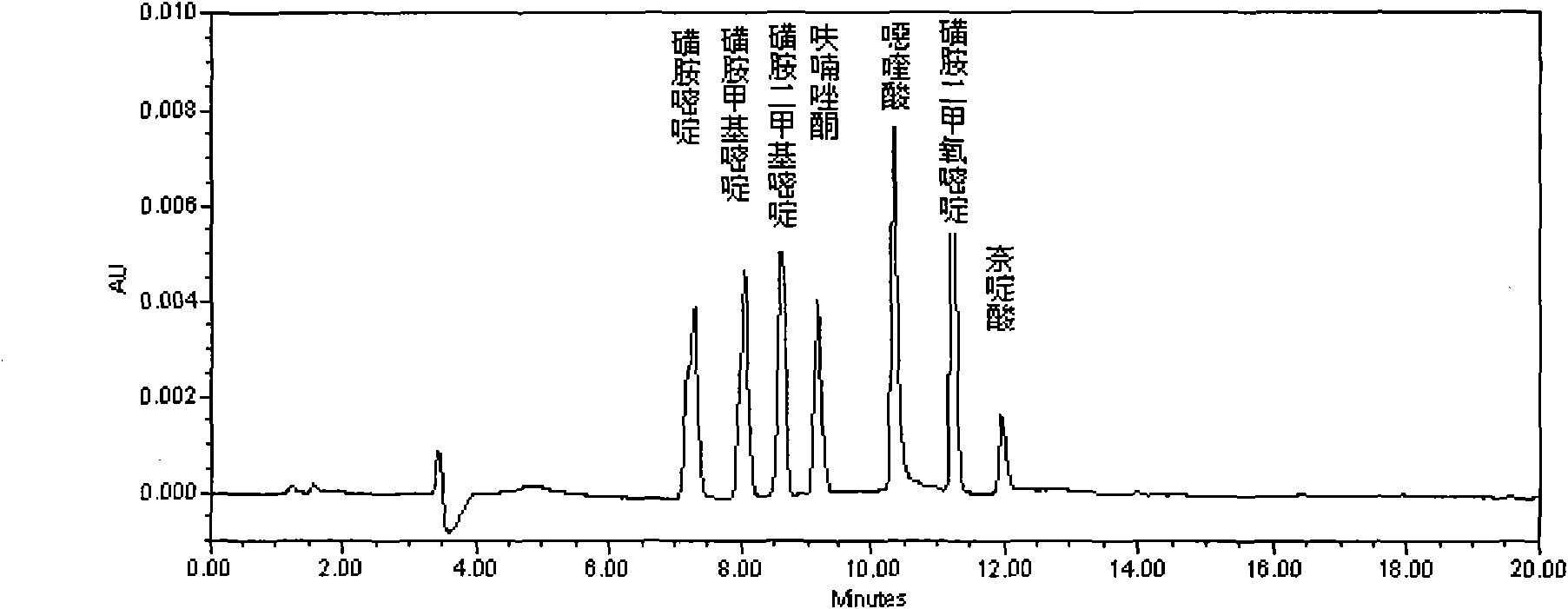
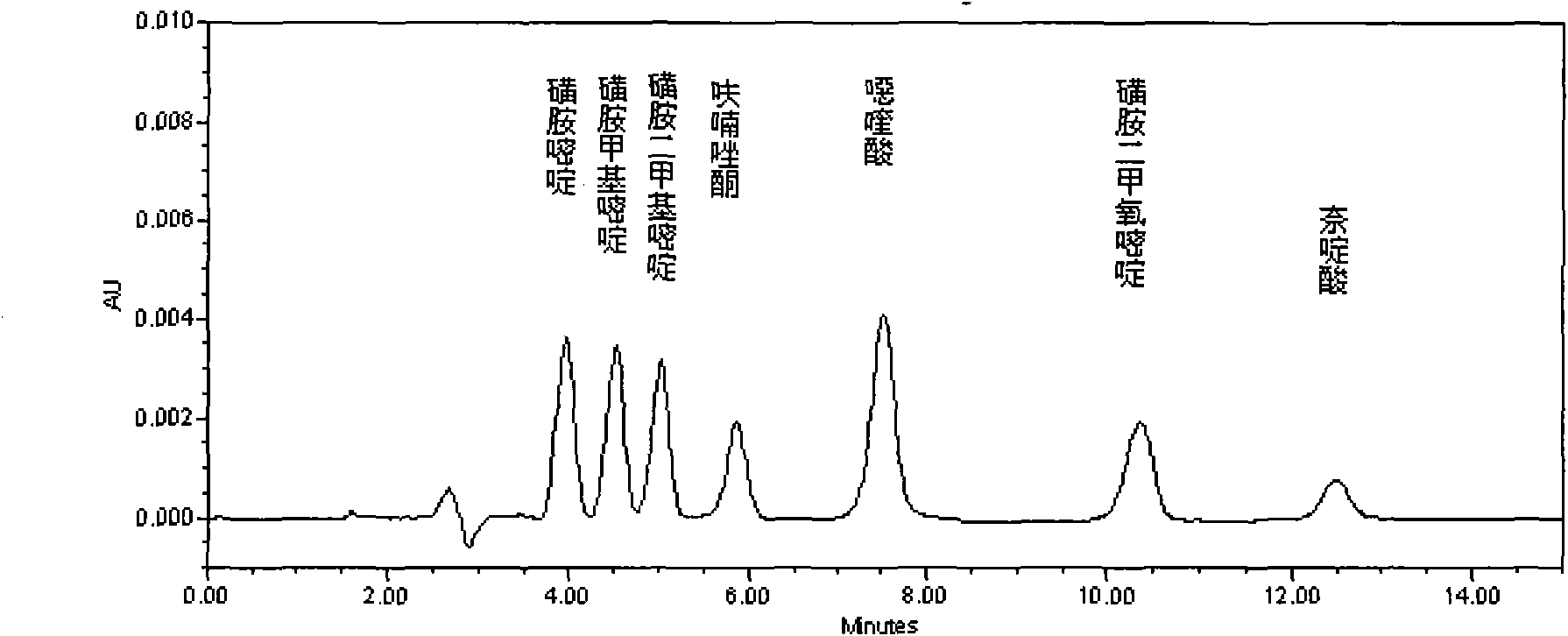
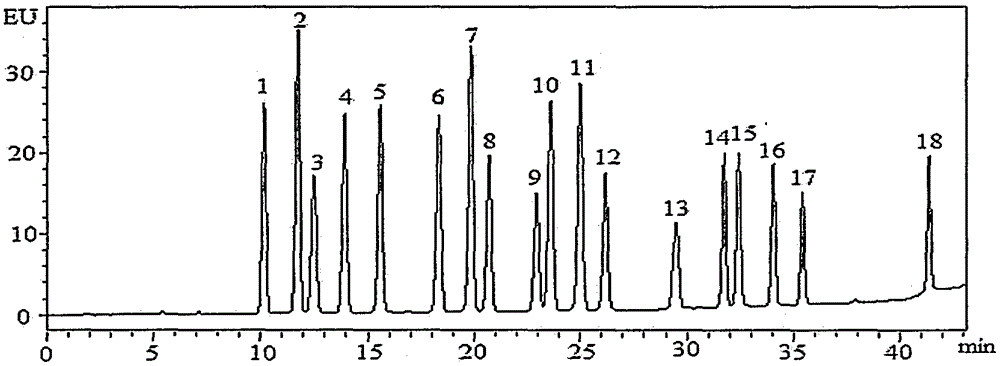
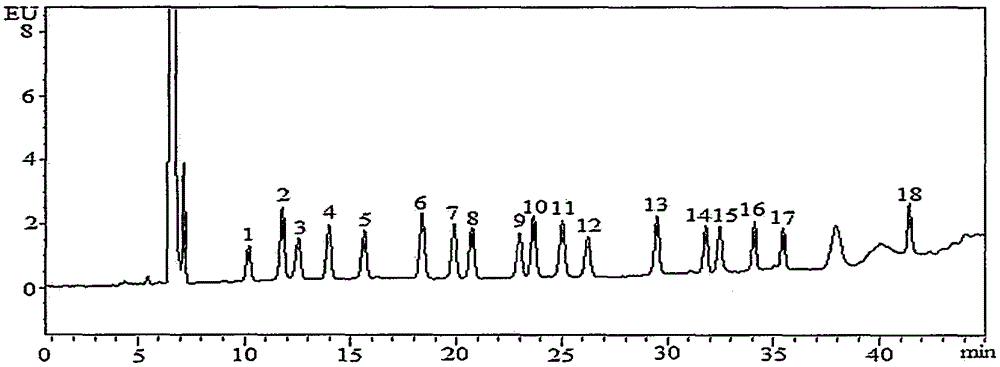
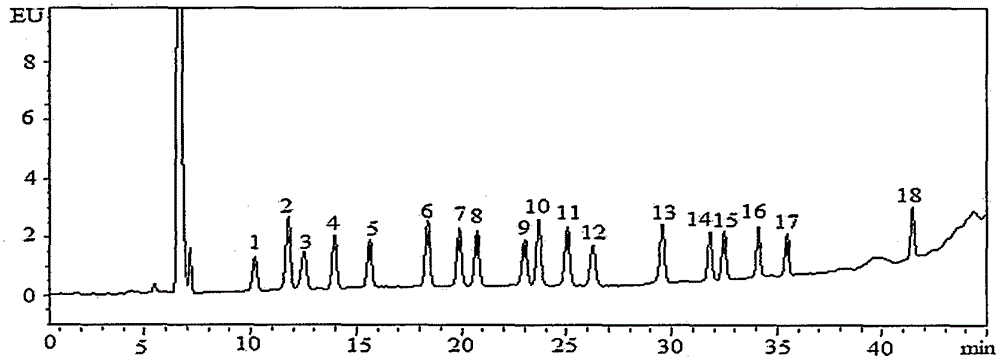
Method for detecting residual quantity of 18 sulfanilamide drugs in beef and mutton
InactiveCN105259277AShorten the timeHigh detection sensitivityComponent separationSulfur drugSodium acetate
The invention discloses a method for detecting residual quantity of 18 sulfanilamide drugs in beef and mutton. The method comprises taking 5g of uniform beef and mutton, adding anhydrous magnesium sulfate, sodium acetate and acetonitrile acetate solutions into the beef and mutton, carrying out high-speed homogenized extraction, carrying out centrifugation, carrying out vortex mixing purification impurity-removal on the supernatant by an adsorbent containing a plurality of purification materials, carrying out pressure reduction or nitrogen blowing condensation on the supernatant subjected to re-centrifugation until drying, redissolving the residues, after shaking-up, filtering the solution by a microfiltration membrane with pore sizes of 0.22 micrometers to obtain a sample solution, carrying out separation by a reversed phase column filled with C18 as a filler, carrying out ultraviolet light on-line rapid derivation, carrying out detection by a fluorescence detector, carrying out qualitative analysis according chromatographic peak retention time, carrying out quantification by an external standard method and calculating residual quantity of 18 sulfanilamide drugs in beef and mutton. The method has simple processes, utilizes less amount of a reagent, has high detection sensitivity, releases simultaneous rapid detection of 18 sulfanilamide drugs by one step, greatly shortens analysis time and has a good application prospect.
Owner:CHINA ACAD OF SCI NORTHWEST HIGHLAND BIOLOGY INST
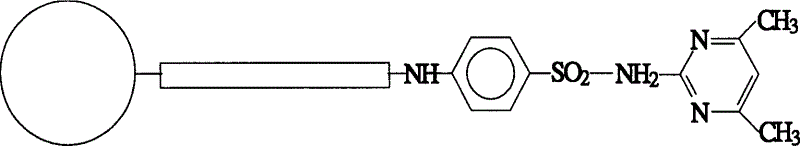
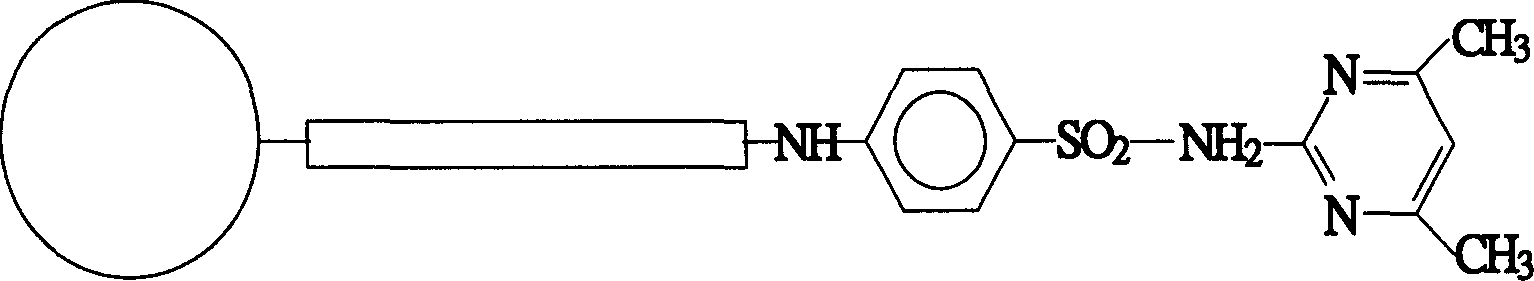
Affinity chromatographic stuffing with sulfadimidine as ligand
InactiveCN1548224AHigh affinityImprove securityIon-exchange process apparatusOther chemical processesAntigenSulfanilamide
The present invention is one kind of affinity chromatographic stuffing with sulfadimidine as ligand specially for separating antigen globin. Sulfadimidine is one kind of widely applied sterilizing medicine, and its amino group may be utilized to bond to various carrier to prepare affinity chromatographic stuffing. The stuffing is used in specifically separating gamma antigen globin from plasma, fermented liquid, tissue homogenizing liquid and other matter containing gamma antigen globin, with purity reaching 91 %. The immobilized sulfadimidine and human IgG have interaction dissociation constant up to 3E(-6) mol / L. The new affinity chromatographic stuffing has high affinity, high safety, low cost and other features.
Owner:INST OF CHEM CHINESE ACAD OF SCI
Analyzing method of residues of sulfanilamide and antibiotic medicaments in aquatic product
InactiveCN101639466AReduce pollutionConform to the requirements of veterinary drug residue analysis methodComponent separationPreparing sample for investigationSulfanilamideAquatic product
The invention relates to a detection method of residues in tissue of an aquatic product, in particular to an analyzing method of residues of sulfanilamide and antibiotic medicaments in an aquatic product, which comprises the following steps: adding filler and EDTA sodium salt to the tissue of an aquatic product to be detected and mixing the components to obtain a mixed material; drip washing the mixed material by a drip washing agent; eluting the obtained mixed material by an eluting agent to obtain an eluant; concentrating the eluant obtained in the eluting step, dissolving the concentrated eluant by a solvent and then filtering the eluant to obtain a filter liquor; and using a buffer solution as a flowing phase to carry out HPLC measurement on the filter liquor. The method can simultaneously detect the residues of sulfanilamide and tetracycline medicaments in a sample to be detected.
Owner:赖克强 +3
Method for rapid detection of residual amount of sulfanilamide in food
ActiveCN103760141AAchieving High Sensitivity DetectionReduce distractionsFluorescence/phosphorescenceFluorescamineSulfanilamide
The invention discloses a method for rapid detection of the residual mount of sulfanilamide in food. The method comprises the following steps: firstly enabling the sulfanilamide and fluorescamine to perform derivation reaction, further enriching derivative products by using cloud point extraction, adding a sample enrichment and a standard plate prepared from a sulfanilamide standard product on a thin layer silica gel plate, comparing the fluorescence intensities of the two under an ultraviolet lamp to judge the concentration range of the fluorescamine in a sample, and determining the residual amount of the sulfanilamide in the food, wherein a detection limit of the method is 0.08 micron g / mL. The method disclosed by the invention has the advantages of simplicity in operation, small using amount of organic solvent, high detection sensitivity, short detection time, strong specificity and capability of realizing effective separation from interfering substances, is a simple, convenient, fast and accurate analytical method, does not need large-scale instruments, only needs to configure small-scale instruments and equipment, and further has extensive application prospects.
Owner:KUNMING UNIV OF SCI & TECH
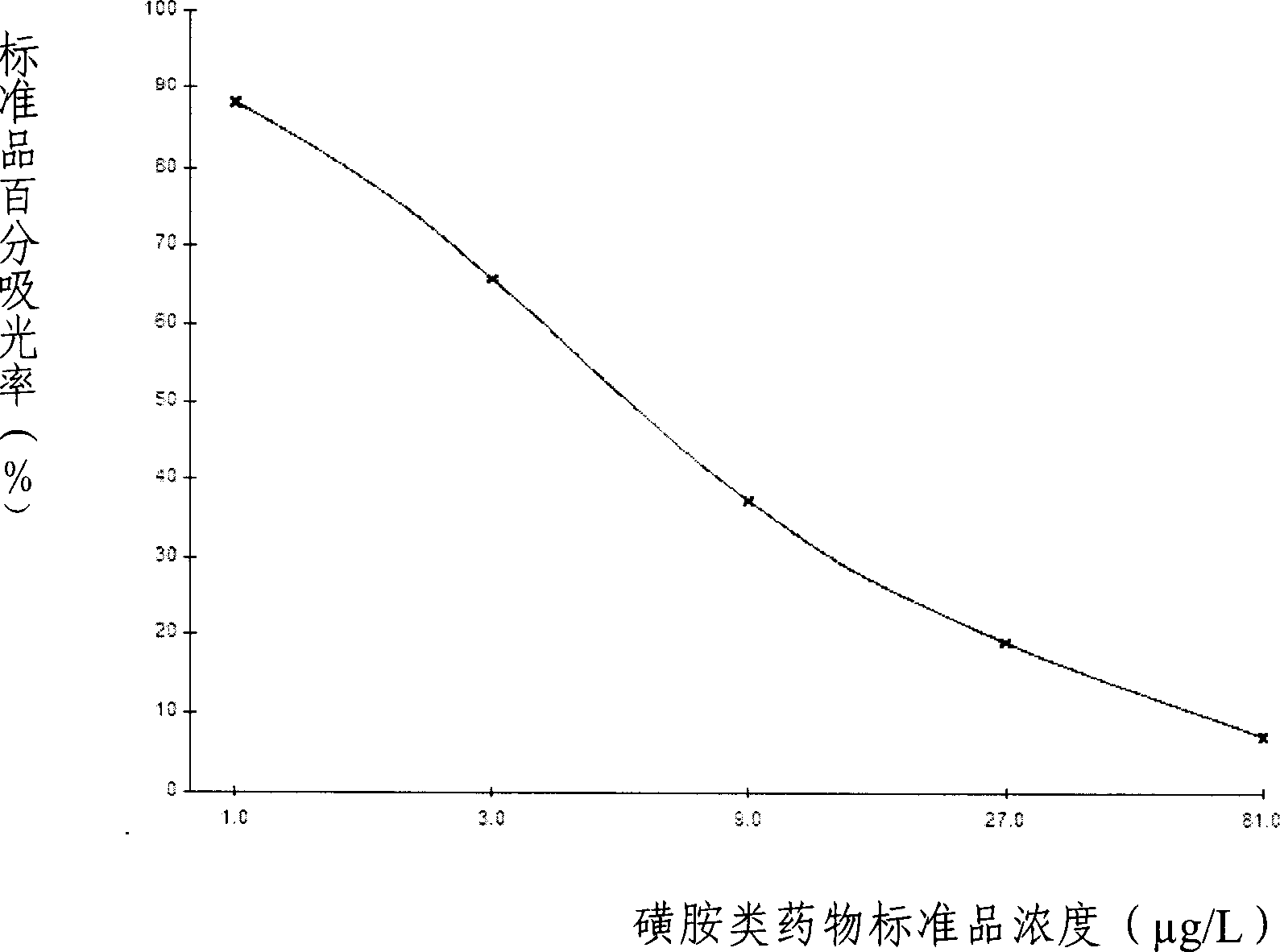
ELISA kit for detecting sulfanilamides residue in animal derived food
The invention provides an enzyme immune agent box for detecting sulpha drugs of animal organization, which uses enzyme immune method to detect the preprocessed animal organization, honey, urine, milk. The enzyme immune agent box comprises: enzyme mark plate which coats sulpha drugs antigen or antibody, sulpha drugs mouse monoclonal antibody working solution, enzyme mark antibody or sulpha drugs antigen solution, sulpha drugs standard solution, base material color developing solution, compression cleaning liquid, ending solution and compression extracting solution. The invention also discloses a method for applying the detecting method, which comprises: first doing sample front process, then using the agent box to detect, at last analyzing the detected result.
Owner:BEIJING WANGER BIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com