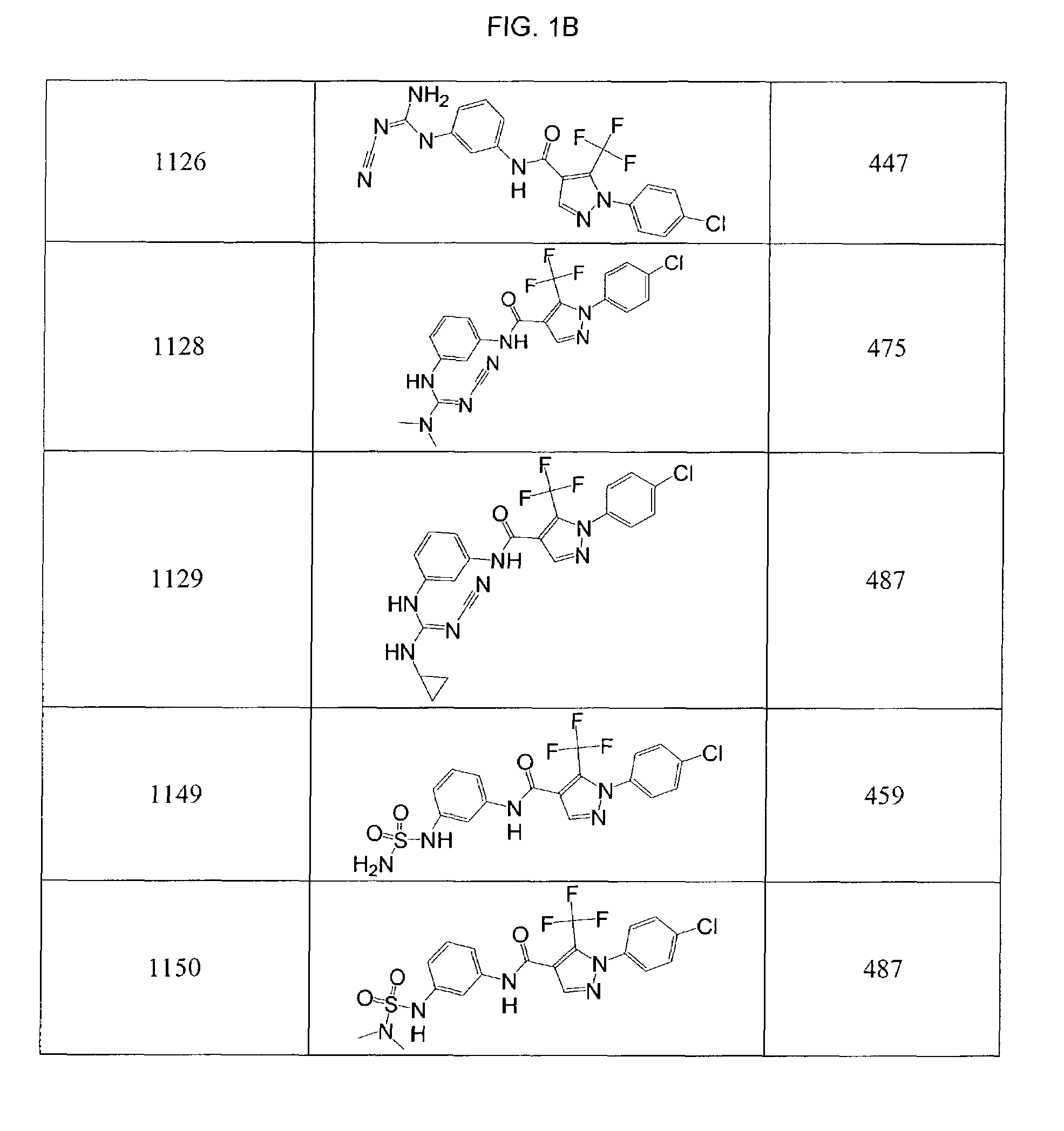
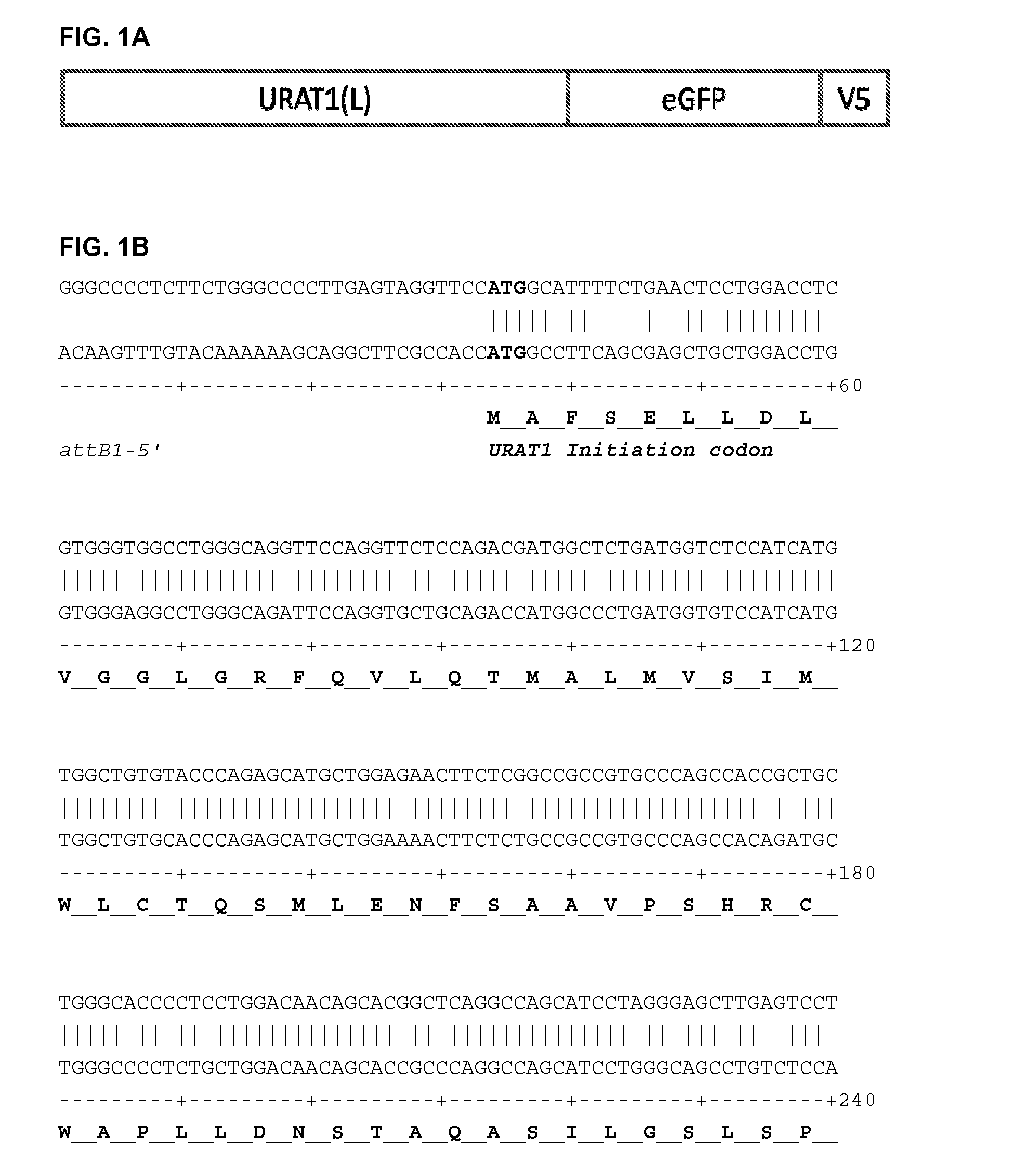
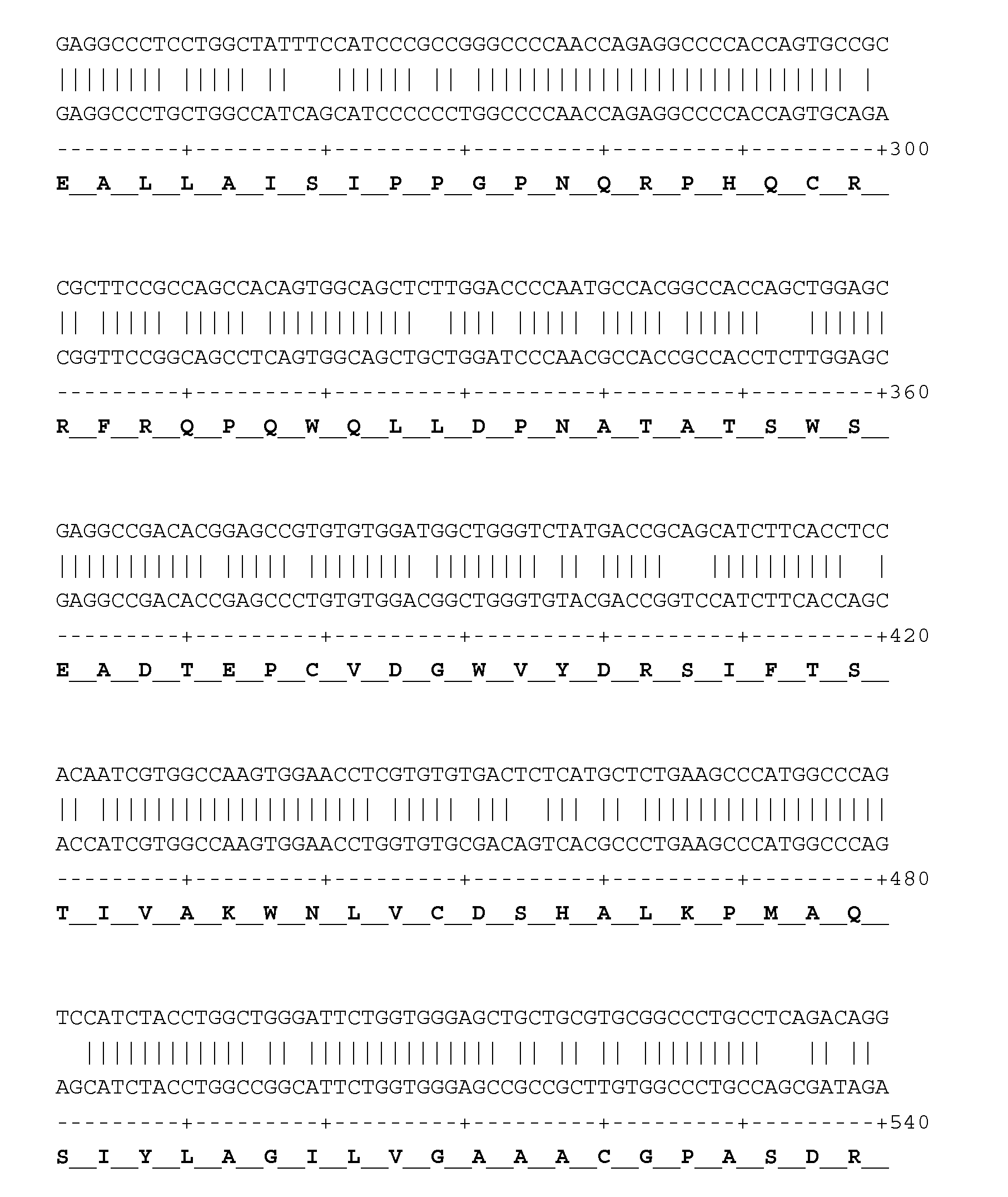
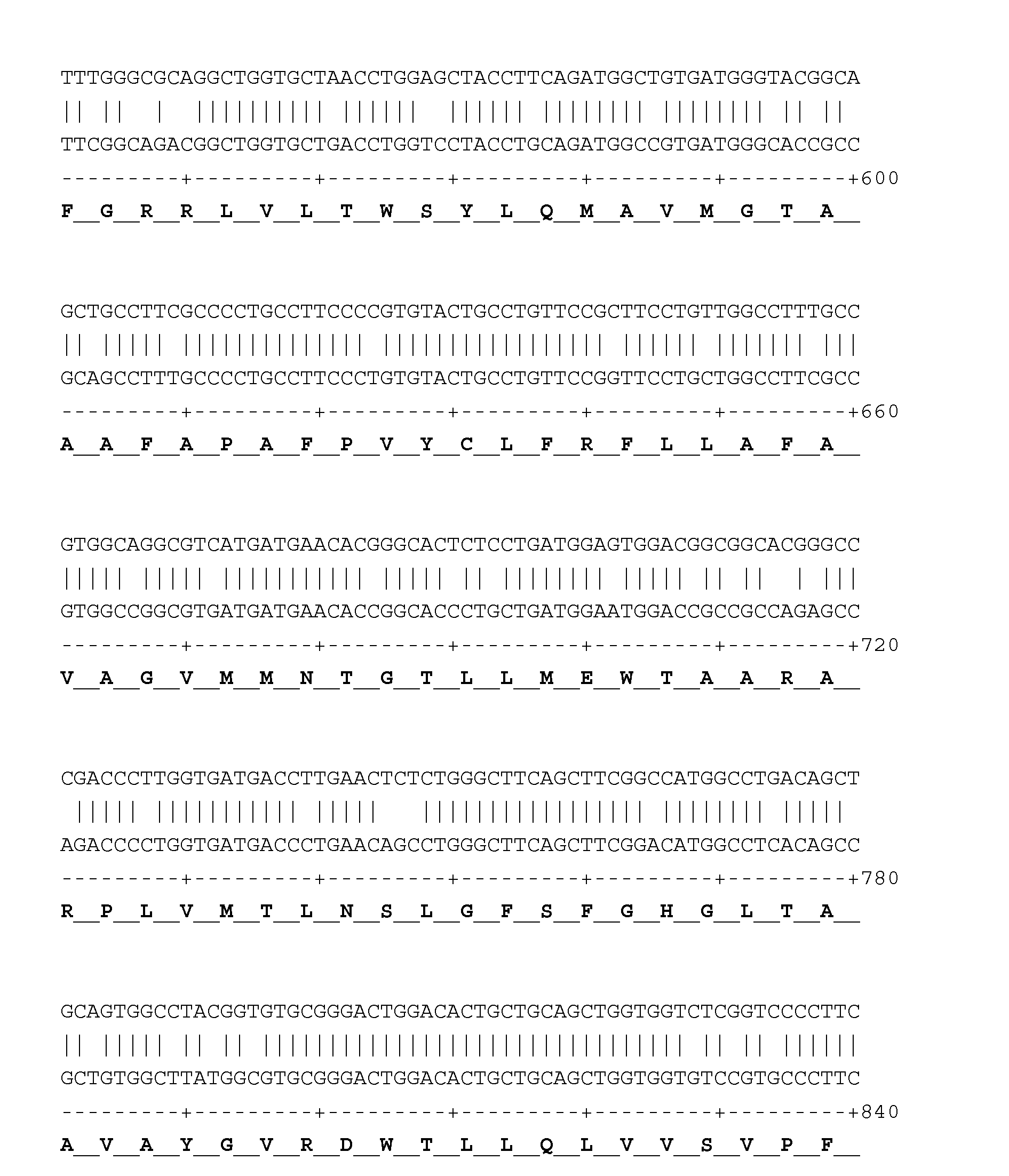
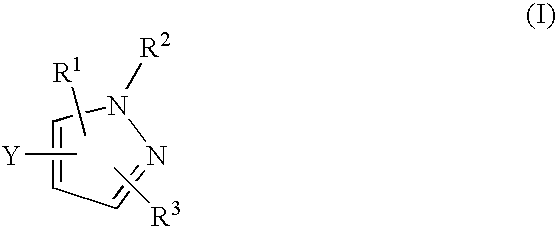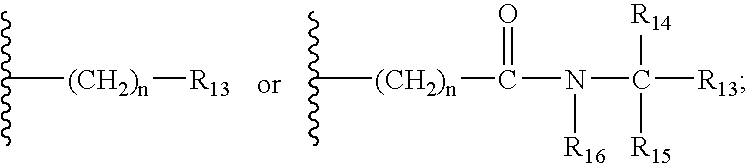Patents
Literature
641 results about "Sulfonilamide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Hepatitis C inhibitor tri-peptides
ActiveUS7091184B2Better pharmacokinetic profileNot significant inhibitory activityBiocideDipeptide ingredientsHcv ns3 proteaseHepatitis C
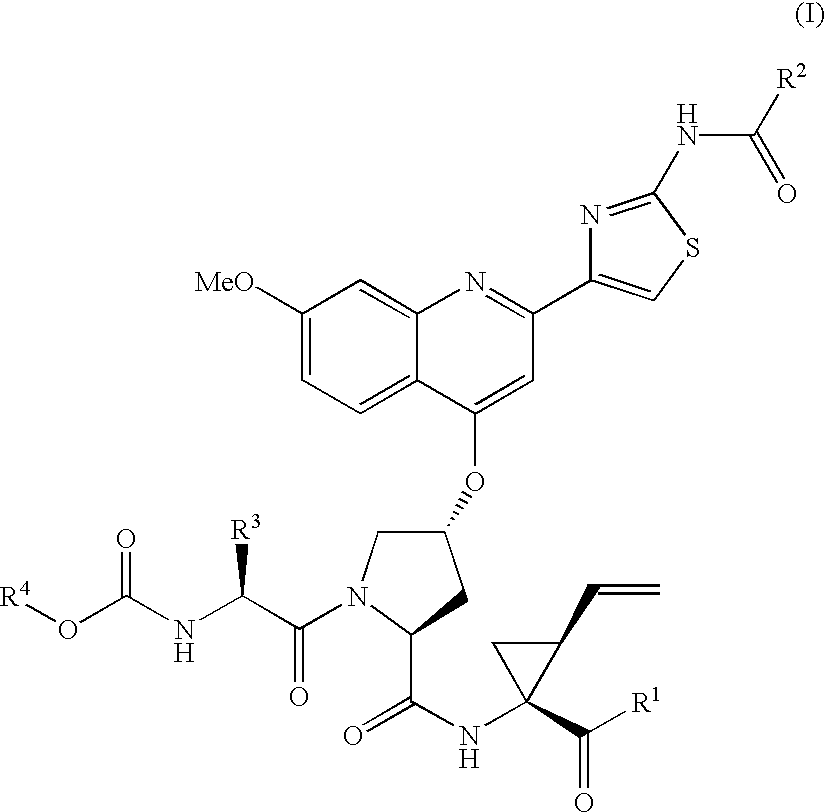
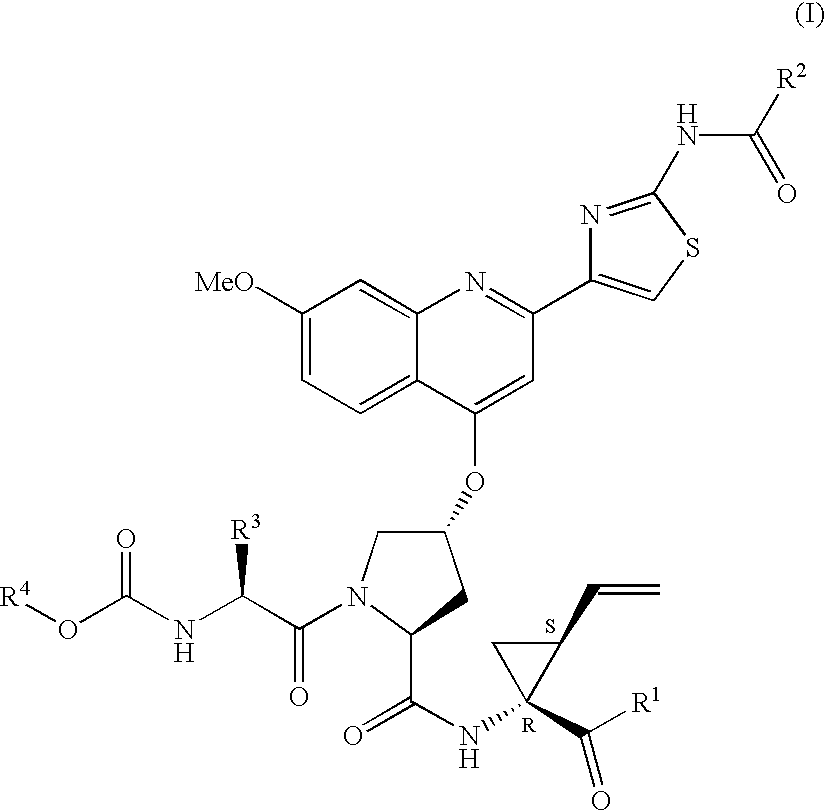
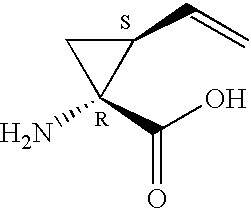
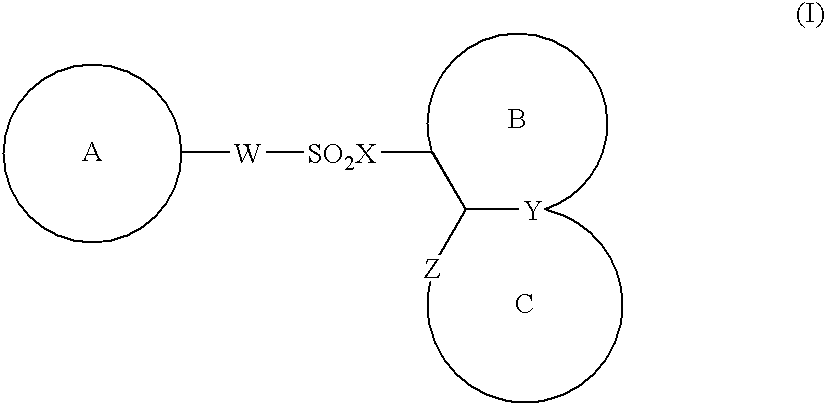
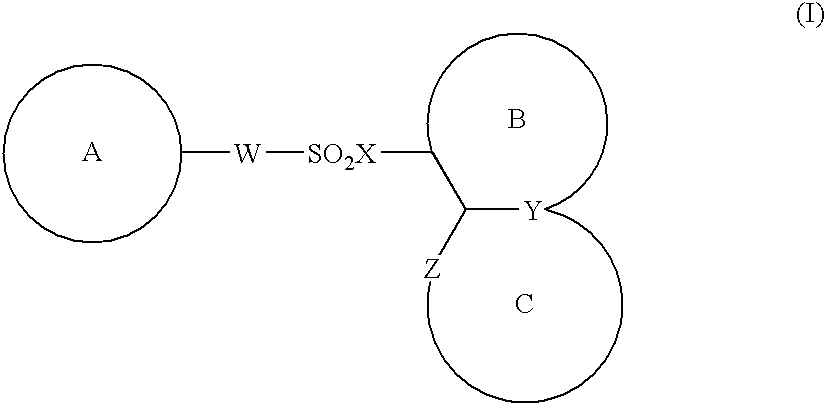
Compounds of formula (I):wherein R1 is hydroxyl or sulfonamide derivative; R2 is t-butyl or —CH2—C(CH3)3 or —CH2-cyclopentyl; R3 is t-butyl or cyclohexyl and R4 is cyclobutyl, cyclopentyl or cyclohexyl; or a pharmaceutically acceptable salt thereof, are described as useful as inhibitor of the HCV NS3 protease.
Owner:BOEHRINGER INGELHEIM INT GMBH
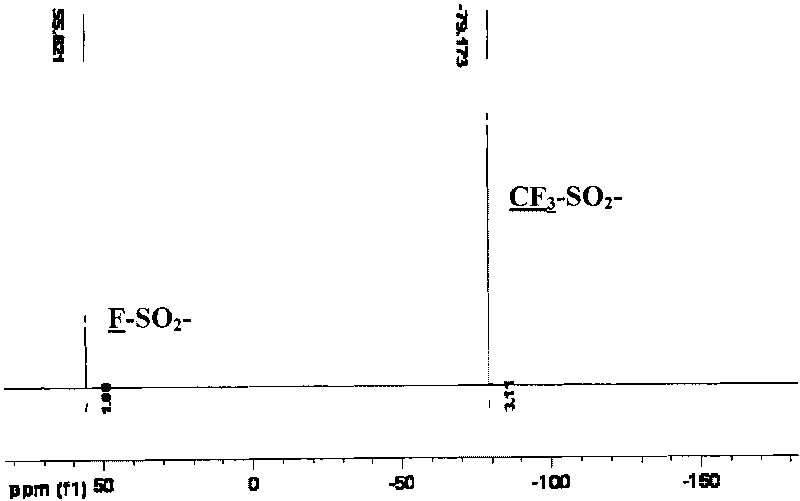
Method for preparing bi-(sulfonyl fluoride) imine and (fluorinated alkyl sulfonyl fluorine sulfonyl) imine alkali metal salt
ActiveCN101747242AAvoid product agglomerationSimple and efficient operationSulfonic acid amide preparationSolventDecomposition
The invention discloses a method for preparing bi-(sulfonyl fluoride) imine and (fluorinated alkyl sulfonyl fluorine sulfonyl) imine alkali metal salt. The method comprises the following steps of: reacting sulfamide and thionyl chloride and chlorosulfonic acid to prepare bi-(sulfonyl chlorine) imine and (fluorinated alkyl sulfonyl chlorine sulfonyl) imine, reacting with antimony trifluoride and potassium carbonate (rubidium or caesium) to obtain corresponding high-purity bi-(sulfonyl fluoride) imine potassium (rubidium or caesium) salt or (fluorinated alkyl sulfonyl fluorine sulfonyl) imine potassium (rubidium or caesium) salt, performing double decomposition exchange reaction using the potassium (rubidium or caesium) salt with the lithium perchlorate (or sodium) or lithium tetrafluoroborate (or sodium) in the aprotic polar solvent to obtain corresponding lithium (or sodium) salt with high purity. The method in the invention has the characteristics of simple operating steps, easy separation and extraction of output, high purity and yield, no environmental pollution, and the like, and is suitable for mass industrial production.
Owner:武汉市瑞华新能源科技有限公司
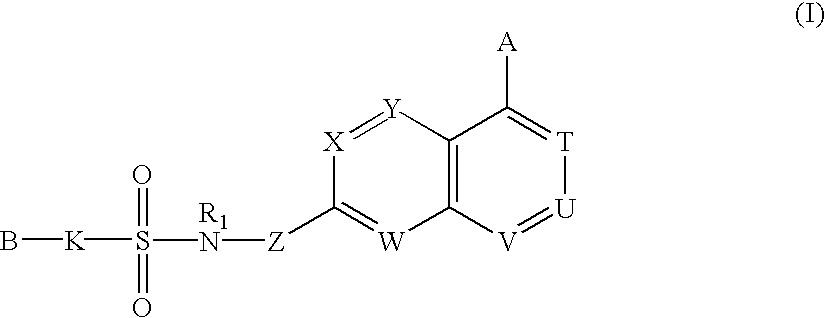
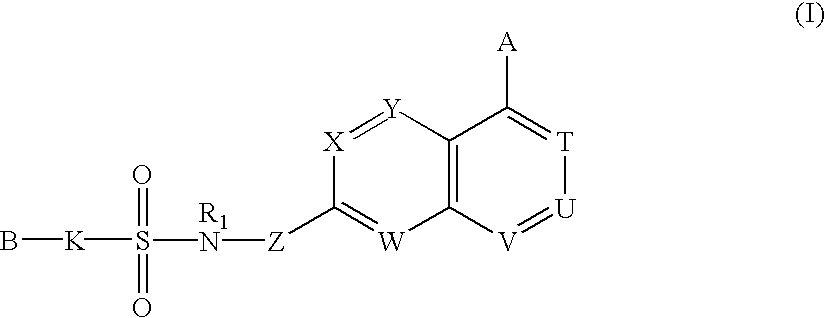
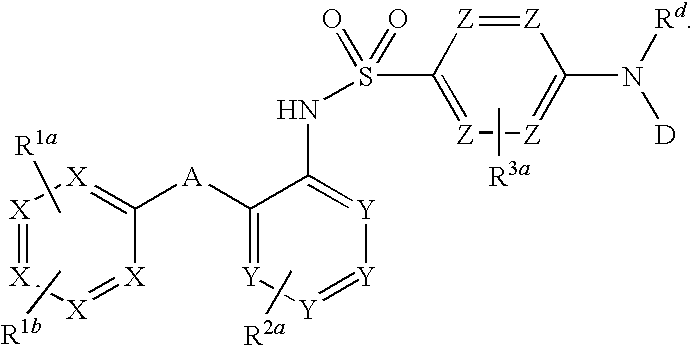
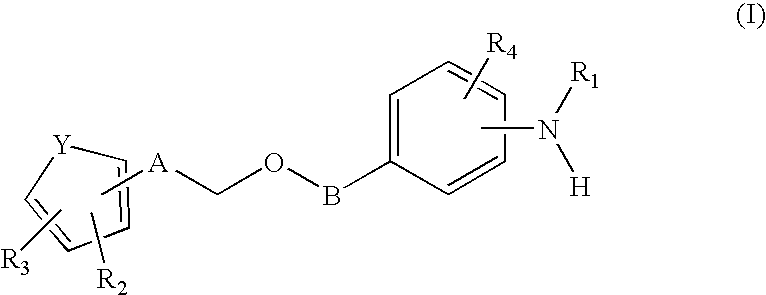
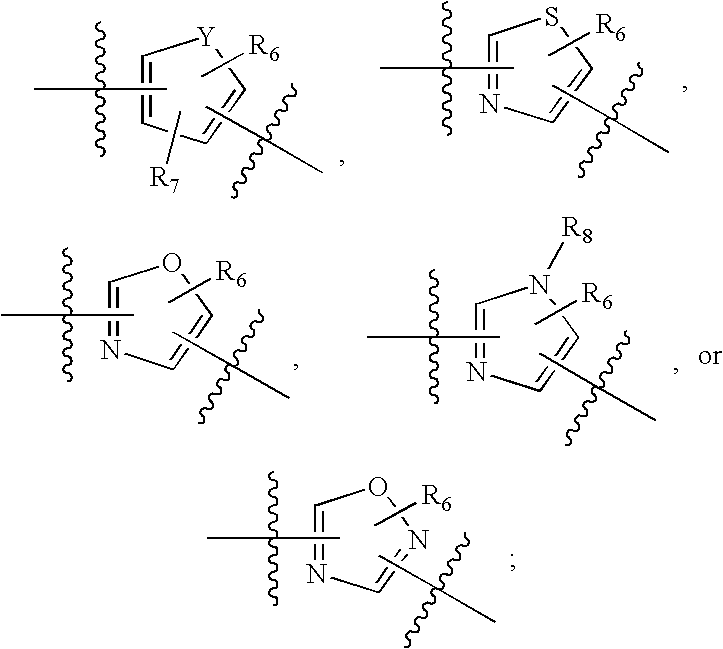
Sulfonamide-containing heterocyclic compounds
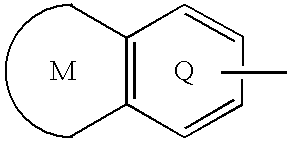
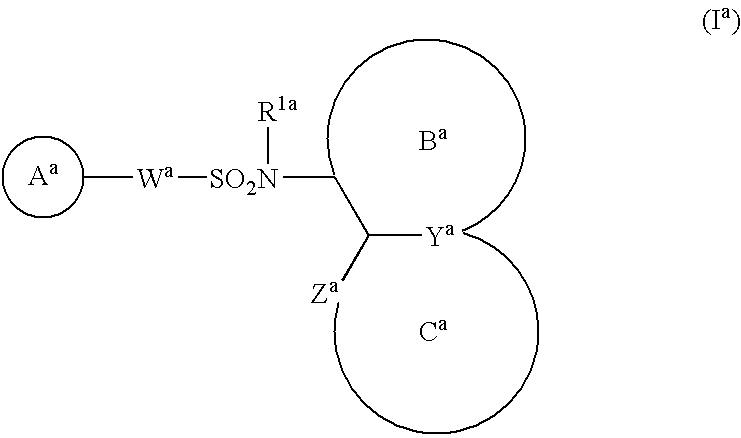
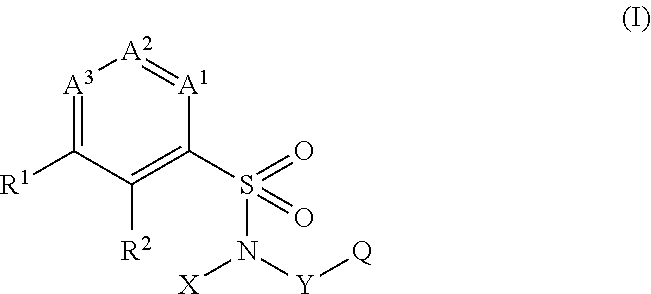
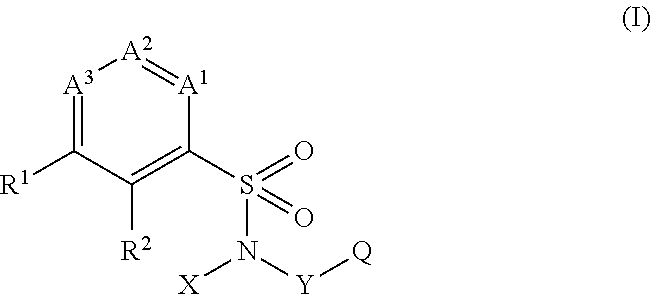
The present invention provides a sulfonamide- or sulfonylurea-containing heterocyclic compounds. Specifically, it provides a heterocyclic compound represented by the formula (I), a pharmacologically acceptable salt thereof or a hydrate of them.In the formula, A is hydrogen atom, a halogen atom, a C1-C4 alkyl or alkoxy group which may be substituted with a halogen atom, or cyano group; B is an optionally substituted aryl group or monocyclic heteroaryl group, or:(wherein, the ring Q is an aromatic ring which may have nitrogen atom; and the ring M is a ring sharing a double bond with the ring Q, which ring may have a heteroatom; and the rings Q and M may share nitrogen atom); K is a single bond; T, W, X and Y are the same as or different from each other and each is =C(D)- (wherein, D is hydrogen or a halogen atom) or nitrogen atom; U and V are the same as or different from each other and each is =C(D)-, nitrogen atom, -CH2-, oxygen atom or -CO-; Z is a single bond or -CO-NH-; and R1 is hydrogen atom, etc.
Owner:EISIA R&D MANAGEMENT CO LTD
Medicinal compositions for concomitant use as anticancer agent
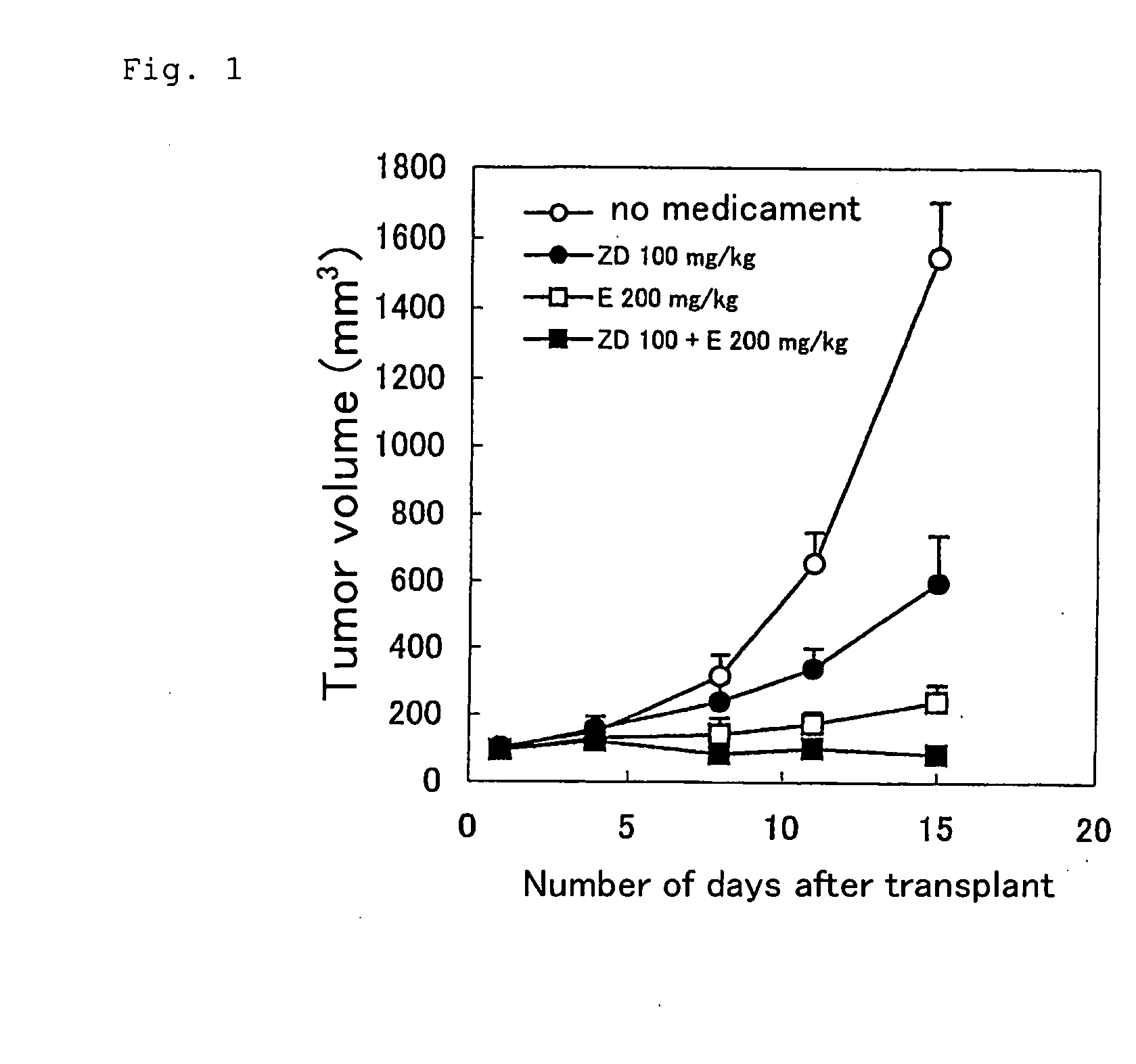
InactiveUS20030215523A1Good synergyEliminate side effectsHeavy metal active ingredientsBiocideCarboplatinAnticarcinogen
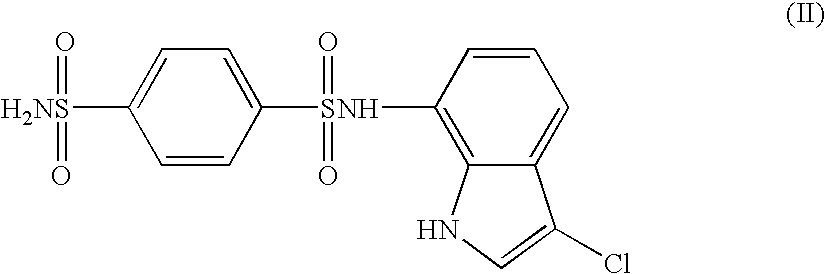
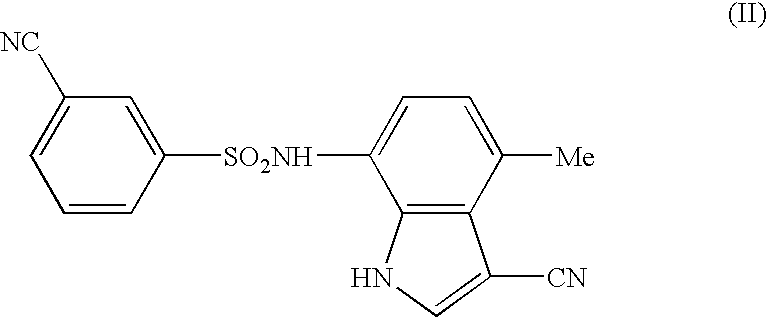
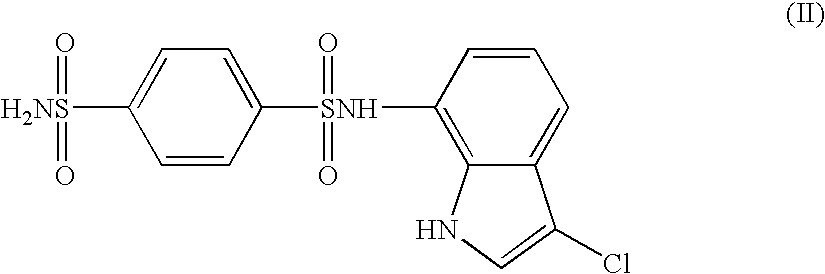
The present invention provides a medicinal composition having an excellent antitumor activity. That is, it provides a medicinal composition comprising a sulfonamide compound, a sulfonate compound or a salt of them, which is represented by the following formula: (wherein ring A represents an aromatic ring which may have a substituent group; ring B represents a 6-membered unsaturated hydrocarbon ring which may have a substituent group etc.; ring C represents a 5-membered hetero-ring containing one or two nitrogen atoms, and the ring C may have a substituent group; W represents a single bond or -CH=CH-; X represents -NH- etc.; and Y represents a carbon atom or a nitrogen atom; and Z represents -NH- etc.), particularly N-(3-chloro-1H-indol-7-yl)-4-sulfamoylbenzenesulfonamide or a salt thereof, combined with at least one substance selected from (1) irinotecan hydrochloride trihydrate; (2) mitomycin C; (3) 5-fluorouracil; (4) cisplatin; (5) gemcitabine hydrochloride; (6) doxorubicin; (7) taxol; (8) carboplatin; (9) oxaliplatin; (10) capecitabine; and (11) a salt of the above-mentioned (1) to (10).
Owner:EISIA R&D MANAGEMENT CO LTD
Pyrazole-amides and -sulfonamides
Owner:ICAGEN INC
Hydroxyethylamino sulphonamides useful as retroviral protease inhibitors
The invention relates to sulfonamide-containing hydroxyethylamine protease inhibitor compounds, their process of making, composition and method of use for inhibiting retroviral proteases such as human immunodeficiency virus.
Owner:GD SEARLE & CO
Antitumor agent comprising combination of sulfonamide-containing heterocyclic compound with an angiogenesis inhibitor
InactiveUS20050119303A1Efficient combinationBiocideAnimal repellantsAbnormal tissue growthAngiogenesis growth factor
The present invention provides a composition and a kit for treating tumors, which permits a sulfonamide-containing heterocyclic compound to exhibit its angiogenesis inhibitory activity and antitumor activity more effectively. According to the present invention, the sulfonamide-containing heterocyclic compound can be used in treating cancers more effectively by combination with a VEGF inhibitor / FGF inhibitor.
Owner:EISIA R&D MANAGEMENT CO LTD
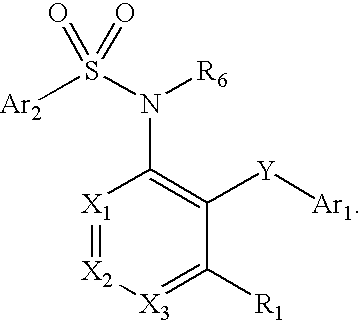
Heterocyclic sulfonamides as modulators of cardiac sarcomeres
Certain substituted sulfonamide derivatives of Formula I selectively modulate the cardiac sarcomere, for example by potentiating cardiac myosin, and are useful in the treatment of systolic heart failure including congestive heart failure.
Owner:CYTOKINETICS INC
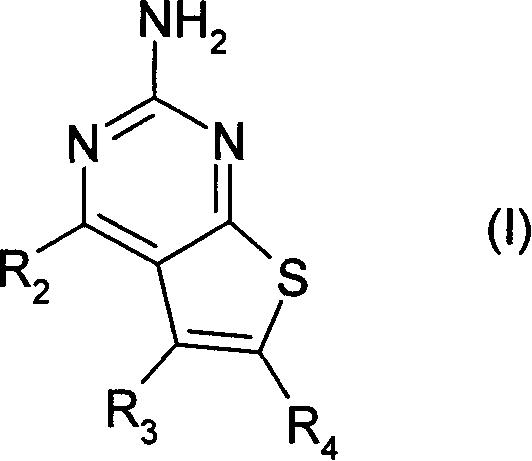
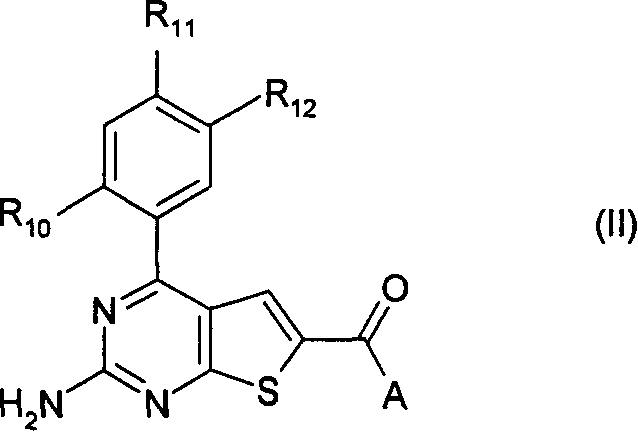
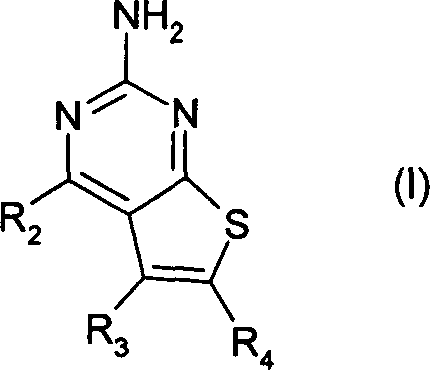
Pyrimidothiophene compounds
InactiveCN1842532APrevent proliferationOrganic active ingredientsOrganic chemistryArylCarboxylic ester
Compounds of formula (1) are inhibitors of HSP90 activity in vitro or in vivo, and of use in the treatment of cancer: wherein R2 is a group of formula -(Ar<1>)m-(Alk<1>)P-(Z)r-(Alk2)S-Q wherein Ar<1> is an optionally substituted aryl or heteroaryl radical, Alk' and Alk 2 are optionally substituted divalent C1-C3 alkylene or C2-C3 alkenylene radicals, m, p, r and s are independently 0 or 1, Z is -0-, -S-, -(C=O)-, -(C=S)-, -S02-, -C(=O)O-, -C(=O) NR - , -C(=S)NR -, -S02NR -, -NR C(=O)_, -NR S02- or-NR -wherein R is hydrogen or C1-C6 alkyl, and Q is hydrogen or an optionally substituted carbocyclic or heterocyclic radical; R3 is hydrogen, an optional substituent, or an optionally substituted (C1-C6)alkyl, aryl or heteroaryl radical; and R4 is a carboxylic ester, carboxamide or sulfonamide group.
Owner:VERNALIS (R&D) LTD +1
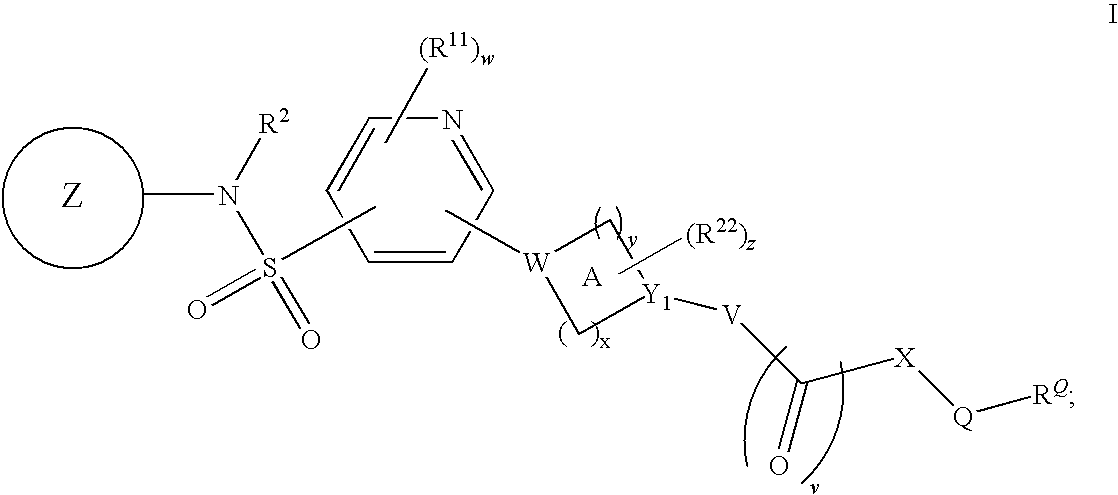
Pyridyl sulfonamides as modulators of ion channels
The present invention relates to pyridyl sulfonamide derivatives useful as inhibitors of ion channels. The invention also provides pharmaceutically acceptable compositions comprising the compounds of the invention and methods of using the compositions in the treatment of various disorders.
Owner:VERTEX PHARMA INC
Triazolyl phenyl benzenesulfonamides
Compounds are provided that act as potent antagonists of the CCR2 or CCR9 receptor. Animal testing demonstrates that these compounds are useful for treating inflammation, a hallmark disease for CCR2 and CCR9. The compounds are generally aryl sulfonamide derivatives and are useful in pharmaceutical compositions, methods for the treatment of CCR2-mediated diseases, CCR9-mediated diseases, as controls in assays for the identification of CCR2 antagonists and as controls in assays for the identification of CCR9 antagonists.
Owner:CHEMOCENTRYX INC
Fluorescent ion probe and its application in ion detecting
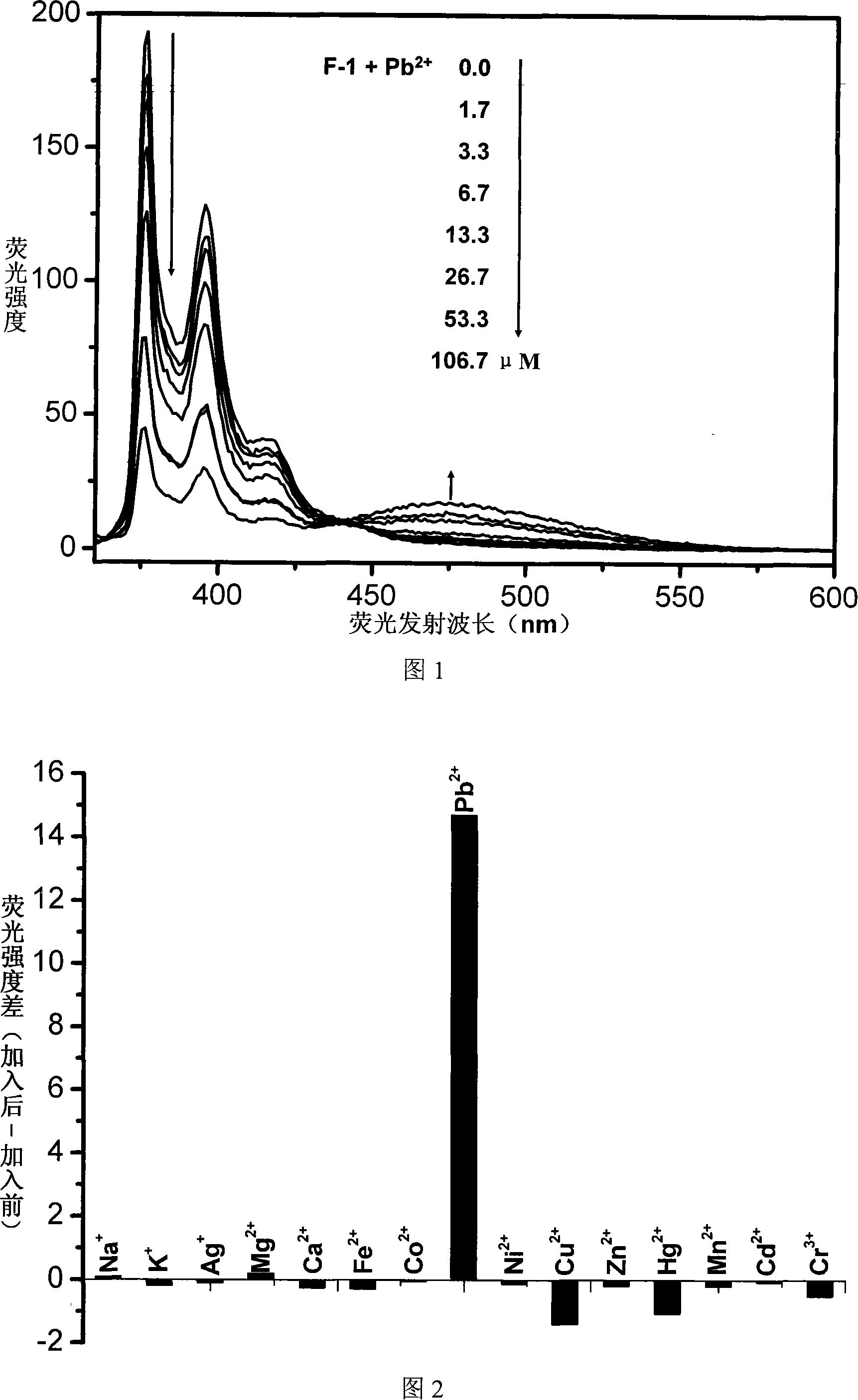
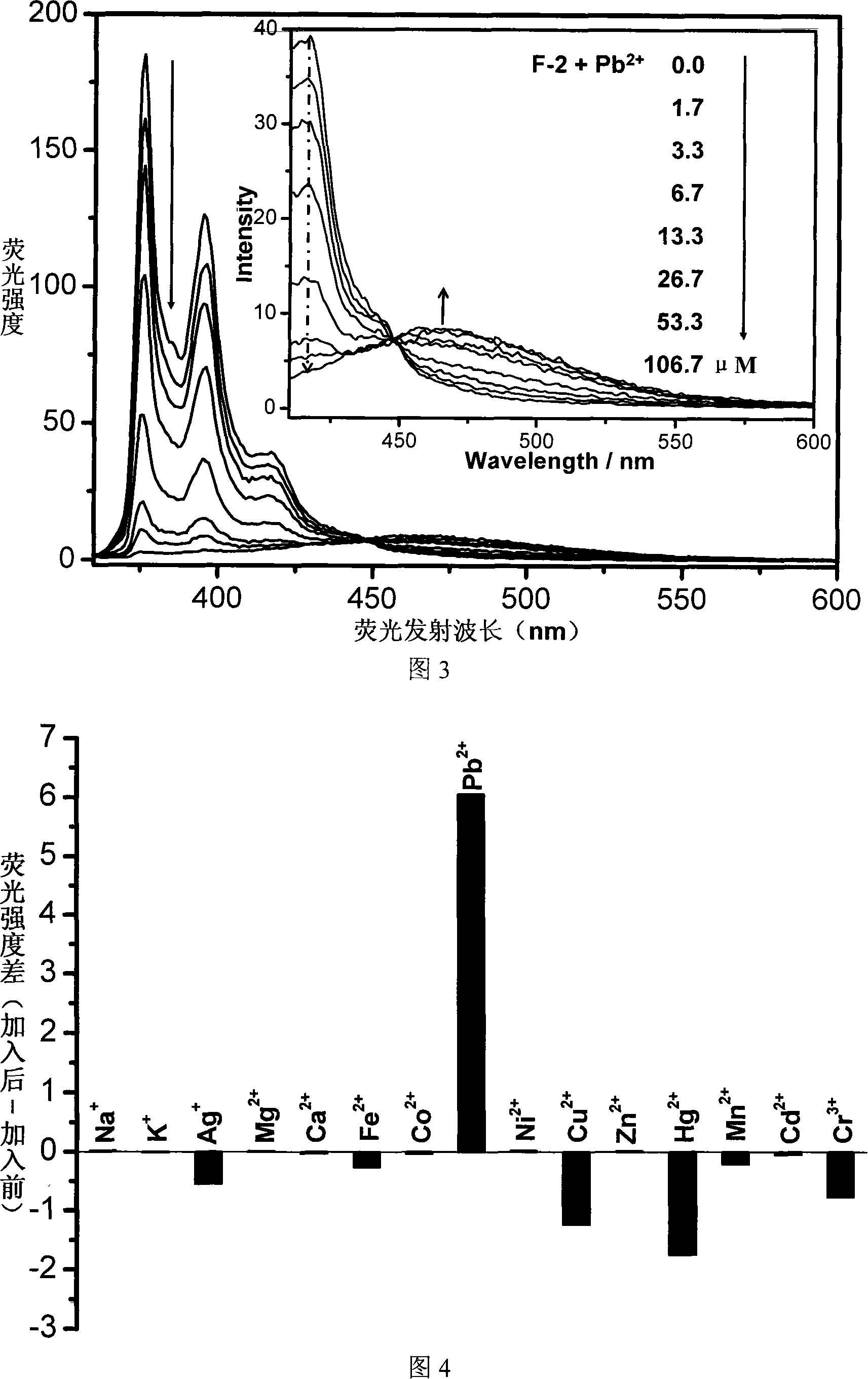
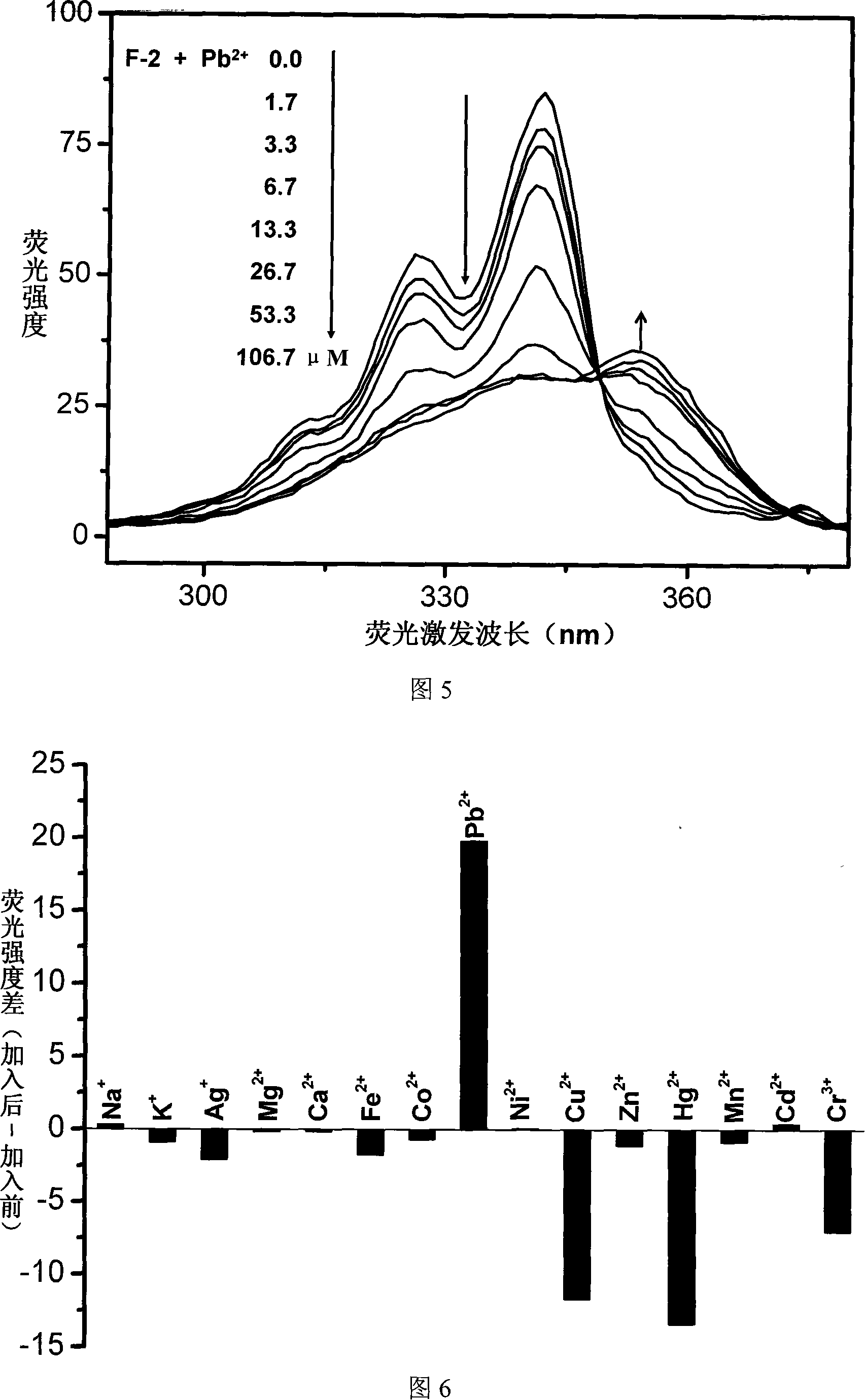
InactiveCN101153848AEasy to synthesizeSimple structureChemiluminescene/bioluminescenceLuminescent compositionsBenzoxazoleFluorophore
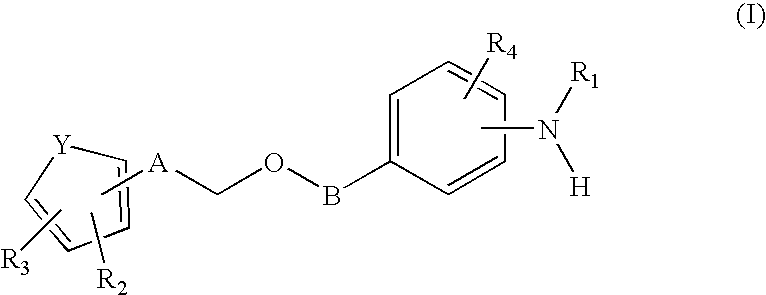
The invention relates to a fluorescent ion probe (I) with high selectivity and high sensitivity in detection and the application of the fluorescent ion probe in identifying and detecting heavy metal ion and transition metal ion, wherein, the Y is an organic conjugate group with fluorescence transmitting function such as pyrene, naphthalene, 4-amidogen-1, 8-naphthyl imide, Dan sulfonamide, anthracene, carbazole, benzimidazoins, benzoxazoles, boron fluoride bipyrrole (BODIPY), fluorescein, 3, 4, 9, 10-perylenetetracarboxylic diimide or rhodamine B; the X is acylamino group, sulfoamino group or ester group. The fluorescent ion probe uses the fluorescence peak of fluorescence chromophore aggregate as the response signal to identify metal ion, thereby effectively avoiding the quenching effect of transition metal ion and heavy metal ion on fluorophore; moreover, the fluorescent ion probe realizes selective identification of heavy metal ion and transition metal ion in various solvents and aqueous solution in particular.
Owner:JILIN UNIV
Aryl substituted 3-ethoxy phenyl trifluoromethane sulfonamides for the treatment of non-insulin dependent diabetes mellitus (NIDDM)
This invention provides compounds of formula I, having the structure wherein R1, R2, R3, R4, A, and B are as defined in the specification, or a pharmaceutically acceptable salt thereof, that are useful in treating metabolic disorders mediated by insulin resistance or hyperglycemia.
Owner:WYETH
Planographic printing plate precursor
InactiveUS20050064325A1Improve solubilityHighly lipophilicPhotosensitive material auxillary/base layersPhotosensitive materials for photomechanical apparatusImideRecording layer
The planographic printing plate precursor of the invention includes a recording layer capable of forming an image upon irradiation with infrared rays, wherein the recording layer comprises (A) an alkali-soluble resin having, in a main chain, a structural unit containing at least one type of bond selected from an amide bond, urea bond, urethane bond and ester bond and having at least one type of acid group selected from a phenolic hydroxyl group, sulfonamide group and active imide group, and (B) an infrared absorbing agent. The invention provides a positive-working planographic printing plate precursor excellent in printing durability and chemical resistance.
Owner:FUJIFILM CORP +1
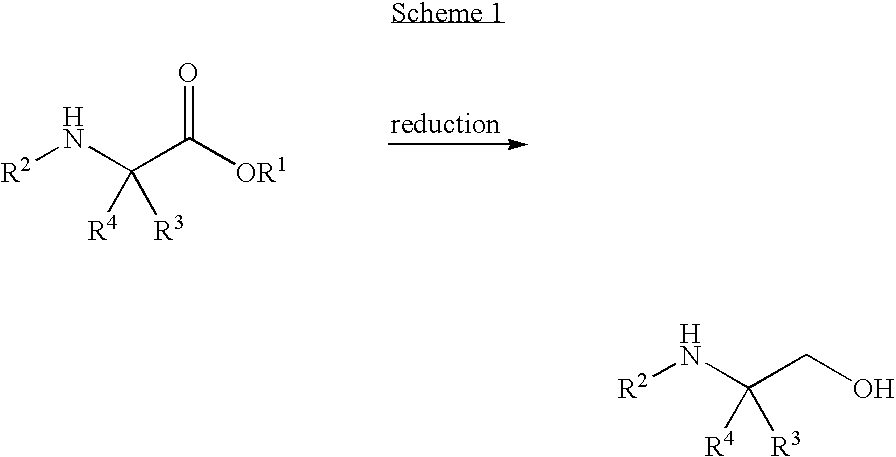
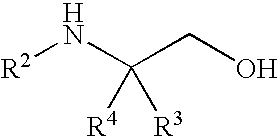
Methods for preparing sulfonamide substituted alcohols and intermediates thereof
InactiveUS7687666B2Readily apparentNervous disorderOrganic compound preparationAlcoholStereochemistry
Processes for preparing amino alcohols or salts thereof and sulfonamide substituted alcohol compounds are provided. Desirably, the sulfonamide substituted alcohol compounds are heterocyclic sulfonamide trifluoroalkyl-substituted alcohol compounds or phenyl sulfonamide trifluoroalkyl-substituted alcohol compounds.
Owner:WYETH LLC
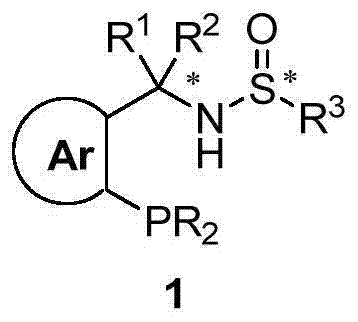
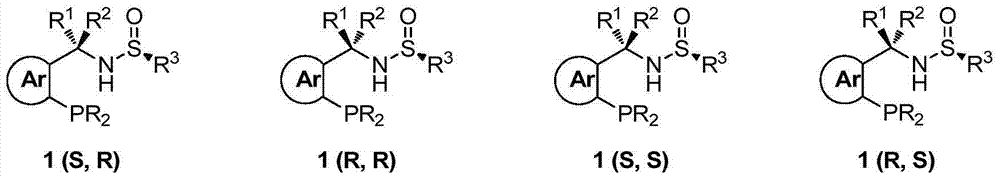
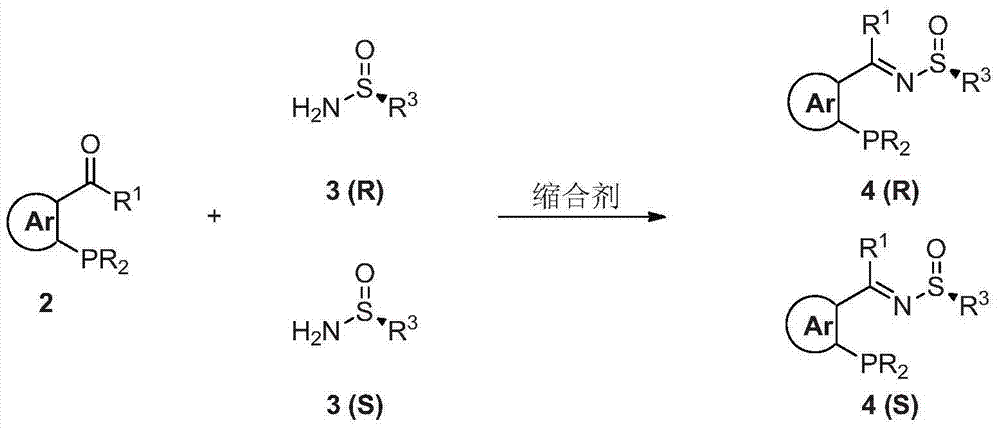
Chiral sulfinylamine monophosphine, and full-configuration preparation method and application thereof
The invention provides a chiral sulfinylamine monophosphine and a preparation method thereof. The preparation method comprises the following steps: carrying out a condensation reaction of 2-disubstituted phosphinoaryl(heteroaryl)formaldehyde (ketone) 2 and chiral sulfonamide 3 to obtain a compound 4, and reacting the compound 4 with a nucleophilic reagent to prepare a compound 1; or carrying out a condensation reaction of aldehyde (ketone) 5 and the chiral sulfonamide 3 to obtain imine 6, and reacting the imine 6 with a 2-disubstituted phosphinoaryl(heteroaryl) metal reagent to obtain the compound 1; or reacting the imine 6 with the 2-disubstituted phosphinoaryl(heteroaryl) metal reagent to obtain a compound, and reducing the compound to obtain the compound 1; or carrying out a condensation reaction of 2-substituted phosphinoaryl(heteroaryl)formaldehyde (ketone) 8 and the chiral sulfonamide 3 to obtain an imine compound 9, reacting the imine compound 9 with the nucleophilic reagent to obtain a compound 7, and reducing the compound 7 to obtain the compound 1. Different chiral sulfenamides and different metal reagents are used to conveniently obtain the optically pure compound of four configurations comprising (R,R), (R,S), (S,S) and (S,R). The above ligand has the advantages of simple skeleton, synthesis convenience and easy reconstruction, can be applied in various metal-catalyzed asymmetric reactions, and has a very high reaction activity and stereoselectivity.
Owner:苏州凯若利新材料科技有限公司
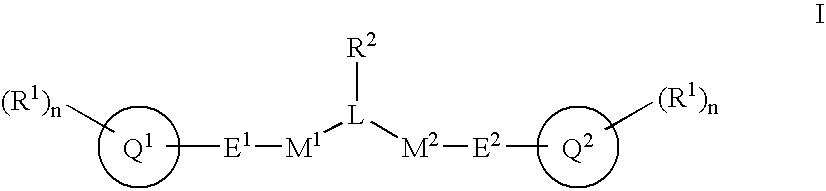
Alpha, beta-unsaturated sulfones, sulfoxides, sulfonimides, sulfinimides, acylsulfonamides and acylsulfinamides and therapeutic uses thereof
α,β-Unsaturated sulfones, sulfoxides, sulfonimides, sulfinimides, acylsulfonamides and acylsulfinamides of Formula I:wherein R1, R2, M1, M2, L, E1, E2, Q1, Q2 and n are as defined herein, are useful as anti-angiogenesis agents, as agents for treatment of age related senile dementia, and as antiproliferative agents including, for example, as anticancer agents.
Owner:TEMPLE UNIVERSITY
Medicinal compositions for cocominant use as anticancer agent
The present invention provides a medicinal composition having an excellent antitumor activity. That is, it provides a medicinal composition comprising a sulfonamide compound, a sulfonate compound or a salt of them, which is represented by the following formula: wherein ring A represents an aromatic ring which may have a substituent group; ring B represents a 6-membered unsaturated hydrocarbon ring which may have a substituent group etc.; ring C represents a 5-membered hetero-ring containing one or two nitrogen atoms, and the ring C may have a substituent group; W represents a single bond or -CH=CH-; X represents -NH- etc.; and Y represents a carbon atom or a nitrogen atom; and Z represents -NH- etc.), particularly N-(3-chloro-1H-indol-7-yl) -4-sulfamoylbenzenesulfonamide or a salt thereof, combined with at least one substance selected from (1) irinotecan hydrochloride trihydrate; (2) mitomycin C; (3) 5-fluorouracil; (4) cisplatin; (5) gemcitabinehydrochloride; (6) doxorubicin; (7) taxol; and (8) a salt of the above-mentioned (1) to (7).
Owner:EISIA R&D MANAGEMENT CO LTD
Sulfonamide aryl acetylene compound and use thereof
The present invention discloses a sulfonamide aryl acetylene compound having structure characteristics of formula I and a use thereof, and belongs to the technical field of chemical medicines. The compound has an activity of targetedly inhibiting protein kinase, and is able to conduct target treatment of hyperproliferative diseases in human, other mammal tumors, etc. caused by abnormal protein kinase.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
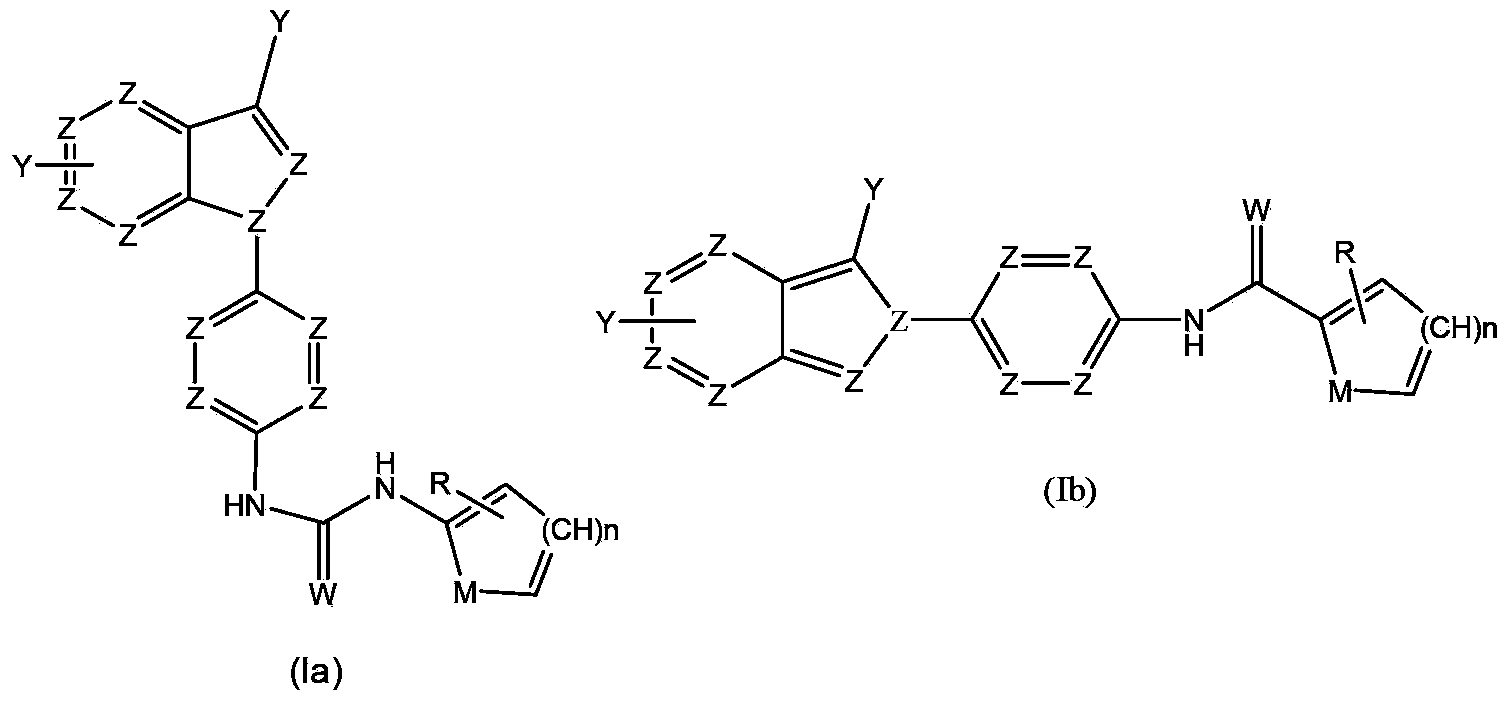
Anti-tumor medicine in double-aryl urea structure based on indazole, indole, azaindazole or azaindole
The invention belongs to the field of medicines, relates to anti-tumor medicines, and in particular relates to anti-tumor medicines in double-aryl urea structures based on indazole, indole, azaindazole or azaindole. The structural formulae of the anti-tumor medicines are shown in formula (Ia) and formula (Ib), wherein Z is N atom or C atom; W is O; M is O, S, N or CH; n is 1 or 2; Y and R are halogen atoms, H, R1, CF3, OCF3, OH, OR2, OCOR3, NH2, NHR4, NR52, NHCOR6, carboxyl group, ester group, cyano group, mercapto group, alkyl sulphanyl, sulfuryl, sulfoxide, sulfonic group, sulfamate, sulfonamide, ketonic group, aldehyde group, nitro group or nitroso group. Pharmacodynamic experiments prove that the medicines have good anti-tumor effects on human lung cancer, human kidney cancer, human colon cancer, human liver cancer, human gastric cancer, human breast cancer, melanoma and the like.
Owner:JINAN HAILE MEDICAL TECH DEV
.alpha.- and .beta.-amino acid hydroxyethylamino sulfonamides useful as retroviral protease inhibitors
InactiveUS6172082B1BiocidePeptide/protein ingredientsProteinase activityRetroviral protease inhibitor
.alpha.- and .beta.-amino acid hydroxyethylamino sulfonamide compounds are effective as retroviral protease inhibitors, and in particular as inhibitors of HIV protease.
Owner:GD SEARLE & CO
Layered resin molding and multilayered molded article
InactiveUS6893729B2Envelopes/bags making machinerySynthetic resin layered productsPolymer sciencePolyamide
The present invention is to provide a laminated resin molding comprising a polyamide-based resin composition as an outer layer and being excellent in interlayer adhesion strength, in particular a laminated resin molding comprising a fluorine-containing resin as an inner layer.The present invention is a laminated resin molding which comprises a layer (A) comprising a polyamide-based resin composition and a layer (B) laminated to said layer (A), said layer (B) comprising a fluorine-containing ethylenic polymer having a carbonyl group, and said polyamide-based resin composition having a functional group, in addition to an amide group, selected from the group consisting of hydroxyl group, carboxyl group, ester group and sulfonamide group in a total amount of 0.05 to 80 equivalent percent relative to the amide group.
Owner:DAIKIN IND LTD
Silicon-containing composition having sulfonamide group for forming resist underlayer film
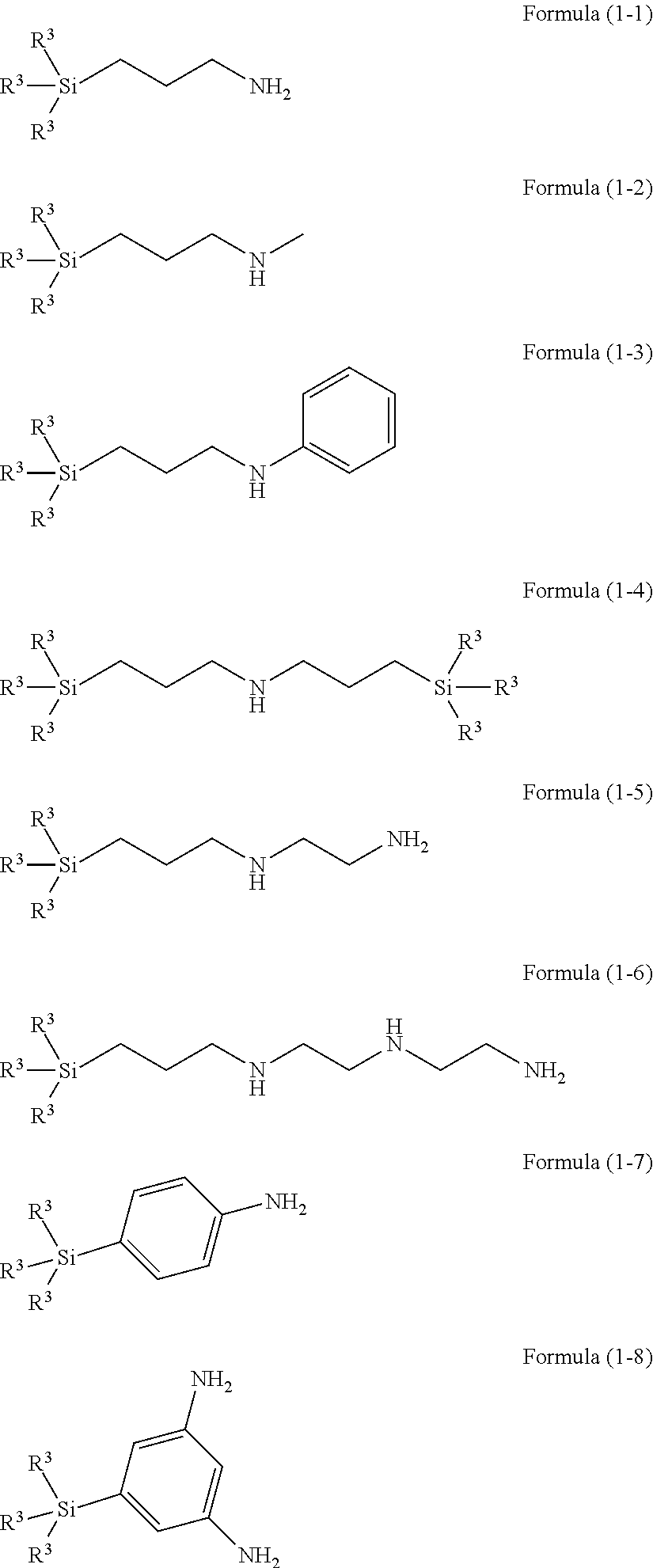
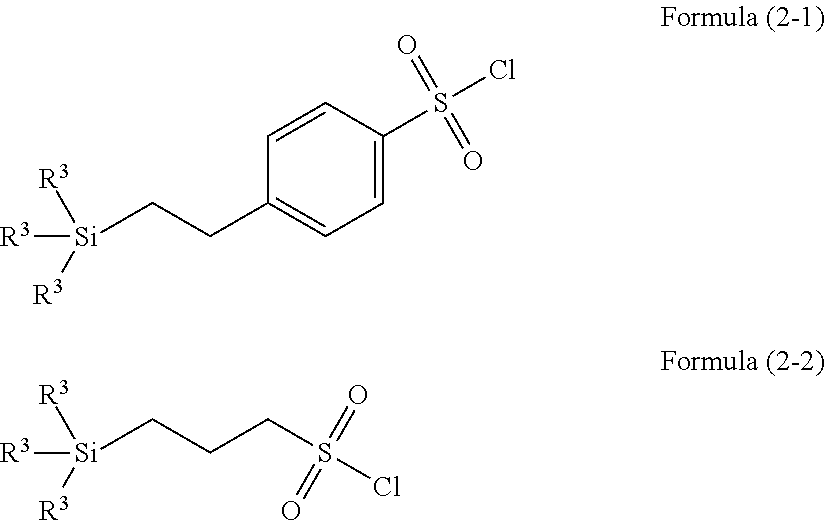
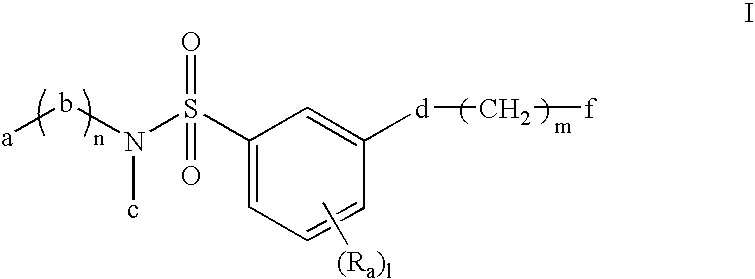
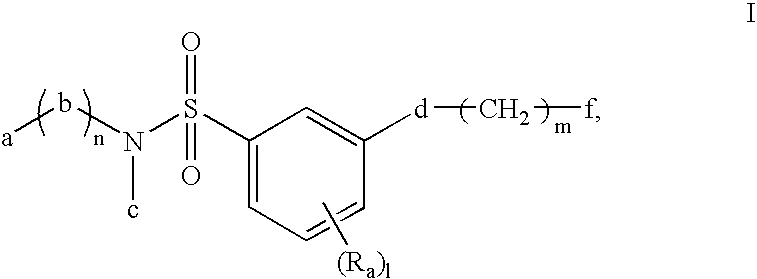
ActiveUS20120178261A1Improve adhesionGood resist shapeSilicon organic compoundsPhotoprinting processesSilane compoundsSilicon
There is provided a lithographic resist underlayer film-forming composition for forming a resist underlayer film which can be used as a hard mask. A lithographic resist underlayer film-forming composition including a silane compound having sulfonamide group, wherein the silane compound having sulfonamide group is a hydrolyzable organosilane having a sulfonamide group in the molecule, a hydrolyzate thereof, or a hydrolytic condensation product thereof. The composition including a silane compound having sulfonamide group and a silane compound lacking a sulfonamide group, wherein the silane compound having sulfonamide group is present within the silane compounds overall in a proportion of less than 1 mol %, for example 0.1 to 0.95 mol %.
Owner:NISSAN CHEM IND LTD
Sulfonyl amide inhibitors of calcium channel function
ActiveUS20050245535A1Blood pressureReduce blood pressureBiocideOrganic chemistryCalcium channelStereochemistry
Owner:BRISTOL MYERS SQUIBB CO
Antipsychotic sulfonamide-heterocycles, and methods of use thereof
One aspect of the present invention relates to heterocyclic compounds comprising a sulfonamide moiety. A second aspect of the present invention relates to the use of the heterocyclic compounds comprising a sulfonamide moiety to treat diseases, afflictions or maladies caused at least in part by abnormal activity of one or more GPCRs or ligand-gated ion channels. An additional aspect of the present invention relates to the synthesis of combinatorial libraries of the heterocyclic compounds comprising a sulfonamide moiety, and the screening of those libraries for biological activity, e.g., in animal models of psychosis.
Owner:SEPACOR INC
Aryl-and hetarylsulfonamides as active ingredients against abiotic plant stress
Use of substituted sulfonamides of the formula (I) or salts thereoffor enhancing stress tolerance in plants to abiotic stress, especially for enhancing plant growth and / or for increasing plant yield.
Owner:BAYER INTELLECTUAL PROPERTY GMBH
Thyroid receptor ligands
Thyroid receptor ligands are provided which have the general formula I wherein: R1 is R2 and R3 are the same or different and are hydrogen, halogen, alkyl of 1 to 4 carbons or cycloalkyl of 3 to 5 carbons, provided that at least one of R2 and R3 is other than hydrogen; R4 is R5 and R6 are the same or different and are selected from hydrogen, aryl, heteroaryl, alkyl, cycloalkyl, aralkyl or heteroaralkyl. R7 is aryl, heteroaryl, alkyl, aralkyl, or heteroaralkyl; R8 is aryl, heteroaryl, or cycloalkyl; R9 is R7 or hydrogen; R10 is hydrogen, halogen, cyano or alkyl; R11 and R12 are each independently selected from the group consisting of hydrogen, halogen, alkoxy, hydroxy (—OH) cyano, and alkyl; R13 is carboxylic acid (COOH) or esters thereof, phosphonic and phosphinic acid or esters thereof, sulfonic acid, tetrazole, hydroxamic acid, thiazolidinedione, acylsulfonamide, or other carboxylic acid surrogates known in the art; R14 and R15 may be the same or different and are selected from hydrogen and alkyl, or R14 and R15 may be joined together forming a chain of 2 to 5 methylene groups [—(CH2)m-, m=2, 3, 4 or 5], thus forming 3- to 6-membered cycloalkyl rings; R16 is hydrogen or alkyl of 1 to 4 carbons; R17 and R18 are the same or different and selected from hydrogen, halogen and alkyl; n is 0 or an integer from 1 to 4; X is oxygen (—O—), sulfur (—S—), sulfonyl (—SO2—), sulfenyl (—SO—) selenium (—Se—), carbonyl (—CO—), amino (—NH—) or methylene (—CH2-); wherein the substituents are as described herein. In addition, a method is provided for preventing, inhibiting or treating diseases or disorders associated with metabolism dysfunction or which are dependent upon the expression of a T3 regulated gene, wherein a compound as described above is administered in a therapeutically effective amount.
Owner:BRISTOL MYERS SQUIBB CO
Xanthene dyes comprising a sulfonamide group
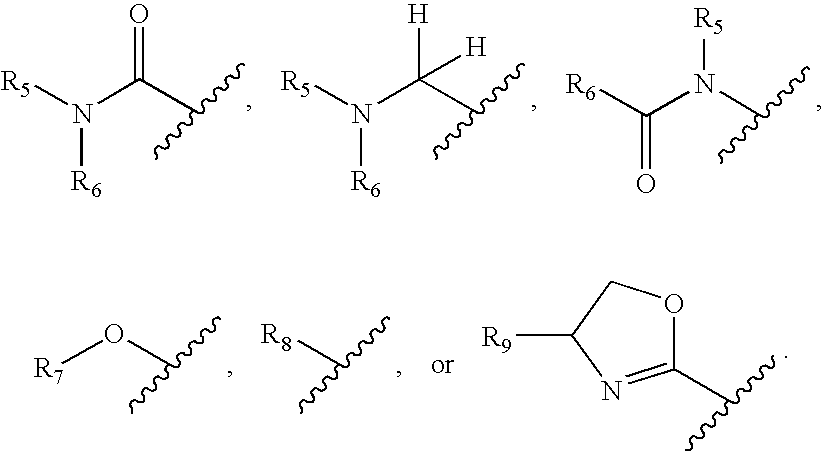
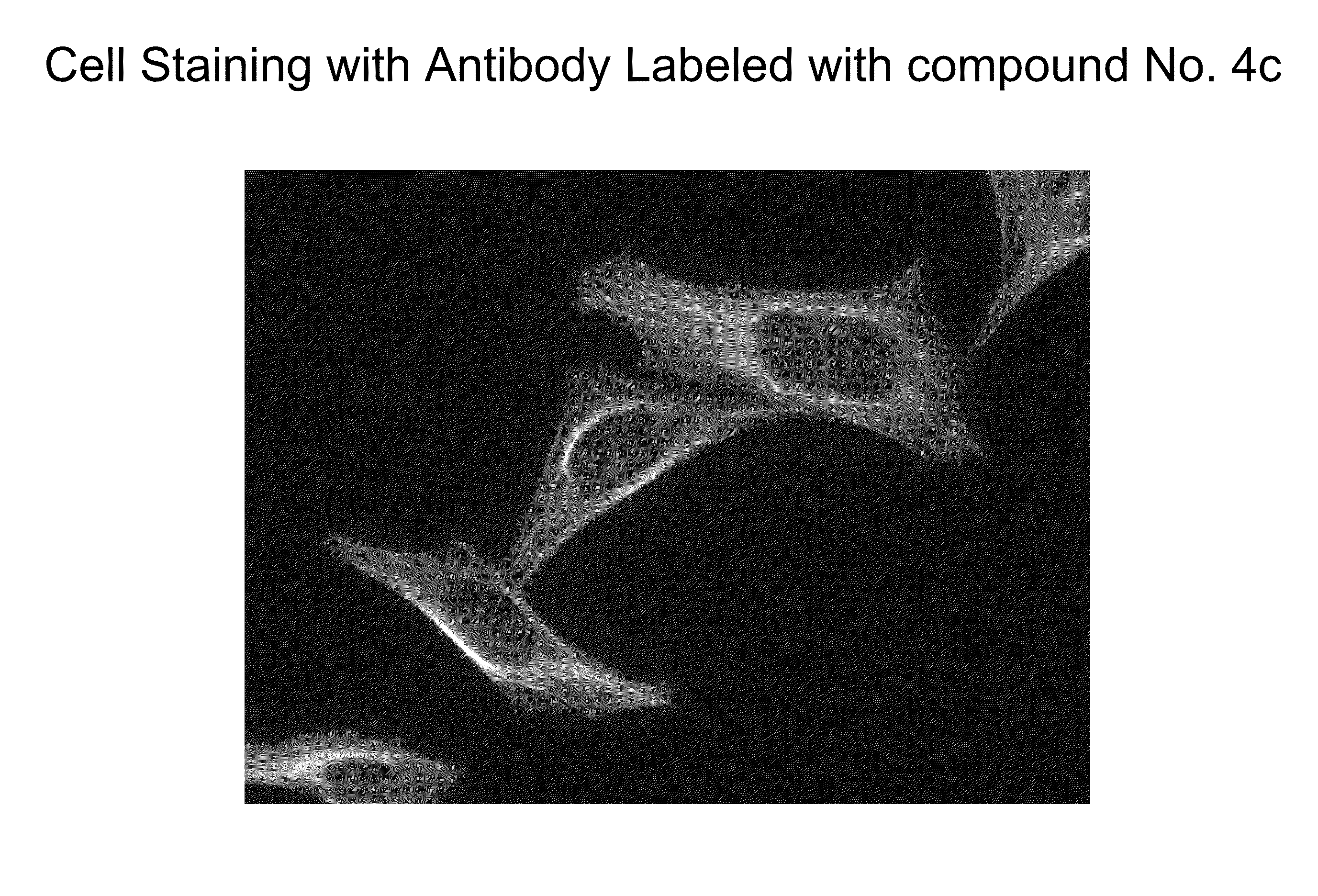
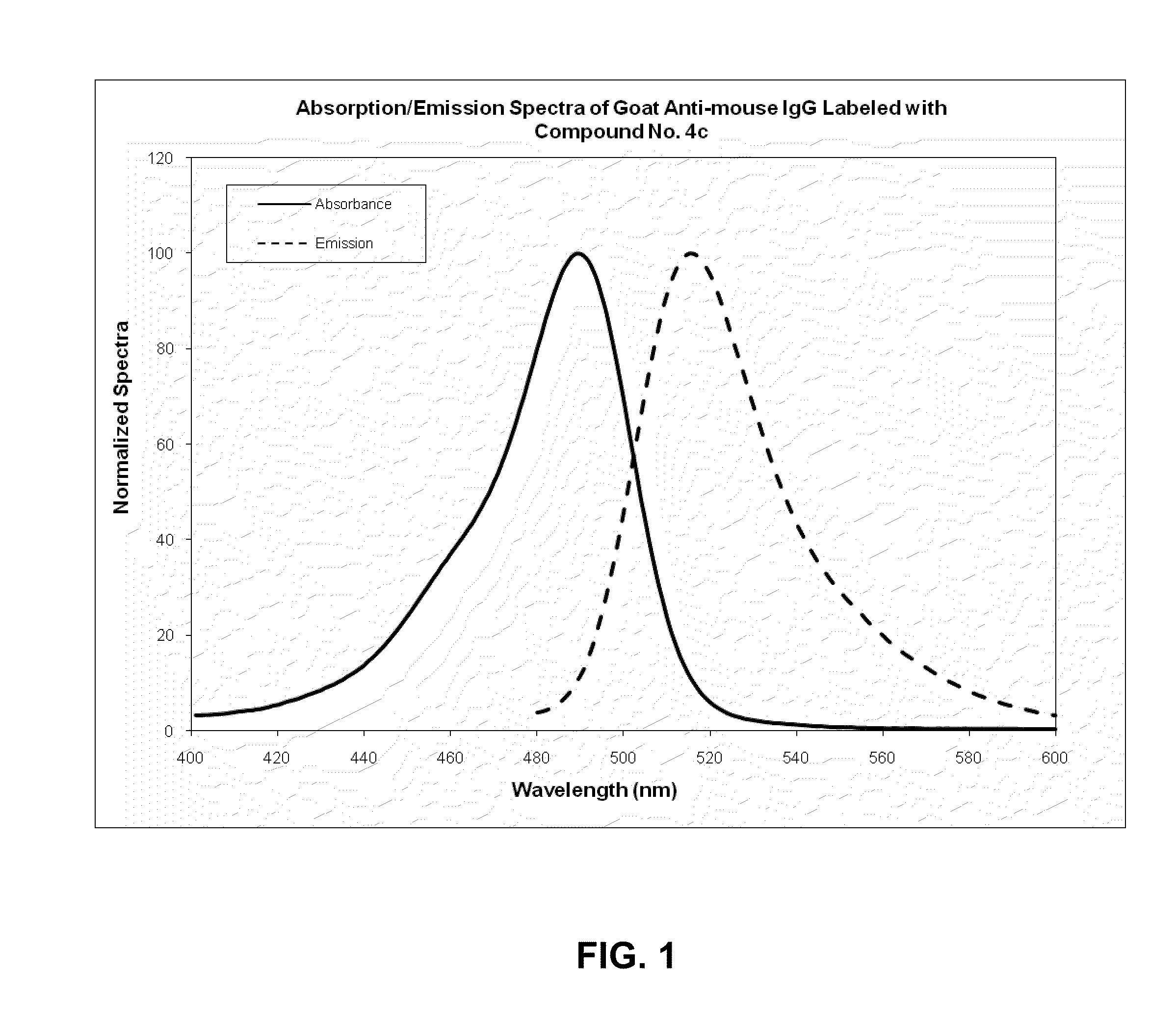
ActiveUS20100197030A1Convenient and effective labelingHigh fluorescence quantum yieldSugar derivativesPeptide preparation methodsDiseaseMicroorganism
The present invention relates to fluorescent dyes in general. The present invention provides a wide range of fluorescent dyes and kits containing the same, which are applicable for labeling a variety of biomolecules, cells and microorganisms. The present invention also provides various methods of using the fluorescent dyes for research and development, forensic identification, environmental studies, diagnosis, prognosis, and / or treatment of disease conditions.
Owner:BIOTIUM INC
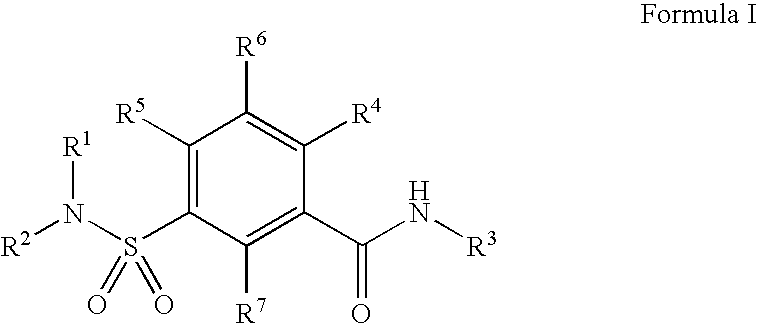
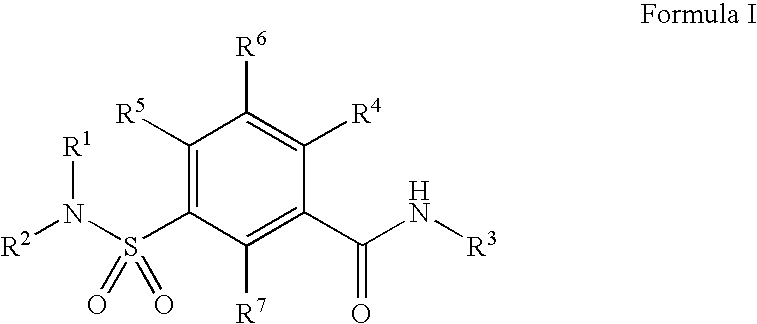
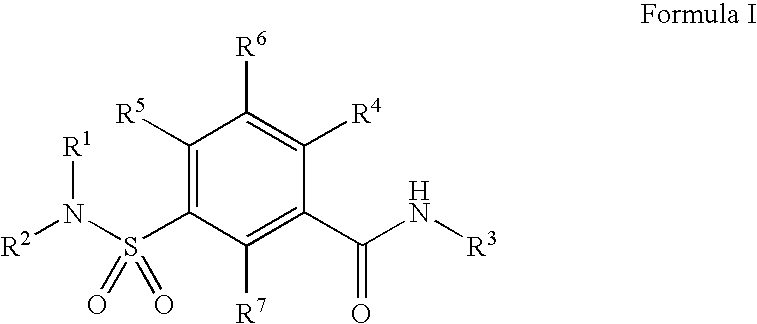
Sulfonamide compound as well as preparation method and application thereof
ActiveCN113773300AImprove securityStrong inhibitory activityOrganic chemistryAntipyreticDiseaseMetabolite
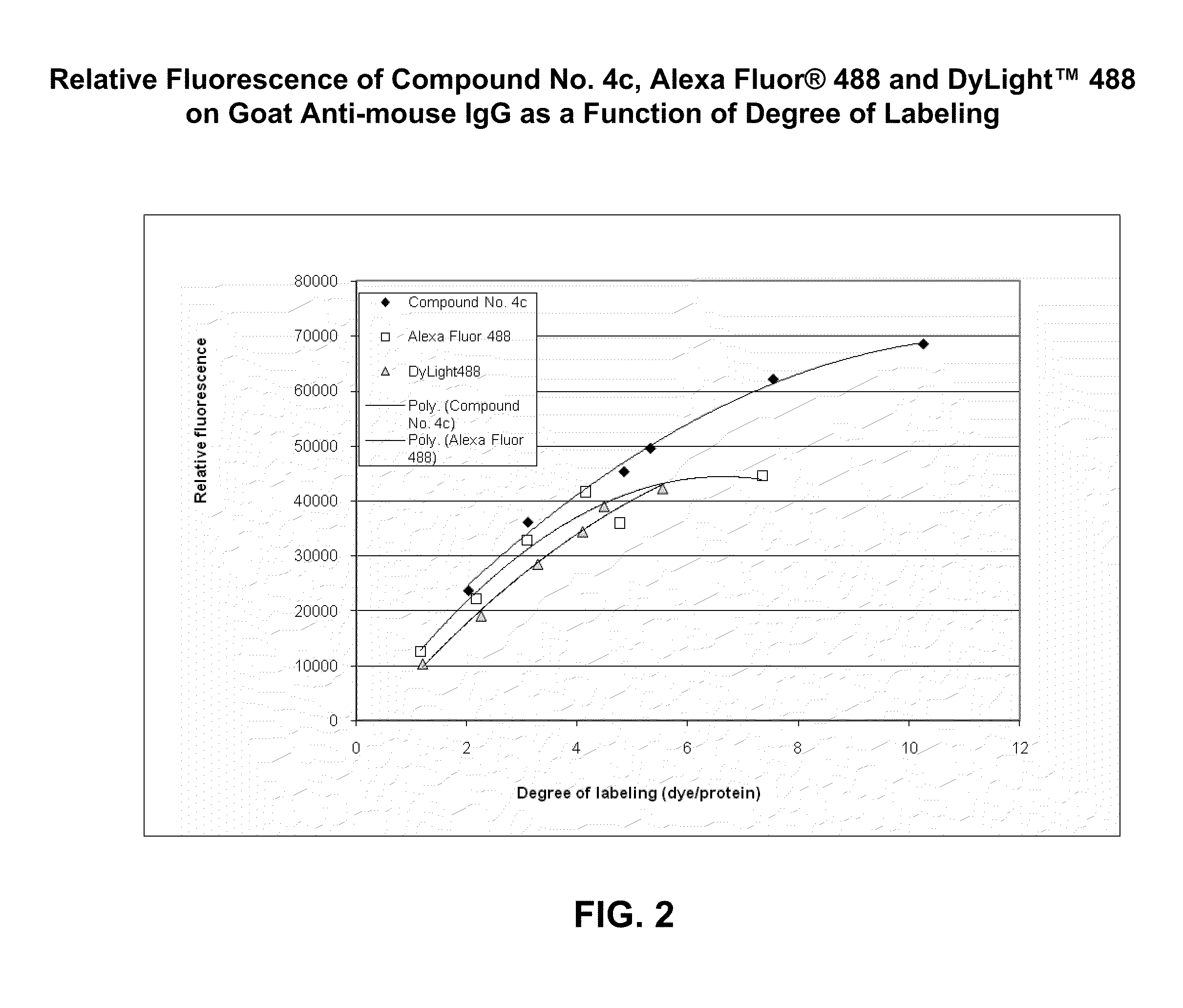
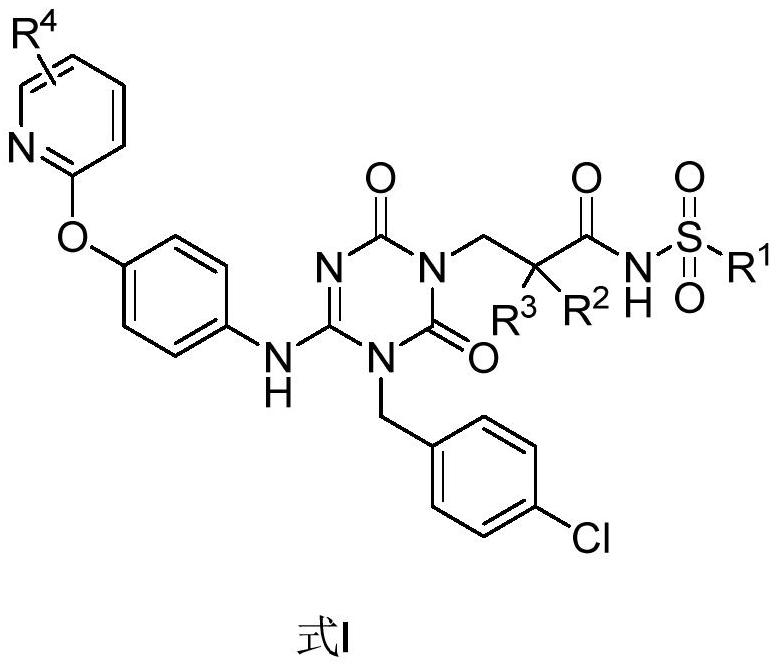
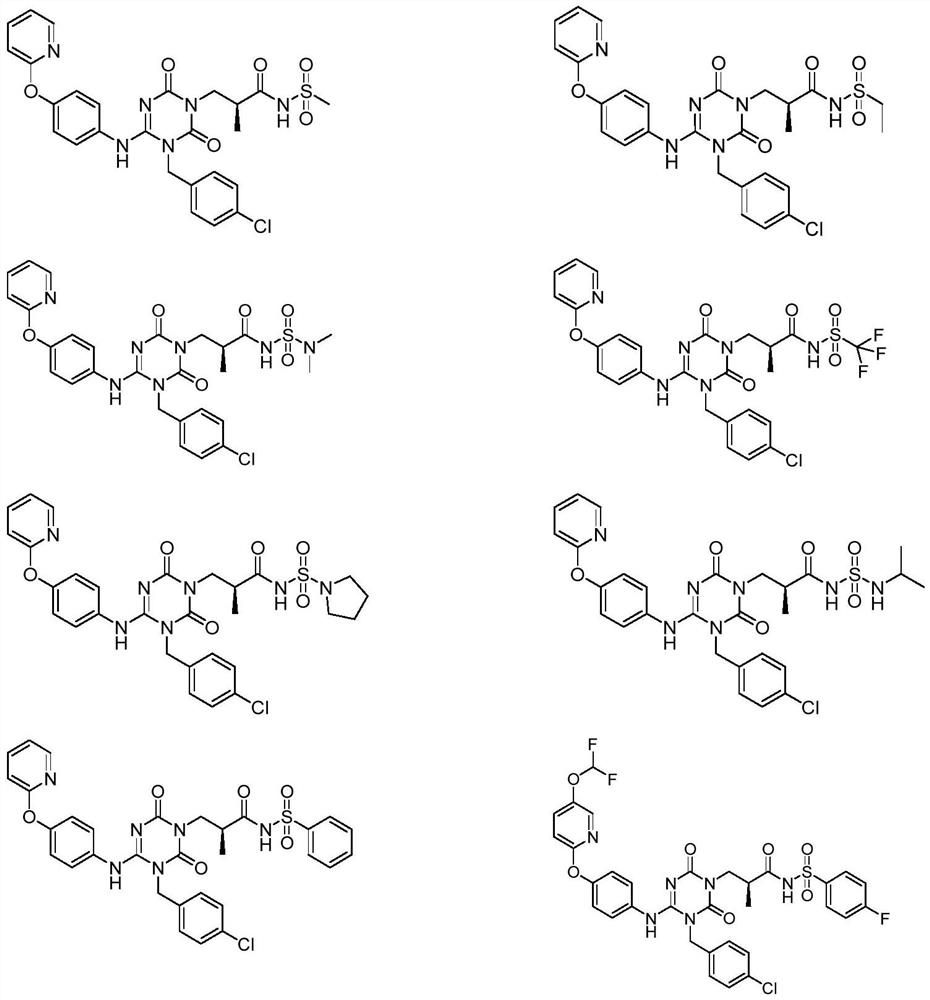
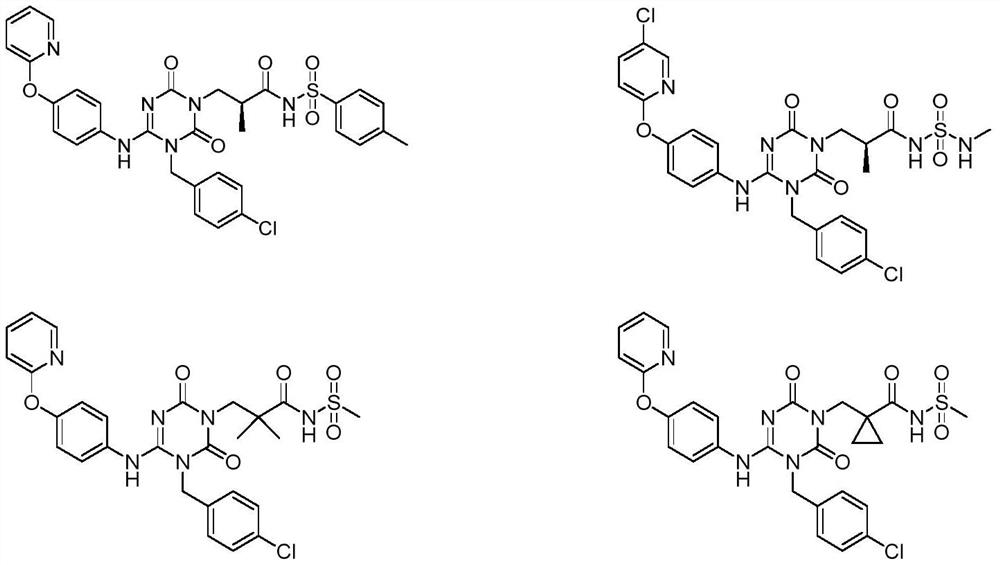
The invention discloses a sulfonamide compound as well as a preparation method and application thereof, and belongs to the technical field of medicinal chemistry. The structure of the sulfonamide compound is shown as a formula I in the specification. The invention also discloses a preparation method of the compound as shown in the formula I. The invention provides application of a compound as shown in the formula I or a salt, a solvate, an allomer, a metabolite, nitrogen oxide and a prodrug of the compound in preparation of drugs for treating or preventing P2X3 and / or / P2X2 / 3 receptor related diseases. The sulfonamide compound provided by the invention has almost no influence on the taste of mice, and has a significant statistical difference from a positive control drug gefapixant. The antitussive effect strong positive drug has an antitussive effect time obviously prolonged compared with that of a contrast 1 compound, and the inhibitory activity of the antitussive drug pair on P2X3 is superior to that of the contrast 1 compound and the positive control drug gefapixant.
Owner:CHENGDU SHIBEIKANG BIOLOGICAL MEDICINE TECH CO LTD
Chemical compounds
InactiveUS20140315878A1Good metabolic stabilityProlong half-life in vivoBiocideOrganic chemistryChemical compoundMedicinal chemistry
Owner:PFIZER LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com