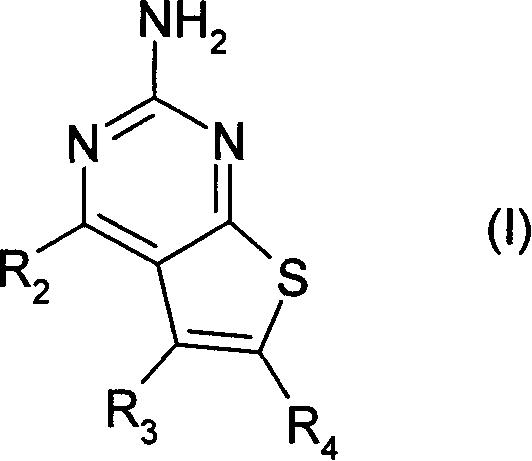
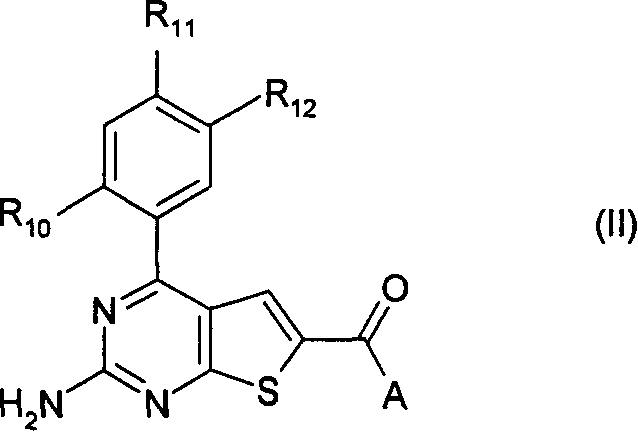
Pyrimidothiophene compounds
A technology of compounds and oxides, applied in 24] The present invention relates to the field of using a class of substituted thieno[2, substituted bicyclic thieno[2
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0134] 2-Amino-4-phenyl-thieno[2,3-d]pyrimidine-6-carboxylic acid ethyl ester
[0135]
[0136] step 1
[0137] 2-Amino-4-chloro-thieno[2,3-d]pyrimidine-6-carboxylic acid ethyl ester
[0138]
[0139] To a mixture of 2-amino-4,6-dichloro-5-formyl-pyridine (1 eq) and potassium carbonate (1 eq) in acetonitrile was added ethyl 2-mercaptoacetate (0.95 eq) with stirring at ambient temperature ) and the mixture was stirred at ambient temperature for 3 hours, then heated at 80 °C for 1 hour. After cooling the mixture was concentrated to dryness under vacuum. Column chromatography on silica, eluting with ethyl acetate and hexanes, gave a sample of Example 1 as a yellow powder.
[0140] LC-MS retention time: 2.371 minutes, [M+H] + 258.0
[0141] Step 2 (Suzuki reaction):
[0142] 2-Amino-4-phenyl-thieno[2,3-d]pyrimidine-6-carboxylic acid ethyl ester
[0143]
[0144] Nitrogen was passed into the solution of 1,4-dioxane and water (3.5:1) of the sample (1 equivalent) of s...
Embodiment 2
[0148] 2-Amino 4-(4-trifluoromethyl-phenyl)-thieno[2,3-d]pyrimidine-6-carboxylic acid ethyl ester
[0149]
[0150] Prepared according to Example 1.
[0151] LC-MS retention time: 2.768 minutes, [M+H] + 368.1
[0152] 1 H NMR (400MHz, d6-DMSO): δ = 1.07 (3H, t, J = 7.1Hz), 4.09 (2H, q, J = 7.1Hz), 7.25 (2H, br s), 7.68 (1H, s) , 7.76 (2H, d, J = 8.0 Hz), 7.85 (2H, d, J = 8.0 Hz).
[0153] This compound has activity 'B' using the fluorescence polarization assay described below.
[0154] The compounds in Table 1 below were synthesized and tested using the fluorescence polarization analysis described below. Suzuki reaction was carried out according to step 2 of Example 1. The reductive amination reaction was carried out as in Example 33 below:
Embodiment 33
[0154] The compounds in Table 1 below were synthesized and tested using the fluorescence polarization analysis described below. Suzuki reaction was carried out according to step 2 of Example 1. The reductive amination reaction was carried out as in Example 33 below:
[0155] 2-Amino-4-(4-piperidin-1-ylmethyl-phenyl)-thieno[2,3-d]pyrimidine-6-carboxylic acid ethyl ester (Example 33 of Table 1)
[0156]
[0157] To a suspension of ethyl 2-amino-4-(4-formyl-phenyl)-thieno[2,3-d]pyrimidine-6-carboxylate (1 eq) in methanol was added pyrrolidine (5 eq) . The reaction mixture was heated to reflux for 3.5 hours and then cooled to room temperature. Sodium borohydride (3 equiv) was added and stirred for 10 minutes. The mixture was concentrated in vacuo then partitioned between ethyl acetate and water. The phases were separated and the organic layer was washed with brine, dried and evaporated to a yellow oil. The crude product was purified by preparative HPLC.
[0158] LC-MS re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com