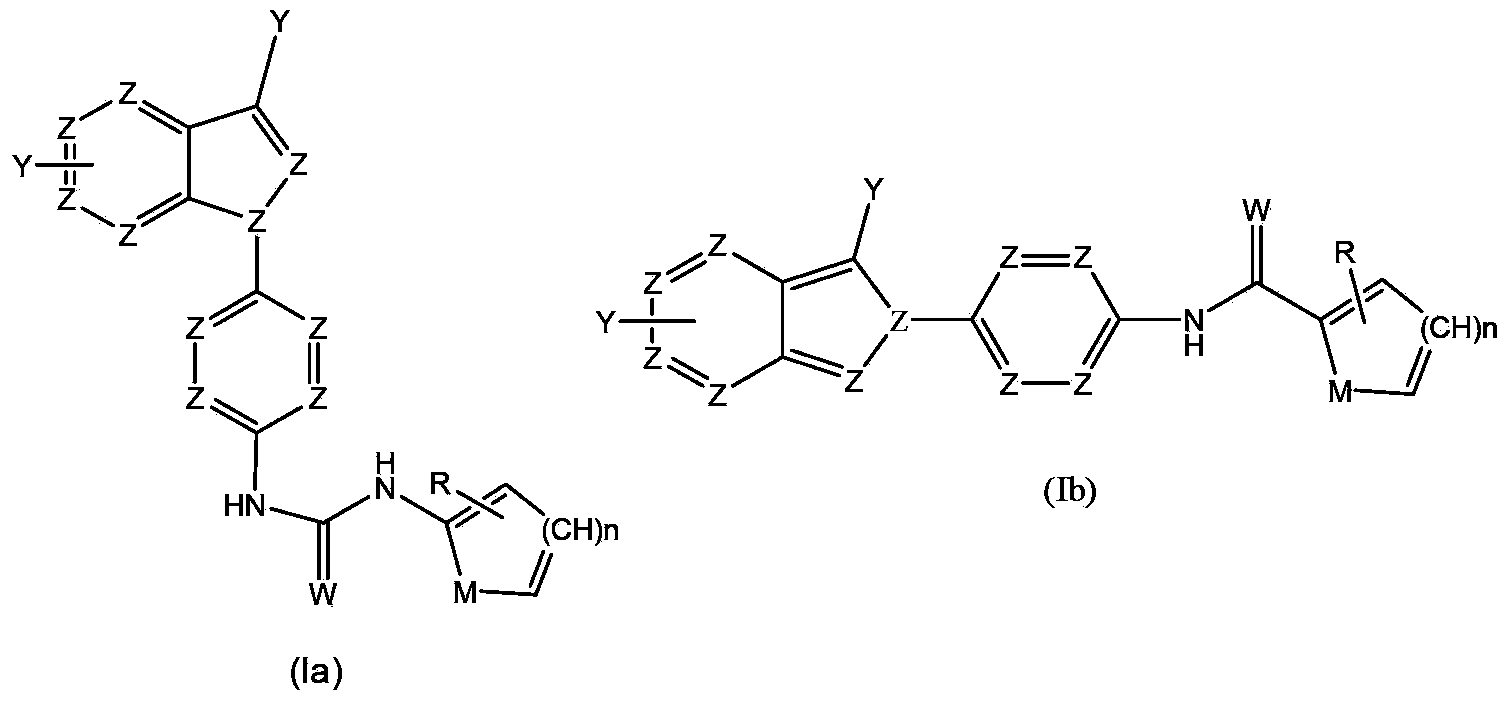
Anti-tumor medicine in double-aryl urea structure based on indazole, indole, azaindazole or azaindole
An anti-tumor drug, the technology of azaindole, which is applied in the field of anti-tumor drugs and anti-tumor drugs with bisaryl urea structure, can solve the problems of anti-tumor drugs with bis-aryl urea structure that have not been found yet
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
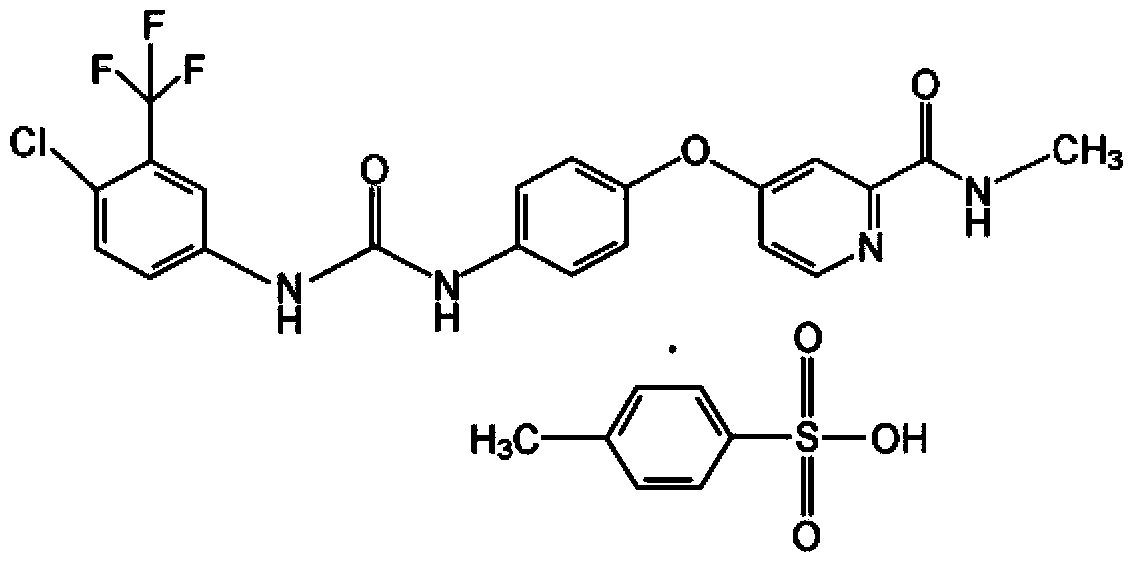
[0079] Example 1: N-{5-[2-(4-chloro-1-indazolyl)]pyridyl}-N'-(4-chloro-3-trifluoromethylphenyl)urea p-toluenesulfonic acid Salt (1):
[0080]
[0081] 2.0 g (13 mmol) of 4-chloroindazole and 2.1 g (13 mmol) of 2-chloro-5-nitropyridine were dissolved in 20 mL of DMF, 5.2 g (16 mmol) of cesium carbonate was added, and the reaction was stirred at 50°C for 5 h. The reactant was poured into ice water, and a yellow precipitate was precipitated. Suction filtration, washing with water, and drying gave 3.2 g of 4-chloro-1-(5-nitro-2-pyridyl)indazole with a yield of 90%, which was directly used in the next reaction.
[0082] Dissolve the above compound in 40mL of acetic acid, add a solution of 1.7g of ammonium chloride dissolved in 20mL of water, then add 3.4g of reduced iron powder, and reflux for 4h. After filtration, it was concentrated under reduced pressure. The residue was added to water, and a pale yellow solid was precipitated. The solid was dissolved in ethyl acetate and...
Embodiment 2
[0085] Example 2: N-{5-[2-(1-indolyl)]pyridyl}-N'-(4-chloro-3-trifluoromethylphenyl)urea p-toluenesulfonate (2) :
[0086]
[0087] Dissolve 2.34g (20mmol) of indole and 3.18g (20mmol) of 2-chloro-5-nitropyridine in 40mL DMF, add 7.82g (24mmol) of cesium carbonate, and stir the reaction at 50°C for 5h. After the reaction was completed, the reactant was poured into ice water, and a yellow precipitate was precipitated. Suction filtration, washing with water, and drying yielded 4.32 g of 1-(5-nitro-2-pyridyl)indole, which was directly used in the next reaction.
[0088] The above compound was dissolved in 50mL of ethanol, a solution of 3.24g (60mmol) of ammonium chloride dissolved in 20mL of water was added, and 6.7g (120mmol) of reduced iron powder was added, and the reaction was refluxed for 6h. After the reaction was completed, the temperature was lowered, filtered and concentrated under reduced pressure. Extract with ethyl acetate, dry over anhydrous magnesium sulfate, ...
Embodiment 3
[0090] Example 3: N-{2-[5-(4-chloro-1-indazol)yl]pyridyl}-N'-(4-chloro-3-trifluoromethylphenyl)urea (3):
[0091]
[0092] 1.5g (10mmol) of 4-chloroindazole and 1.59g (10mmol) of 5-chloro-2-nitropyridine were dissolved in 20mL DMF, 3.5g (11mmol) of cesium carbonate was added, and the reaction was stirred at 50°C for 5h. The reactant was poured into ice water, and a yellow precipitate was precipitated. Suction filtration, washing with water, and drying gave 2.4 g of 1-(4-nitro-5-pyridyl)-4-chloroindazole with a yield of 90%, which was directly used in the next reaction.
[0093] The above compound was dissolved in 40 mL of ethanol, a solution of 1.62 g of ammonium chloride dissolved in 20 mL of water was added, and 3.4 g of reduced iron powder was added, and the reaction was refluxed for 4 h. After filtration, it was concentrated under reduced pressure. The residue was added to water, and 0.73 g of light yellow solid 1-(2-amino-5-pyridyl)-4-chloroindazole was precipitated,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com