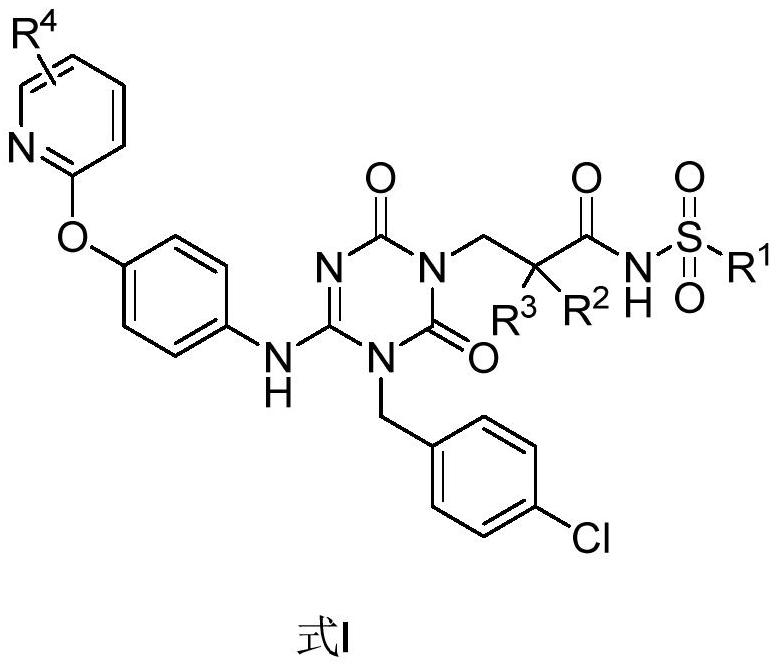
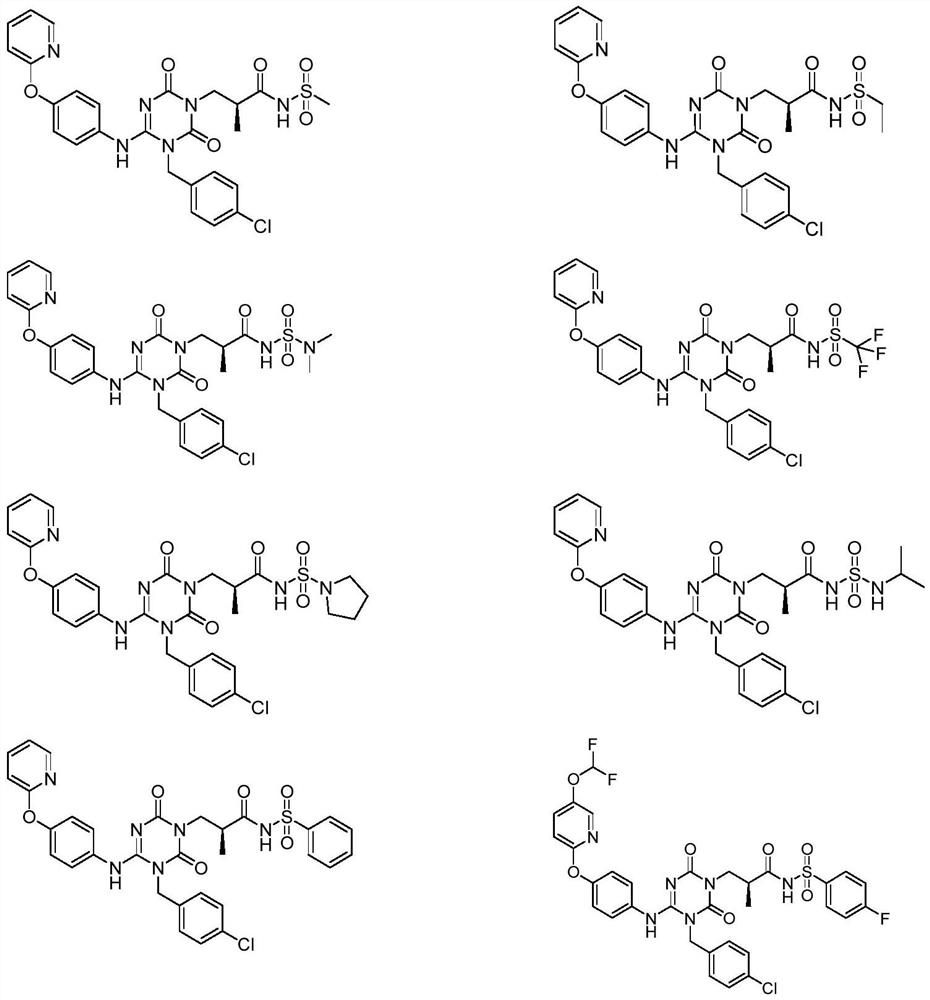
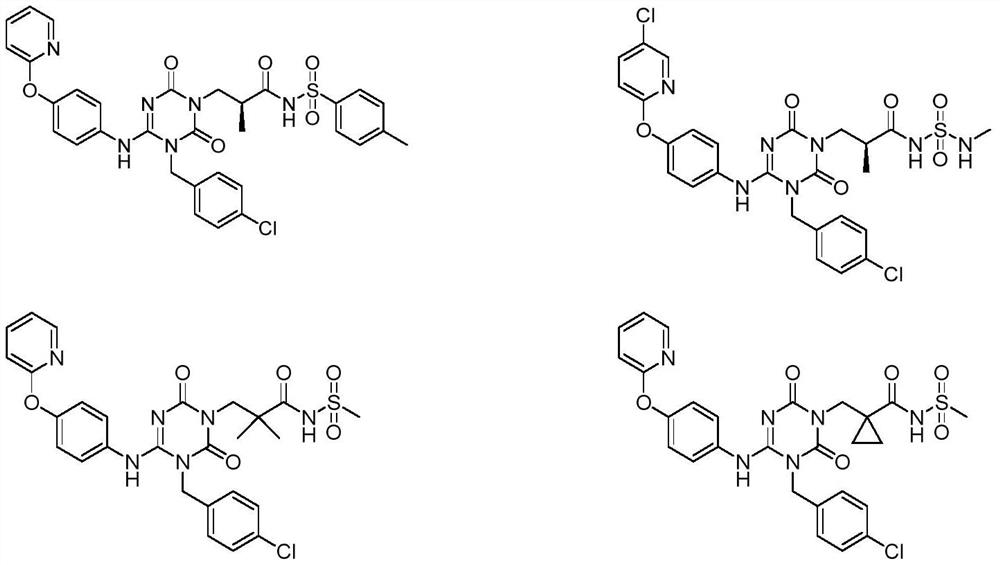
Sulfonamide compound as well as preparation method and application thereof
A compound and solvate technology, applied in the field of medicinal chemistry, can solve the problems of failure to reach the primary efficacy endpoint, high incidence of taste-related adverse events, high frequency of drug withdrawal in the 45mg group, and achieve good P2X3 receptor antagonism and good safety Sexuality, the effect of prolonging the time of antitussive action
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048]Example 1: (S)-3-(3-(4-chlorobenzyl)-2,6-dioxo-4-(4-(pyridine-2-oxyl)phenyl)amino)-3,6 - Preparation of dihydro-1,3,5-triazin-1(2H)-yl)-2-methyl-N-(methylsulfonyl)propanamide (compound 1):
[0049] Step 1: Preparation of 4-(pyridine-2-oxyl)aniline (compound b-1)
[0050]
[0051] 2-fluoropyridine (97.1g, 1.0mol) and p-aminophenol (108.1g, 0.99mol) were dissolved in dimethyl sulfoxide (600ml), cesium carbonate (620g, 1.96mol) was added to obtain a reaction mixture, and mechanically stirred The reaction mixture was stirred evenly. Then the internal temperature of the reaction system was raised to 80° C. for 3 h. Thin-layer chromatography tracked the reaction progress. After the reaction was complete, the reaction mixture was added to 2L of water and kept stirring. Then extract the product three times with ethyl acetate, combine and dry the ethyl acetate and then concentrate to obtain the crude product. The crude product is slurried with 500ml of water for 1h and filt...
Embodiment 2
[0075] Example 2: (S)-3-(3-(4-chlorobenzyl)-2,6-dioxo-4-(4-(pyridine-2-oxyl)phenyl)amino)-3,6 Preparation of -dihydro-1,3,5-triazin-1(2H)-yl)-N-(ethylsulfonyl)-2-methylpropanamide (compound 2)
[0076] Compared with Example 1, the preparation method of this example is different in that the compound m-1 in step 6: methanesulfonamide is replaced by equimolar ethylsulfonamide, and the rest of the conditions are the same. (S)-3-(3-(4-chlorobenzyl)-2,6-dioxo-4-(4-(pyridine-2-oxy)phenyl)amino)-3 was obtained as a white solid, 6-dihydro-1,3,5-triazin-1(2H)-yl)-N-(ethylsulfonyl)-2-methylpropanamide (compound 2), yield: 77.0%, purity is 99.13%.
[0077] ESI-MS:m / z=599.2(M+H) + .
[0078] 1H NMR (400MHz, DMSO-d6) δ11.53(s,1H),8.60(s,1H),8.15(dd,J=5.0,2.0Hz,1H),7.90–7.79(m,1H),7.45– 7.37(m,2H),7.30(d,J=8.4Hz,2H),7.15(m,4H),7.14–7.09(m,1H),7.03(d,J=8.3Hz,1H),5.42–5.15 (m,2H), 3.88(m,2H), 3.33(m,2H), 2.69(m,1H), 1.27(m,3H), 0.98(m,3H).
Embodiment 3
[0079] Example 3: (S)-3-(3-(4-chlorobenzyl)-2,6-dioxo-4-(4-(pyridine-2-oxyl)phenyl)amino)-3,6 Preparation of -dihydro-1,3,5-triazin-1(2H)-yl)-N-(N,N-dimethylaminosulfonyl)-2-methylpropionamide (compound 3)
[0080] Compared with Example 1, the preparation method of this example is different in that the compound m-1 in step 6: methanesulfonamide is replaced by equimolar N,N-dimethylaminosulfonamide, and the rest of the conditions are the same. (S)-3-(3-(4-chlorobenzyl)-2,6-dioxo-4-(4-(pyridine-2-oxy)phenyl)amino)-3 was obtained as a white solid, 6-dihydro-1,3,5-triazin-1(2H)-yl)-N-(N,N-dimethylaminosulfonyl)-2-methylpropanamide (compound 3), yield: 78.6% with a purity of 98.12%.
[0081] ESI-MS:m / z=614.2(M+H) + .
[0082] 1 H NMR (400MHz, DMSO-d6) δ11.28(s,1H),8.60(s,1H),8.15(dd,J=5.0,2.0Hz,1H),7.90–7.79(m,1H),7.45– 7.37(m,2H),7.30(d,J=8.4Hz,2H),7.15(m,4H),7.14–7.09(m,1H),7.03(d,J=8.3Hz,1H),5.42–5.15 (m,2H), 3.88(m,2H), 2.79(s,6H), 2.69(m,1H), 0.98(m,3H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com