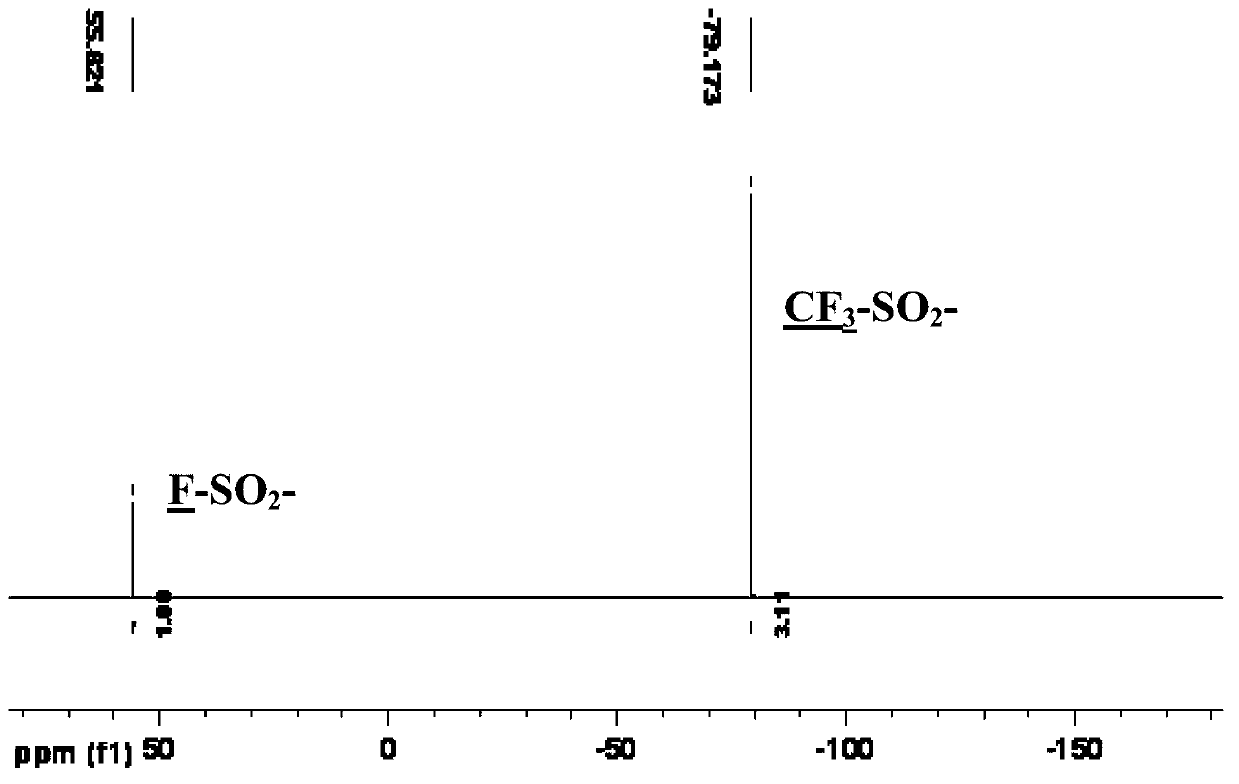
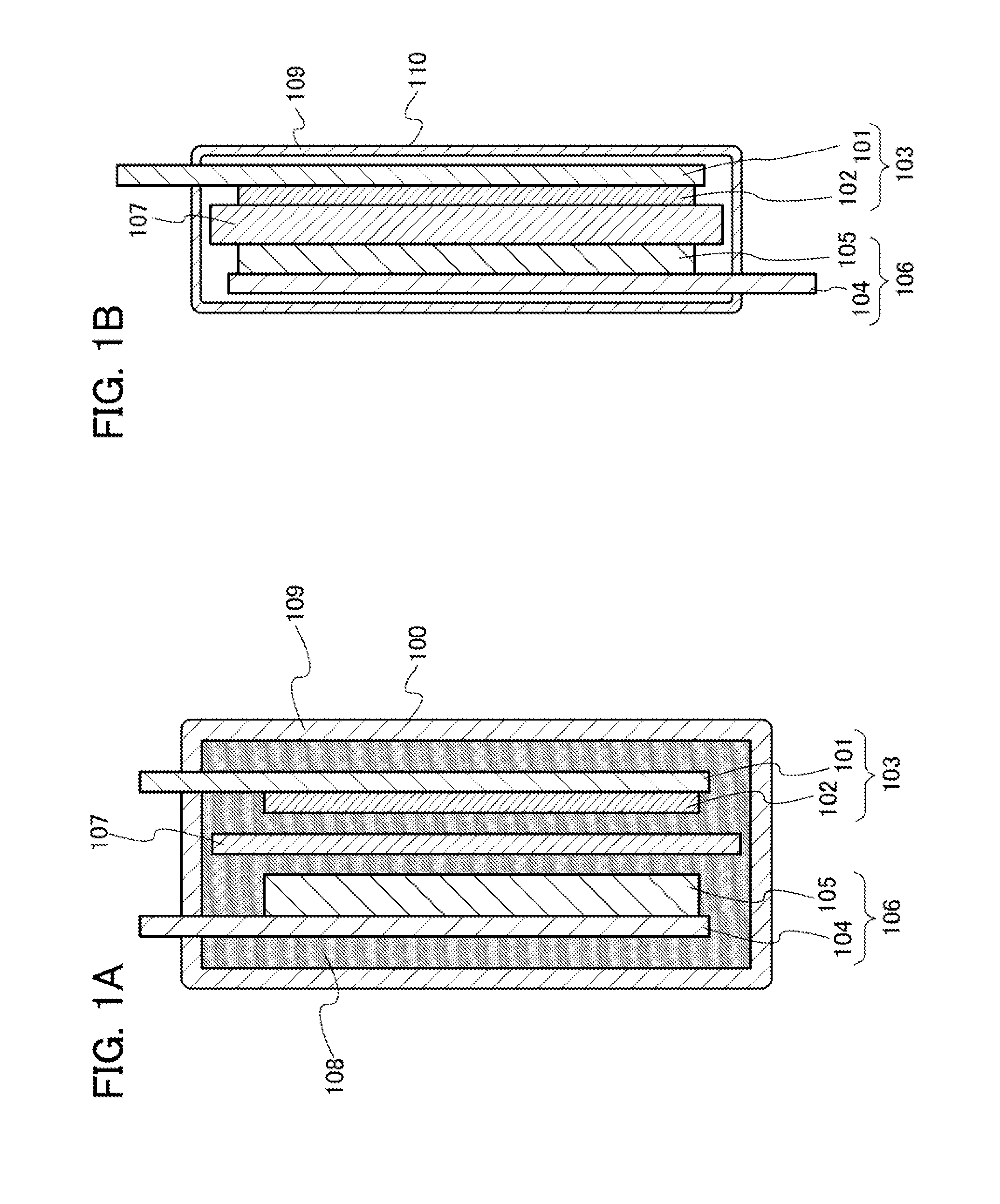
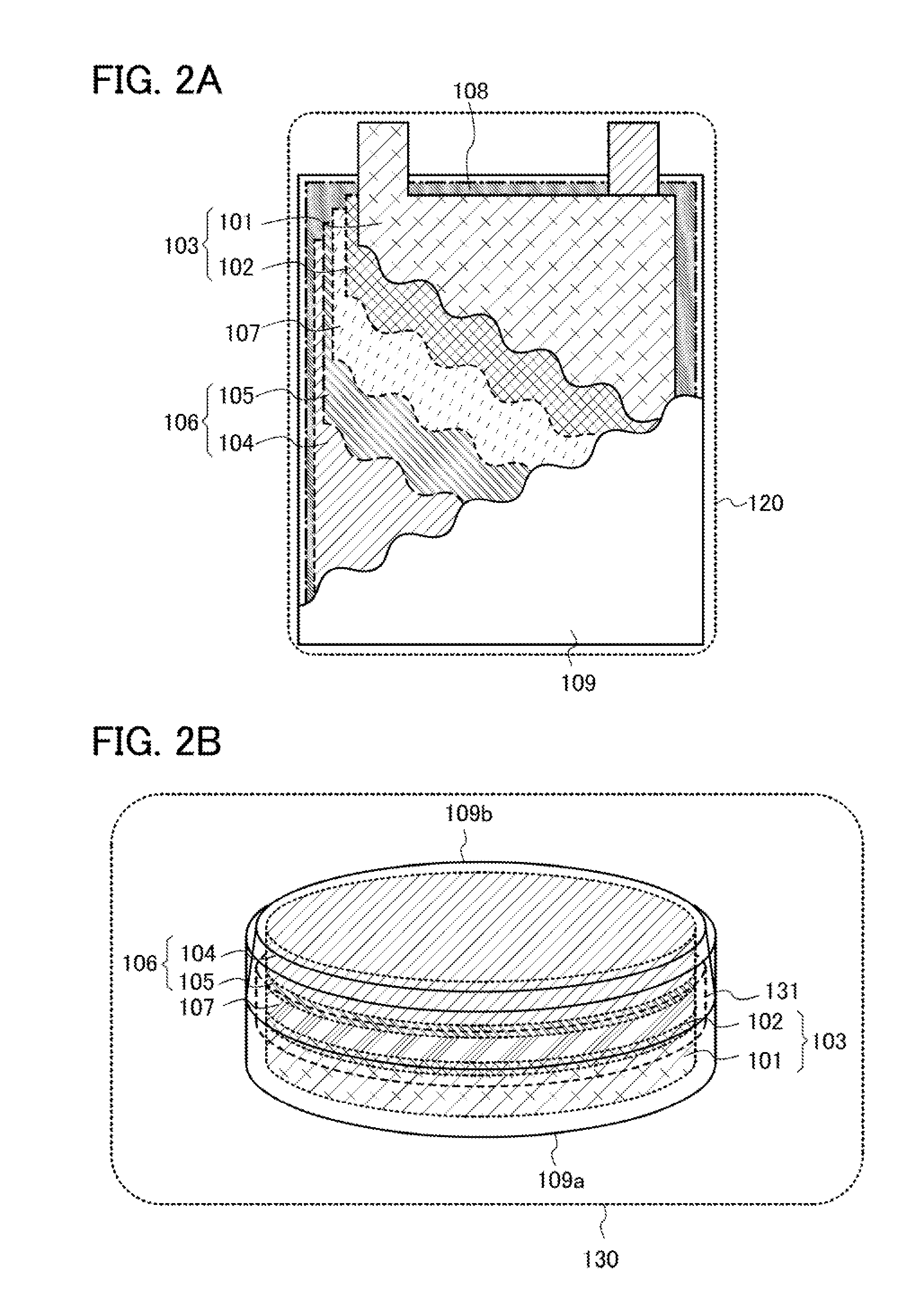
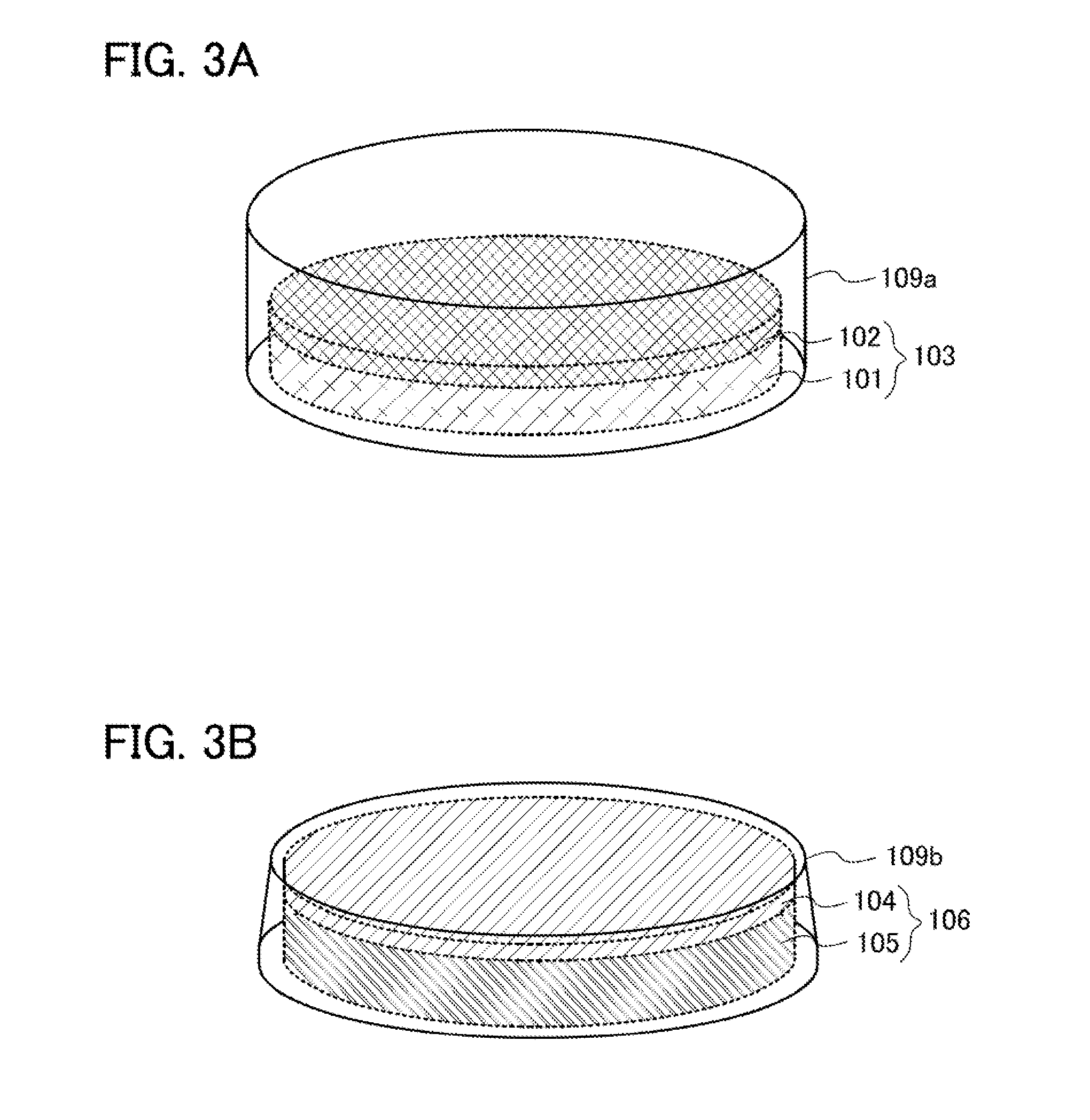
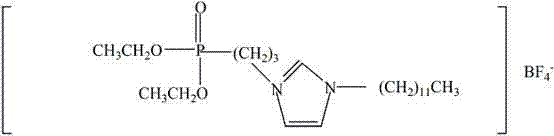
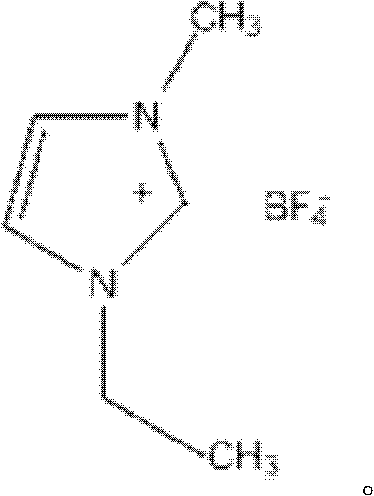
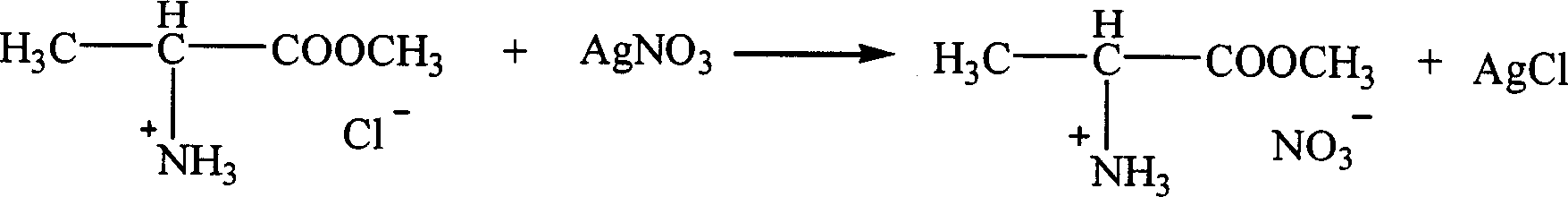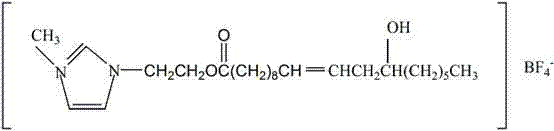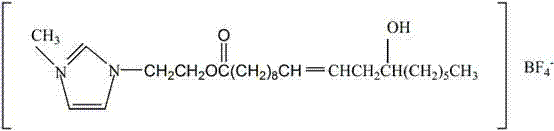Patents
Literature
931 results about "Tetrafluoroborate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
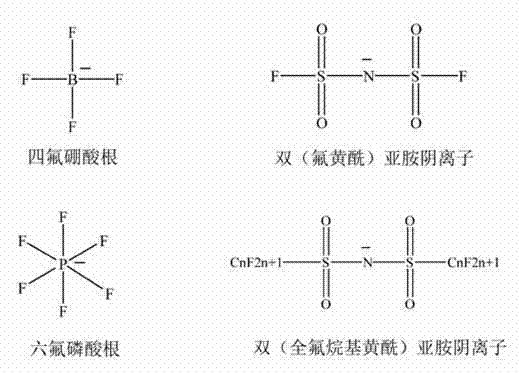
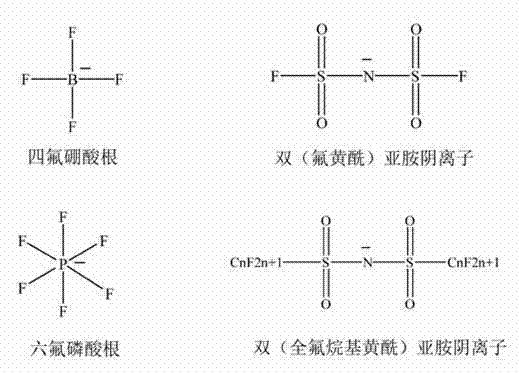
Tetrafluoroborate is the anion BF⁻₄. This tetrahedral species is isoelectronic with tetrafluoroberyllate (BeF²⁻₄), tetrafluoromethane (CF₄), and tetrafluoroammonium (NF⁺₄) and is valence isoelectronic with many stable and important species including the perchlorate anion, ClO⁻₄, which is used in similar ways in the laboratory. It arises by the reaction of fluoride salts with the Lewis acid BF₃, treatment of tetrafluoroboric acid with base, or by treatment of boric acid with hydrofluoric acid.
Method for continuously preparing regenerated cellulose fibre
InactiveCN101328626AReduce manufacturing costReduce the temperatureArtificial filament recoveryFibre treatmentPolymer scienceTetrafluoroborate
The invention discloses a method for continuously preparing regenerated cellulose fibers through the solvent method, comprising the following steps that: a cellulose raw material is dissolved into an ion liquid to prepare a spinning liquid; gel type regenerated cellulose fibers are obtained through spinning; and the regenerated cellulose fibers are obtained through cleaning, rear draft and drying, wherein, the ion liquid is selected from one or a plurality among the following ion liquids: a). an ion liquid with 1, 3-dialkyl imidazole as a cation and formiate radical, radical vinegar or propionate radical as an anion; and b). an ion liquid with 1-R1-3-R2- dialkyl imidazole as the cation and chlorine, bromine, iodine, formiate radical, radical vinegar, sulfate radical, nitrate radical, tetrafluoroborate radical, thiocyanate radical, hexafluorophosphate radical, p-toluenesulfonate radical or trifluoromethanesulfonic acid radical as the anion. The method has the advantages of wide technological range, mild temperature condition, adequate pressure, quick spinning speed and so on, can prepare the regenerated cellulose fibers with superior performance and complete specifications, and has low production cost, high production efficiency and wide application prospect.
Owner:INST OF CHEM CHINESE ACAD OF SCI +1
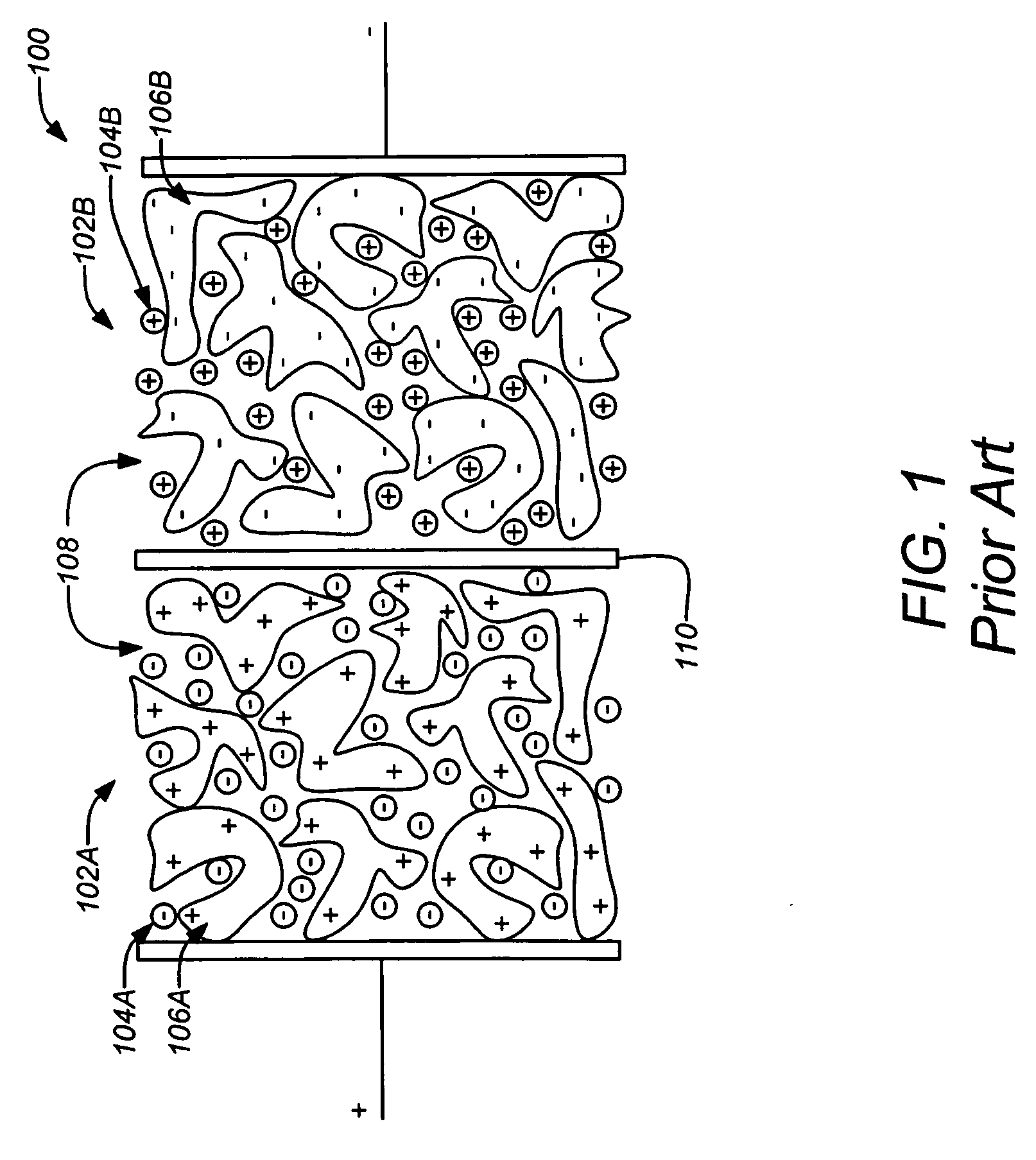
Electrical double-layer capacitor
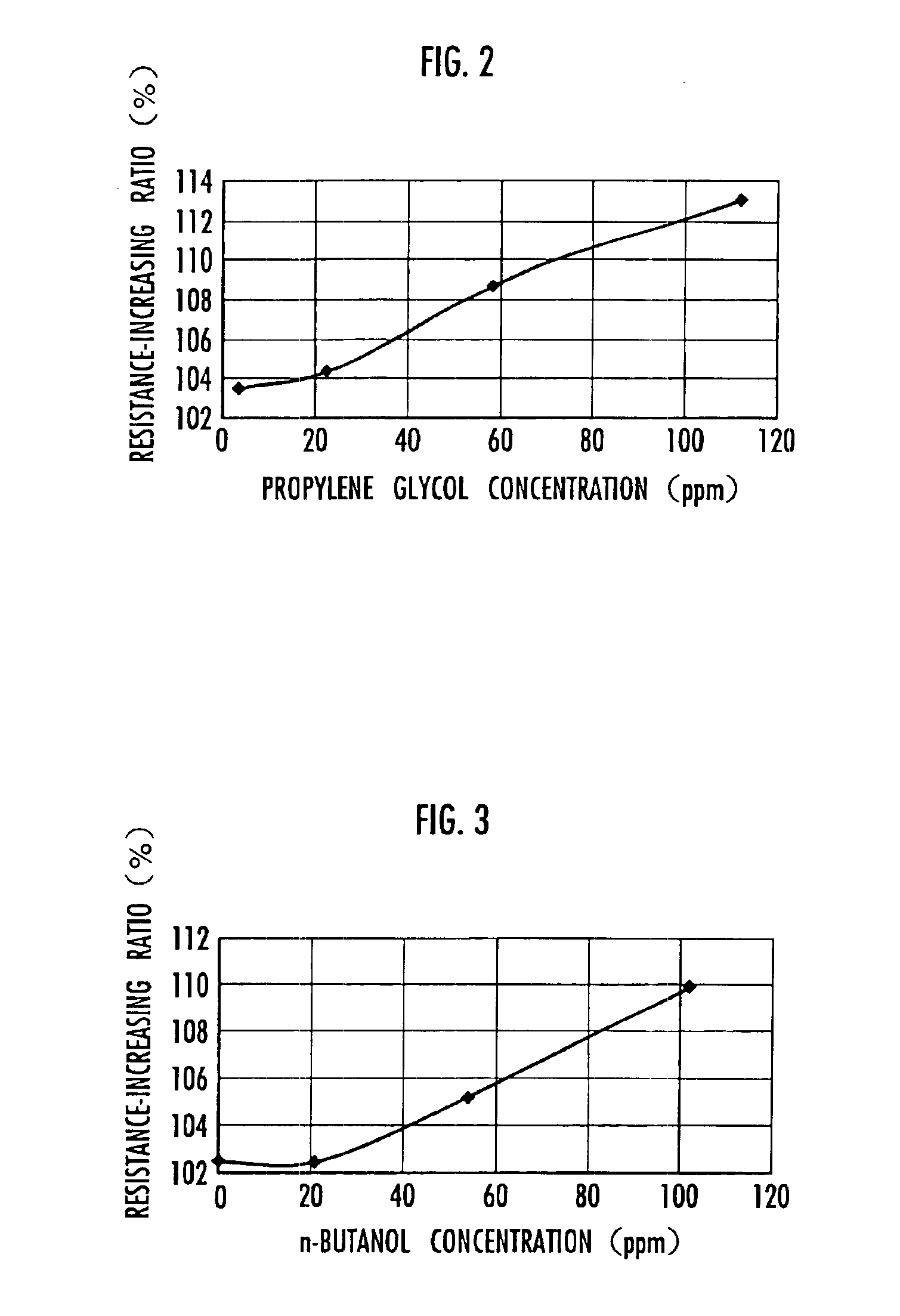
ActiveUS6914768B2Small increase in resistanceIncrease the residual rateHybrid capacitor electrolytesProtecting/adjusting hybrid/EDL capacitorQuaternary ammonium cationTetrafluoroborate
An electrical double-layer capacitor showing a sleight increase of resistance when used under continuous application of high voltage and maintaining high energy residual ratio after standing for a long time includes an electrode element including a pair of electrodes disposed opposite to each other with a separator interposed therebetween, and is impregnated with a nonaqueous electrolyte solution prepared by dissolving quaternary ammonium salts into cyclic carbonates and containing impurities of 30 ppm or less of glycols, 30 ppm or less of primary alcohols and less than 20 ppm of tertiary amines. The water content may be 50 ppm or less. The quaternary ammonium salt may be triethylmethylammonium tetrafluoroborate. The cyclic carbonate may be propylene carbonate. The nonaqueous electrolyte solution may have a concentration of 0.1 to 2.5 mol / liter. The electrode may be a polarizable electrode composed of activated carbon.
Owner:MU IONIC SOLUTIONS CORP +1
Method for preparing ion liquid type gel polymer electrolyte and battery by in situ polymerization
ActiveCN101475663ASimple preparation processSimple manufacturing processFinal product manufactureSecondary cellsPolymer scienceTetrafluoroborate
The invention relates to a method for preparing ionic liquid type gel polymer electrolyte through home position polymerization. The method comprises the following steps: taking acrylonitrile and polyethylene glycol dimethyl acrylic acid ester as monomers, taking ethylene carbonate as an organic plasticizer, taking azo diisobutyl cyanogen as an initiator, taking lithium perchlorate as a lithium salt, adding ionic liquid 1-butyl-3-methylimidazole tetrafluoborate as the component of the electrolyte, and adopting a free radical initiation and home polymerization mode to prepare the stable ionic liquid type gel polymer electrolyte. The home polymerization mode has a simple and feasible process, and is capable of directly assembling a lithium cell while simultaneously preparing the electrolyte. The prepared ionic liquid type gel polymer electrolyte has higher room temperature conductivity, good dimensional stability and mechanic properties, and can also be applied to dye sensitization solar cells. The prepared ionic liquid type gel polymer electrolyte cell can avoid the leakage and volatilization of the electrolyte and improve the safety of the cell.
Owner:CHANGZHOU INST OF ENERGY STORAGE MATERIALS &DEVICES
Preparation method for ion liquid assisted hydrothermal synthesis of MoS2 microsphere
InactiveCN1994895AImprove performanceMild reaction conditionsMolybdenum sulfidesTetrafluoroborateMolybdate
The invention discloses a making method of MoS2 microball synthesized by ionic liquid auxiliary water, which comprises the following steps: dissolving molybdate in the deionized water to form 0.05-0.1m solution; adding thioacetamide or sulfourea as sulfur source with molar rate of thioacetamide or sulfourea and molybdate at 31-51; stirring evenly; adding 1-butyl-3-methyl imidazole tetrafluoride borate as ionic liquid with the bulk rate of ionic liquid and synthetic solution at 1300-1 50; stirring completely; transmitting solution into water heat reacting autoclave to react under 200-240 deg. c for 20-24h; cooling naturally; separating; washing; drying to obtain the product.
Owner:ZHEJIANG UNIV
Electrolyte
ActiveUS20100046142A1Good operational characteristicImprove stabilityHybrid capacitor electrolytesOrganic electrolyte cellsTetrafluoroborateSolvent
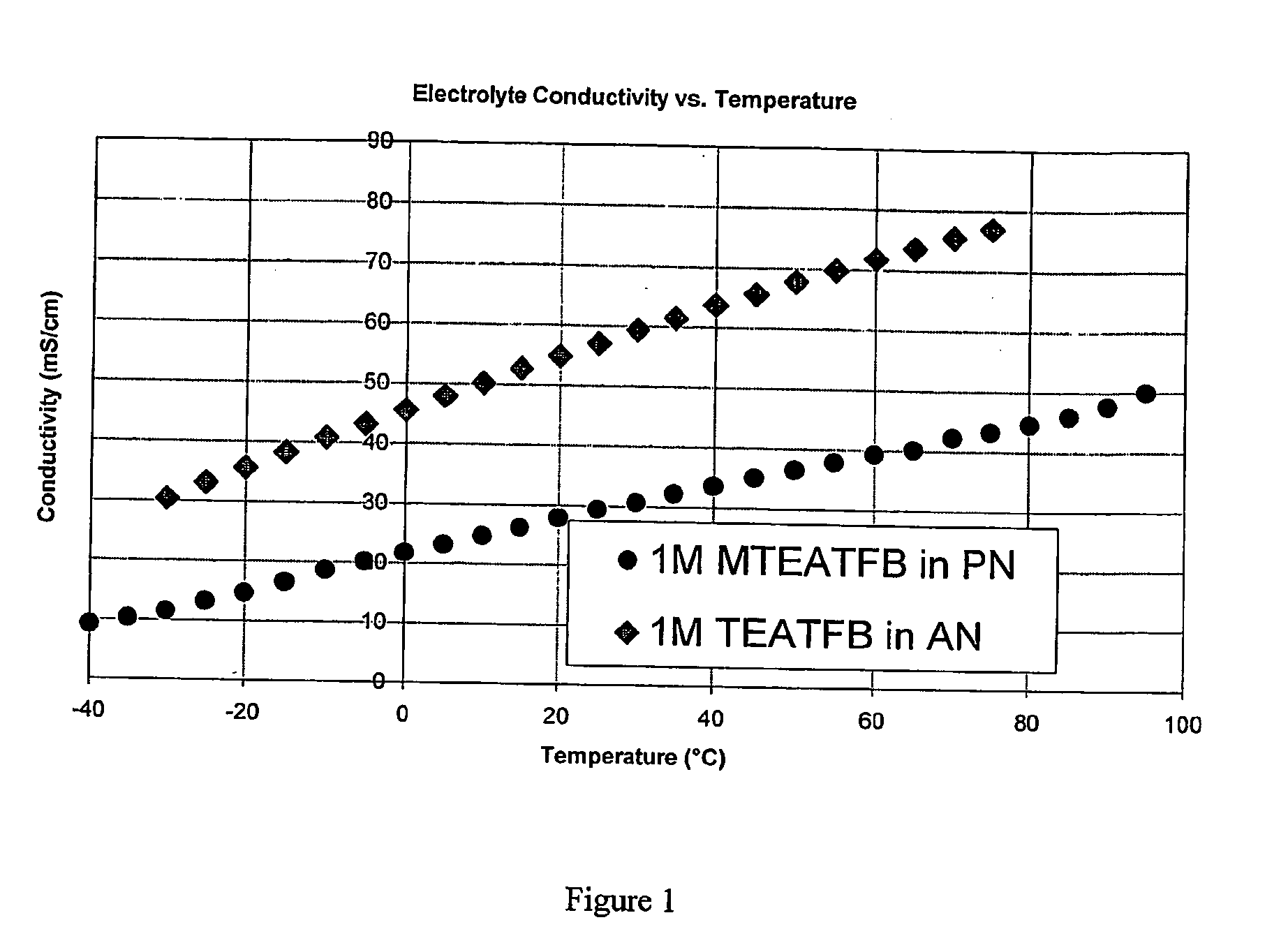
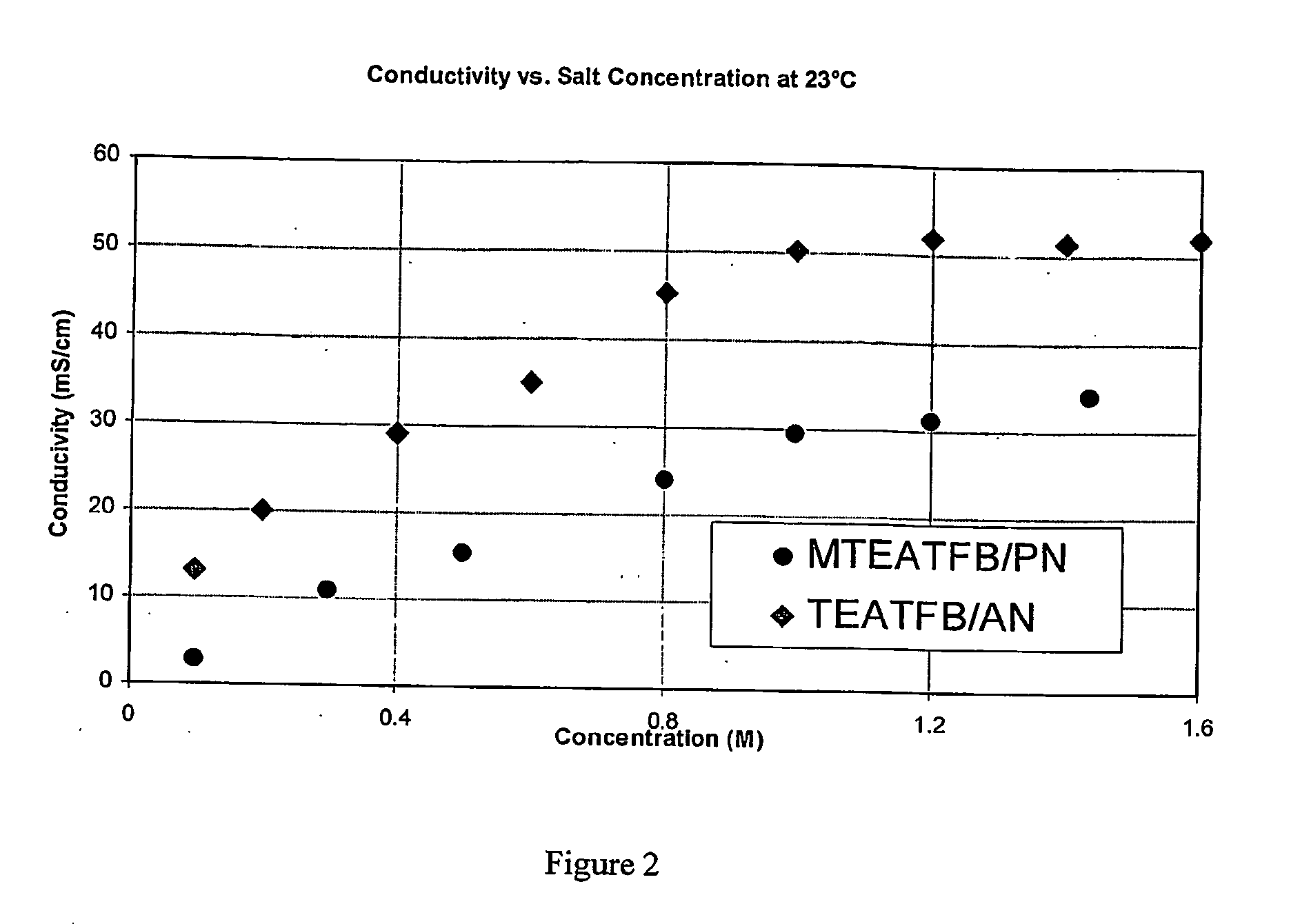
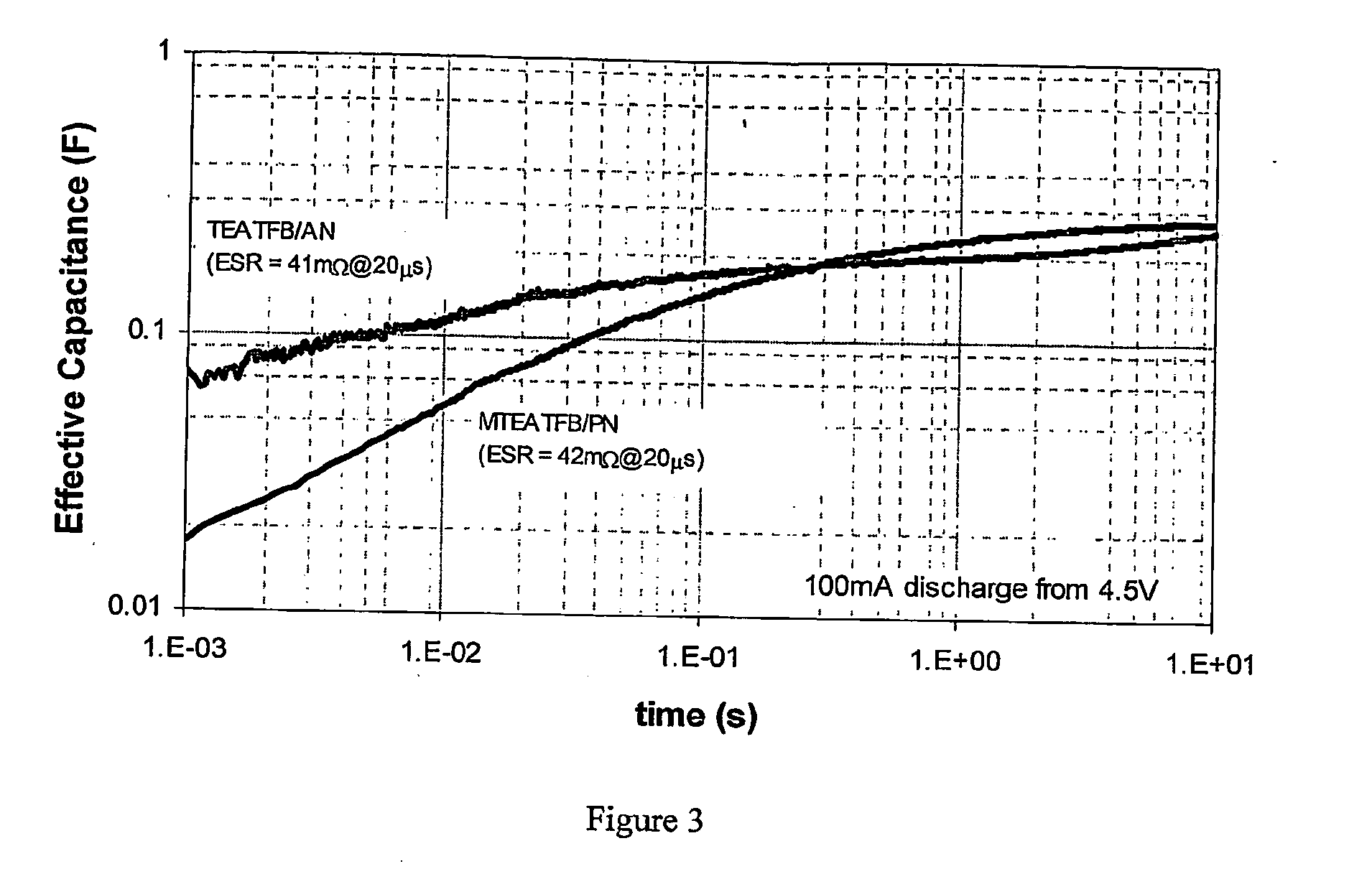
Electrolyte for use in an energy storage device such as a capacitor or supercapacitor which comprises a solvent (preferably propionitrile) and an ionic species (preferably methyltriethylammonium tetrafluoroborate). The electrolytes provide a low ESR rise rate, a high voltage and permit operation over a wide range of temperatures, which makes them beneficil for use in a range of energy storage devices such as digital wireless devices, wireless LAN devices, mobile telephones, computers, electrical or hybrid electrical vehicles.
Owner:CAP XX LTD +1
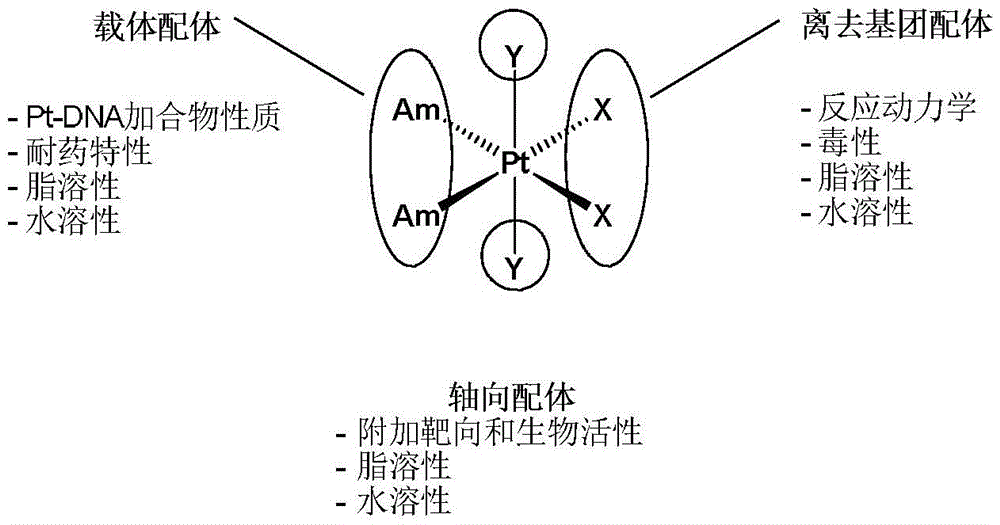
Tetravalent platinum complex with bioactive group and preparation method of tetravalent platinum complex
The invention discloses a tetravalent platinum complex with a bioactive group and a preparation method of the tetravalent platinum complex. The tetravalent platinum complex is a platinum (IV) complex and has the structure shown in the formula II (please see the formula in the description), wherein in the formula II, Y is OH or Cl, and Bio represents the bioactive group. The platinum (IV) complex is prepared according to the equation in the formula III (please see the formula in the description), wherein in the formula III, Y is OH or Cl, Bio-OH represents a compound with bioactivity, TBTU represents a coupling agent O-(benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium tetrafluoroborate, TEA represents a catalyst triethylamine, DMF represents solvent N,N-dimethyl formamide, and DMSO represents solvent dimethylsulfoxide. Cis-platinum is adopted for the bottom face of an octahedron, a small-molecular targeted or medicine active group is introduced to one axial position, a hydroxyl group or helium atom is introduced into another axial position, and the anti-tumor tetravalent platinum complex overcoming cisplatin resistance is provided, so the high-efficiency and low-toxin platinum (IV) complex is obtained.
Owner:SOUTHEAST UNIV
Printable Ionic Gel Separation Layer for Energy Storage Devices
InactiveUS20140017571A1Low vapor pressureHybrid capacitor separatorsAlkaline accumulatorsElectrical conductorTetrafluoroborate
Representative embodiments provide a liquid or gel separator utilized to separate and space apart first and second conductors or electrodes of an energy storage device, such as a battery or a supercapacitor. A representative liquid or gel separator comprises a plurality of particles, typically having a size (in any dimension) between about 0.5 to about 50 microns; a first, ionic liquid electrolyte; and a polymer. In another representative embodiment, the plurality of particles comprise diatoms, diatomaceous frustules, and / or diatomaceous fragments or remains. Another representative embodiment further comprises a second electrolyte different from the first electrolyte; the plurality of particles are comprised of silicate glass; the first and second electrolytes comprise zinc tetrafluoroborate salt in 1-ethyl-3-methylimidalzolium tetrafluoroborate ionic liquid; and the polymer comprises polyvinyl alcohol (“PVA”) or polyvinylidene fluoride (“PVFD”). Additional components, such as additional electrolytes and solvents, may also be included.
Owner:NTHDEGREE TECH WORLDWIDE
Diatomaceous Ionic Gel Separation Layer for Energy Storage Devices and Printable Composition Therefor
InactiveUS20140017558A1Low vapor pressureHybrid capacitor separatorsHybrid capacitor electrolytesElectrical conductorTetrafluoroborate
Representative embodiments provide a liquid or gel separator utilized to separate and space apart first and second conductors or electrodes of an energy storage device, such as a battery or a capacitor. A representative liquid or gel separator comprises a plurality of particles selected from the group consisting of: diatoms, diatomaceous frustules, diatomaceous fragments, diatomaceous remains, and mixtures thereof; a first, ionic liquid electrolyte; and a polymer or, in the printable composition, a polymer or a polymeric precursor. Another representative embodiment further comprises a second electrolyte different from the first electrolyte; the first and second electrolytes comprise zinc tetrafluoroborate salt in 1-ethyl-3-methylimidalzolium tetrafluoroborate ionic liquid; and the polymer comprises polyvinyl alcohol (“PVA”) or polyvinylidene fluoride (“PVFD”). Additional components, such as additional electrolytes and solvents, may also be included.
Owner:NTHDEGREE TECH WORLDWIDE
Ionic liquid mixed electrolyte for lithium ion battery
InactiveCN103094610AImprove solubilityImprove thermal stabilitySecondary cellsTetrafluoroborateQuaternary ammonium cation
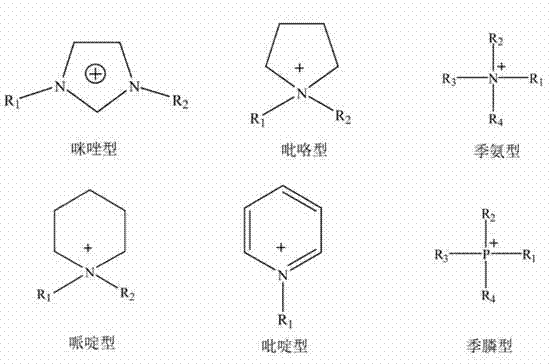
The invention discloses an ionic liquid mixed electrolyte for a lithium ion battery. The ionic liquid mixed electrolyte comprises lithium salt, ionic liquid and a non-aqueous organic solvent, wherein the lithium salt is one or mixture of two of lithium bis borate and lithium difluoroborate; cations in the ionic liquid are selected from one of imidazole cations, piperidine cations, pyridine cations, pyrrole cations, quaternary ammonium cations and quaternary phosphine cations; anions in the ionic liquid are selected from one of tetrafluoroborate radicals, hexafluorophate radicals, difluorosulfimide anions and diperfluoroalkylsulfimide anions; and the non-aqueous organic solvent is selected from any one or mixture of several of linear carbonate, cyclic carbonate, linear ether and cyclic ether. The ionic liquid mixed electrolyte is high in thermal stability, wide for an electrochemical stability window, high in conductivity, low in viscosity and good in compatibility with an anode material and a cathode material of the lithium ion battery at the same time.
Owner:JIANGXI YOULI NEW MATERIALS
Electrodeposition method, electrodeposition solution and method for preparation of rare earth permanent magnetic material by electrodeposition
InactiveCN105839152AFast depositionSave time in electrodeposition processInductances/transformers/magnets manufactureMagnetic materialsRare-earth elementTetrafluoroborate
Owner:BEIJING ZHONG KE SAN HUAN HI TECH
Ion thermal growth method of near infrared light upper conversion fluoride nano crystal
InactiveCN101476151ALow melting pointNon-volatilePolycrystalline material growthFrom normal temperature solutionsSolubilityLuminous intensity
The invention relates to an ion thermal growth method for converting fluoride nano crystal near infrared light which includes steps as follows: weighing some solid yttrium nitrate (lanthanum nitrate), ytterbium nitrate, erbium nitrate (thulium nitrate or holmium nitrate) pro rata. Mol ratio of the rare earth ion is that yttrium ion (lanthanum ion) : ytterbium ion : erbium ion (thulium ion, holmium ion) equal to 70-90 : 0 : 0.001-15; adding tetrafluoroborate type ion liquor into the mixing solid, selective adding some NaCl solid according with various basic, then placing the mixing solution into a high pressure reaction kettle with polyfluortetraethylene lining, placing into an oven for heating reacting, finally, washing, centrifugating, drying and obtaining the product. The prepared nano upper converting fluorescence material has small and uniform granule, strong lighting strength, better water-solubility and can satisfy need of biomolecule fluorescence mark material.
Owner:CHANGCHUN INST OF OPTICS FINE MECHANICS & PHYSICS CHINESE ACAD OF SCI
Boriding agent
A boriding agent for generating boride layers on metallic workpieces, containing boron-releasing substances, activating substances and, in the remainder, refractory, inert extender. The activating substance is a combination of 1 to 5 wt. % potassium tetrafluoroborate and 5 to 40 wt. % calcium fluoride, relative to the total quantity of the boriding agent. With this boriding agent it is possible for single-phase, Fe2B-containing boride layers to be generated on workpieces made of ferrous materials. The agent results in lower emissions of fluorine and fluoride.
Owner:HOUGHTON DURFERRIT GMBH
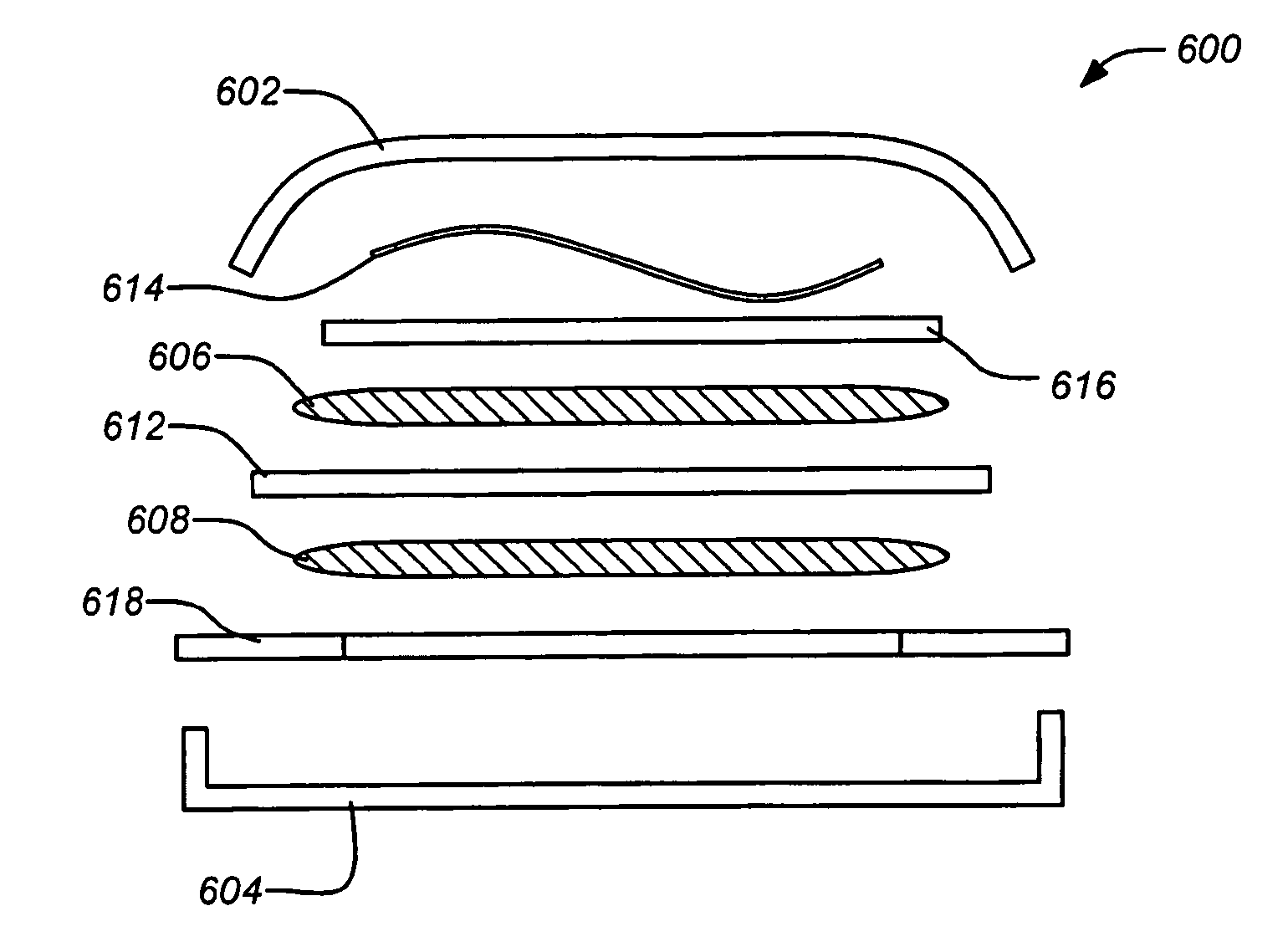
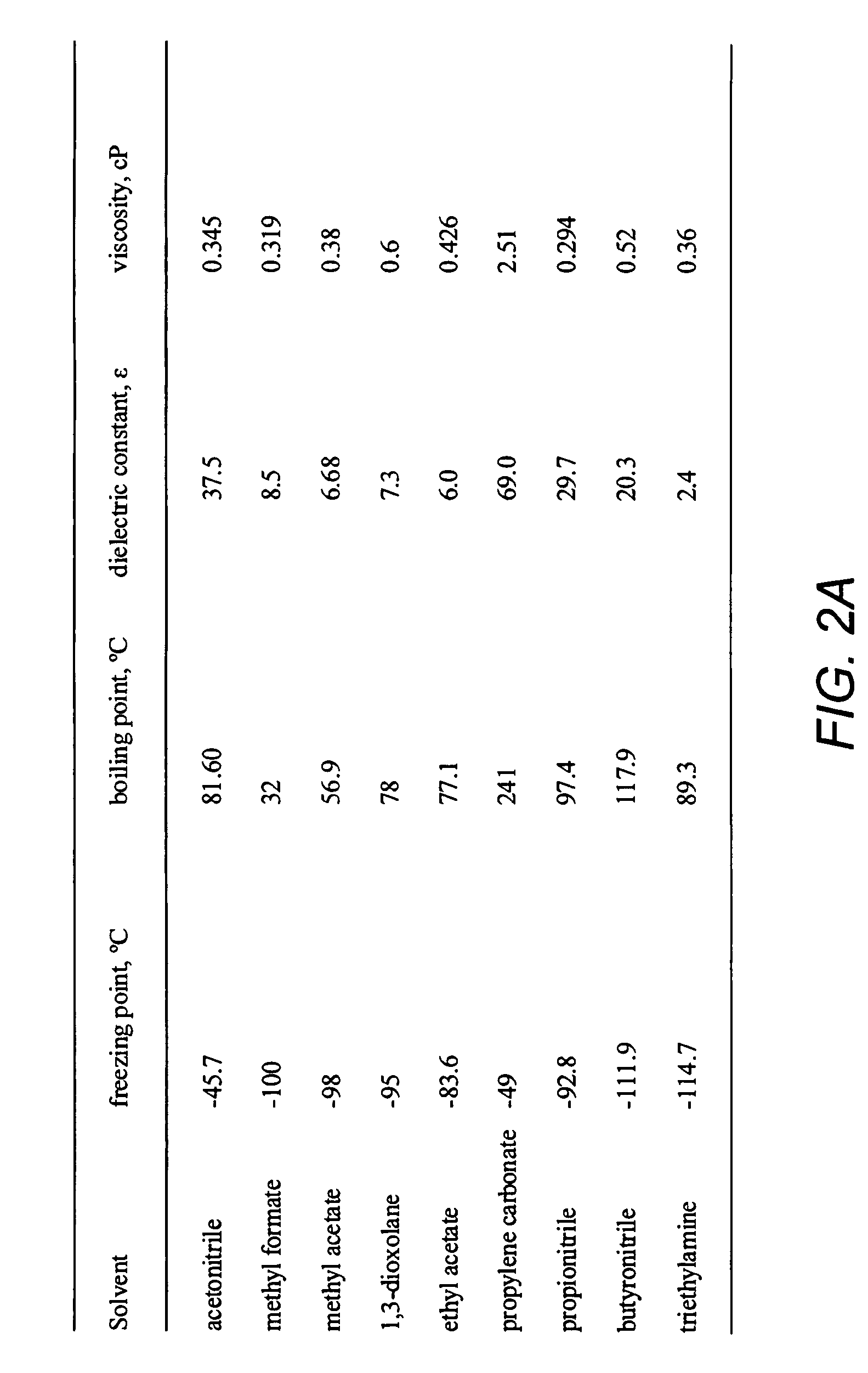
Low temperature double-layer capacitors
ActiveUS20080304207A1Low melting pointHybrid capacitor electrolytesClosuresTetrafluoroborateDevice form
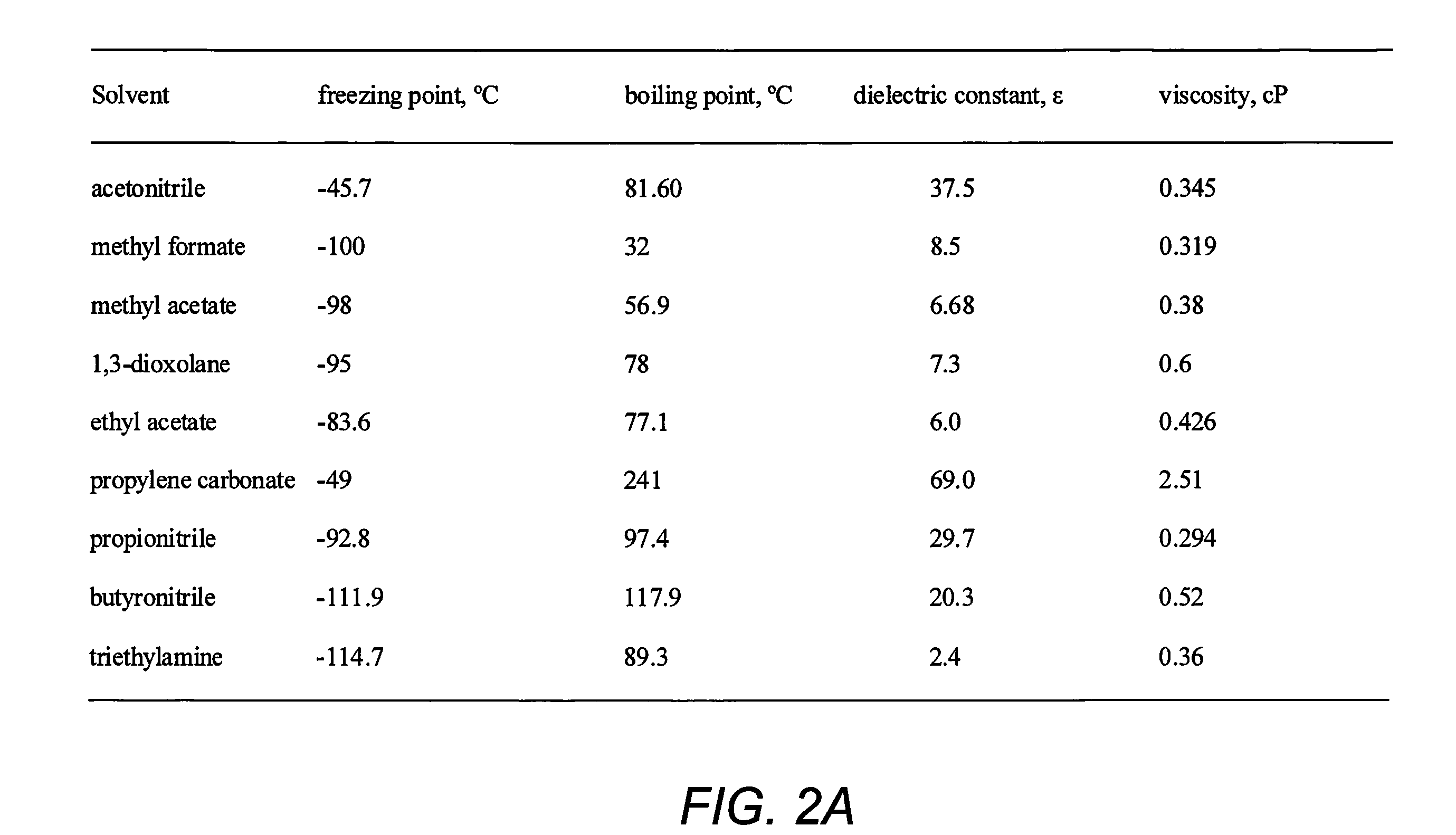
Double-layer capacitors capable of operating at extremely low temperatures (e.g., as low as −75° C.) are disclosed. Electrolyte solutions combining a base solvent (e.g., acetonitrile) and a cosolvent are employed to lower the melting point of the base electrolyte. Example cosolvents include methyl formate, ethyl acetate, methyl acetate, propionitrile, butyronitrile, and 1,3-dioxolane. An optimized concentration (e.g., 0.10 M to 0.75 M) of salt, such as tetraethylammonium tetrafluoroborate, is disolved into the electrolyte solution. In some cases (e.g., 1,3-dioxolane cosolvent) additives, such as 2% by volume triethylamine, may be included in the solvent mixture to prevent polymerization of the solution. Conventional device form factors and structural elements (e.g., porous carbon electrodes and a polyethylene separator) may be employed.
Owner:CALIFORNIA INST OF TECH
Ion liquid of amino acid ester cation and its preparation method
InactiveCN1621152AEasy to getLow priceOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsTetrafluoroborateAmino acid
The present invention is ion liquid of amino acid ester cation and its preparation process, and belongs to the field of new chemical material and its preparation technology. The ion liquid of amino acid ester cation is prepared with amino acid ester hydrochloride and through the substitution reaction with nitrate, tetrafluoroborate, hexafluorophosphate, bistrifluoromesyl imine salt or thiocyanate or the direct addition reaction with aluminum trichloride, ferric trichloride or zinc chloride, and the separation. The ion liquid thus prepared has the characteristic of chiral matter except the ion liquid characteristics, has low cost, simple preparation process and no pollution. The present invention is suitable for industrial production and is expected to become important green chemical material.
Owner:PEKING UNIV
Supported catalyst for preparing aldehyde by olefin hydroformylation
InactiveCN1736602AIncreased space-time yieldSimple compositionOrganic-compounds/hydrides/coordination-complexes catalystsPreparation by carbon monoxide reactionMolecular sieveTetrafluoroborate
Disclosed is a supported catalyst mainly containing sulfonated triphenylphosphine- rhodium complex and is used for preparing aldehyde by hydroformylation of olefin, and relates to an ionic liquid catalyst. It contains solid oxide, sulfonated triphenylphosphine- rhodium complex, sulfonated triphenylphosphine ligand and ionic liquid. The solid oxide is one from hole molecular sieve, SiO2, TiO2, gamma- Al2O3; sulfonated triphenylphosphine- rhodium complex is single-sulfonated triphenylphosphine- rhodium complex, di- sulfonated triphenylphosphine- rhodium complex and tri- sulfonated triphenylphosphine- rhodium complex; and the ionic liquid is 1, 1, 3, 3, - tetramethyl guanidine lactate, 1- butyl- 3- methyl imidazolium tetrafluorborate and 1- butyl- 3- methyl imidazolium hexafluorophosphate. By mass ratio, the solid oxide is among 50%- 90%, the ionic liquid 8%- 49%, and rhodium 0.05%- 2%, and by molecular ratio phosphine to rhodium is 3- 200.
Owner:XIAMEN UNIV
Preparation method of bis(sulfonyl fluoride) imine and (perfluoroalkyl sulfonyl fluorine sulfonyl) imine alkali metal salt
ActiveCN102786452AOvercome operabilityOvercome fatal shortcomings such as difficult product purificationSulfonic acid amide preparationTetrafluoroborateDecomposition
The invention discloses a method for preparing bis(sulfonyl fluoride) imine and (perfluoroalkyl sulfonyl fluorine sulfonyl) imine alkali metal salt. According to the method, sulfamide is utilized to take reaction with thionyl chloride and chlorosulfonic acid for preparing bis(sulfonyl fluoride) imine or (perfluoroalkyl sulfonyl fluorine sulfonyl) imine, then, the bis(sulfonyl fluoride) imine or (perfluoroalkyl sulfonyl fluorine sulfonyl) imine takes reaction with antimony trifluoride and potassium (rubidium or caesium and the like) carbonate, and corresponding high-purity bis(sulfonyl fluoride) imine potassium (rubidium or caesium) salt or (perfluoroalkyl sulfonyl fluorine sulfonyl) imine potassium (rubidium or caesium) salt can be obtained; and the double decomposition exchange reaction of the potassium (rubidium or caesium) salt and lithium (or sodium) perchlorate or lithium (or sodium) tetrafluoroborate and the like in aprotic polar solvents is utilized to obtain corresponding high-purity lithium (or sodium) salt. The method provided by the invention has the characteristics that the operation step is simple, the products can be easily separated and purified, the purity and the yield are high, the environment pollution is avoided, the method is suitable for industrial mass production, and the like.
Owner:武汉市瑞华新能源科技有限公司
Ionic liquid and power storage device including the same
ActiveUS20120308882A1Improve electrochemical stabilityLow melting pointHybrid capacitor separatorsHybrid capacitor electrolytesTetrafluoroborateElectrochemistry
An ionic liquid having high electrochemical stability and a low melting point. An ionic liquid represented by the following general formula (G0) is provided.In the general formula (G0), R0 to R5 are individually any of an alkyl group having 1 to 20 carbon atoms, a methoxy group, a methoxymethyl group, a methoxyethyl group, and a hydrogen atom, and A− is a univalent imide-based anion, a univalent methide-based anion, a perfluoroalkyl sulfonic acid anion, tetrafluoroborate, or hexafluorophosphate.
Owner:SEMICON ENERGY LAB CO LTD
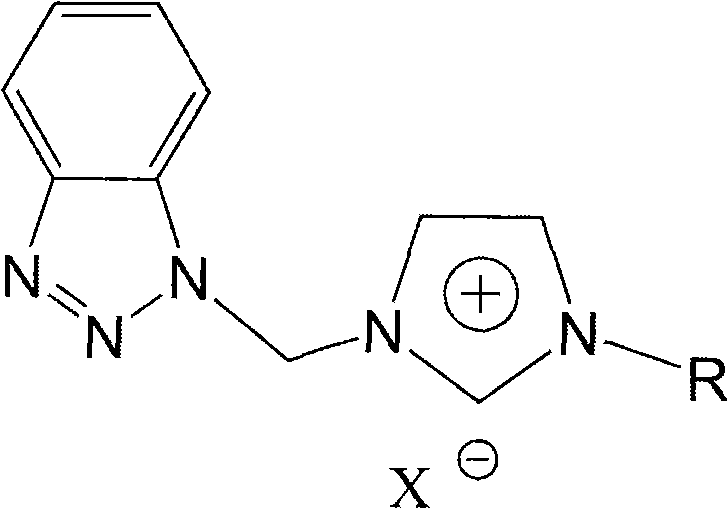
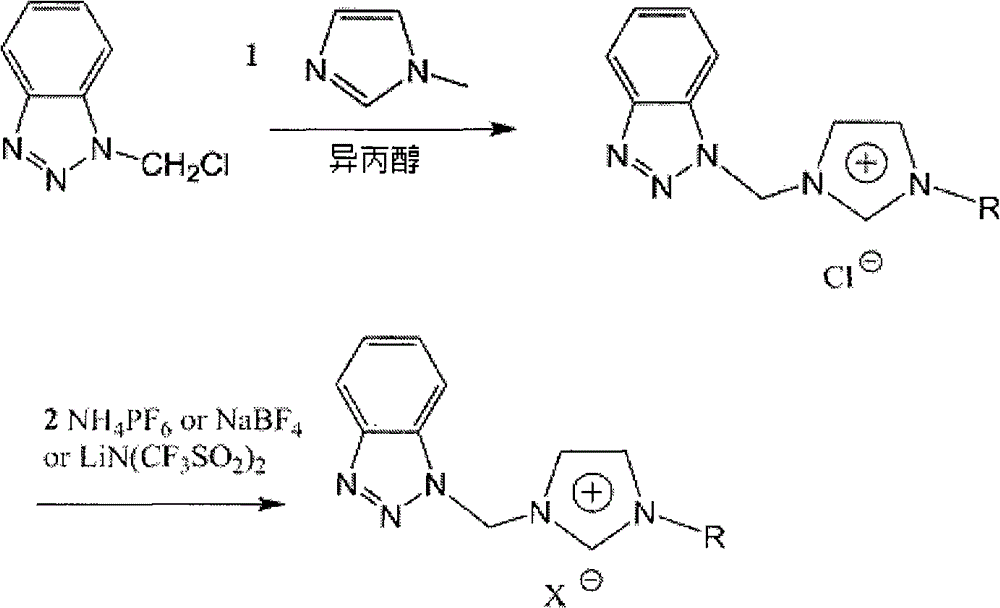
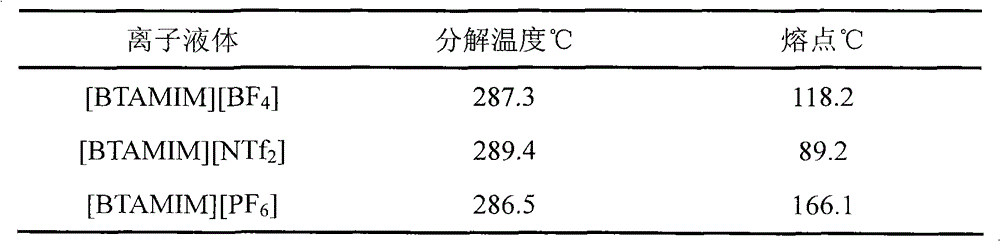
Benzotriazole group-containing ionic liquid and its preparation method and use
InactiveCN102746279AImprove thermal stabilityCorrosion resistanceOrganic chemistryAdditivesImideBenzene
The invention discloses a benzotriazole group-containing ionic liquid and its preparation method and use. The benzotriazole group-containing ionic liquid is characterized in that cations are imidazolium cations; anions are hexafluorophosphate radical, tetrafluoroborate radical or bis(trifluoromethylsulfonyl)imide anions; and an imidazole ring-substituted end group contains a benzotriazole group. The benzotriazole group-containing ionic liquid can be used as a lubricant additive or a lubricating grease additive.
Owner:LANZHOU INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Composite extreme pressure anti-wear agent and total-synthesis anti-wear hydraulic oil containing same
ActiveCN102776053AExcellent friction reductionImprove wear resistanceAdditivesTetrafluoroborateBase oil
The invention discloses a composite extreme pressure anti-wear agent and total-synthesis anti-wear hydraulic oil containing the composite extreme pressure anti-wear agent, belonging to the field of production of lubricating oil. The hydraulic oil is mainly prepared by mixing aqueous base oil and the composite extreme pressure anti-wear agent, wherein the composite extreme pressure anti-wear agent is a compound of two kinds of imidazolyl tetrafluoroborate ion liquid. Compared with the prior art, the total-synthesis anti-wear hydraulic oil has the characteristics of excellent lubricating performance, energy saving, environment friendliness and the like and has high popularization and application values.
Owner:SHANDONG YUANGEN PETROLEUM CHEM
Ionic liquid for assisting oil-sand separation and separation method
ActiveCN102391185ALow viscosityReduce transportation energy consumptionOrganic chemistryLiquid hydrocarbon mixture productionTetrafluoroborateOrganic solvent
The invention relates to ionic liquid for assisting oil-sand separation and a separation method. According to the separation method, the ionic liquid is 1-ethyl-3-methylimidazole tetrafluoroborate; the range of the mass of added ionic liquid is 1-5 times of the mass of oil sand, and the ratio of the volume of an organic solvent to the mass of the oil sand is 1-12 ml / g so as to extract asphalt; the organic solvent and the ionic liquid simultaneously enter an extraction device so as to carry out extraction separation on the oil sand, and the asphalt separation is carried out under a temperature range of 15-60 DEG C; the organic solvent is collected through distilling at a temperature of 70-200 DEG C, and the rest organic product after distilling is an asphalt product; the residual sand and the ionic liquid are subjected to water cleaning by a small amount of water after oil-sand separation, the residual sand product is cleanly discharged; and the ionic liquid and the water can be recycled through fractionation by distillation. The residual sand and the ionic liquid are cleaned by the small amount of water after the oil-sand separation, thus, water is saved, and further, the loss of ionic liquid reagents is little; and in addition, the assisting separation method has high efficiency for the recovery of the asphalt which can reach 95%, and the residual organic matters in the oil sand is little.
Owner:TIANJIN UNIV
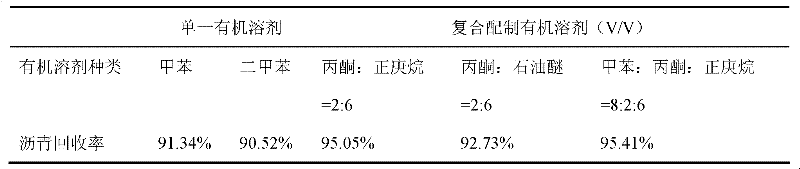
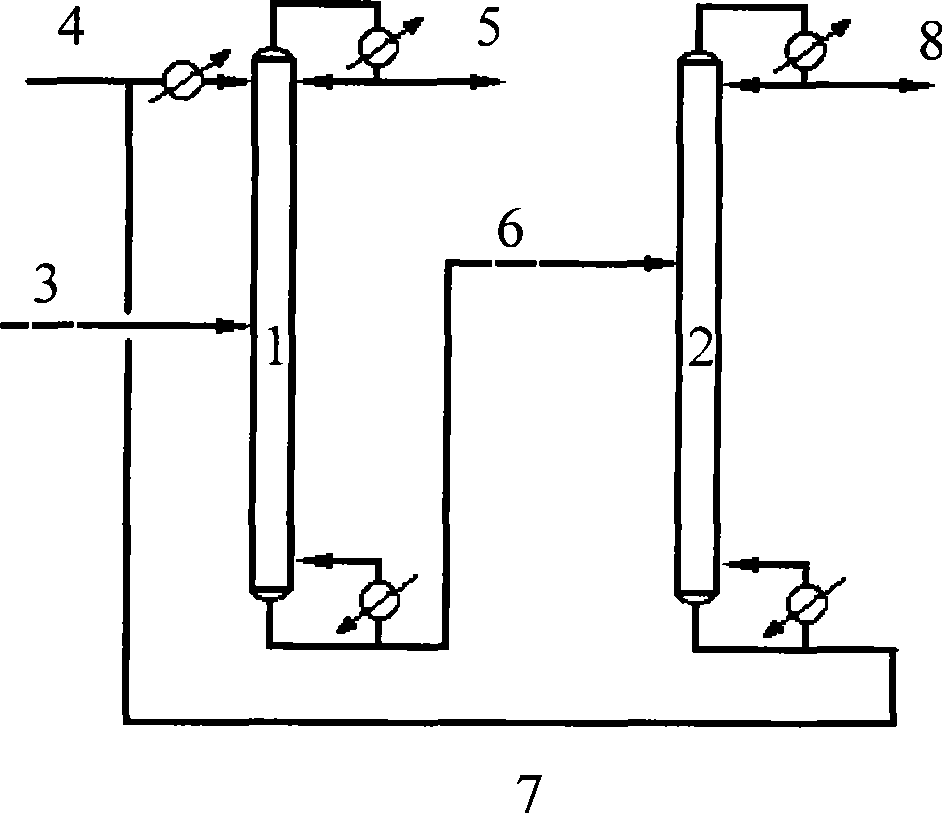
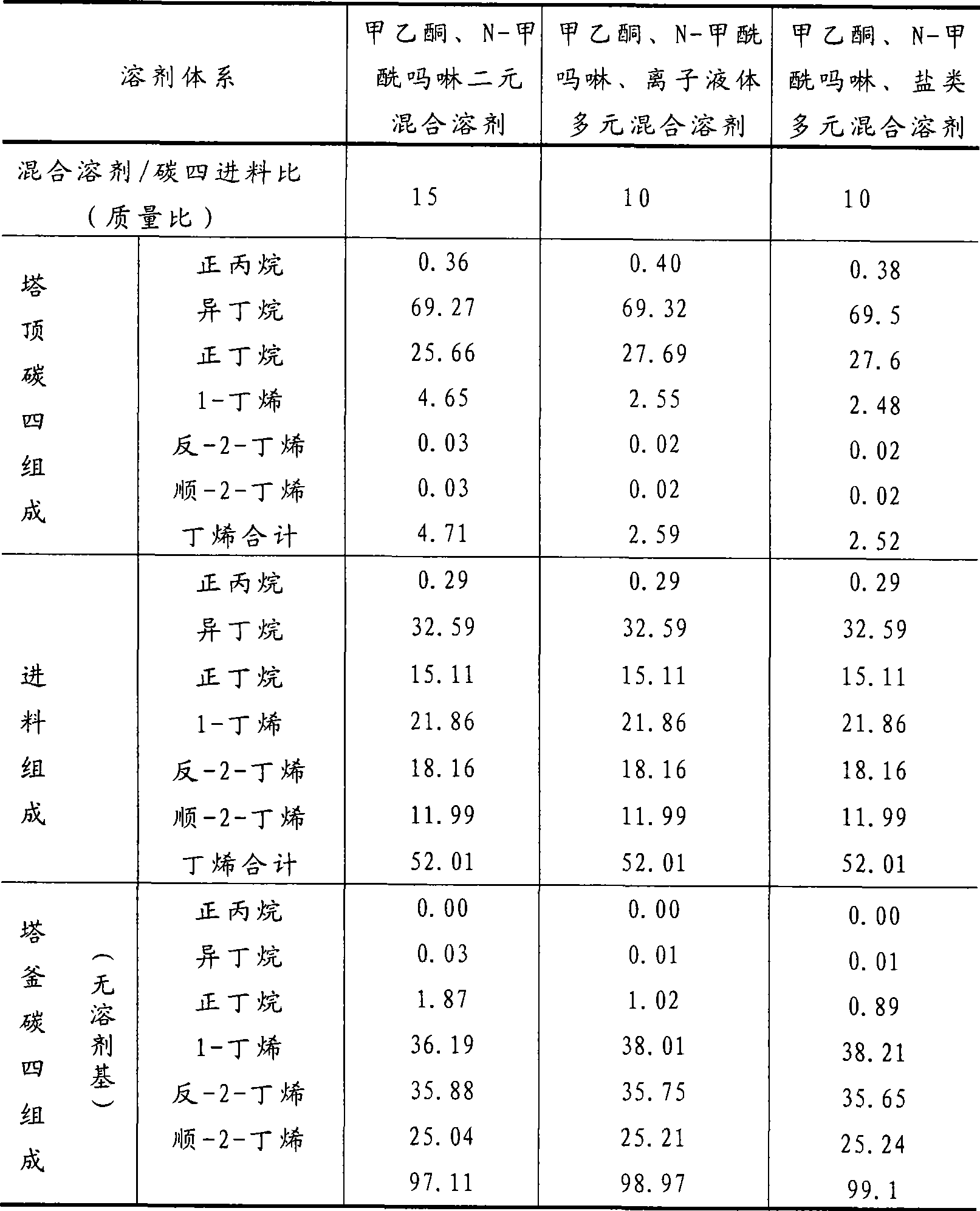
Method for separating butane and butene by using multiple mixed solvent
InactiveCN101417913ARetain solubilityImprove solubilityDistillation purification/separationBulk chemical productionSodium sulfocyanateTetrafluoroborate
The invention relates to a method for spearing butane from butylene with the multicomponent compound of ionic liquid, saline, ethyl methyl ketone and N-formylmorpholine. The content of ionic liquid in the multicomponent compound is 1.0 to 95 percent; the content of the saline in the multicomponent compound is 0.5 to 20 percent; the electropositive ion of the ionic liquid is iminazole electropositive ion, alkyl imidazole electropositive ion or alkyl quaternary ammonium ion, or the compound thereof; the electronegative ion is tetrafluoroborate electronegative ion, hexafluorophosphoric acid electronegative ion, nitrate ion, tetrachloro aluminic acid iron, heptachlor bi aluminic acid iron, chloride ion, bromine electronegative ion or the mixture; and the ionic liquid can be dimethylformamide potassium thiocyanate compound salt, dimethylformamide sodium sulfocyanate compound salt, or the compound; the saline is potassium thiocyanate, sodium sulfocyanate, ammonium thiocyanate, sodium nitrate, potassium nitrate, sodium iodide, potassium iodide, zinc chloride, copper chloride, zinc chloride, potassium bromide, or the compound thereof.
Owner:YANTAI UNIV
Molding composition and method, and molded article
A curable method useful for encapsulating solid state devices includes (A) an epoxy resin; (B) an effective amount of a cure catalyst comprising (B1) a first latent cationic cure catalyst comprising a diaryl iodonium hexafluoroantimonate salt; (B2) a second latent cationic cure catalyst comprising (B2a) a diaryl iodonium cation, and (B2b) an anion selected from perchlorate, imidodisulfurylfluoride anion, unsubstituted and substituted (C1-C12)-hydrocarbylsulfonates, (C2-C12)-perfluoroalkanoates, tetrafluoroborate, unsubstituted and substituted tetra-(C1-C12)-hydrocarbylborates, hexafluorophosphate, hexafluoroarsenate, tris(trifluoromethylsulfonyl)methyl anion, bis(trifluoromethylsulfonyl)methyl anion, bis(trifluoromethylsulfuryl)imide anion, and combinations thereof; and (B3) a cure co-catalyst selected from free-radical generating aromatic compounds, peroxy compounds, copper (II) salts of aliphatic carboxylic acids, copper (II) salts of aromatic carboxylic acids, copper (II) acetylacetonate, and combinations thereof; and (C) about 70 to about 95 weight percent of an inorganic filler, based on the total weight of the curable composition. The composition's cure catalyst allows the use of increased filler loadings, which in turn reduces moisture absorption and thermal expansion of the cured composition.
Owner:SABIC INNOVATIVE PLASTICS IP BV
Carbon electrodes and electrochemical capacitors
InactiveUS7924549B1Hybrid capacitor electrodesElectrolytic capacitorsHigh concentrationTetrafluoroborate
Carbon electrodes for a capacitor having conditioned carbon elements in combination with a high concentration of an electrolyte tetrafluoroborate salt and a non-aqueous aprotic solvent to provide an operational voltage up to 4.5V and capacitors used with the carbon electrodes.
Owner:SMITH W NOVIS +1
Drilling fluid and use of imidazole ionic liquid therein
ActiveCN105038739AImprove high temperature rheologyExcellent high temperature rheologyDrilling compositionCarboxymethyl celluloseTetrafluoroborate
The invention relates to drilling fluid containing 0.02-0.08% by weight of ionic liquid 1-alkyl-3-methylimidazol tetrafluoroborate, 0.1-0.5% by weight of polymers, 1-6% by weight of bentonite and the balance of aqueous solution, wherein the polymers are selected from one or more of partially hydrolyzed polyacrylamide, high-viscosity sodium carboxymethyl cellulose and xanthan gum, and the carbon atom number of alkyl is 4-8. The drilling fluid has an obviously-improved system high-temperature rheological property.
Owner:CHINA UNIV OF GEOSCIENCES (BEIJING)
Ionic liquid polymer electrolyte and preparation method thereof
InactiveCN104466240AEasy to prepareImprove conductivityElectrolyte accumulators manufactureChemical LinkagePolyethylene oxide
The invention discloses an ionic liquid polymer electrolyte and a preparation method thereof. An ionic liquid polymer is synthesized through using a polymer chemical functionalization process, and the structure of the ionic liquid polymer is represented by formula (1) shown in the specification. The ionic liquid polymer is formed through connecting N-methylimidazole with a polymer by chemical bonds, wherein the polymer can be a linear polymer and a hyperbranched polymer, the linear polymer is polyethylene oxide and polyepoxy chloropropane preferably, and the hyperbranched polymer is hyperbranched polyether preferably; and anion a can be halogen, tetrafluoroborate, hexafluorophosphate and ditrifluoromethyl sulfimide, and N-methylimidazole in the formula (1) can be substituted by a pyridine ring, tributyl amine and a pyrrole ring. The ionic liquid polymer electrolyte is composed of the ionic liquid polymer and a lithium salt, and contains no volatile solvents, and the conductivity at room temperature of the ionic liquid polymer electrolyte can reach 3.5*10<-4>Scm<-1>. The polymer electrolyte can be used in lithium ion secondary batteries, electrochromic devices, supercapacitors and other electrochemical devices.
Owner:UNIVERSITY OF CHINESE ACADEMY OF SCIENCES
Tetrafluoroborate compounds, compositions and related methods of use
InactiveUS8389453B2Non-corrosive to skinLow transportation costSoap detergents with inorganic compounding agentsDetergent mixture composition preparationTetrafluoroborateNitrogenous base
Owner:VITECH INT
Low temperature double-layer capacitors using asymmetric and spiro-type quaternary ammonium salts
InactiveUS20110170237A1Improve performanceHybrid capacitor electrolytesHybrid capacitor electrodesTetrafluoroborateQuaternary ammonium cation
Double-layer capacitors capable of operating at extremely low temperatures (e.g., as low as −80° C.) are disclosed. Electrolyte solutions combining a base solvent (e.g., acetonitrile) and a cosolvent are employed to lower the melting point of the base electrolyte. Example cosolvents include methyl formate, ethyl acetate, methyl acetate, propionitrile, butyronitrile, and 1,3-dioxolane. A quaternary ammonium salt including at least one of triethylmethylammonium tetrafluoroborate (TEMATFB) and spiro-(1,1′)-bipyrrolidium tetrafluoroborate (SBPBF4), is used in an optimized concentration (e.g., 0.10 M to 0.75 M), dissolved into the electrolyte solution. Conventional device form factors and structural elements (e.g., porous carbon electrodes and a polyethylene separator) may be employed.
Owner:CALIFORNIA INST OF TECH
Preparation method of purified vanadium adsorption material
InactiveCN105833854AImprove adsorption capacityNot easy to loseOther chemical processesTetrafluoroborateIodide
The invention relates to a preparation method of a purified vanadium adsorption material. Bis(cyclopentadiene) vanadium iodide and octadecendioic acid are introduced in the polymerization process; 1-allyl-3-buthylimidazole tetrafluoroborate is used for polymerization and preparation.
Owner:王金明
Method of removing and recovering boron trifluoride with metal fluoride and process for polyolefin production using the same
InactiveCN1289344AImprove efficiencyHigh desorption recoveryIon-exchange process apparatusIon-exchanger regenerationOligomerPolyolefin
Boron trifluoride can be recovered in a reusable state by a method that is economical and does not cause environmental pollution, which method comprises the steps of bringing a fluid containing boron trifluoride or its complex into contact with metal fluoride so as to selectively adsorb and remove boron trifluoride in the complex and heating the resultant metal tetrafluoroborate at a temperature in the range of 100 to 600 DEG C to separate it into boron trifluoride and metal fluoride. By applying the method to a process for producing polybutene or olefin oligomer using boron trifluoride complex catalyst, the catalyst can be recovered with retaining its activity and reused effectively.
Owner:NIPPON PETROCHEMICAL CO LTD
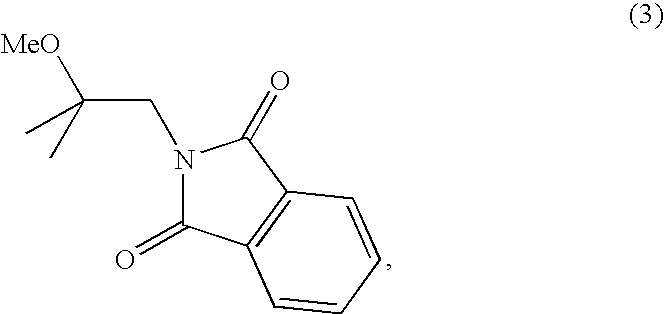
Methods for preparing 2-methoxyisobutylisonitrile and tetrakis(2-methoxyisobutylisonitrile)copper(i) tetrafluoroborate
ActiveUS20080102028A1Isocyanic acid derivatives preparationCarboxylic acid nitrile preparationTechnetiumTetrafluoroborate
The present invention relates to new synthetic methods for preparing 2-methoxyisobutylisonitrile and metal isonitrile complexes, such as tetrakis(2-methoxyisobutylisonitrile)copper(I) tetrafluroroborate, which are used in the preparation of technetium (99mTc) Sestamibi, and novel intermediate compounds useful in such methods.
Owner:JUBILANT DRAXIMAGE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com