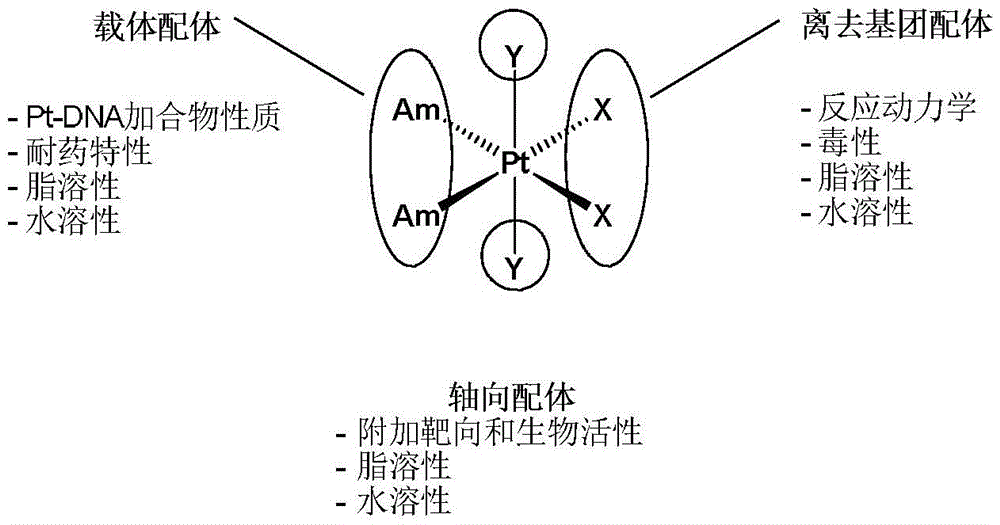
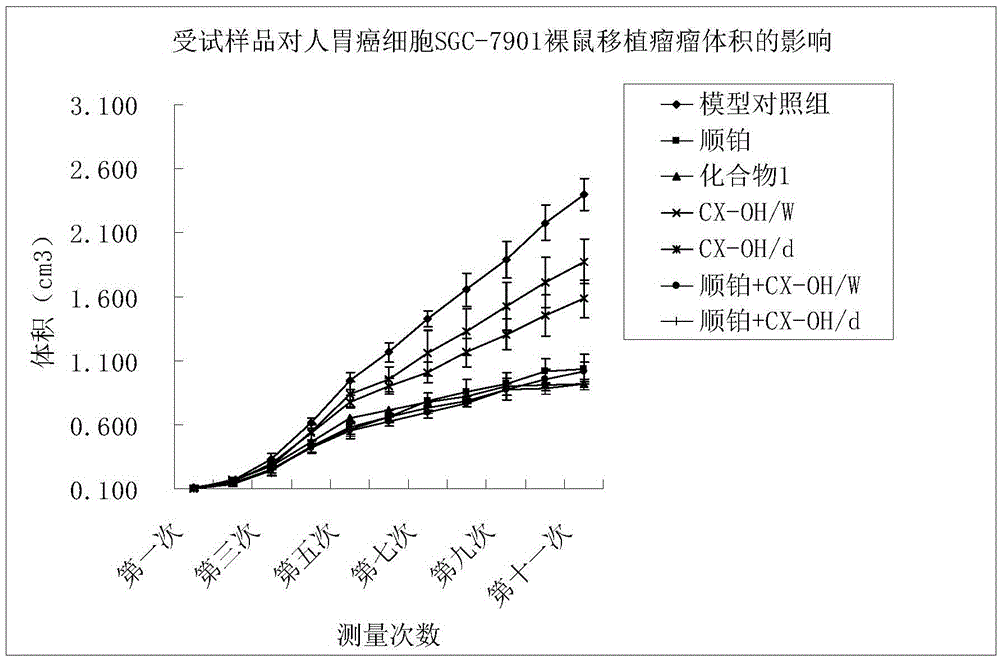
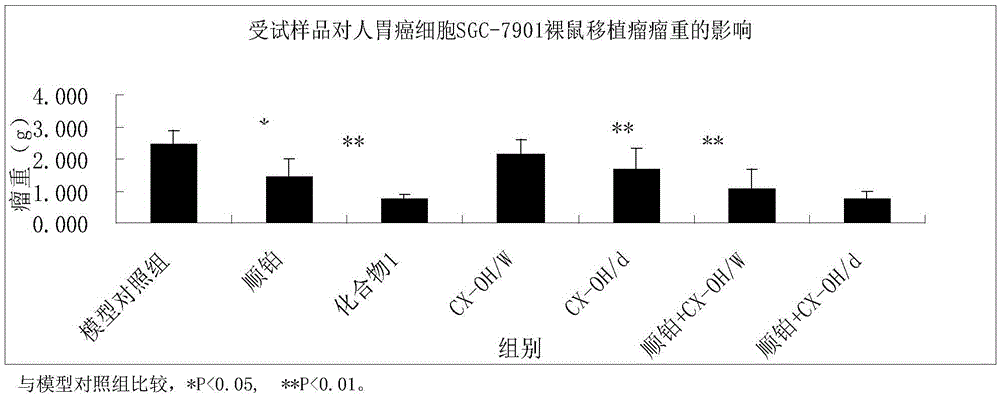
Tetravalent platinum complex with bioactive group and preparation method of tetravalent platinum complex
A technology of biological activity and complexes, applied in the direction of platinum organic compounds, platinum group organic compounds, compounds containing elements of group 8/9/10/18 of the periodic table, etc., can solve toxicity, nephrotoxicity, and drug resistance And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0061] The preparation of CUT-OH is carried out by the reaction formula shown in formula V,
[0062]
[0063] The preparation of CUT-OH adopts the following preparation method:
[0064] 1. Add equimolar 2,4-dihydroxybenzaldehyde and acetylglycine and three times the amount of sodium acetate into a certain volume of acetic anhydride, stir and reflux for 4 hours, cool the reaction solution to room temperature, pour it into ice water, and obtain yellow The precipitate was filtered, washed twice with ice water, and the solid was dried to obtain intermediate M1;
[0065] 2. Add M1 to the mixed solution of concentrated hydrochloric acid and ethanol (2:1), reflux for 1h, cool the reaction solution to room temperature, pour it into ice water, and then slowly add twice the amount of sodium nitrite under ice bath conditions After adding and stirring for 15min, slowly add three times the amount of sodium azide, continue to stir for 15min, filter out the brown precipitate, wash with w...
Embodiment 1
[0072] Embodiment 1. Preparation of compound 1
[0073]Dissolve 157.4 mg (0.45 mmol) of CX-OH and 144.5 mg (0.45 mmol) of TBTU in 15 mL of anhydrous DMF, stir at room temperature for 10 min, then add 45.5 mg (0.45 mmol) of TEA, continue stirring for 5 min, and then add 150.0 mg (0.45 mmol) cis, cis, anti-[Pt(NH 3 ) 2 Cl 2 (OH) 2 ], reacted at 50°C for 48h under the protection of nitrogen. The reaction solution was concentrated, and the concentrated solution was separated by silica gel column chromatography, and the eluent was a mixed solvent of dichloromethane and methanol (10:1) to obtain 79.9 mg of a yellow product with a yield of 40%.
[0074] 1 HNMR (DMSO-d6, ppm): δ4.23 (s, 6H), 7.13 (d, J = 7.9, 1H), 7.44 (t, J = 8.0, 1H), 7.97 (d, J = 8.4, 1H) , 8.07(d, J=8.6, 1H), 8.27(m, 2H), 8.46(t, J=2.0, 1H), 8.61(d, J=5.9, 1H), 8.82(d, J=8.5, 1H ), 9.14(d, J=5.9, 1H), 9.7(s, 1H), 10.2(s, 1H). 13 CNMR (DMSO-d6, ppm): δ116.73, 119.53, 120.53, 122.58, 122.75, 122.92, 124.37, ...
Embodiment 2
[0075] Embodiment 2. Preparation of Compound 2
[0076] With reactants cis, cis, trans-[Pt(NH 3 ) 2 Cl 2 (OH)Cl] instead of cis, cis, anti-[Pt(NH 3 ) 2 Cl 2 (OH) 2 ] Prepared with reference to the method described in Example 1 to obtain a yellow product with a yield of 38%.
[0077] 1 HNMR (DMSO-d6, ppm): δ6.38 (s, 6H), 7.16 (d, J = 8.1, 1H), 7.49 (t, J = 8.2, 1H), 7.99 (d, J = 8.4, 1H) , 8.08(d, J=8.4, 1H), 8.3(m, 2H), 8.61(d, J=5.6, 1H), 8.85(d, J=8.5, 1H), 9.01(d, J=5.6, 1H ), 9.7(s, 1H), 10.2(s, 1H). 13 CNMR(DMSO-d6,ppm):δ116.87,119.53,120.52,121.85,122.29,122.60,124.28,125.38,127.58,128.77,130.62,133.28,135.44,142.40,143.48,147.77,148.22,150.41,173.19.ESI -MS: [M-H] + =681.9
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com