Benzotriazole group-containing ionic liquid and its preparation method and use
A benzotriazole and ionic liquid technology, applied in the petroleum industry, additives, organic chemistry, etc., to achieve the effects of excellent anti-friction and anti-wear properties, high thermal stability, and high bearing capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
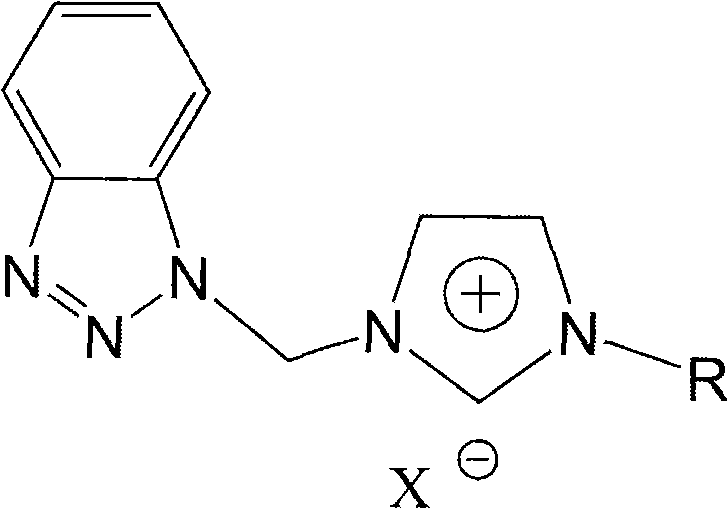
[0031] Synthesis of 1-Benzotriazole Methyl-3-Methylimidazolium Hexafluorophosphate Ionic Liquid
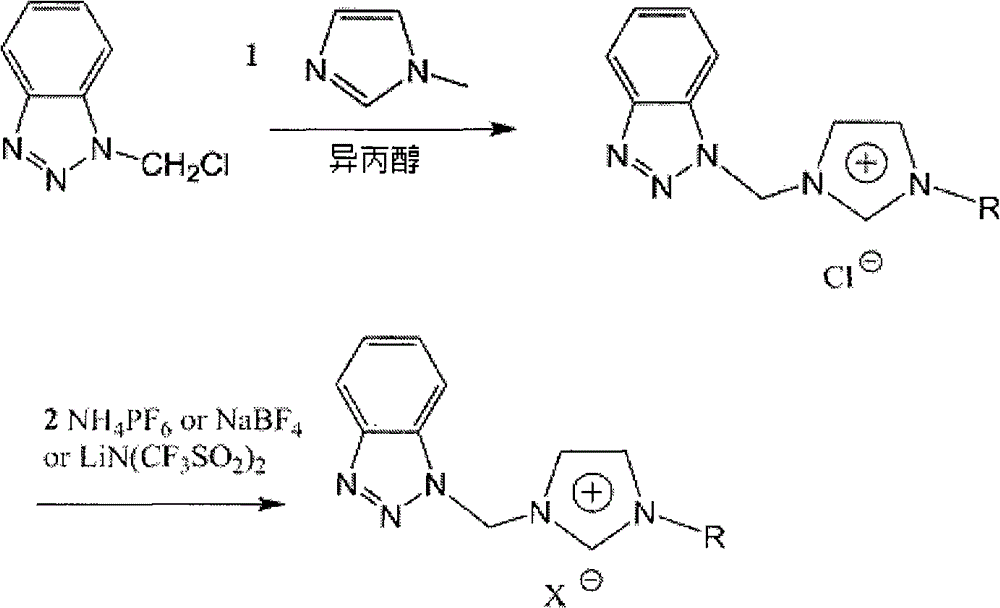
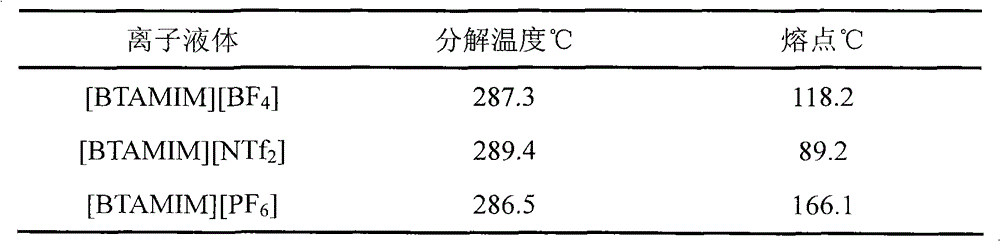
[0032]Add 4.105 grams of N-methylimidazole to a 100ml three-necked bottle and add 15ml of isopropanol to dissolve it, slowly add 8.358 grams of 1-chloromethylbenzotriazole dissolved in 20ml of isopropanol, and pass through nitrogen protection, 60-70 Reaction at ℃ for 15-20 hours. After the reaction, the solvent was spun off to obtain 11.42 g of white or light yellow powder with a yield of 91%. Add 4.994 grams of 1-benzotriazole methyl-3-methylimidazolium chloride salt into a 250ml single-mouth bottle and add an appropriate amount of distilled water to dissolve it, then add 3.91 grams of ammonium hexafluorophosphate dissolved in an appropriate amount of distilled water drop by drop, the solution changes rapidly Turbidity and white solids are formed, exchange for 16-24 hours after the dropwise addition. After the reaction, it was filtered and dried to obtain 6.39 g of white powder...
Embodiment 2
[0034] Synthesis of Ionic Liquids of 1-Benzotriazole Methyl-3-Methylimidazolium Tetrafluoroborate
[0035] The preparation method of 1-benzotriazole methyl-3-methylimidazolium chloride salt is the same as that in Example 1. Add 4.994 grams of 1-benzotriazole methyl-3-methylimidazolium chloride salt into a 250ml single-mouth bottle and add an appropriate amount of distilled water to dissolve it, then add 2.64 grams of sodium tetrafluoroborate dissolved in an appropriate amount of distilled water drop by drop, and the solution changes rapidly Turbidity and white solids are formed, exchange for 16-24 hours after the dropwise addition. After the reaction, it was filtered and dried to obtain 5.4189 g of white powdery solid, and 5.21 g of white crystals were obtained by silica gel column chromatography with a yield of 96%. 1 H-NMR (400MHz, CD 3 COCD 3 , ppm): δ=9.43 (1H, s, N=CH), 8.16 (1H, s, CH-C), 8.09 (1H, s, CH-C), 8.01 (1H, s, N-CH), 7.76 (1H, s, CH-N), 7.70 (1H, s, CH=CH)...
Embodiment 3
[0037] Synthesis of Ionic Liquids of 1-Benzotriazole Methyl-3-Methylimidazolium Bis(trifluoromethylsulfonyl)amine Salt
[0038] The preparation method of 1-benzotriazole methyl-3-methylimidazolium chloride salt is the same as that in Example 1. Add 4.994 grams of 1-benzotriazole methyl-3-methylimidazolium chloride salt into a 250ml single-mouth bottle and add an appropriate amount of distilled water to dissolve it, and add 6.89 grams of bis(trifluoromethylsulfonyl) dissolved in an appropriate amount of distilled water drop by drop ) Lithium amide, the solution quickly becomes turbid and a white solid is formed, exchange for 16-24 hours after the dropwise addition. After the reaction, it was filtered and dried to obtain 9.19 g of white powdery solid, and 8.46 g of white crystals were obtained by silica gel column chromatography, with a yield of 92%. 1 H-NMR (400MHz, CD 3 COCD 3 , ppm): δ=9.50 (1H, s, N=CH), 8.12 (1H, s, CH-C), 8.10 (1H, s, CH-C), 8.07 (1H, s, N-CH), 7.80 (1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com