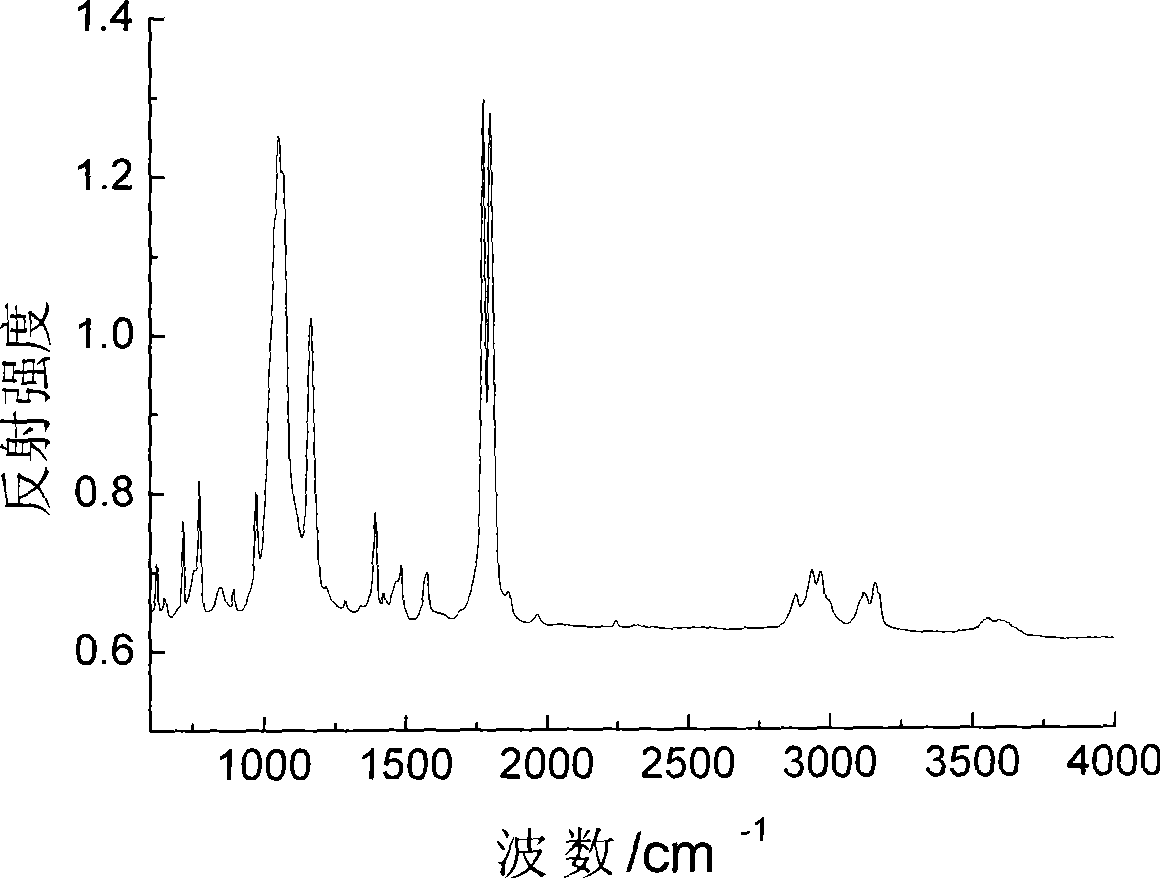
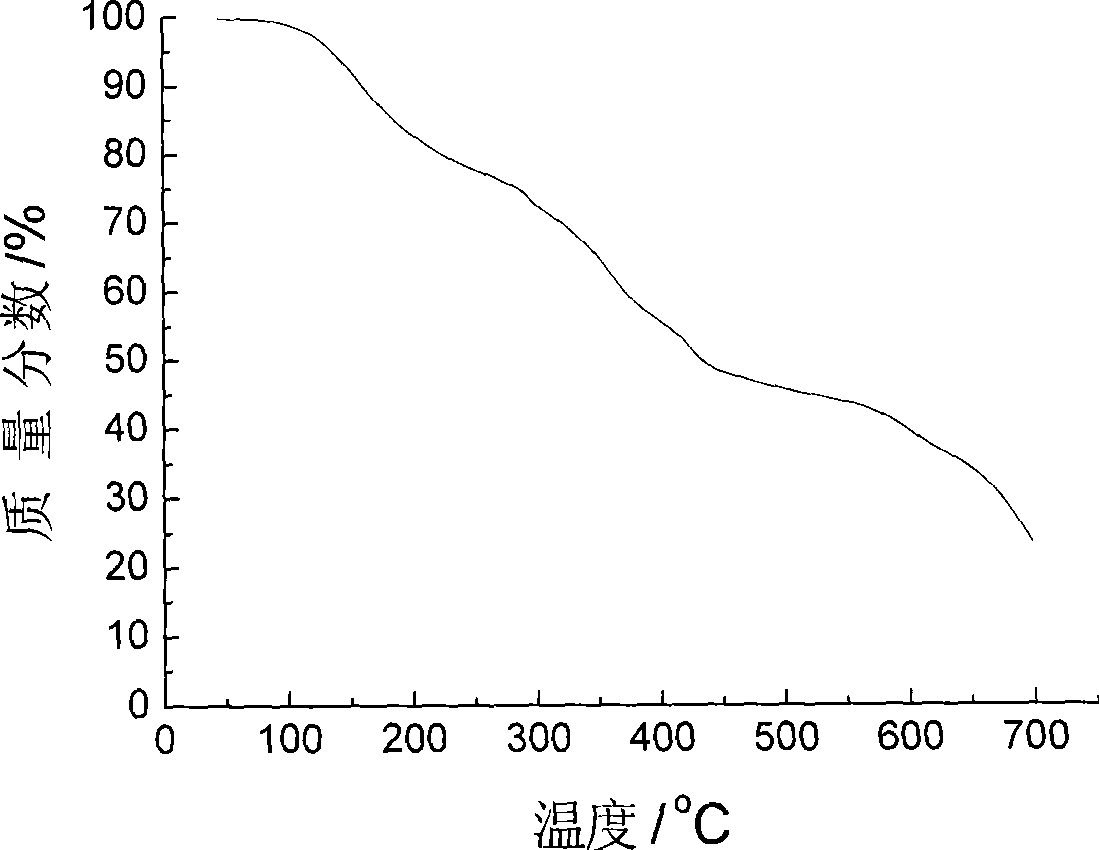
Method for preparing ion liquid type gel polymer electrolyte and battery by in situ polymerization
A gel polymer, ionic liquid technology, used in secondary batteries, circuits, electrical components, etc., can solve the problem of ionic liquid gel polymer electrolytes and batteries. There are no patent publications and article reports, lithium-ion battery preparation The method is complex, the process requirements are high, and the effect of good application prospect, avoidance of leakage and simple preparation process is achieved.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Dissolve 0.1g of polyethylene glycol dimethacrylate in 1.9g of acrylonitrile as component A; dissolve 1.0g of ethylene carbonate in component A as component B; weigh 1.0g of 1- Butyl-3-methylimidazolium tetrafluoroborate and 0.01g of azobisisobutyrocyanide initiator are directly mixed with component B and stirred at 25°C. Polymerized for 72 hours to obtain ionic liquid gel polymer electrolyte. Conductivity at room temperature: 1.69×10 -4 S cm -1 . It is also found that under this proportion, the prepared ionic liquid gel polymer electrolyte has good dimensional stability, and satisfies the requirement of ion conductivity as an electrolyte for lithium-ion batteries.
Embodiment 2
[0034] Dissolve 0.1g of polyethylene glycol dimethacrylate in 1.9g of acrylonitrile as component A; dissolve 1.0g of ethylene carbonate and 0.17g of lithium perchlorate in component A as component B ;;Weigh 1.0g of 1-butyl-3-methylimidazolium tetrafluoroborate and 0.01g of azobisisobutyrocyanide initiator, mix it directly with component B, and stir at 25°C, After stirring evenly, polymerize in situ at 60° C. for 72 hours to obtain an ionic liquid gel polymer electrolyte. Conductivity at room temperature: 9.86×10 -5 S cm -1 . It is also found that under this proportion, the prepared ionic liquid gel polymer electrolyte has good dimensional stability, and satisfies the requirement of ion conductivity as an electrolyte for lithium-ion batteries.
Embodiment 3
[0036] Dissolve 0.1g of polyethylene glycol dimethacrylate in 1.9g of acrylonitrile to form component A; dissolve 1.0g of ethylene carbonate and 0.2128g of lithium perchlorate in component A to become component B; Weigh 1.0g of 1-butyl-3-methylimidazolium tetrafluoroborate and 0.01g of azobisisobutyronitrile initiator, mix it directly with component B, and stir at 25°C, After stirring evenly, polymerize in situ at 60° C. for 72 hours to obtain an ionic liquid gel polymer electrolyte. Conductivity at room temperature: 1.72×10 -4 S cm -1 . It is also found that under this proportion, the prepared ionic liquid gel polymer electrolyte has good dimensional stability, and satisfies the requirement of ion conductivity as an electrolyte for lithium-ion batteries.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conductivity at room temperature | aaaaa | aaaaa |
| Conductivity | aaaaa | aaaaa |
| Conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com