Method of removing and recovering boron trifluoride with metal fluoride and process for polyolefin production using the same
A technology of boron trifluoride and fluoride, applied in chemical instruments and methods, separation methods, chemical/physical processes, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
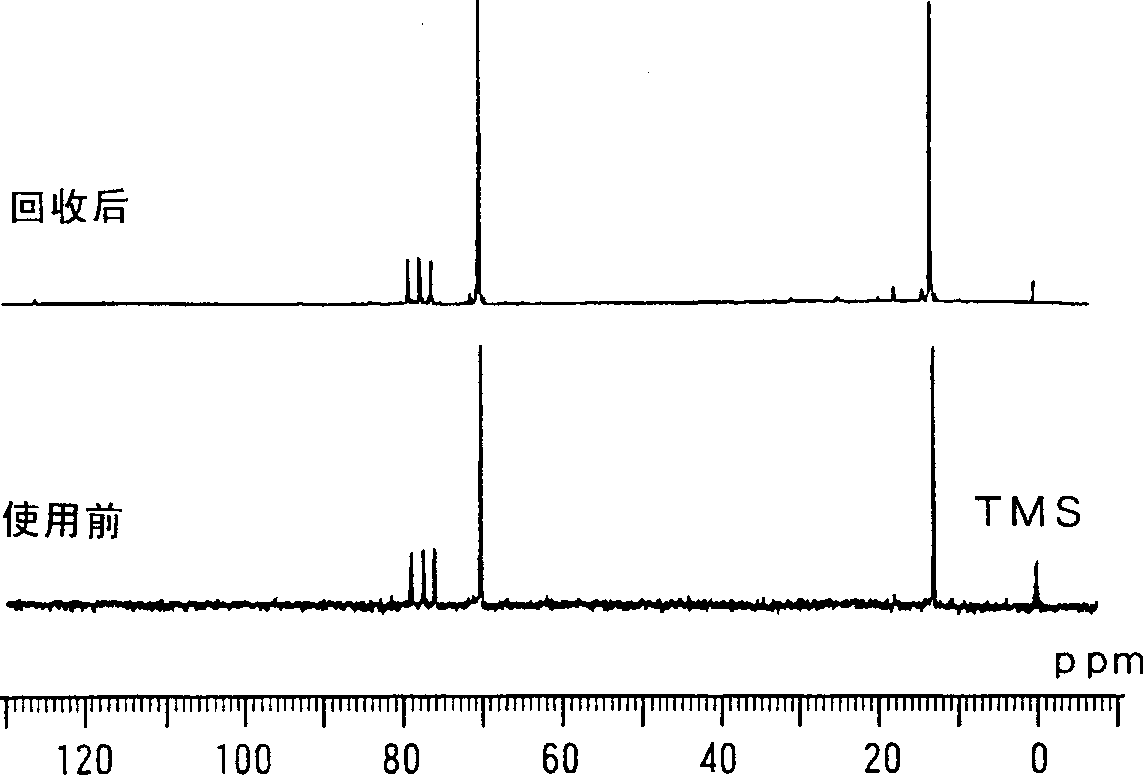
Image
Examples
Embodiment 1
[0193] (Absorptive removal of boron trifluoride)
[0194] Under nitrogen flow, 100 g of the dispersion liquid (boron trifluoride 0.25% (weight)) that boron trifluoride diethyl ether complex is dispersed in hexane is put into the Erlenmeyer flask with stirrer, at a constant temperature of 25 ℃ and While maintaining stirring, 1.91 g (equivalent to 20 times the mole of boron trifluoride) of a commercially available special-grade reagent lithium fluoride (purity 99% or more, manufactured by Soegawa Rika Co., Ltd.) was added, and stirred for 30 minutes under nitrogen flow.
[0195] Stop stirring, let the reaction solution stand still, use the difference in specific gravity to separate the adsorbent and the organic liquid into two phases, and separate the organic phase into another container by decantation. An aqueous solution of calcium chloride was added to the above-mentioned organic phase, and the concentration of boron trifluoride remaining in the organic phase was measured by ...
Embodiment 2
[0205] (Absorptive removal of boron trifluoride)
[0206] 18.00 g (694 mmol) of lithium fluoride used in Example 1 was filled in a stainless steel tubular container with a diameter of 20 mm and a length of 200 mm. The above-mentioned tube was kept at a constant temperature of 25° C., and in the same manner as in Example 1, a dispersion solution in which boron trifluoride diethyl ether complex was dispersed in hexane was supplied at a constant flow rate of 10 ml / hour (boron trifluoride 16.5 mg / hour). Liquid (boron trifluoride 0.25% (weight)). The concentration of boron trifluoride in the outlet fluid is 0, indicating that boron trifluoride has been completely removed by adsorption.
[0207] The adsorption operation was continued, and 120 hours after the supply of the dispersion solution was started, the supply of the dispersion solution was stopped when a trace amount of boron trifluoride was detected from the outlet fluid. After the supply of the dispersion solution was stop...
Embodiment 3
[0214] The boron trifluoride diethyl ether complex used in Example 1 is changed into a boron trifluoride phenoxide complex, and the dispersion liquid (boron trifluoride 0.25% (weight)) that is dispersed in hexane is used, Other than that, the adsorption and desorption tests of boron trifluoride were carried out in the same manner as in Example 1, and the results are shown in Table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| adsorption capacity | aaaaa | aaaaa |
| adsorption rate | aaaaa | aaaaa |
| adsorption rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com