Preparation method of bis(sulfonyl fluoride) imine and (perfluoroalkyl sulfonyl fluorine sulfonyl) imine alkali metal salt
An alkali metal salt and sulfonamide technology, applied in the field of fluorine chemical synthesis, can solve the problems of difficult product purification, cumbersome operation, and environmental pollution, and achieve the effects of avoiding product agglomeration, easy operation, high yield and purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
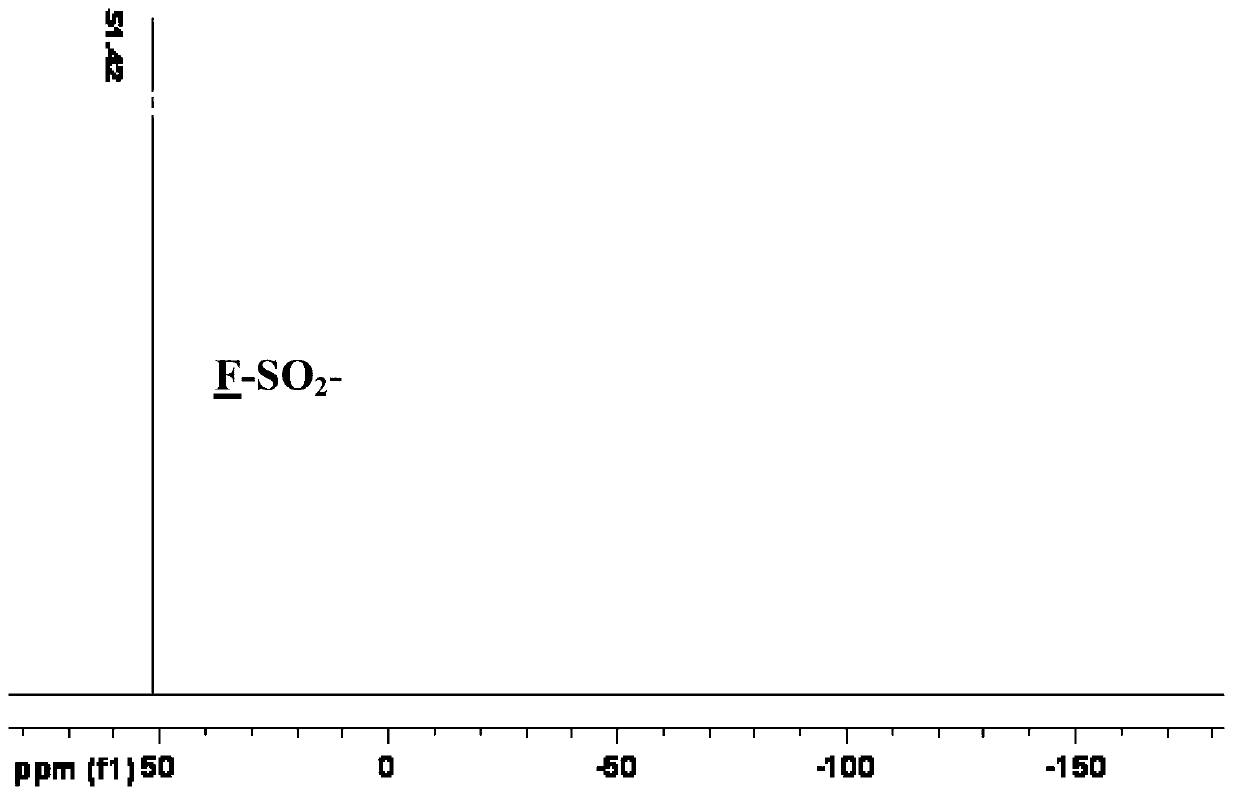
[0044] Example 1: Preparation of potassium bis(fluorosulfonyl)imide (K[FSI])
[0045] The synthesis reaction scheme is as follows:
[0046] HOSO 2 NH 2 +SOCl 2 +HOSO 2 Cl→H[N(SO 2 Cl) 2 ]+SO 2 ↑+HCl↑
[0047]
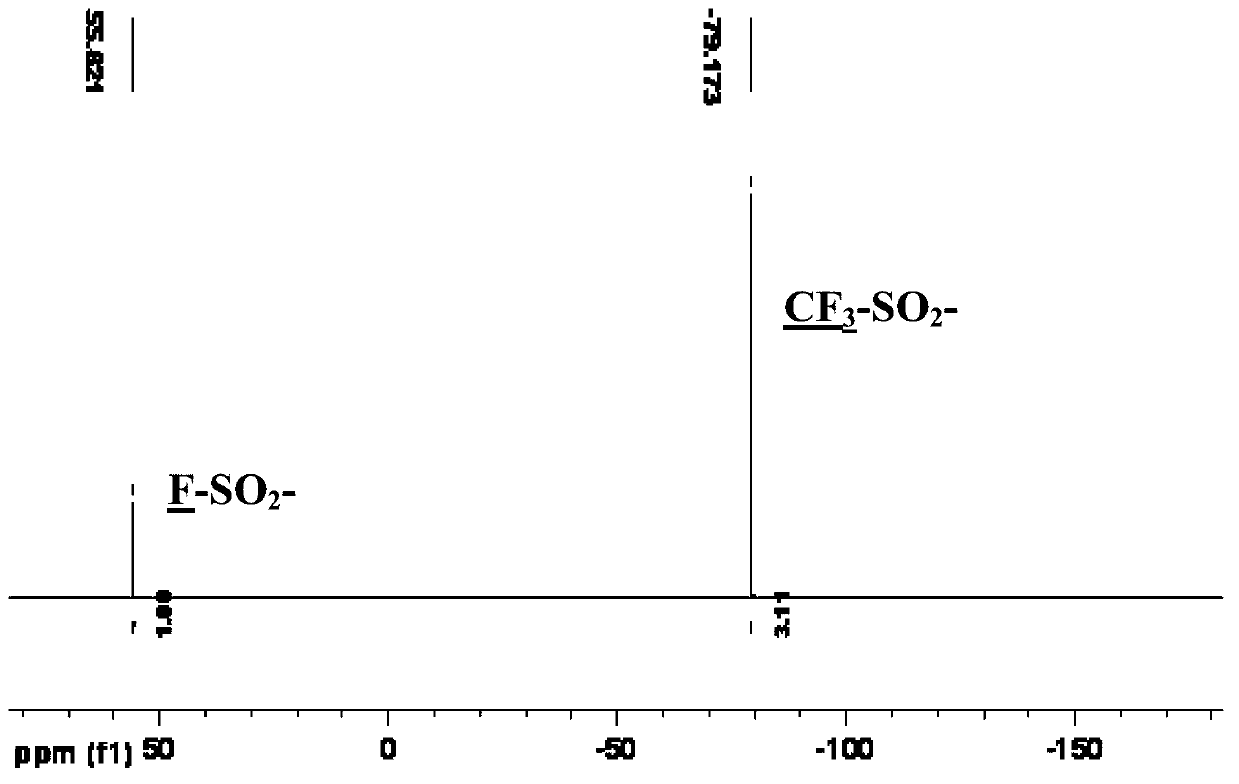
[0048] Under the protection of nitrogen, add 480 grams (5mol) of sulfamic acid, 1780 grams (15mol) of thionyl chloride, and 580 grams (0.5mol) of chlorosulfonic acid into a 5000mL reaction flask in sequence, and stir and react at 130°C for 24 hours , Atmospheric pressure distillation removes excessive low-boiling point reactant, then carries out vacuum distillation, collects the cut of 112-114 ℃ / 2mmHg, obtains two (chlorosulfonyl)imides (HN(SO 2 Cl) 2 ) 880 g (4.1 mol) of colorless crystals, yield 82%.
[0049] Under magnetic stirring and nitrogen protection, 96 grams (0.45mol) of bis(chlorosulfonyl)imide and 54 grams (0.3mol) of anhydrous antimony trifluoride were placed in a 500mL three-necked flask, and stirred at room temperature After reacting for 12 ...
Embodiment 2
[0051] Embodiment 2: Preparation of bis(fluorosulfonyl)imide cesium (Cs[FSI])
[0052] The synthesis reaction scheme is as follows:
[0053]
[0054] Under magnetic stirring and nitrogen protection, 9.6 grams (0.045mol) of bis(chlorosulfonyl)imide (prepared according to the operation of Example 1) and 5.4 grams (0.03mol) of anhydrous antimony trifluoride were placed in 100mL In a three-necked flask, after stirring for 12 hours at room temperature, 40 mL of acetonitrile was added to the reaction flask. After most of the solids were dissolved, 8.9 g (0.03 mol) of anhydrous cesium carbonate was added in portions under stirring, and the reaction was continued for 12 hours after the addition was complete. Then, the pH value of the system was adjusted to neutral with 2M HCl. Filter under reduced pressure to remove solid insoluble matter, concentrate the filtrate to about 10 mL, add an equal volume of CH 2 Cl 2 Perform recrystallization. After filtration, washing and drying, ...
Embodiment 3
[0055] Embodiment 3: Preparation of bis(fluorosulfonyl)imide lithium (Li[FSI])
[0056] The synthesis reaction scheme is as follows:
[0057]
[0058] In a vacuum glove box, add 91.5 g (0.34 mol) of potassium bis(fluorosulfonyl)imide (K[FSI]) and 250 mL of anhydrous acetonitrile into a 500 mL three-necked flask in sequence, stir to dissolve, and slowly Lithium perchlorate (LiClO 4 ) of acetonitrile solution 150mL (containing 36.2 grams of LiClO 4 ), stirred and reacted at room temperature for 24 hours, stood still overnight, and filtered under reduced pressure to remove insoluble potassium perchlorate (KClO 4 ), the filtrate was concentrated to about 60mL, and an equal volume of CH was added 2 Cl 2 Perform recrystallization. Filtration, CH 2 Cl 2 After washing and vacuum drying, 62 g (0.33 mol) of white solid powder Li[FSI] was obtained. 19 F NMR (acetone-d 6 , CCl 3 F, 376.5MHz): δ=51.8ppm(s).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com