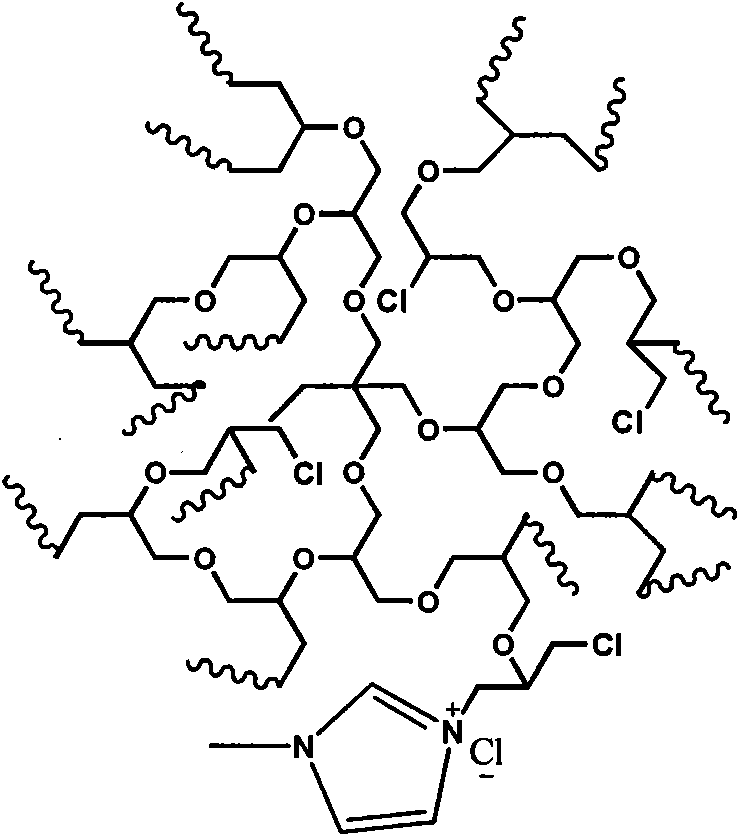
Ionic liquid polymer electrolyte and preparation method thereof
An ionic liquid and polymer technology, which is applied in the manufacture of electrolyte batteries, non-aqueous electrolyte batteries, circuits, etc., can solve the problems of high room temperature conductivity, wide electrochemical window, and low room temperature conductivity, which limit practical use, and achieve high conductivity. efficiency, simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] In this embodiment, the polymerization reaction used is anionic polymerization. After baking the constant pressure funnel, spherical condenser, distillation device and 100ml three-neck flask in an oven for 3 hours, add 0.12g (1mmol) trimethylolpropane to it, remove oxygen and nitrogen, repeat three times, add 0.55ml Water, methanol and 0.45ml potassium methoxide solution were stirred and reacted for 0.5 hours to distill off the methanol. Raise the temperature to 90°C, then add 12ml glycidol dropwise within 12h, then continue heating and stirring for 12h, add a certain amount of methanol and then evaporate to dryness, put it in a vacuum oven at 45°C for 12h. This was obtained as a clear, viscous, colorless liquid. According to elemental analysis: C49.00%, H8.51%, 042.49%. The number average molecular weight measured by GPC was 1719, and the molecular weight distribution was 1.37. Each hyperbranched molecule contains 24 hydroxyl molecules.
Embodiment 2
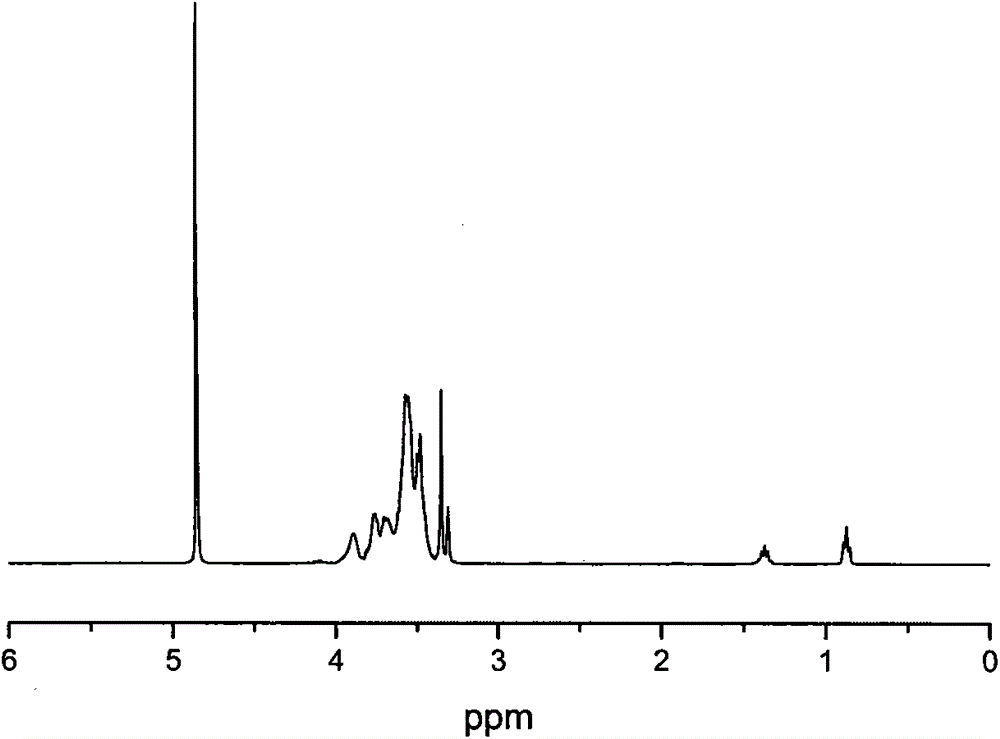
[0036] Add 10g of hyperbranched polyglycidol (HPG) in Example 1 to a 500ml dry single-necked bottle equipped with a magnet, add 300ml of thionyl chloride, under nitrogen protection, heat and reflux at 80°C for 24h, then distill out unreacted under reduced pressure Thionyl chloride was dried in a vacuum oven for 24 hours to obtain a yellow viscous liquid. 1 H NMR calculations showed that the hydroxyl groups were all chlorinated.
Embodiment 3
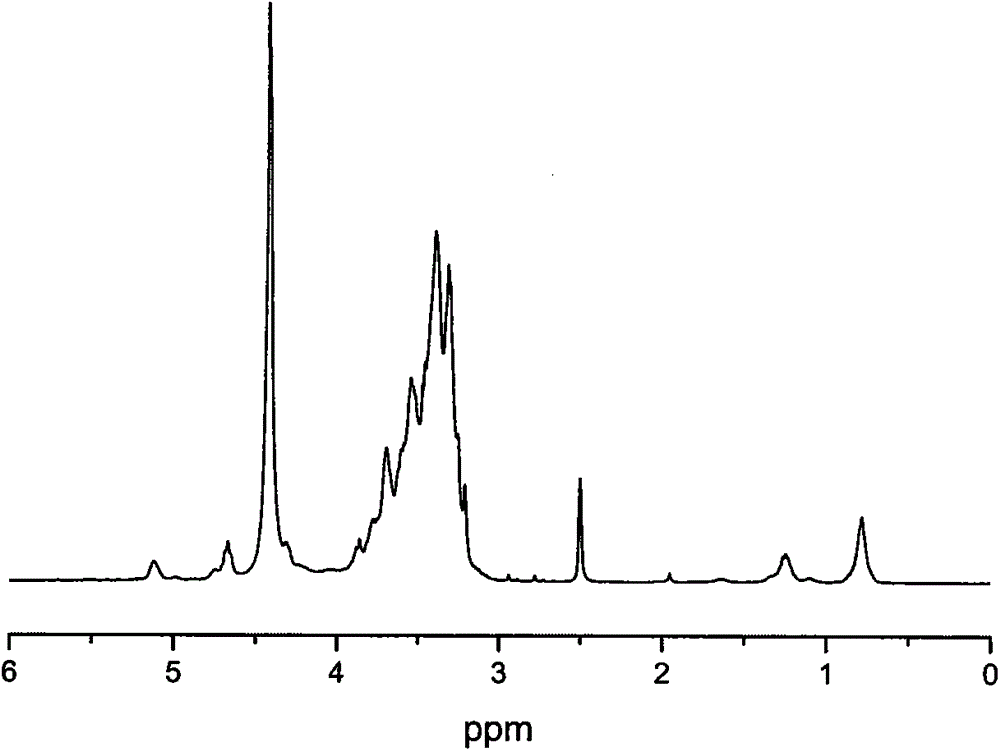
[0038] Add the chlorinated hyperbranched polyglycidol (HPG-C1) that 5g embodiment 2 prepares in the 250ml two-necked flask that magnetron is housed, add 20mlN, N-dimethylformamide, cool in ice-water bath, under nitrogen condition Slowly add N-methylimidazole ([MmIm] / [C1]=1.5:1), then stir and heat for 8 hours, cool to room temperature, steam out N,N-dimethylformamide under pressure, and use appropriate amount of acetone The crude product was washed twice, filtered, and dried in vacuo to obtain yellow polymer [HPG-MeIm]Cl with low viscosity. The glass transition temperature was -18°C as measured by DSC, and the initial decomposition temperature was 169°C as measured by TGA.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Initial decomposition temperature | aaaaa | aaaaa |
| Conductivity | aaaaa | aaaaa |
| Glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com