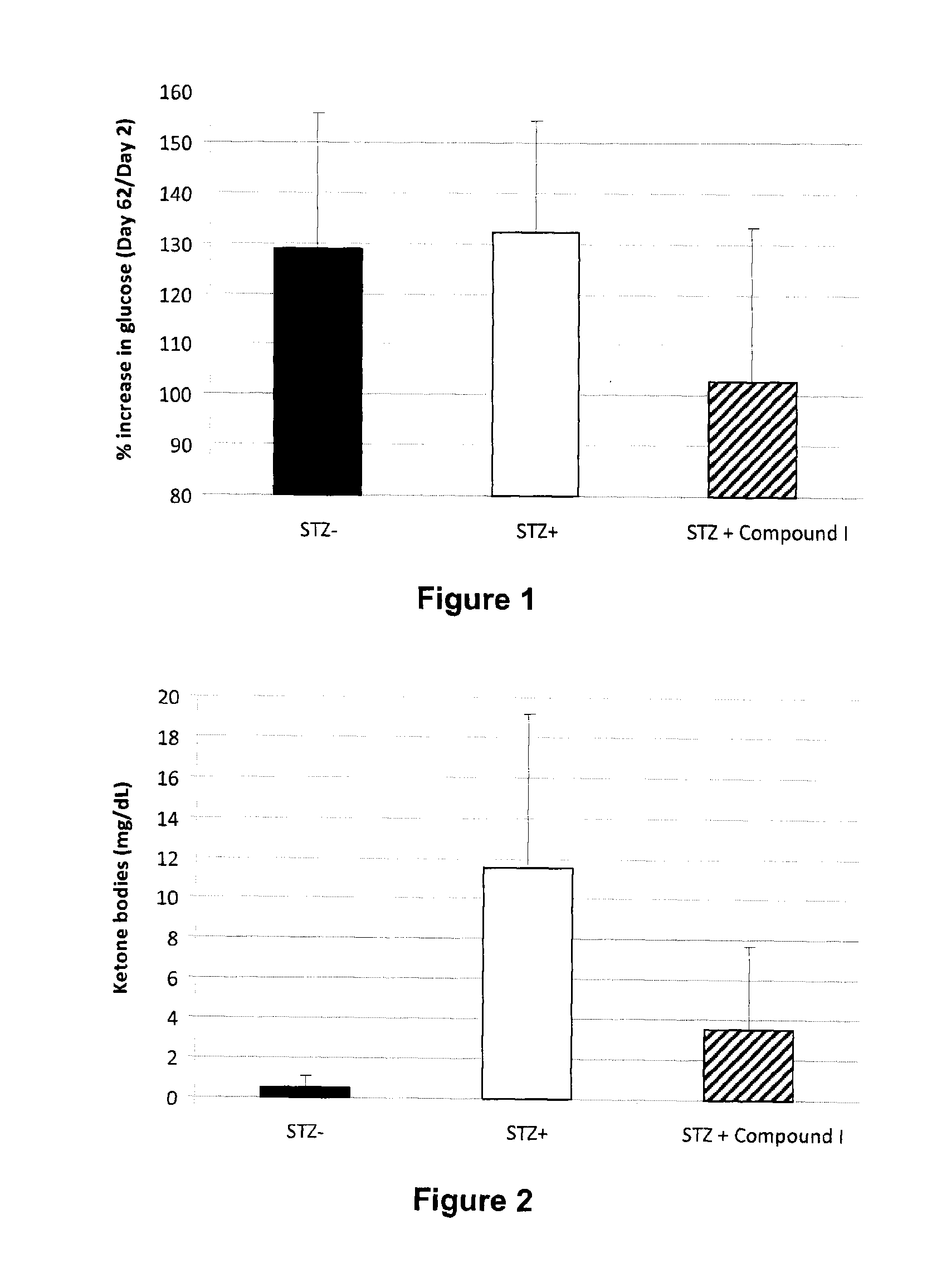
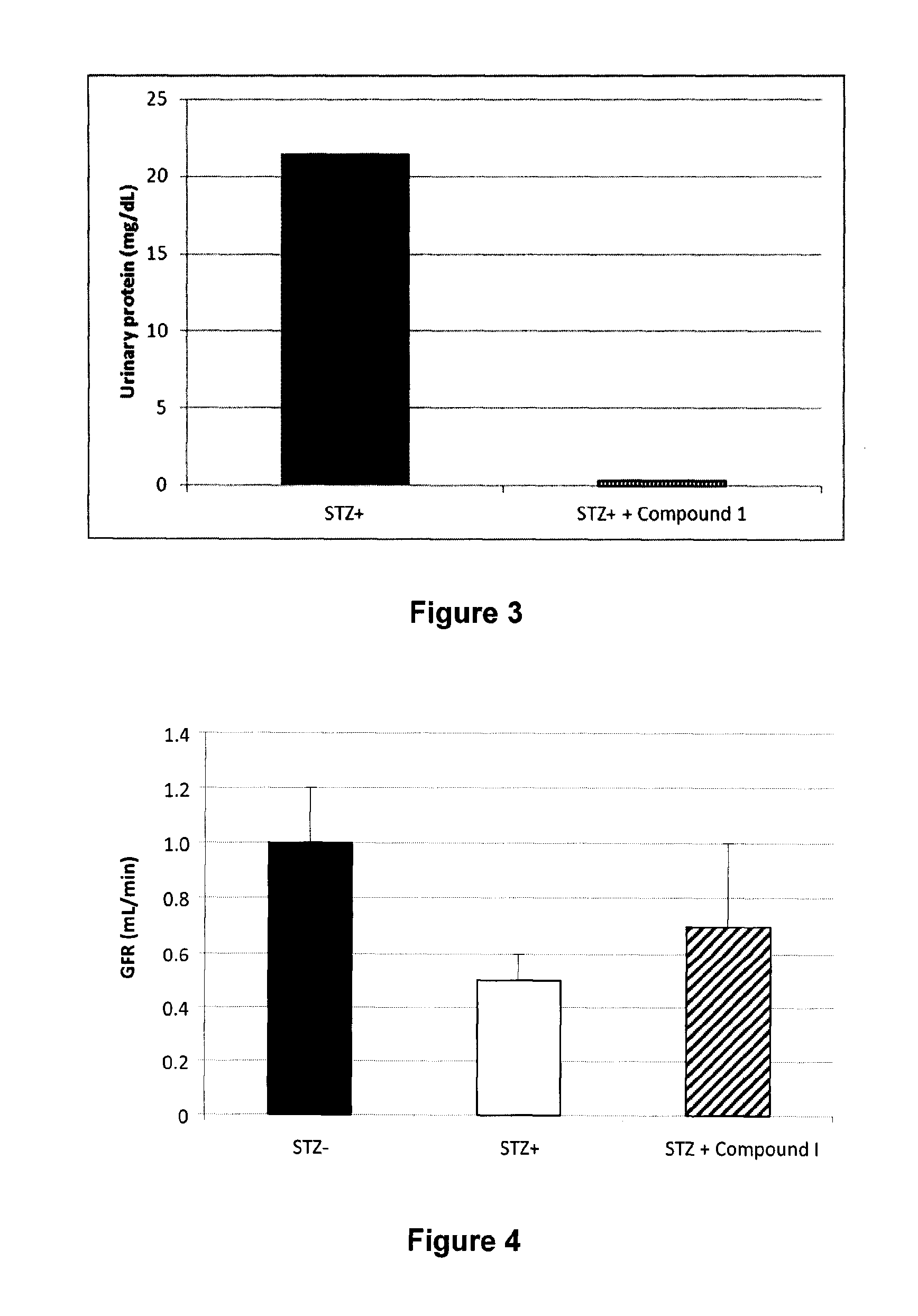
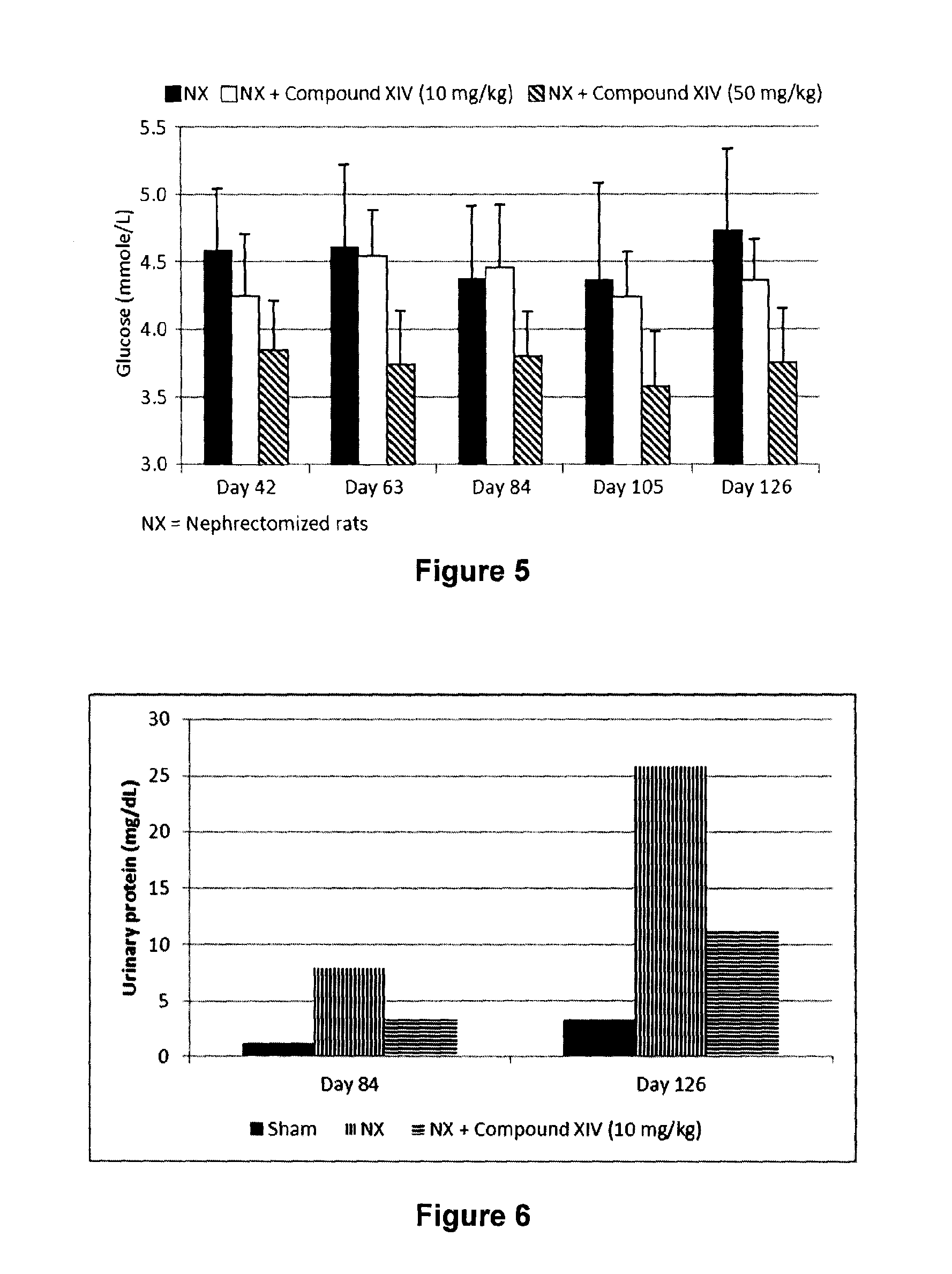
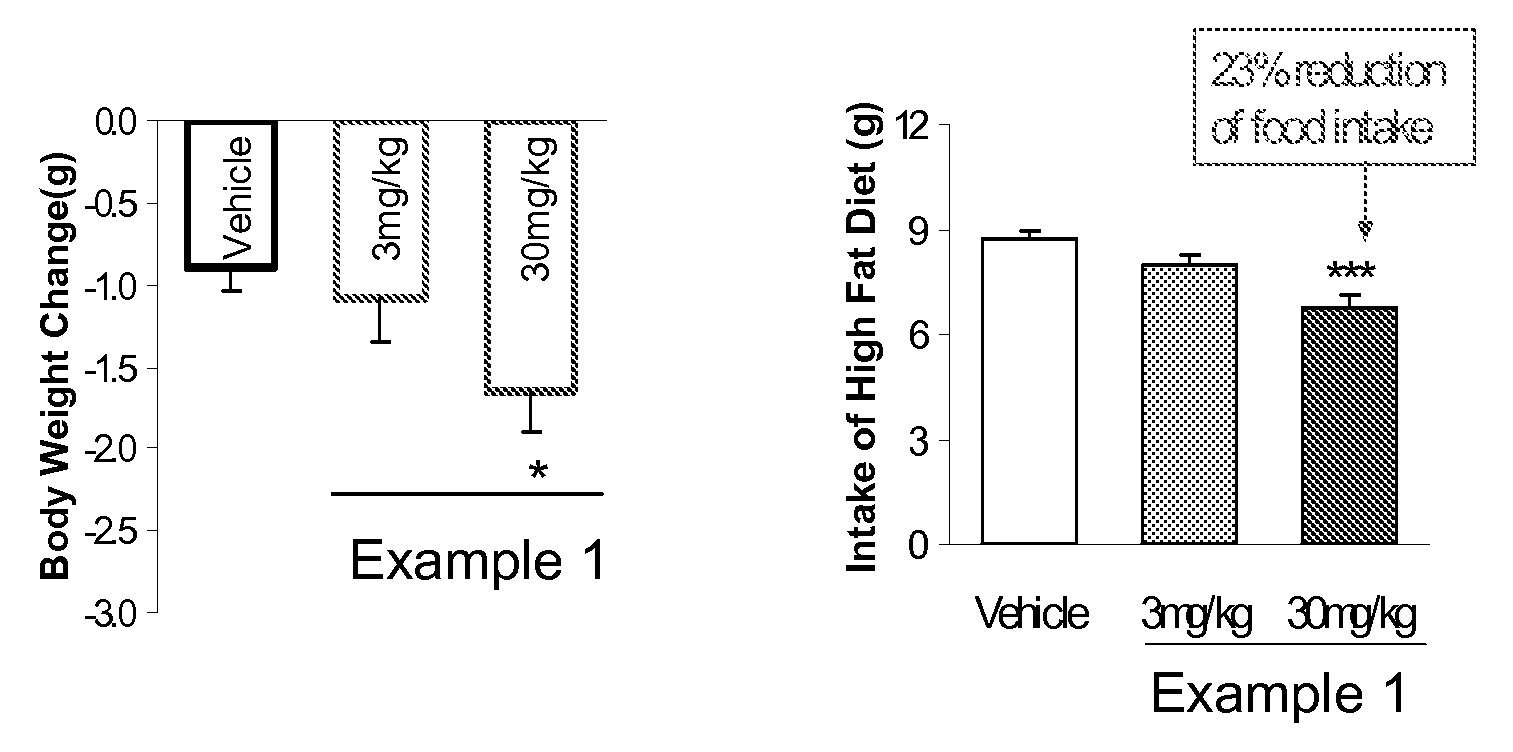
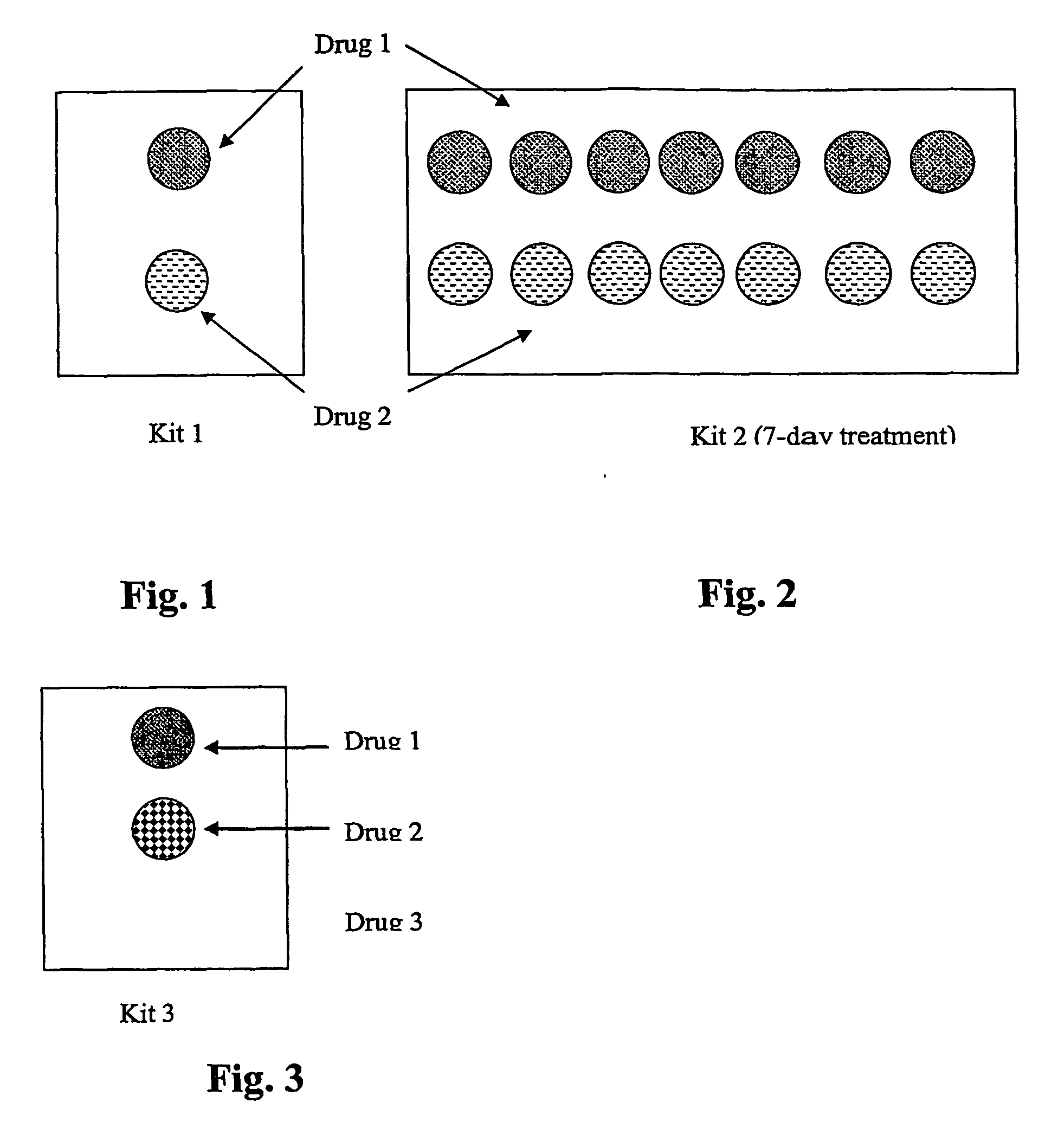
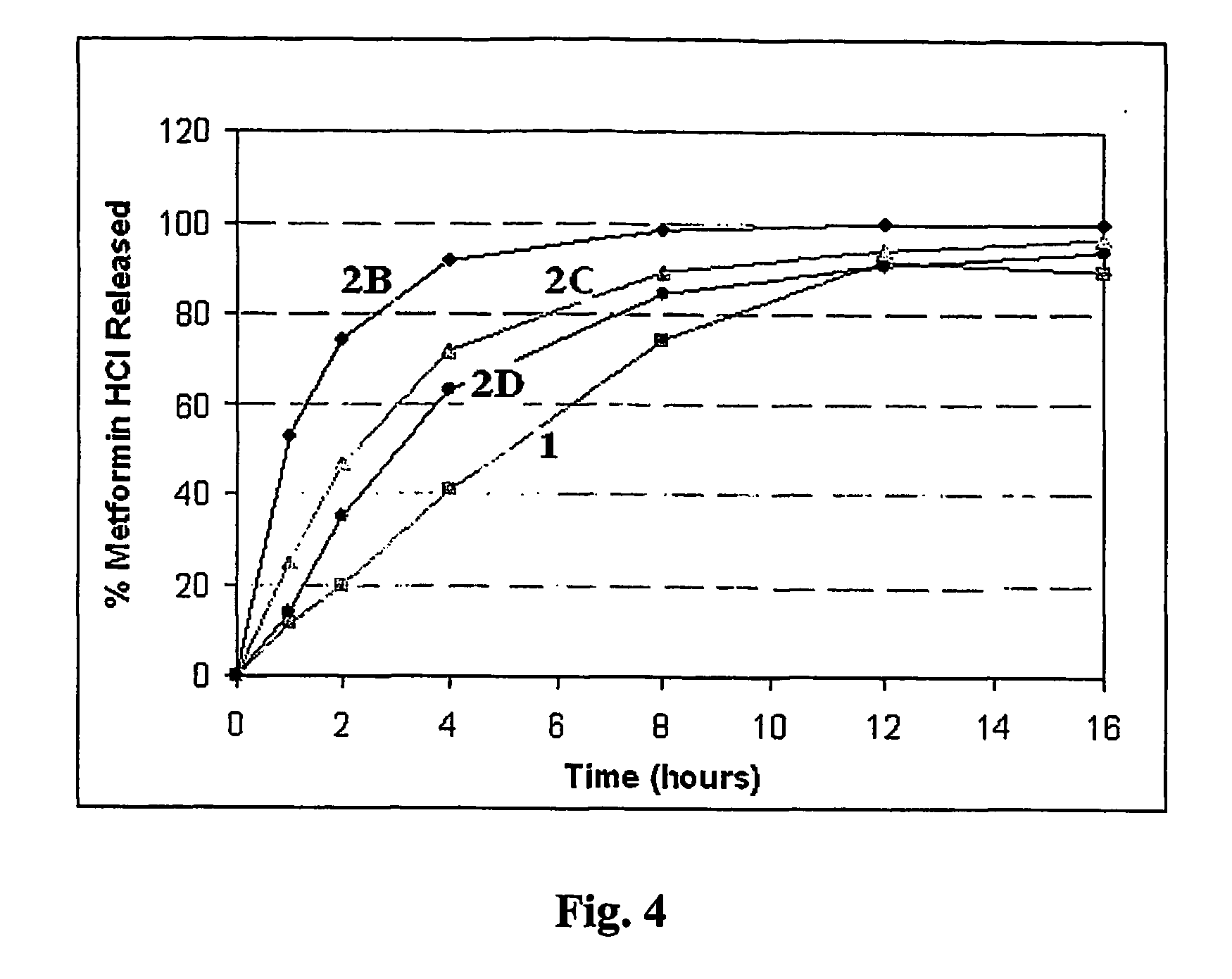
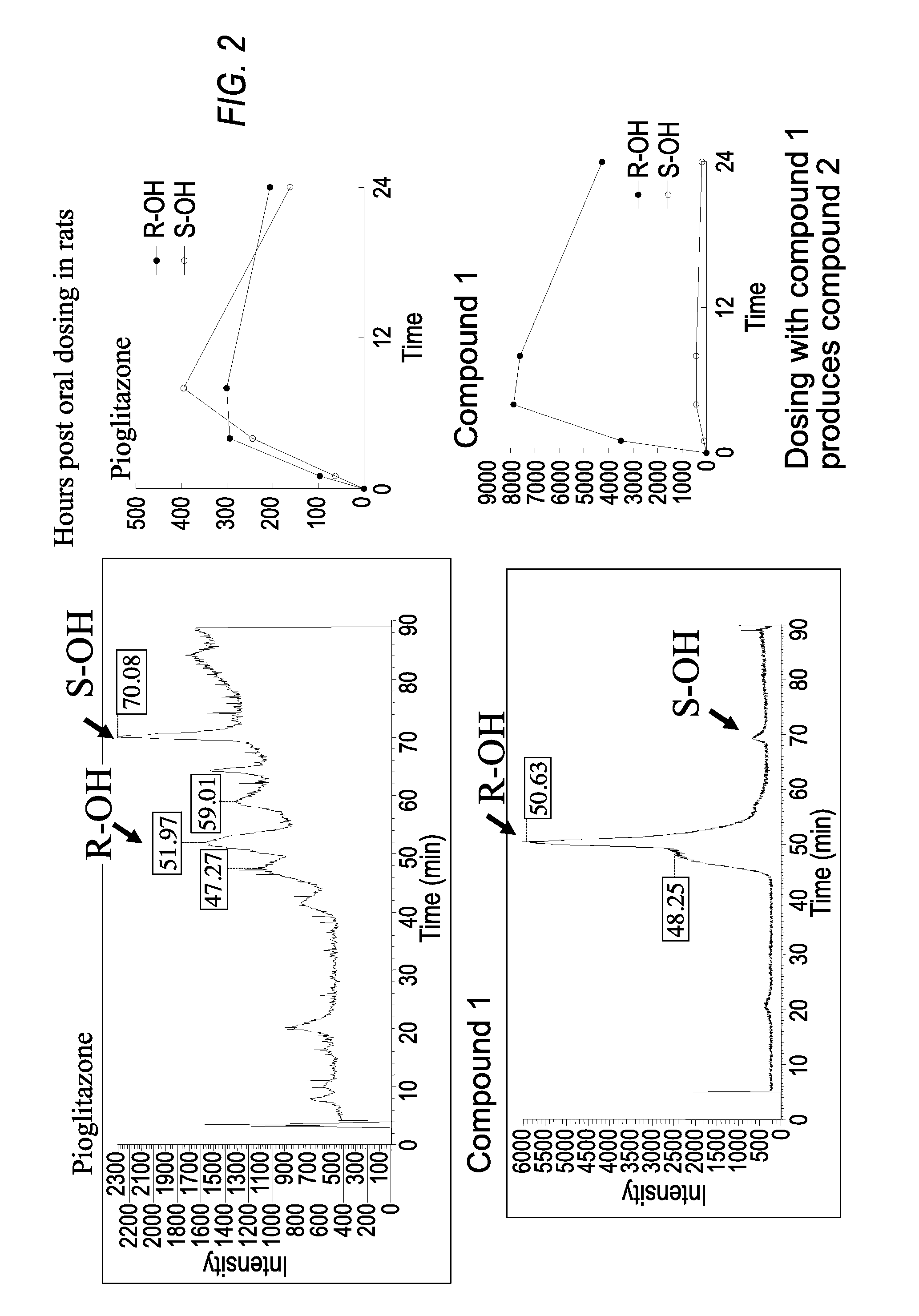
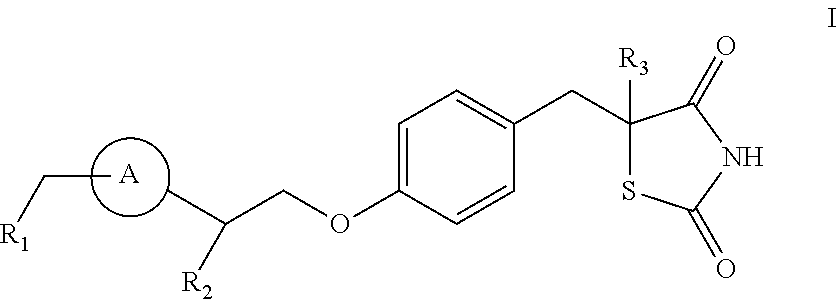
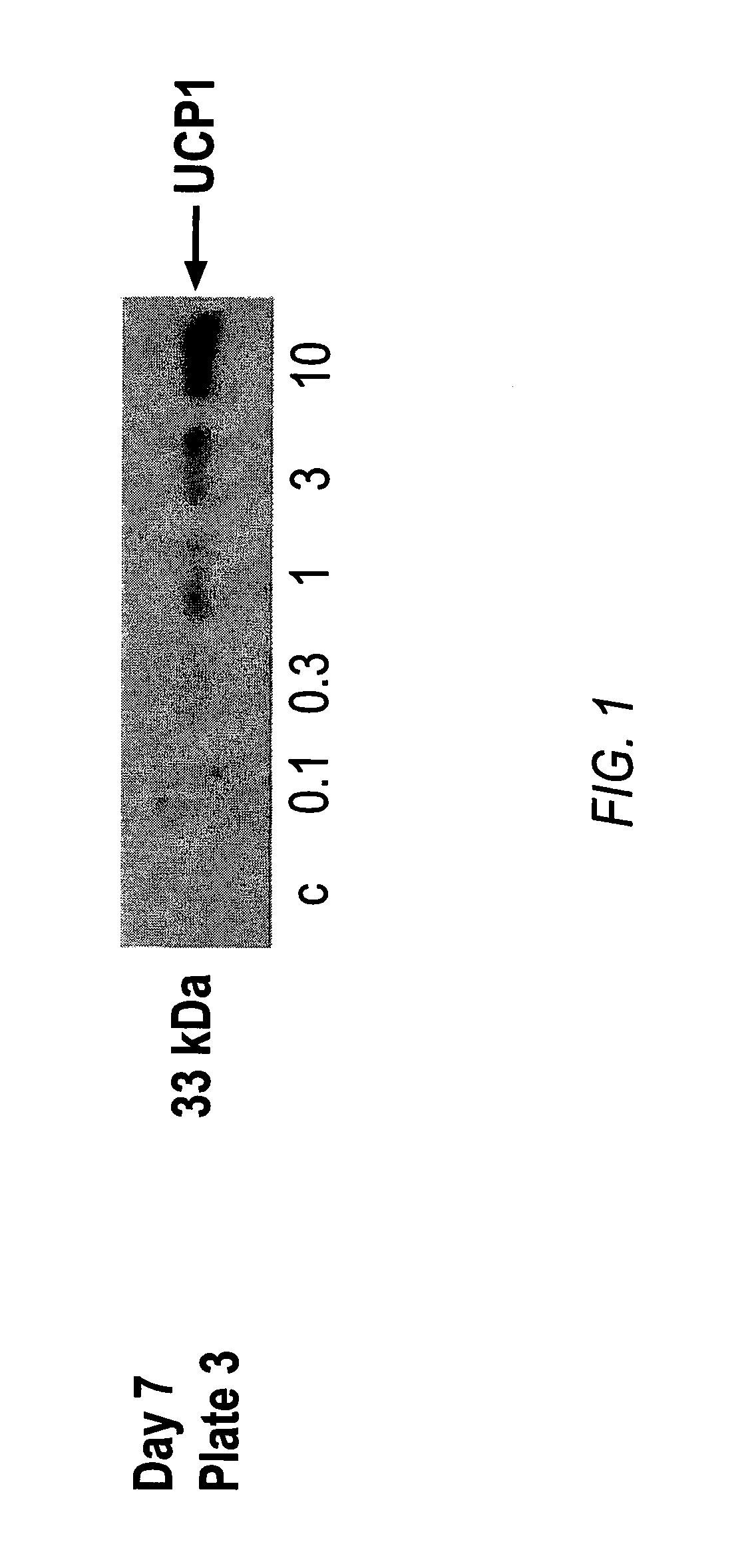
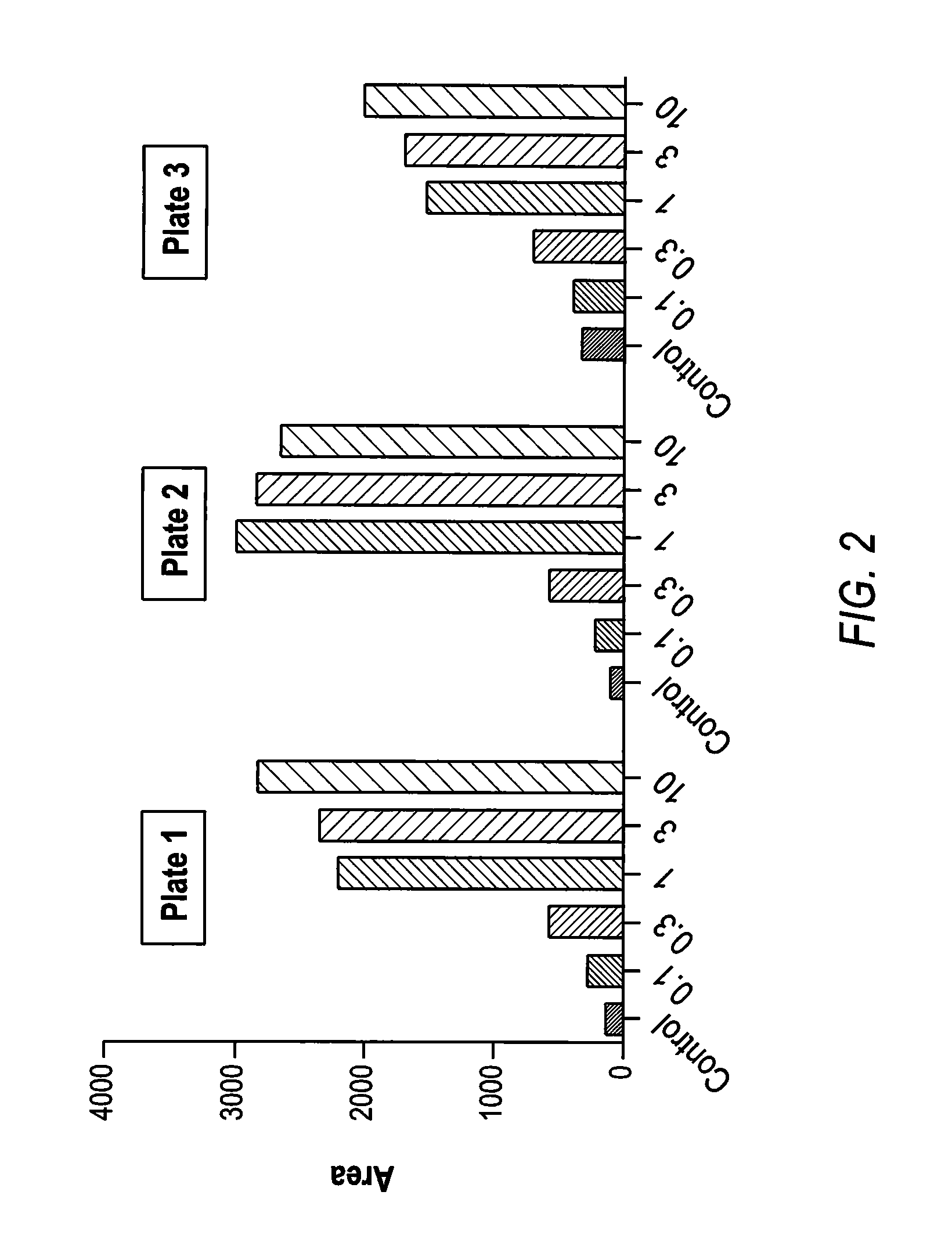
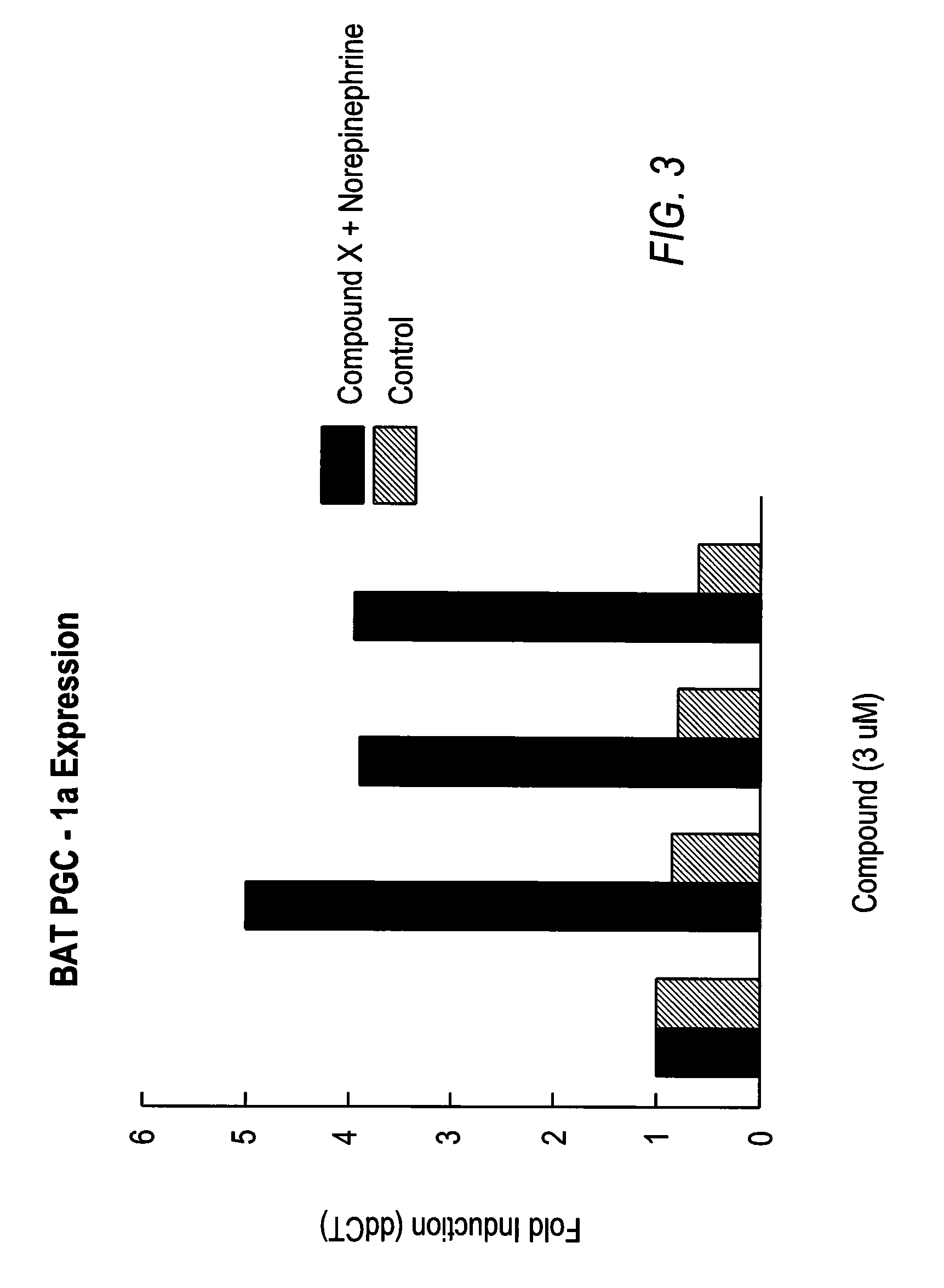
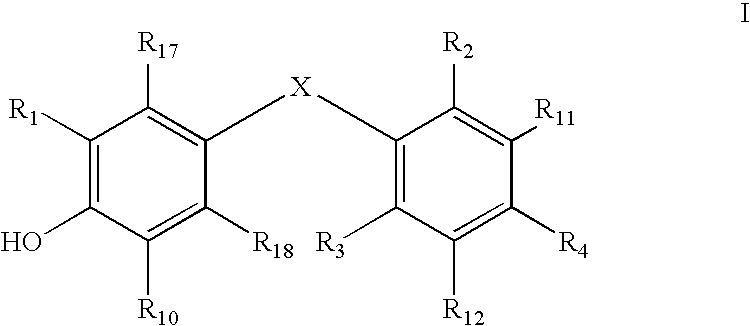
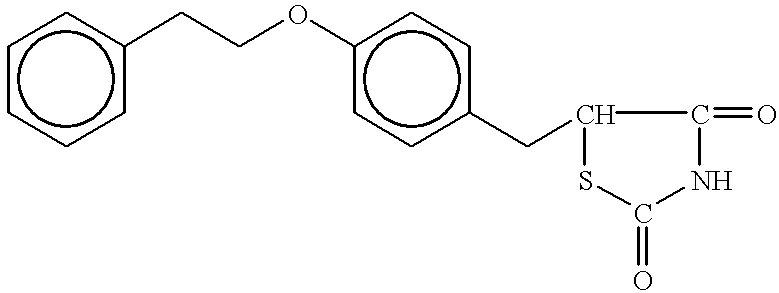
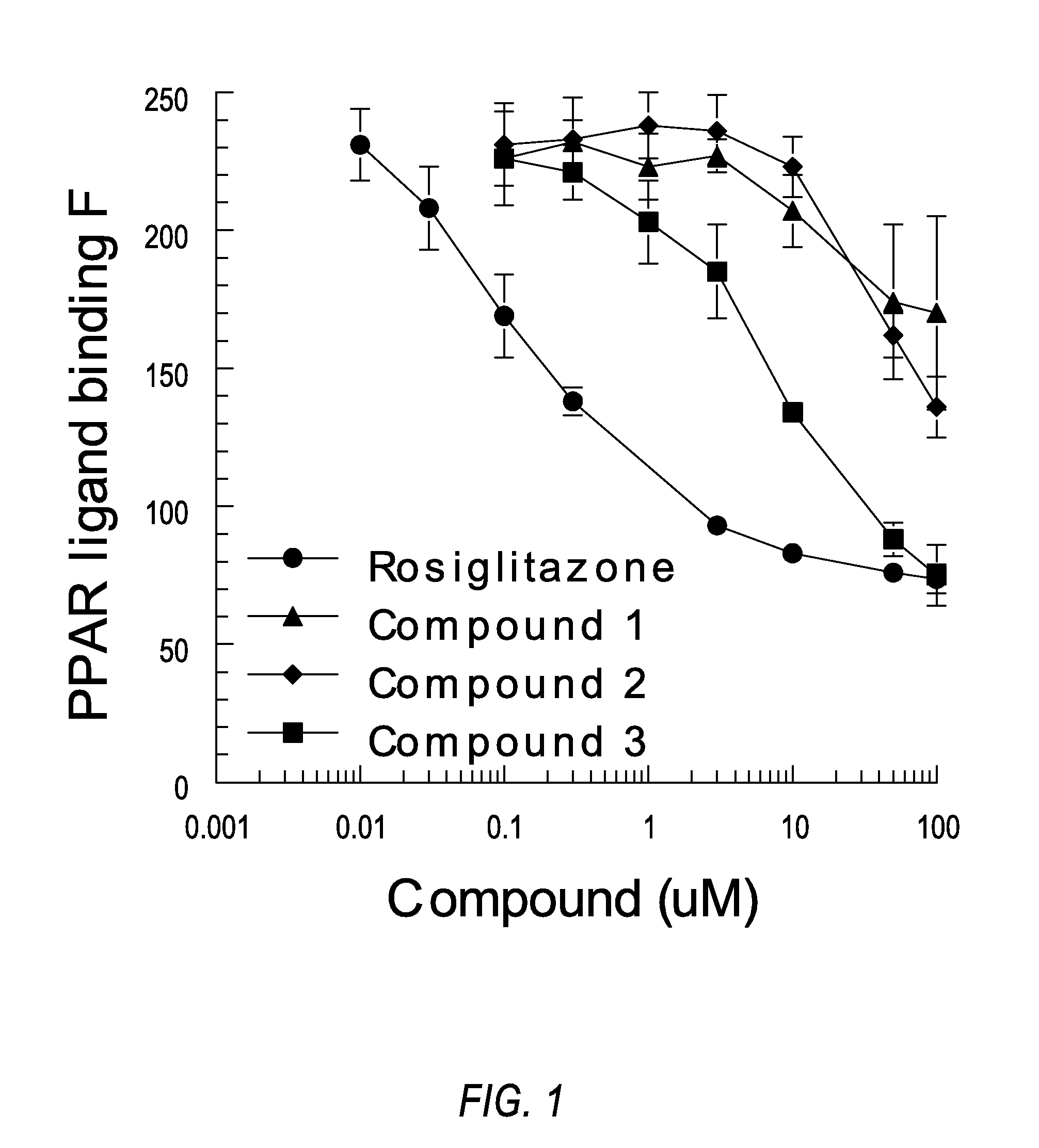
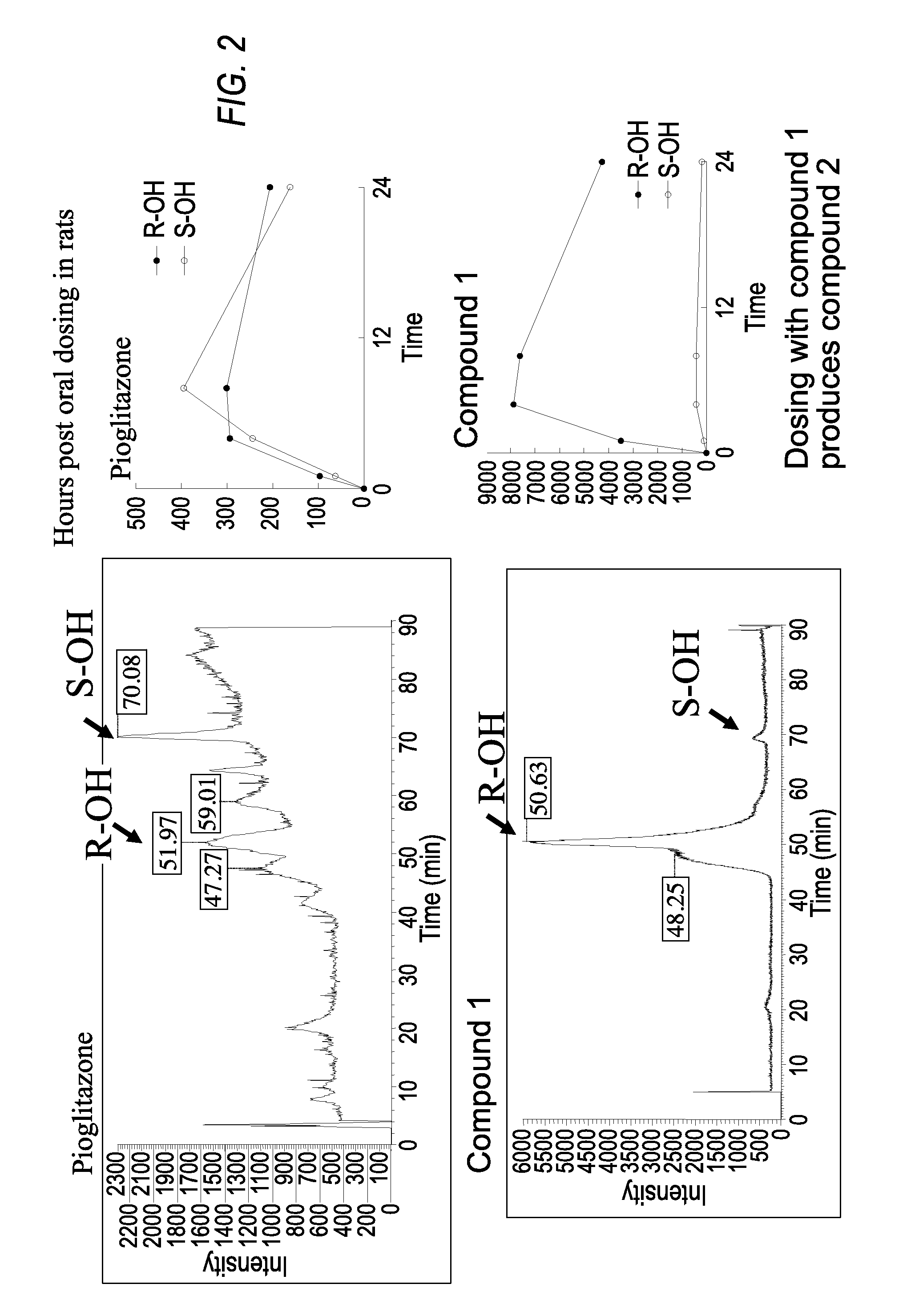
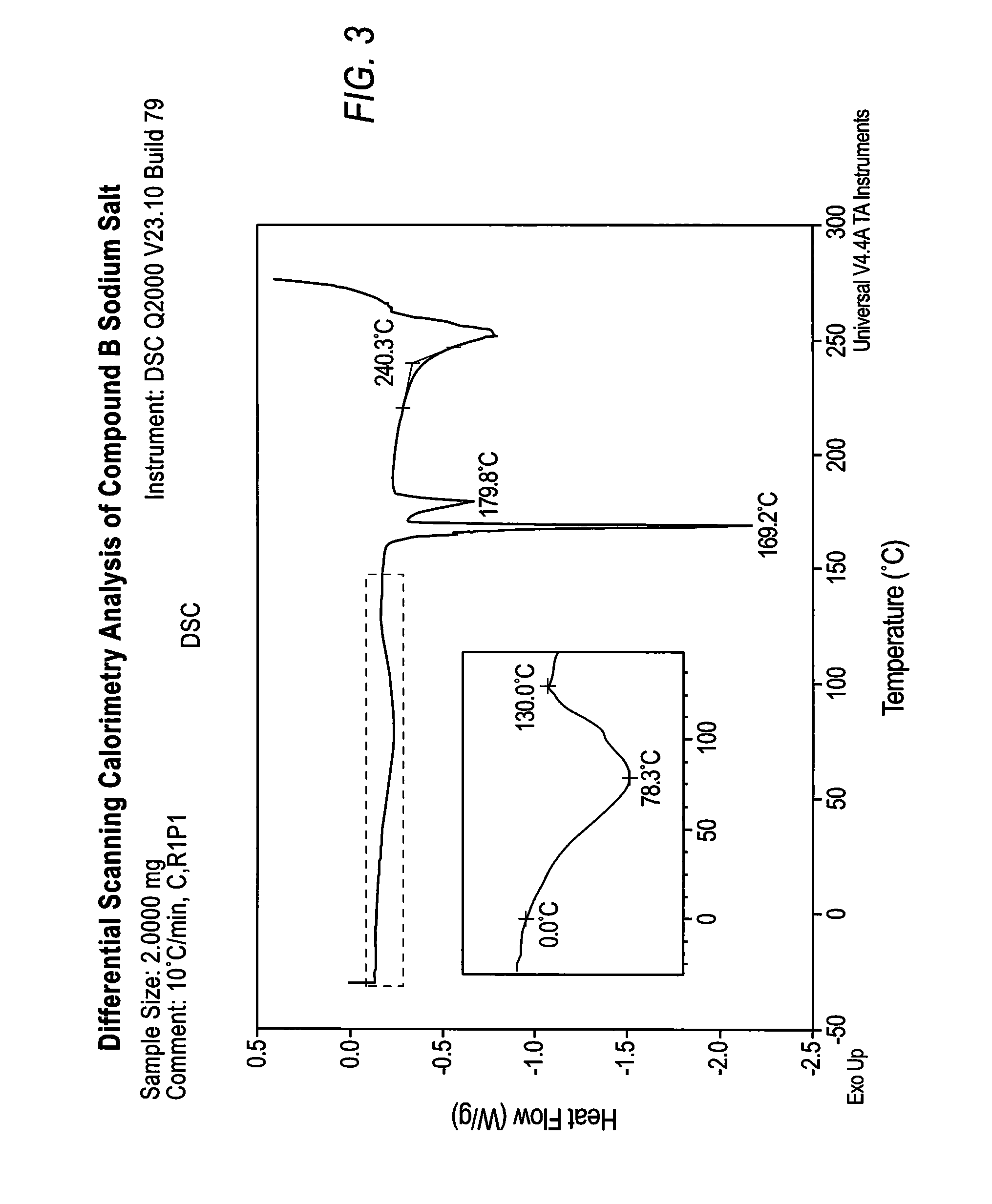
Patents
Literature
296 results about "Thiazolidinedione" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
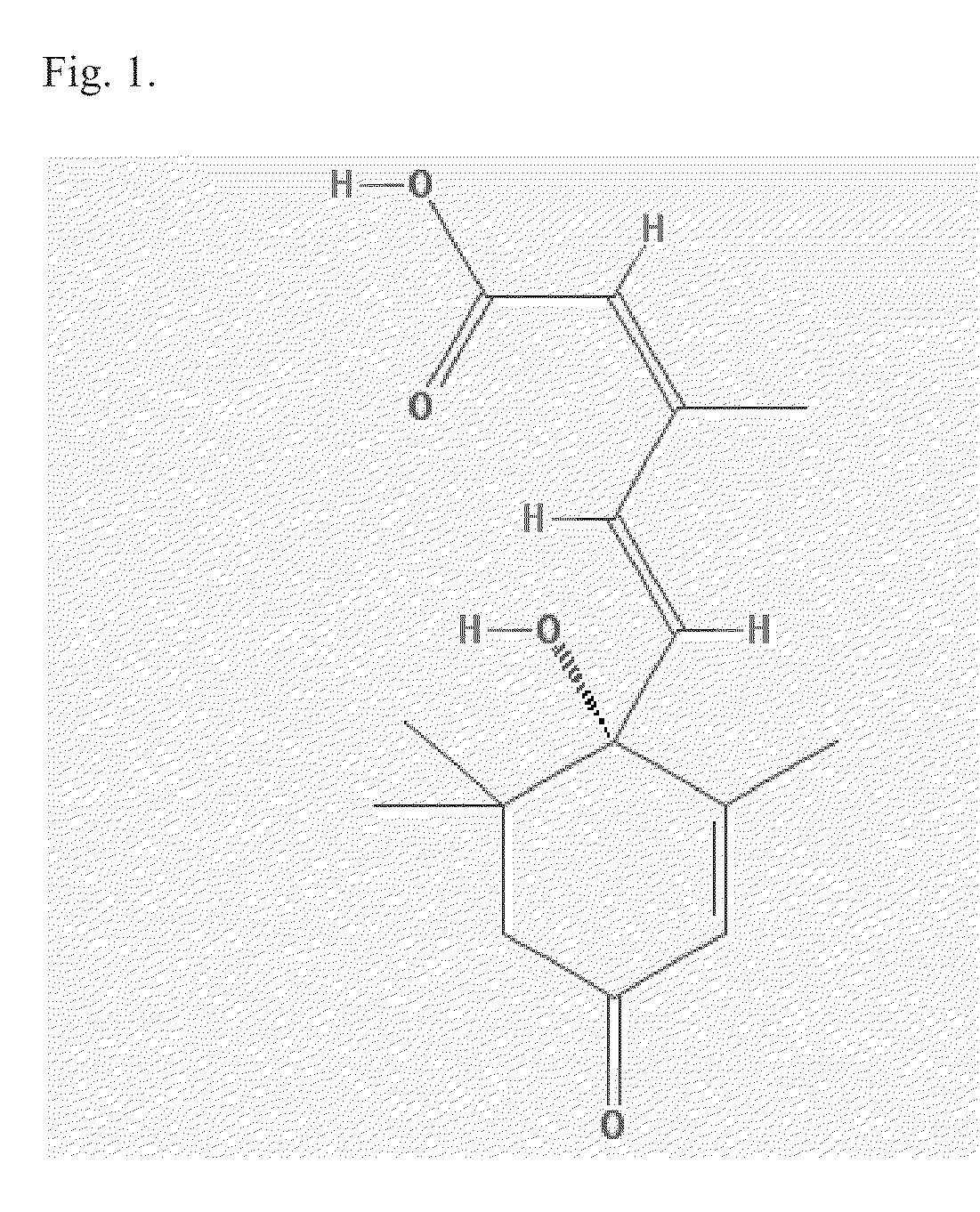
The thiazolidinediones /θaɪ.əˌzoʊlɪdiːnˈdaɪ.oʊn/, abbreviated as TZD, also known as glitazones after the prototypical drug ciglitazone, are a class of heterocyclic compounds consisting of a five-membered C₃NS ring. The term usually refers to a family of drugs used in the treatment of diabetes mellitus type 2 that were introduced in the late 1990s.
Synergistic use of thiazolidinediones with glucagon-like peptide-1 and agonists thereof to treat metabolic instability associated with non-insulin dependent diabetes
InactiveUS7223728B2Lower blood sugar levelsIncrease secretionPeptide/protein ingredientsMetabolism disorderInsulin dependent diabetesSide effect
Thiazolidinedione (TZD) and its pharmacologically active derivatives can be used, in combination with agonists of glucagon-like peptide-1 (GLP-1), to treat non-insulin dependent diabetes mellitus, optionally with other therapies, by improving glycemic control while minimizing side effects, such as heart hypertrophy and elevated fed-state plasma glucose, which are associate with both TZD and GLP-1 monotherapies. Thus, the co-administration of TZD and GLP-1 helps regulate glucose homeostasis in Type II diabetic patients.
Owner:ELI LILLY & CO
Methods and compositions for the treatment of alopecia and other disorders of the pilosebaceous apparatus
Insulin sensitivity increasing substances (ISIS), including but not limited to D-chiro-inositol, thiazolidinedione and derivatives, and biguanides, are useful in the treatment of hair loss and other disorders of the pilosebaceous apparatus (hirsutism, acne, etc.) associated with conditions of excess insulin and / or insulin resistance. The treatment comprises administering to a mammal, such as a human, at least one ISIS either alone or in combination with at least one agent, such as an androgen receptor blocker (ARB) and / or a steroid enzyme inhibitor or inducer (STI). Additionally, an activity enhancing agent may be included for topical administration.
Owner:ORENTREICH FOUND FOR THE ADVANCEMENT OF SCI
Peroxisome proliferator-acitvated receptor gamma ligand eluting medical device
Implantable medical devices having an anti-restenotic coatings are disclosed. Specifically, implantable medical devices having coatings of peroxisome proliferator-activated receptor gamma (PPAR.gamma.) agonists are disclosed. The anti-restenotic PPAR.gamma. ligands include thiazolidinedione compounds including ciglitazone. The anti-restenotic medial devices include stents, catheters, micro-particles, probes and vascular grafts. The medical devices can be coated using any method known in the art including compounding the thiazolidinedione with a biocompatible polymer prior to applying the coating. Moreover, medical devices composed entirely of biocompatible polymer-thiazolidinedione blends are disclosed. Additionally, medical devices having a coating comprising at least one thiazolidinedione in combination with at least one additional therapeutic agent are also disclosed. Furthermore, related methods of using and making the anti-restenotic implantable devices are also disclosed.
Owner:MEDTRONIC AVE
Multilayer tablets containing thiazolidinedione and biguanides and methods for producing them
InactiveUS20060057202A1Facilitated releaseEfficient compressionBiocideOrganic active ingredientsAcute hyperglycaemiaImmediate release
A novel patient-convenient, cost effective pharmaceutical composition, comprising of thiazolidinediones and biguanide for controlling hyperglycemia manufactured as multilayer tablet and its process of manufacturing, for immediate release of thiazolidinediones or thiazolidinediones and biguanide and prolonged release of the biguanide only, the tablet comprising of minimum two layers wherein one outer layer comprises of a mixture of excipients and thiazolidinediones or thiazolidinediones and biguanide allowing immediate release of thiazolidinediones or thiazolidinediones and biguanide respectively and the other layer arranged in contact with the immediate release layer which comprises of a novel composition of excipients and a minimum one or more non-biodegradable, inert polymer(s) and the biguanide allowing pH independent prolonged release of the biguanide up to a period of 8-12 hours. The tablets are for once a day dosing. The tablets may optionally be film coated or enrobed by soft gelatin ribbons for additional protection against oxidation, photodegradation, identification, ease of swallowing, taste masking and for aesthetic appeal without altering the dissolution profile.
Owner:INVENTIA HEALTHCARE LTD
Lanthionine synthetase component c-like proteins as molecular targets for preventing and treating diseases and disorders
ActiveUS20110275558A1Organic active ingredientsPeptide/protein ingredientsThiazolidinedioneAutoimmune disease
The present invention relates to the field of medical treatments for diseases and disorders. More specifically, the present invention relates to the use of the lanthionine synthetase component C-like (LANCL) proteins as therapeutic targets for novel classes of anti-inflammatory, immune regulatory and antidiabetic drugs. This includes but it is not limited to abscisic acid (ABA), ABA analogs, benzimidazophenyls, repurposed drugs or drug combinations, including thiazolidinediones (TZDs); naturally occurring compounds such as conjugated diene fatty acids, conjugated triene fatty acids, isoprenoids, and natural and synthetic agonists of peroxisome proliferator-activated receptors that activate this receptor through an alternative mechanism of action involving LANCL2 or other membrane proteins to treat or prevent the common inflammatory pathogenesis underlying type 2 diabetes, atherosclerosis, cancer, some inflammatory infectious diseases such as influenza and autoimmune diseases including but not limited to inflammatory bowel disease (Crohn's disease and Ulcerative colitis), rheumatoid arthritis, multiple sclerosis and type 1 diabetes and other chronic inflammatory conditions.
Owner:VIRGINIA TECH INTPROP INC
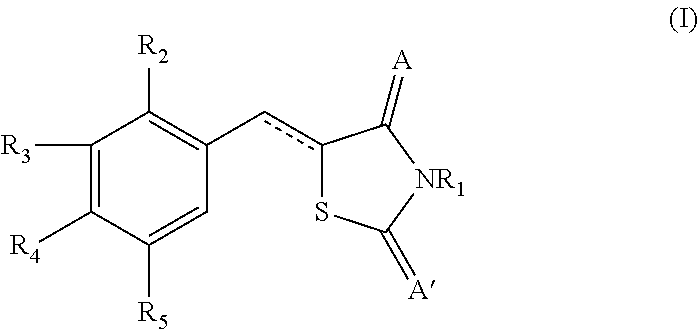
Novel thiazolidinedione derivative and use thereof
InactiveUS20110269954A1Inhibiting hair lossPromote growthOrganic active ingredientsOrganic chemistryWound healingVascular disease
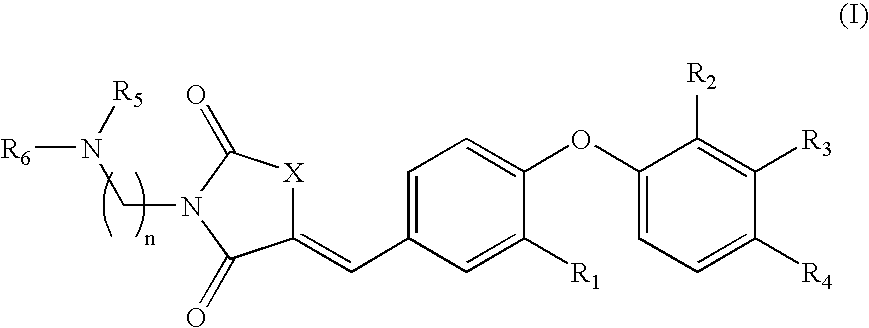
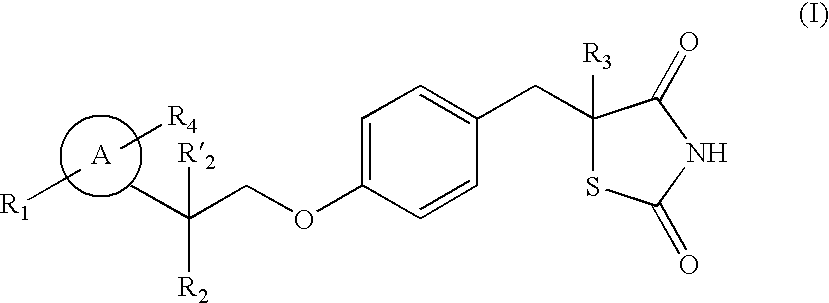
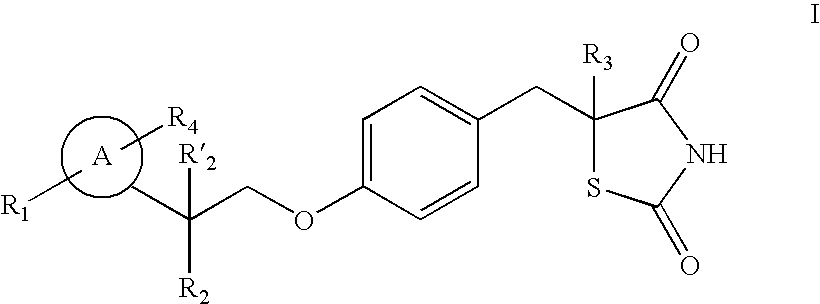
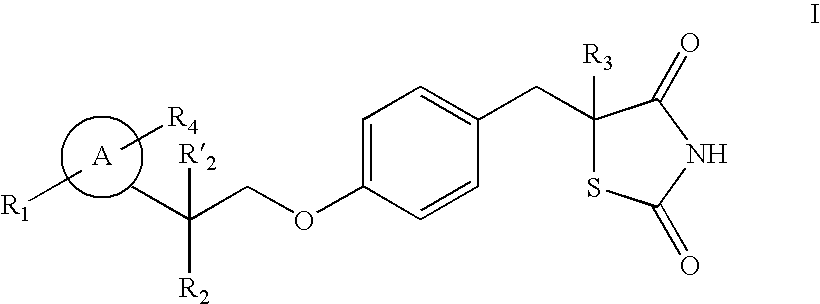
The present invention relates to novel thiazolidinedione derivatives expressed by the following formula (I) and the uses thereof. More specifically, the present invention relates to novel thiazolidinedione derivatives expressed by the following formula (I) and a pharmaceutical composition comprising the same. The novel thiazolidinedione derivatives of formula (I) according to the present invention can be effectively used for the prevention or treatment of cardiovascular disease, gastrointestinal disease and renal disease by inhibiting the activity of 15-hydroxyprostaglandin dehydrogenase (15-PGDH) that decomposes prostaglandins as well as useful for the prevention of hair loss and the stimulation of hair growth, and osteogenic stimulation and wound healing.
Owner:IND ACADEMIC CORP FOUND YONSEI UNIV
Compounds and Pharmaceutical Compositions for Uses in Diabetes
InactiveUS20130225683A1Improve degradation rateReduce rate of ascentBiocideMetabolism disorderDiabetic retinopathyDiabetic kidney
New uses for phenylketone carboxylate compounds and substituted aromatic compounds of Formula I, Formula I, IA, IB and IC, and their pharmaceutical acceptable salts are described for prevention or treatment of diabetes or a diabetes-related disorder in a subject in need thereof. Diabetes and diabetes-related disorder include Type I diabetes, Type II diabetes, maturity-onset diabetes of the young, latent autoimmune diabetes of adults (LADA), gestational diabetes, diabetic nephropathy, proteinuria, ketonuria, obesity, hyperglycemia, glucose intolerance, insulin resistance, hyperinsulinemia, hypercholesterolemia, hypertension, hyperlipoproteinemia, hyperlipidemia, hypertriglyceridemia, dyslipidemia, metabolic syndrome, syndrome X, diabetic neuropathy, diabetic retinopathy, hypoglycemia, cardiovascular disease, atherosclerosis, diabetic kidney disease, ketoacidosis, thrombotic disorders, sexual dysfunction, dermatopathy, edema, metabolic syndrome and renal disorders. The related pharmaceutical compositions and methods are also described. These compounds can be used in combination with comprising a therapeutic agent for lowering or controlling blood glucose level such as metformin or a thiazolidinedione.
Owner:PROMETIC PHARMA SMT LTD
SUBSTITUTED PHENOXY THIAZOLIDINEDIONES AS ESTROGEN RELATED RECEPTOR-alpha MODULATORS
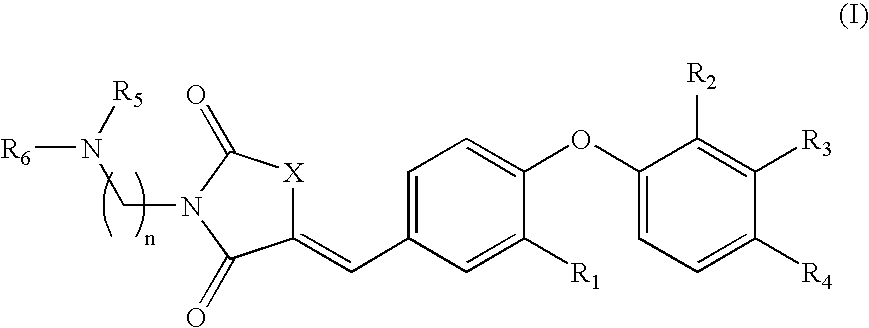
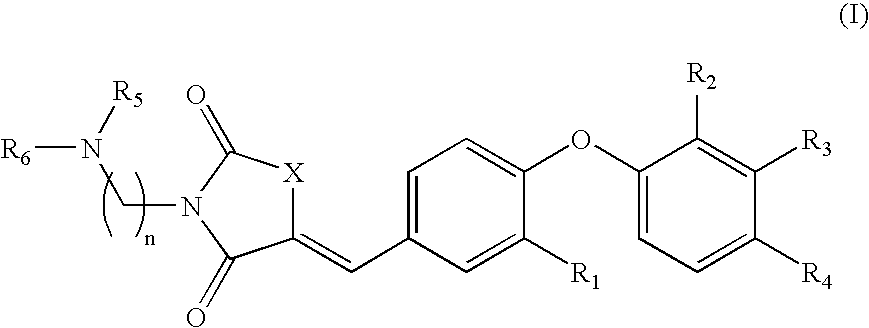
The present invention relates to compounds of Formula (I),methods for preparing these compounds, compositions, intermediates and derivatives thereof and for treating a condition including but not limited to ankylosing spondylitis, artherosclerosis, arthritis (such as rheumatoid arthritis, infectious arthritis, childhood arthritis, psoriatic arthritis, reactive arthritis), bone-related diseases (including those related to bone formation), breast cancer (including those unresponsive to anti-estrogen therapy), cardiovascular disorders, cartilage-related disease (such as cartilage injury / loss, cartilage degeneration, and those related to cartilage formation), chondrodysplasia, chondrosarcoma, chronic back injury, chronic bronchitis, chronic inflammatory airway disease, chronic obstructive pulmonary disease, diabetes, disorders of energy homeostasis, gout, pseudogout, lipid disorders, metabolic syndrome, multiple myeloma, obesity, osteoarthritis, osteogenesis imperfecta, osteolytic bone metastasis, osteomalacia, osteoporosis, Paget's disease, periodontal disease, polymyalgia rheumatica, Reiter's syndrome, repetitive stress injury, hyperglycemia, elevated blood glucose level, and insulin resistance.
Owner:JANSSEN PHARMA NV
Combination therapy for endothelial dysfunction, angina and diabetes
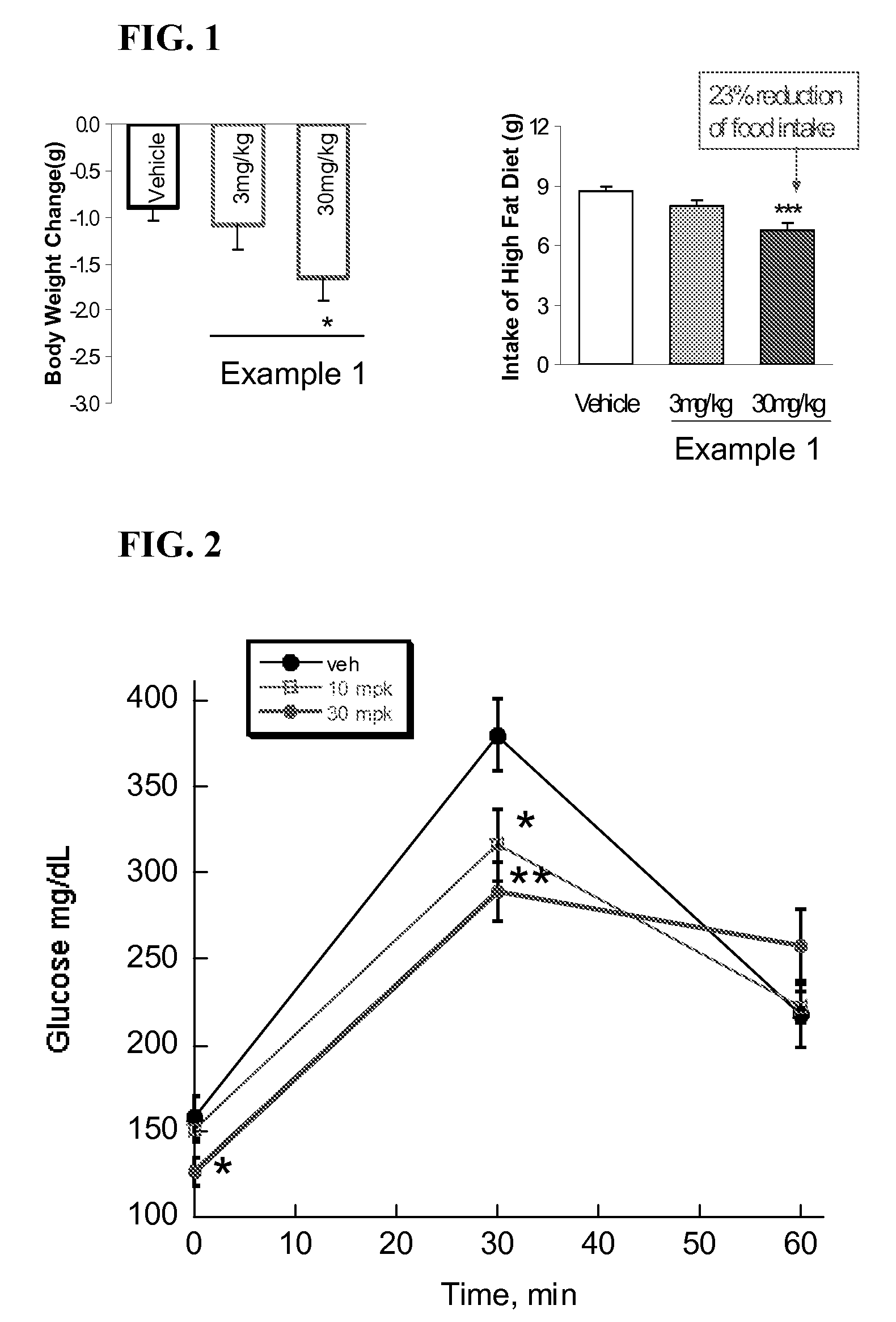
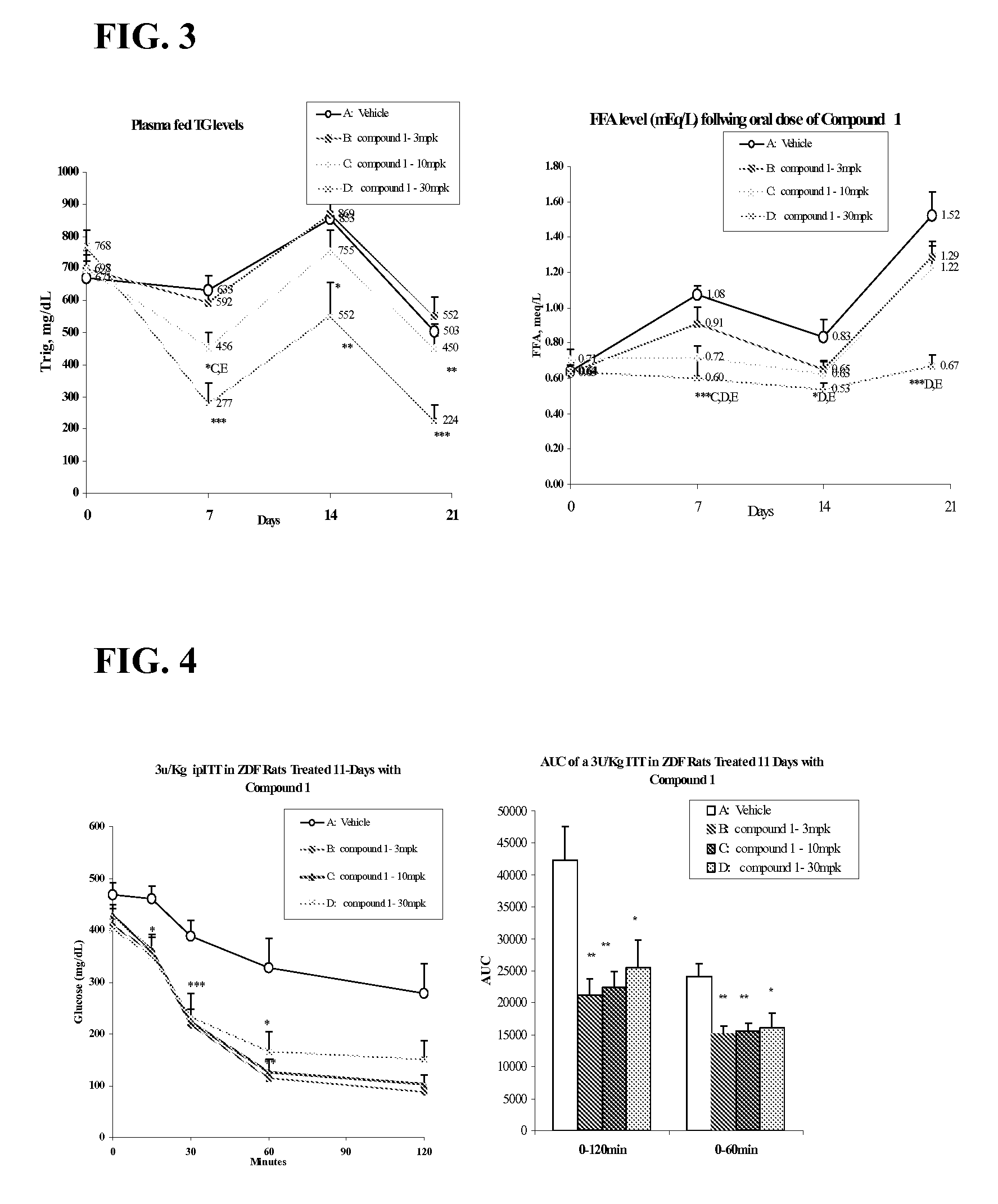
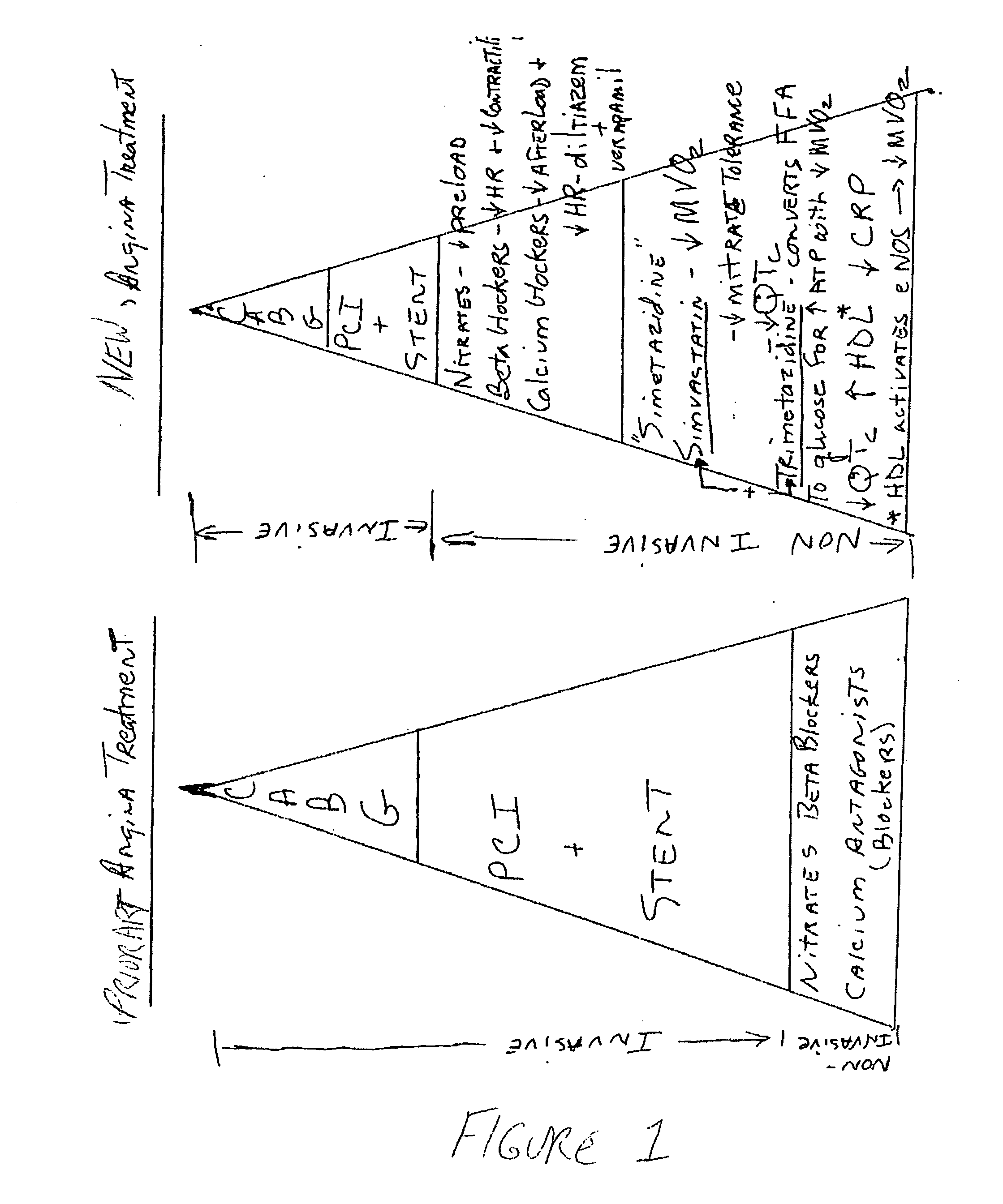
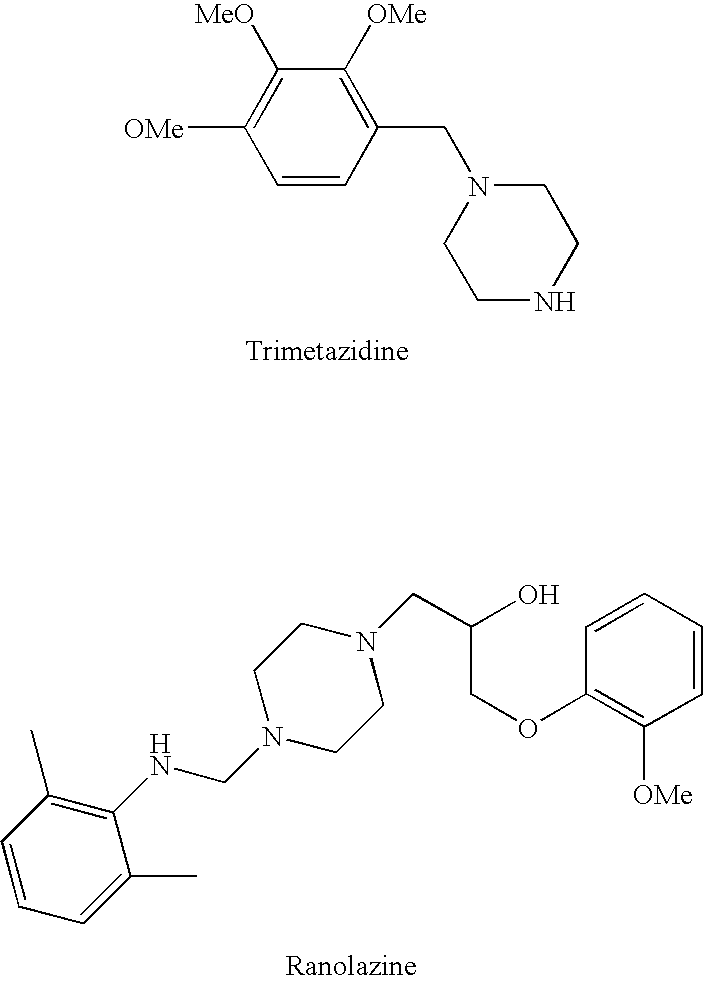
InactiveUS20060205727A1Control blood sugar levelsIncrease productionBiocideMetabolism disorderHMG-CoA reductaseTrimetazidine
The combination of a HMG CoA reductase inhibitor like a statin, such as simvastatin, with a pFox inhibitor such as trimetazidine (“Simetazidine”) is particularly advantageous for treatment of end-stage complications, such as acute coronary syndrome (ACS) and chronic angina, especially in type II diabetics. The combination therapy is also useful in the treatment and / or prevention of chronic heart failure (CHF) and peripheral arterial disease (PAD). The combination of a nitric oxide (NO) mechanism with increased NO production with pFox inhibition simultaneously treats both the effect and the cause of angina. One or more oral hypoglycemic compounds (biguanides, insulin sensitizers, such as thiazolidinediones, α-glucosidase inhibitors, insulin secretagogues, and dipeptidyl peptidase IV inhibitors), protein kinase C (PKC) inhibitors, and acetyl-CoA carboxylase inhibitors can also be used in combination with the HMG CoA reductase inhibitors and / or pFox inhibitors, especially in type II diabetics, to control glucose levels and treat endothelial dysfunction. The drugs can be given in combination (e.g. a single tablet) or in separate dosage forms, administered simultaneously or sequentially. In the preferred form the statin is given in a dose of between 5 and 80 mg / day in two separate doses, and the pFox inhibitor is administered in a sustained or extended dosage formulation at a dose of 20 mg three times a day or 35 mg two times a day. The dose of the oral hypoglycemic, PKC inhibitor, or acetyl-CoA carboxylase inhibitor varies with the type of drug used.
Owner:HONG KONG NITRIC OXIDE
Substituted phenoxy n-alkylated thiazolidinediones as estrogen related receptor-alpha modulators
The present invention relates to compounds of Formula (I),methods for preparing these compounds, compositions, intermediates and derivatives thereof and for treating a condition including but not limited to ankylosing spondylitis, artherosclerosis, arthritis (such as rheumatoid arthritis, infectious arthritis, childhood arthritis, psoriatic arthritis, reactive arthritis), bone-related diseases (including those related to bone formation), breast cancer (including those unresponsive to anti-estrogen therapy), cardiovascular disorders, cartilage-related disease (such as cartilage injury / loss, cartilage degeneration, and those related to cartilage formation), chondrodysplasia, chondrosarcoma, chronic back injury, chronic bronchitis, chronic inflammatory airway disease, chronic obstructive pulmonary disease, diabetes, disorders of energy homeostasis, gout, pseudogout, lipid disorders, metabolic syndrome, multiple myeloma, obesity, osteoarthritis, osteogenesis imperfecta, osteolytic bone metastasis, osteomalacia, osteoporosis, Paget's disease, periodontal disease, polymyalgia rheumatica, Reiter's syndrome, repetitive stress injury, hyperglycemia, elevated blood glucose level, and insulin resistance.
Owner:JANSSEN PHARMA NV
Co-formulations or kits of bioactive agents
Provided, among other things, is a formulation or kit comprising: (a) a pharmaceutically effective dosage of one or more a glucose-level-controlling bioactive agents selected from an α-glucodase inhibitor, sulfonylurea, meglitinide, thiazolidinediones, biguanide, insulin, dual PPARα / γ agonist, PPARγ agonist or insulin secretagogue; and (b) a pharmaceutically effective dosage of (i) one or more of an antihypertensive bioactive agent selected from an ACE inhibitor, calcium channel blocker, beta blocker, angiotension II receptor antagonist or diuretic, or (ii) one or more of an anti-dyslipidemia bioactive agent selected from a HMG-CoA reductase inhibitor, bile acid sequestrant, fibric acid derivative, sterol, cholesterol absorption inhibitor, MTP inhibitor or nicotinic acid derivative; wherein: in the case of (i) a combination of a first bioactive agent of group (a) that is metformin with a second bioactive agent of group (b), or (ii) a combination of a first bioactive agent of group (a) that is a thiazolidinedione or dual PPARα / γ agonist with an angiotension II receptor antagonist, one or more of the following applies: (I) one of the first bioactive agent or the second bioactive agent is formulated for sustained release, and the other is formulated for immediate release, each formulated for once-a-day dosing; or (II) the co-formulation or kit comprises (A) a biguanide and a thiazolidinedione and (B) one or more group (b) bioactive agents.
Owner:ABEILLE PHARMA
Sugar-reducing medicine composition
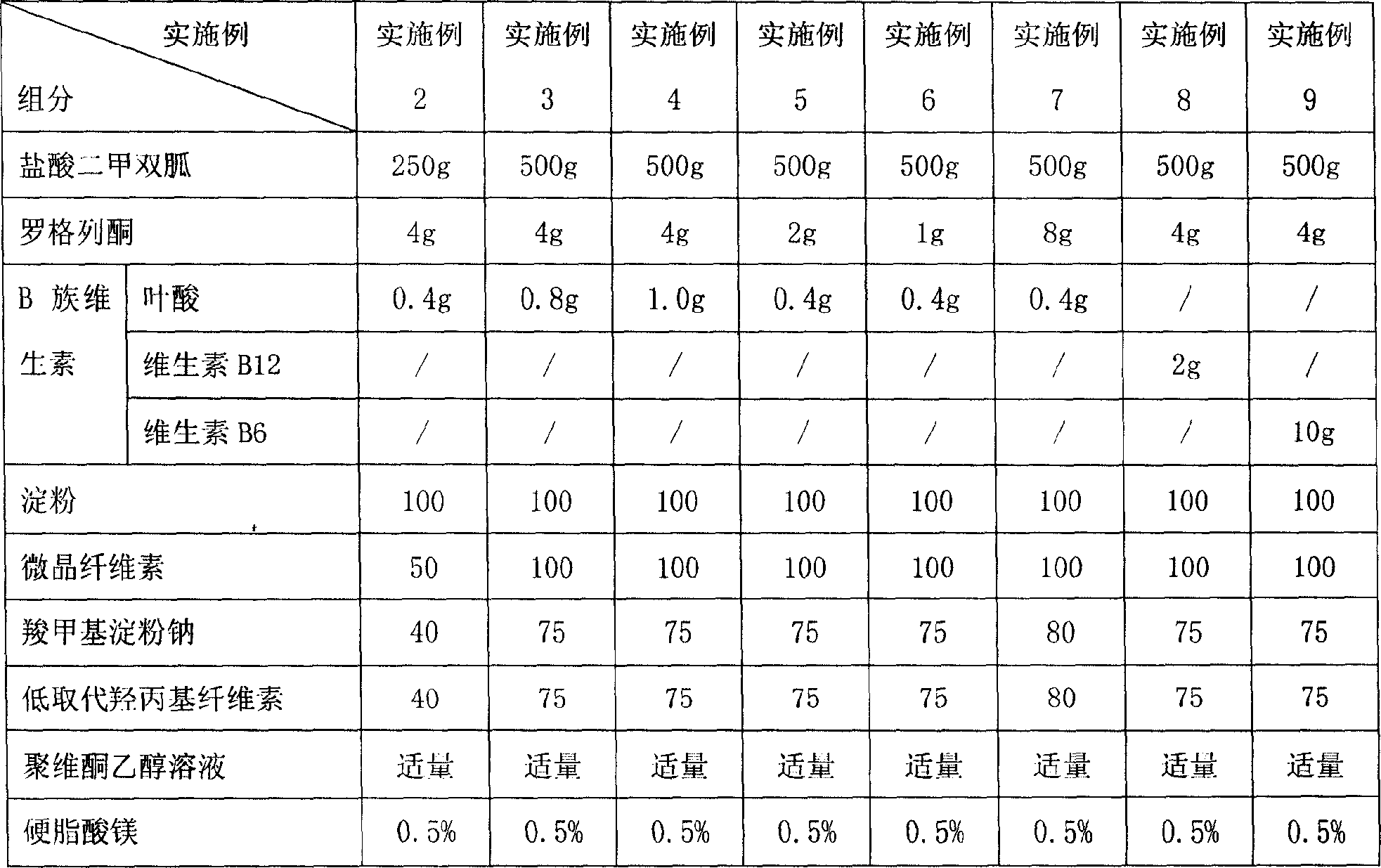
InactiveCN101069745AReduce complicationsAvoid complicationsMetabolism disorderPill deliveryDiabetes Mellitus ComplicationsThiazole
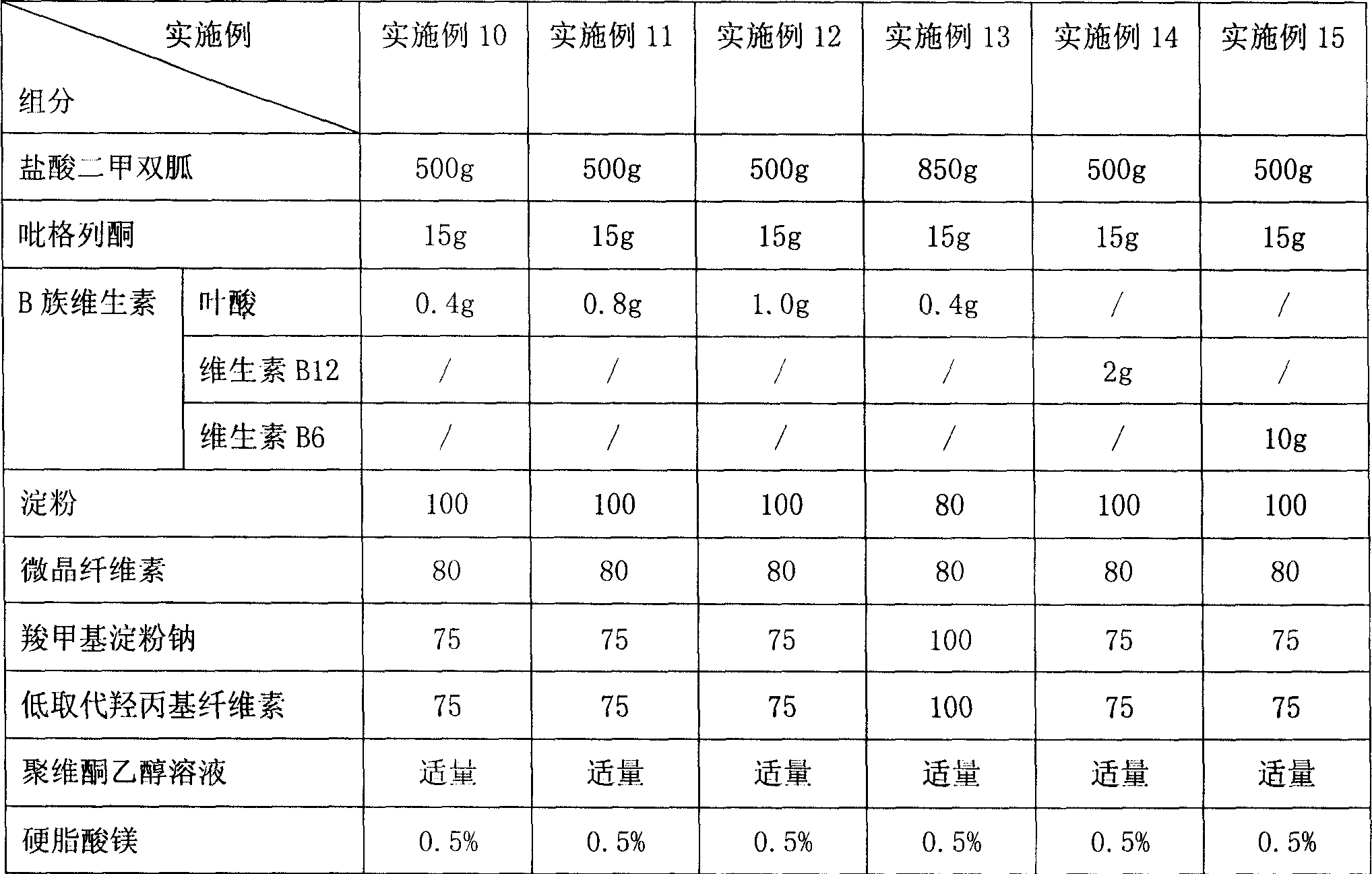
The present invention relates to a new-type hypoglycemic medicine composition. Said new-type hypoglycemic medicine composition contains biguanide hypoglycemic medicine or its medicinal salt with effective dose, thiazolidione hypoglycemic medicine or its medicinal salt with effective dose, vitamins B with effective dose and medicinal carrier or excipient. Said invention also provides the application of said medicine composition in preparation of medicine for preventing and / or curing diabetes and its complication.
Owner:SHENZHEN AUSA PHARMA
Novel pharmaceutical formulation containing a biguanide and a thiazolidinedione derivative
A pharmaceutical dosage form comprising a controlled release component comprising an antihyperglycemic drug in combination with a second component comprising a thiazolidinedione derivative is herein disclosed and described.
Owner:ACTAVIS +1
Modulation of Peripheral Clocks in Adipose Tissue
InactiveUS20090202659A1Improve responseBiocideNervous disorderCircadian clock geneGlycogen synthase I
Genes encoding the transcription factors controlling the core circadian oscillator (BMAL, Clock, NPAS, Per) and their regulatory targets (Rev-erbα, Rev-erb) have been found in adipose tissue. The circadian pattern of these genes was entrained using restricted feeding. The circadian gene expression profiles were examined in mice and in undifferentiated and adipocyte-differentiated human adipose stem cells following exposure to nuclear hormone receptor ligands (dexamethasone or thiazolidinedione) or 30% fetal bovine serum. All three agents induced the initiation of a cyclic expression profile in representative circadian genes in the human adipose stem cells. The circadian genes studied displayed an oscillatory expression profile, characterized by both a zenith and nadir within a 24-28 hr phase. The circadian gene pattern has been lengthened with use of an inhibitor of glycogen synthase kinase 3 beta. Modulation of the circadian pattern to lengthen or shorten can be used to affect weight gain or loss, respectively.
Owner:BOARD OF SUPERVISORS OF LOUISIANA STATE UNIV & AGRI & MECHANICAL COLLEGE
Use of fgf-21 and thiazolidinedione for treating type 2 diabetes
A method for treating type 2 diabetes and metabolic syndrome comprising administering an effective amount of fibroblast growth factor 21 in combination with a thiazolidinedione.
Owner:ELI LILLY & CO
Thiazolidinedione analogues
InactiveUS20090143442A1Reduce the binding forceReduce activationBiocideNervous disorderDiseaseMetabolic inflammation
The present invention relates to thiazolidinedione analogues that are useful for treating metabolic inflammation mediated diseases such as diabetes.
Owner:METABOLIC SOLUTIONS DEVMENT
Thiazolidinedione analogues
ActiveUS8389556B2Reduced binding and activationMore effective to treat and prevent diabetesBiocideNervous disorderMedicineThiazolidinedione
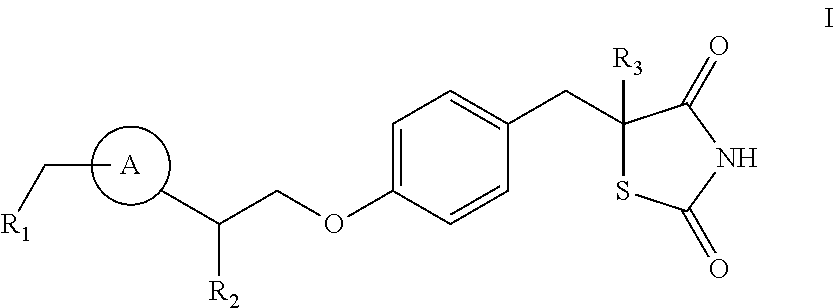
The present invention relates to methods of using thiazolidinedione analogues of formula I:for treating Alzheimer's disease The terms R1, R2 and R3 are herein defined.
Owner:METABOLIC SOLUTIONS DEVMENT
Ppar-sparing thiazolidinediones and combinations for the treatment of neurodegenerative diseases
InactiveUS20130053350A1Increase in cAMPReduce the binding forceBiocideNervous disorderThiazolidinedioneNeuro-degenerative disease
The present invention relates to thiazolidinedione analogues and pharmaceutical compositions that are useful for treating and / or preventing neurodegenerative disorders.
Owner:CIRIUS THERAPEUTICS INC
Thyroid receptor ligands
Thyroid receptor ligands are provided which have the general formula I wherein: R1 is R2 and R3 are the same or different and are hydrogen, halogen, alkyl of 1 to 4 carbons or cycloalkyl of 3 to 5 carbons, provided that at least one of R2 and R3 is other than hydrogen; R4 is R5 and R6 are the same or different and are selected from hydrogen, aryl, heteroaryl, alkyl, cycloalkyl, aralkyl or heteroaralkyl. R7 is aryl, heteroaryl, alkyl, aralkyl, or heteroaralkyl; R8 is aryl, heteroaryl, or cycloalkyl; R9 is R7 or hydrogen; R10 is hydrogen, halogen, cyano or alkyl; R11 and R12 are each independently selected from the group consisting of hydrogen, halogen, alkoxy, hydroxy (—OH) cyano, and alkyl; R13 is carboxylic acid (COOH) or esters thereof, phosphonic and phosphinic acid or esters thereof, sulfonic acid, tetrazole, hydroxamic acid, thiazolidinedione, acylsulfonamide, or other carboxylic acid surrogates known in the art; R14 and R15 may be the same or different and are selected from hydrogen and alkyl, or R14 and R15 may be joined together forming a chain of 2 to 5 methylene groups [—(CH2)m-, m=2, 3, 4 or 5], thus forming 3- to 6-membered cycloalkyl rings; R16 is hydrogen or alkyl of 1 to 4 carbons; R17 and R18 are the same or different and selected from hydrogen, halogen and alkyl; n is 0 or an integer from 1 to 4; X is oxygen (—O—), sulfur (—S—), sulfonyl (—SO2—), sulfenyl (—SO—) selenium (—Se—), carbonyl (—CO—), amino (—NH—) or methylene (—CH2-); wherein the substituents are as described herein. In addition, a method is provided for preventing, inhibiting or treating diseases or disorders associated with metabolism dysfunction or which are dependent upon the expression of a T3 regulated gene, wherein a compound as described above is administered in a therapeutically effective amount.
Owner:BRISTOL MYERS SQUIBB CO
Thiazolidinedione analogues for the treatment of metabolic diseases
ActiveUS8067450B2Reduce activationEffective to preventOrganic active ingredientsBiocideDiseaseAlkoxy group
The present invention relates to thiazolidinedione analogues that are useful for treating hypertension, diabetes, and inflammatory diseases. Formula (I), wherein: each of R1 and R4 is independently selected from H, halo, aliphatic, and alkoxy, wherein the aliphatic and alkoxy are optionally substituted with 1-3 of halo; R2 is halo, hydroxy, or optionally substituted aliphatic, and R′2 is H, or R2 and R′2 together form oxo; R3 is H; and Ring A is a phenyl.
Owner:CIRIUS THERAPEUTICS INC
Novel heterocyclic analogs of diphenylethylene compounds
Novel diphenylethylene compounds and derivatives thereof containing thiazolidinedione or oxazolidinedione moieties are provided which are effective in lowering blood glucose level, serum insulin, triglyceride and free fatty acid levels in animal models of Type II diabetes. The compounds are disclosed as useful for a variety of treatments including the treatment of inflammation, inflammatory and immunological diseases, insulin resistance, hyperlipidemia, coronary artery disease, cancer and multiple sclerosis.
Owner:THERAKOS INC
Thiazolidinedione, oxazolidinedione and oxadiazolidinedione derivatives
Novel thiazolidinedione, oxazolidinedione and oxadiazolidinedione derivatives, process for their manufacture, pharmaceutical preparations containing them and the use of the compounds in conditions associated with insulin resistance.
Owner:ASTRAZENECA AB
Thiazolidinedione analogues
ActiveUS20120015982A1Reduced binding and activationMore effective to treat and prevent diabetesBiocideNervous disorderDiseaseMedicine
The present invention relates to thiazolidinedione analogues that are useful for treating metabolic inflammation mediated diseases such as diabetes.
Owner:METABOLIC SOLUTIONS DEVMENT
Ppar-sparing thiazolidinediones and combinations for the treatment of obesity and other metabolic diseases
The present invention relates to thiazolidinedione analogues and pharmaceutical compositions that are useful for treating and / or preventing obesity or diabetes, optionally in combination with a second treatment therapy such a diet restriction or an increase in duration or exertion in physical activity.
Owner:METABOLIC SOLUTIONS DEVMENT
Novel process to prepare pioglitazone via several novel intermediates
InactiveUS20080004446A1High yieldEasy to operateOrganic chemistryMetabolism disorderThiazolidinedioneMedicinal chemistry
A process for preparing thiazolidinediones, preferably pioglitazone, is described. Also described are intermediates involved in synthesizing thiazolidinediones, and processes for preparation and use in medicine.
Owner:CADILA HEALTHCARE LTD
Ppar-sparing thiazolidinedione salts for the treatment of metabolic diseases
ActiveUS20120309975A1Reduce the binding forceReduce activationOrganic active ingredientsNervous disorderDiabetes mellitusThiazole
The present invention relates to novel salts of thiazolidinediones and other pharmaceutical agents that are useful for treating and / or preventing metabolic diseases (e.g., diabetes, or neurodegenerative diseases (e.g., Alzheimer's Disease).
Owner:CIRIUS THERAPEUTICS INC
Methods and compositions for locally increasing body fat
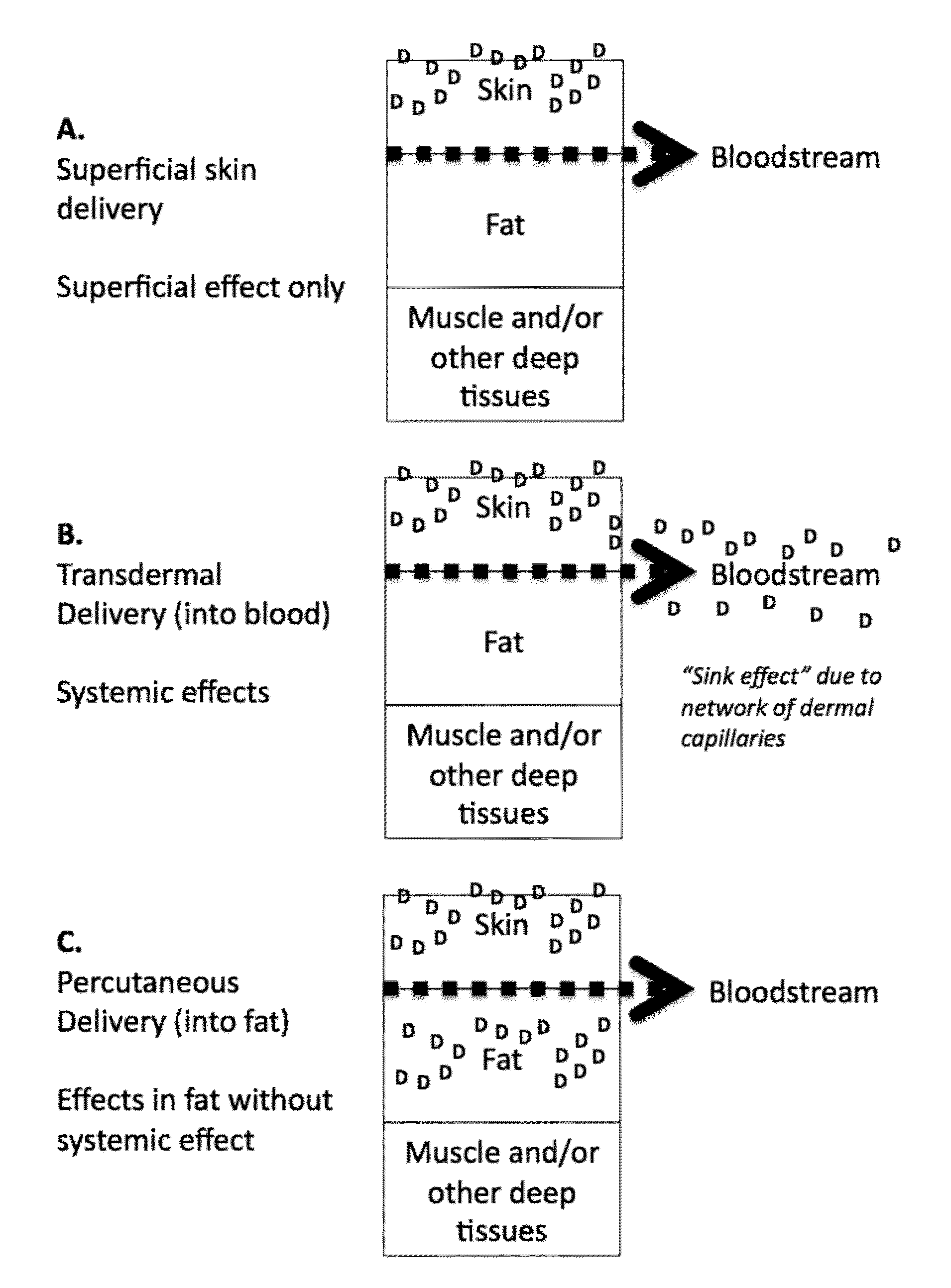
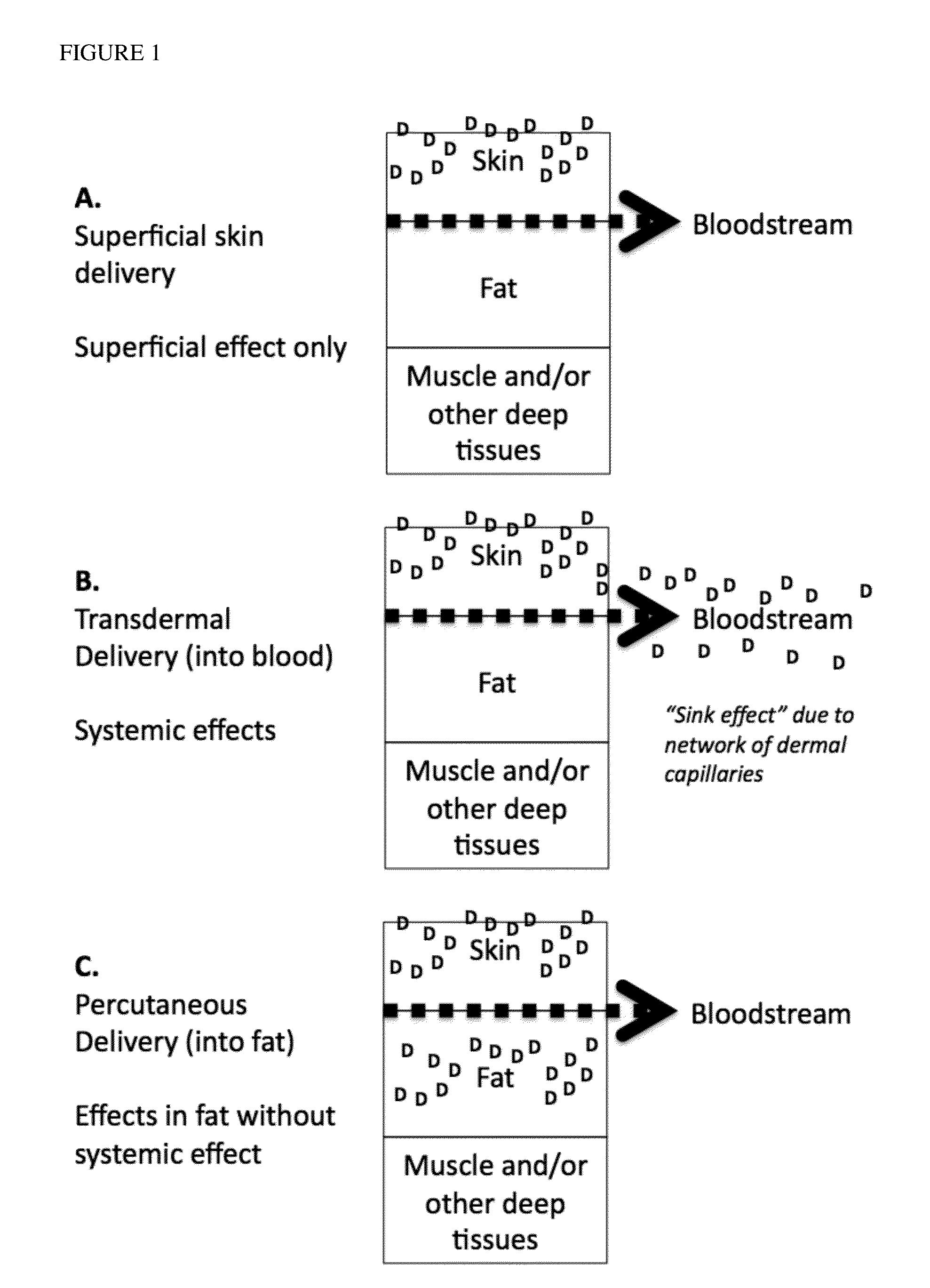
InactiveUS8778981B2Efficient implementationMinimize appearanceCosmetic preparationsBiocideThiazolidinedioneSubcutaneous fat
Provided are methods for increasing fat locally in a body of a subject in need thereof comprising percutaneously administering to the subcutaneous fat of the subject a thiazolidinedione or an orexigenic compound, or a pharmaceutically acceptable salt or prodrug thereof, optionally delivered as a composition comprising a pharmaceutically acceptable carrier, as described herein. In certain embodiments, the pharmaceutically acceptable carrier comprises a percutaneous carrier, as described herein. Further provided are compositions comprising a thiazolidinedione or an orexigenic compound, or a pharmaceutically acceptable salt or prodrug thereof, for use according to the invention.
Owner:VELAKINE THERAPEUTICS INC
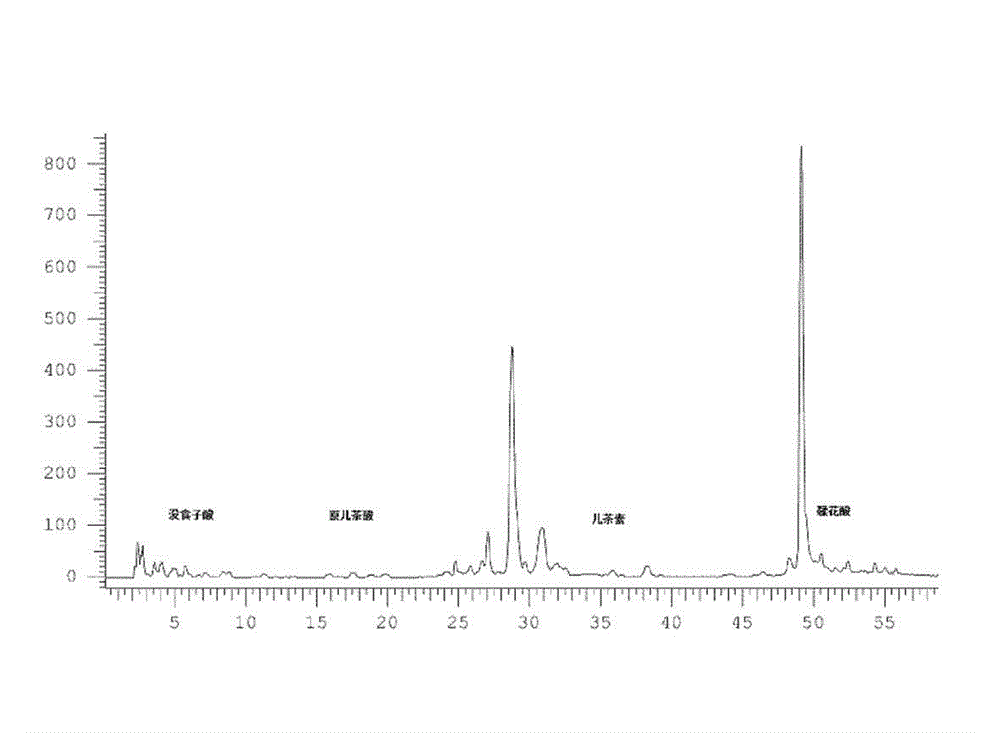
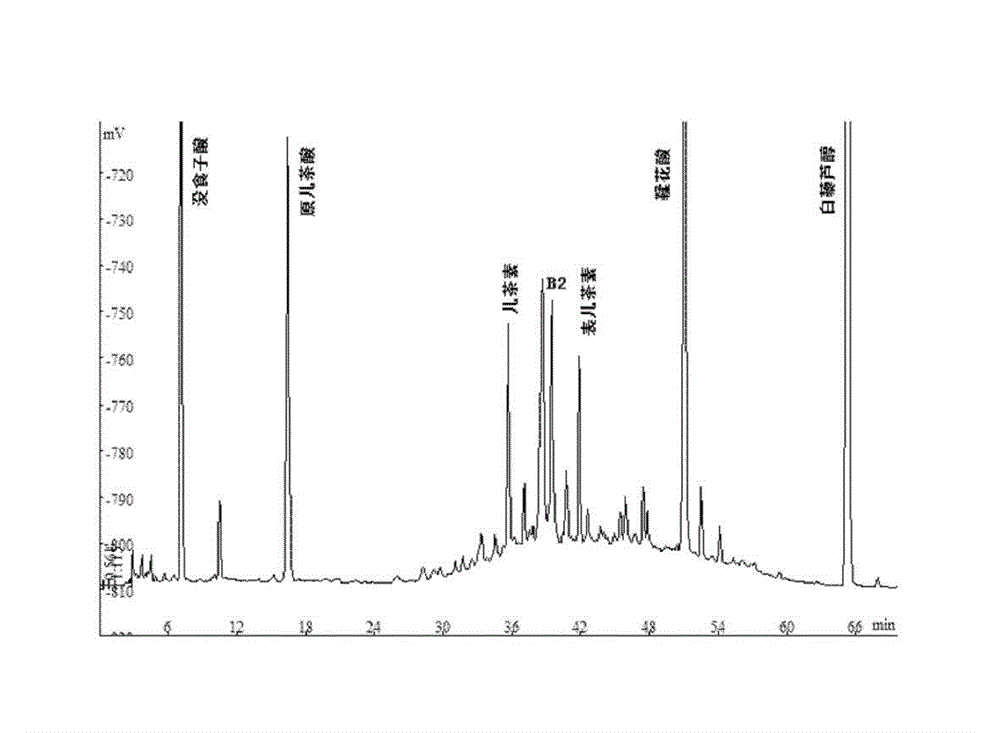
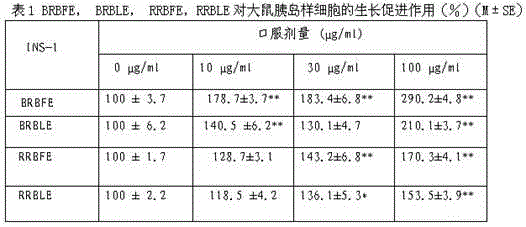
Application of black raspberry extract in preparing anti-diabetic medicine
ActiveCN103142720AGood hypoglycemic effectImprove elevated blood sugar levelsMetabolism disorderPlant ingredientsThiazolidinedioneDiabrezide
The invention relates to an application of a black raspberry extract in preparing an anti-diabetic medicine, and an application of the black raspberry extract in preventing type-II diabetes mainly characterized by glucose metabolic disorders, beta islet cell damage and insulin resistance. A novel anti-diabetic medicine preparation provided by the invention takes the black raspberry extract as an active ingredient, and the total effective dose is 1-100mg / kg / d. According to the novel anti-diabetic medicine preparation provided by the invention, the black raspberry extract can be compounded with the diabetes treatment medicines on the current market including sulfonylureas, biguanides or thiazolidinediones to prepare a compound preparation. The black raspberry extract can promote islet cell multiplication and inhibit the PTP1B activity; and meanwhile, in-vivo study finds that the black raspberry extract can be used for improving the increase of the mice blood glucose level caused by alloxan and has an obvious effect in reducing blood glucose. Through verification, the blood glucose reducing effect of the black raspberry fruit or leaf extract is superior to that of the selected positive medicine diaformin.
Owner:赵红石
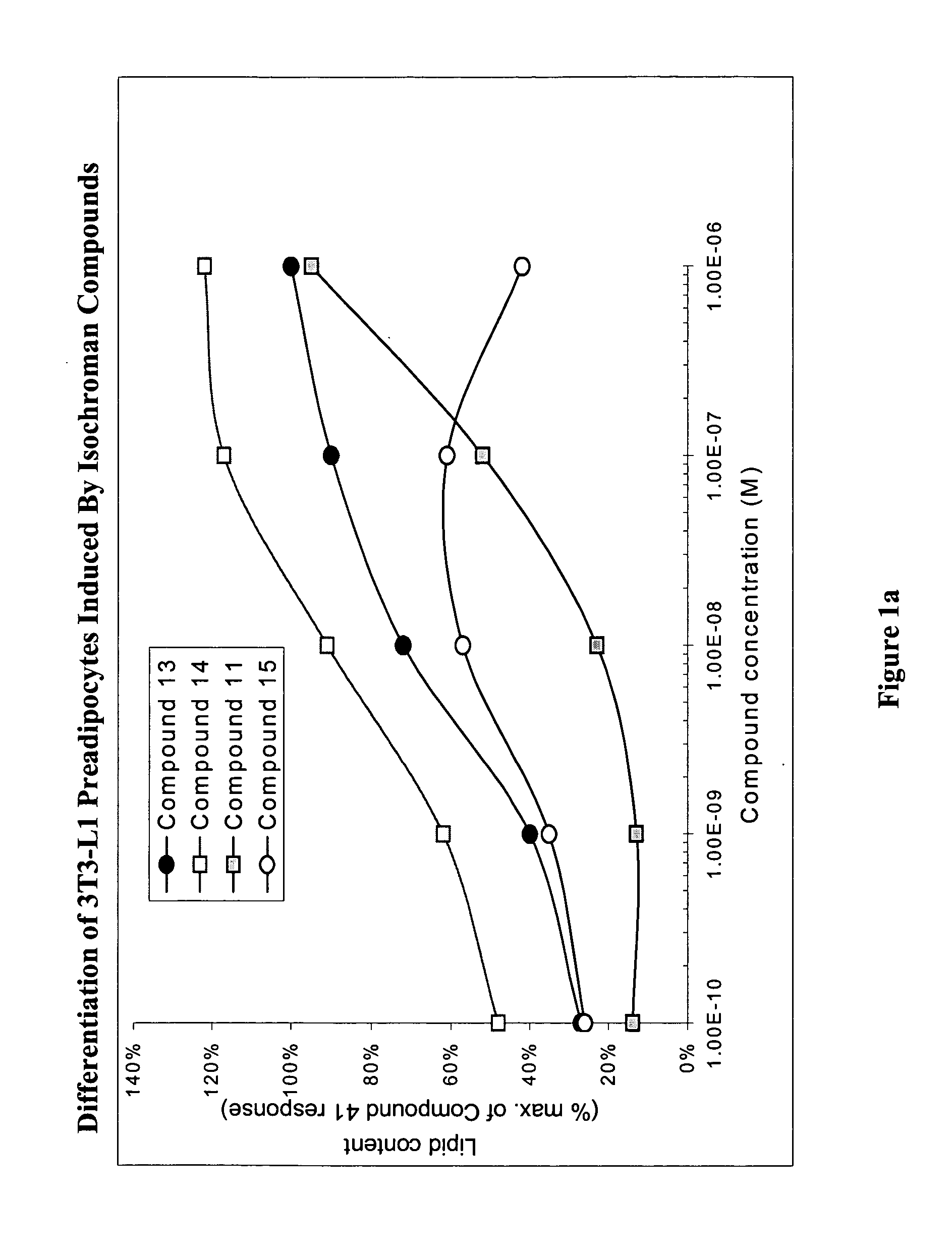
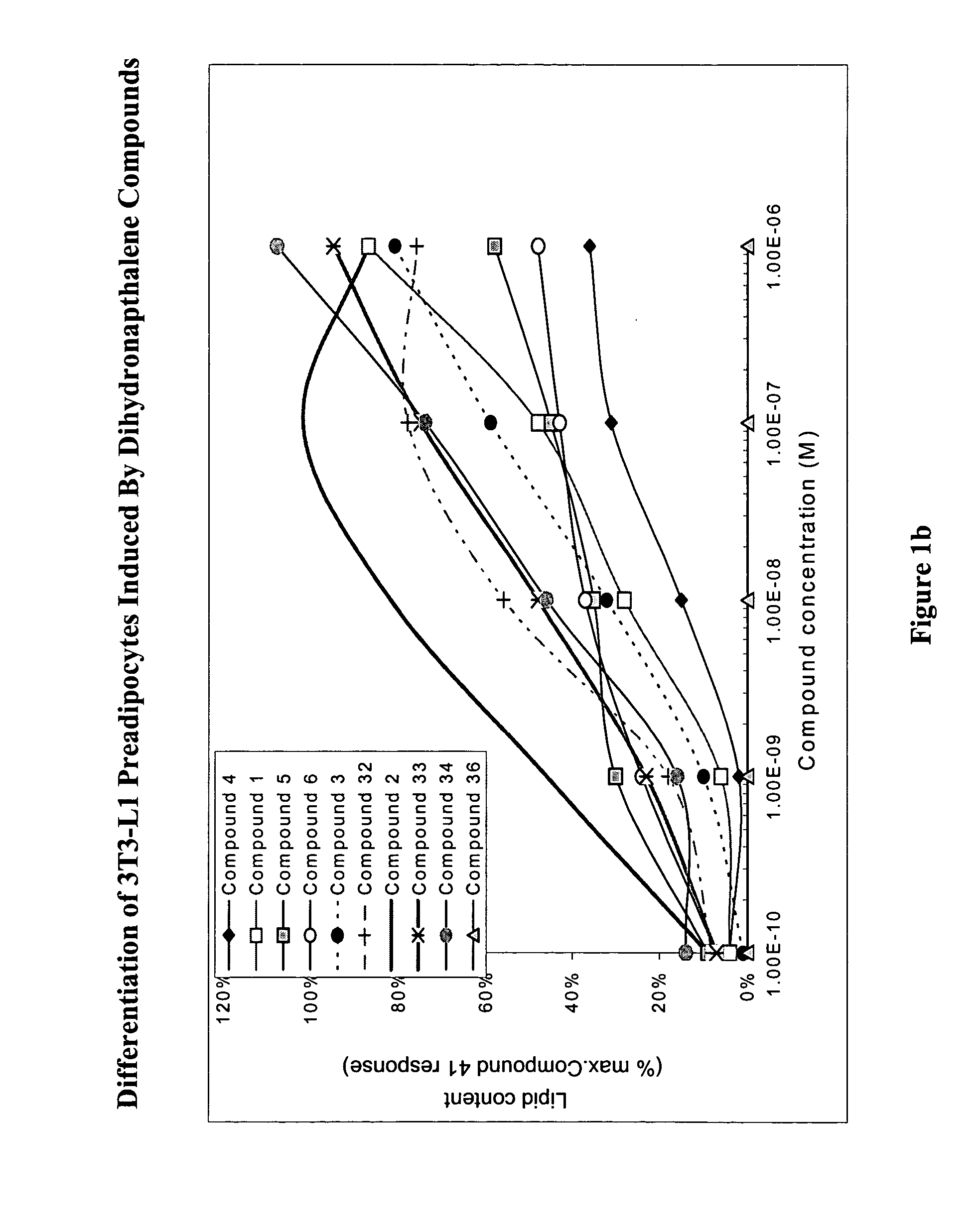
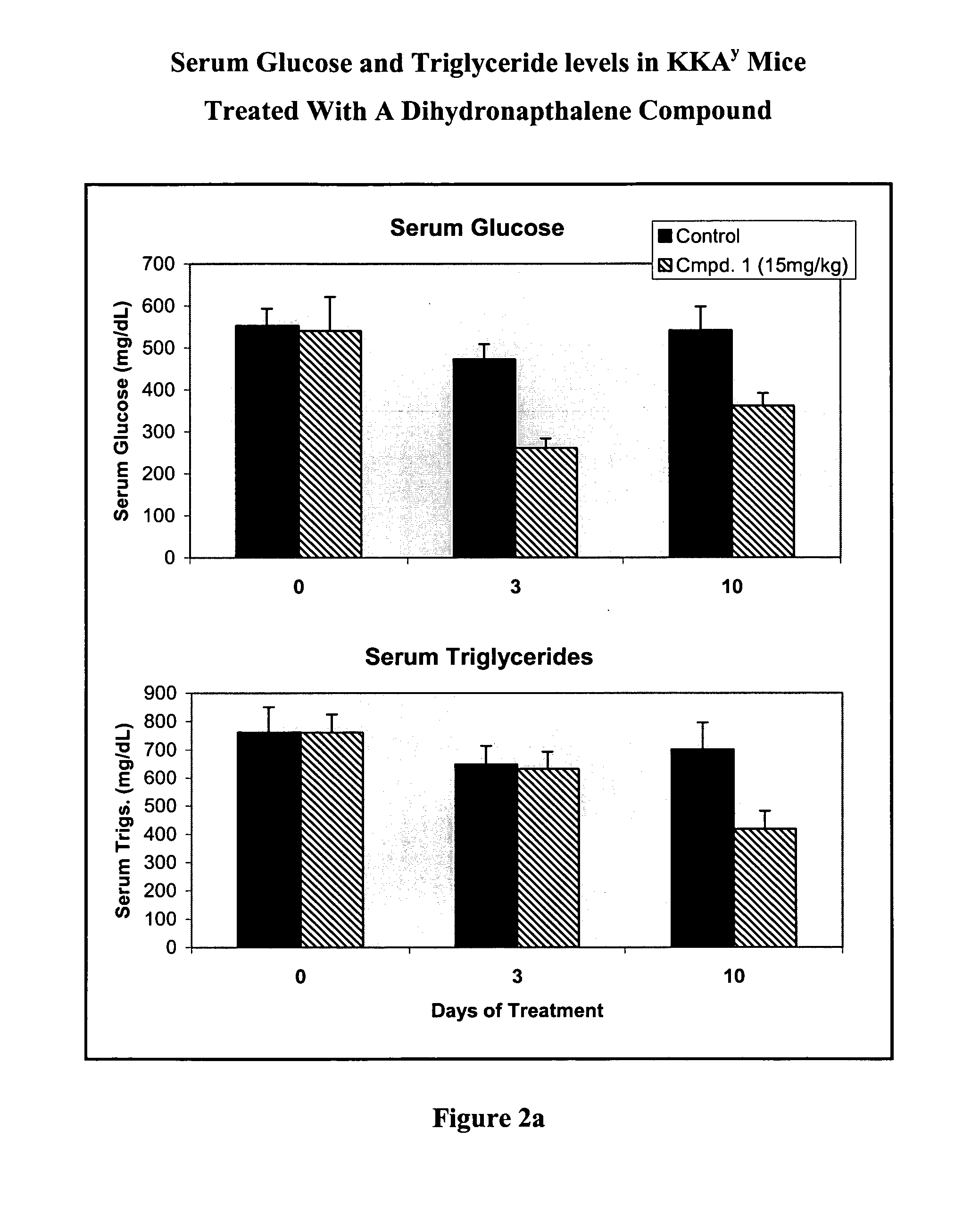
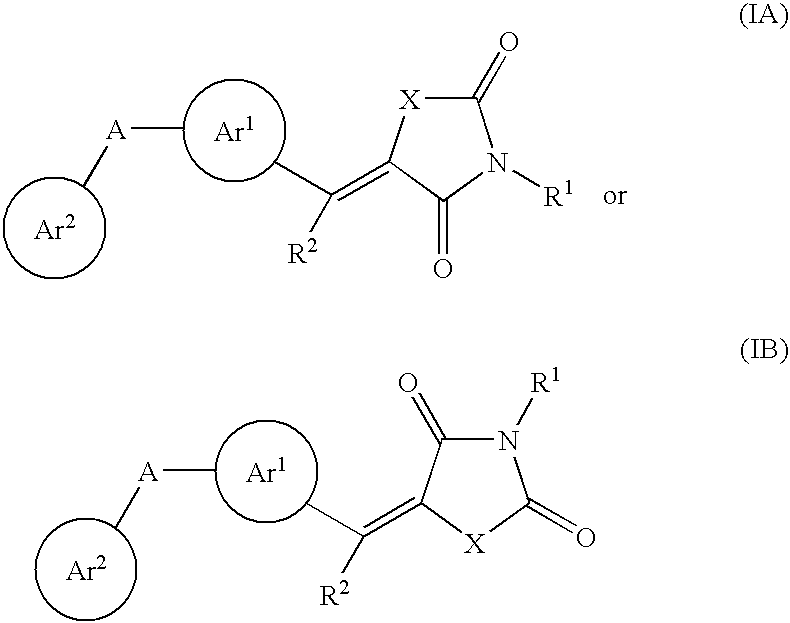
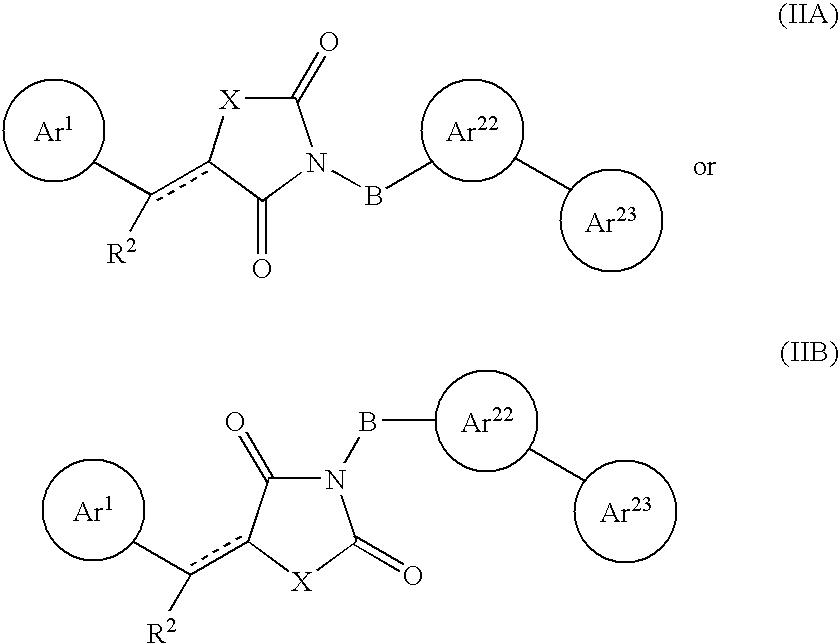
Substituted dihydronaphthalene and isochroman compounds for the treatment of metabolic disorders, cancer and other diseases
InactiveUS20050038098A1Superior physical property and bioavailabilityLower Level RequirementsBiocideOrganic chemistryThiazolidinedioneDrug biological activity
The invention relates to novel heterocyclic compounds having the structure illustrated by Formula (I) wherein the Ar1 radicals are substituted dihydronapthalene or isochroman radicals, the Ar2 radicals are aryl or heteroaryl radicals; and HAr is a 2,4-thiazolidinedione, 2-thioxo-thiazolidine-4-one, 2,4-imidazolidinedione or 2-thioxo-imidazolidine-4-one radical. The compounds of Formula (I) can have biological activity for advantageously regulating carbohydrate metabolism, including serum glucose level, and lipid metabolism, and can be useful for the treatment of hyperlipidemia and / or hypercholesterolemia, and Type II diabetes. The compounds of Formula (I) can also have utility in the treatment of diseases of uncontrolled proliferation, including cancer.
Owner:INCYTE CORP
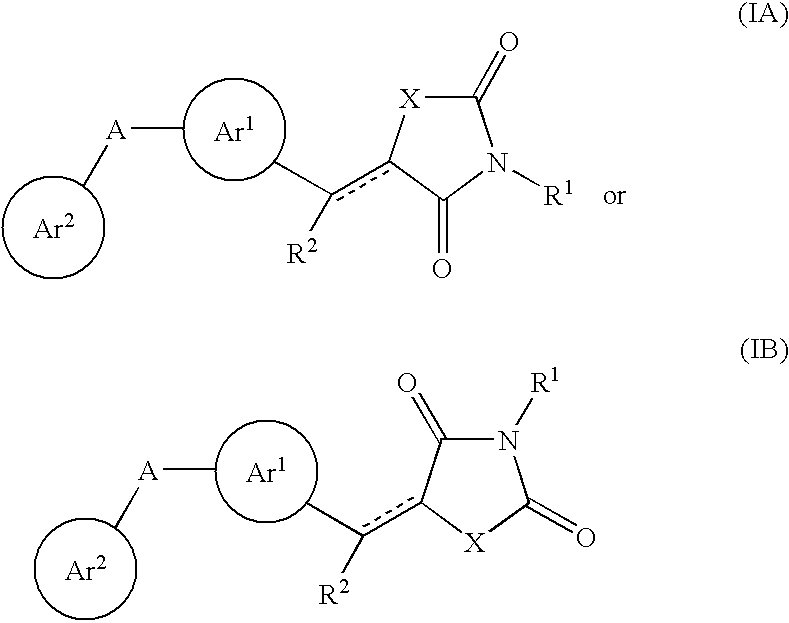
Aryl-link-aryl substituted thiazolidine-dione and oxazolidine-dione as sodium channel blockers
Aryl-link-aryl thiazolidine-dione and aryl-link-aryl oxazolidine-dione compounds are sodium channel blockers; pharmaceutical compositions that include an effective amount of the aryl-link-aryl thiazolidine-dione and aryl-link-aryl oxazolidine-dione compounds and a pharmaceutically acceptable carrier, and a method of treatment of acute pain, chronic pain, visceral pain, inflammatory pain, or neuropathic pain, as well as initable bowel syndrome, Cnohns disease, epilepsy, partial and generalized tonic seizures, multiple sclerosis, bipolar depression, and tachy-arrhythmias by the administration of an effective amount of aryl-link-aryl thiazolidine-dione and aryl-link-aryl oxazolidine-dione compounds are described.
Owner:MERCK SHARP & DOHME CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com