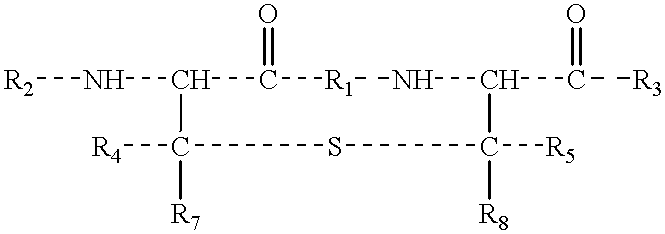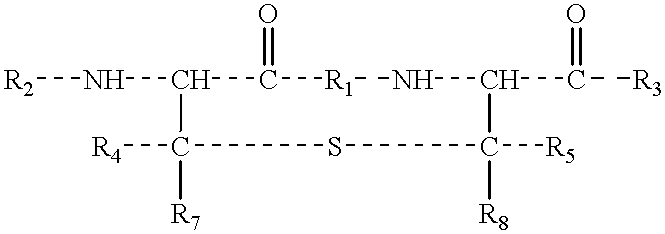Patents
Literature
35 results about "Lanthionine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
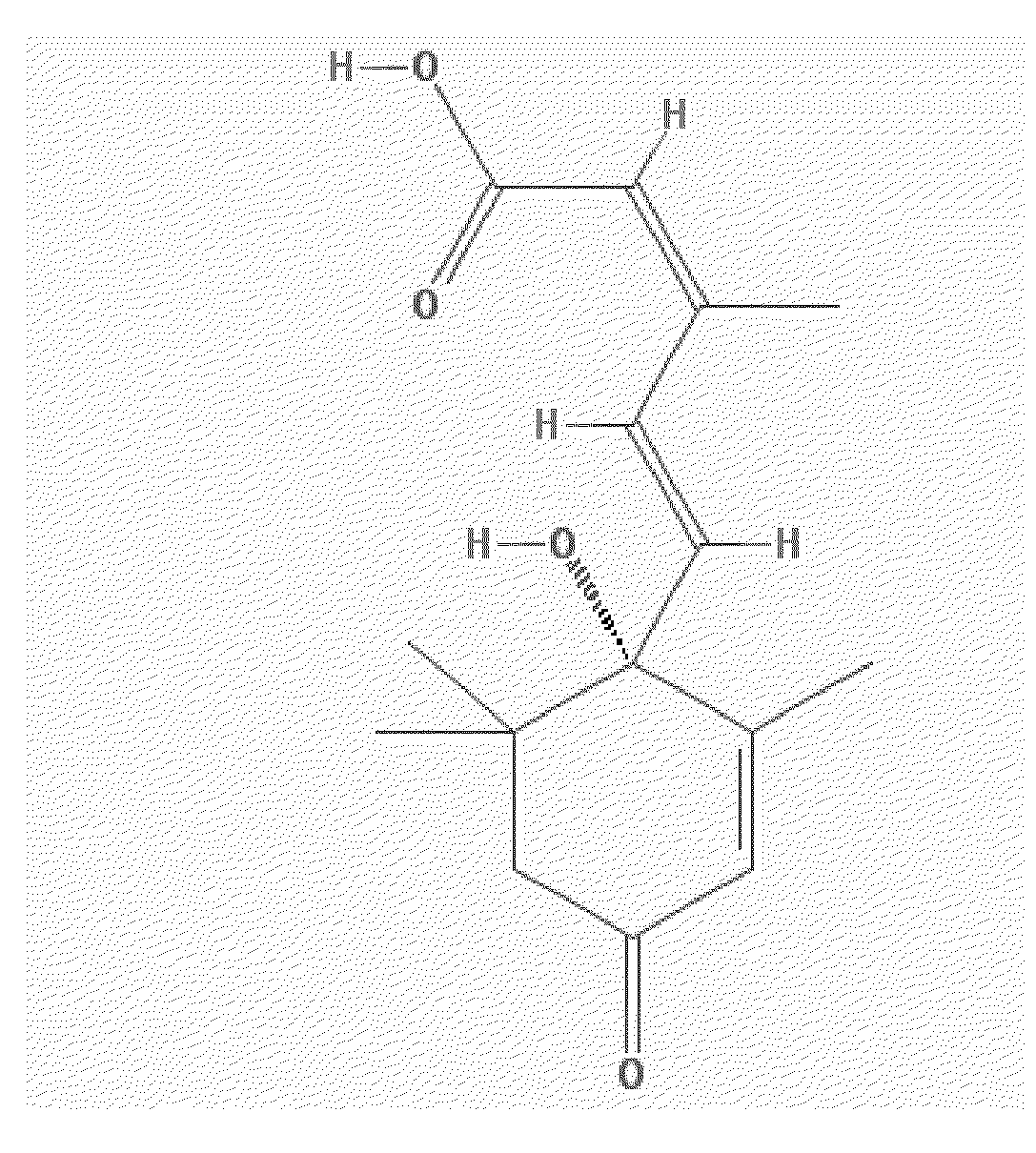
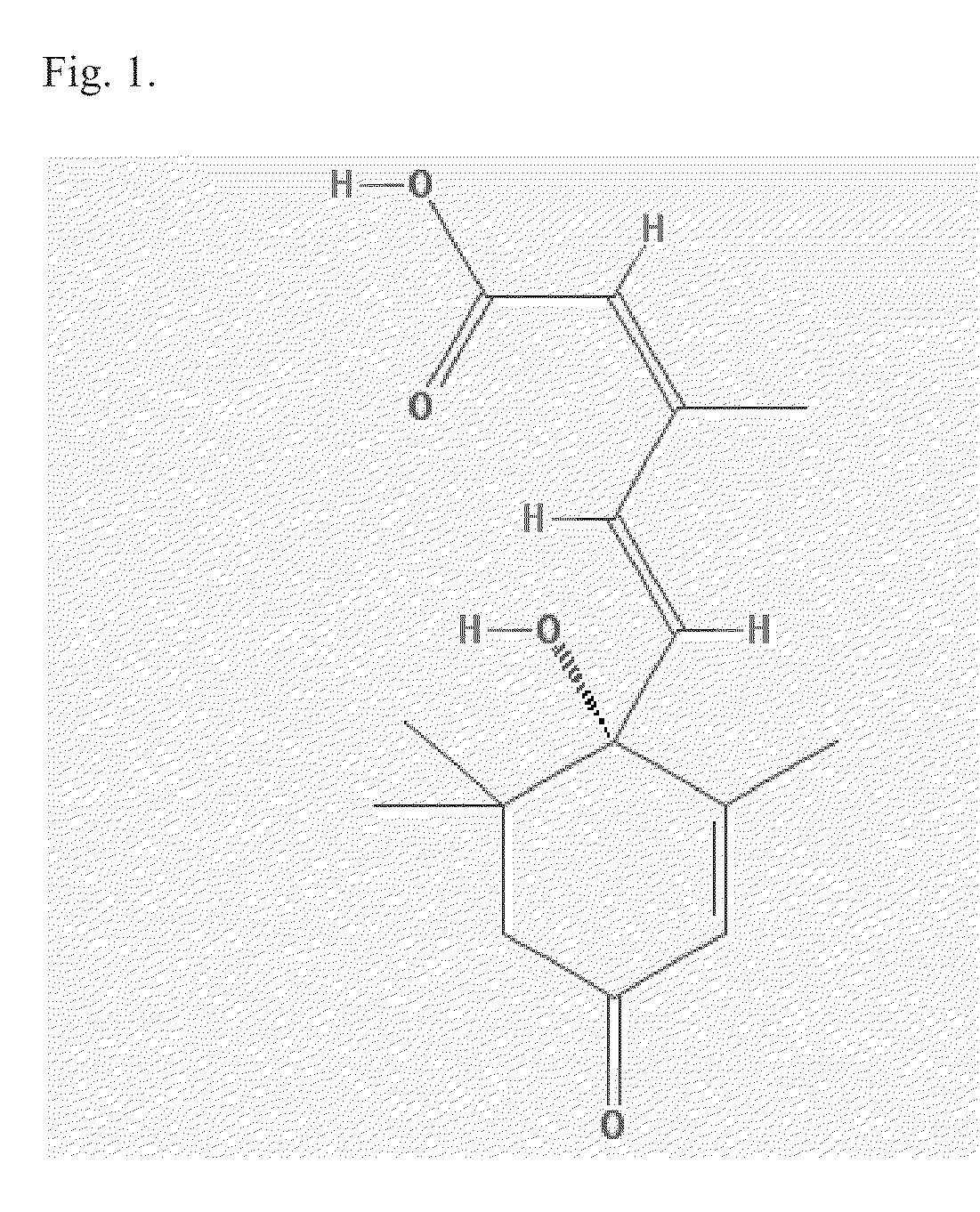
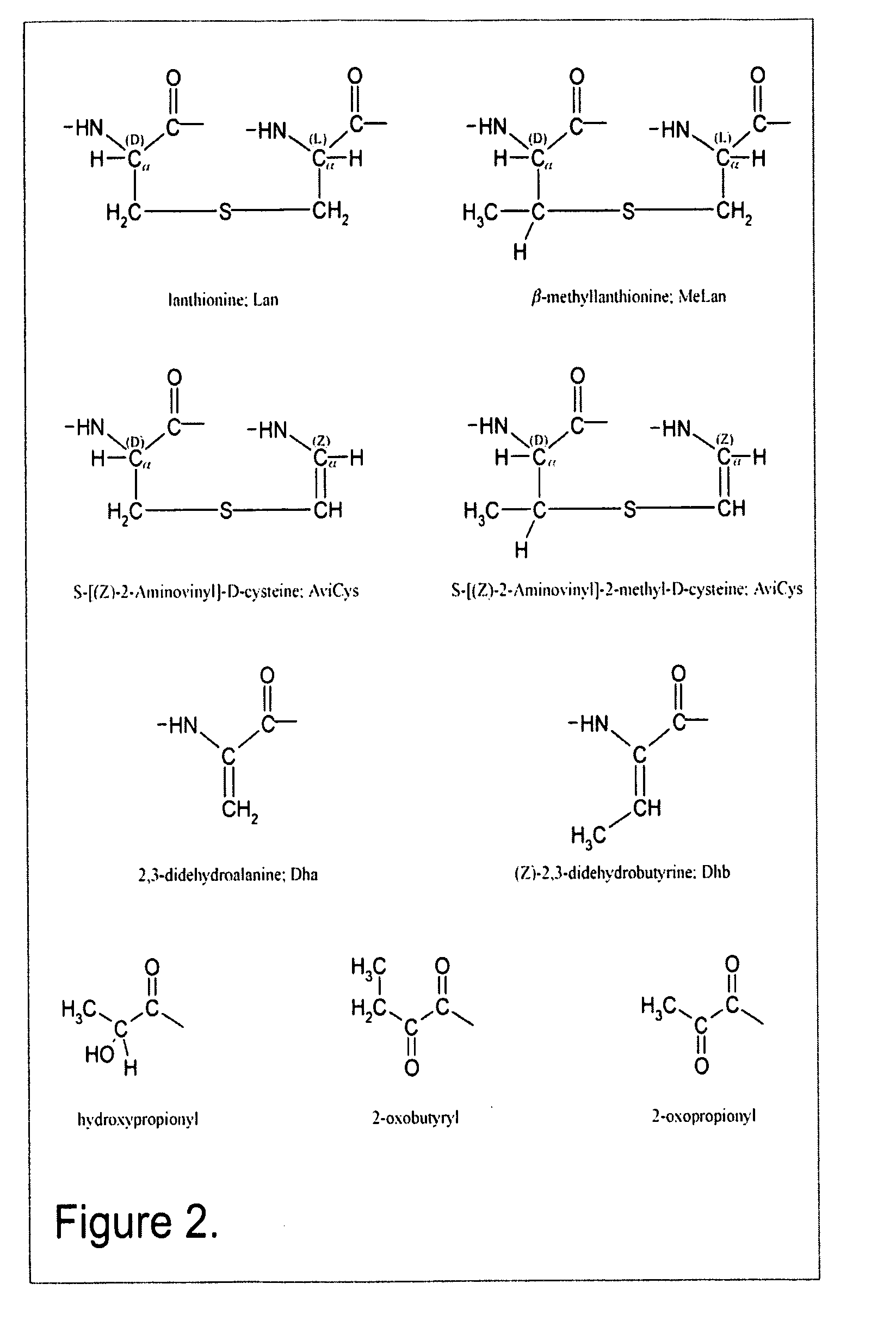
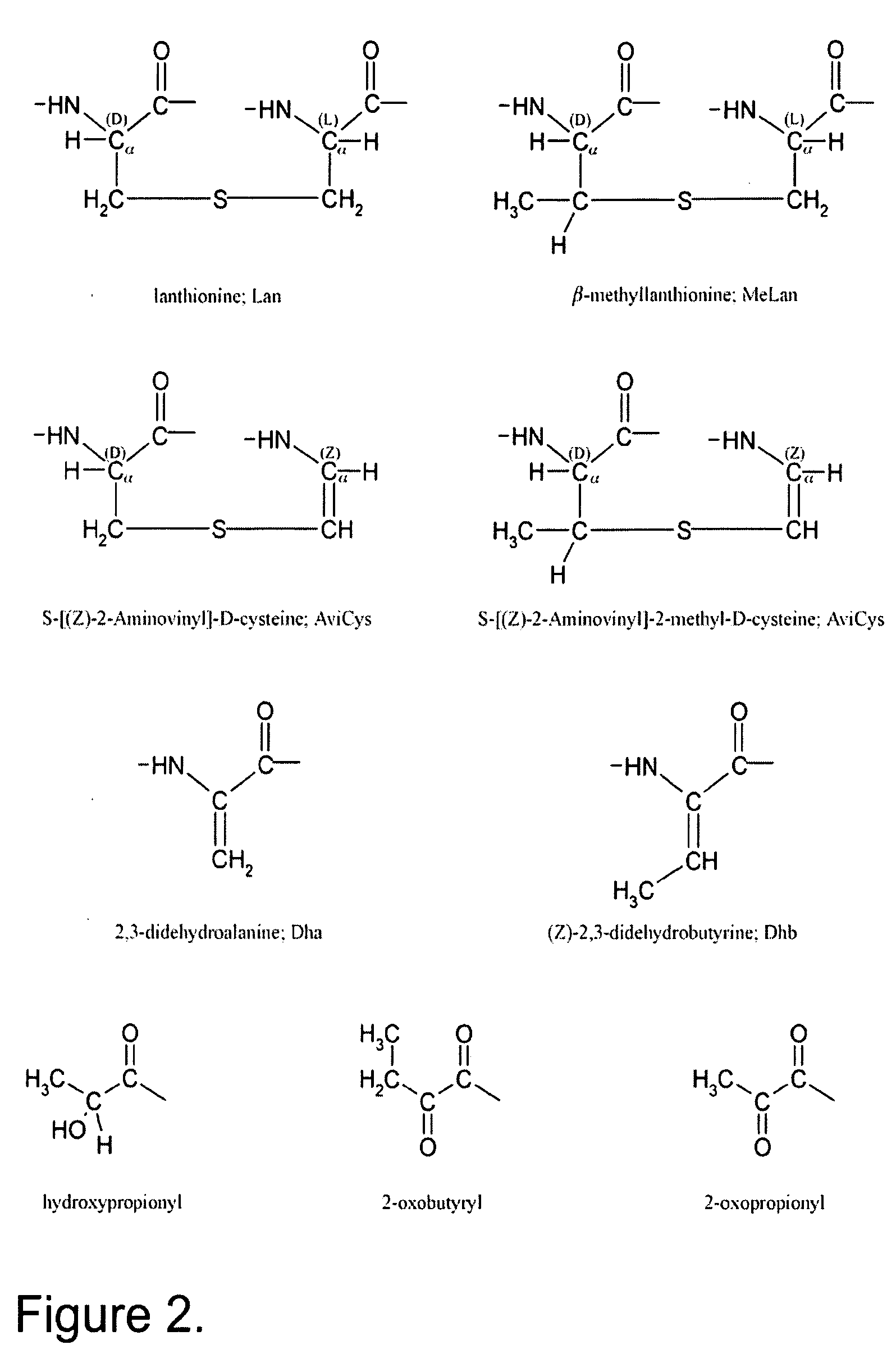
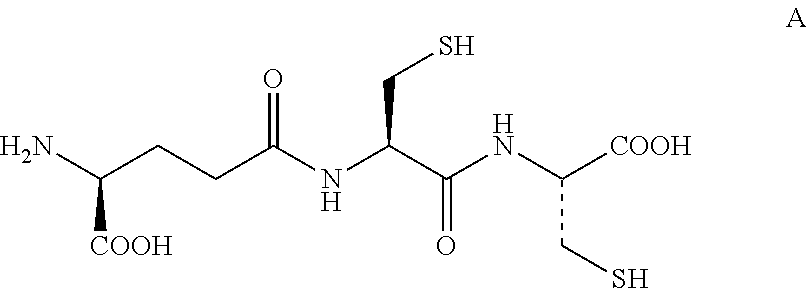
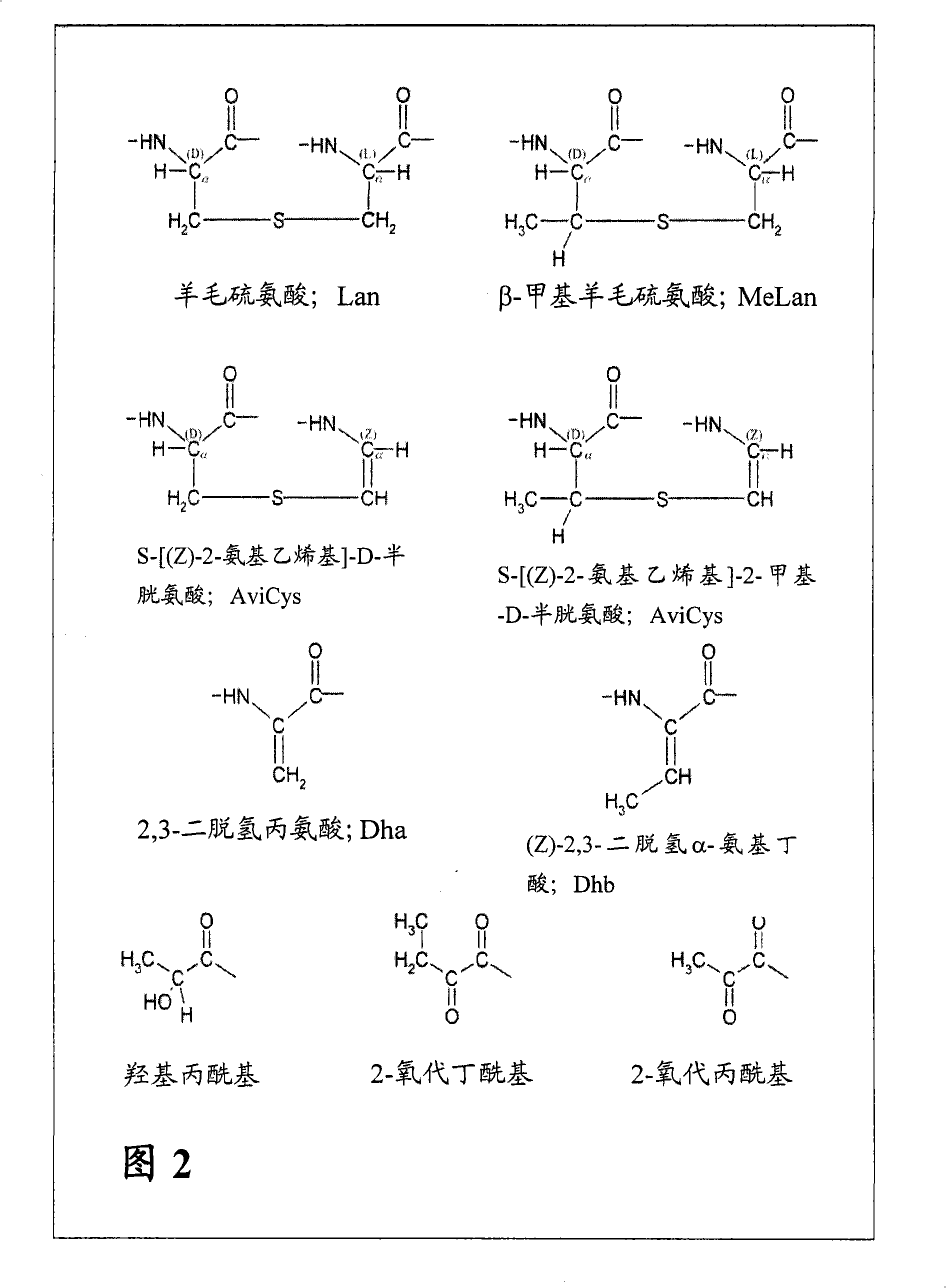
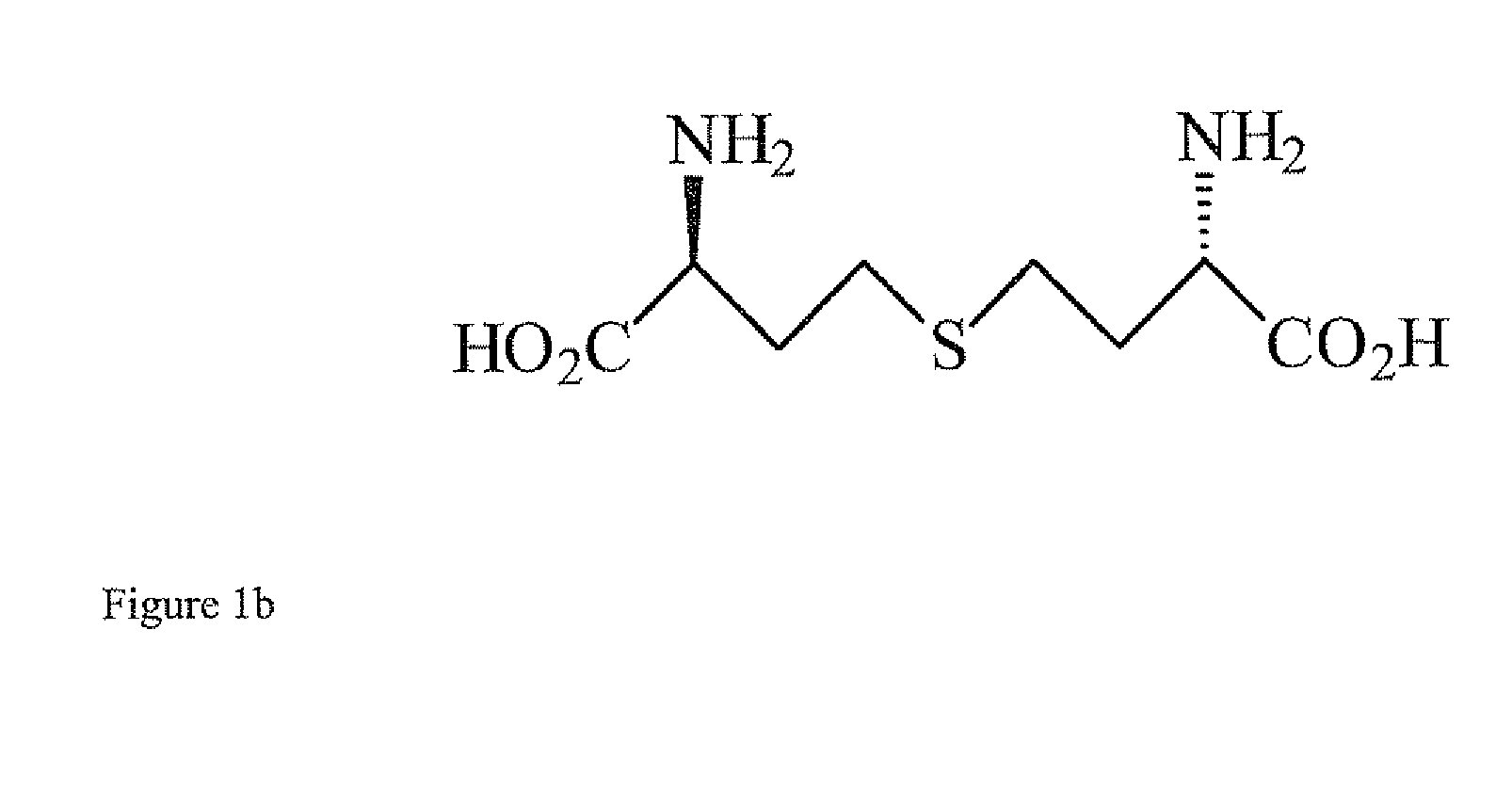
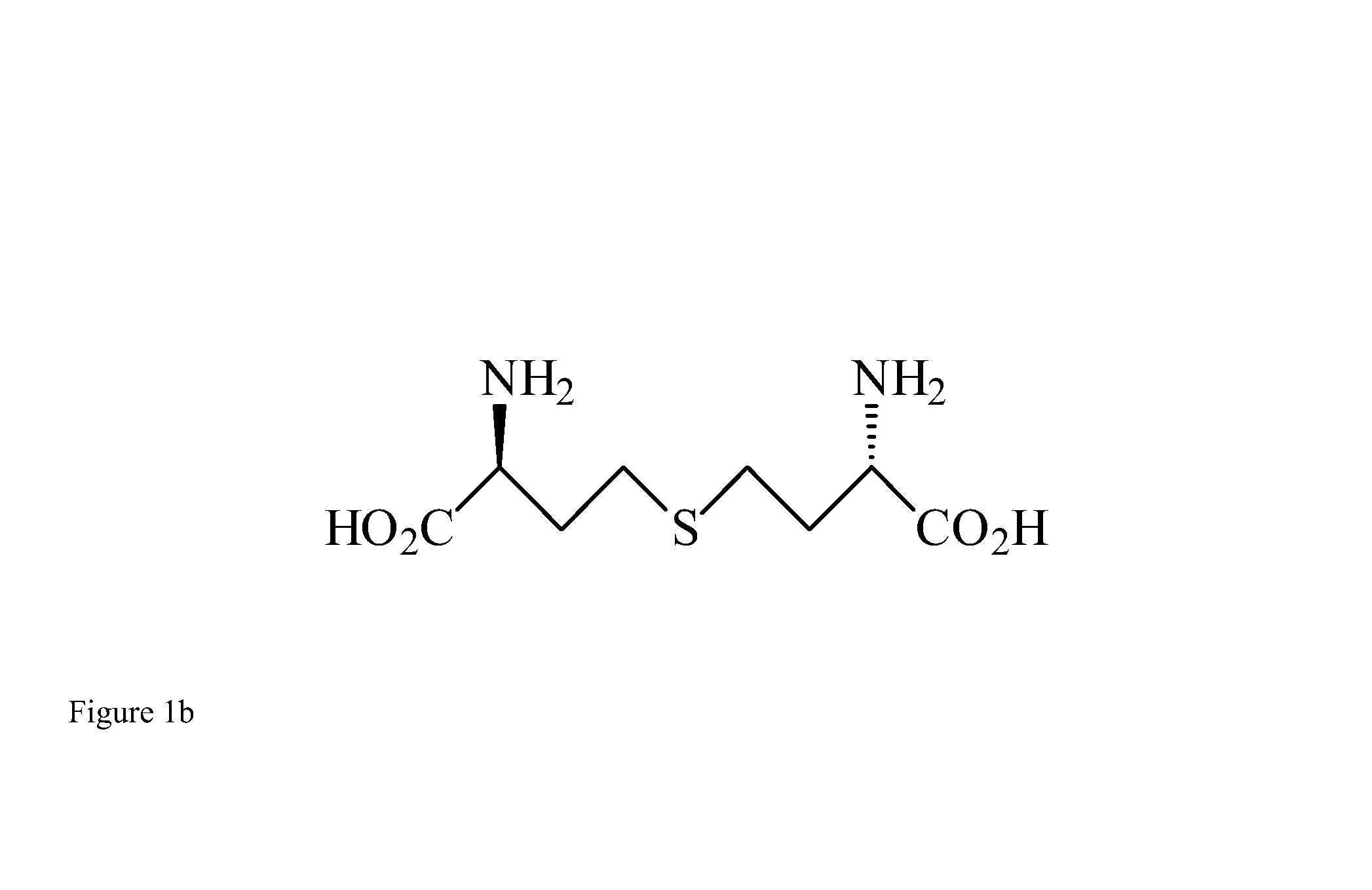
Lanthionine is a nonproteinogenic amino acid with the chemical formula (HOOC-CH(NH₂)-CH₂-S-CH₂-CH(NH₂)-COOH). It is a thioether dimer of cysteine, composed of two alanine residues that are crosslinked on their β-carbon via a sulfur atom. Despite its name, lanthionine does not contain the element lanthanum.
Lanthionine synthetase component c-like proteins as molecular targets for preventing and treating diseases and disorders
ActiveUS20110275558A1Organic active ingredientsPeptide/protein ingredientsThiazolidinedioneAutoimmune disease
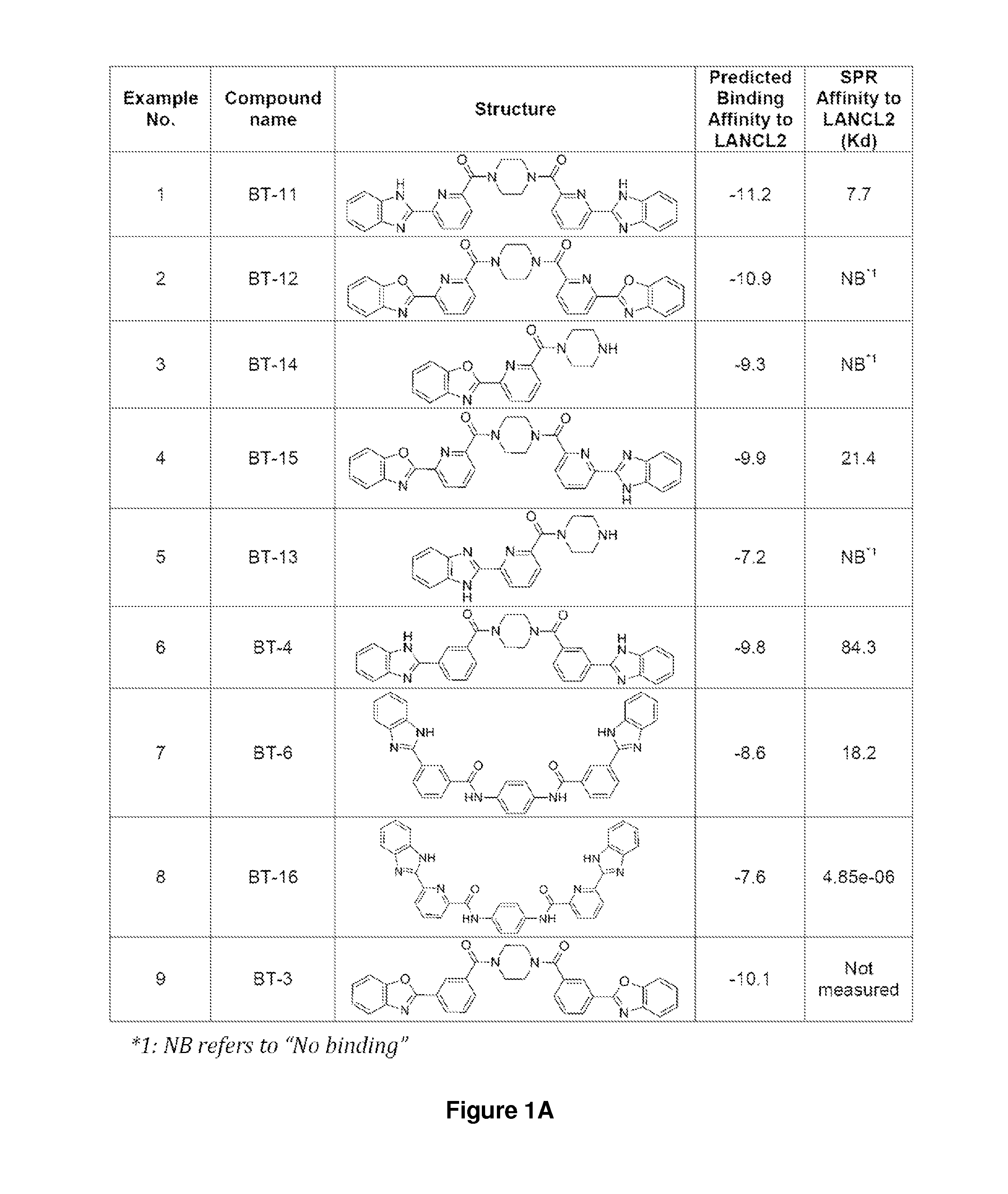
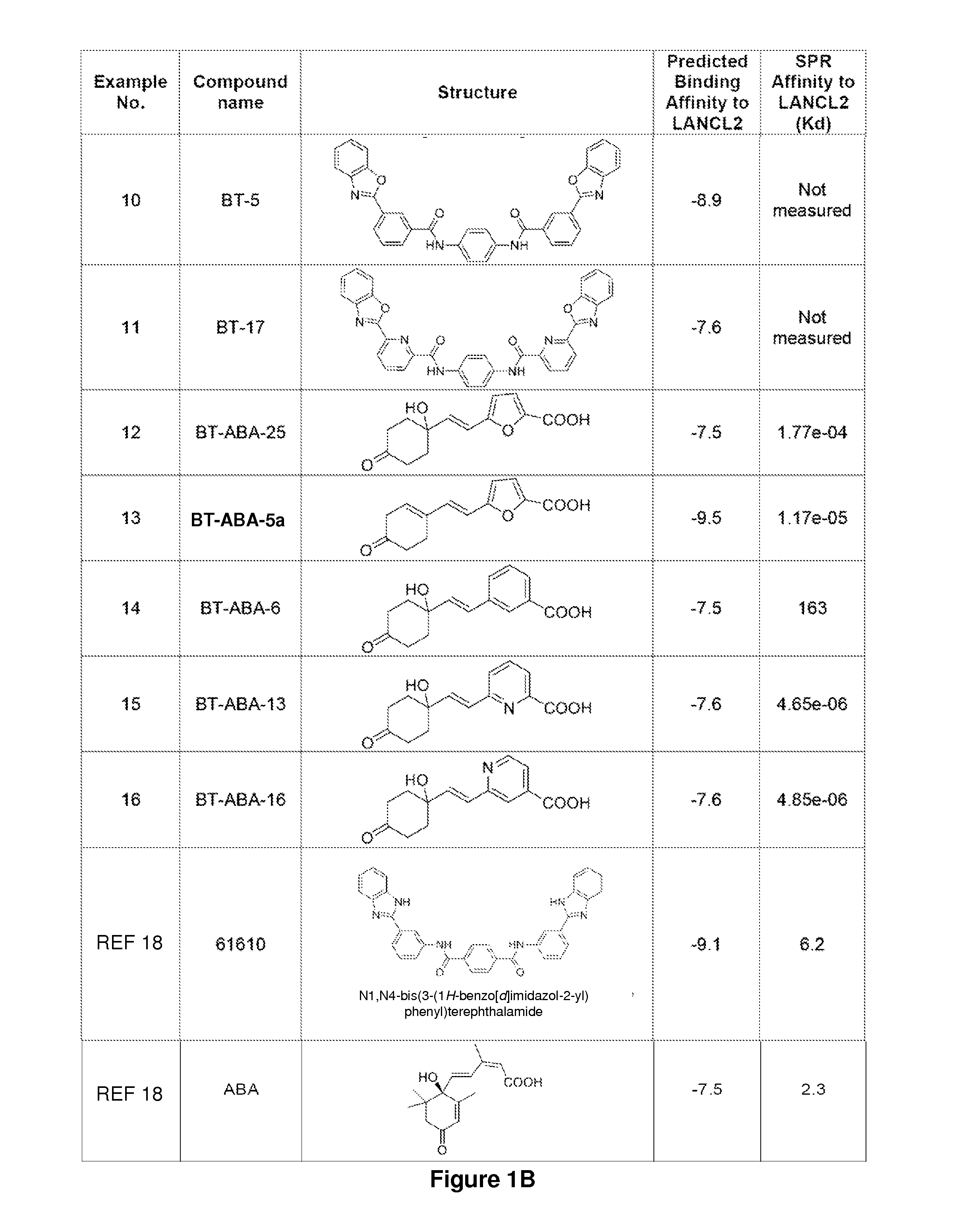
The present invention relates to the field of medical treatments for diseases and disorders. More specifically, the present invention relates to the use of the lanthionine synthetase component C-like (LANCL) proteins as therapeutic targets for novel classes of anti-inflammatory, immune regulatory and antidiabetic drugs. This includes but it is not limited to abscisic acid (ABA), ABA analogs, benzimidazophenyls, repurposed drugs or drug combinations, including thiazolidinediones (TZDs); naturally occurring compounds such as conjugated diene fatty acids, conjugated triene fatty acids, isoprenoids, and natural and synthetic agonists of peroxisome proliferator-activated receptors that activate this receptor through an alternative mechanism of action involving LANCL2 or other membrane proteins to treat or prevent the common inflammatory pathogenesis underlying type 2 diabetes, atherosclerosis, cancer, some inflammatory infectious diseases such as influenza and autoimmune diseases including but not limited to inflammatory bowel disease (Crohn's disease and Ulcerative colitis), rheumatoid arthritis, multiple sclerosis and type 1 diabetes and other chronic inflammatory conditions.
Owner:VIRGINIA TECH INTPROP INC
Lanthionine synthetase C-like 2-based therapeutics
ActiveUS9556146B2High activityReduce inflammationOrganic active ingredientsOrganic chemistryDiabetes mellitusAutoimmune disease
Provided are compounds that target the lanthionine synthetase C-like protein 2 pathway. The compounds can be used to treat a number of conditions, including infectious disease, autoimmune disease, diabetes, and a chronic inflammatory disease.
Owner:NIMMUNE BIOPHARMA INC
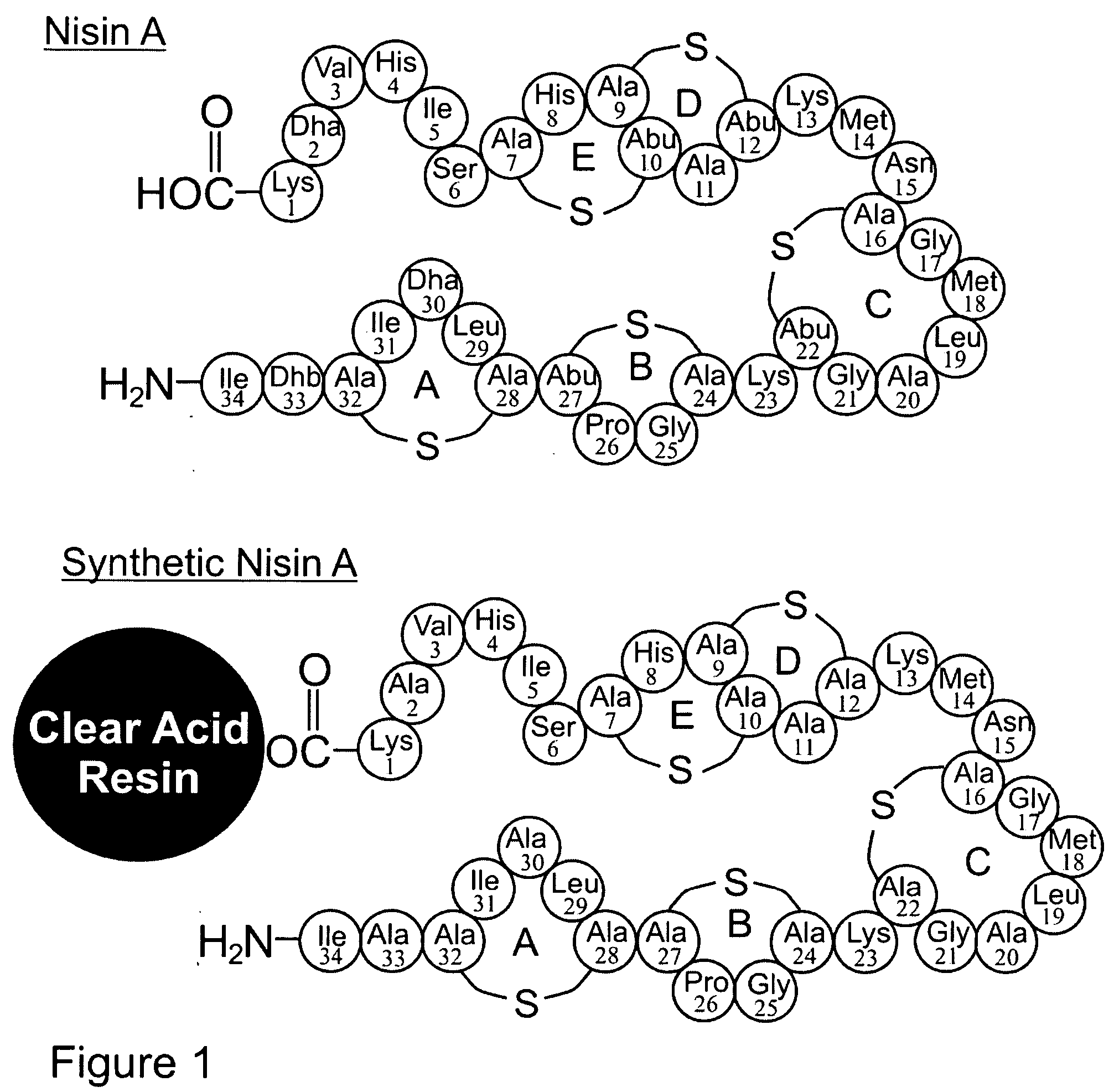
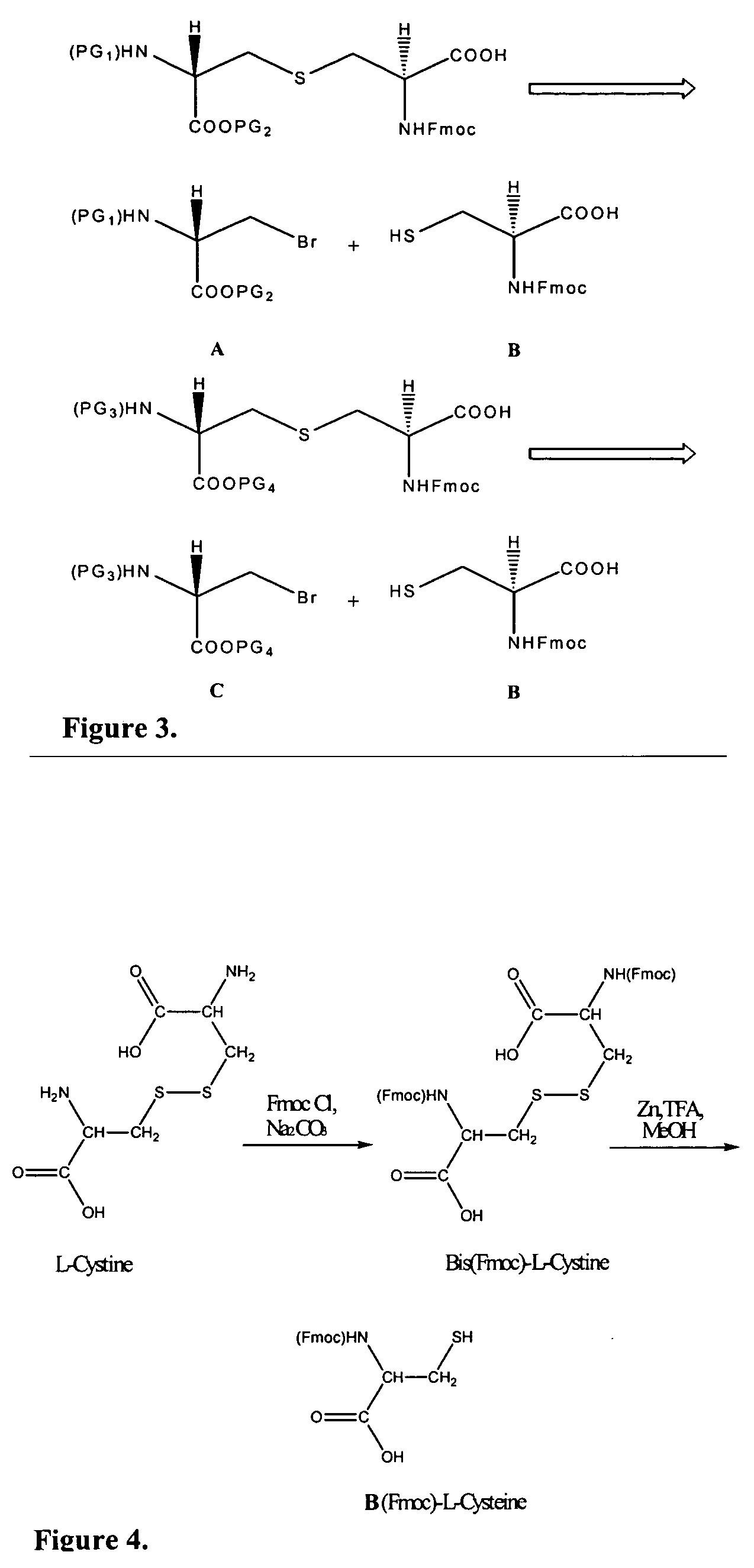
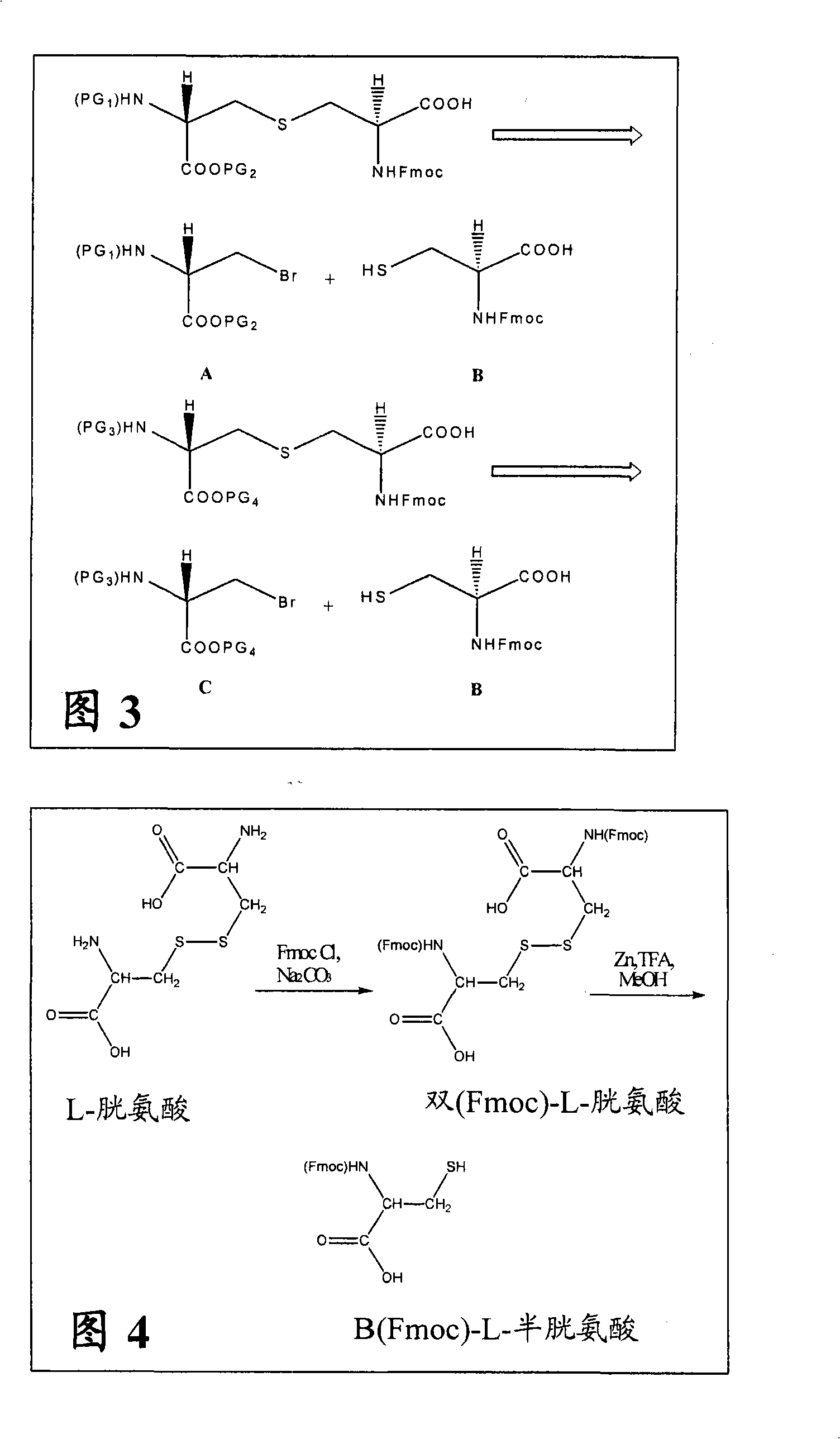
Differentially protected orthogonal lanthionine technology
InactiveUS20090215985A1Antibacterial agentsPeptide/protein ingredientsIntramolecular forceLantibiotics
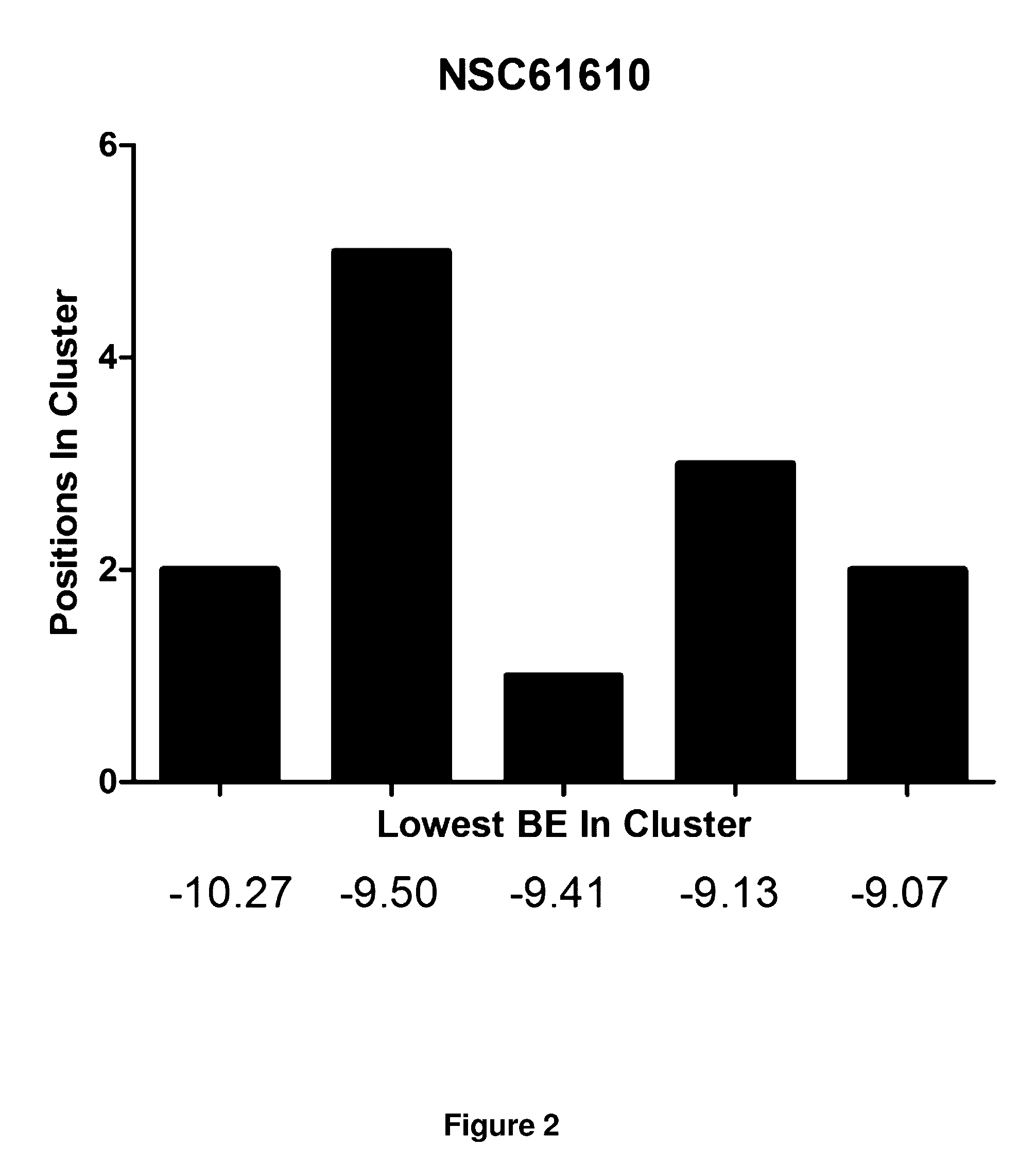
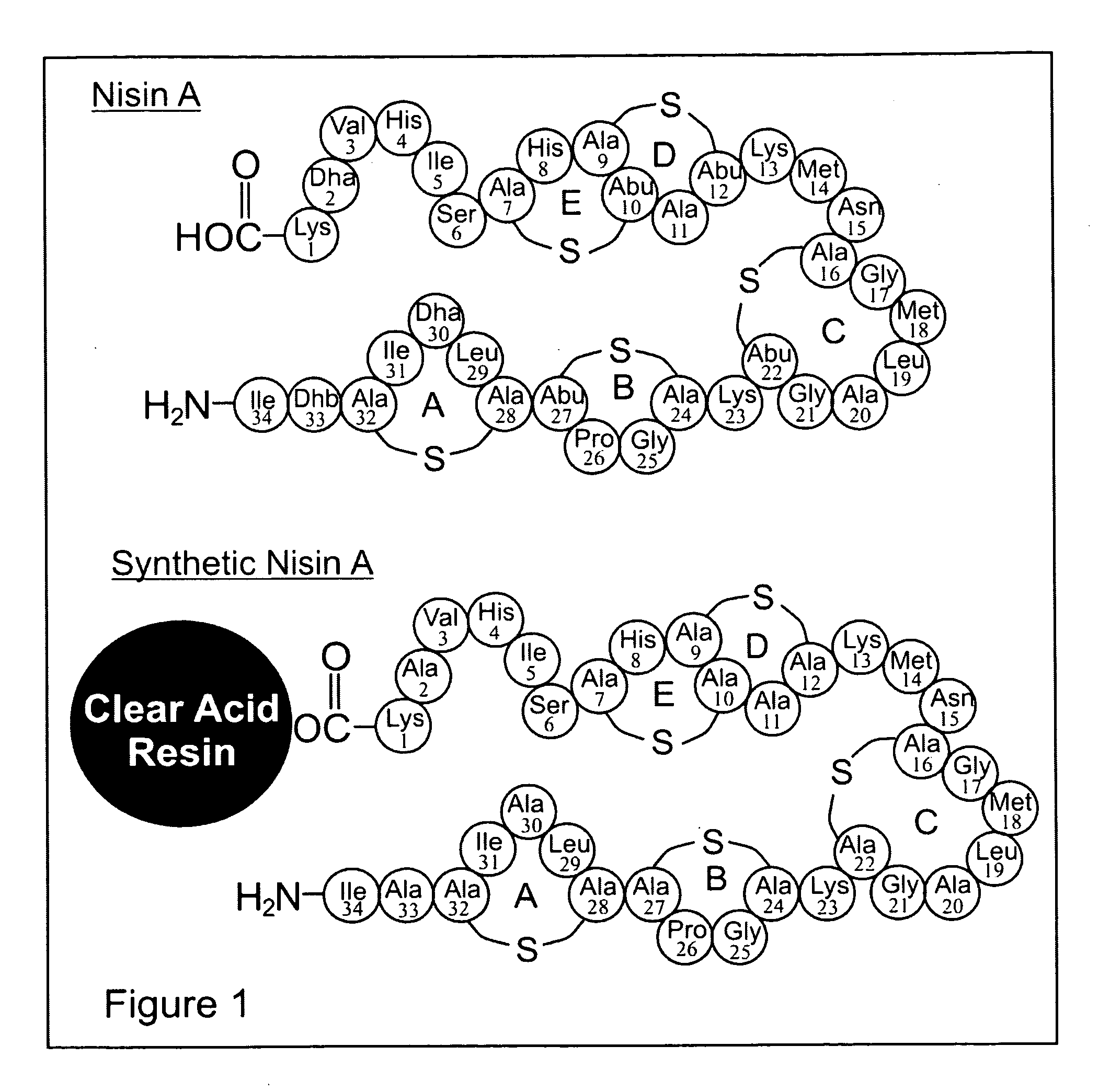
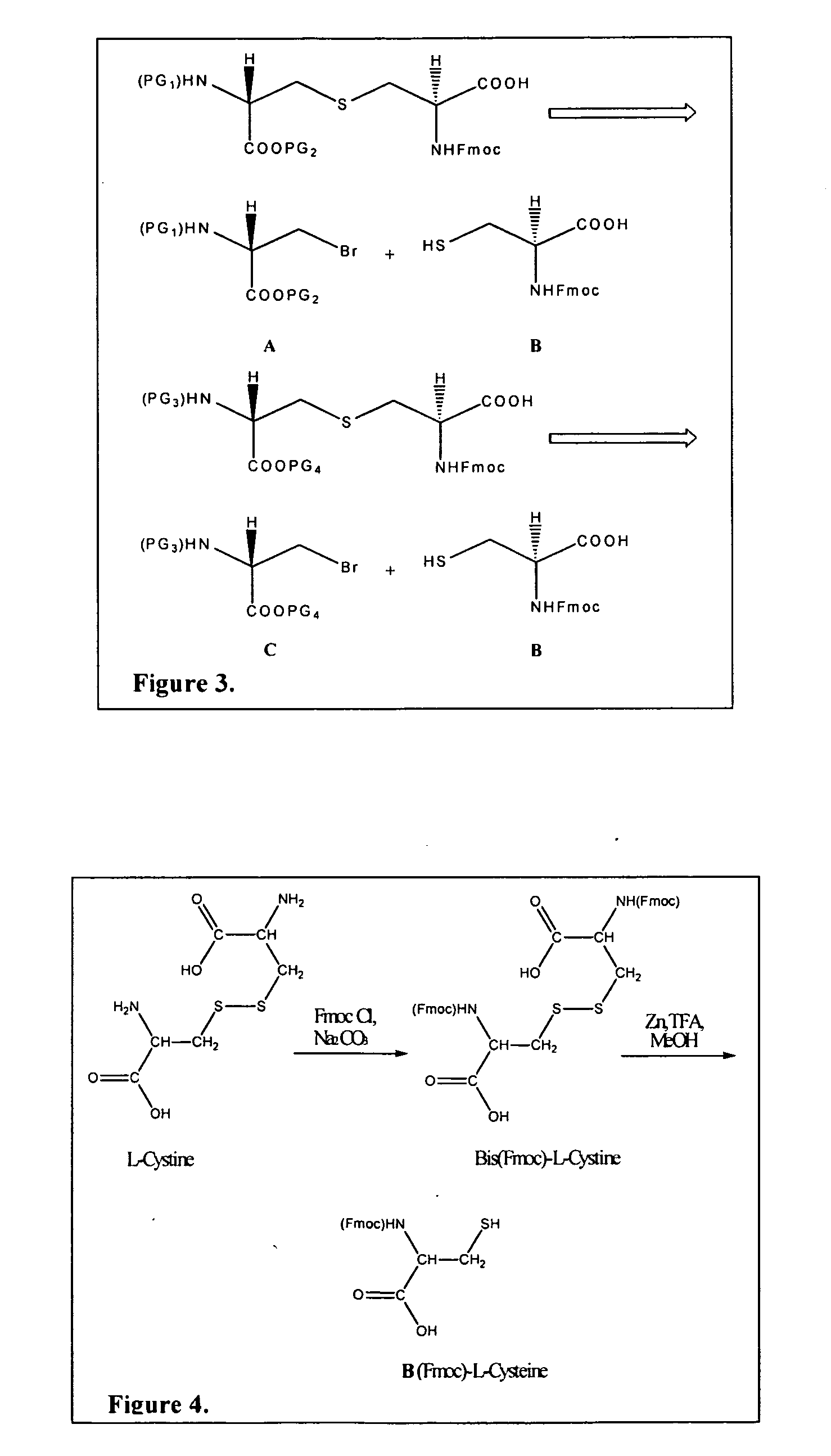
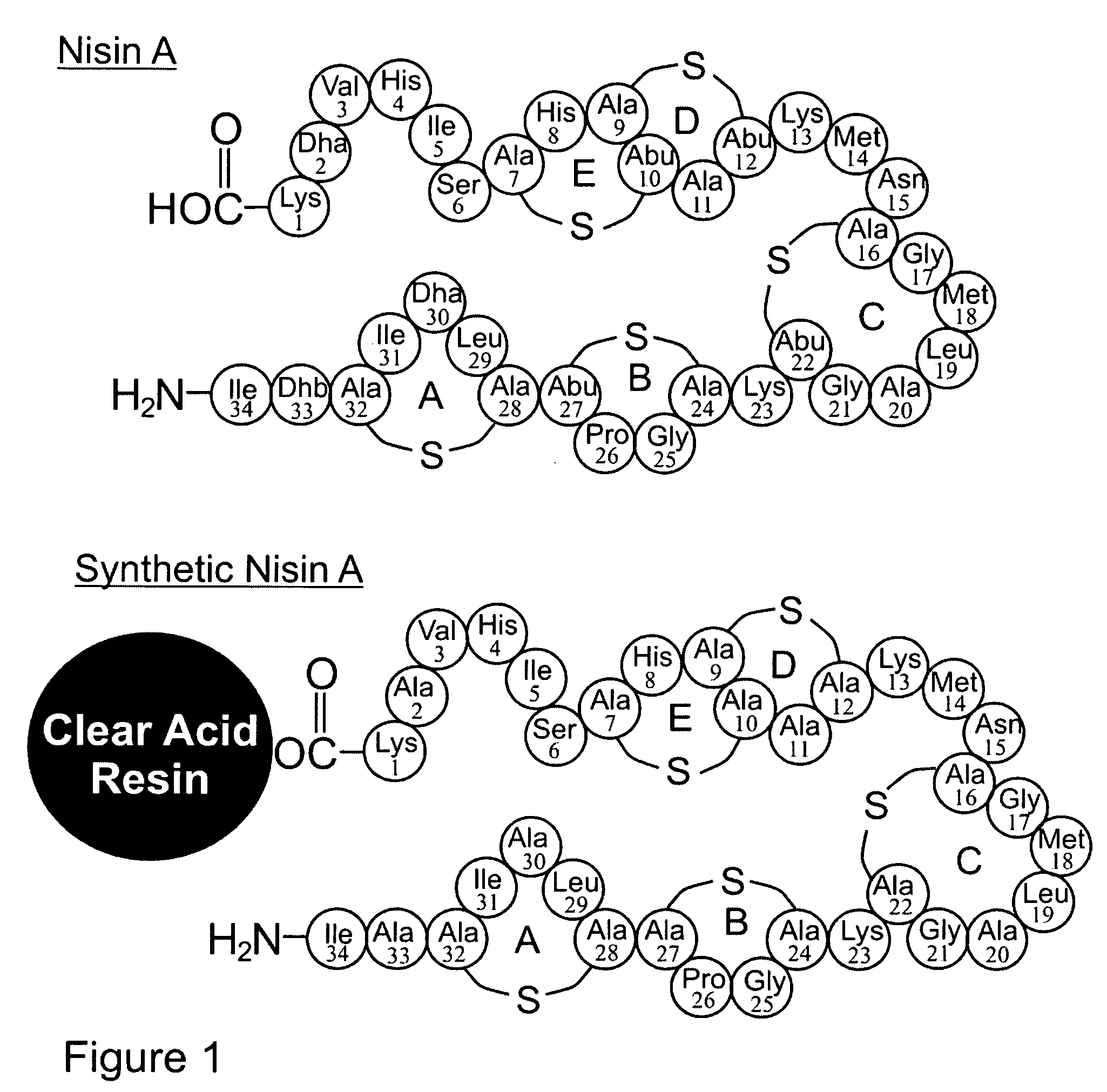
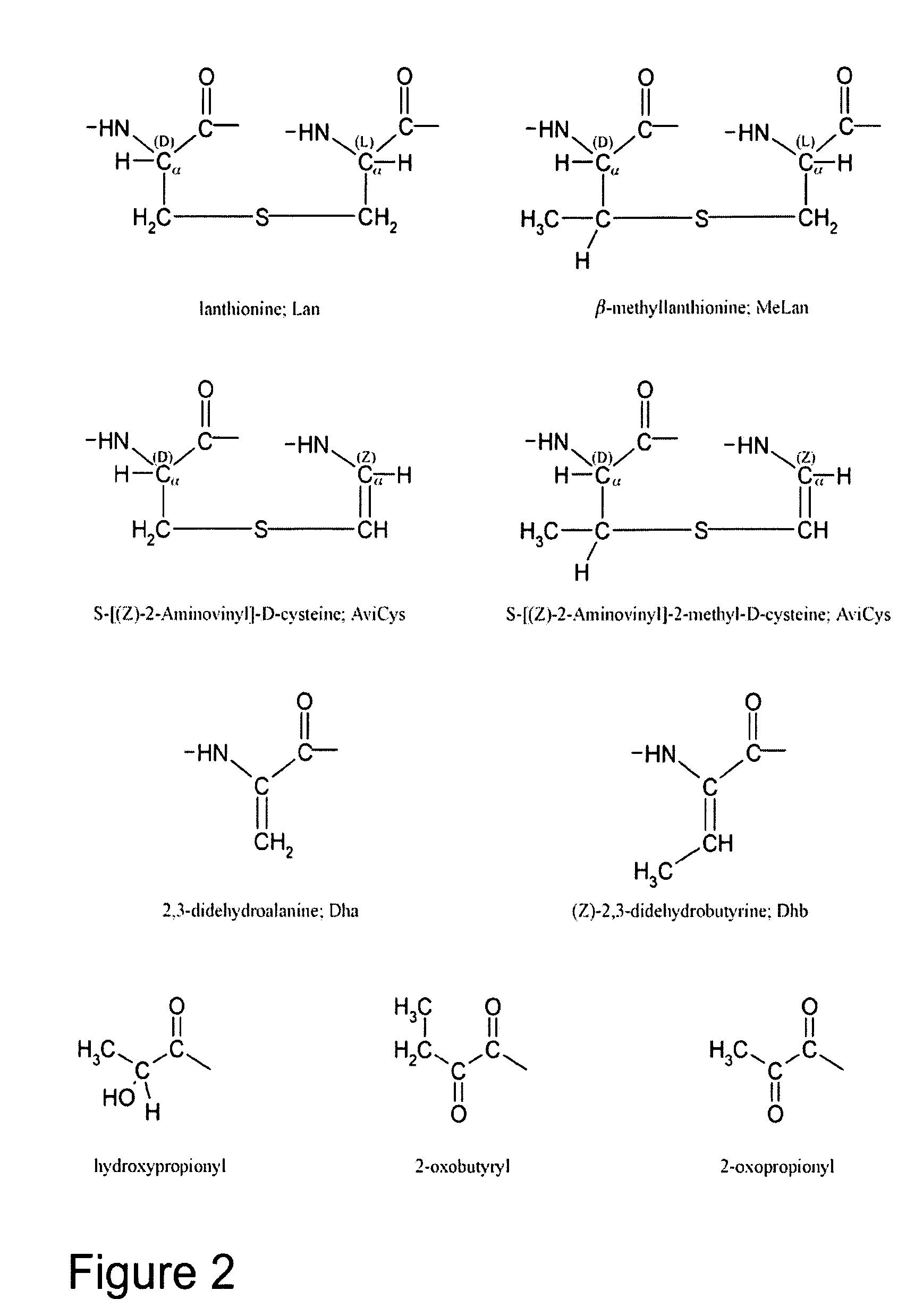
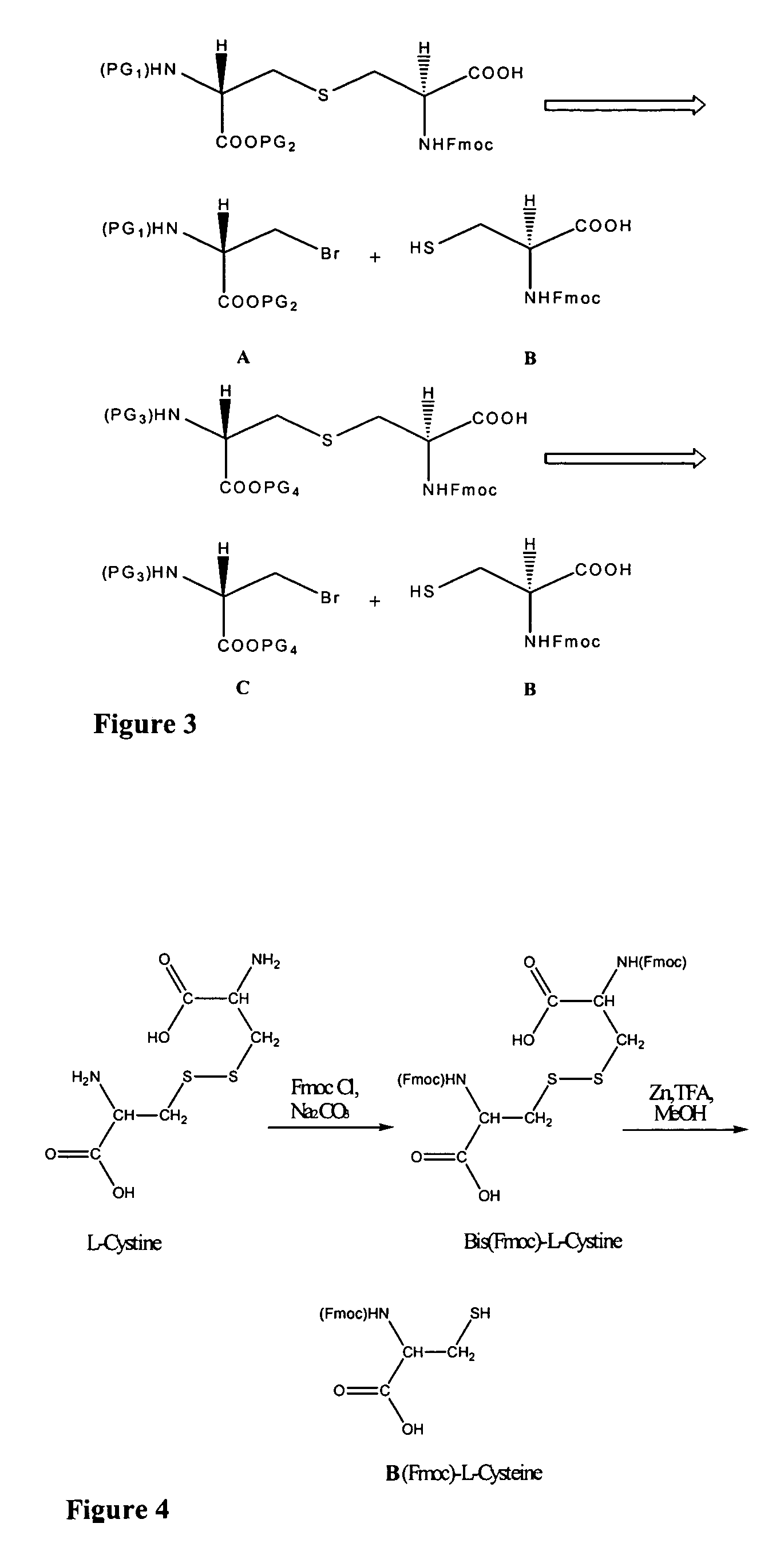
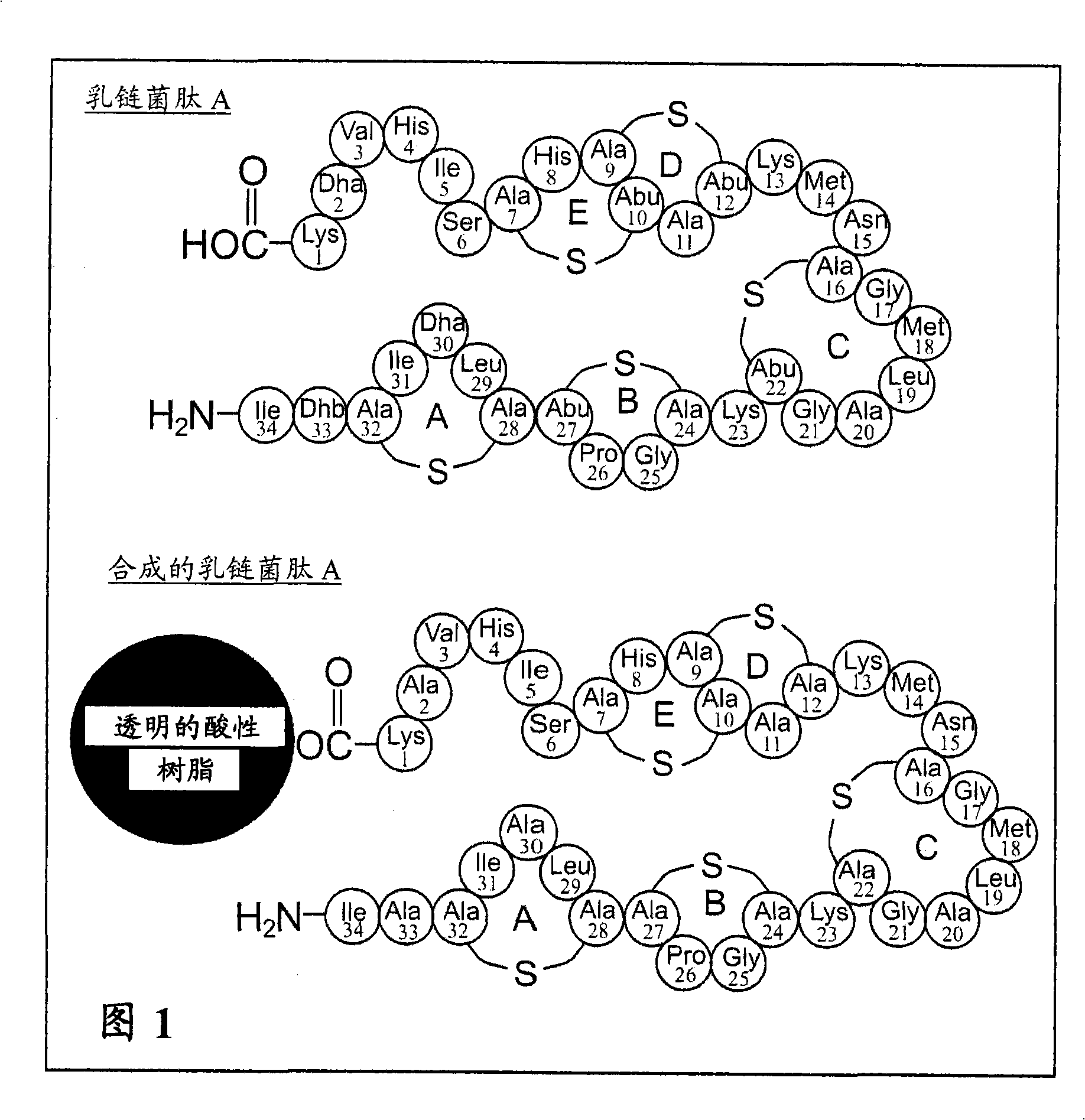
The present invention provides a method of synthesizing an intramolecularly bridged polypeptide comprising at least one intramolecular bridge. The present invention further provides a method of synthesizing an intramolecularly bridged polypeptide comprising two intramolecular bridges, wherein the two intramolecular bridges form two overlapping ring, two rings in series, or two embedded rings. The present invention also provides methods for synthesizing lantibiotics, including Nisin A. Additionally, the invention provides intramolecularly bridged polypeptides synthesized by the methods disclosed herein and differentially protected orthogonal lanthionines.
Owner:ORAGENICS
Lanthionine synthetase component C-like proteins as molecular targets for preventing and treating diseases and disorders
The present invention relates to the field of medical treatments for diseases and disorders. More specifically, the present invention relates to the use of the lanthionine synthetase component C-like (LANCL) proteins as therapeutic targets for novel classes of anti-inflammatory, immune regulatory and antidiabetic drugs. This includes but it is not limited to abscisic acid (ABA), ABA analogs, benzimidazophenyls, repurposed drugs or drug combinations, including thiazolidinediones (TZDs); naturally occurring compounds such as conjugated diene fatty acids, conjugated triene fatty acids, isoprenoids, and natural and synthetic agonists of peroxisome proliferator-activated receptors that activate this receptor through an alternative mechanism of action involving LANCL2 or other membrane proteins to treat or prevent the common inflammatory pathogenesis underlying type 2 diabetes, atherosclerosis, cancer, some inflammatory infectious diseases such as influenza and autoimmune diseases including but not limited to inflammatory bowel disease (Crohn's disease and Ulcerative colitis), rheumatoid arthritis, multiple sclerosis and type 1 diabetes and other chronic inflammatory conditions.
Owner:VIRGINIA TECH INTPROP INC
Differentially protected orthogonal lanthionine technology
The present invention provides a method of synthesizing an intramolecularly bridged polypeptide comprising at least one intramolecular bridge. The present invention further provides a method of synthesizing an intramolecularly bridged polypeptide comprising two intramolecular bridges, wherein the two intramolecular bridges form two overlapping ring, two rings in series, or two embedded rings. The present invention also provides methods for synthesizing lantibiotics, including Nisin A. Additionally, the invention provides intramolecularly bridged polypeptides synthesized by the methods disclosed herein and differentially protected orthogonal lanthionines.
Owner:ORAGENICS
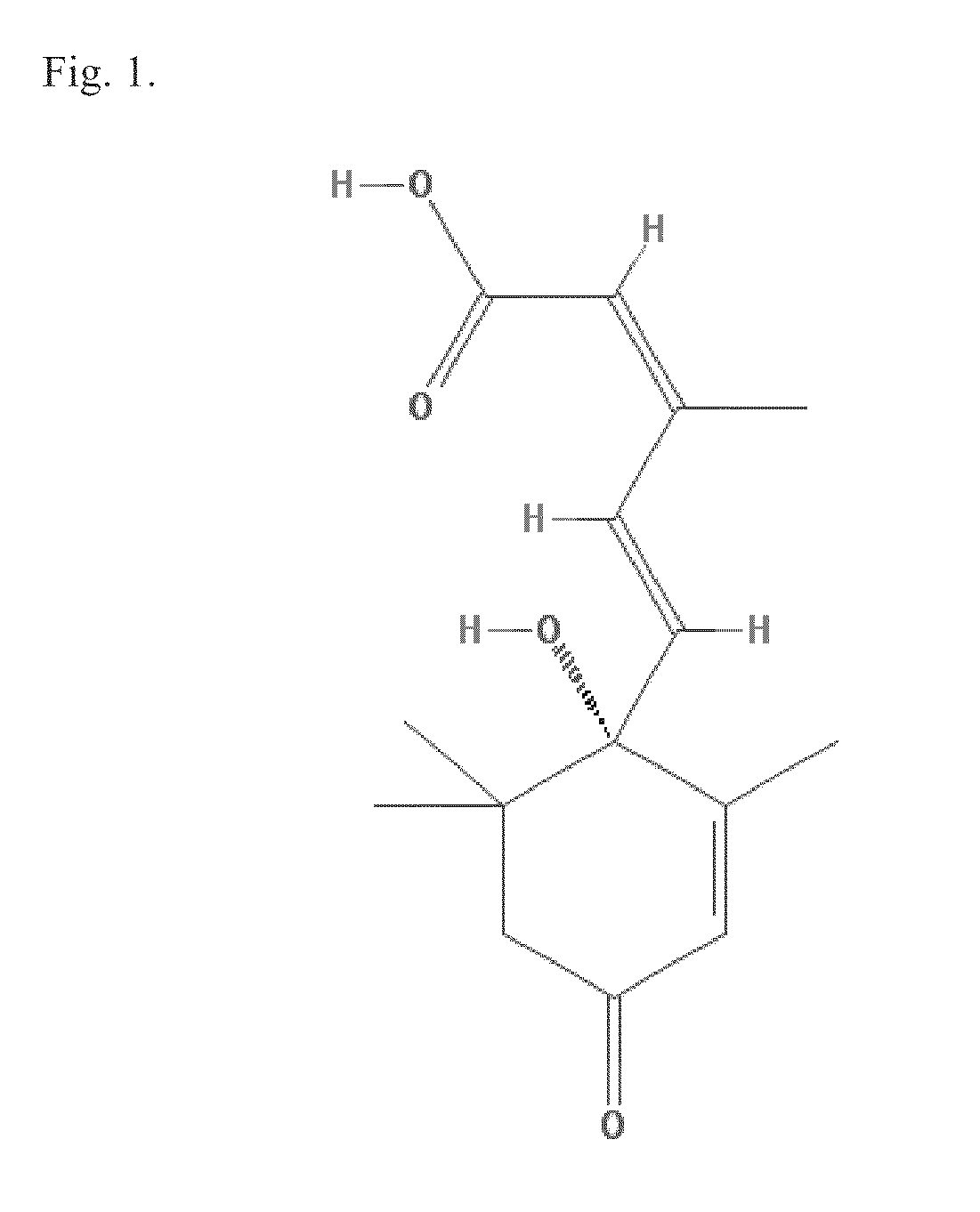
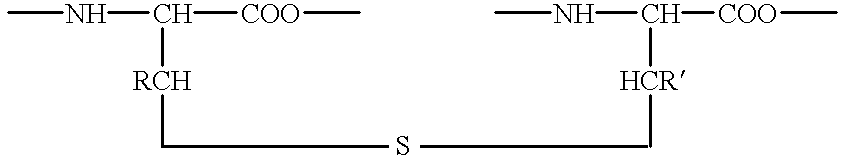
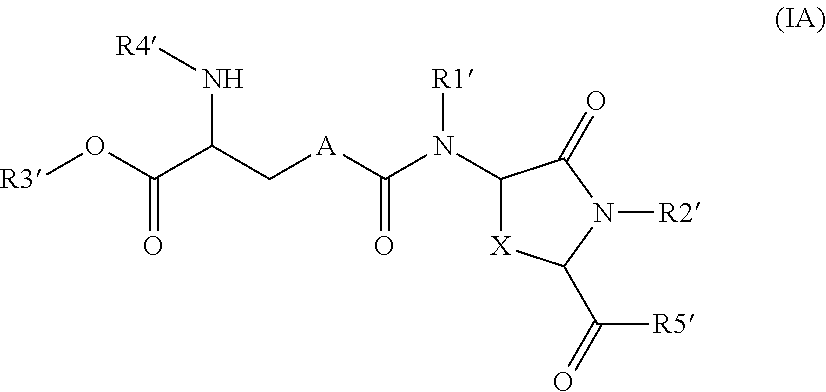
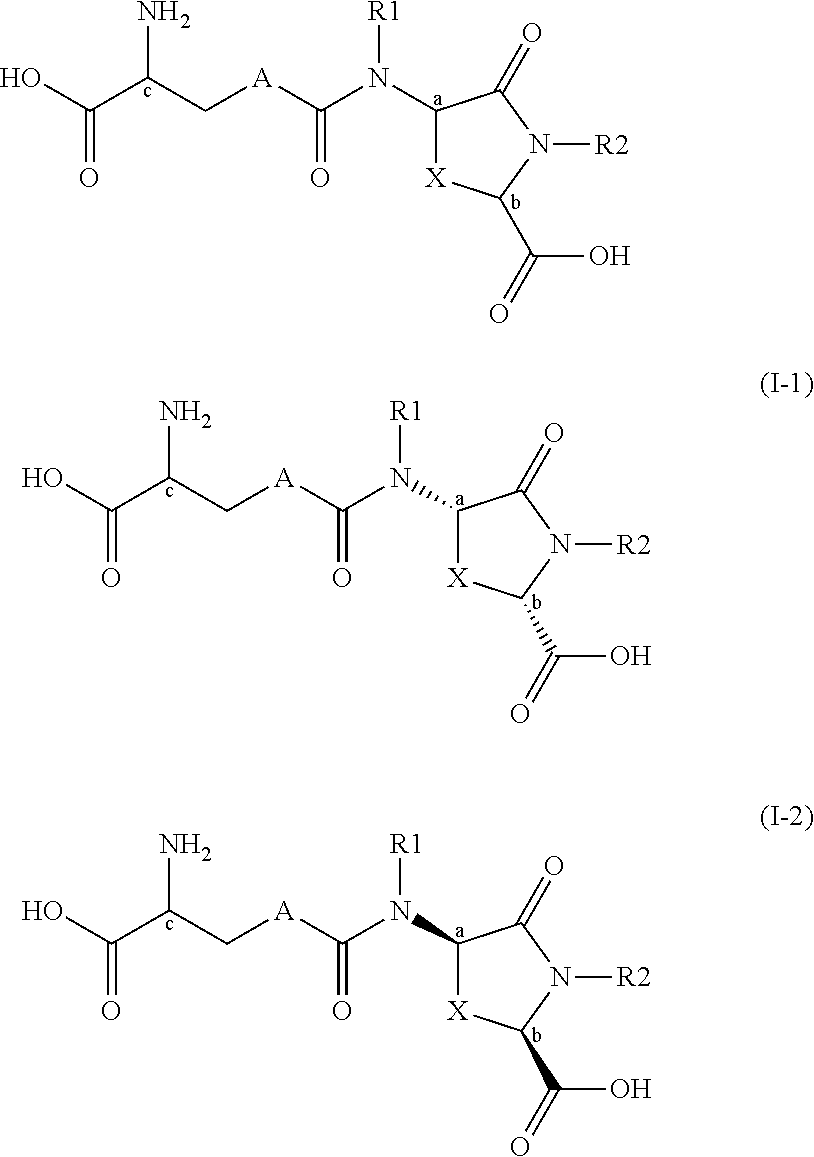
Lanthionine bridged peptides
Disclosed are lanthionine peptides having the structure methods of their preparation and use as pharmacologically active agents.
Owner:WINFRIED KOLBECK
Lanthionine synthetase c-like 2-based therapeutics
ActiveUS20170119762A1High activityReduce inflammationOrganic active ingredientsOrganic chemistryDiabetes mellitusAutoimmune responses
Owner:NIMMUNE BIOPHARMA INC
Differentially protected orthogonal lanthionine technology
The present invention provides a method of synthesizing an intramolecularly bridged polypeptide comprising at least one intramolecular bridge. The present invention further provides a method of synthesizing an intramolecularly bridged polypeptide comprising two intramolecular bridges, wherein the two intramolecular bridges form two overlapping ring, two rings in series, or two embedded rings. The present invention also provides methods for synthesizing lantibiotics, including Nisin A. Additionally, the invention provides intramolecularly bridged polypeptides synthesized by the methods disclosed herein and differentially protected orthogonal lanthionines.
Owner:ORAGENICS
Lanthionine bridged peptides
Disclosed are lanthionine bridged peptides having the structuremethods of their preparation and their use as pharmacologically active agents.
Owner:KOLBECK WINFRIED
Lanthionine-related compounds for the treatment of inflammatory diseases
InactiveUS7683055B2Reduce deliveryOvercome limitationsAntibacterial agentsSenses disorderDiseaseNervous system
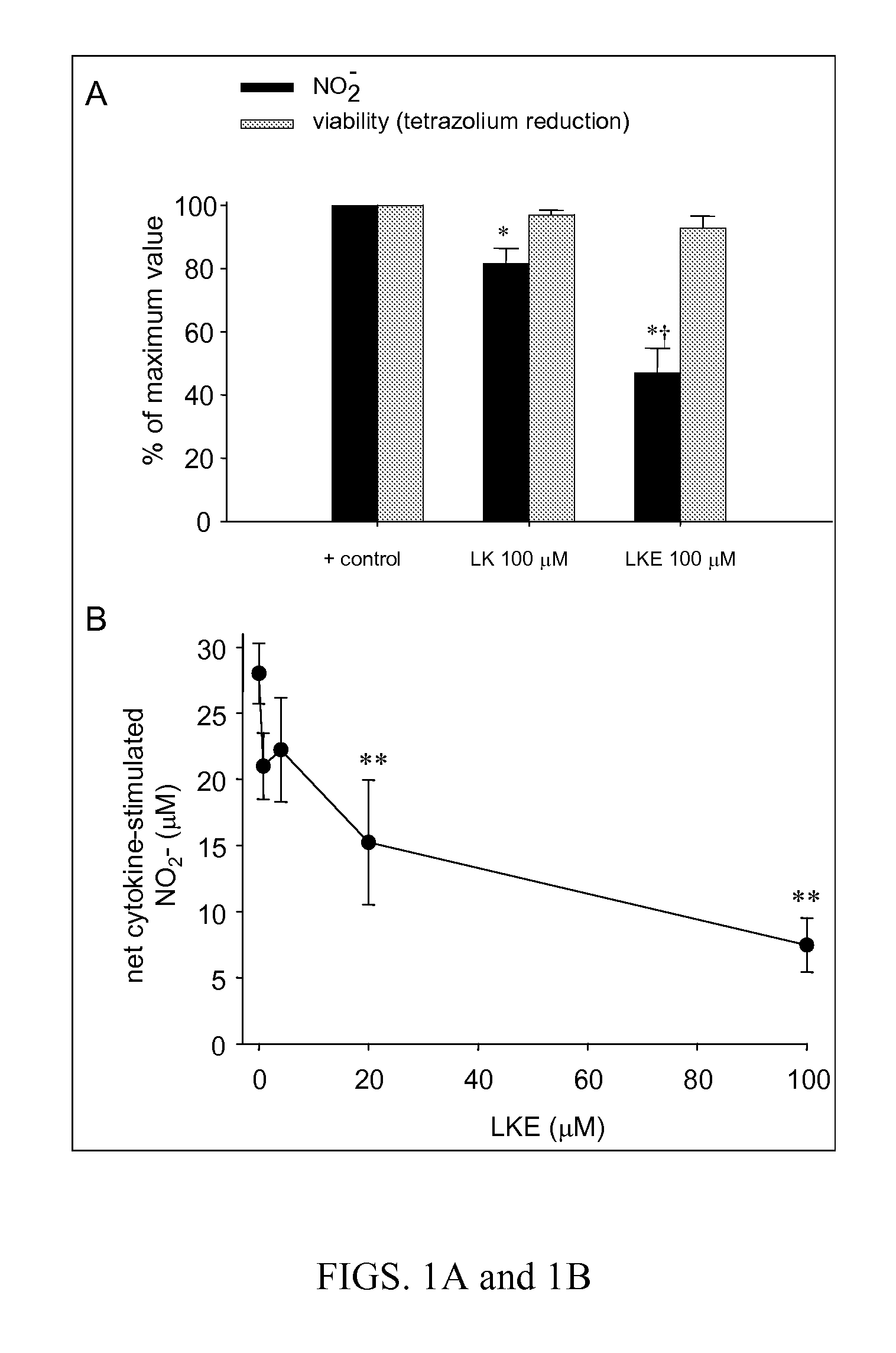
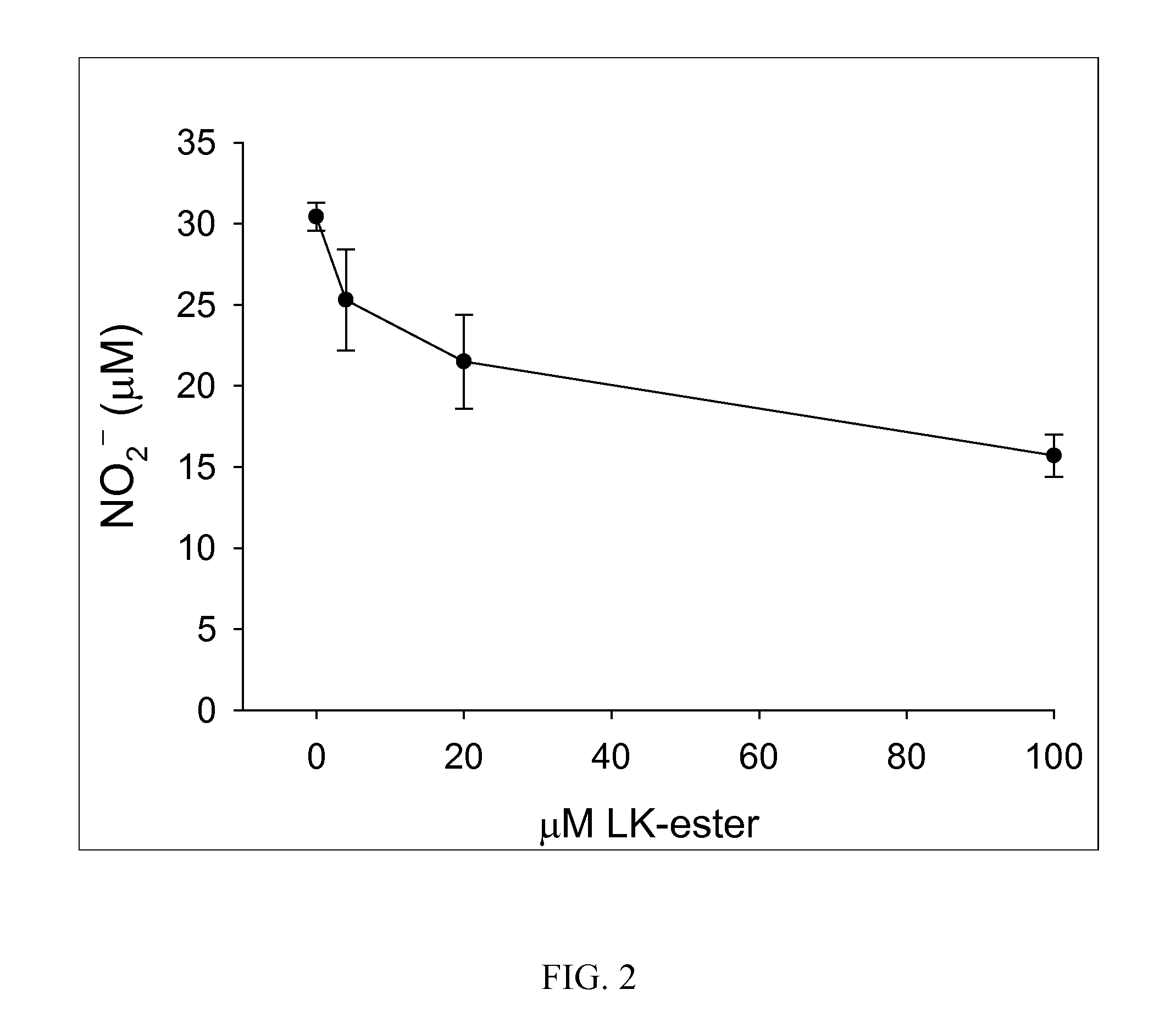
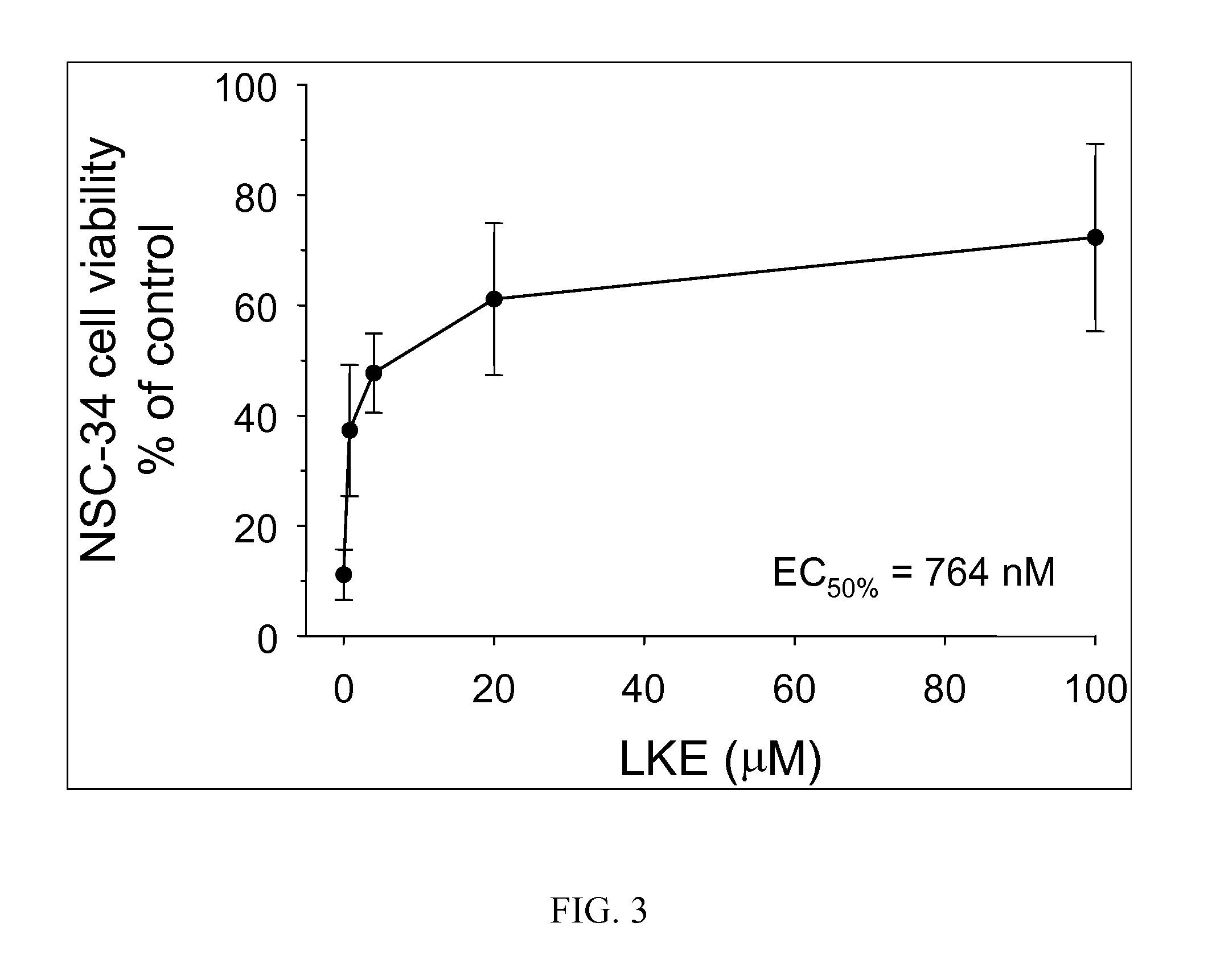
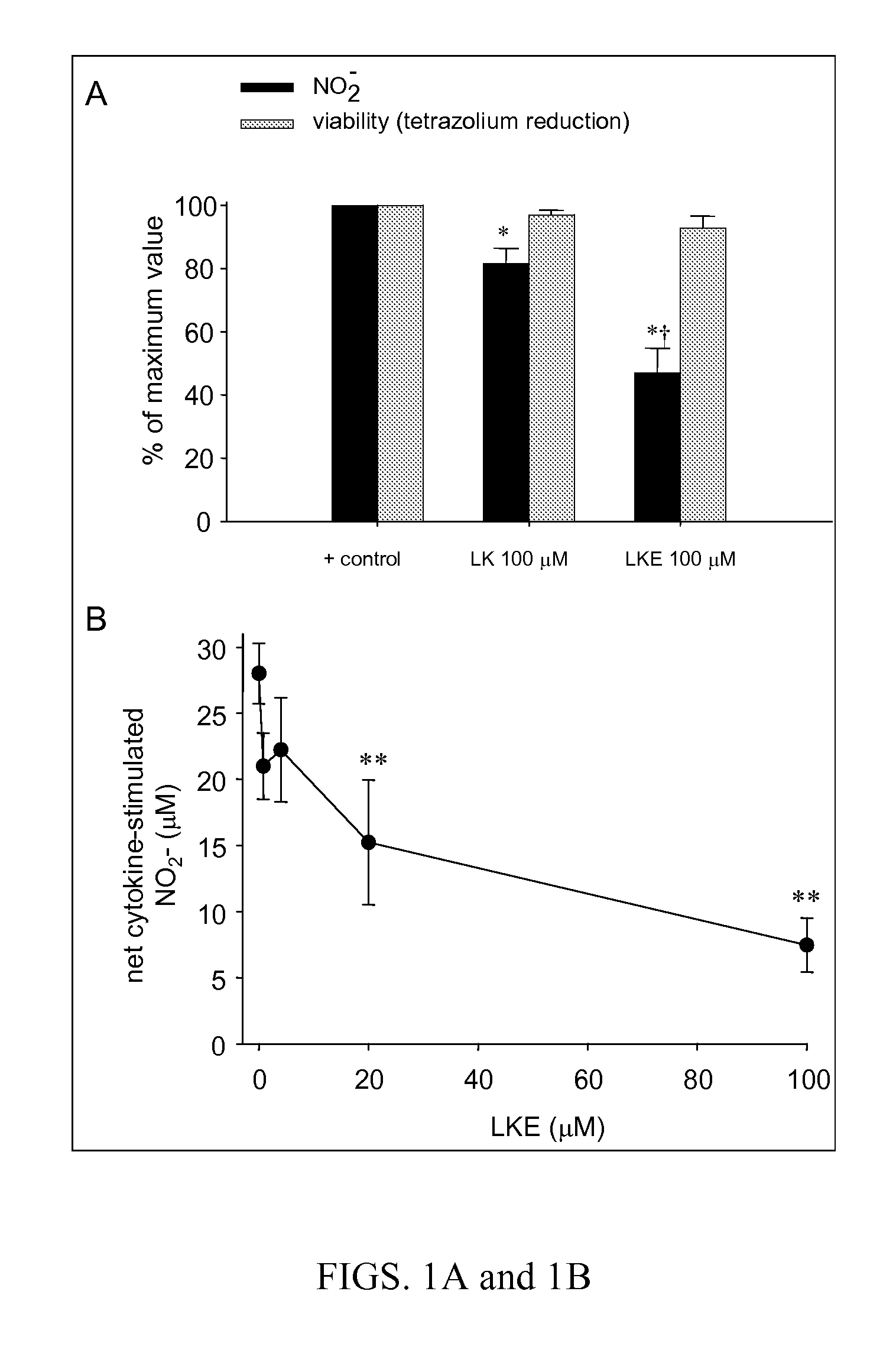
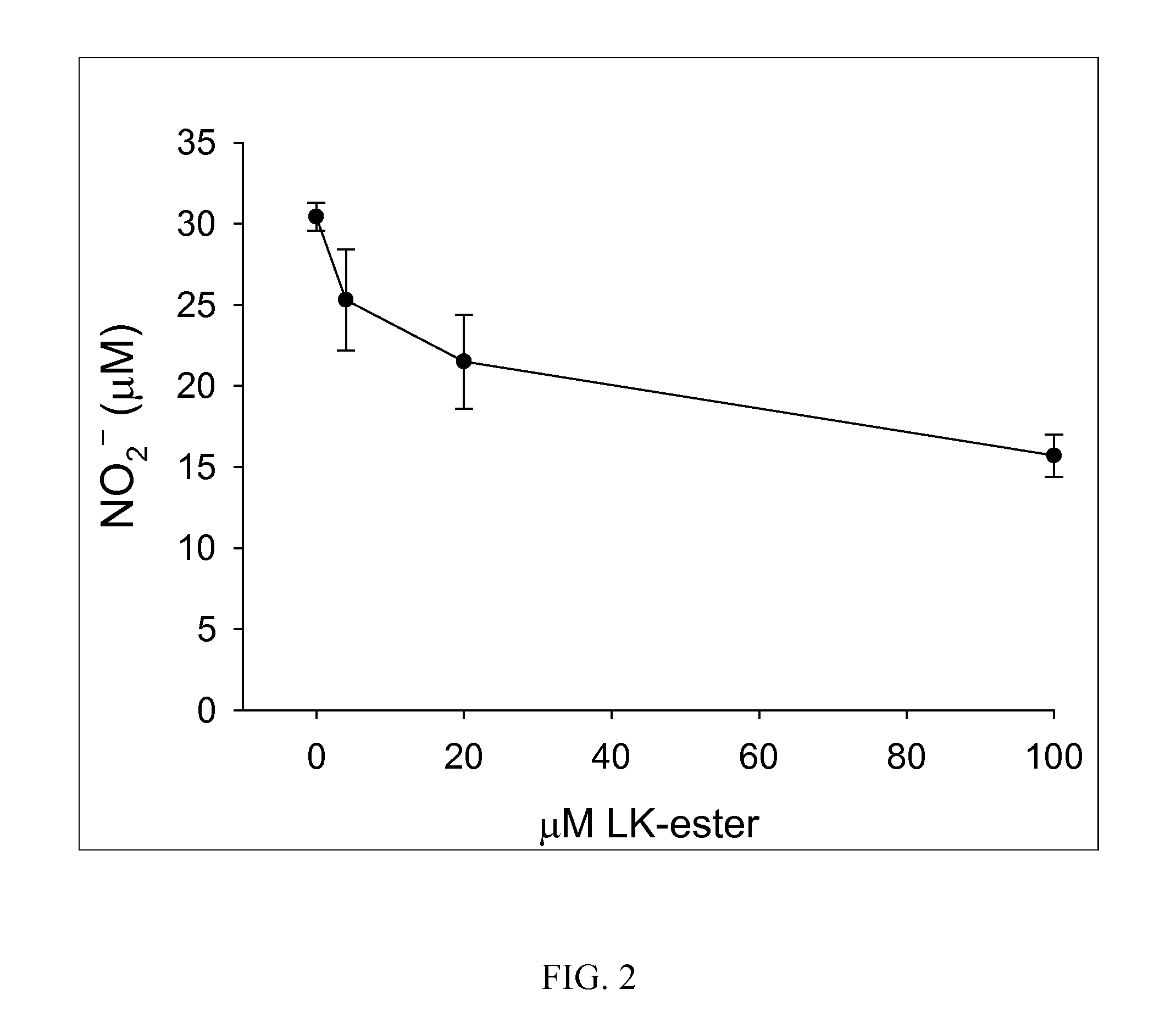
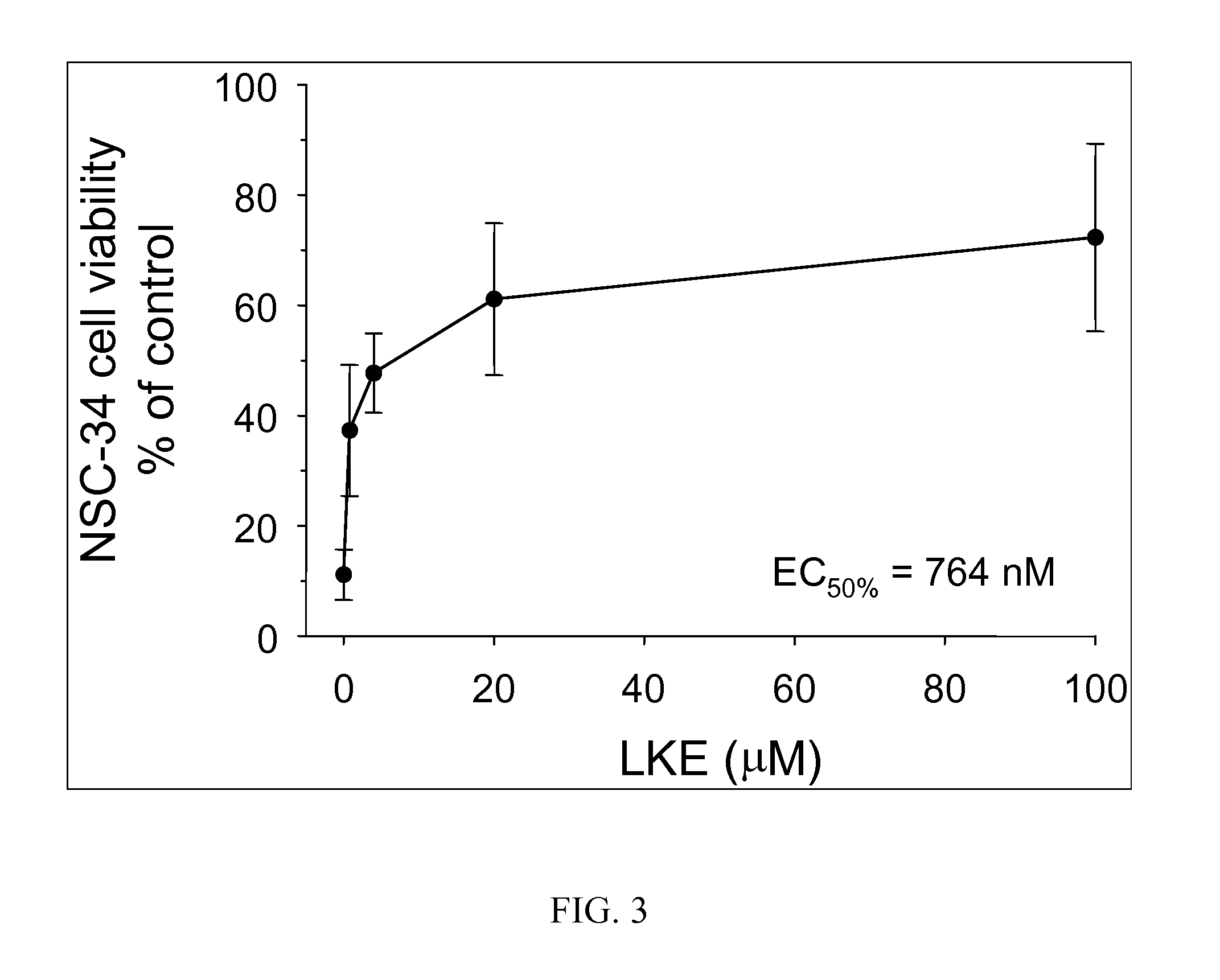
The present invention provides compositions comprising lanthionine ketimine derivatives and thiomorpholine dicarboxylic acid derivatives, as well as processes for the preparation of such compounds. The invention also concerns the use of lanthionine, lanthionine ketimine (LK), LK derivatives, thiomorpholine dicarboxylic acid (TMDCA), and TMDCA derivatives. It concerns the use of these compounds for the treatment and / or prevention diseases, including diseases affecting the central nervous system. The invention provides for compounds and methods having anti-oxidant, anti-neuroinflammatory and neuroprotective activities. It also provides for compounds having the ability to pass through and / or be transported through cellular membranes, such the blood-brain barrier.
Owner:OKLAHOMA MEDICAL RES FOUND
Lanthionine-related compounds for the treatment of inflammatory diseases
InactiveUS20070197515A1Reduce deliveryOvercome limitationsAntibacterial agentsSenses disorderNervous systemAnti oxidant
The present invention provides compositions comprising lanthionine ketimine derivatives and thiomorpholine dicarboxylic acid derivatives, as well as processes for the preparation of such compounds. The invention also concerns the use of lanthionine, lanthionine ketimine (LK), LK derivatives, thiomorpholine dicarboxylic acid (TMDCA), and TMDCA derivatives. It concerns the use of these compounds for the treatment and / or prevention diseases, including diseases affecting the central nervous system. The invention provides for compounds and methods having anti-oxidant, anti-neuroinflammatory and neuroprotective activities. It also provides for compounds having the ability to pass through and / or be transported through cellular membranes, such the blood-brain barrier.
Owner:OKLAHOMA MEDICAL RES FOUND
Lanthionine synthetase c-like 2-based therapeutics
ActiveUS20160115153A1High activityReduce inflammationBiocideOrganic chemistryDiabetes mellitusAutoimmune disease
Provided are compounds that target the lanthionine synthetase C-like protein 2 pathway. The compounds can be used to treat a number of conditions, including infectious disease, autoimmune disease, diabetes, and a chronic inflammatory disease.
Owner:NIMMUNE BIOPHARMA INC
Lanthionizing compositions, systems, and methods
InactiveUS6391293B1High tensile strengthReduced malto-oligosaccharideCosmetic preparationsHair removalLanthionineOligosaccharide
Disclosed is a lanthionizing composition that comprises a lanthionizing agent, such as guanidine hydroxide or sodium hydroxide, and a reduced malto-oligosaccharide. Also disclosed is a method for lanthionization, the method comprising applying to the hair the lanthionizing composition of the invention.
Owner:GRAIN PROCESSING CORP
Method for Producing Lanthionine Derivative
InactiveUS20140120233A1High CaSR agonist activityExcellent kokumi-imparting effectOrganic compound preparationDrug compositionsAgonistLanthionine
The present invention relates to a method for producing a novel lanthionine derivative having a CaSR agonist activity, an intermediate compound of the lanthionine derivative, and use of the intermediate compound for production of a CaSR agonist, a kokumi-imparting agent, and a food and / or beverage ingredient.
Owner:AJINOMOTO CO INC
Differentially protected orthogonal lanthionine technology
The present invention provides a method of synthesizing an intramolecularly bridged polypeptide comprising at least one intramolecular bridge. The present invention further provides a method of synthesizing an intramolecularly bridged polypeptide comprising two intramolecular bridges, wherein the two intramolecular bridges form two overlapping ring, two rings in series, or two embedded rings. The present invention also provides methods for synthesizing lantibiotics, including Nisin A. Additionally, the invention provides intramolecularly bridged polypeptides synthesized by the methods disclosed herein and differentially protected orthogonal lanthionines.
Owner:ORAGENICS
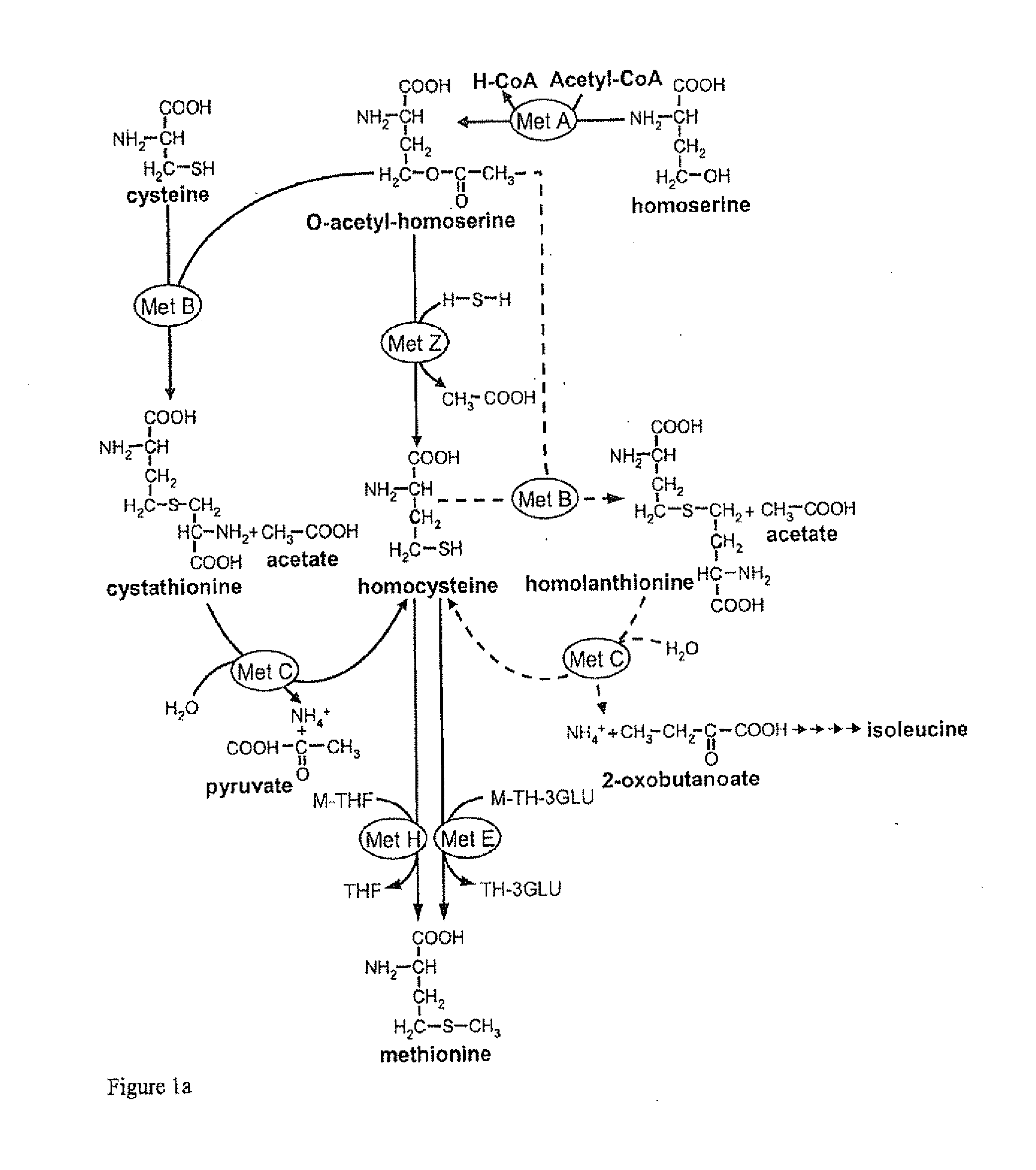
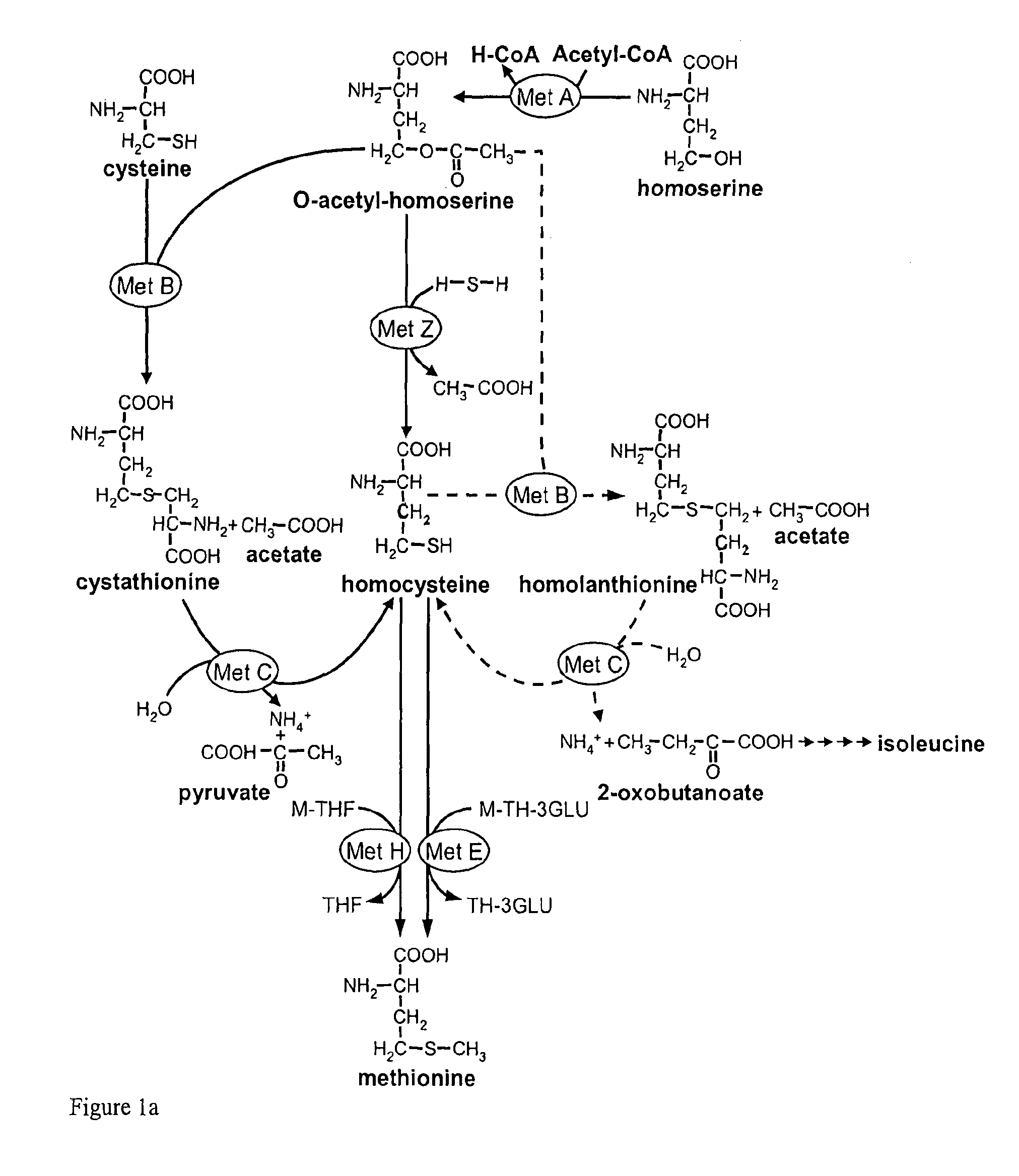
Microorganism and process for the preparation of l-methionine
InactiveUS20090298137A1Efficient production of L-methionineEfficient productionBacteriaAnimal feeding stuffMethionine biosynthesisLanthionine
The present invention relates to microorganisms and processes for the efficient preparation of L-amino acids such as L-methionine. In particular, the present invention relates to microorganisms and processes in which the formation and / or accumulation of homolanthionine in the methionine pathway is reduced and / or prevented.
Owner:EVONIK DEGUSSA GMBH
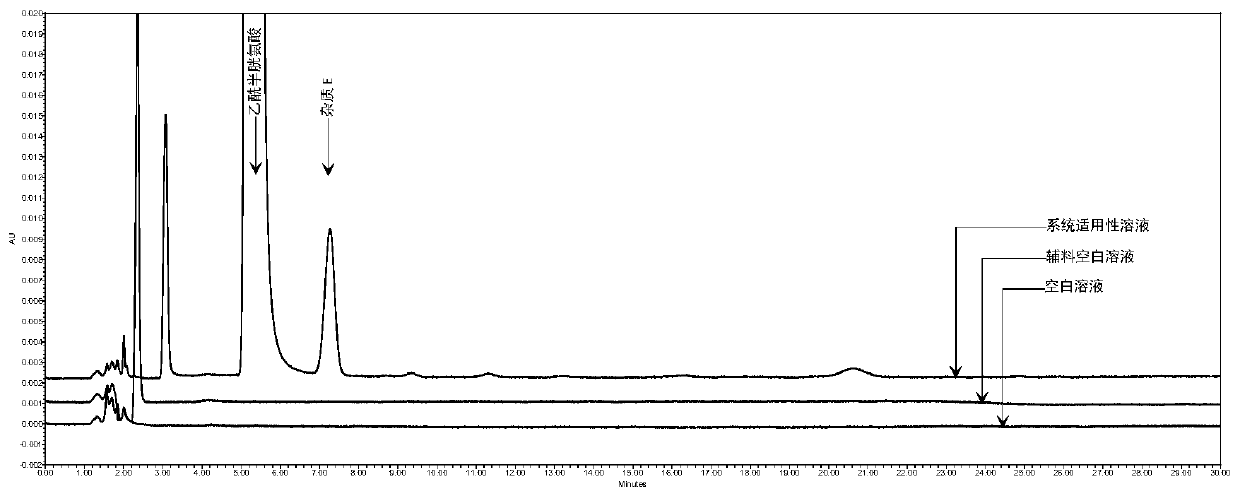
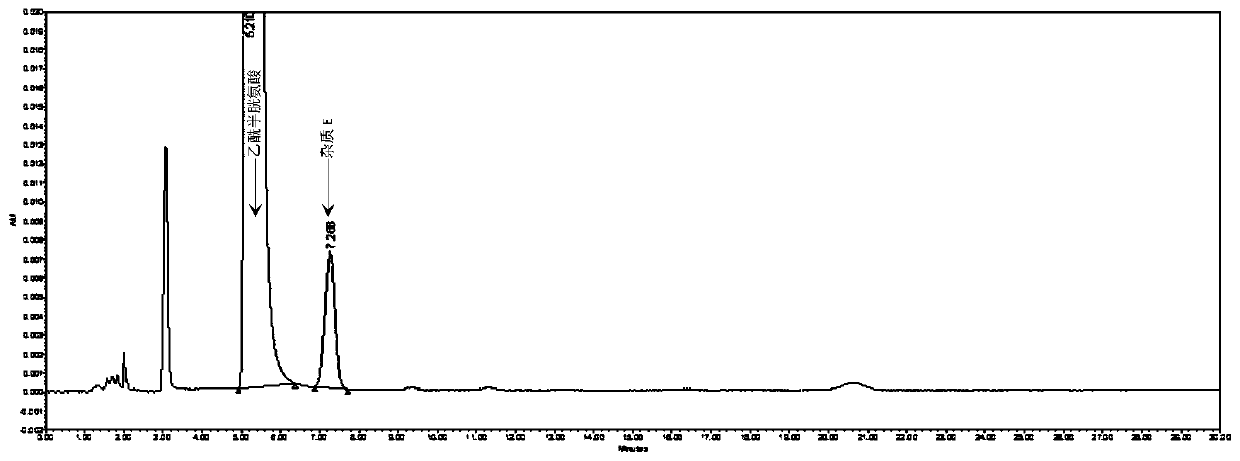
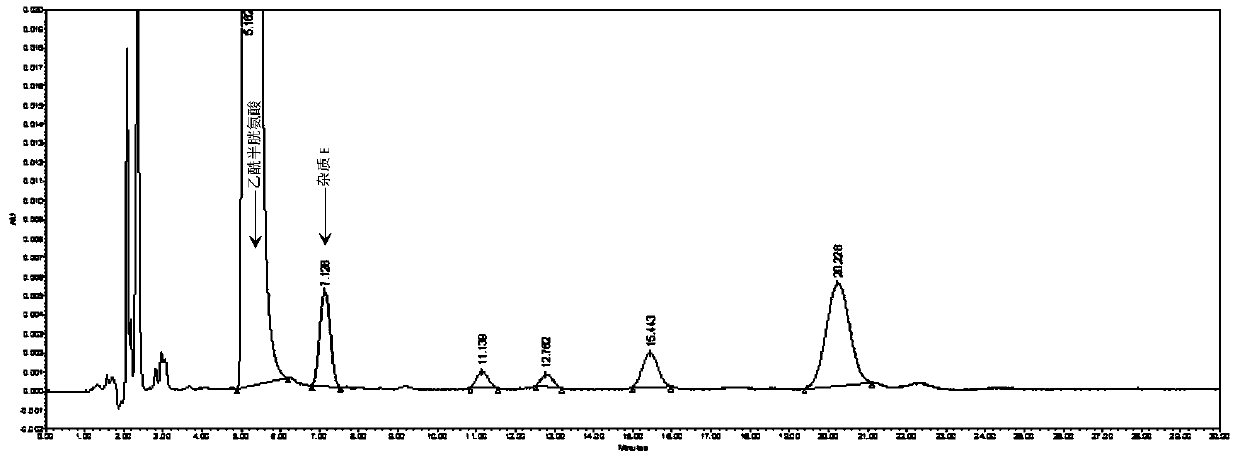
Method for detecting N,N-diacetyl lanthionine in acetylcysteine solution
The invention discloses a method for detecting N,N-diacetyl lanthionine in an acetylcysteine solution, comprising the steps of: respectively using a mobile phase to dilute the acetylcysteine solutionto be tested and dissolving an N,N-diacetyl lanthionine reference substance to obtain a test solution and a reference substance solution, respectively performing high performance liquid chromatographydetection, and calculating the content of the N,N-diacetyl lanthionine according to the detection result, wherein chromatographic conditions are as follows: chromatographic column: octadecyl silane bonded silica gel column, detection wavelength: 205 + / - 2 nm, and mobile phase: 6.8 g / L of phosphate buffer-methanol solution with a volume ratio of 97.4%: 2.6% to 98.6%: 1.4%. By adopting the method disclosed by the invention, the N,N-diacetyl lanthionine in the acetylcysteine solution can be effectively detected, furthermore, there is no interference, and the specificity is high; the detection limit and the quantification limit are respectively 8 ng and 32 ng, and quantitative detection can be realized; detection accuracy is relatively good; and the application prospect is broad.
Owner:武汉兴华智慧医药科技有限公司
Microorganism and process for the preparation of L-methionine
InactiveUS8148117B2Efficient production of L-methionineEfficient productionBacteriaAnimal feeding stuffMicroorganismMethionine biosynthesis
Owner:EVONIK DEGUSSA GMBH
Lanthionine c-like protein 2 ligands, cells prepared therewith, and therapies using same
ActiveUS11117881B2Organic active ingredientsOrganic chemistryAutoimmune conditionInfectious Disorder
Provided are compounds that target the lanthionine synthetase C-like protein 2 pathway. The compounds can be used to treat a number of conditions, including autoimmune diseases, inflammatory diseases, chronic inflammatory diseases, diabetes, and infectious diseases, such as lupus, Sjögren's syndrome, rheumatoid arthritis, type 1 diabetes, inflammatory bowel disease, viral diseases, and nonalcoholic steatohepatitis. The compounds can also be used to generate cells, such as immune cells, for treating the conditions.
Owner:NIMMUNE BIOPHARMA INC
Cyclic galanin analogs and uses thereof
ActiveCN107531803BPolypeptide with localisation/targeting motifHormone peptidesCyclic peptidePharmaceutical medicine
Owner:LANTHIO PEP
Lanthionine derivatives
ActiveUS8568814B2High activityFunction increaseMilk preparationOrganic active ingredientsLanthionineChemistry
The present invention provides a variety of compounds having a CaSR agonist activity which possesses a superior kokumi-imparting function, and more particularly provides a kokumi-imparting composition, which contains the foregoing compound, and / or another substance having a CaSR agonist activity, in combination. The present invention also provides a kokumi-imparting composition which includes a lanthionine derivative and / or another substance having a CaSR agonist activity.
Owner:AJINOMOTO CO INC
Hair straightening composition
Owner:UNILEVER NV
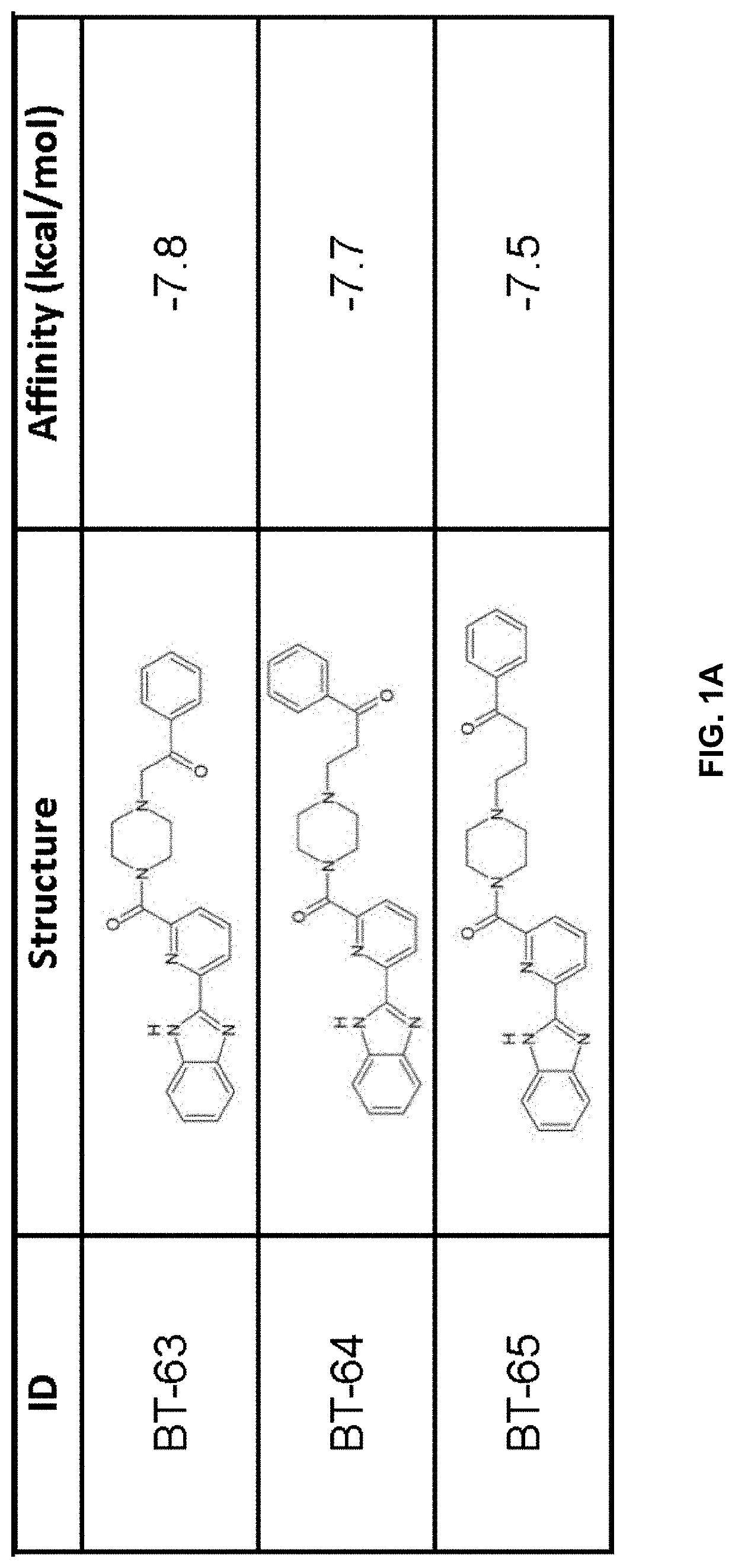
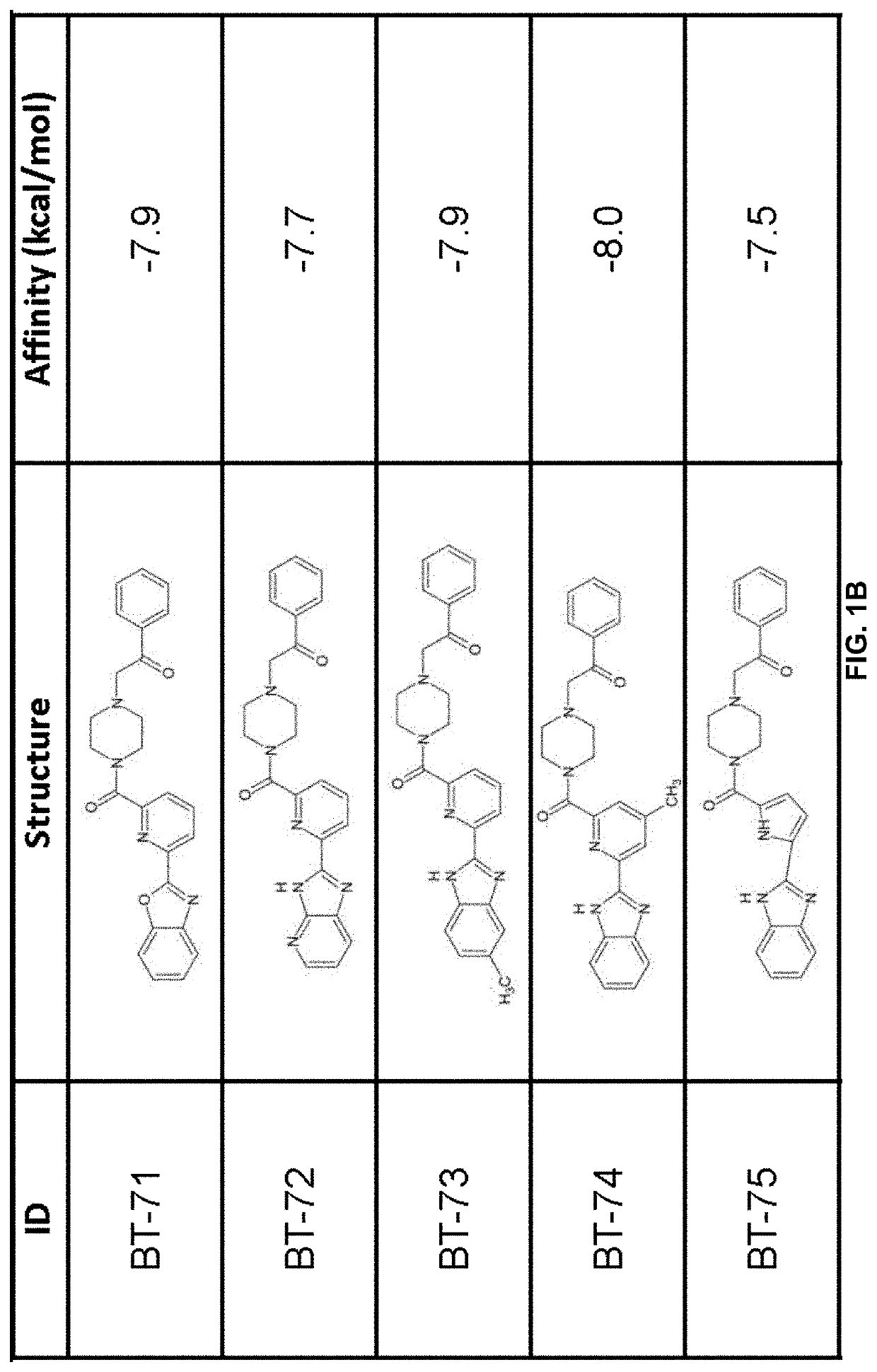
LANCL ligands
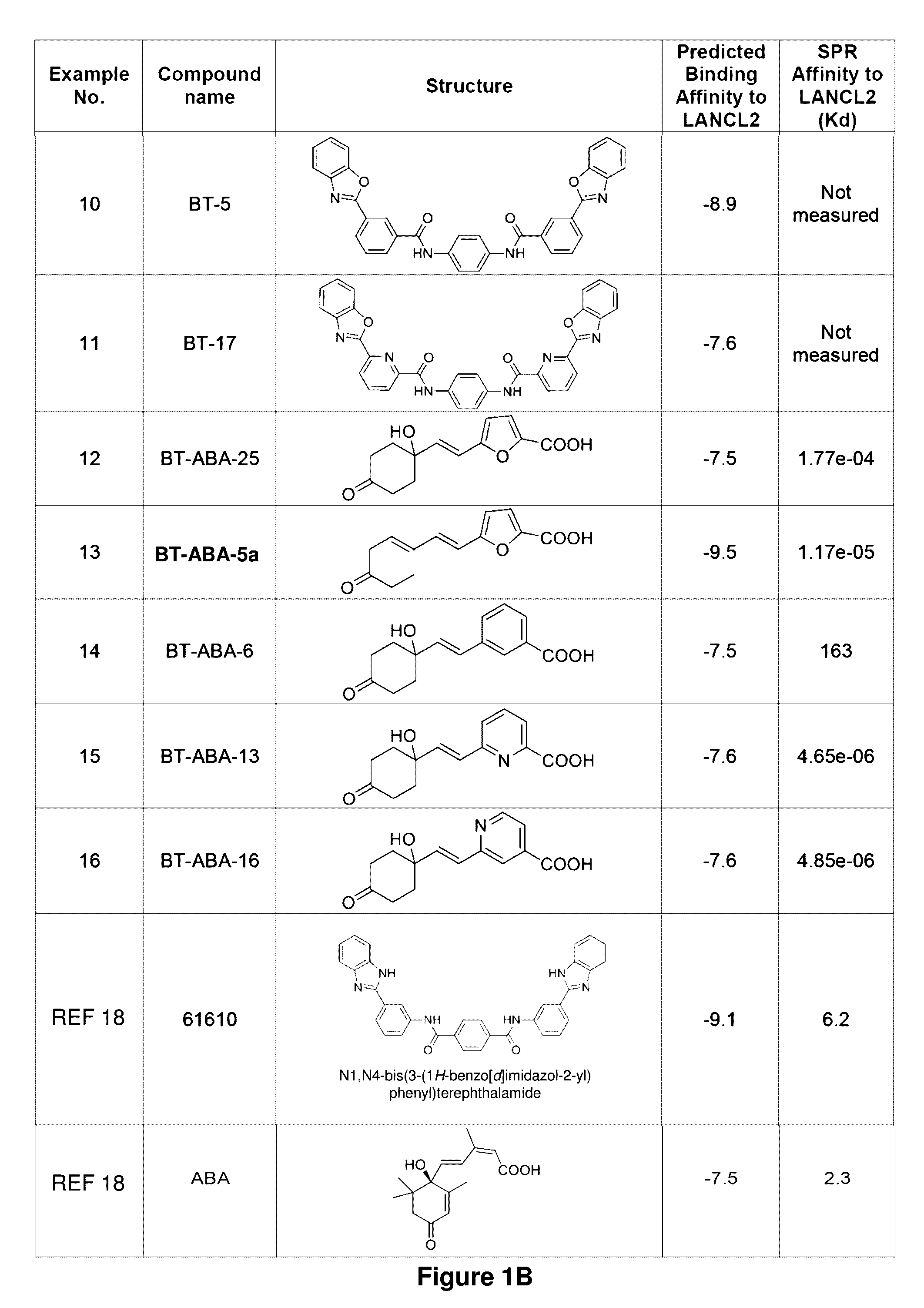
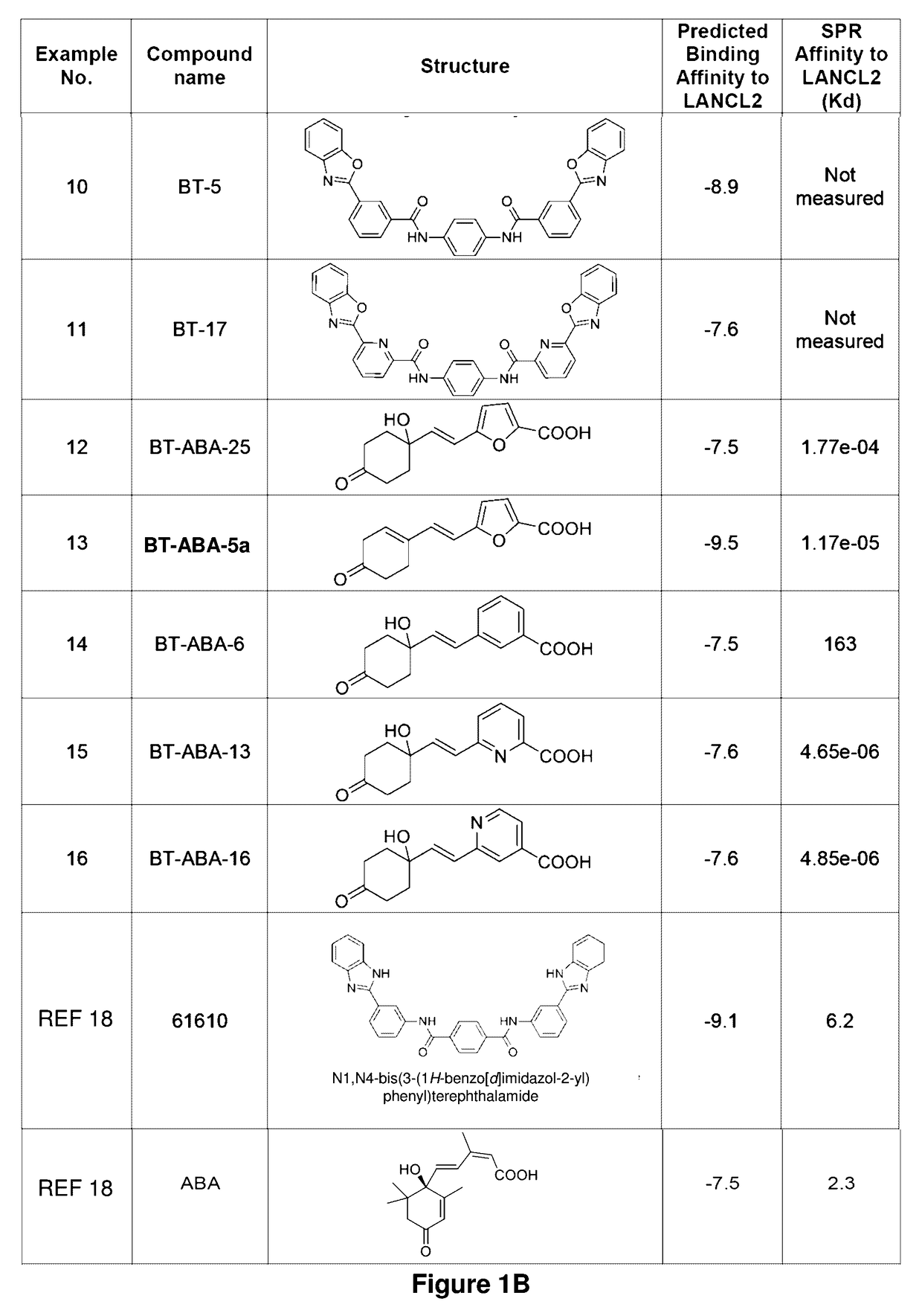
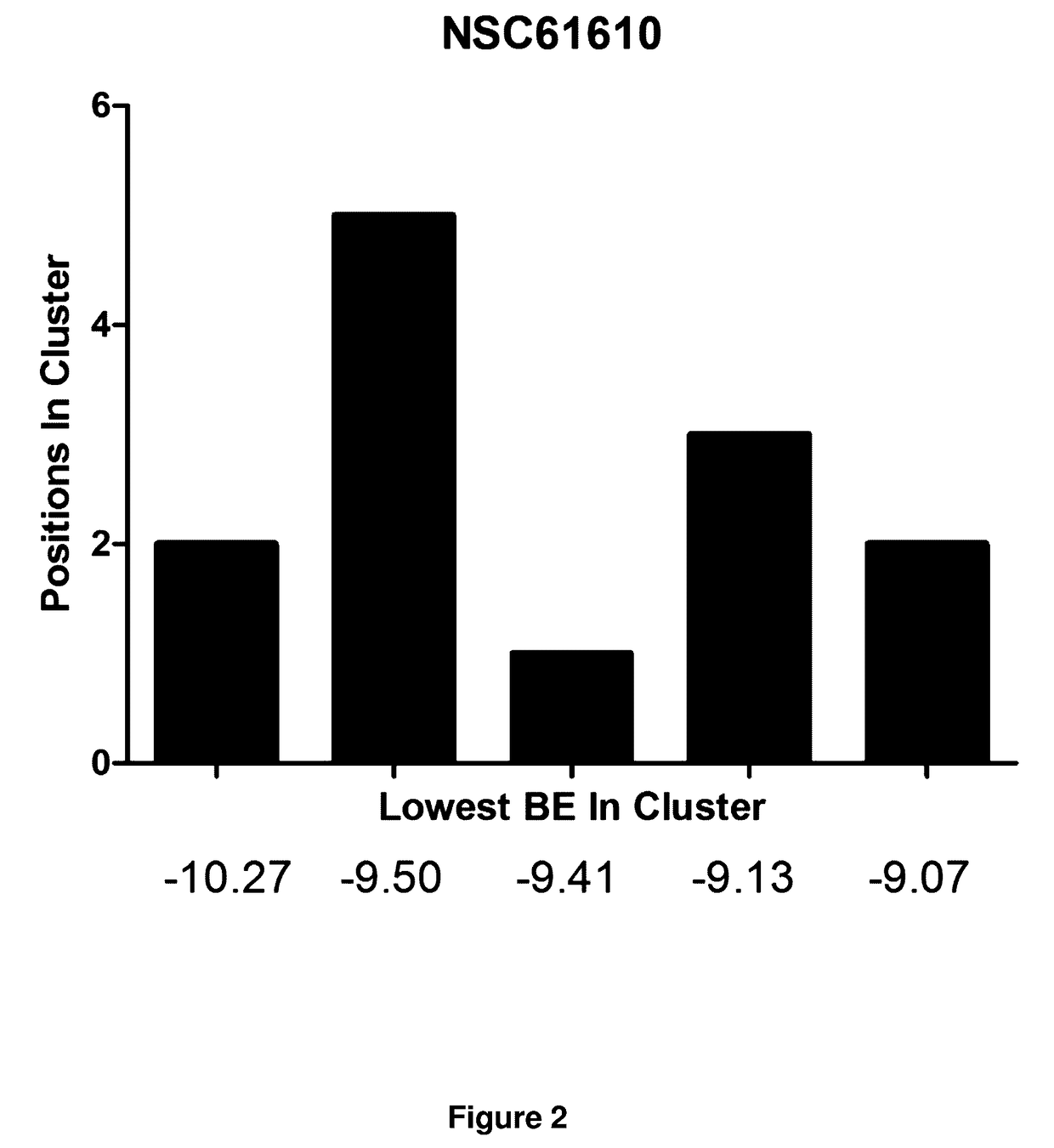
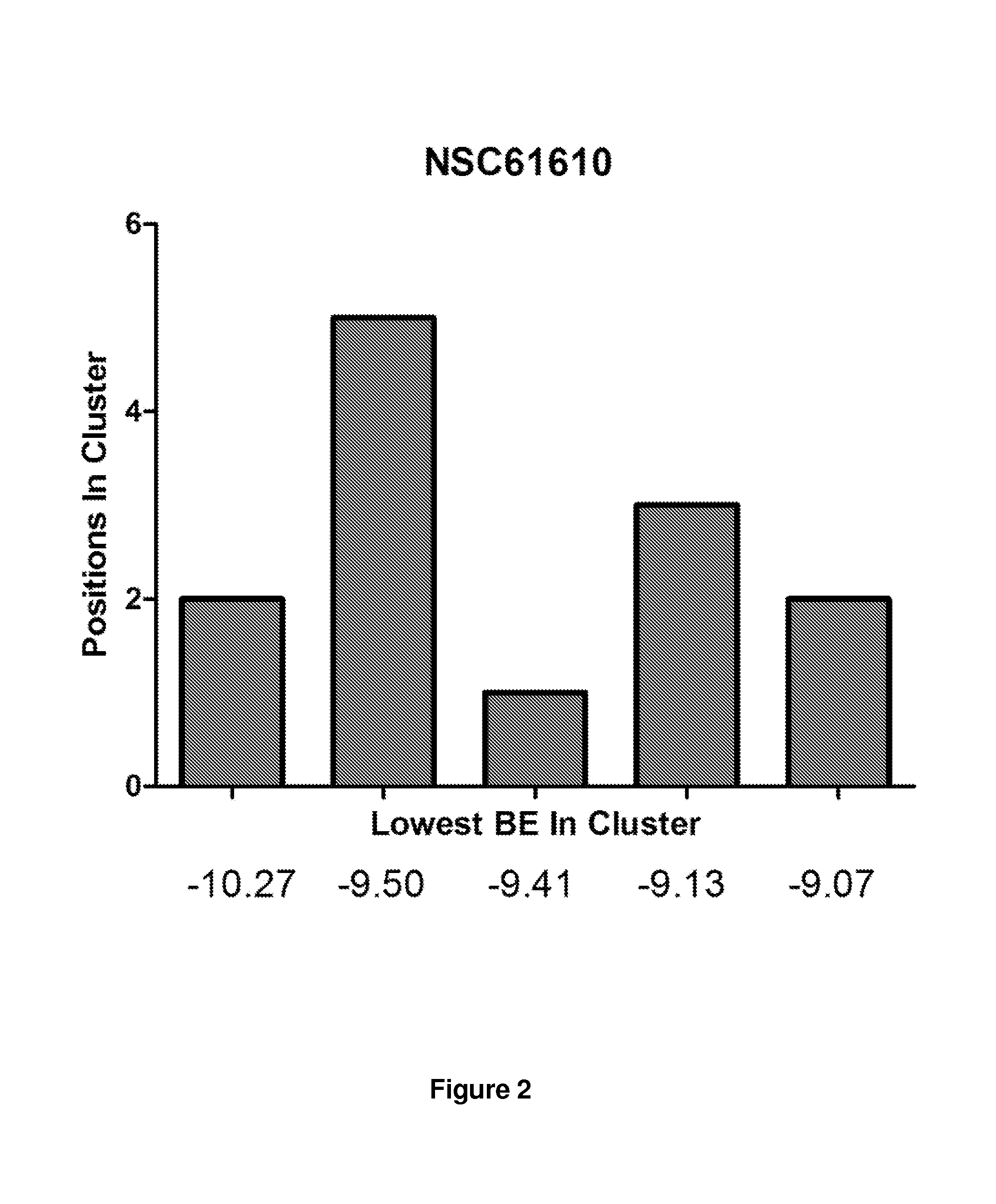
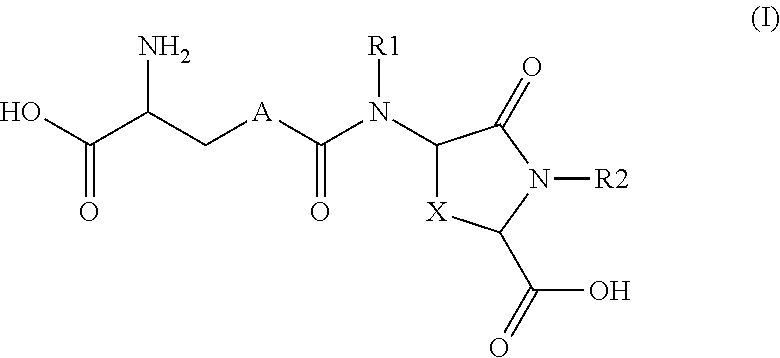
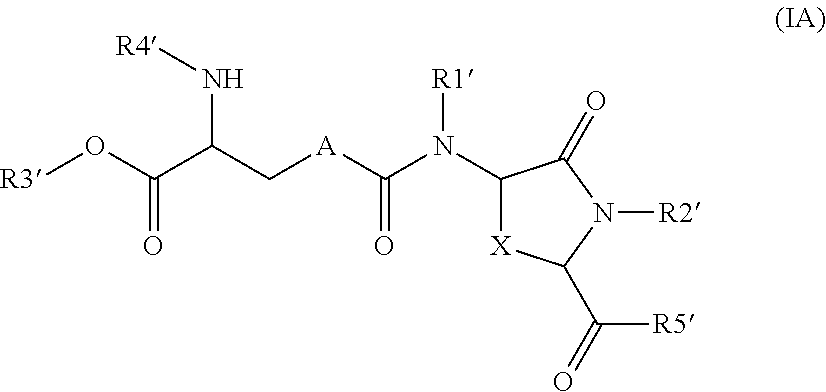
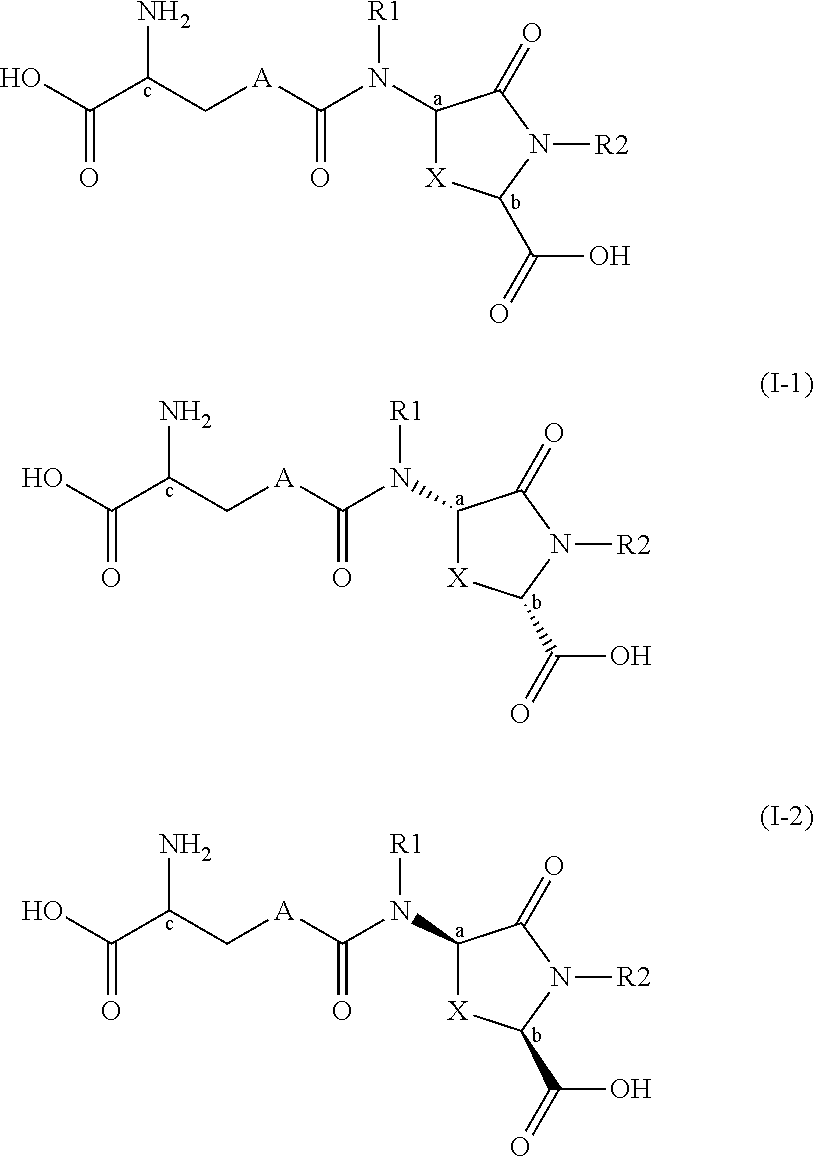
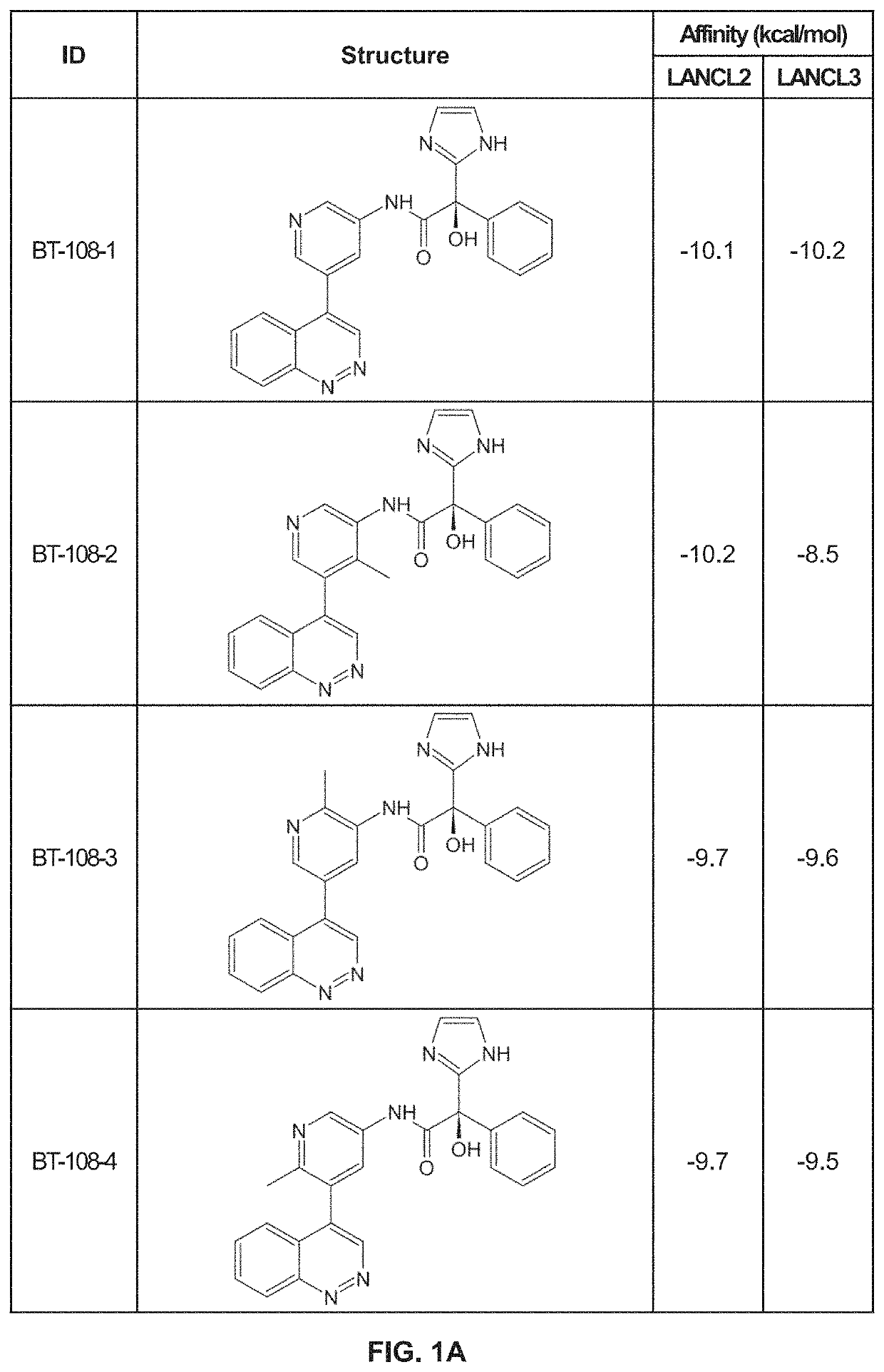
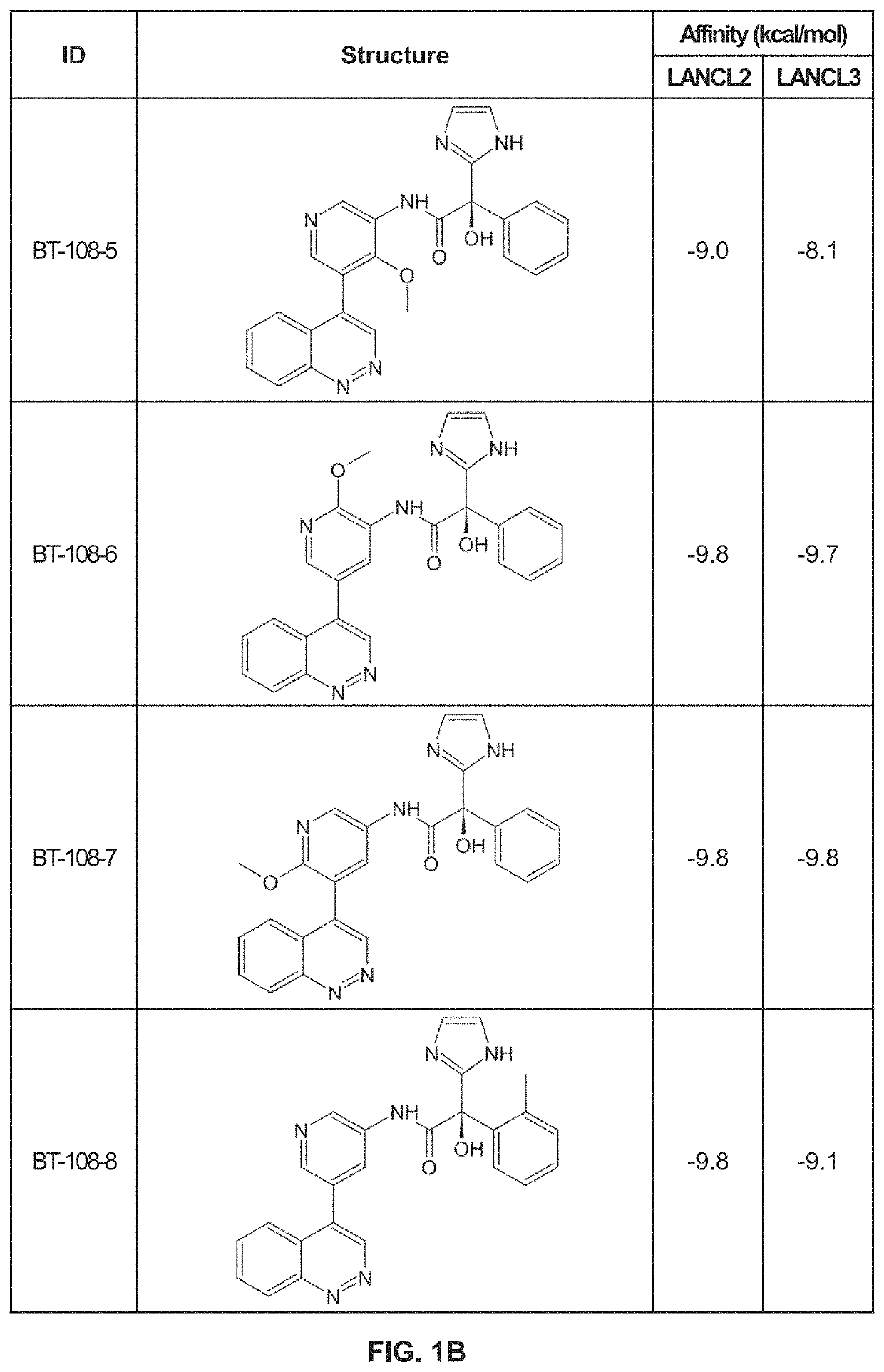
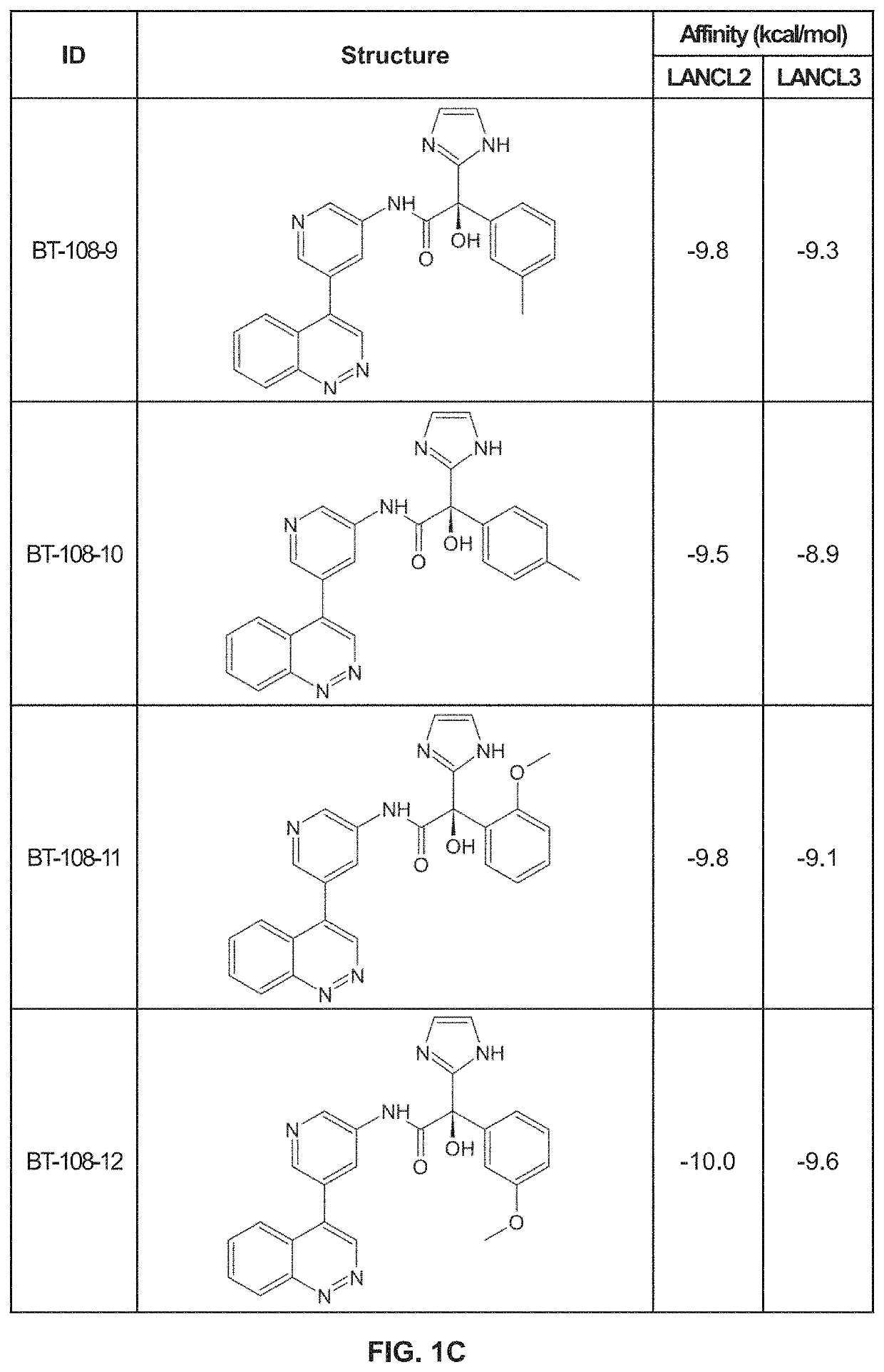
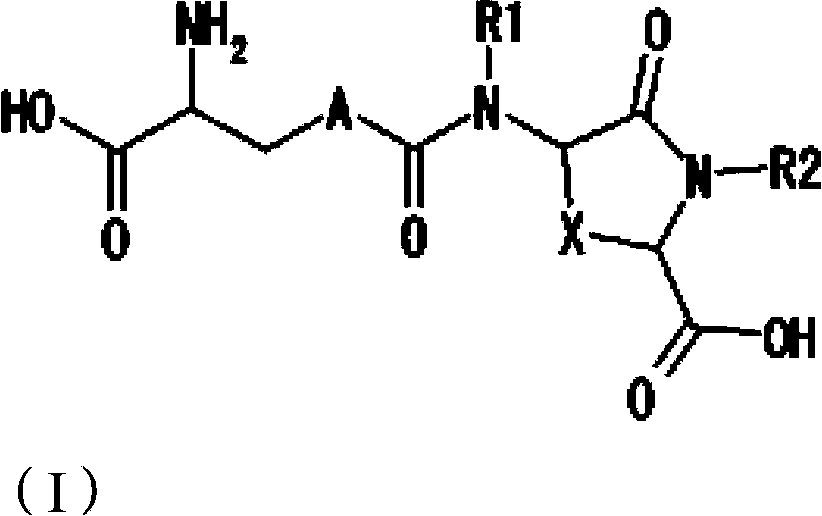
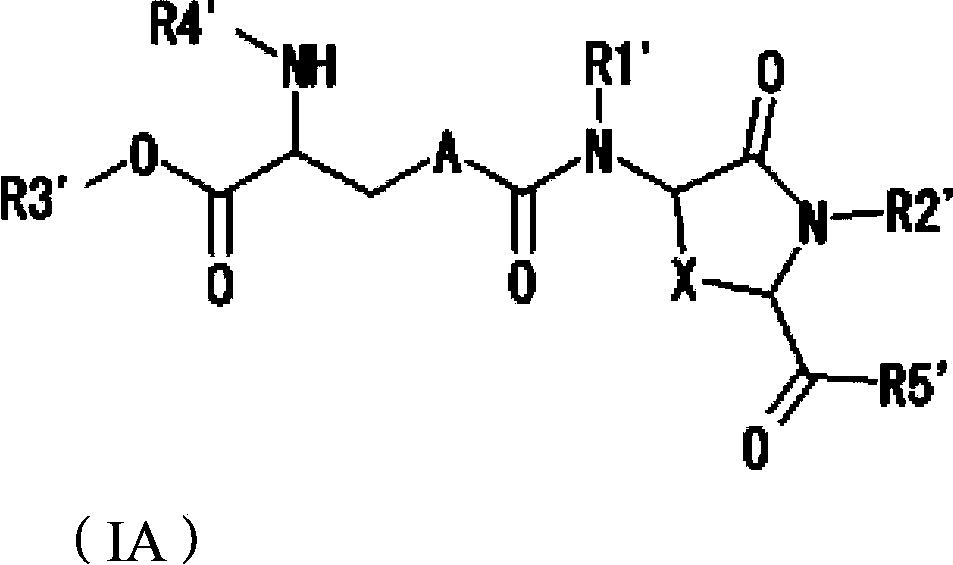
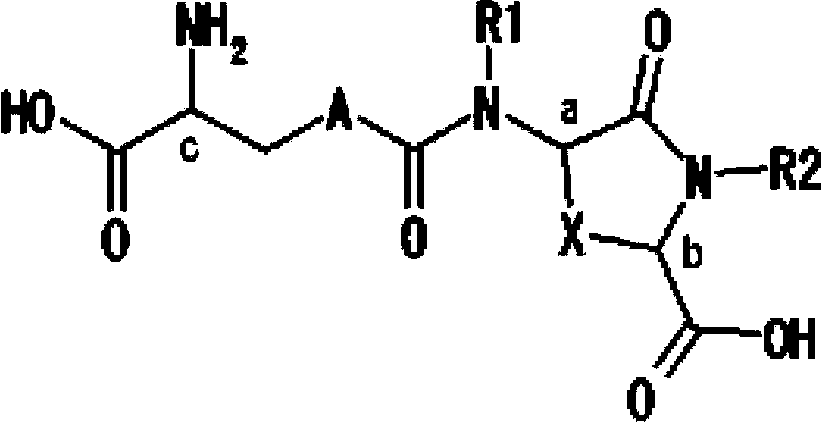
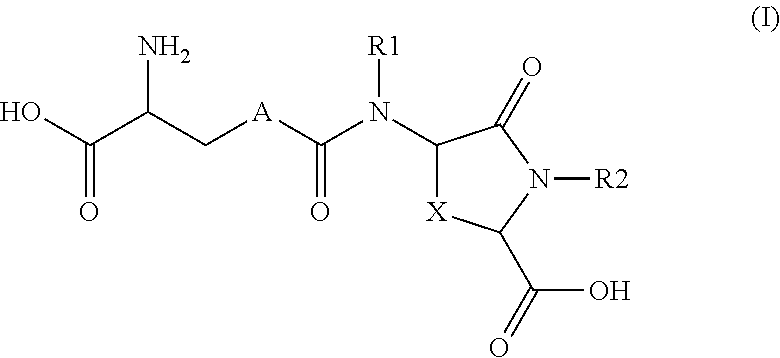
Provided are compounds of Formula (I):The compounds target the lanthionine synthetase C-like (LANCL) family of proteins, including LANCL2 and LANCL3. The compounds can be used to treat conditions such as inflammatory diseases, metabolic diseases, autoimmune diseases, cancers, and infectious diseases. Exemplary conditions include inflammatory conditions of the liver, such as nonalcoholic fatty liver disease, nonalcoholic steatohepatitis, and cirrhosis; inflammatory conditions of the bile duct, such as primary biliary cholangitis, primary sclerosing cholangitis; inflammatory bowel disease, such as Crohn's disease and ulcerative colitis; lupus, such as systemic lupus erythematosus, lupus nephritis, and cutaneous lupus; arthritis, such as rheumatoid arthritis; hyperglycemia, such as type 1 diabetes, type 2 diabetes, and prediabetes and associated conditions such as atherosclerosis and diabetic kidney disease; psoriasis; and multiple sclerosis.
Owner:NIMMUNE BIOPHARMA INC
Lanthionine derivative
To search a number of variation compounds having a CaSR agonist activity in order to obtain a substance having a more excellent kokumi-imparting function, and more particularly to provide a kokumi-imparting agent, which contains the foregoing substance, and a composite kokumi-imparting agent, which likewise contains the aforementioned substance and another substance having a CaSR agonist activity as well, in combination a kokumi-imparting agent comprises a lanthionine derivative and a composite kokumi-imparting agent comprising the lanthionine derivative and another substance having a CaSR agonist activity.
Owner:AJINOMOTO CO INC
Compounds, compositions, and methods for treating inflammatory or immune-mediated conditions of surface tissues
PendingUS20220152019A1Effective treatmentOrganic active ingredientsAntipyreticPsoriasis universalisInflammation
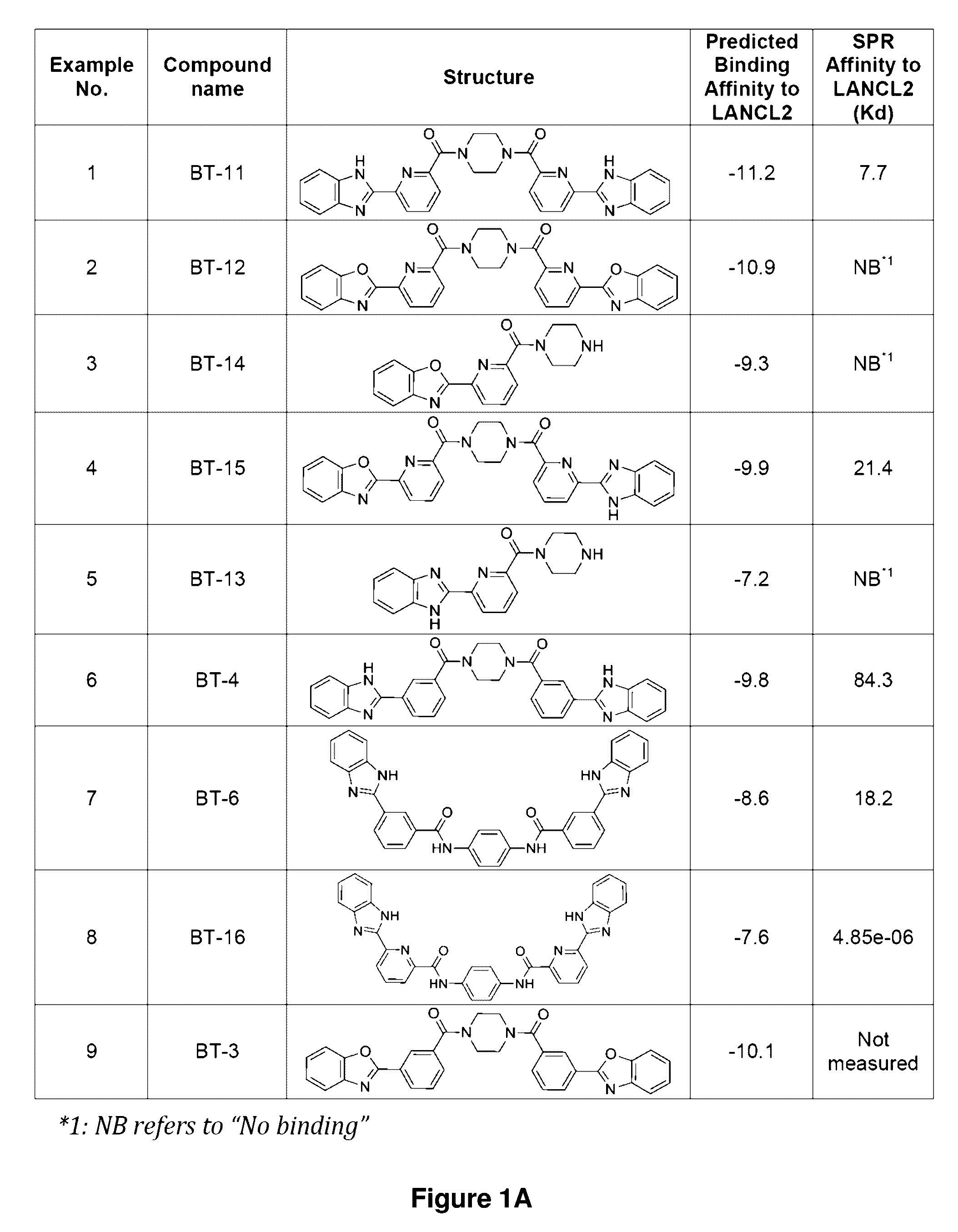
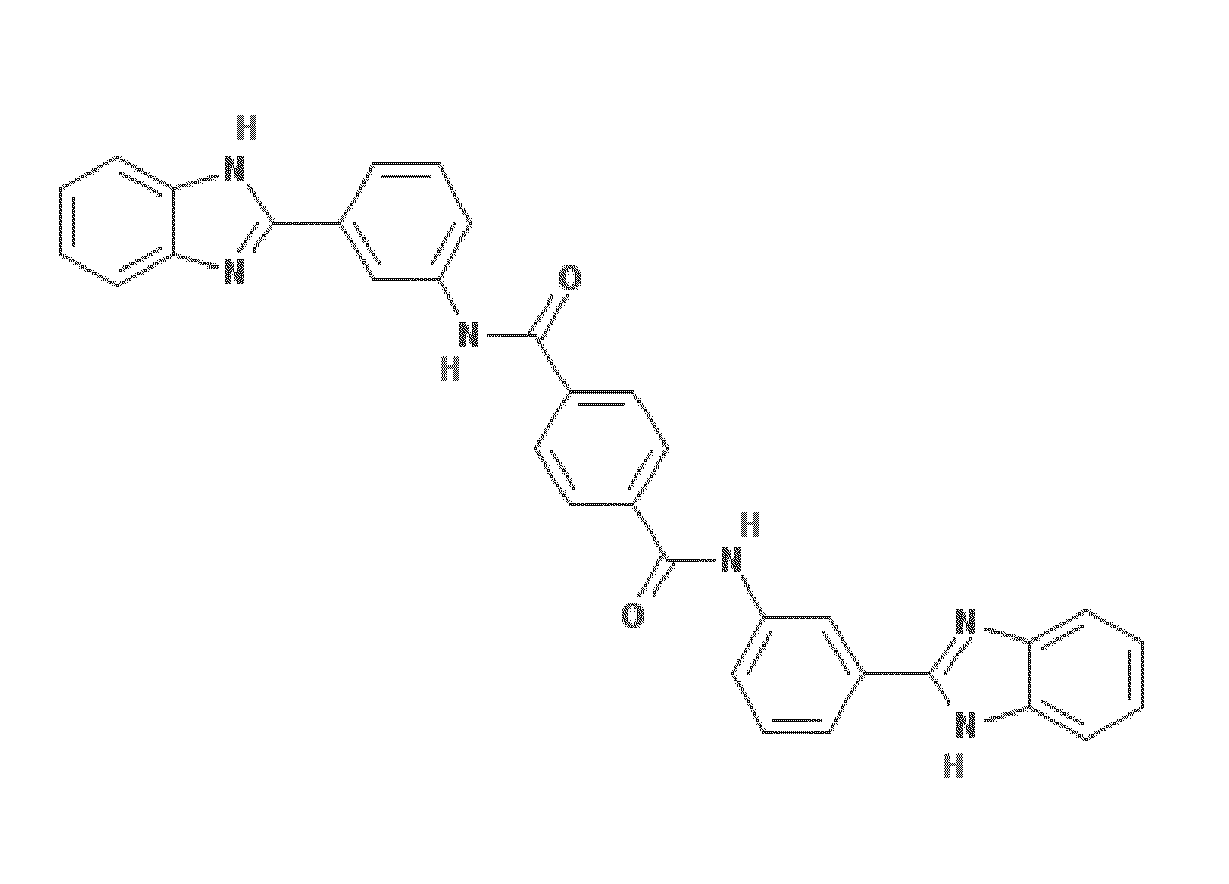
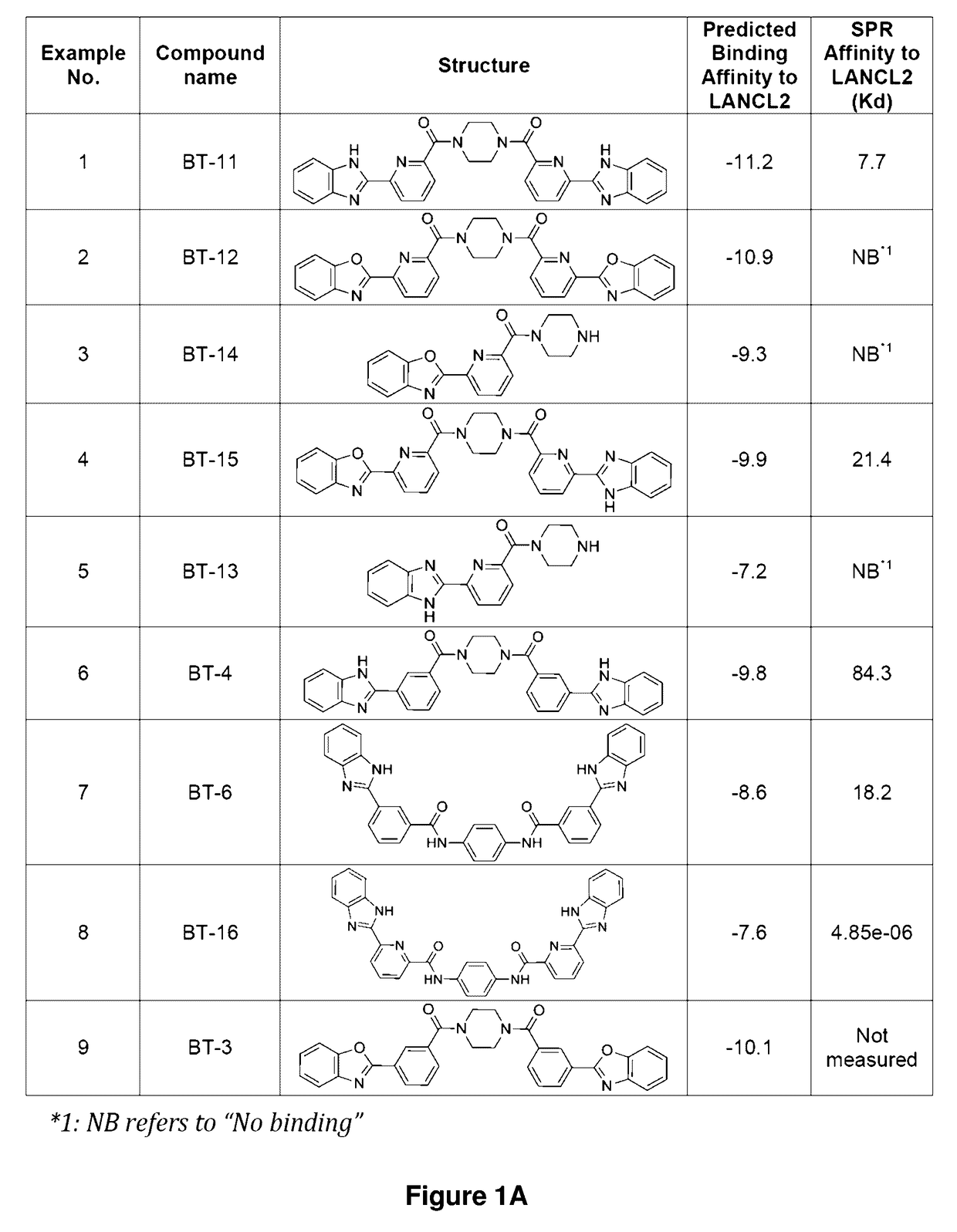
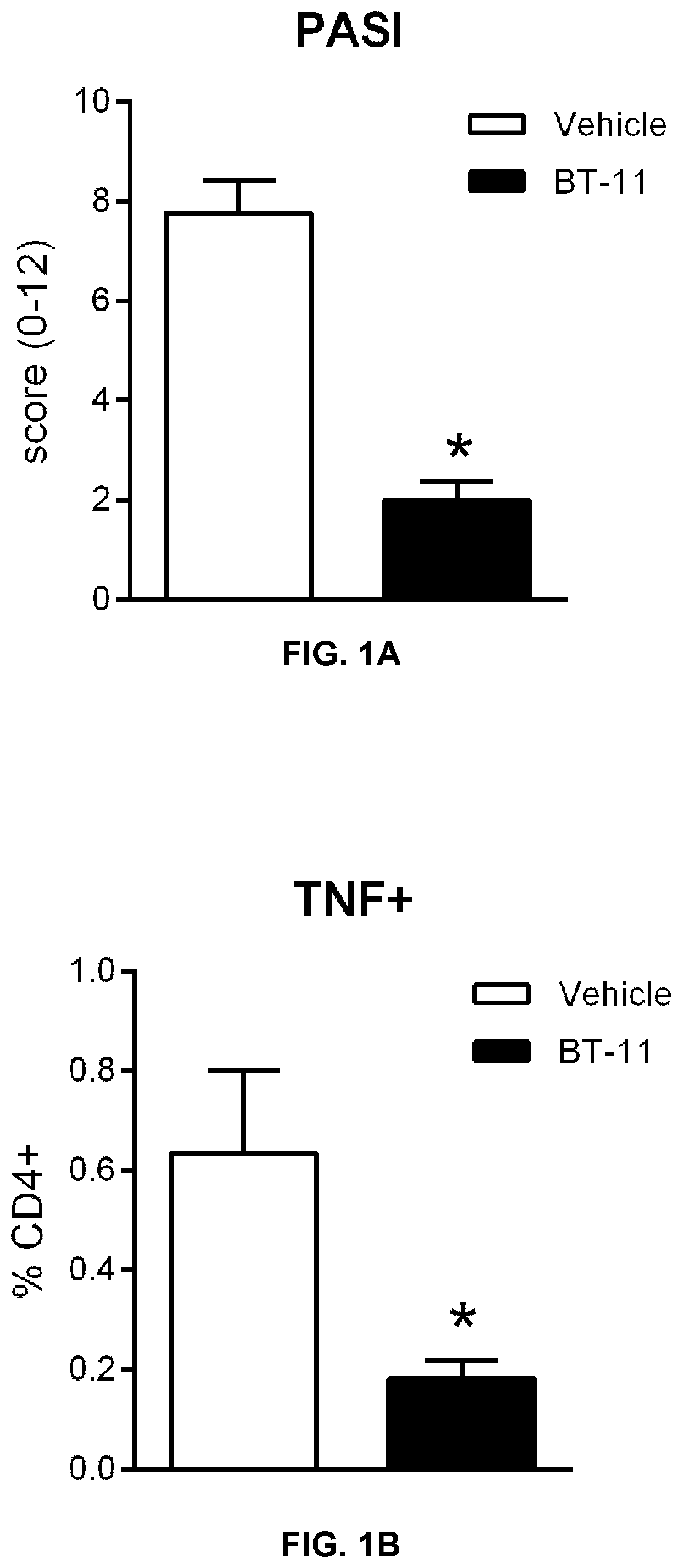
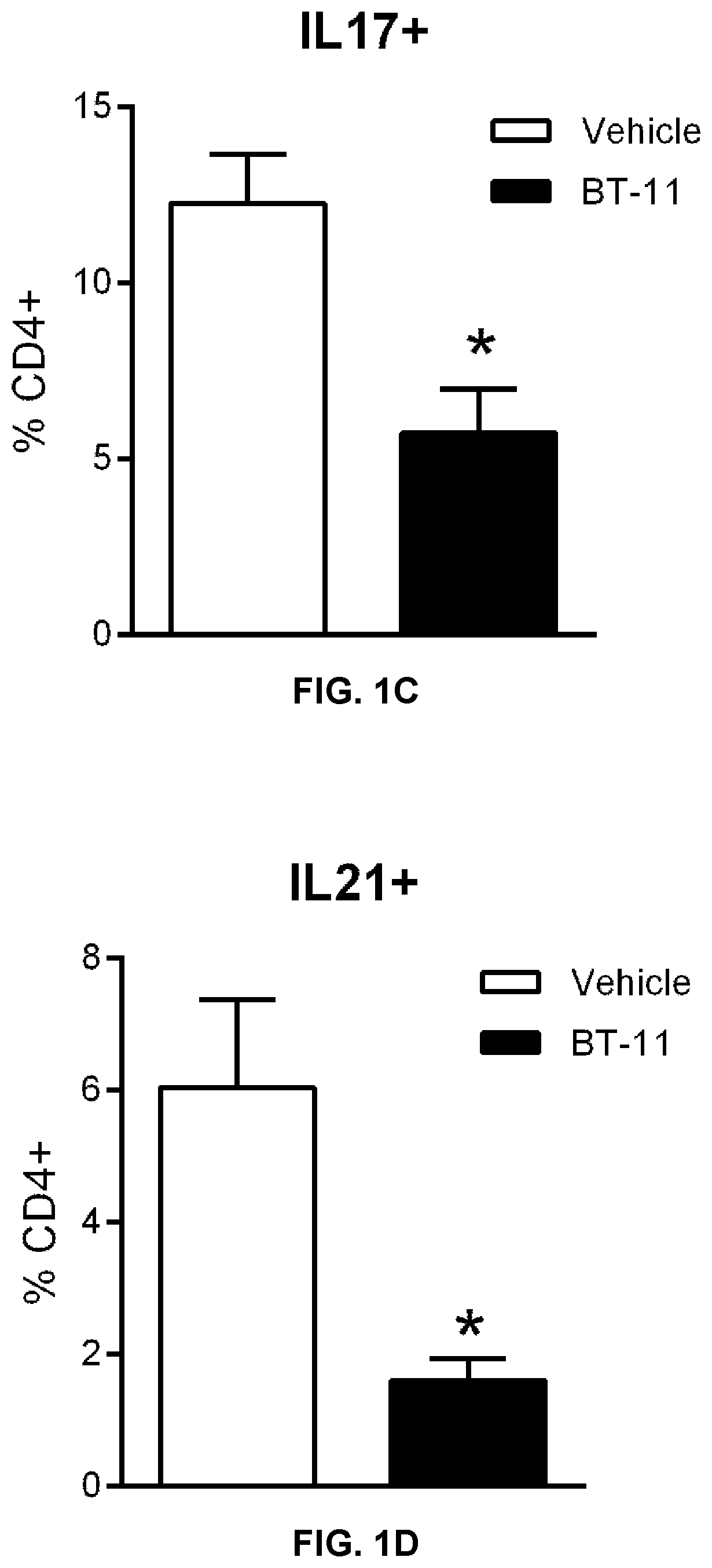
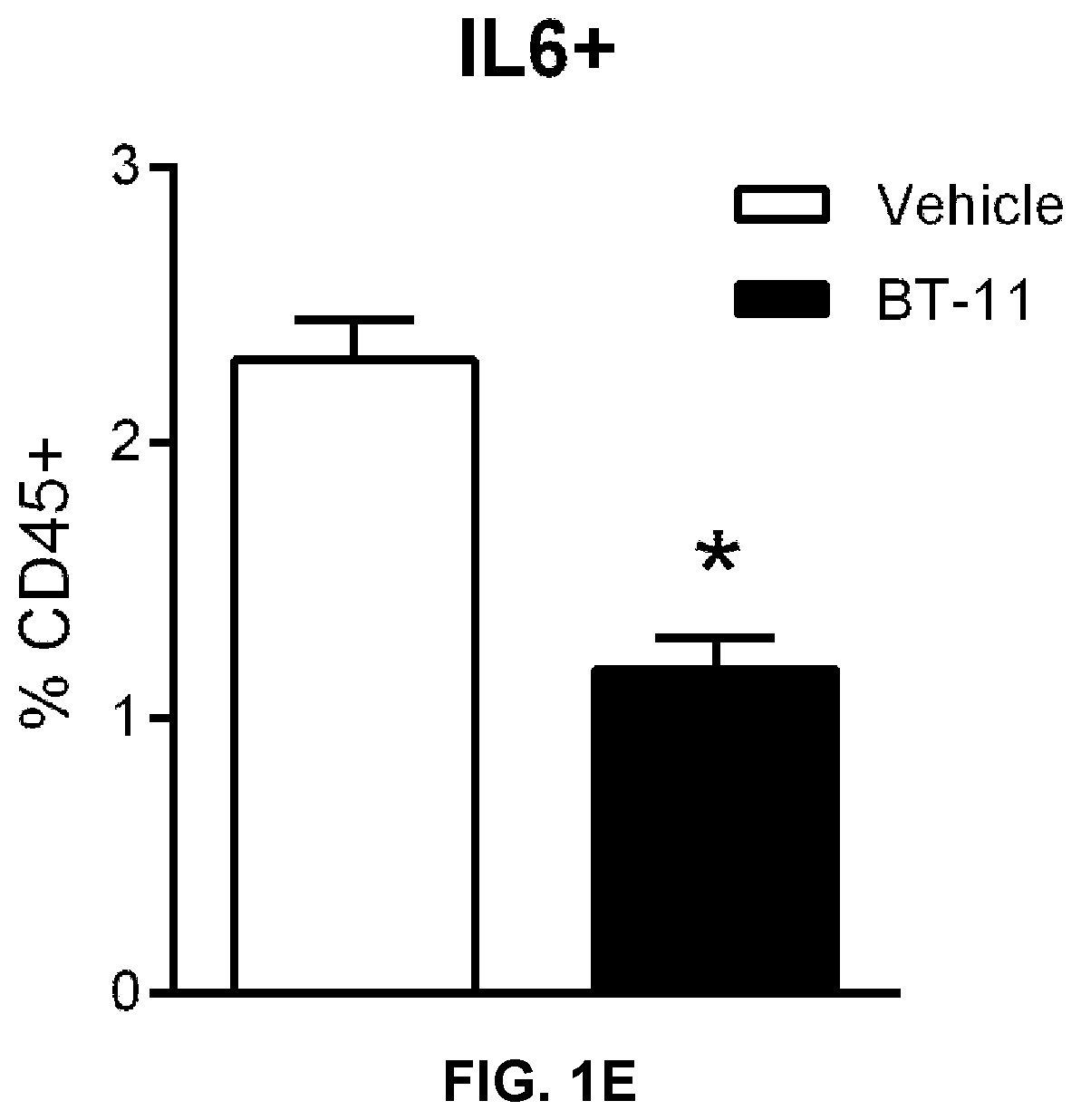
Compounds, compositions, and methods for treating inflammatory or immune-mediated conditions of surface tissues. The methods preferably involve topically administering to a surface tissue an amount of a compound effective to treat an inflammatory or immune-mediated condition. The compounds include lanthionine synthetase C-like 2 (LANCL2) agonists, such as piperazine-1,4-diylbis((6-(1H-benzo[d]imidazo-2-yl)pyridine-2-yl)methanone) and salts and analogs thereof. The surface tissues include the skin and the mucosa, such as the oral mucosa. The inflammatory or immune-mediated conditions include psoriasis and dermatitis (such as atopic dermatitis), among others.
Owner:NIMMUNE BIOPHARMA INC
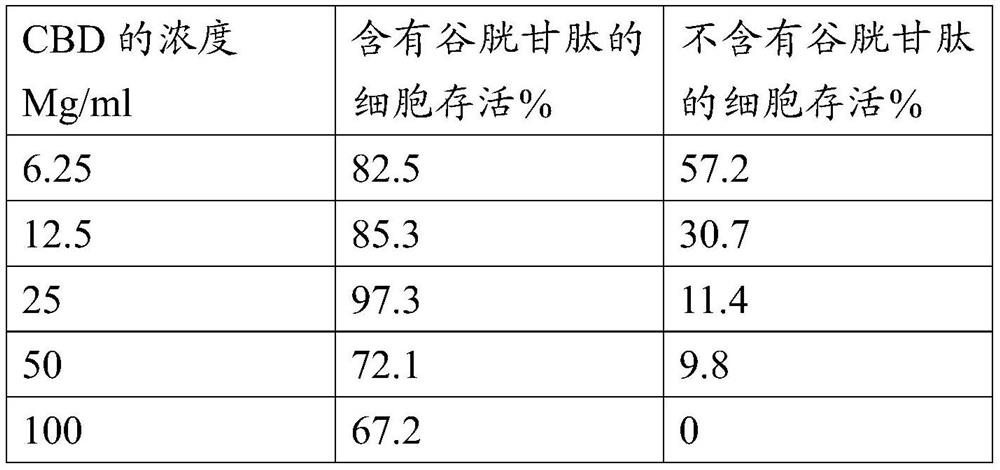
Pharmaceutical composition comprising cannabinoid
PendingCN114615997AHydroxy compound active ingredientsPharmaceutical delivery mechanismPenicillaminePharmaceutical Substances
The present invention provides a pharmaceutical composition consisting of: a first pharmaceutically active part consisting of one or more cannabinoids; a second pharmaceutically active part consisting of one or more of glutathione, cysteine, acetylcysteine, alliin, briximine, carboxy methylcysteine, mucine, cat urine, lantidine, mesine hydrochloride, penicillamine cysteine disulfide, or any functional equivalent thereof; and a pharmaceutical composition comprising the first pharmaceutically active part and the second pharmaceutically active part, wherein the second pharmaceutically active part consists of one or more of glutathione, cysteine, acetylcysteine, alliin, briximine, carboxy methylcysteine, mucine, cat urine, lantidine, mesine hydrochloride, penicillamine cysteine disulfide or any functional equivalent thereof. And optionally one or more excipients; wherein the molar ratio of the second pharmaceutically active moiety to the first pharmaceutically active moiety is at least 0.5: 1, advantageously at least 1: 1. The composition of the present invention is advantageous in that it can prevent hepatotoxicity that may be caused by ingestion of isolated cannabinoids. The present invention also provides a method of manufacturing a pharmaceutical composition comprising the steps of: extracting a first fraction comprising one or more cannabinoids from cannabis plant material using a polar solvent; extracting a second fraction comprising glutathione from the same cannabis plant material using water; and drying and combining the first fraction and the second fraction to form the pharmaceutical composition.
Owner:BLACKHAWK PARTNERS LTD
Lanthionine derivatives
ActiveUS20120282386A1Function increaseEasy to prepareOrganic active ingredientsBiocideLanthionineChemistry
The present invention provides a variety of compounds having a CaSR agonist activity which possesses a superior kokumi-imparting function, and more particularly provides a kokumi-imparting composition, which contains the foregoing compound, and / or another substance having a CaSR agonist activity, in combination. The present invention also provides a kokumi-imparting composition which includes a lanthionine derivative and / or another substance having a CaSR agonist activity.
Owner:AJINOMOTO CO INC
Lanthionine c-like protein 2 ligands, cells prepared therewith, and therapies using same
ActiveUS20210355101A1Organic active ingredientsOrganic chemistryAutoimmune conditionInfectious Disorder
Owner:NIMMUNE BIOPHARMA INC
Activated Carbon Relaxer System
InactiveUS20190029933A1Small low-volume poresIncrease surface areaCosmetic preparationsHair cosmeticsFiberActivated carbon
Straightening the hair with the activated carbon relaxer system drastically reduces or, eliminates post perm hair odor (PPO) caused by lanthionization, which occurs in all hydroxide relaxer systems. During the relaxing process, the activated carbon component of the compound directly absorbs the malodorous sulfur gases and byproducts that are released from deep within the hair fiber. After allowing the relaxer system adequate processing time to straighten the hair, the compound along with the absorb sulfur gases and byproducts, are removed from the hair by rinsing the hair with water and neutralizing shampoo, thereby preventing the released gases from entering the air and causing PPO.
Owner:WILLIAMS JR RAFE
Hair straightening composition
A hair straightening composition comprising: i) gamma- thiobutyrolactone; ii) an acid or salt thereof; and iii) an amine. A method for lanthionizing keratin fibres to achieve relaxation of said keratin fibres in which said composition is applied to the hair.
Owner:UNILEVER NV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com