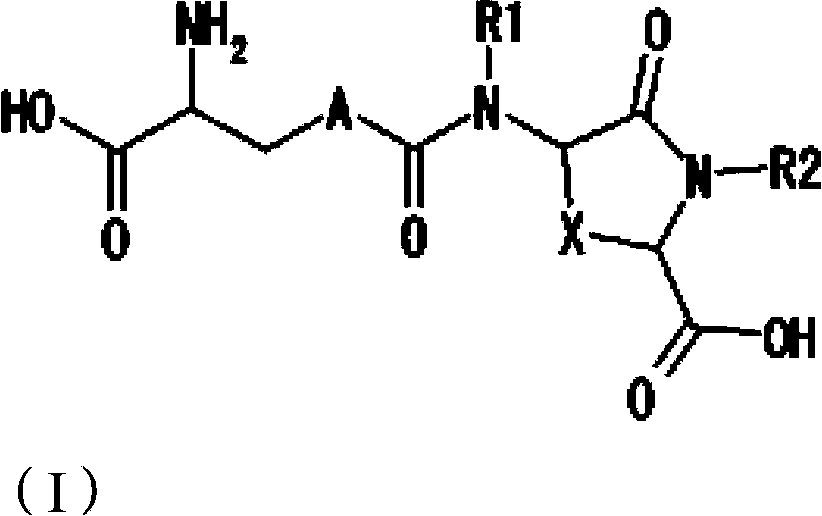
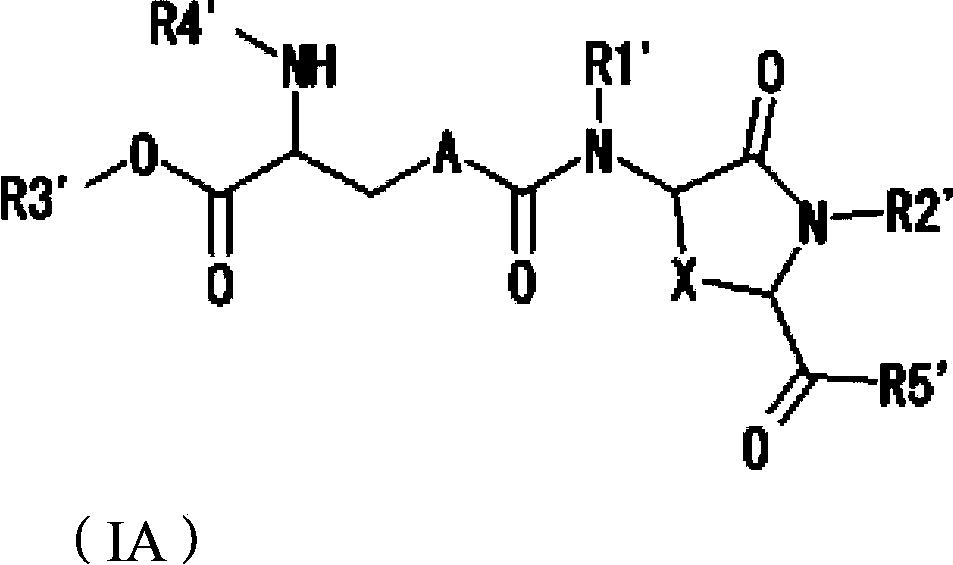
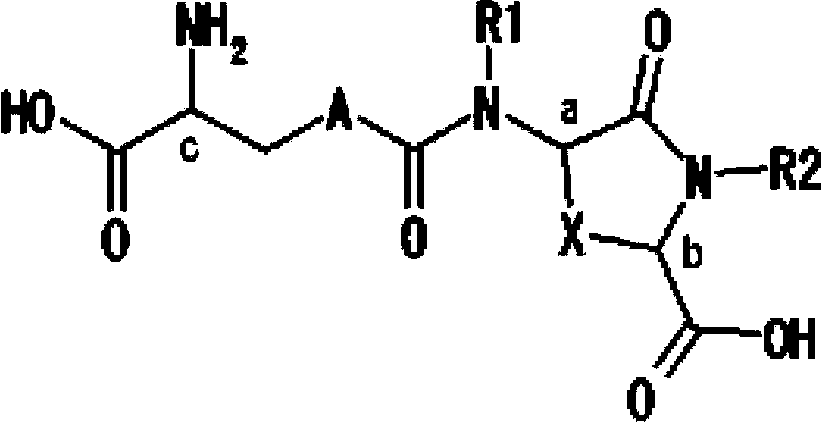
Lanthionine derivative
一种硫基、化合物的技术,应用在羊毛硫氨酸衍生物领域,能够解决稳定性、气味问题等问题,达到稳定性良好、低成本的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1)
[0179] (Example 1) compound 1 Synthesis
[0180] (Fmoc-L-Cys-Ot-Bu) 2 (N,N'-difluorenylmethoxycarbonyl-L-cystine di-t-butylester, 4.81mmol) was dissolved in tetrahydrofuran (58.5mL) and water (1.5mL). Tributylphosphine (5.28 mmol) was added under ice-cooling to return to room temperature, and stirred for 4 hours. The reaction liquid was cooled, and 10% aqueous citric acid solution (60 mL) was added. The cloudy solution was returned to room temperature, and extracted with ethyl acetate (60 mL). The organic layer was washed with 60 mL of brine, and concentrated to obtain an oily residue. The residue was purified with a silica gel column (n-hexane-ethyl acetate) to obtain the compound as an oil 1 .
[0181] The yield is 97%.
[0182] ESI MS m / z 422.4 (M+Na) +
[0183] 1 H NMR (400MHz, CDCl 3 )δ1.50(9H,s),2.99(2H,m),4.23(1H,t,J=6.8Hz),4.41(2H,m),4.54(1H,m),5.68(1H,d,J =7.2Hz),7.32(2H,m),7.41(2H,t,J=7.2Hz),7.61(2H,d,J=7.6Hz),7.77(2H,d,J=7.2Hz).
[0184] [chemical formu...
Embodiment 2)
[0186] (Example 2) compound 2 Synthesis
[0187] compound 1 (6.04mmol) was dissolved in dimethylformamide (60mL), after adding Boc-iodo-D-Ala-OMe (N-t-butoxycarbonyl-3-iodo-D-alanine methylester) (6.20mmol), adding cerium carbonate ( 6.02 mmol), stirred overnight at room temperature. The reaction solution was cooled, 10% citric acid aqueous solution (50 mL) and water (30 mL) were added, extracted with ethyl acetate (60 mL), and then the aqueous layer was extracted with ethyl acetate (60 mL) again. The organic layers were combined, washed successively with 10% citric acid aqueous solution (50 mL) and brine (50 mL), and the organic layer was concentrated. The obtained oily residue was purified with a silica gel column (n-hexane-ethyl acetate) to obtain the compound as an oil 2 .
[0188] Yield 66%.
[0189] ESI MS m / z 601.2(M+H) +
[0190] 1 H NMR (400MHz, CDCl 3 )δ1.45(9H,s),1.49(9H,s),3.01(4H,m),3.73(3H,s),4.24(1H,t,J=7.2Hz),4.39(2H,d,J =7.2Hz),4.48-4.55(2H,m),5.37(...
Embodiment 3)
[0193] (Example 3) compound 4 Synthesis
[0194] (Step 1) Compound 2 (4.01 mmol) was dissolved in 70 mL of dichloromethane, trifluoroacetic acid (70 mL) was added, stirred at room temperature for 1 hour, and the reaction solution was concentrated to obtain a residue containing 3. Assuming a yield of 100%, it was directly used in the next reaction.
[0195] [chemical formula 20]
[0196]
[0197] (Step 2) Under ice cooling, anhydrous dimethylformamide (60 mL) was added to compound 3 (corresponding to 4.01 mmol) to form a homogeneous liquid, and diisopropylethylamine (8.04 mmol) was added dropwise thereto. After returning to room temperature, carbonylbiimidazole (8.10 mmol) was added, and the mixture was stirred overnight. Under the condition of cooling and stirring, 10% citric acid aqueous solution (50 mL) was added to the reaction solution, and after returning to room temperature, it was extracted with ethyl acetate (100 mL), and the aqueous layer was extracted with ethy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com