Pharmaceutical composition comprising cannabinoid
A composition, cannabinoid technology, applied in the directions of drug delivery, active ingredients of hydroxyl compounds, medical preparations containing active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
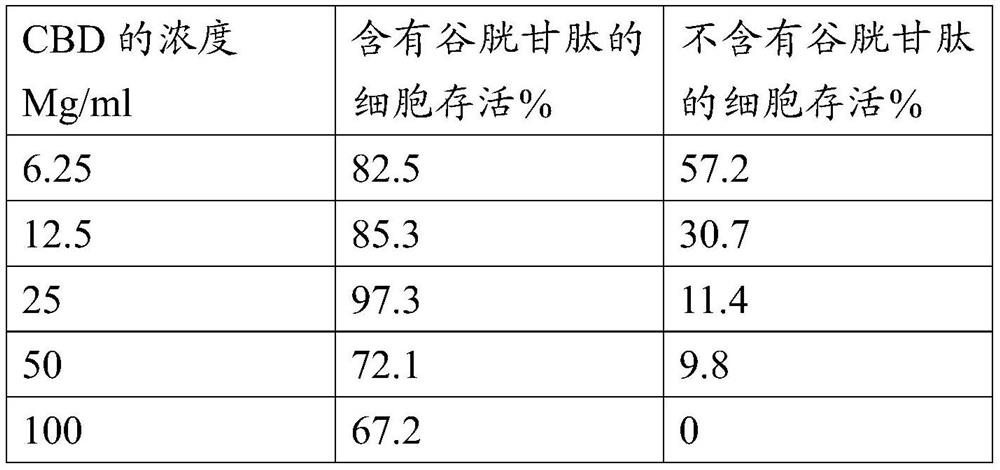
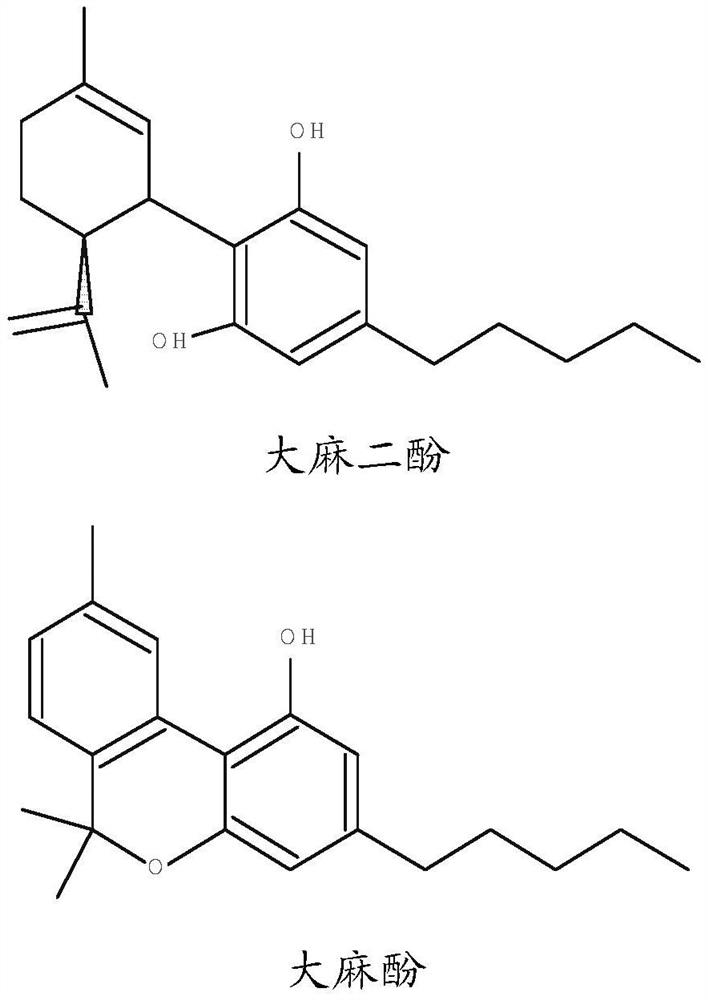
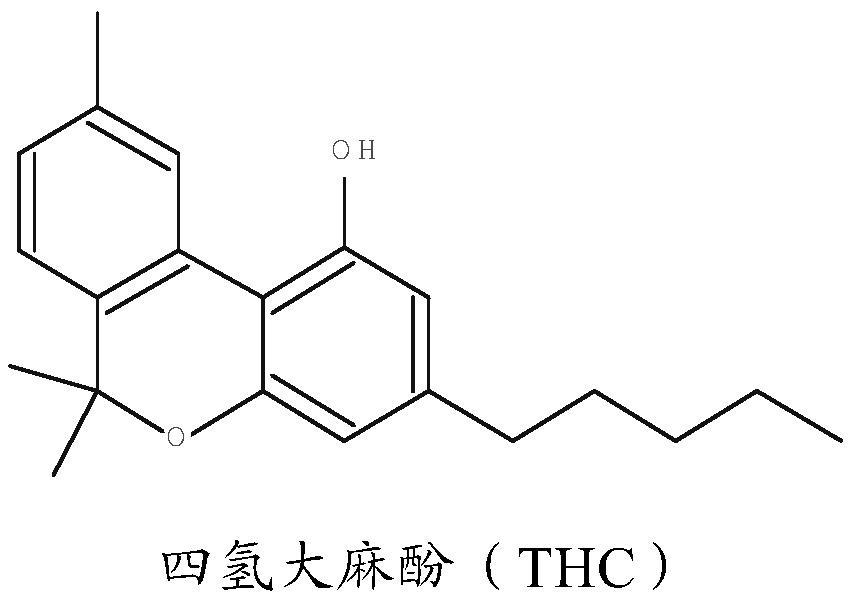
[0050] Solvent extraction of cannabinoids from cannabis plants and isolation of specific cannabinoids is well known in the art. One such method is disclosed in US 6,403,126, and the method disclosed in this patent is applicable to the present invention. In particular, the method of US 6,403,126 is suitable for the extraction of cannabinoids from cannabis sativa.
[0051] In this method, cannabis powder is extracted with a solvent such as ethanol for a period of two to four hours. The solvent can then be evaporated to leave a residue. The extracted residue may then be passed through a chromatography column arranged to fractionate the at least one cannabinoid. The fractionated cannabinoid(s) can then be used to form pharmaceutical compositions.
[0052] ii) Extraction of glutathione
[0053] Material: 10g dried cannabis leaves
[0054] 100ml deionized water
[0055] 250ml erhlingmeyer flask
[0056] Magnetic Stirrers and Rods
[0057] Filter paper
[0058] filter funnel...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com