Methods for site-specific pegylation
A chemical and free aldehyde technology, applied in the preparation method of peptides, chemical instruments and methods, specific peptides, etc., can solve the problems of non-site selectivity and achieve high site selectivity, easy characterization, and small content differences.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
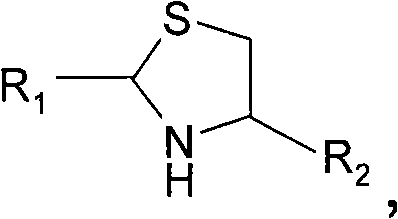
[0145] Embodiment 1) preparation H-NMeCys-Lys-Phe-NH 2
[0146]
[0147] Rink amide MBHA resin (211 mg, 0.152 mmol) (Novabiochem, San Diego, Calif.) was swollen in dichloromethane (DCM) and washed with dimethylformamide (DMF). The resin was debolcked by treatment with 25% piperidine / DMF (10 mL) solution 2 x 10 min. The resin was washed three times with DMF (10 mL). By using Fmoc-Phe-OH (Novabiochem, San Diego, Calif.) (235 mg, 0.606 mmol), 1-hydroxybenzotriazole (HOBt) (92.3 mg, 0.606 mmol) and diisopropylcarbodiene A solution of amine (DIC) (77 mg, 0.606 mmol) in N-methylpyrrolidone (NMP) (2 mL) was treated for 1 hour to couple the first amino acid to the resin. The resin was filtered and washed three times with DMF (10 mL).
[0148] The Fmoc protecting group was removed by treatment with 25% piperidine / DMF (10 mL) solution for 2 x 10 min, and the resin was washed three times with DMF (10 mL). Fmoc-Lys(Boc)-OH (Novabiochem, San Diego, Calif.) (285 mg, 0.606 mol) c...
Embodiment 2
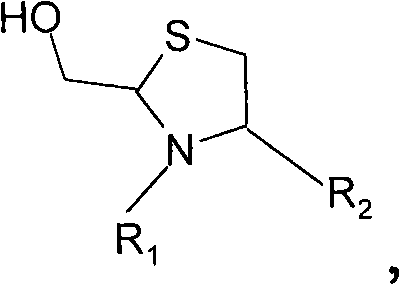
[0152] Embodiment 2) prepare mPEG-Tmc-Lys-Phe-NH 2
[0153] mPEG has CH here 3 O(CH 2 CH 2 O) n -(CH 2 ) 2 -The structure of which n is a positive integer.
[0154]
[0155]The peptide product of Example 1 (0.5 mg, 1.22 micromol) was dissolved in 1.0 mL of pH 4 buffer (20 mmol NaOAc, 150 mmol NaCl and 1 mmol EDTA). mPEG-aldehyde (1.5 equivalents, average molecular weight 31378 Daltons, NOF Corp., Tokyo, Japan) was added to the resulting solution. According to the reverse phase analytical HPLC system (Vydac C 18 5 μ peptide / protein column, 4.6 x 250 mm), the reaction was approximately 90% complete after 27 hours at room temperature. Load the reaction mixture onto 5 mL Zeba TM Desalting spin columns (Pierce Biotechnology, Rockford, IL). A white foam (36.7 mg) was obtained after lyophilization.
Embodiment 3
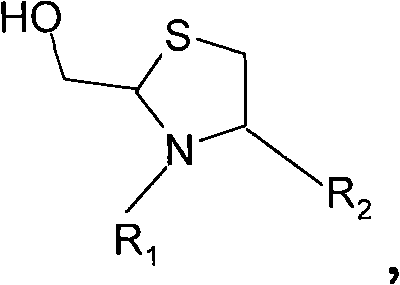
[0156] Embodiment 3) preparation H-NMeCys (Prd-PEG)-Lys-Phe-NH 2
[0157]
[0158] The peptide product of Example 1 (0.5 mg, 1.22 micromol) was dissolved in 1.0 mL of pH 7 buffer (20 mmol NaOAc). α-(3-(3-Maleimido-1-oxopropyl)amino)propyl-ω-methoxy-polyoxyethylene (1.5 equivalents, average molecular weight 11962 Daltons, NOF Corp. ., Tokyo, Japan) and 2 equivalents of tris(2-carboxyethyl)phosphine hydrochloride (TCEP) were added to the resulting solution. According to the reverse phase analytical HPLC system (Vydac C 18 5 μ peptide / protein column, 4.6 x 250 mm), the reaction was completed after 1 hour at room temperature. Load the reaction mixture onto 5 mL Zeba TM Desalting spin columns (Pierce Biotechnology, Rockford, IL). A white foam (15.1 mg) was obtained after lyophilization. Put this product in the High Trap TM Further purification was performed on a SPXL cation exchange column (GE Healthcare, Piscataway, NJ). The molecular weight distribution of the pu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com