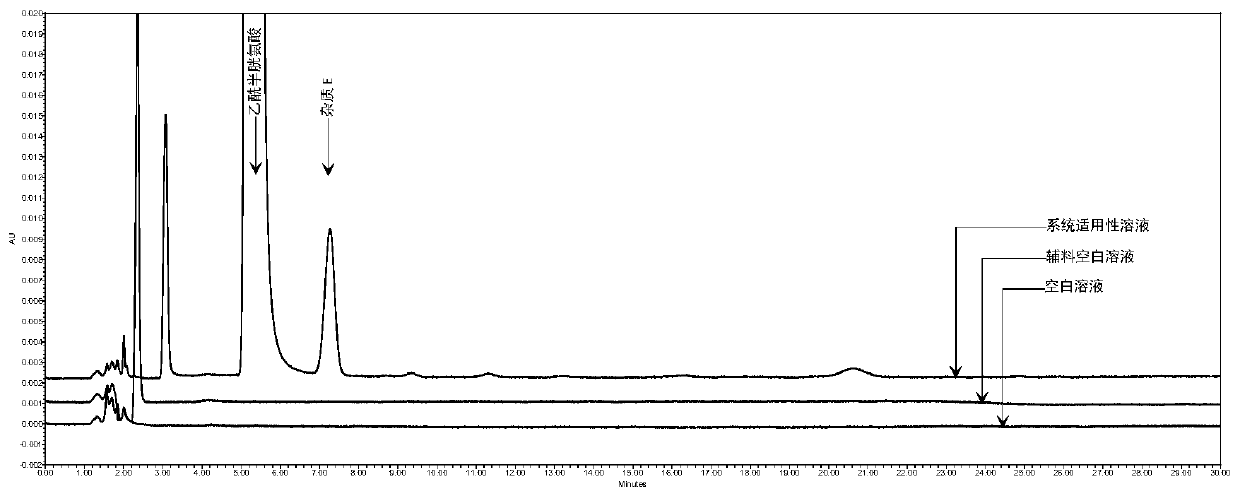
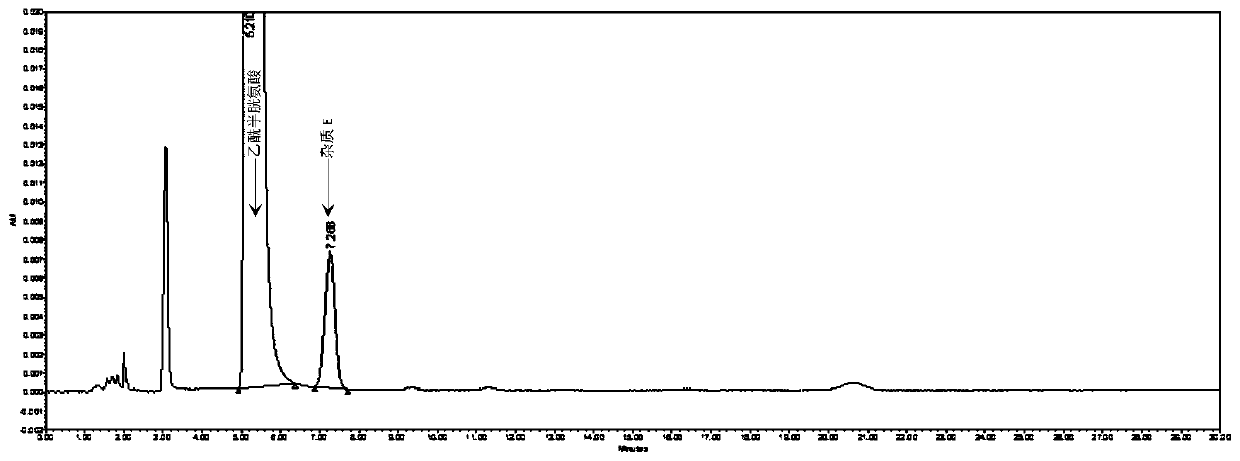
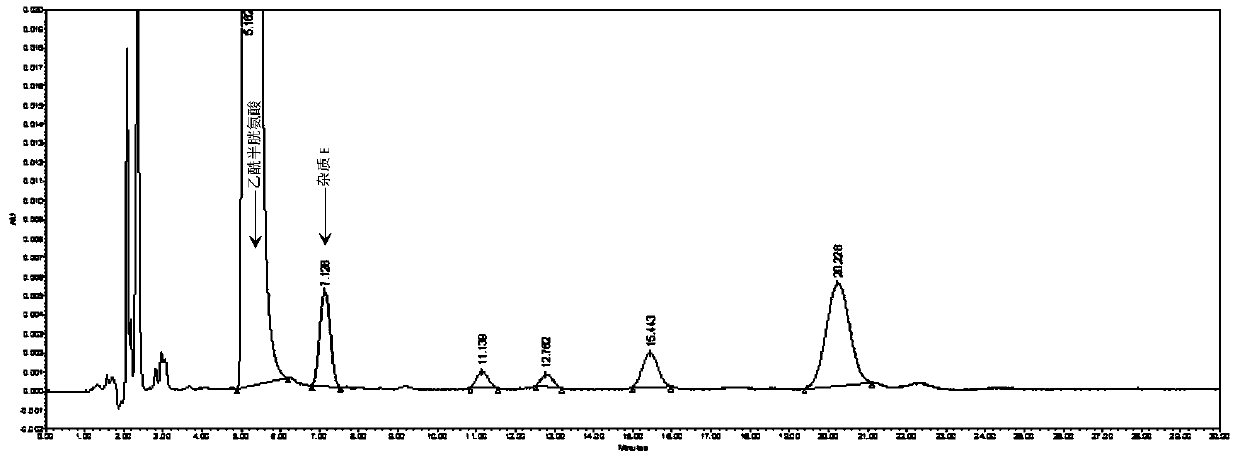
Method for detecting N,N-diacetyl lanthionine in acetylcysteine solution
A technology of acetylcysteine and lanthionine, applied in the field of analysis and testing, can solve problems such as unseen and unseen, and achieve the effects of reducing solvent effect, strong method specificity, and good detection accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] 1. Detection conditions of high performance liquid chromatography:
[0053] Mobile phase: 6.8g / L phosphate buffer-methanol, the volume ratio is 98:2. Among them, the configuration method of 6.8g / L phosphate buffer is as follows: weigh 6.8g of potassium dihydrogen phosphate, add 1000ml of purified water to dissolve , adjust the pH value to 2.0 with phosphoric acid;
[0054] Column: Octadecylsilane bonded silica gel column with an inner diameter of 3.9 mm, a length of 300 mm, and a particle size of 10 μm;
[0055] Detection wavelength: 205nm;
[0056] Column temperature: 25°C;
[0057] Flow rate: 1.5ml / min;
[0058] Injection volume: 20 μl.
[0059] 2. Solution preparation:
[0060] Test solution: Precisely pipette 1ml of acetylcysteine solution (specification: 3ml: 0.3g) for inhalation, dilute with mobile phase so that the content of acetylcysteine per 1ml is 4mg, specific reference, precise pipetting Put 1ml of acetylcysteine solution for inhalation into a...
Embodiment 2
[0122] Only the flow rate in the liquid chromatography detection conditions in Example 1 was adjusted from 1.5ml / min to 1.4ml / min, and the preparation of the solution was the same as in Example 1.
Embodiment 3
[0124] Only the flow rate in the liquid chromatography detection conditions in Example 1 was adjusted from 1.5ml / min to 1.6ml / min, and the preparation of the solution was the same as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com