Differentially protected orthogonal lanthionine technology
A technology of differential and protective groups, applied in the technical field of differentially protected orthogonal lanthionine, can solve the problem of not allowing the construction of molecules with overlapping rings and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
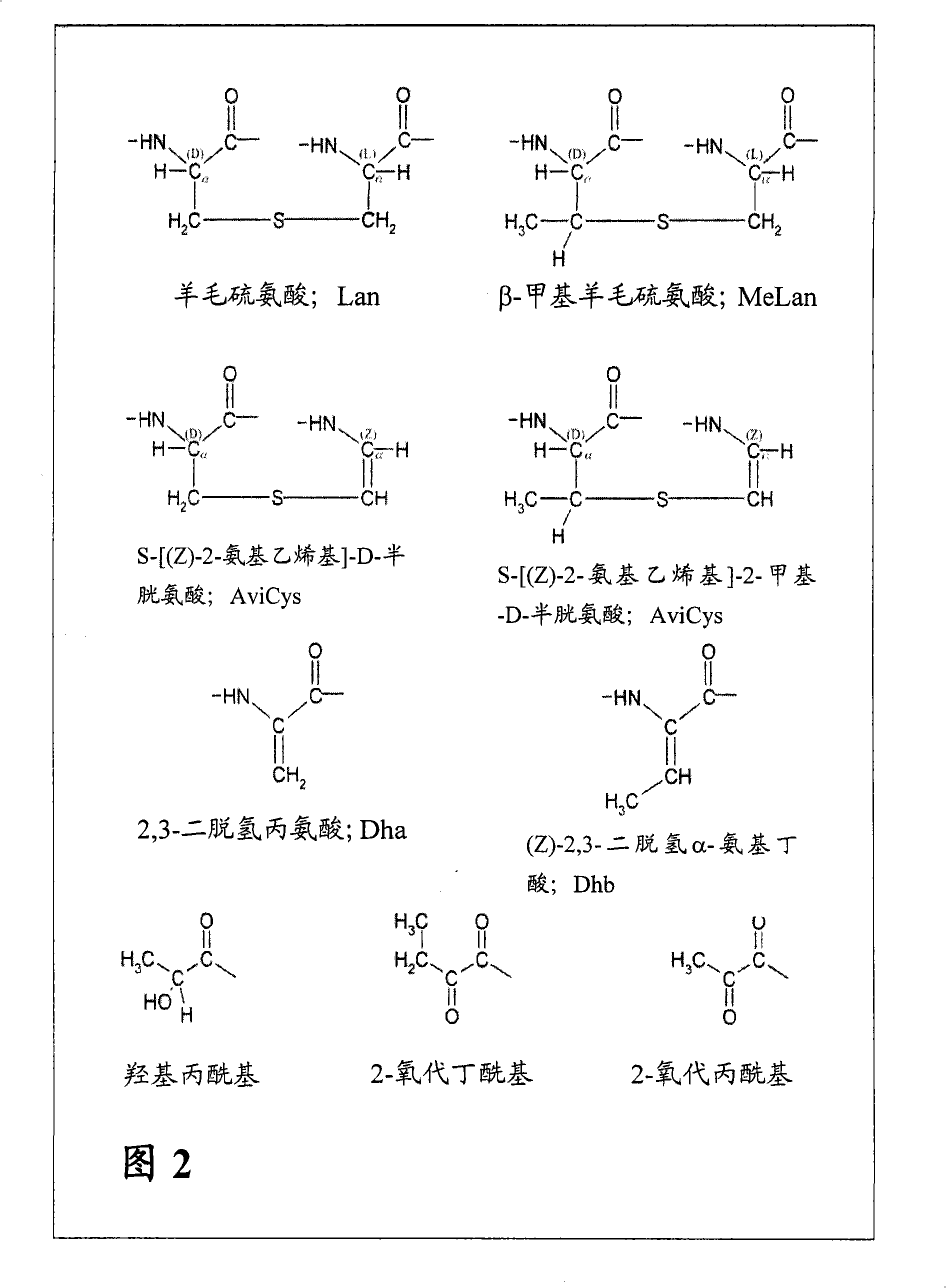
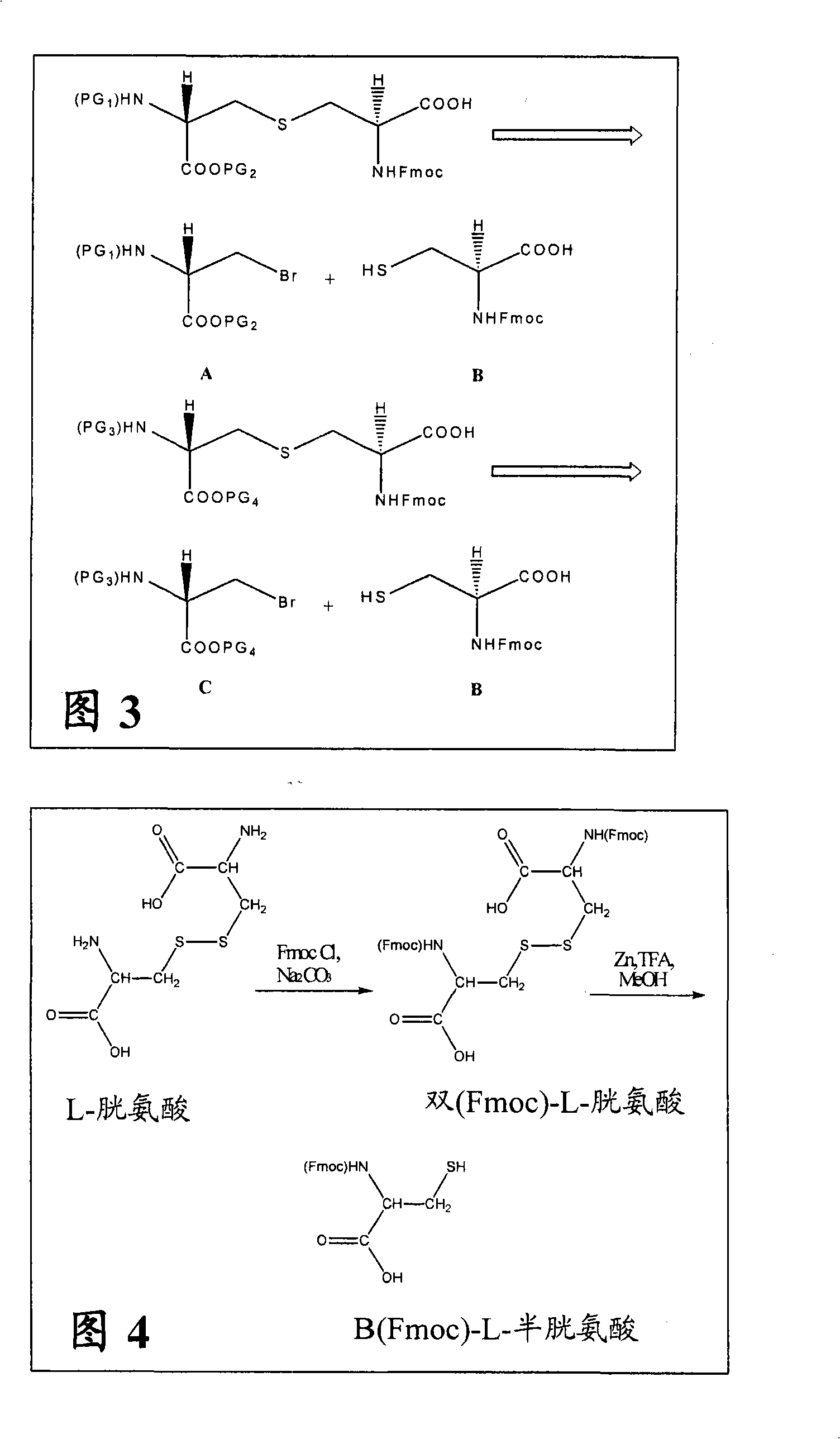
[0160] Example 1: Synthesis of Differentially Protected Orthogonal Lanthionines
[0161] A. Synthesis of Fmoc-Cys
[0162] Fmoc-protected cysteine ( FIG. 3 , structure B) was synthesized from L-cystine in a two-step sequence as outlined in FIG. 4 . Sodium carbonate (4.6 g, 43.6 mmol) and L-cystine (5.0 g, 20.8 mmol) were dissolved in water (200 mL). The obtained solution was cooled to 10°C. FmocCl (11.85 g, 45.8 mmol) was dissolved in dioxane (80 mL), and the obtained solution was added dropwise to an aqueous solution of L-cystine. The solution was stirred at 10°C for 2 hours and allowed to gradually warm to room temperature. A sticky white precipitate was obtained which was filtered onto a sintered glass funnel. The product was triturated with diethyl ether (50 mL) and dried under vacuum for 2 days. N,N'-bis(Fmoc)-L-cystine (14.0 g, yield 98%) was obtained as a white powder.
[0163] N,N'-bis(Fmoc)-L-cystine (12.0 g, 17.5 mmol) was dissolved in methanol (300 mL). G...
Embodiment 2
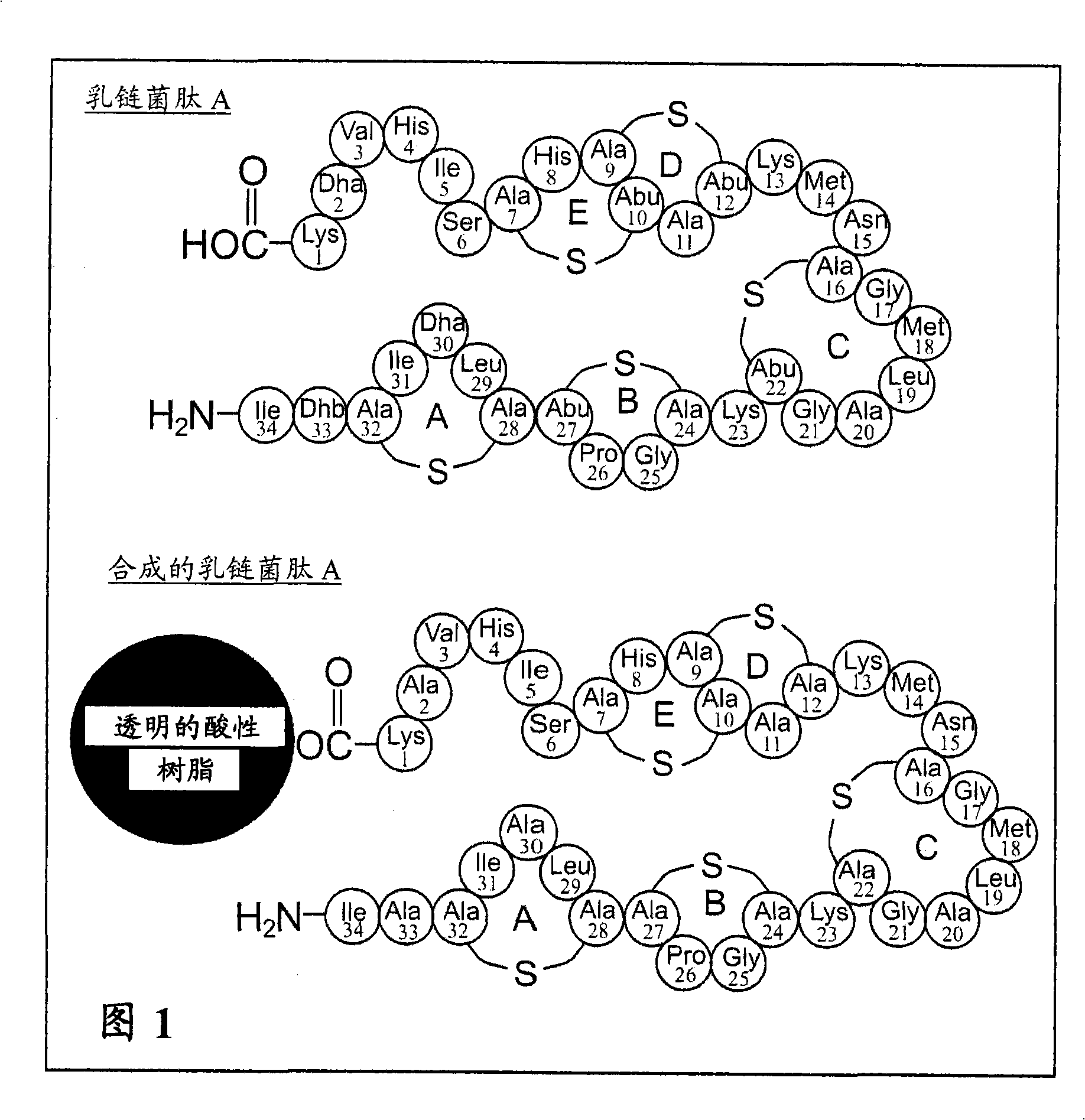
[0177] Example 2: Synthesis of the lantibiotic nisin A using lanthionine 1 and 2 thing
[0178] A. Solid-phase peptide synthesis of nisin A analogs
[0179] The synthesis of a nisin A analog [SEQ ID NO: 2] according to the present invention is outlined below. This analog contains alanine substitutions to dehydroa-aminobutarine at position 33 and dehydroalanine at positions 30 and 2. Considerable evidence shows that this has no significant effect on the activity spectrum and potency of the product relative to native nisin A (Kuipers et al., (1996); Devos et al., (1995), Molecular Microbiology 17, 427-437; Sahl et al., (1995 ), European Journal of Biochemistry 230, 827-853; Bierbaum et al., (1996), Applied and Environmental Microbiology 62, 385-392).
[0180] Unless otherwise stated, all protocols are standard FmocSPPS methods reported in the literature. White (2003) Fmoc Solid Phase Peptide Synthesis, Apractical Approach, Oxford University Press, Oxford. Nisin A is synt...
Embodiment 3
[0208] Example 3: Structural and Biological Analysis of Purified Nisin A Analogs
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com