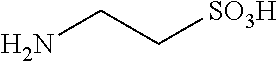Patents
Literature
116 results about "Prediabetes" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The time period before development of type 2 diabetes where blood sugar is high, but no other symptoms of diabetes are seen.
Use of angiotensin II receptor antagonists
InactiveUS20050070594A1Lower Level RequirementsGood blood pressureBiocideMetabolism disorderBlood pressurePrediabetes
The invention relates to the use of angiotensin II receptor antagonists for treating people in whom type 2 diabetes mellitus has been diagnosed or who are suspected of prediabetes, for preventing diabetes or for treating metabolic syndrome and insulin resistance in patients with normal blood pressure.
Owner:BOEHRINGER INGELHEIM INT GMBH
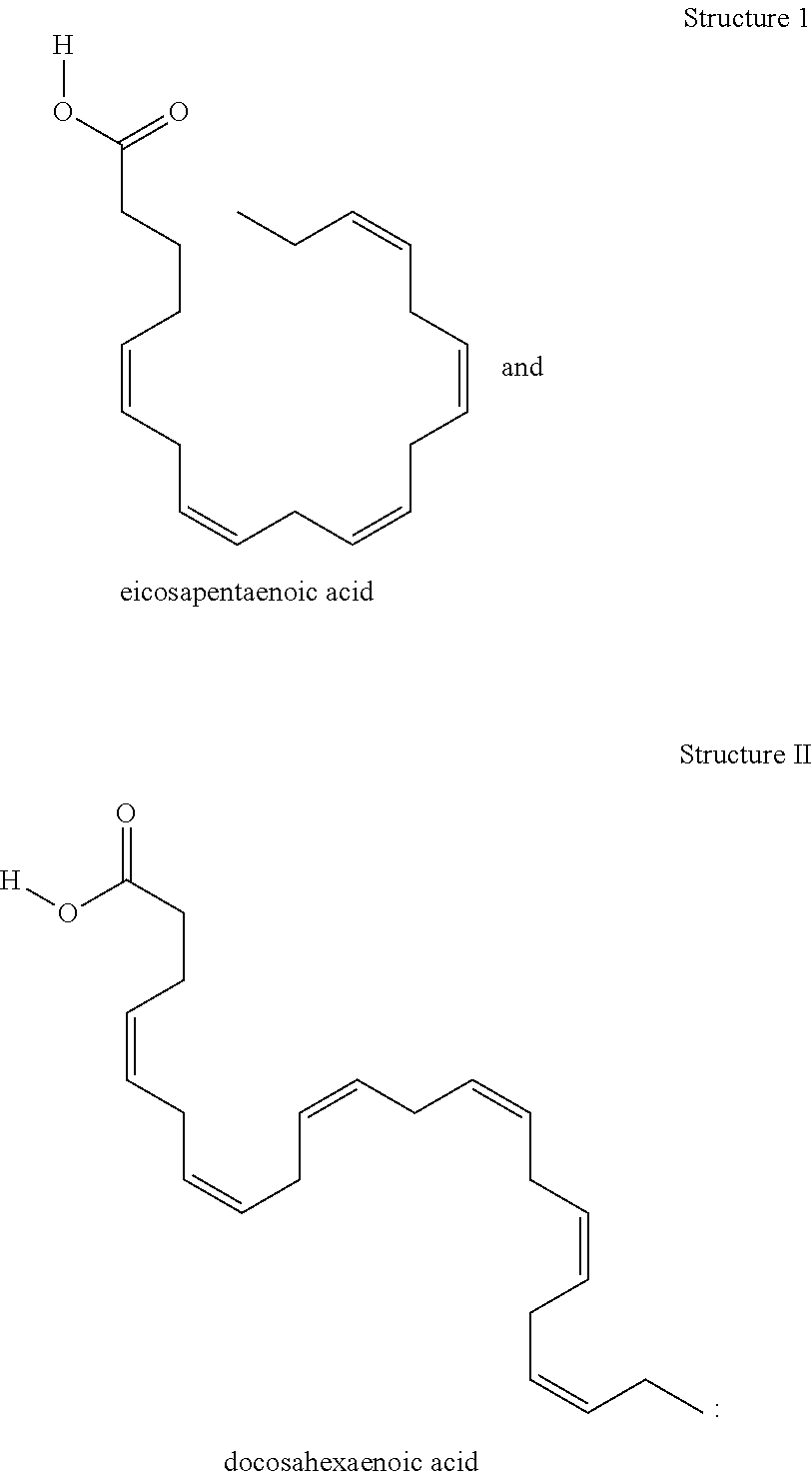
Glycemic control for prediabetes and/or diabetes Type II using docosahexaenoic acid
InactiveUS20040092590A1Improve blood sugar controlReduce drug side effectsBiocidePeptide/protein ingredientsDocosahexaenoic acidPrediabetes
This invention is directed to methods of treating patients with metabolic syndrome, prediabetes and / or Type II diabetes mellitus by administering docosahexaenoic acid (DHA) alone or in combination with diabetes-related medications.
Owner:MARTEK BIOSCIENCES CORP
Method for Treatment of Diabetes and Prediabetes with Low-Level Laser Therapy
InactiveUS20110087312A1Lower Level RequirementsSurgical instrument detailsLight therapyDiabetes mellitusPrediabetes
Low-level laser therapy is applied externally through the skin of the patient to targeted areas of a of a patient's body to treat diabetes or prediabetes. A patient is first diagnosed with diabetes or prediabetes, preferably by blood draws that measure insulin, A1C, or glucose levels. A therapeutic amount of laser energy to apply is determined from a patient's biological factors such as body mass index. The therapeutic amount of laser energy is applied one or more times over a two week time period. The patient is then retested for diabetes or prediabetes. If necessary, a new therapeutic amount of laser energy is determined according to any changes in the patient's diabetes or prediabetes diagnosis and any changes in the patient's biological factors. Adjusted treatments with low-level laser energy continue until the diabetes or prediabetes diagnosis is removed.
Owner:ERCHONIA CORP
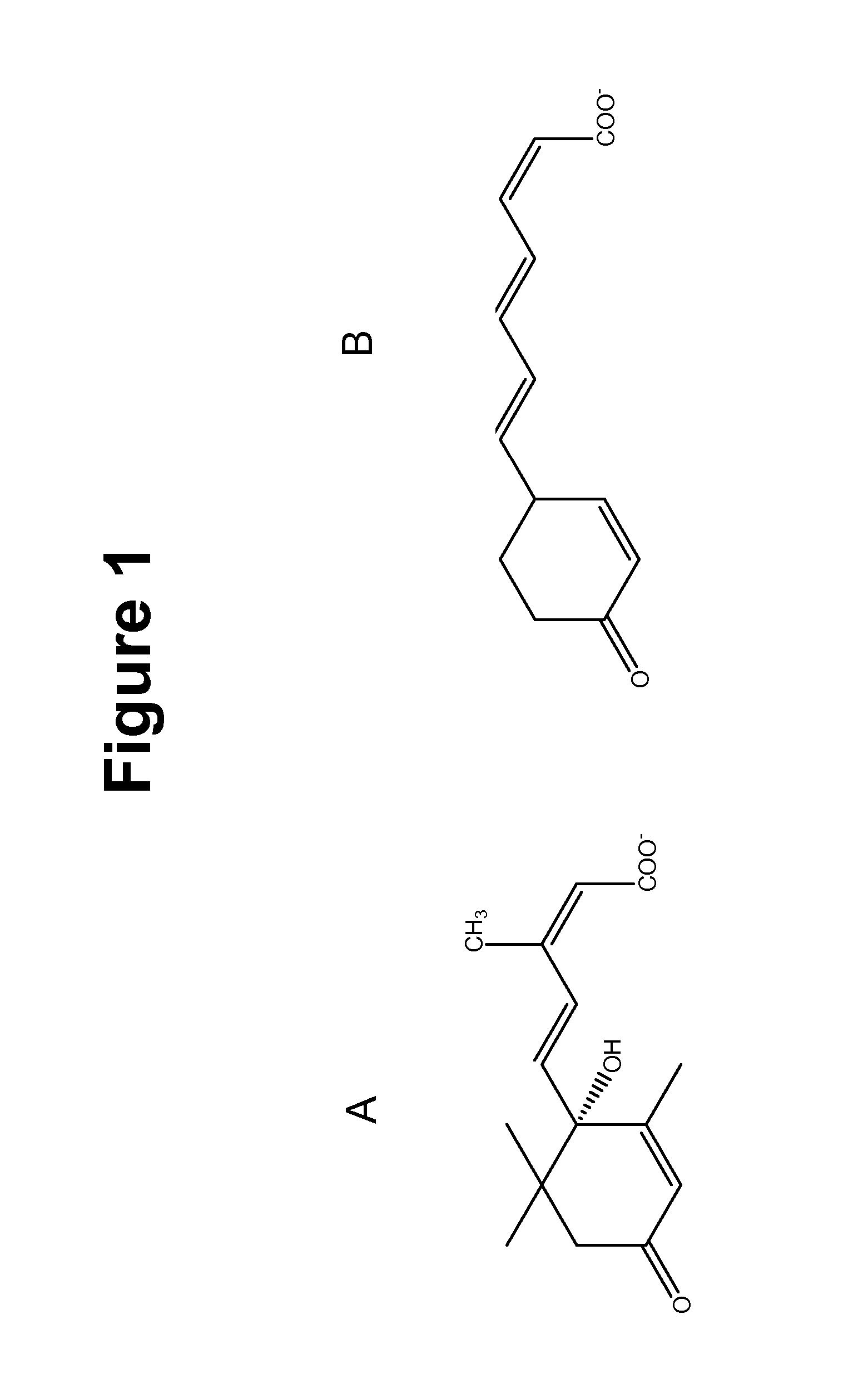
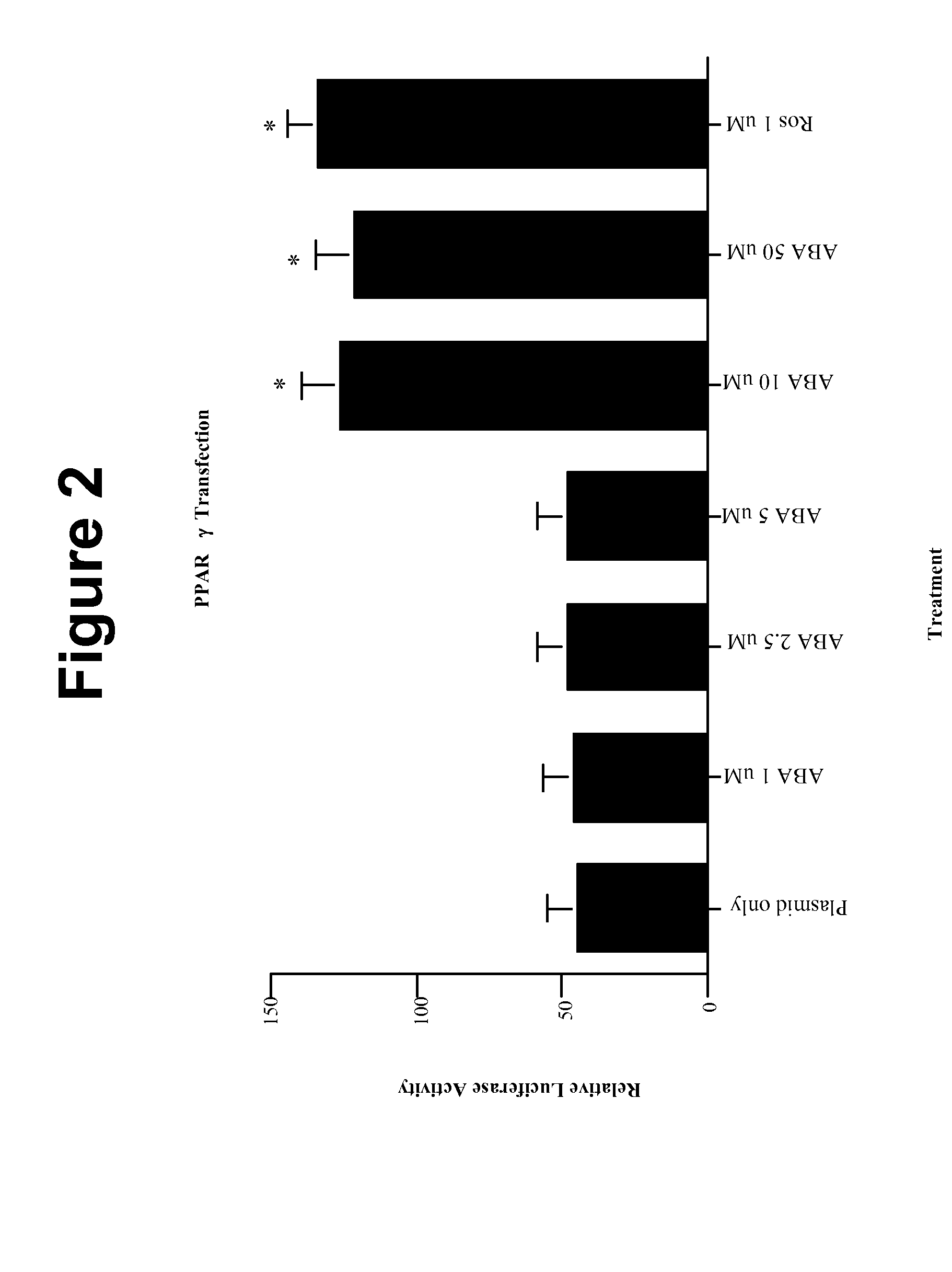
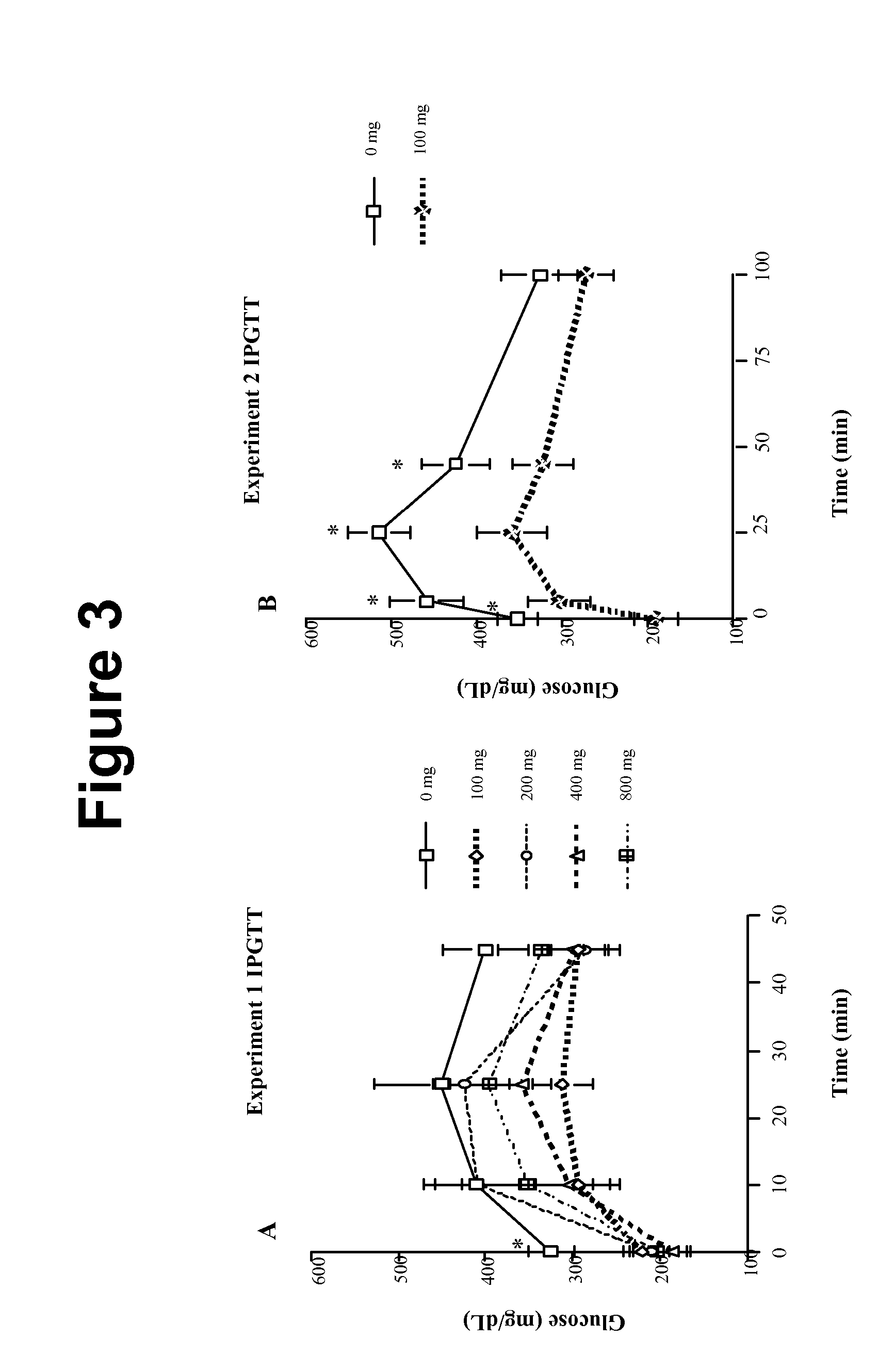
Method of using abscisic acid to treat diseases and disorders
The present invention provides compositions and methods for treating and / or preventing diseases and disorders associated with expression of PPAR γ and / or infiltration of macrophages into skeletal muscle tissue and / or white adipose tissue. The method treats such diseases and disorders with abscisic acid (ABA). Exemplary diseases and disorders include diabetes, including type 2 diabetes, prediabetes, glucose intolerance insulin resistance, and diseases and disorders involving the immune system, such as inflammation, including obesity-related inflammation, inflammatory bowel disease, type 1 diabetes, multiple sclerosis, allergies, asthma, cardiovascular disease, and arthritis.
Owner:VIRGINIA TECH INTPROP INC
Pharmaceutical composition, methods for treating and uses thereof
InactiveUS20140315832A1Good effectFew complianceBiocidePeptide/protein ingredientsPrediabetesInternal medicine
The present invention relates to certain SGLT-2 inhibitors for treating, preventing, protecting against and / or delaying the progression of chronic kidney disease in patients, for example patients with prediabetes, type 1 or type 2 diabetes mellitus.
Owner:BOEHRINGER INGELHEIM INT GMBH
Novel PPAR ligands that do not cause fluid retention, edema or congestive heart failure
Methods are provided for treating or prophylactically preventing metabolic disorders in humans without causing, promoting, or aggravating fluid retention, peripheral edema, pulmonary edema, or congestive heart failure, by administration of a therapeutically effective amount of a compound sufficient to partially or fully activate peroxisome proliferator activated receptors (PPARs) and partially or fully inhibit, antagonize or block the activity of angiotensin II type 1 receptors. Metabolic disorders that can be treated or prevented include but are not limited to type 2 diabetes, the metabolic syndrome, prediabetes, and other insulin resistance syndromes. Compounds are provided that antagonize or block the angiotensin II type 1 (AT1) receptor, function as partial or full activators of peroxisome proliferator activated receptors (PPARs), can be used to treat or prevent diseases known to be treatable or preventable by PPAR activators and were not previously recognized to be therapeutic targets for angiotensin II receptor antagonists.
Owner:BETHESDA PHARMA
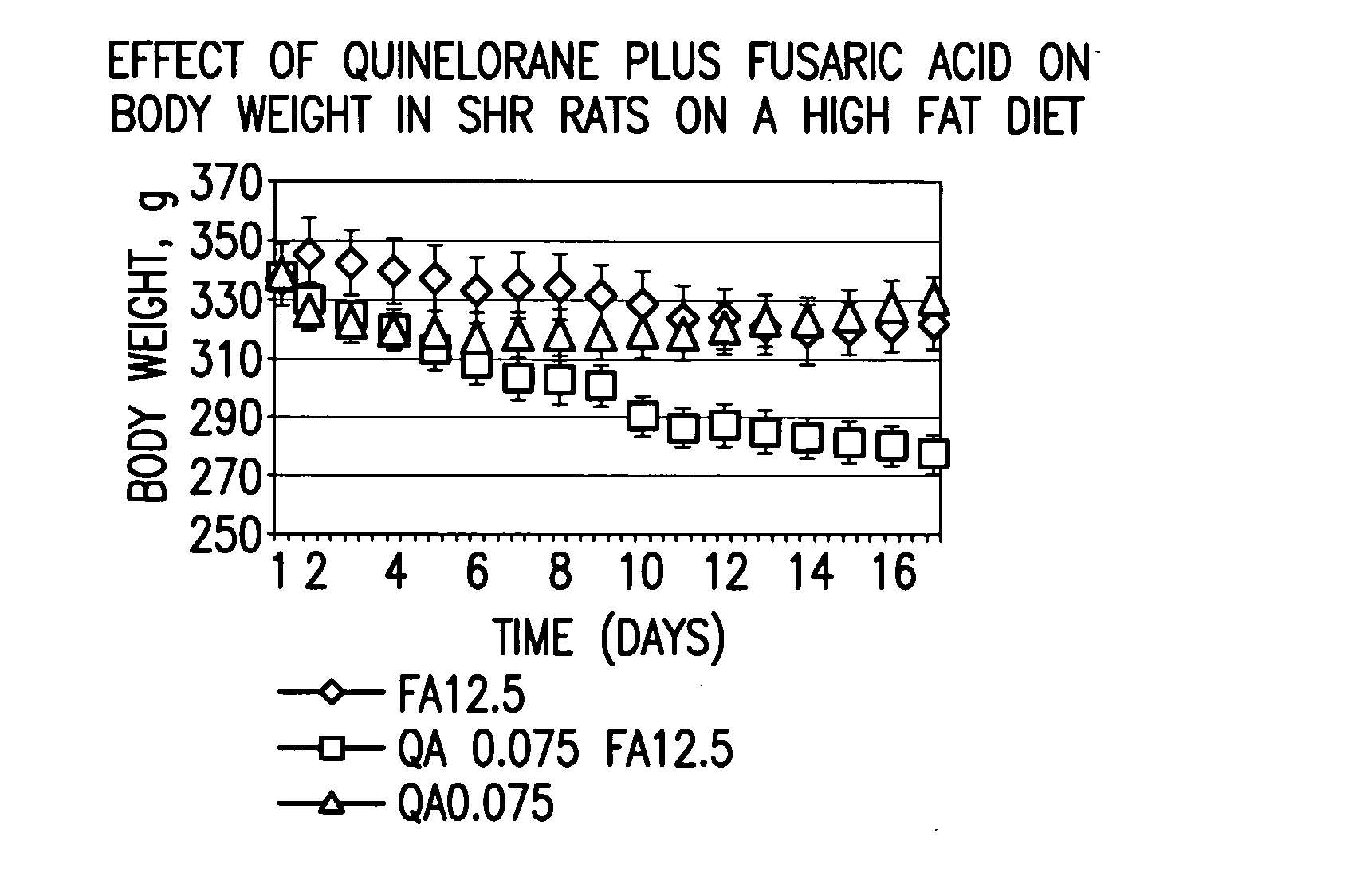
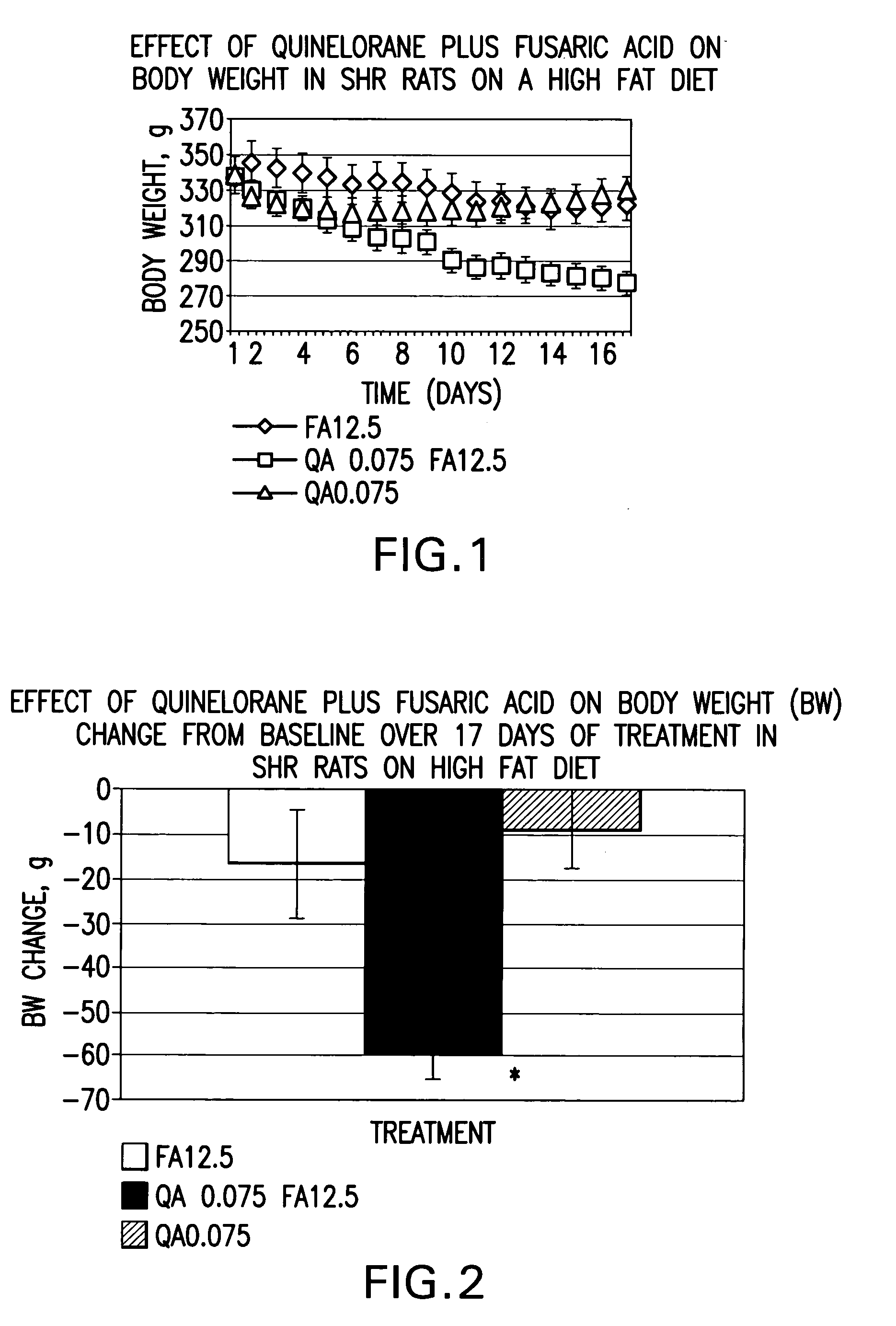
Therapeutic treatment for metabolic syndrome, type 2 diabetes, obesity, or prediabetes
ActiveUS20080293735A1Increases central neuronal dopamine activityReduced activityBiocidePeptide/protein ingredientsNoradrenergic neuronsPrediabetes
The present invention is directed to a method for treating a patient suffering from the metabolic syndrome, Type 2 diabetes, obesity, or prediabetes, comprising the step of increasing the ratio of dopaminergic neuronal to noradrenergic neuronal activity within the central nervous system and particularly the hypothalamus of the central nervous system of the patient.In another aspect, the present invention is directed to a method for treating a patient suffering from a metabolic disorder such as the metabolic syndrome, Type 2 diabetes, obesity, or prediabetes, and the metabolic sequale of these diseases including cardiovascular, cerebrovascular, renal and hepatic diseases, comprising the step of: administering to a patient suffering from the metabolic syndrome, Type 2 diabetes, obesity, or prediabetes a pharmaceutical composition comprising (1) at least one compound that stimulates an increase in central dopaminergic neuronal activity level in the subject, and (2) at least one compound that stimulates a decrease in central noradrenergic neuronal activity level in the subject. The present invention is also directed to pharmaceutical compositions that include the above compounds and a pharmaceutically acceptable carrier.
Owner:VEROSCI
Metabolic markers of diabetic conditions and methods of use thereof
Novel methods for assessing the state of a diabetic condition of a subject are described, comprising determining the amount of a metabolite in a sample from a body fluid or tissue of the subject. The methods may be used, for example, in diagnosing and monitoring insulin resistance, prediabetes, or the response to a drug which alters a diabetic condition.
Owner:TETHYS BIOSCI
Metabolic markers of diabetic conditions and methods of use thereof
Novel methods for assessing the state of a diabetic condition of a subject are described, comprising determining the amount of a metabolite in a sample from a body fluid or tissue of the subject. The methods may be used, for example, in diagnosing and monitoring insulin resistance, prediabetes, or the response to a drug which alters a diabetic condition.
Owner:TRUE HEALTH IP LLC
Lipid-lowering antidiabetic agent
InactiveUS20120178813A1Improve bioavailabilityHigh degreeBiocideOrganic chemistryTriglyceridePrediabetes
A composition which includes a salt of metformin and the use of the composition for treatment of or use in prediabetes, diabetes, lowering triglycerides and / or other conditions in mammals.
Owner:THETIS PHARMA
Pegylation of Vasoactive Intestinal Peptide (Vip) / Pituitary Adenylate Cyclase Activating Peptide (Pacap) Receptor 2 (Vpac2) Agonists and Methods of Use
InactiveUS20080261863A1Higher effective dosLower blood pressurePeptide/protein ingredientsVasoactive intestinal peptideSide effectTreatment options
This invention relates to modified Vasoactive Intestinal Peptide (VIP) / Pituitary Adenylate Cyclase Activating Peptide (PACAP) Receptor 2 (VPAC2) agonists (VPAC2 agonists) comprising a VPAC2 agonist linked to a polyethylene glycol polymer, as well as related formulations, dosages, and methods of administration thereof for therapeutic purposes. These VPAC2 agonists, compositions, and methods are useful in providing a treatment option for those individuals afflicted with a metabolic disorder such as diabetes, impaired glucose tolerance, metabolic syndrome, or prediabetic states, by inducing glucose-dependent insulin secretion in the absence of the therapeutically limiting side effect of reducing or lowering blood pressure.
Owner:BAYER HEALTHCARE LLC
Therapeutic treatment for the metabolic syndrome, type2 diabetes, obesity, or prediabetes
InactiveUS20050054734A1Raise the ratioOrganic active ingredientsBiocideNervous systemNoradrenergic neurons
The present invention is directed to a method for treating a patient suffering from the metabolic syndrome, Type 2 diabetes, obesity, or prediabetes, comprising the step of increasing the ratio of dopaminergic neuronal to noradrenergic neuronal activity within the hypothalamus of the central nervous system of the patient. In another aspect, the present invention is directed to a method for treating a patient suffering from the metabolic syndrome, Type 2 diabetes, obesity, or prediabetes, comprising the step of: administering to a patient suffering from the metabolic syndrome, Type 2 diabetes, obesity, or prediabetes a pharmaceutical composition comprising (1) at least one compound that stimulates an increase in central dopaminergic neuronal activity level in the subject, and (2) at least one compound that stimulates a decrease in central noradrenergic neuronal activity level in the subject. The present invention is also directed to pharmaceutical compositions that include the above compounds and a pharmaceutically acceptable carrier.
Owner:CINCOTTA ANTHONY H
Therapeutic Treatment for Metabolic Syndrome, Type 2 Diabetes, Obesity, or Prediabetes
InactiveUS20130274246A1Increases central neuronal dopamine activityPrevent arteriosclerosisBiocideMetabolism disorderAdrenergicPrediabetes
The present invention is directed to a method for treating a patient suffering from the metabolic syndrome, Type 2 diabetes, obesity, or prediabetes, comprising the step of increasing the ratio of dopaminergic neuronal to noradrenergic and / or serotonin neuronal activity within the central nervous system and particularly the hypothalamus of the central nervous system of the patient.
Owner:VEROSCI
PPAR Ligands that do not cause fluid retention, edema or congestive heart failure
Methods are provided for treating or prophylactically preventing metabolic disorders in humans without causing, promoting, or aggravating fluid retention, peripheral edema, pulmonary edema, or congestive heart failure, by administration of a therapeutically effective amount of a compound sufficient to partially or fully activate peroxisome proliferator activated receptors (PPARs) and partially or fully inhibit, antagonize or block the activity of angiotensin II type 1 receptors. Metabolic disorders that can be treated or prevented include but are not limited to type 2 diabetes, the metabolic syndrome, prediabetes, and other insulin resistance syndromes. Compounds are provided that antagonize or block the angiotensin II type 1 (AT1) receptor, function as partial or full activators of peroxisome proliferator activated receptors (PPARs), can be used to treat or prevent diseases known to be treatable or preventable by PPAR activators and were not previously recognized to be therapeutic targets for angiotensin II receptor antagonists.
Owner:BETHESDA PHARMA
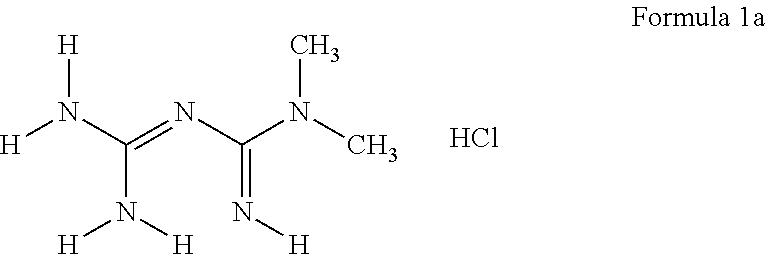
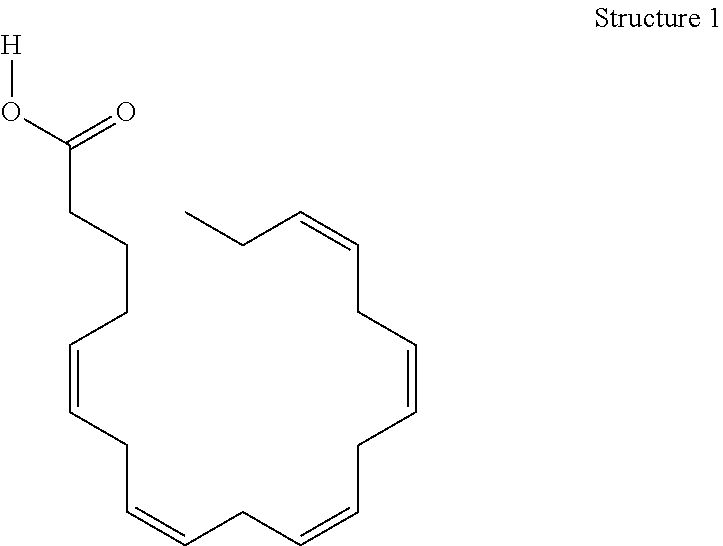
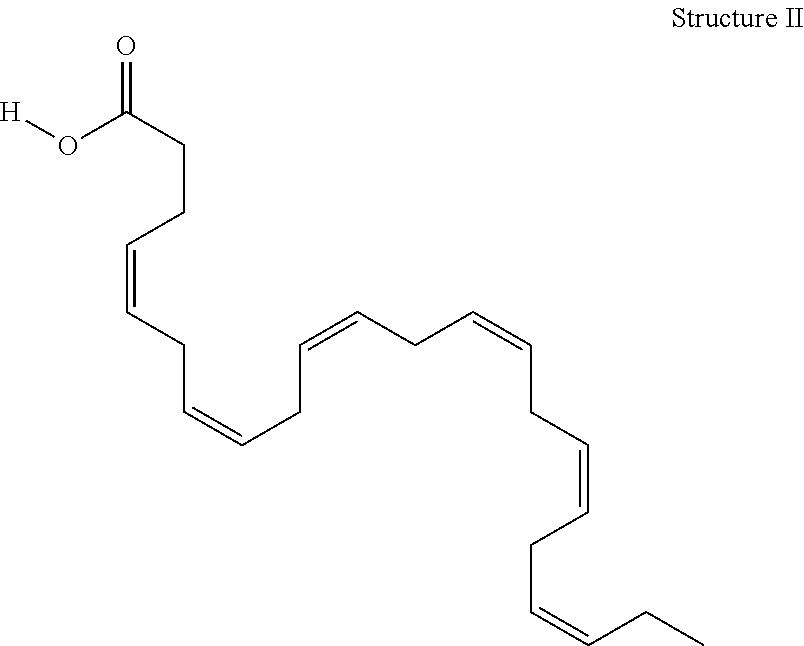
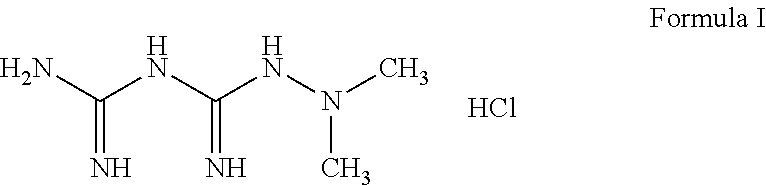
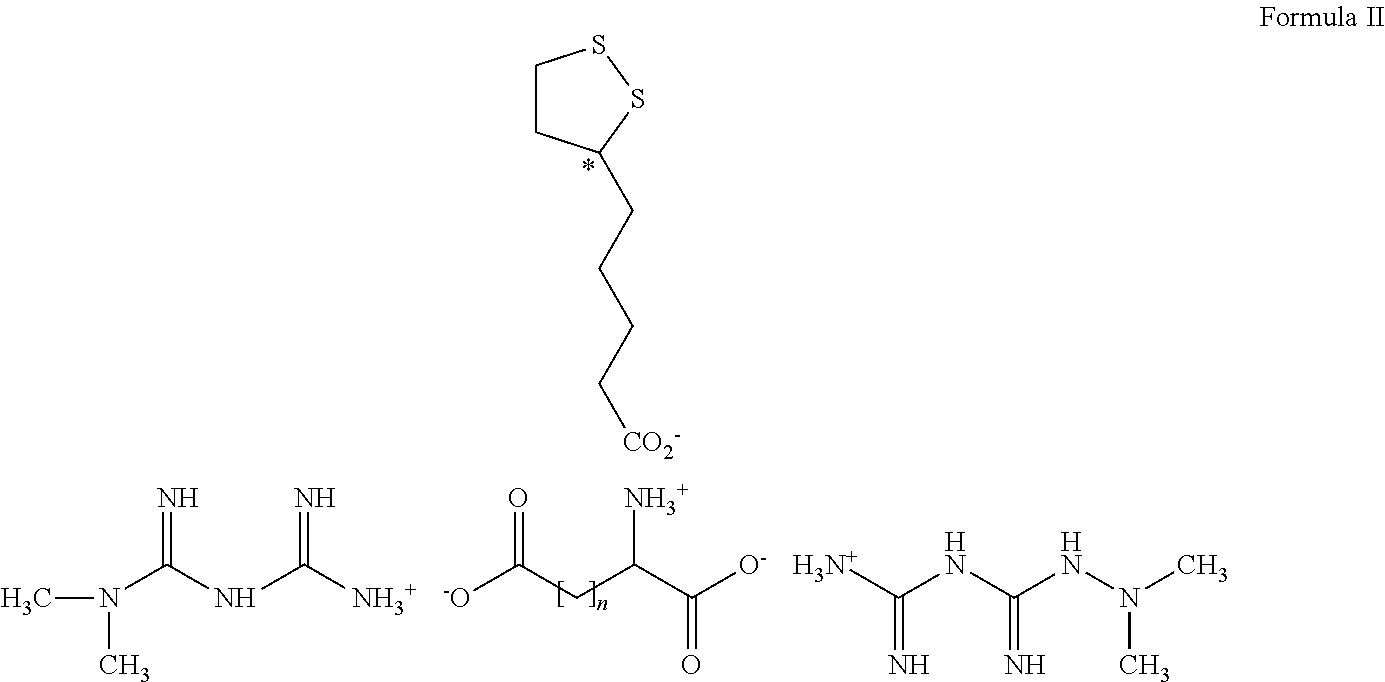
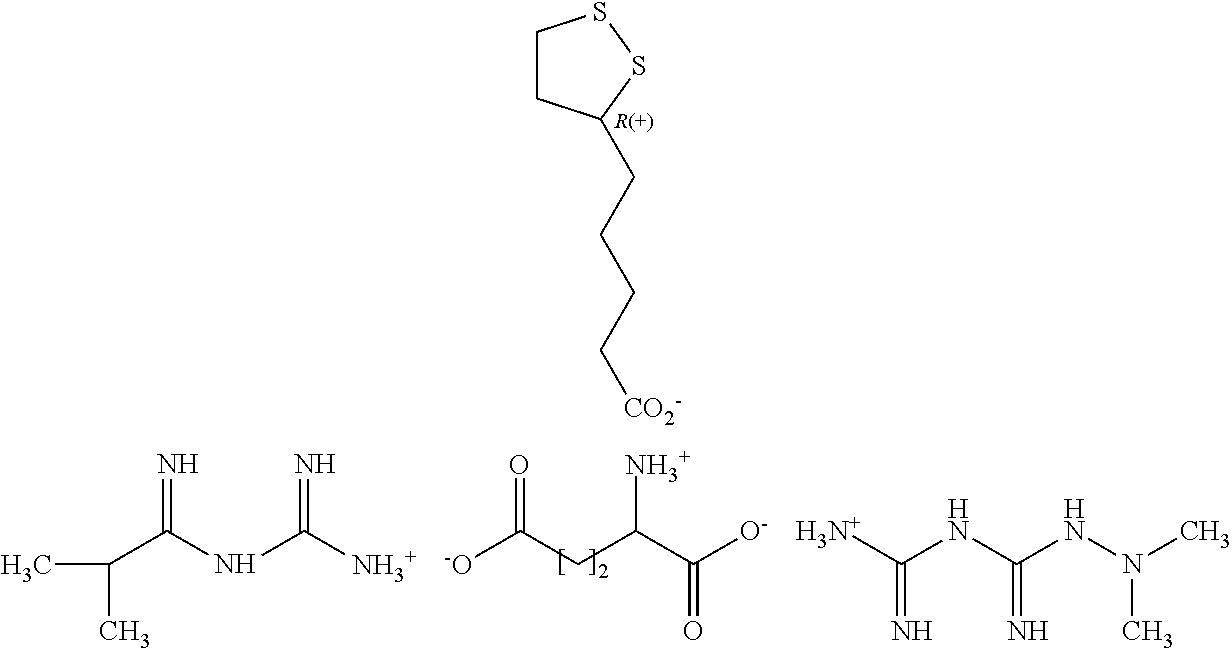
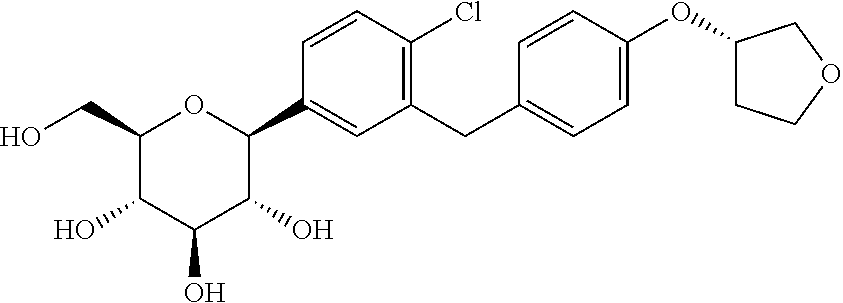
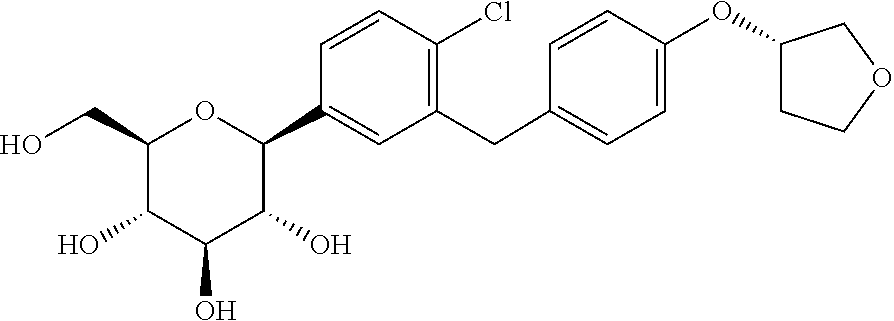
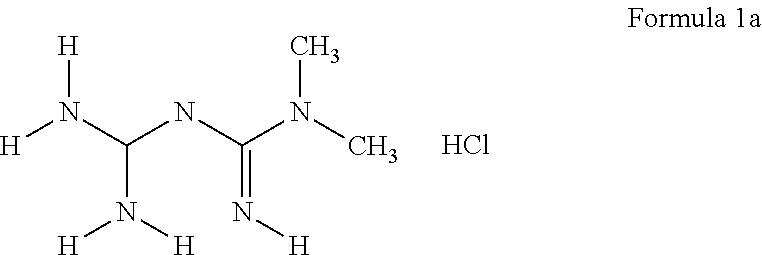
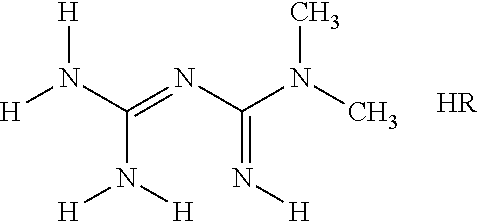
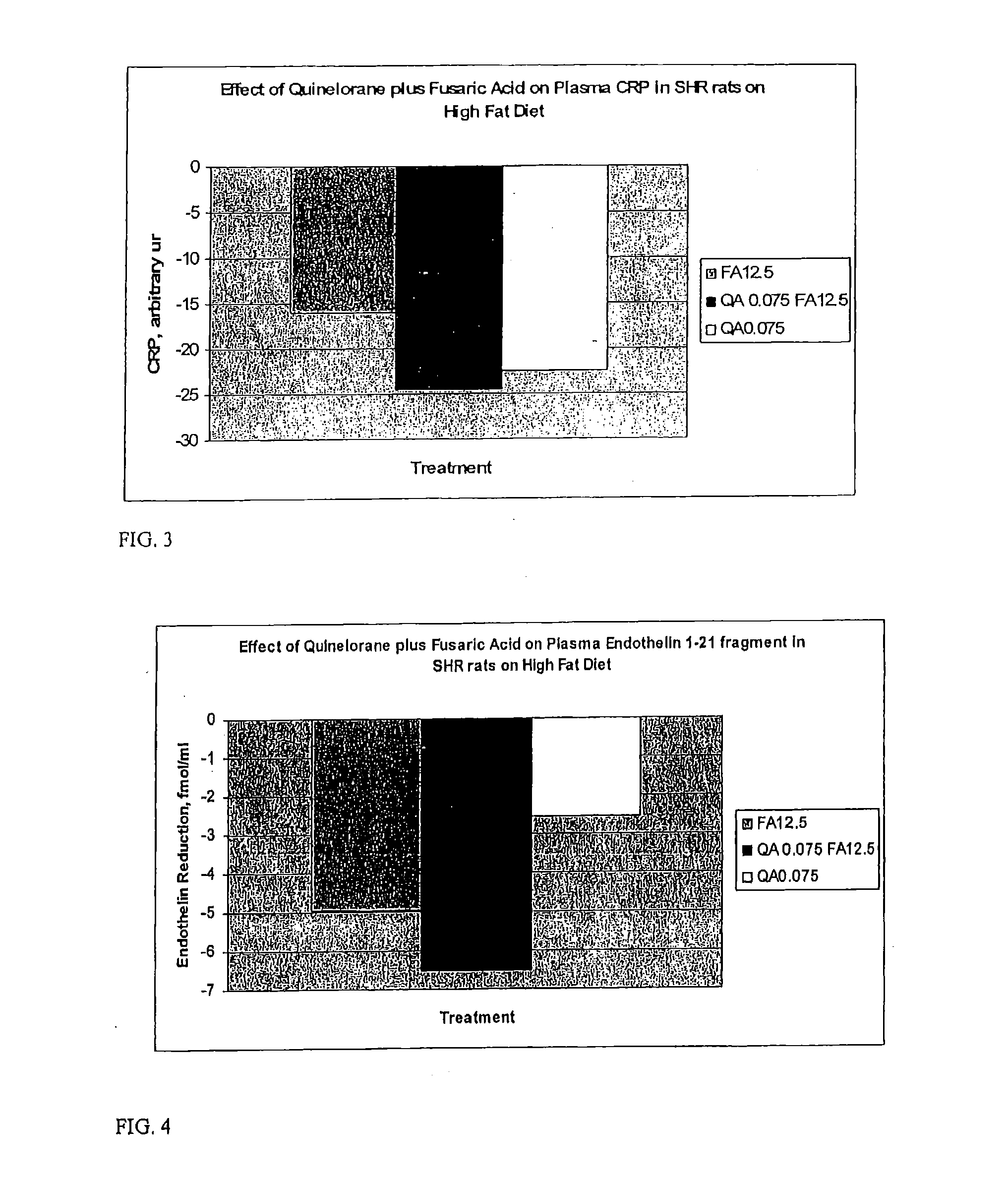
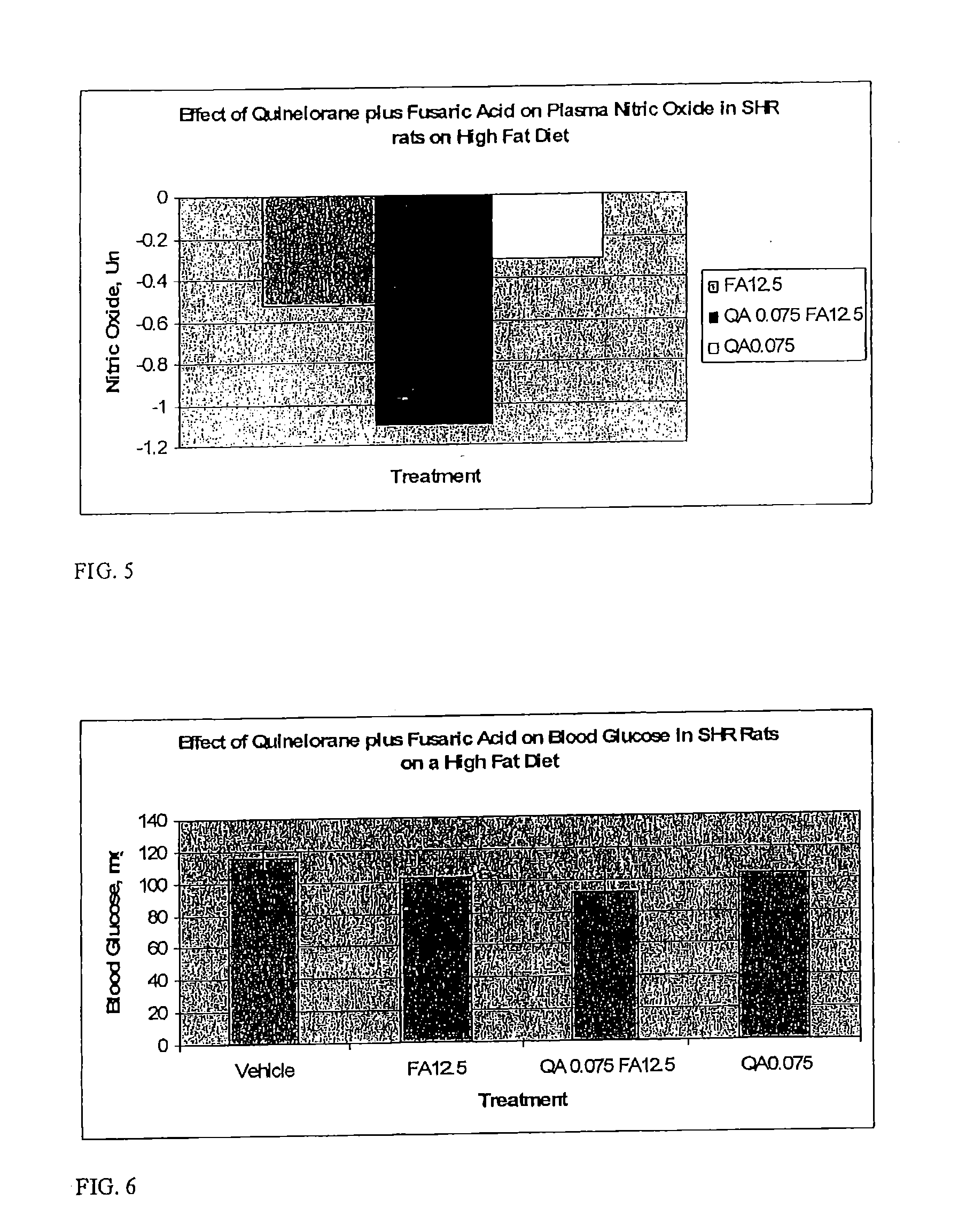
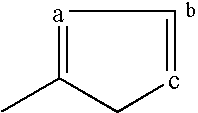
Derivatives of 3-Azabicyclo[3.1.0] Hexane as Dipeptidyl Peptidase-IV Inhibitors
InactiveUS20080300251A1Avoid chemical reactionsBiocideNervous disorderDipeptidyl peptidasePrediabetes
The present invention relates to novel 3-azabicyclo[3.1.0]hexane derivatives as dipeptidyl peptidase-IV inhibitors and the processes for the synthesis of the said compounds. This invention also relates to pharmacological compositions containing the compounds of the present invention, and methods of treating diabetes, especially type 2 diabetes, as well as prediabetes, diabetic dyslipidemia, metabolic acidosis, ketosis, satiety disorders, and obesity. These inhibitors can also be used to treat conditions manifested by a variety of metabolic, neurological, anti-inflammatory, and autoimmune disorders like inflammatory disease, multiple sclerosis, rheumatoid arthritis; viral, cancer and gastrointestinal disorders. The compounds of this invention can also be used for treatment of infertility arising due to polycystic ovary syndrome.
Owner:RANBAXY LAB LTD
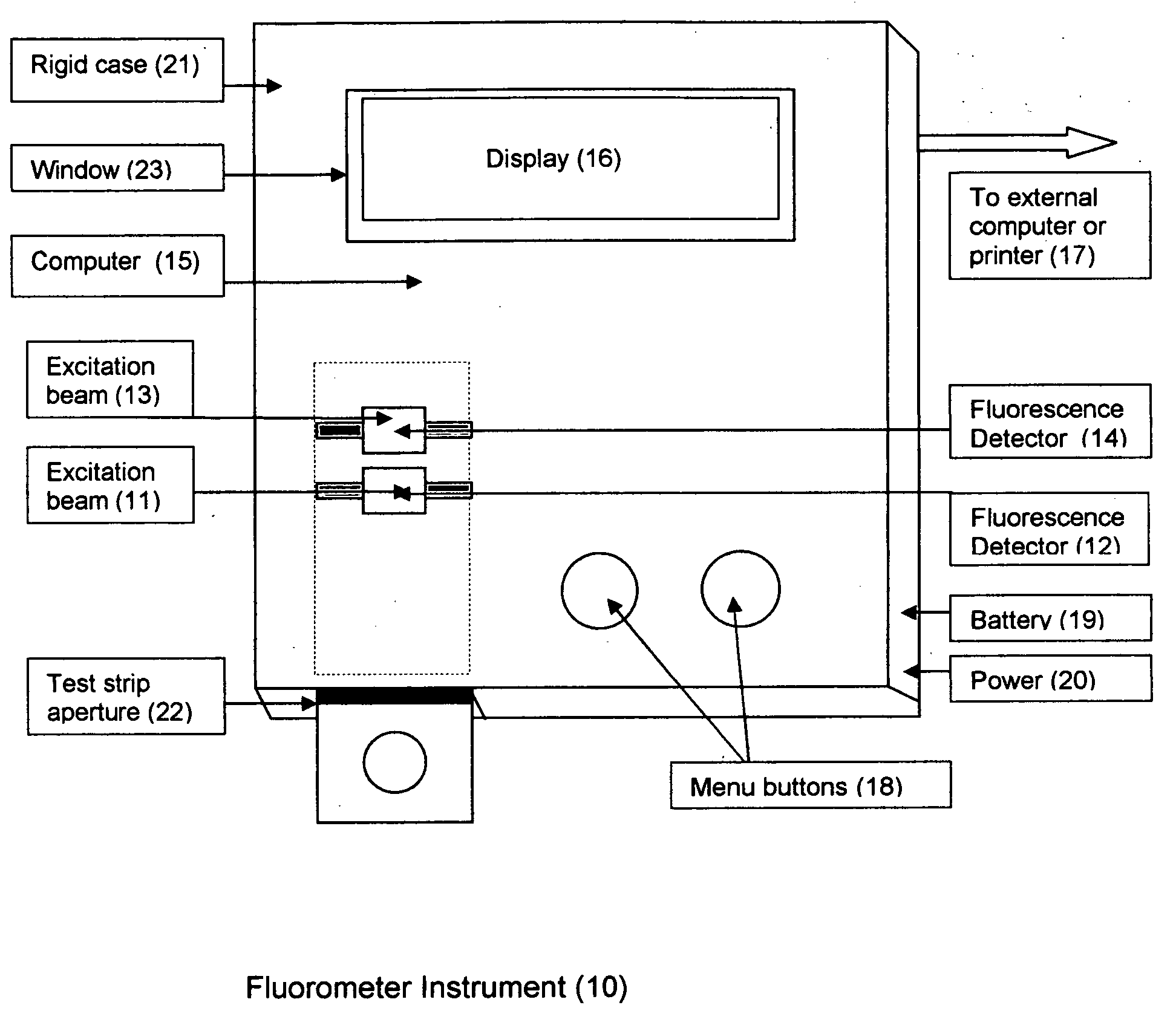
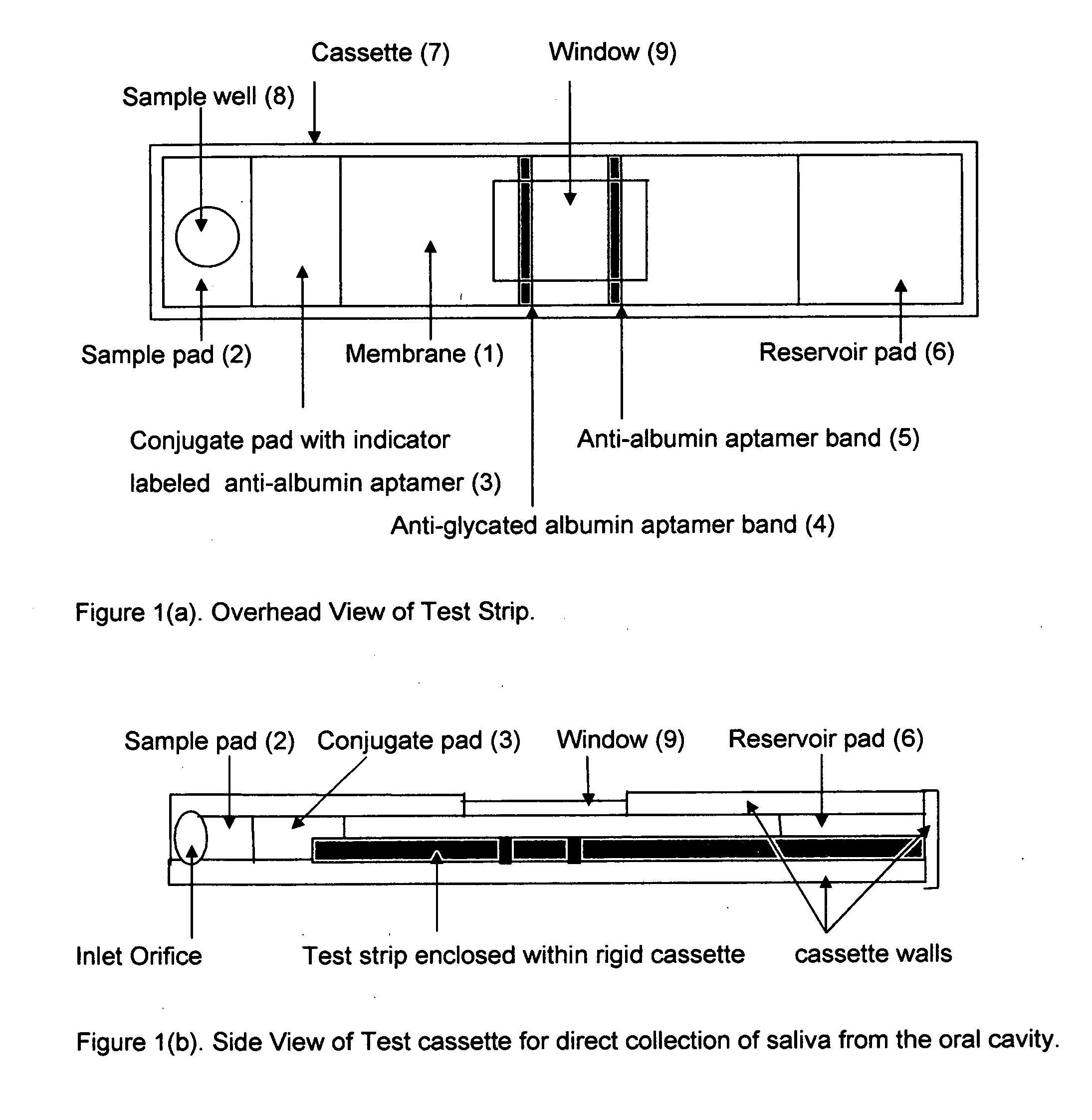
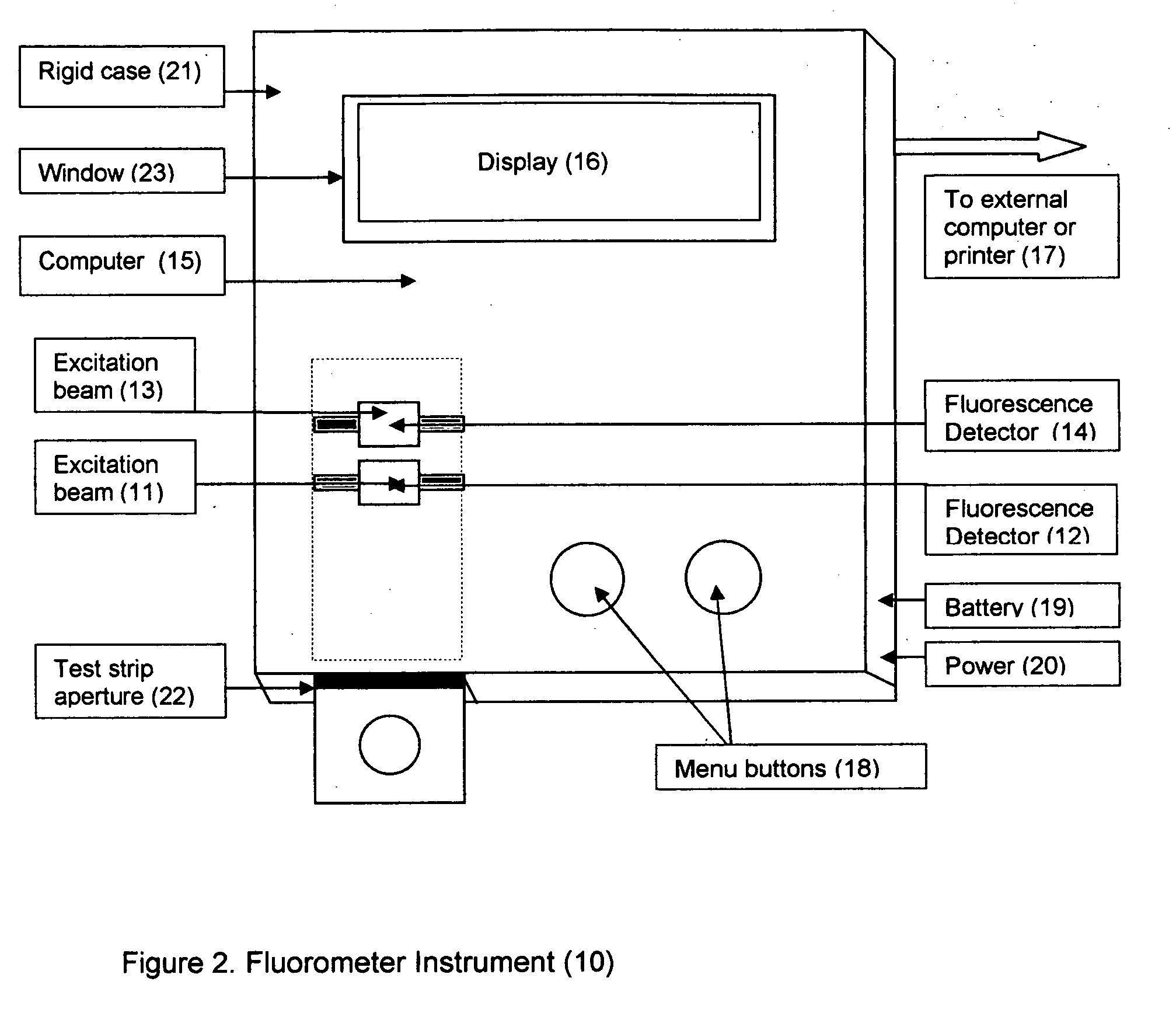
Aptamer based point-of-care test for glycated albumin
InactiveUS20090042237A1Accurate assessmentBioreactor/fermenter combinationsRadiation pyrometryPoint of careMeasuring instrument
This invention describes a point-of-care or home use device for measuring the ratio of glycated albumin to total albumin in saliva and other body fluids. Diabetics and prediabetics may have elevated levels of glucose in their blood that can react with plasma albumin to form glycated albumin. The amount of glycated albumin formed is directly correlated with the level of plasma glucose that the albumin has been exposed to over a period of time. Saliva albumin is derived from plasma albumin and therefore contains glycated and non-glycated albumin fractions that can be measured. The ratio of glycated albumin to total albumin in saliva will provide an indication of the average amount of protein glycation that occurred over the preceding 2-3 week period.The test is performed using a disposable strip or cassette that contains the testing reagents and the results are measured in a measuring instrument that automatically reads, calculates and displays the final result. The results of tests performed over a period of time are stored in the instrument's memory and presented in a numerical or graphical format so that the individual's glycated albumin level can be monitored over time.
Owner:EPINEX DIAGNOSTICS
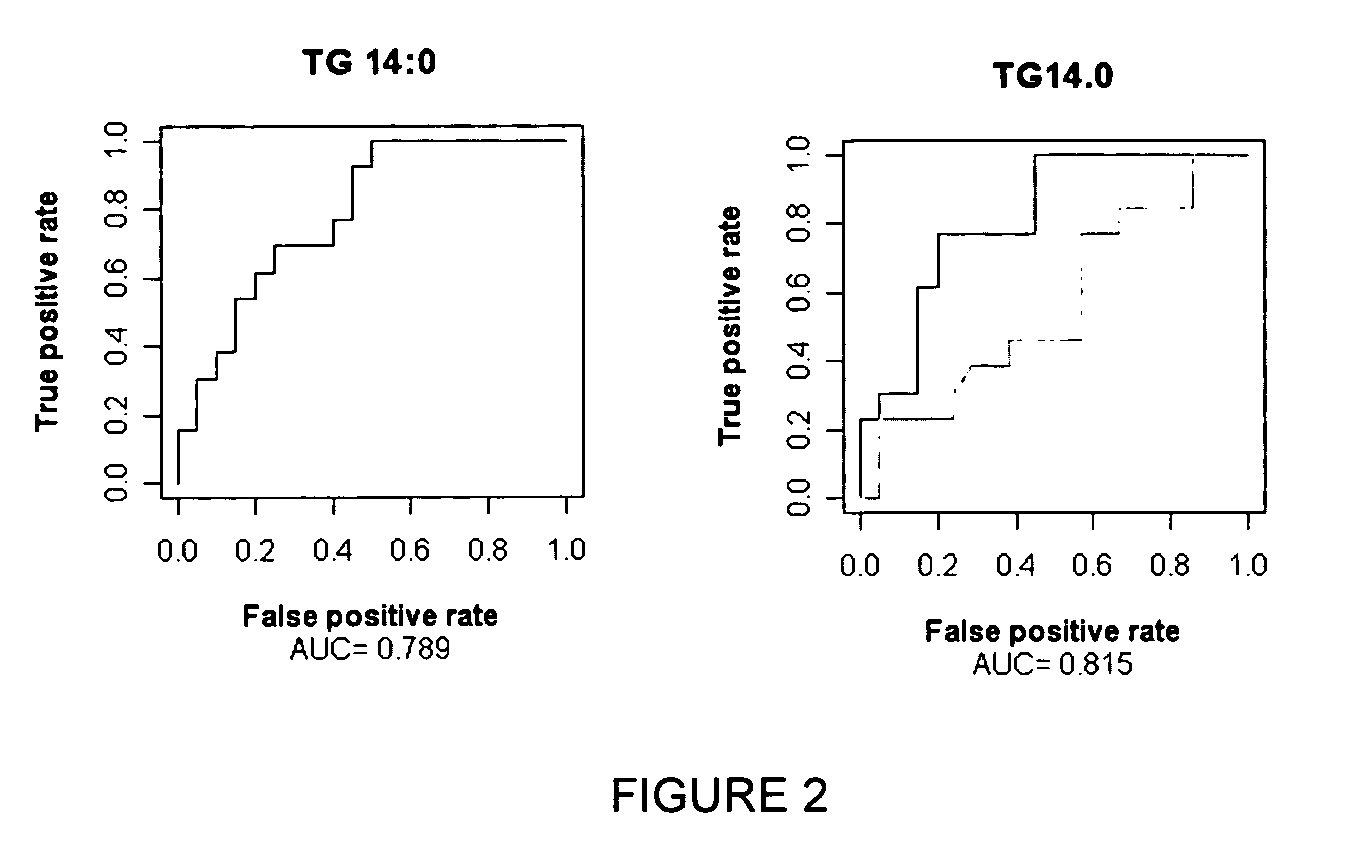
Lipid-lowering antidiabetic agent
InactiveUS20130281535A1Improve bioavailabilityHigh degreeBiocideOrganic chemistryLipid formationTG - Triglyceride
A composition which includes a salt of metformin and the use of the composition for treatment of or use in prediabetes, diabetes, lowering triglycerides and / or other conditions in mammals.
Owner:THETIS PHARMA
Metformin derivatives for treating diabetes and diabetes complications
InactiveUS20140221467A1High blood levelHigh levelBiocideOrganic active ingredientsPrediabetesAcidic amino acids
The invention provides mutual ternary salts of metformin, lipoic acid and acidic amino acids such as aspartic acid and glutamic acid. The invention further provides treatment of prediabetes, diabetes, diabetic complications and / or other conditions in mammals in a method that comprises administering an effective amount of one or more of the foregoing compositions to a mammal in need of such treatment.
Owner:MYLARI BANAVARA L
Pharmaceutical composition, methods for treating and uses thereof
InactiveUS20160038525A1Good effectFew complianceBiocidePeptide/protein ingredientsPrediabetesInternal medicine
The present invention relates to certain SGLT-2 inhibitors for treating, preventing, protecting against and / or delaying the progression of chronic kidney disease in patients, for example patients with prediabetes, type 1 or type 2 diabetes mellitus.
Owner:BOEHRINGER INGELHEIM INT GMBH
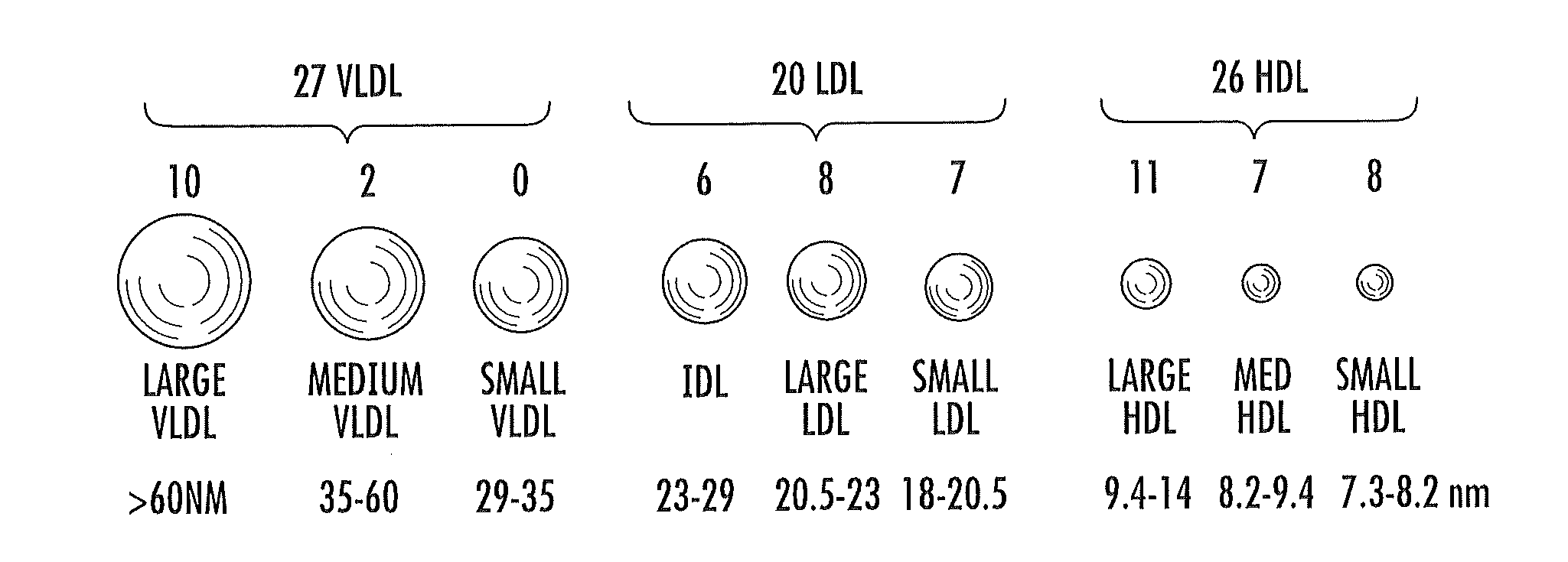
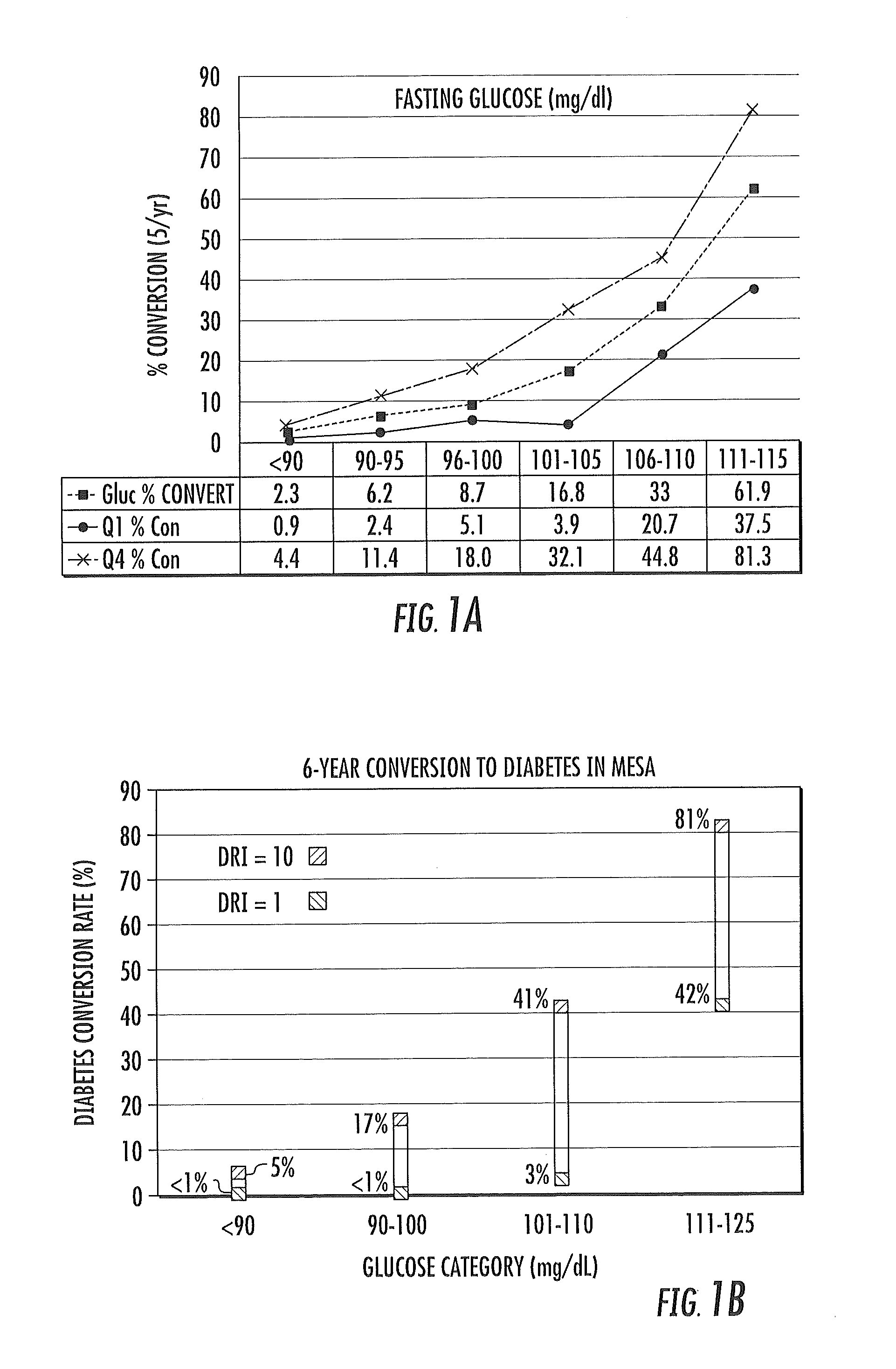
Multi-parameter diabetes risk evaluations
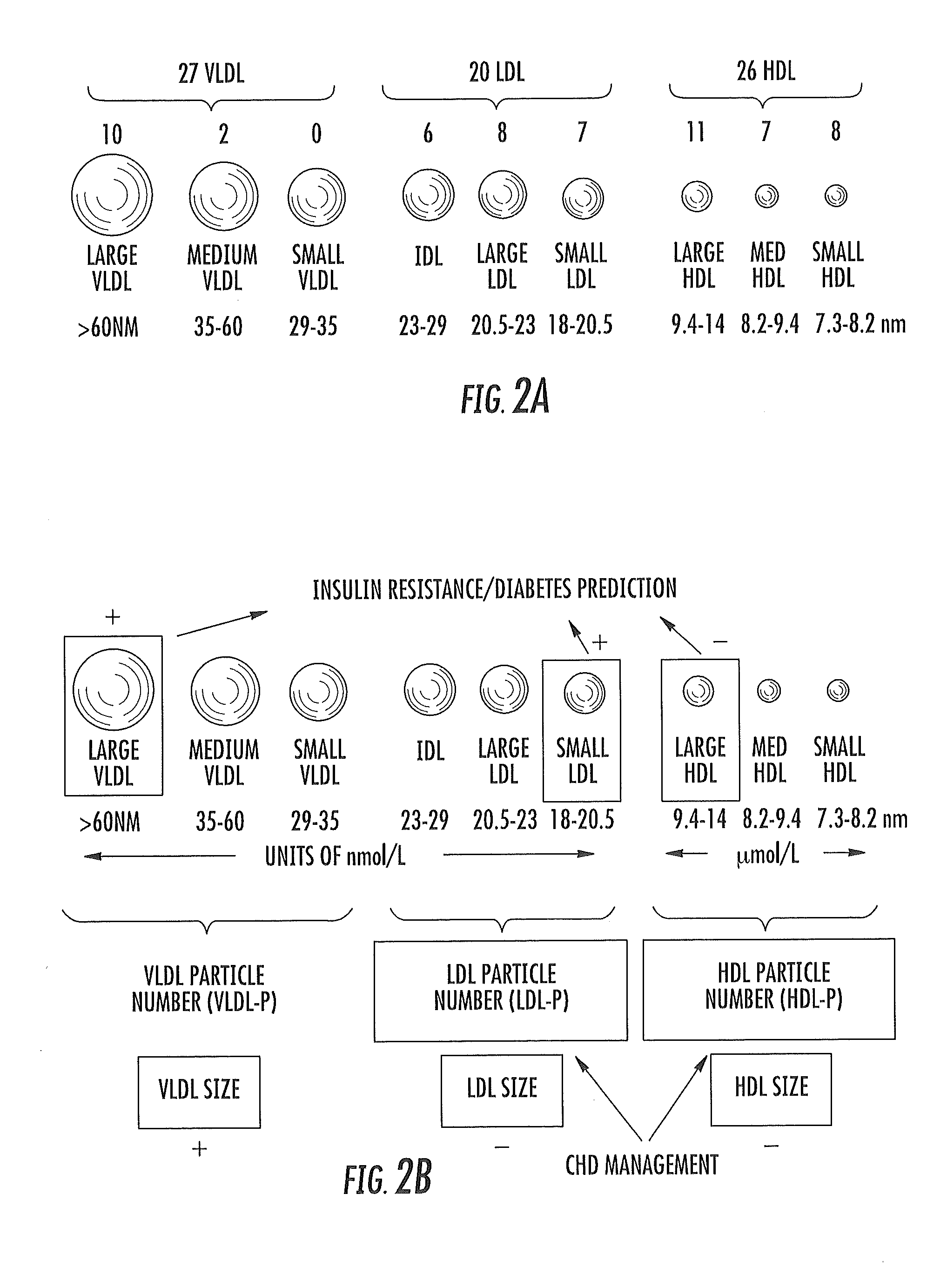
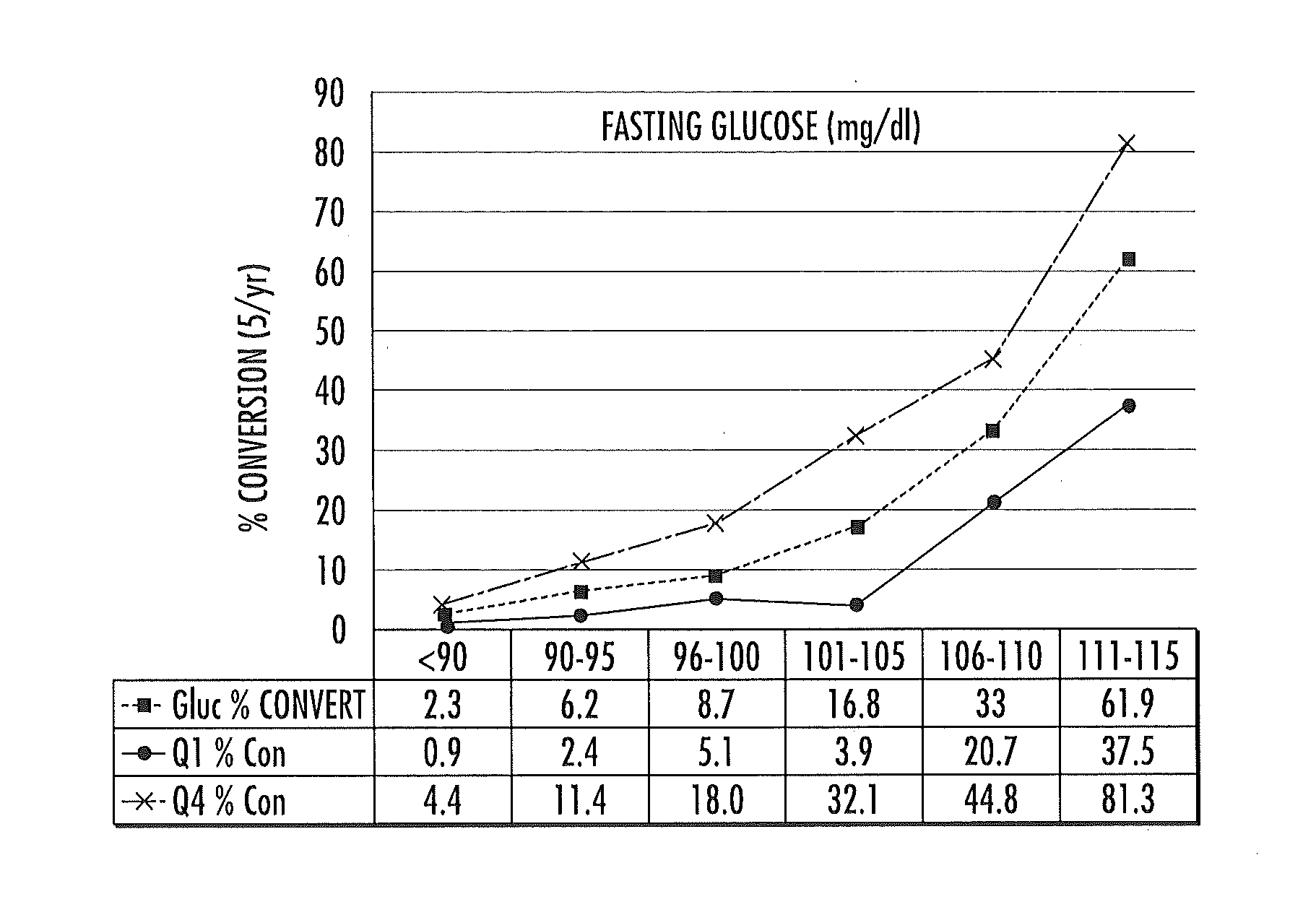
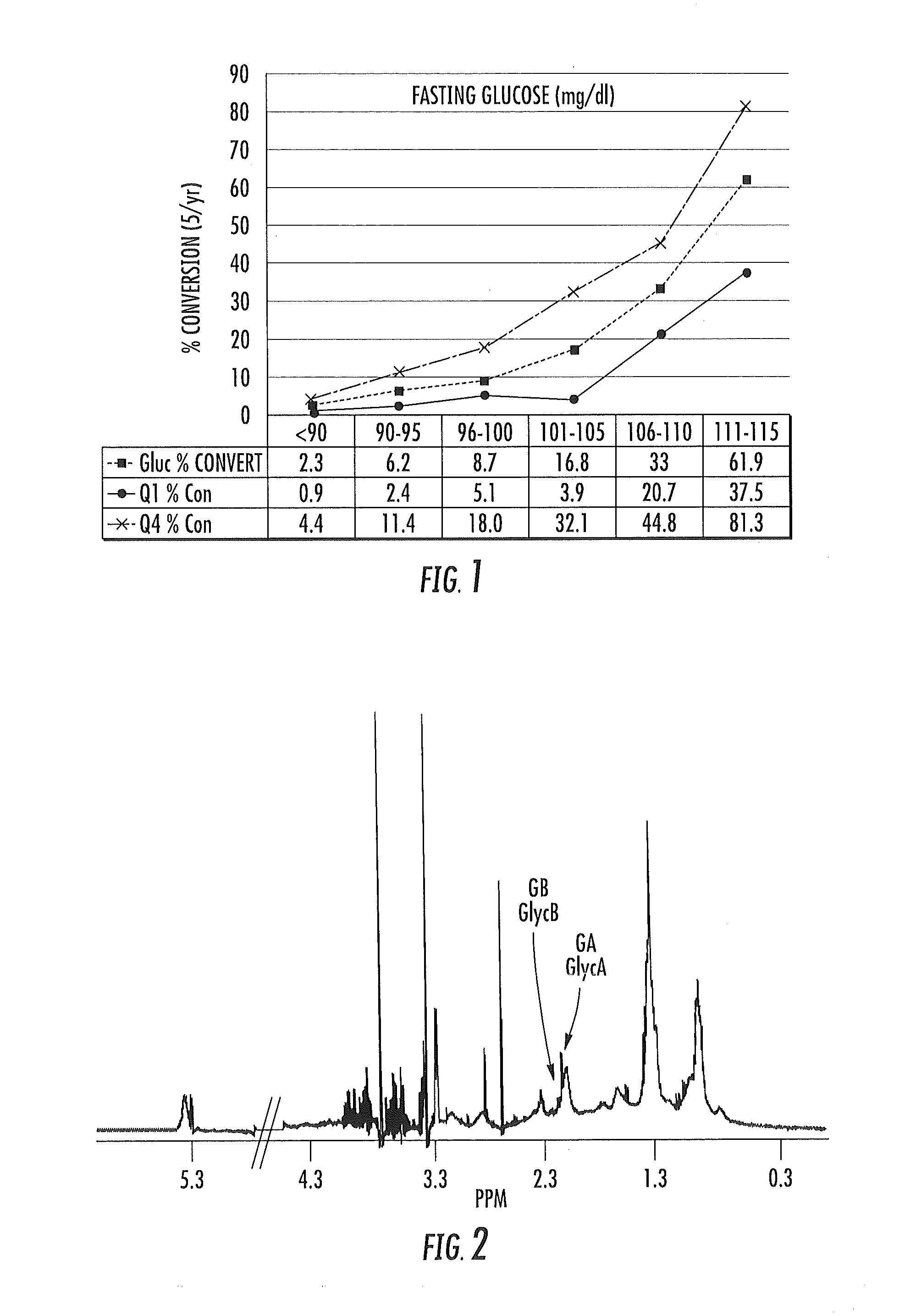
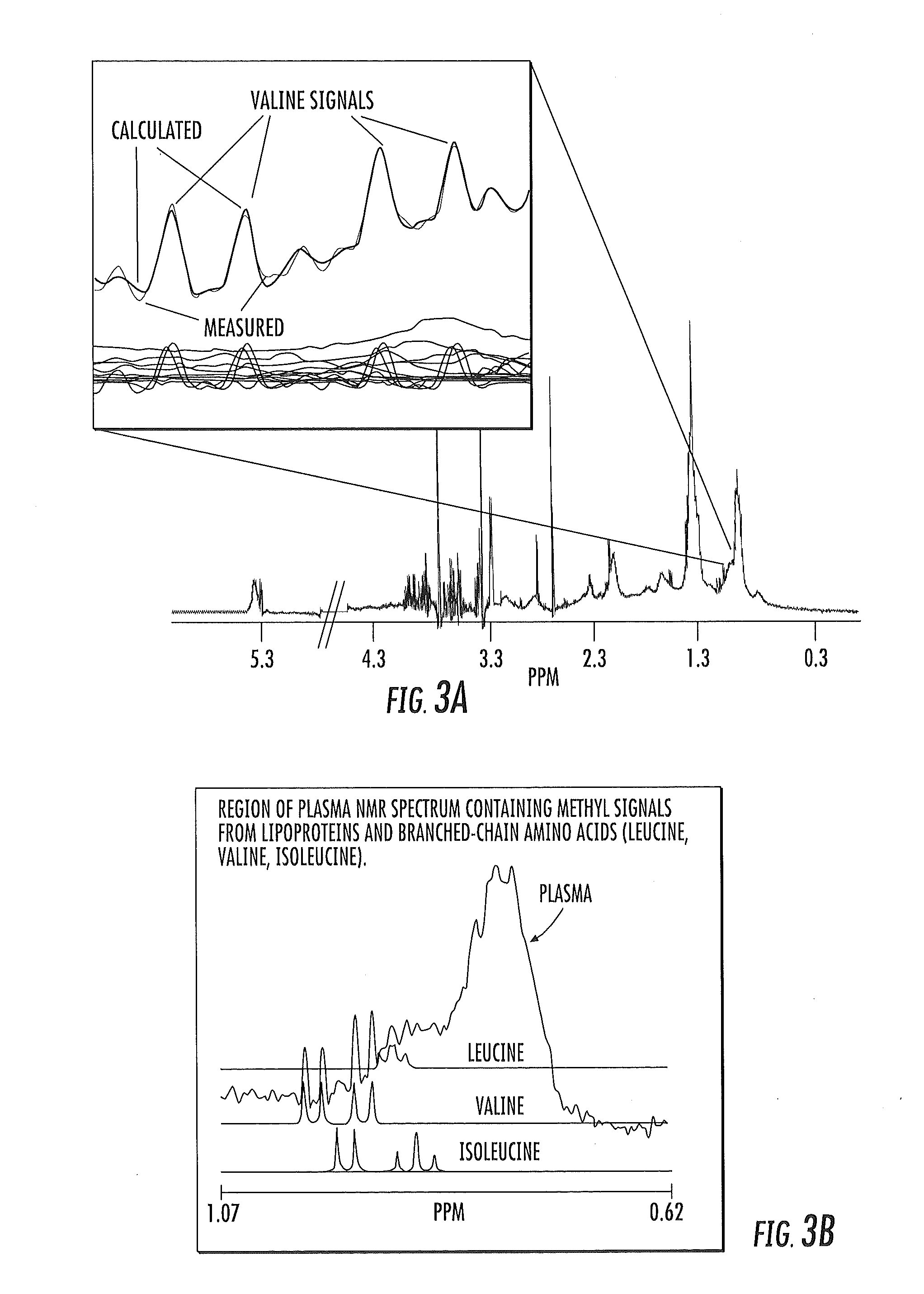
ActiveUS20150149095A1Easy to understandIncreased riskMedical simulationHealth-index calculationMathematical modelPrediabetes
Methods, systems and circuits evaluate a subject's risk of developing type 2 diabetes or having prediabetes using at least one defined mathematical model of risk of progression that can stratify risk for patients having the same glucose measurement. The model may include NMR derived measurements of GlycA and a plurality of selected lipoprotein components of at least one biosample of the subject.
Owner:LIPOSCI
Multi-parameter diabetes risk evaluations
ActiveUS20130332082A1Ease of identifyingEasy to understandMedical simulationHealth-index calculationMedicineMathematical model
Methods, systems and circuits evaluate a subject's risk of developing type 2 diabetes or developing or having prediabetes using at least one defined mathematical model of risk of progression that can stratify risk for patients having the same glucose measurement. The model may include NMR derived measurements of GlycA and a plurality of selected lipoprotein components of at least one biosample of the subject.
Owner:LIPOSCI
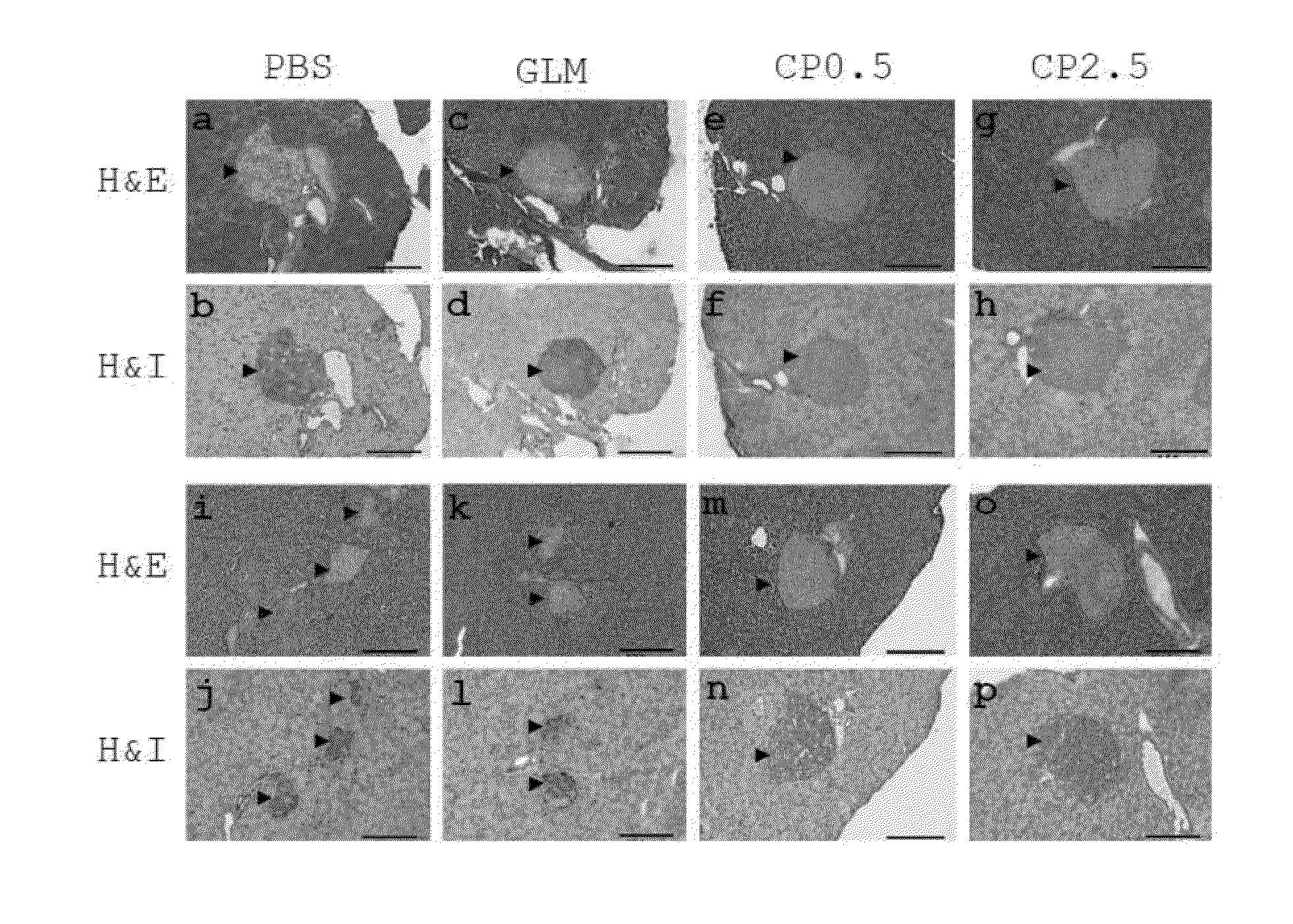
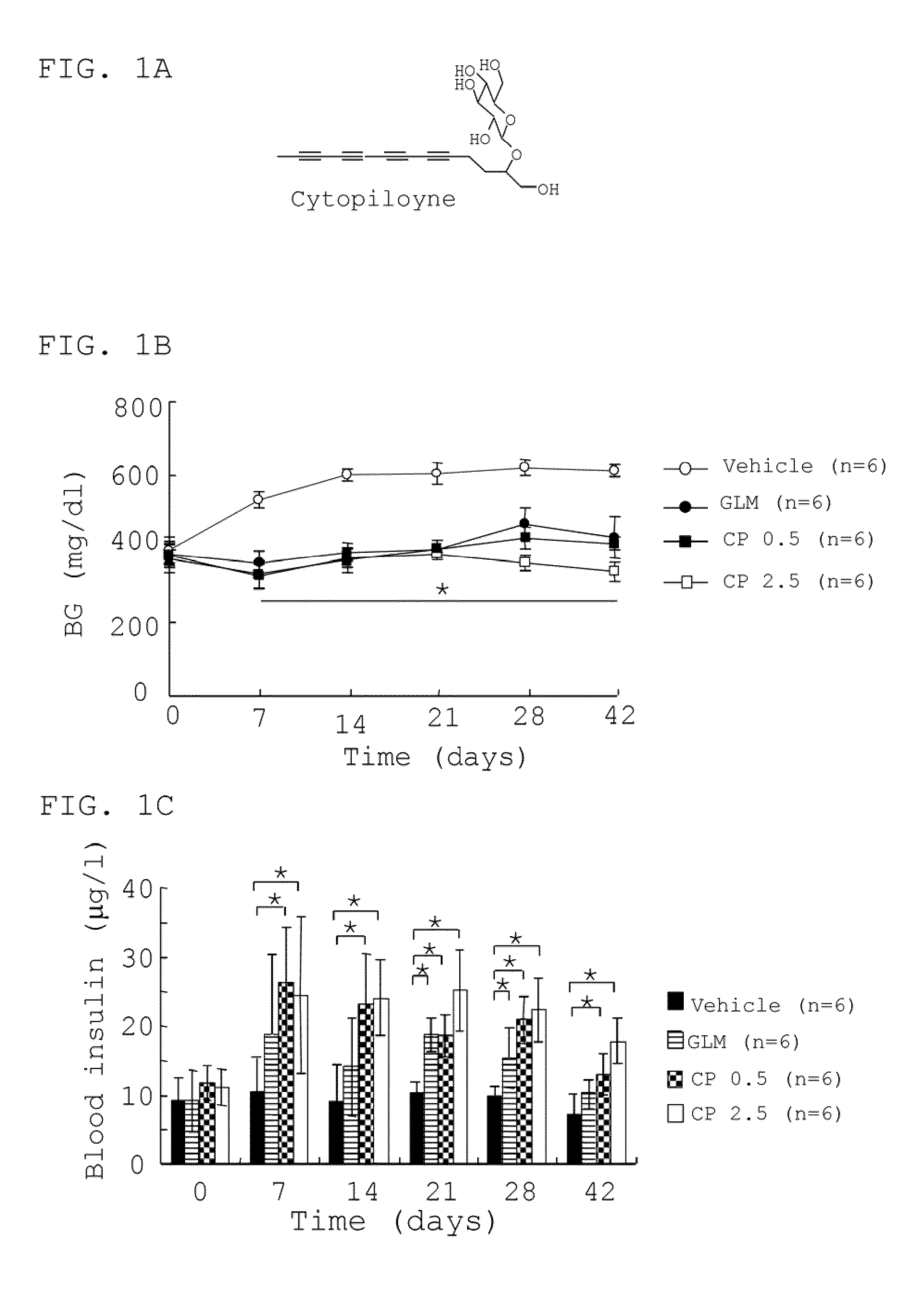
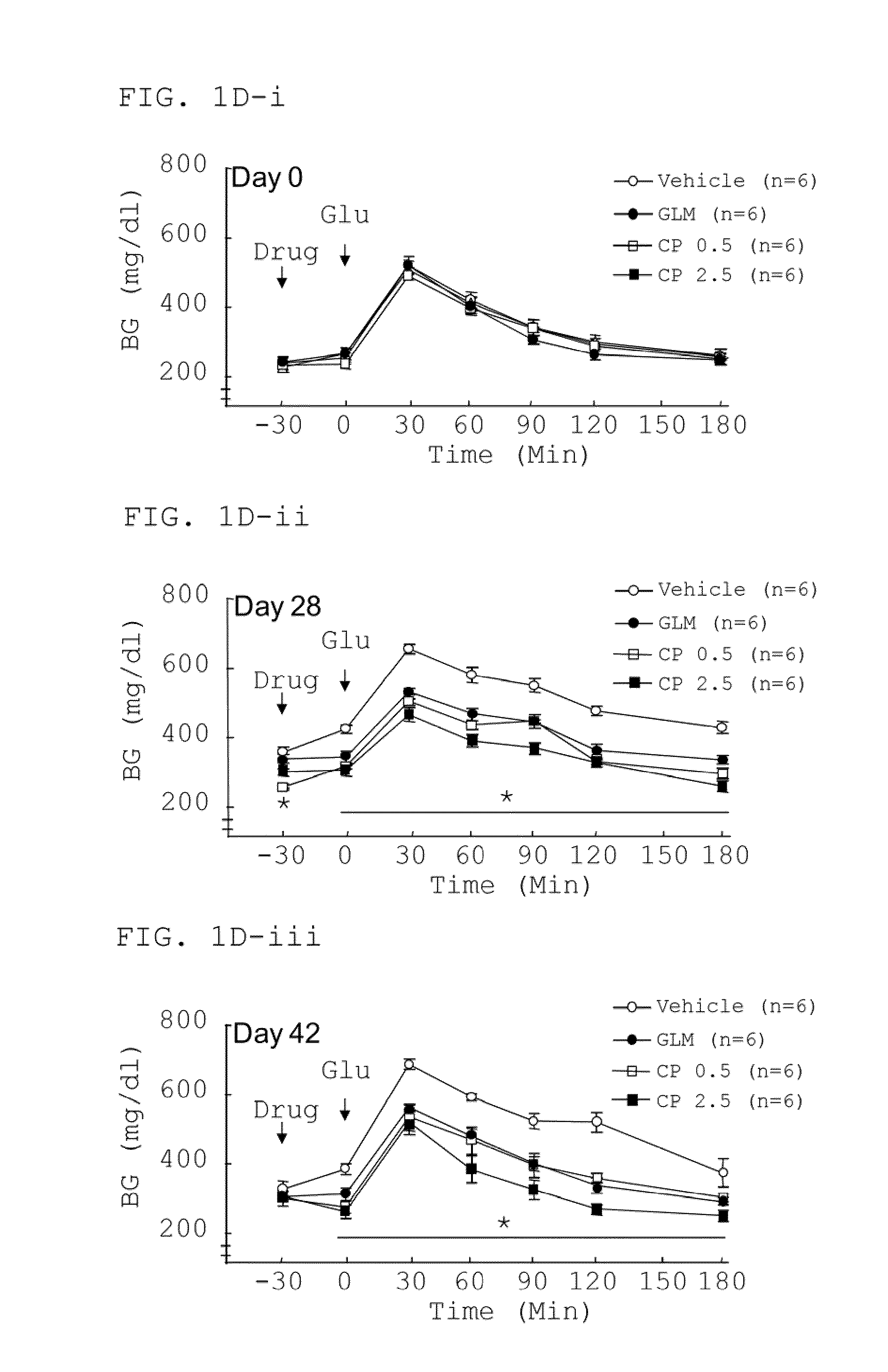
Polyacetylenic compounds for protecting against pancreatic islet atrophy
Pharmaceutical compositions and methods for protecting against atrophy of pancreatic islets in a mammal with metabolic syndrome, prediabetes or diabetes are disclosed. The method comprises administering to the mammal a pharmaceutical composition comprising a compound having a chemical structure of formula (I) in an effective amount and a pharmaceutically acceptable carrier:whereinR is H or COCH2COOH;m=3 or 4;n=0 or 1;o=2; andp=1 or 2.
Owner:ACAD SINIC
Methods for modulating intestinal microbiota
InactiveCN108778309AOrganic active ingredientsPeptide/protein ingredientsIntestinal structureOral medication
The present invention relates to methods for modulating the intestinal microbiota by administering one or more defensins and / or GLP-1 / GLP-1 analogs and methods for prevention or treatment of gut inflammation by oral administration of one or more defensins. GLP-1 analogs such as Liraglutide, as well as mammalian and poultry alfa and beta defensins can cause a change in the composition of the microbiota and metabolome in the intestine and can therefore be used to treat or prevent gut inflammation, colorectal cancer, metabolic syndrome, obesity, prediabetes and diabetes or as lean growth promoters in the meat production.
Owner:DEFENSIN THERAPEUTICS APS
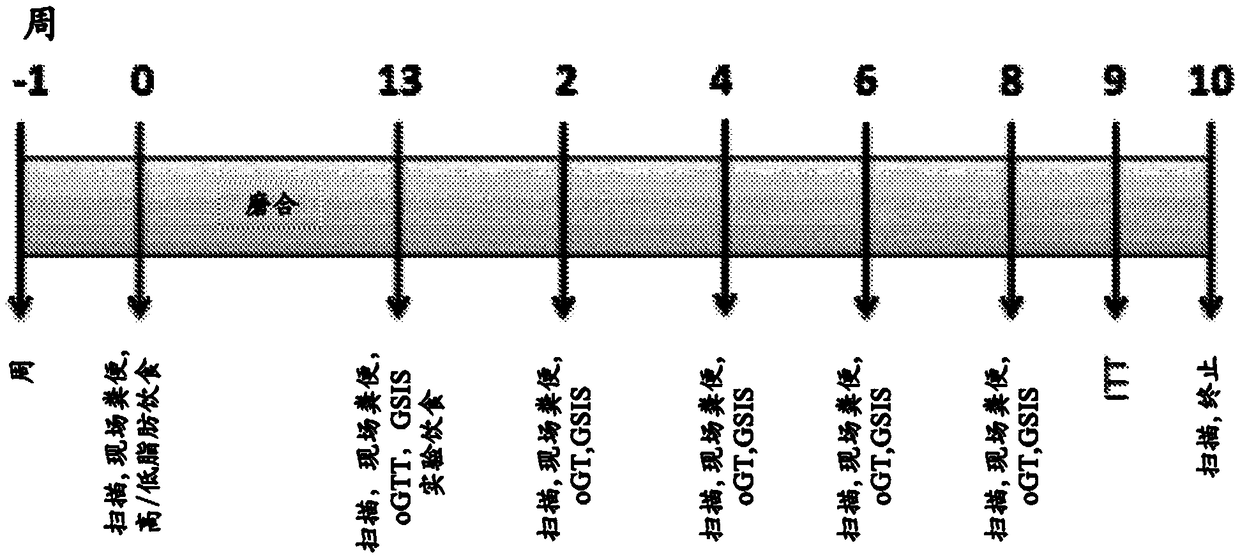
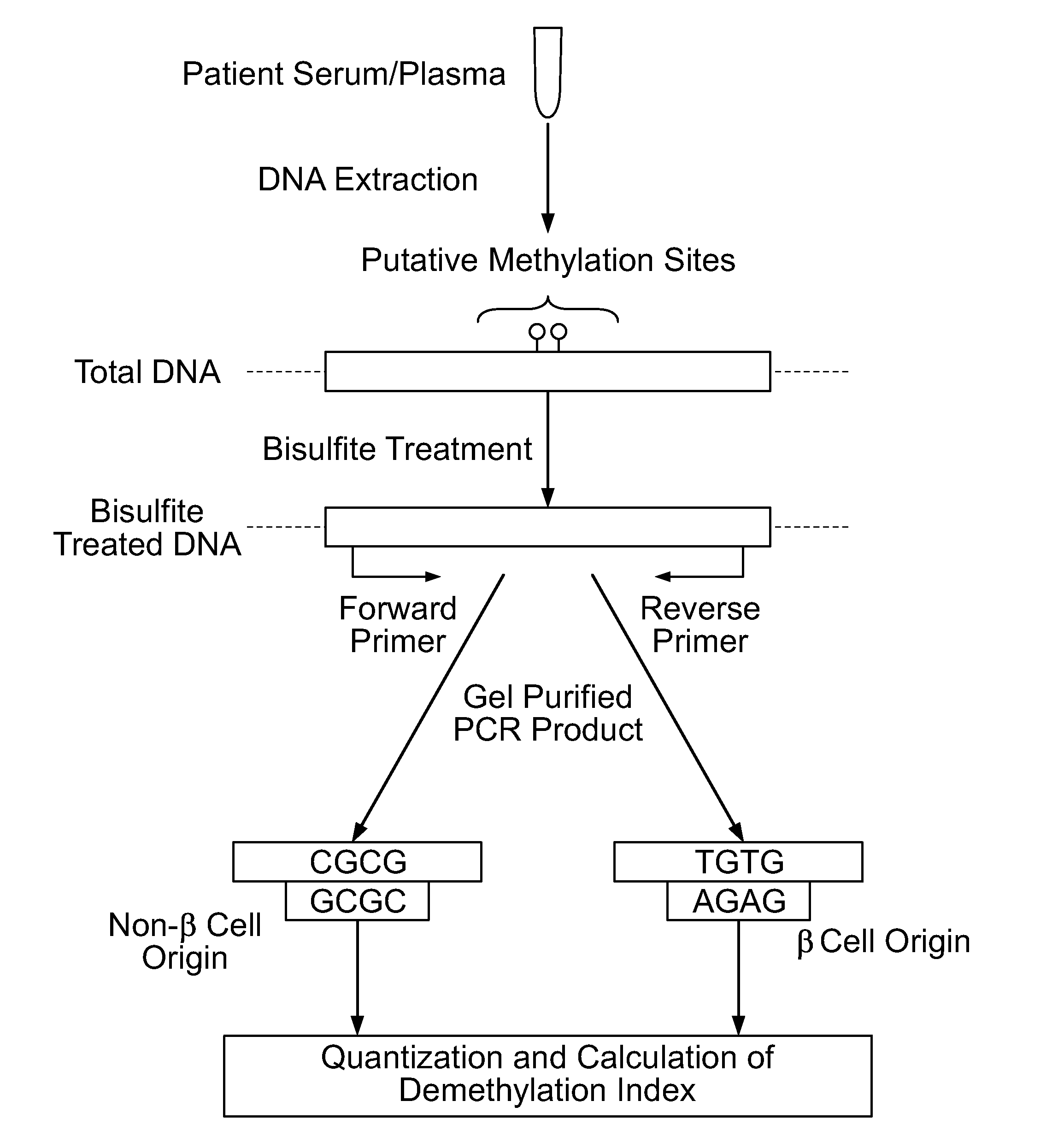
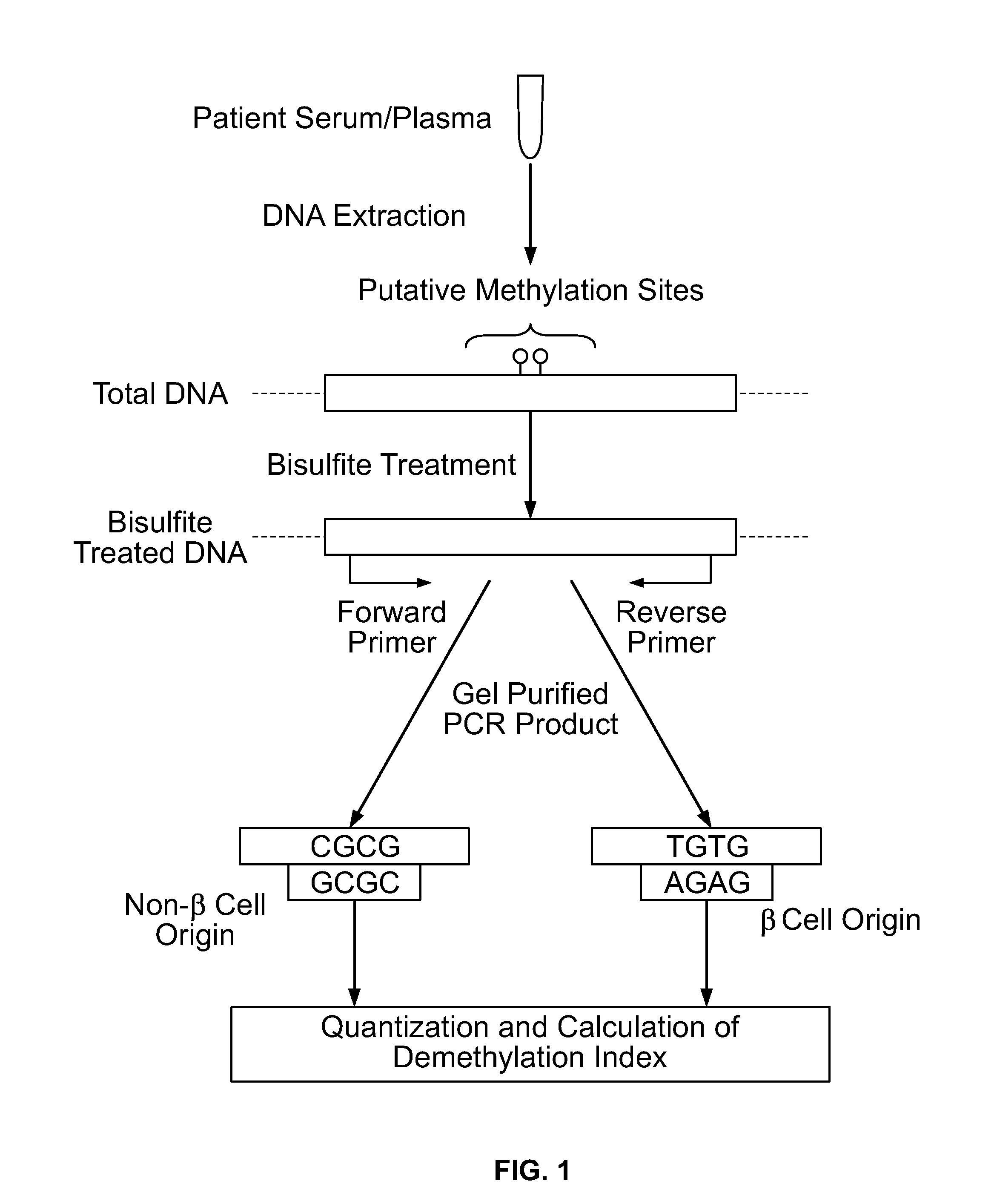
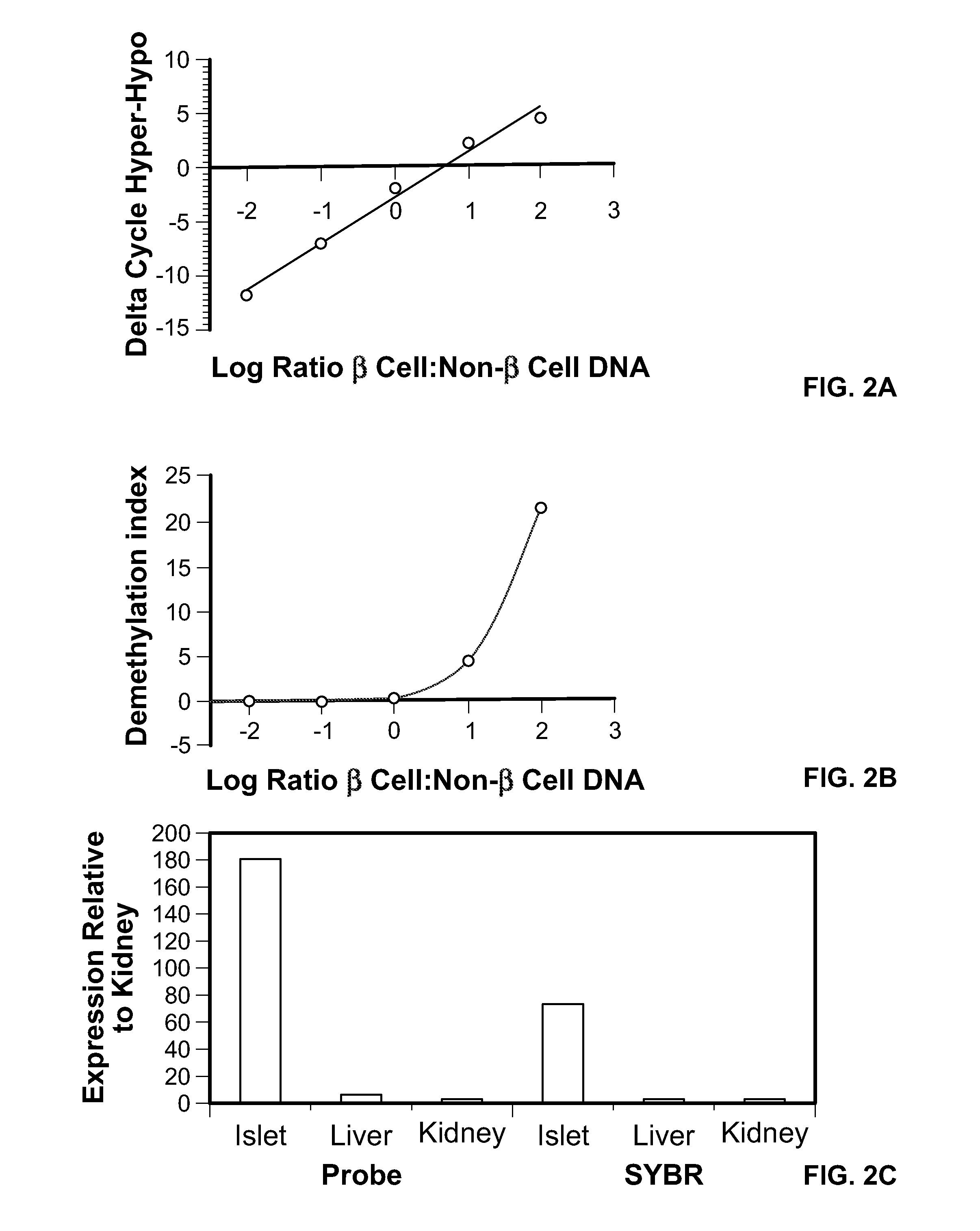
Method for using probe based PCR detection to measure the levels of circulating demethylated beta cell derived DNA as a measure of beta cell loss in diabetes
ActiveUS20130230850A1Ease of evaluationEasy diagnosisMicrobiological testing/measurementCell specificDNA Patterns
A method for measuring blood levels of β cell DNA that is released upon β cell death by using a quantitative probe technology to detect amplified methylated and demethylated forms of the insulin gene DNA, representing normal tissue and β cell specific origin, respectively. Using probes permits the sensitive and specific identification of demethylated insulin DNA patterns that are present only in β cells. The method offers a bioassay for detecting β cell loss in diabetes, useful for screening of prediabetes, monitoring of disease progression, and selection and monitoring of therapies. The technique finds potential use in both Type I and Type II diabetes, as well as gestational diabetes.
Owner:NYU WINTHROP HOSPITAL
Therapeutic treatment for metabolic syndrome, type 2 diabetes, obesity, or prediabetes
ActiveUS9655865B2Minimize desensitizationReducing desensitizationBiocidePeptide/protein ingredientsDiseaseNoradrenergic neurons
The present invention is directed to a method for treating a patient suffering from the metabolic syndrome, Type 2 diabetes, obesity, or prediabetes, comprising the step of increasing the ratio of dopaminergic neuronal to noradrenergic neuronal activity within the central nervous system and particularly the hypothalamus of the central nervous system of the patient.In another aspect, the present invention is directed to a method for treating a patient suffering from a metabolic disorder such as the metabolic syndrome, Type 2 diabetes, obesity, or prediabetes, and the metabolic sequale of these diseases including cardiovascular, cerebrovascular, renal and hepatic diseases, comprising the step of: administering to a patient suffering from the metabolic syndrome, Type 2 diabetes, obesity, or prediabetes a pharmaceutical composition comprising (1) at least one compound that stimulates an increase in central dopaminergic neuronal activity level in the subject, and (2) at least one compound that stimulates a decrease in central noradrenergic neuronal activity level in the subject. The present invention is also directed to pharmaceutical compositions that include the above compounds and a pharmaceutically acceptable carrier.
Owner:VEROSCI
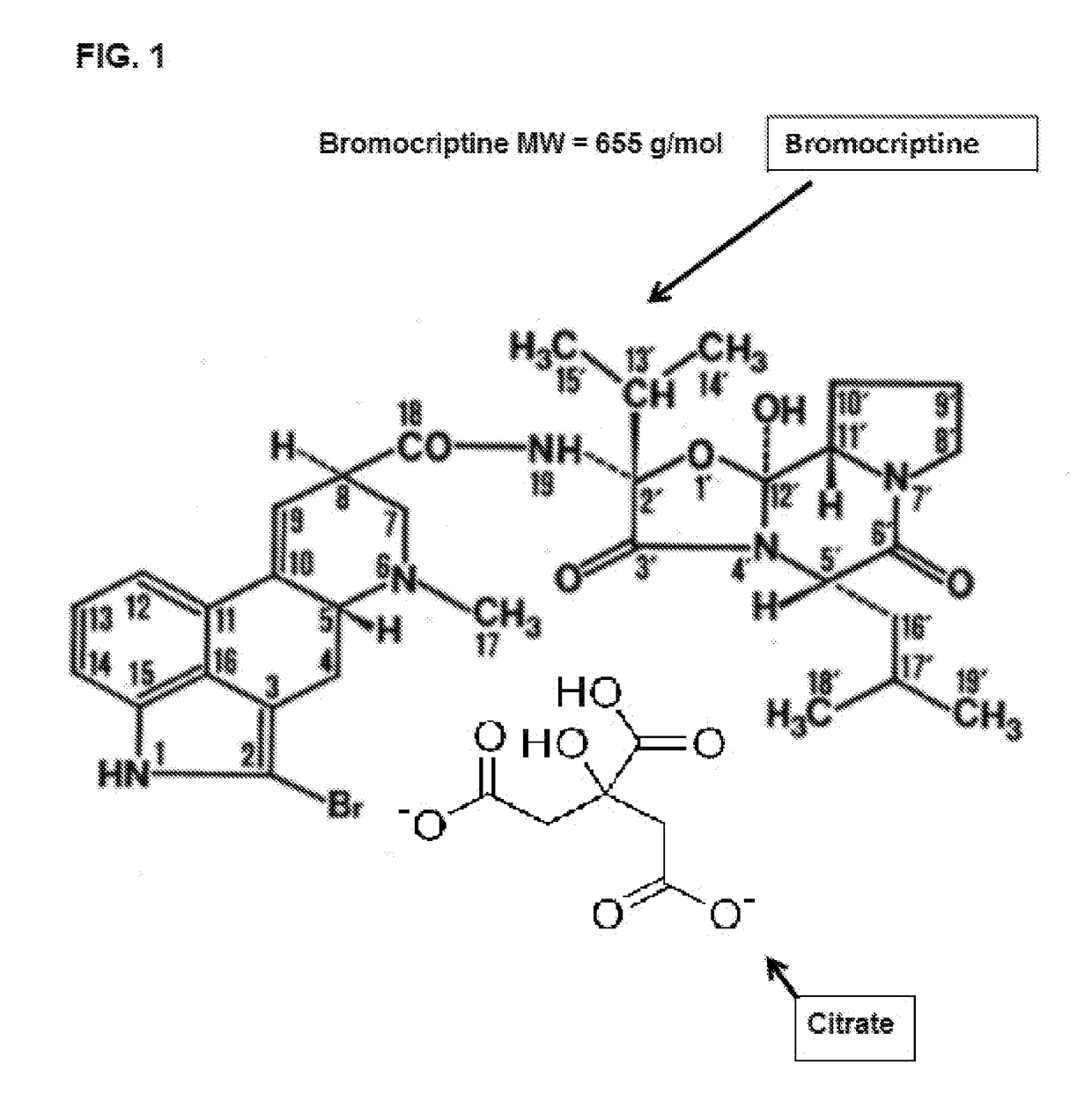
Composition and Method for Treating Metabolic Disorders
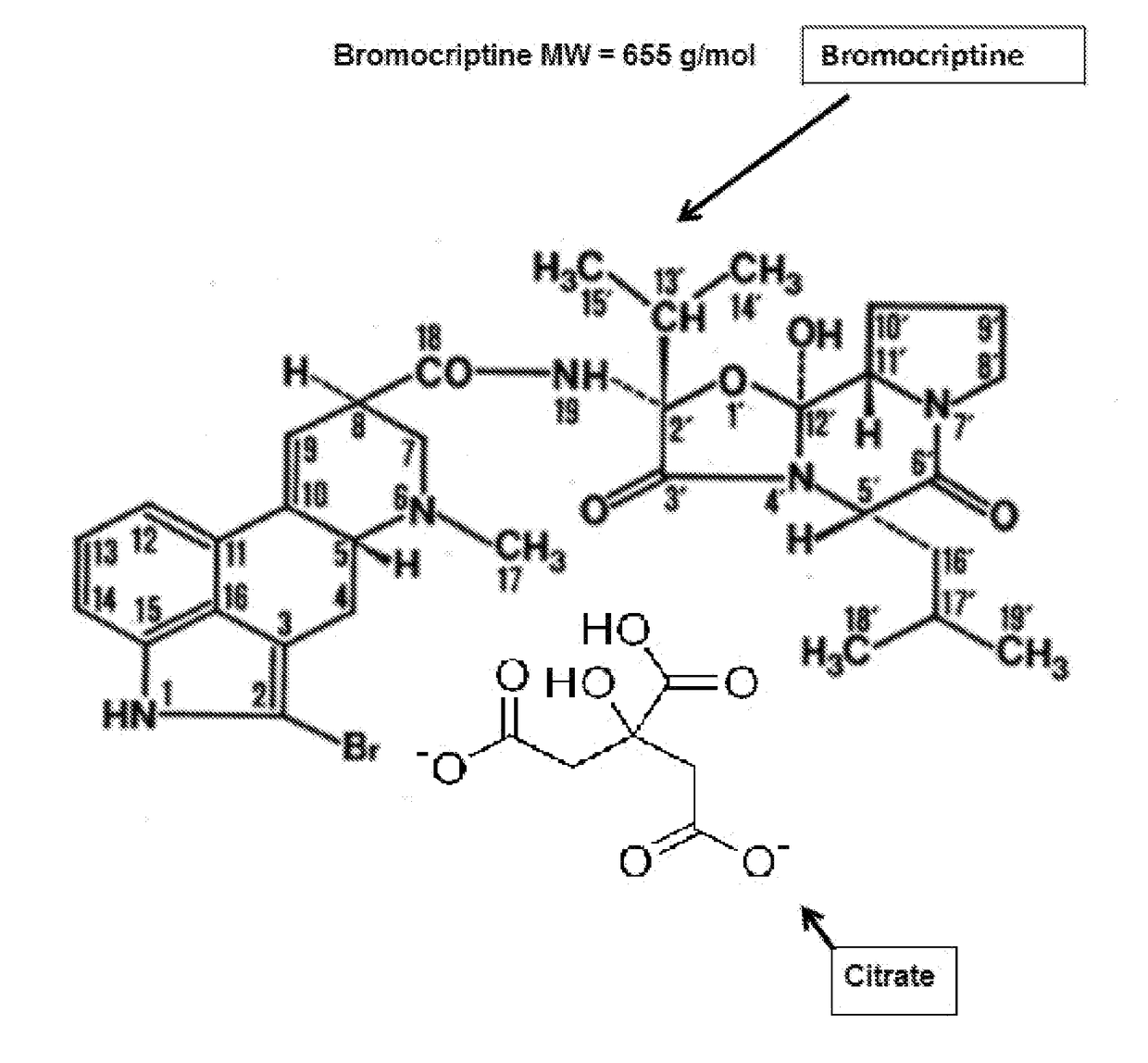
Bromocriptine citrate administered to a vertebrate, animal or human, can be used for any purpose including, e.g., the long-term modification and regulation of metabolic disorders, including prediabetes, obesity, insulin resistance, hyperinsulinemia, hyperglycemia and type 2 diabetes mellitus (T2DM) and / or, e.g., the treatment of other medical disorder(s) including immune or endocrine disorders or diseases. Bromocriptine citrate is administered over a limited or extended period at a time of day dependent on re-establishing the normal circadian rhythm of central dopaminergic activity of healthy members of a similar species and sex. Insulin resistance, hyperinsulinemia and hyperglycemia, T2DM, prediabetes, MS or all, can be controlled in humans on a long term basis by such treatment inasmuch as the daily administration of bromocriptine citrate resets neuronal activity timing in the neural centers of the brain to produce long term effects.
Owner:VEROSCI
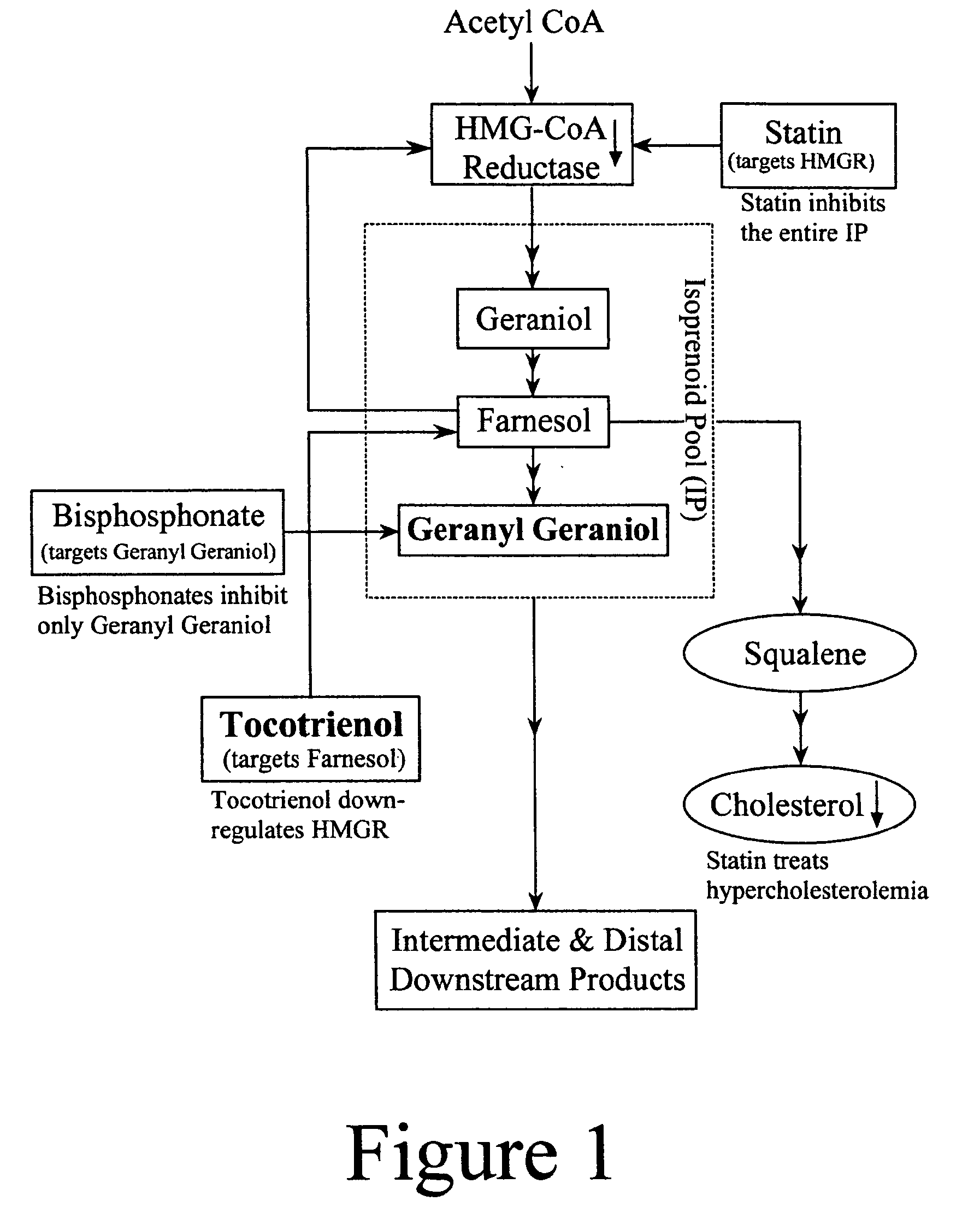
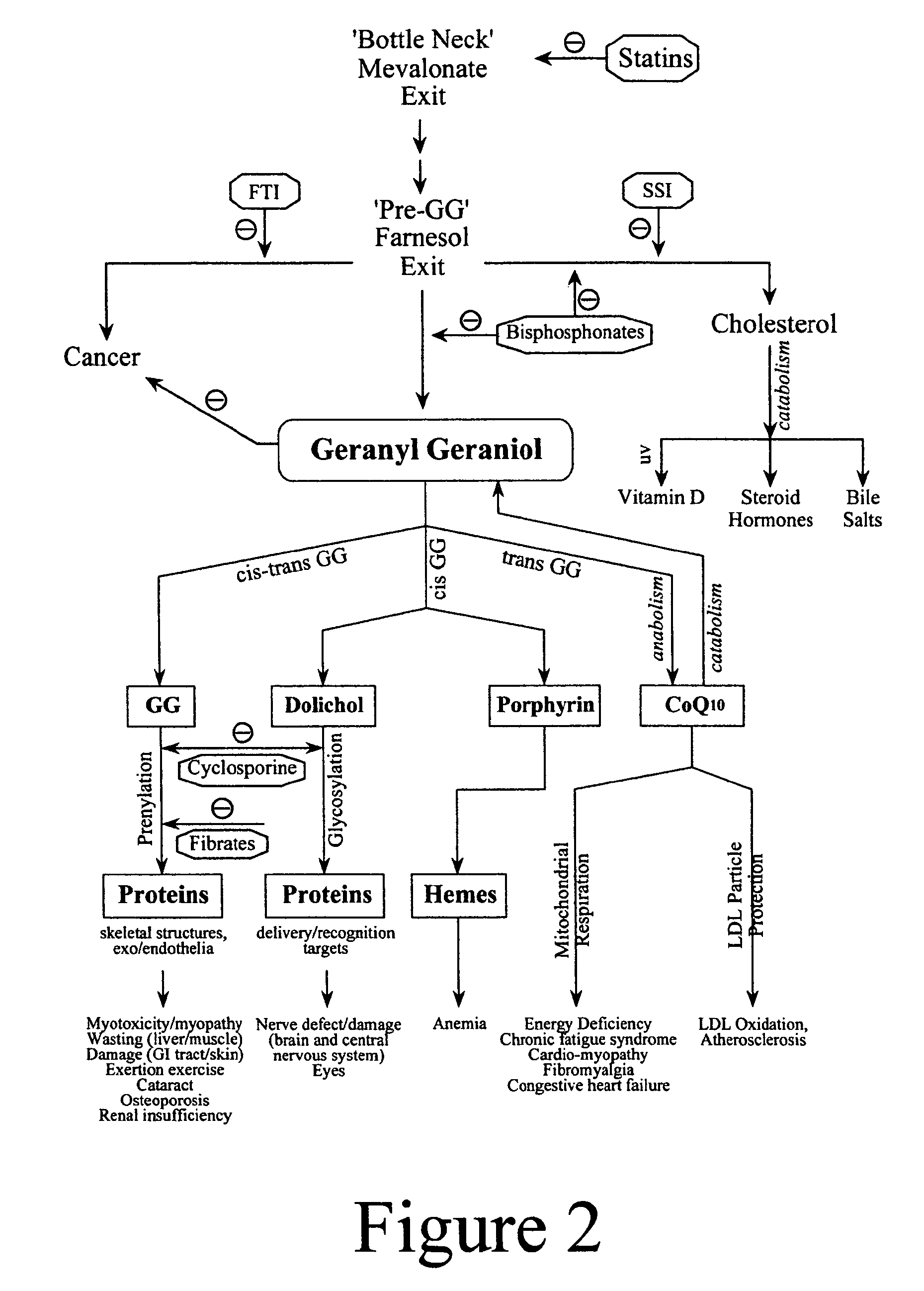
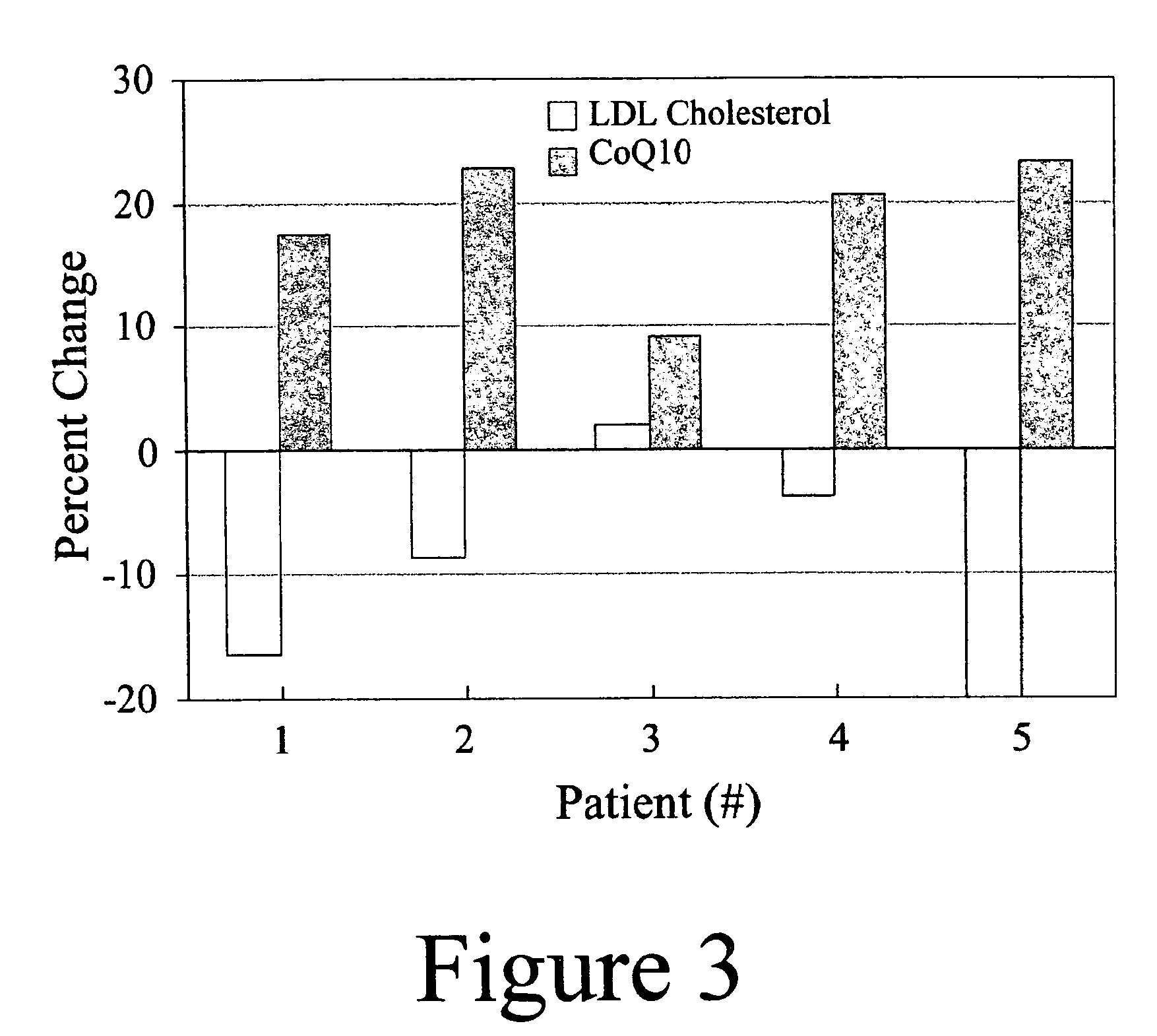
Annatto extract compositions, including geranyl geraniols and methods of use
ActiveUS7989006B2Reduce disease riskIncreasing cellular uptakeBiocideSenses disorderEnergeticsPhysiology
Owner:AMERICAN RIVER NUTRITION LLC
Salivary biomarkers for prediabetes and type 2 diabetes
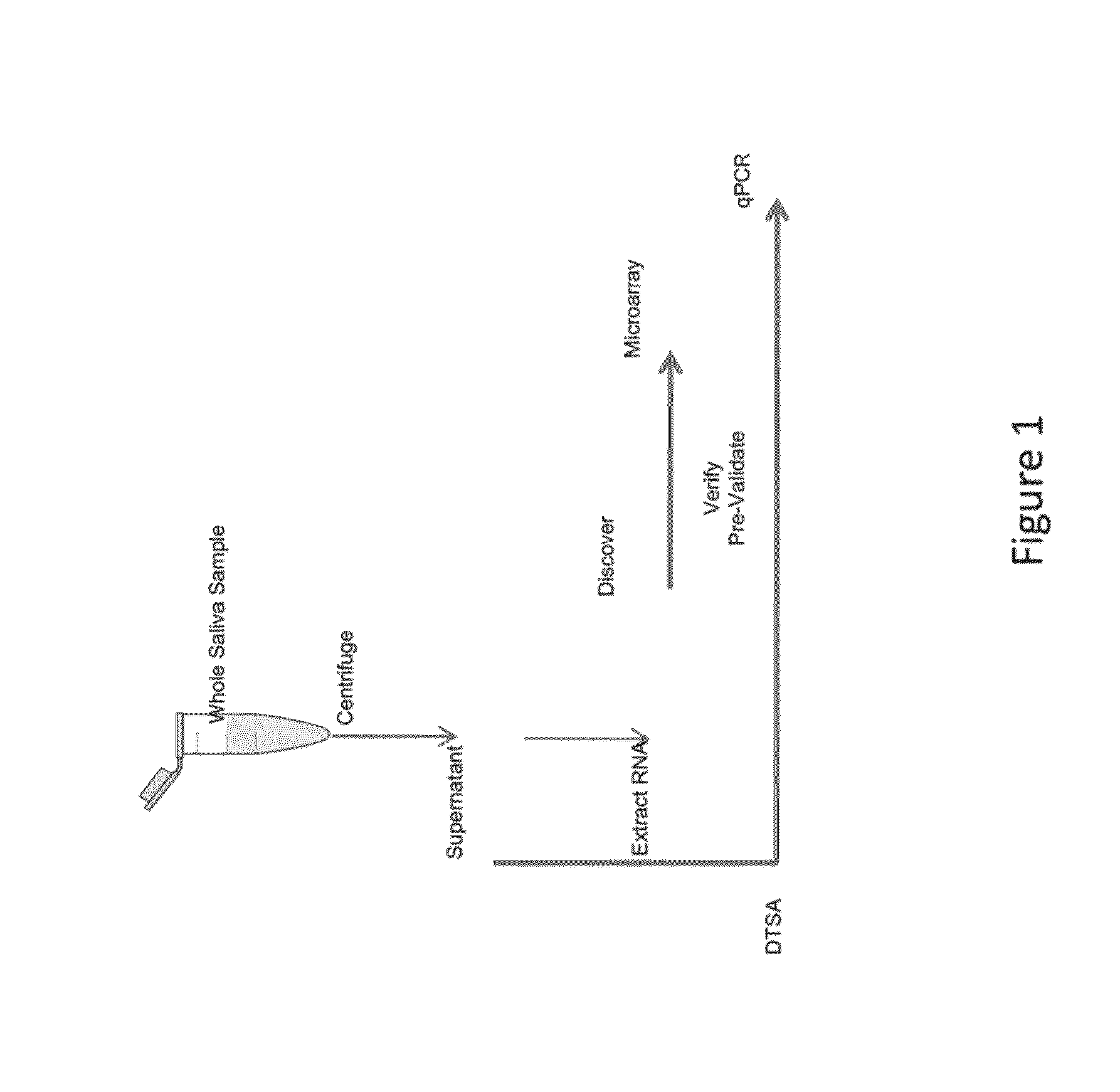
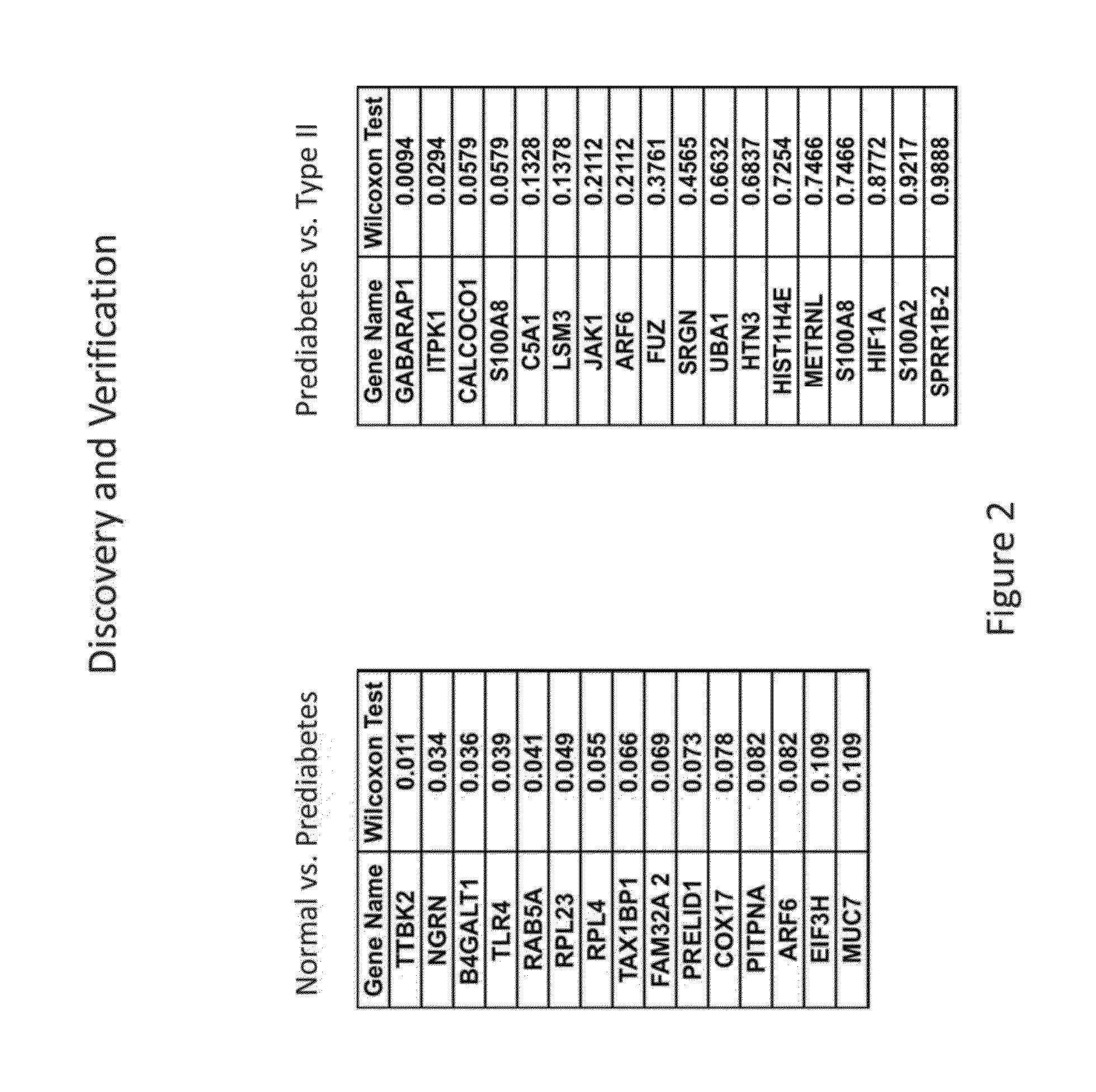
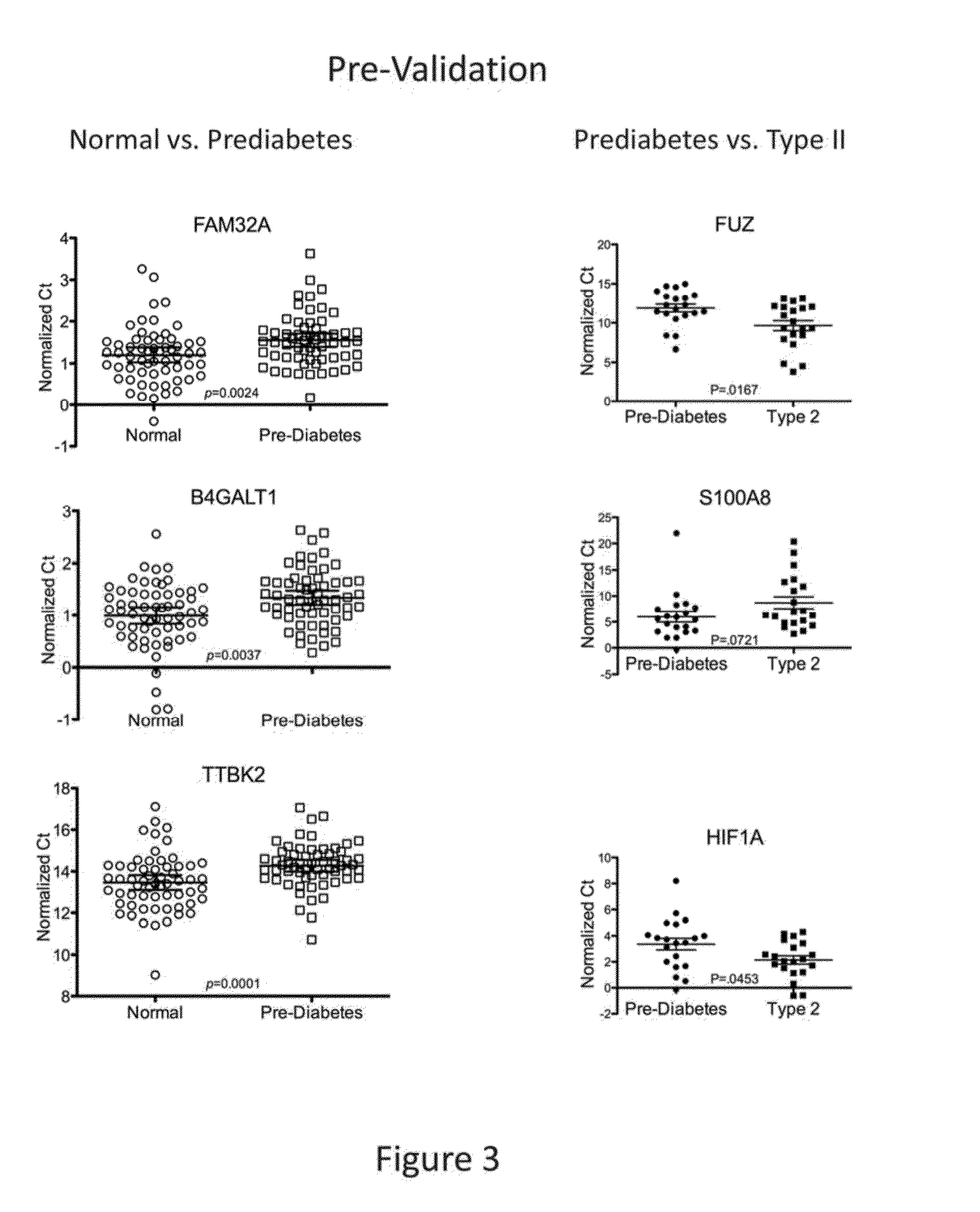
The present invention provides for the first time the identification of salivary protein and RNA factors that can be used in the detection of diabetes. The present invention therefore provides methods of diagnosing and providing a prognosis for diabetes, by examining relevant proteins and RNA in a patient's saliva.
Owner:RGT UNIV OF CALIFORNIA
Methods for modulating intestinal microbiota
InactiveUS20190111107A1Reduce riskImprove glucose toleranceOrganic active ingredientsPeptide/protein ingredientsIntestinal structureOral medication
The present invention relates to methods for modulating the intestinal microbiota by administering one or more defensins and / or GLP-1 / GLP-1 analogs and methods for prevention or treatment of gut inflammation by oral administration of one or more defensins. GLP-1 analogs such as Liraglutide, as well as mammalian and poultry alfa and beta defensins can cause a change in the composition of the microbiota and metabolome in the intestine and can therefore be used to treat or prevent gut inflammation, colorectal cancer, metabolic syndrome, obesity, prediabetes and diabetes or as lean growth promoters in the meat production.
Owner:NOVOZYMES AS
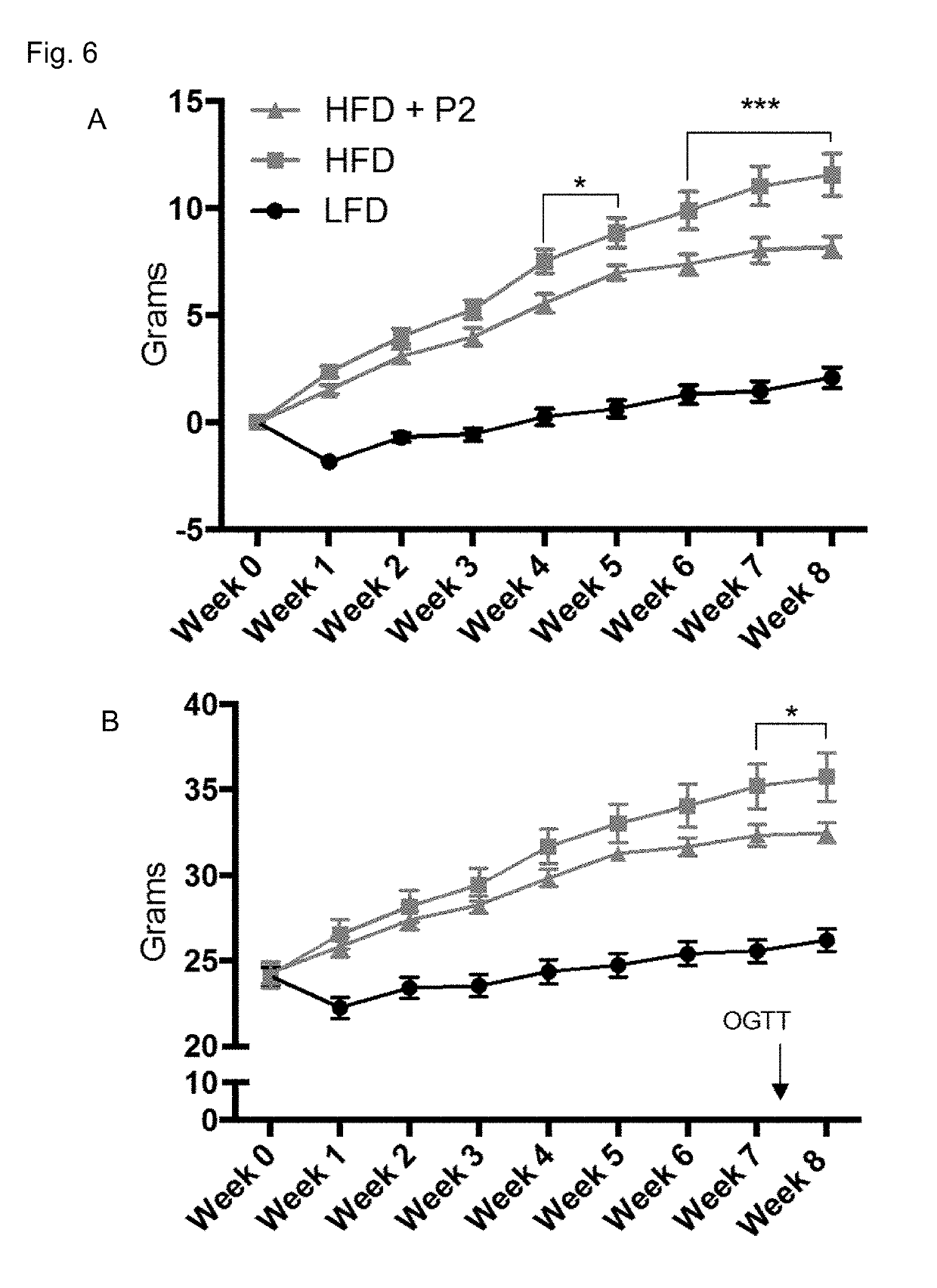
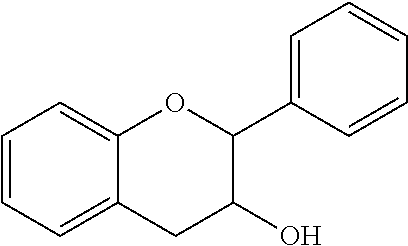
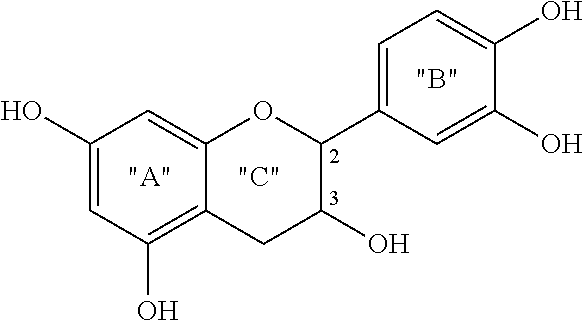
Compositions and Methods for Treatment of Prediabetes
InactiveUS20170312329A1Hydroxy compound active ingredientsAnhydride/acid/halide active ingredientsDiabetes mellitusMedicine
The invention provides compositions and methods to treat prediabetes. In particular, the inventor has discovered that particular combinations of diverse types of antioxidants have synergistic and surprising effects for use against prediabetes. The composition includes: a plurality of heteroatom-based antioxidants having the formula X—CH2—CH(R)—(CH2)m—Z; a plurality of antioxidants with a conjugated segment; an antioxidant that is a flavan-3-ol; and an antioxidant that contains a disulfide bond. The antioxidants are chosen such that at least one has a pro-oxidative effect.
Owner:CROSS III WILLIAM H
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
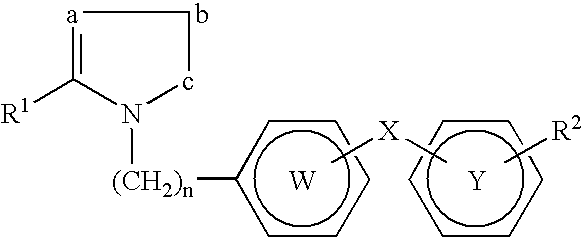
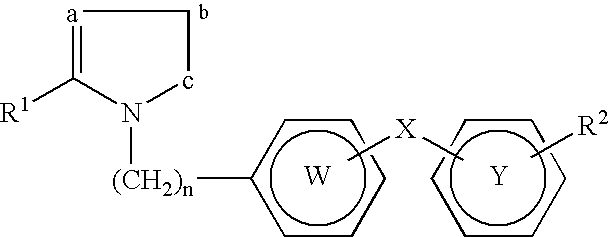
![Derivatives of 3-Azabicyclo[3.1.0] Hexane as Dipeptidyl Peptidase-IV Inhibitors Derivatives of 3-Azabicyclo[3.1.0] Hexane as Dipeptidyl Peptidase-IV Inhibitors](https://images-eureka.patsnap.com/patent_img/b07ea871-988e-445e-b196-080eb11a636d/US20080300251A1-20081204-C00001.png)
![Derivatives of 3-Azabicyclo[3.1.0] Hexane as Dipeptidyl Peptidase-IV Inhibitors Derivatives of 3-Azabicyclo[3.1.0] Hexane as Dipeptidyl Peptidase-IV Inhibitors](https://images-eureka.patsnap.com/patent_img/b07ea871-988e-445e-b196-080eb11a636d/US20080300251A1-20081204-C00002.png)
![Derivatives of 3-Azabicyclo[3.1.0] Hexane as Dipeptidyl Peptidase-IV Inhibitors Derivatives of 3-Azabicyclo[3.1.0] Hexane as Dipeptidyl Peptidase-IV Inhibitors](https://images-eureka.patsnap.com/patent_img/b07ea871-988e-445e-b196-080eb11a636d/US20080300251A1-20081204-C00003.png)