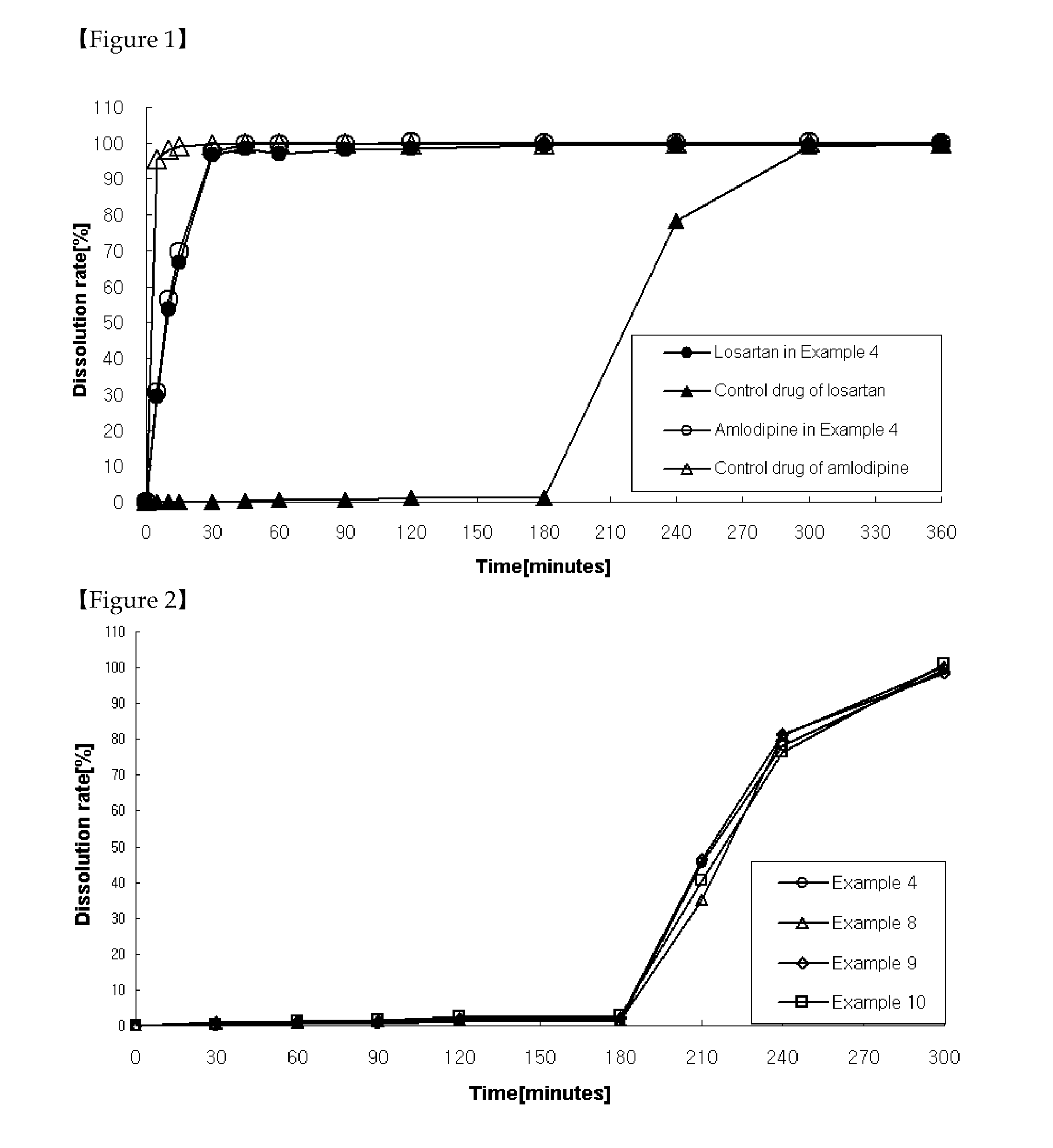
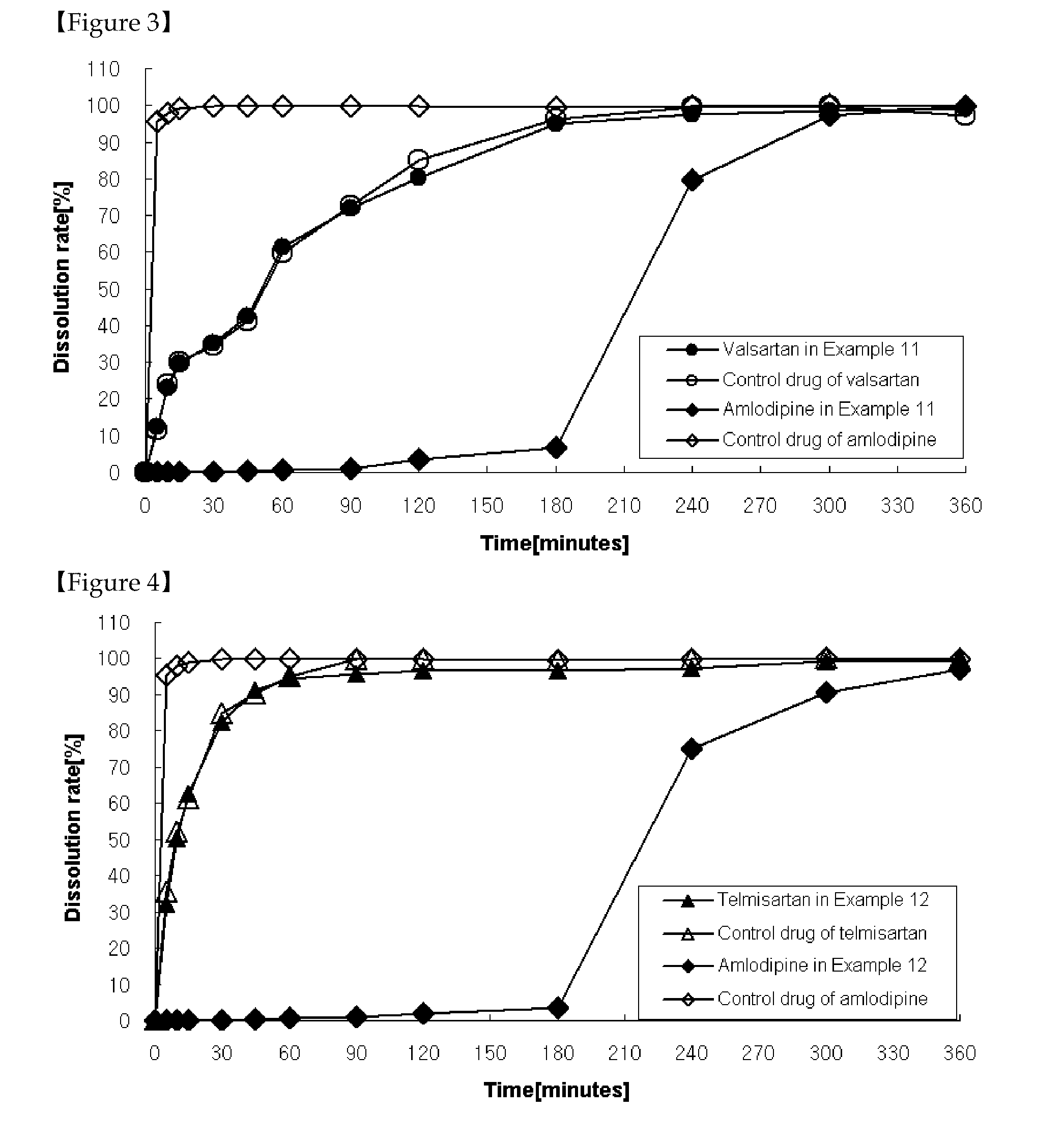
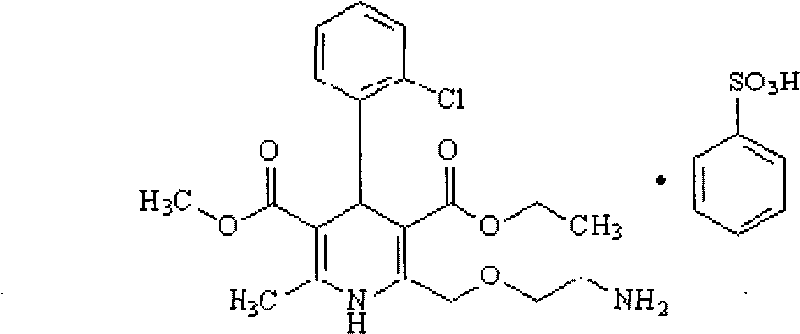
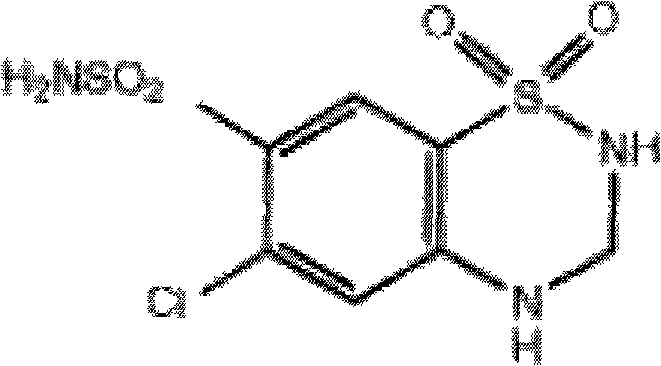
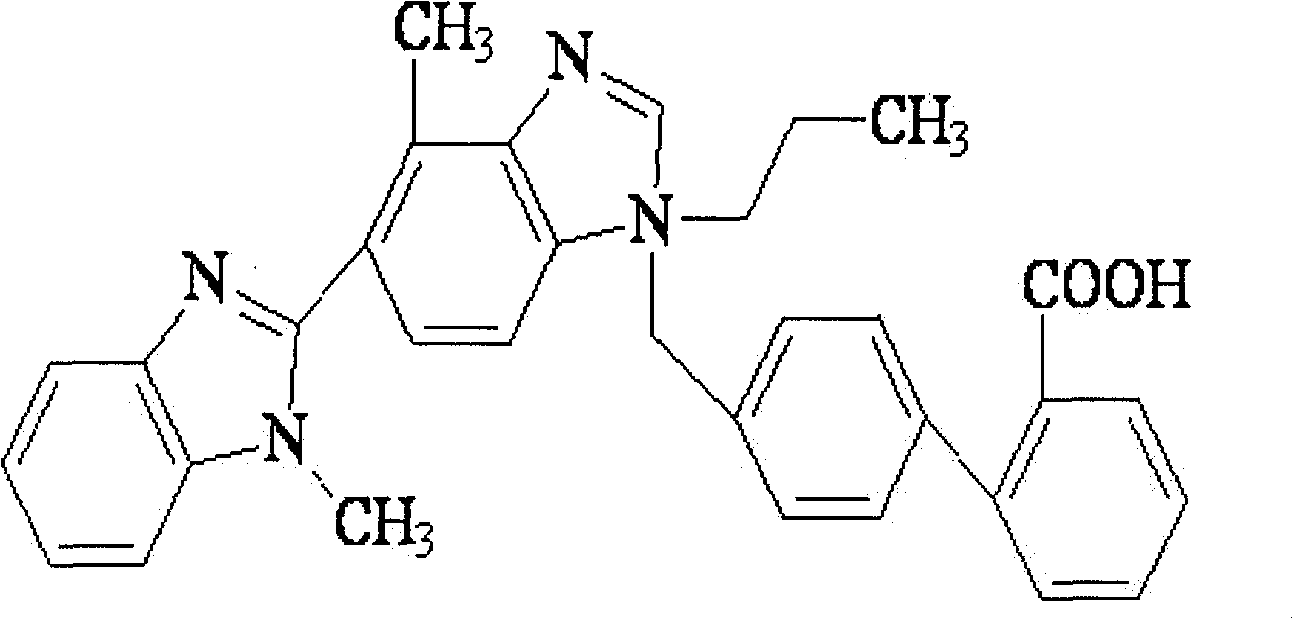
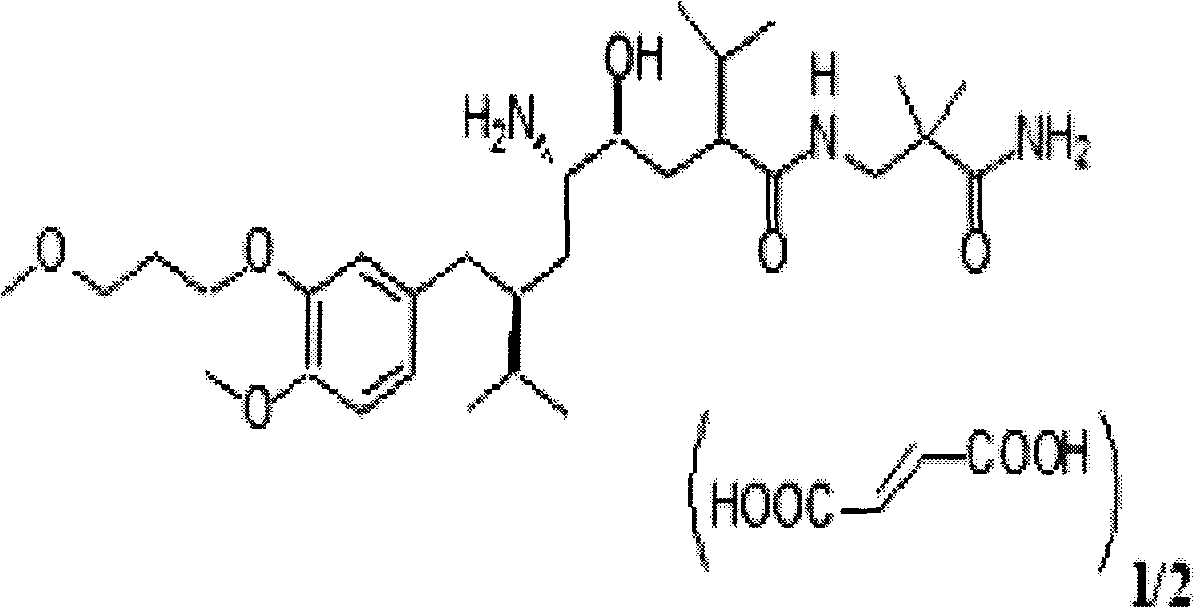
Patents
Literature
113 results about "Angiotensin 2 Receptor Blocker" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
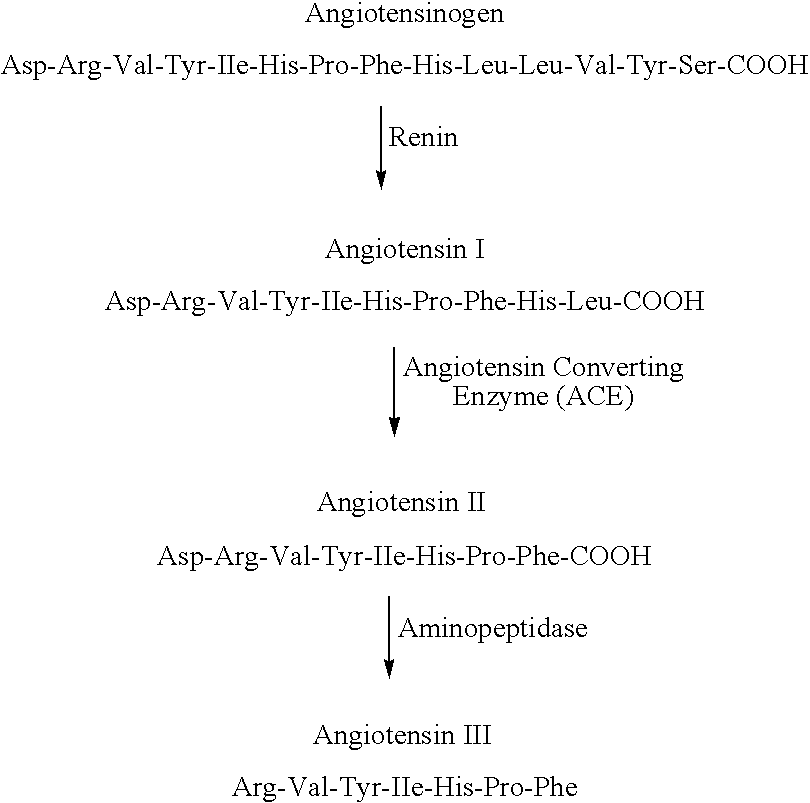
Angiotensin II receptor blocker. Angiotensin II receptor blockers (ARBs), also known as angiotensin II receptor antagonists, AT1 receptor antagonists or sartans, are a group of pharmaceuticals that modulate the renin–angiotensin system.
Use of angiotensin II receptor antagonists
InactiveUS20050070594A1Lower Level RequirementsGood blood pressureBiocideMetabolism disorderBlood pressurePrediabetes
The invention relates to the use of angiotensin II receptor antagonists for treating people in whom type 2 diabetes mellitus has been diagnosed or who are suspected of prediabetes, for preventing diabetes or for treating metabolic syndrome and insulin resistance in patients with normal blood pressure.
Owner:BOEHRINGER INGELHEIM INT GMBH
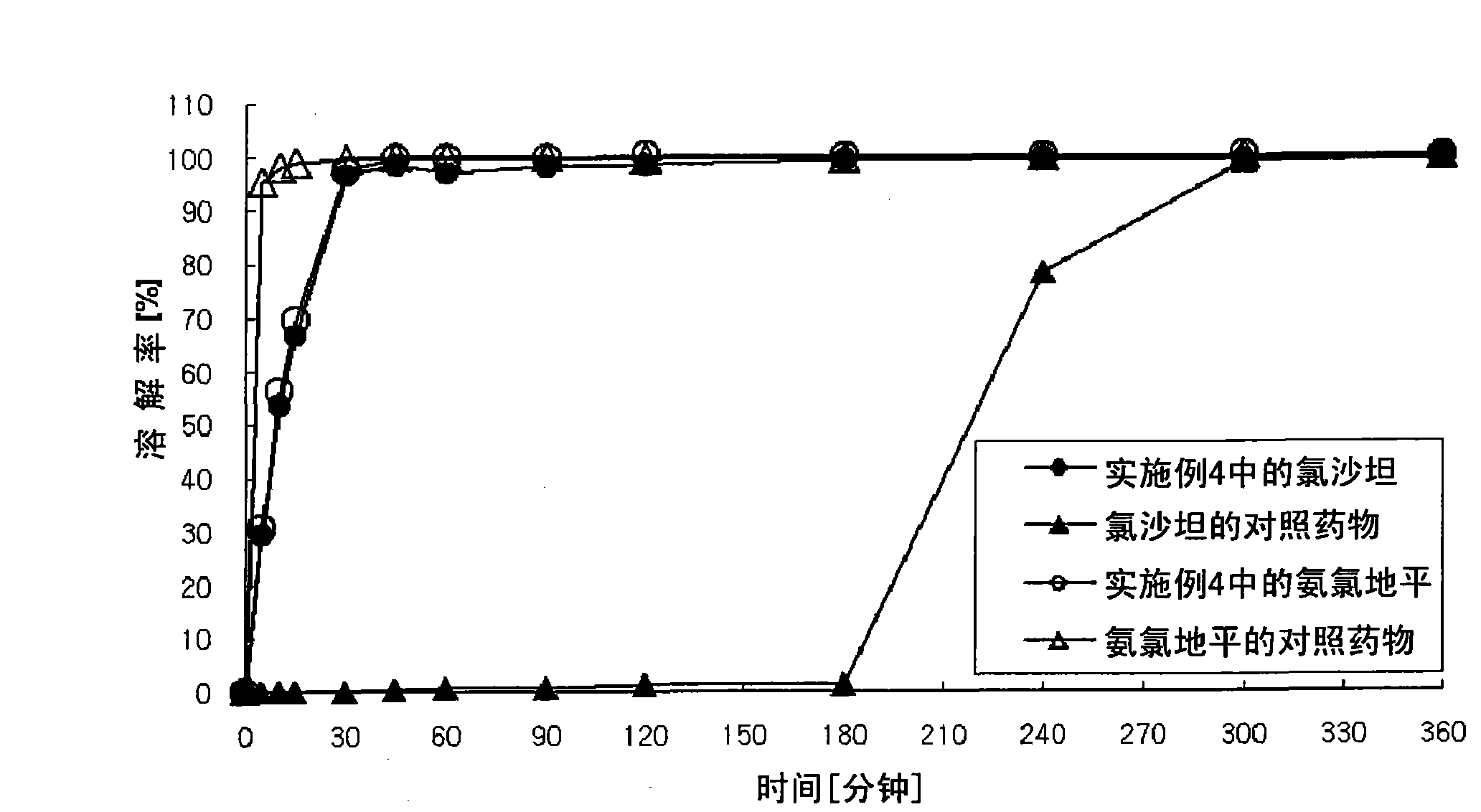
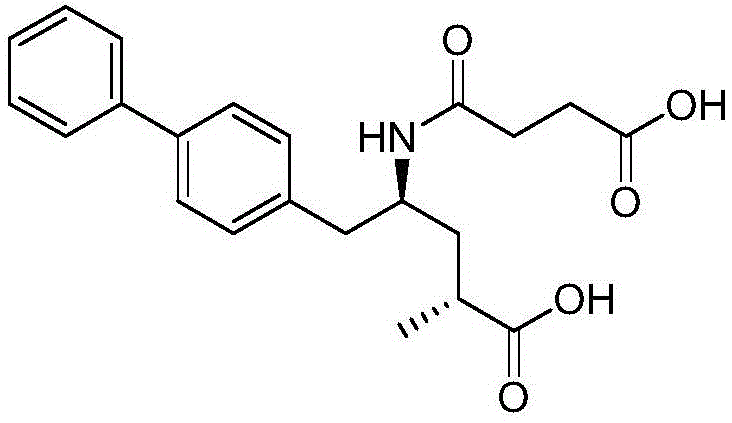
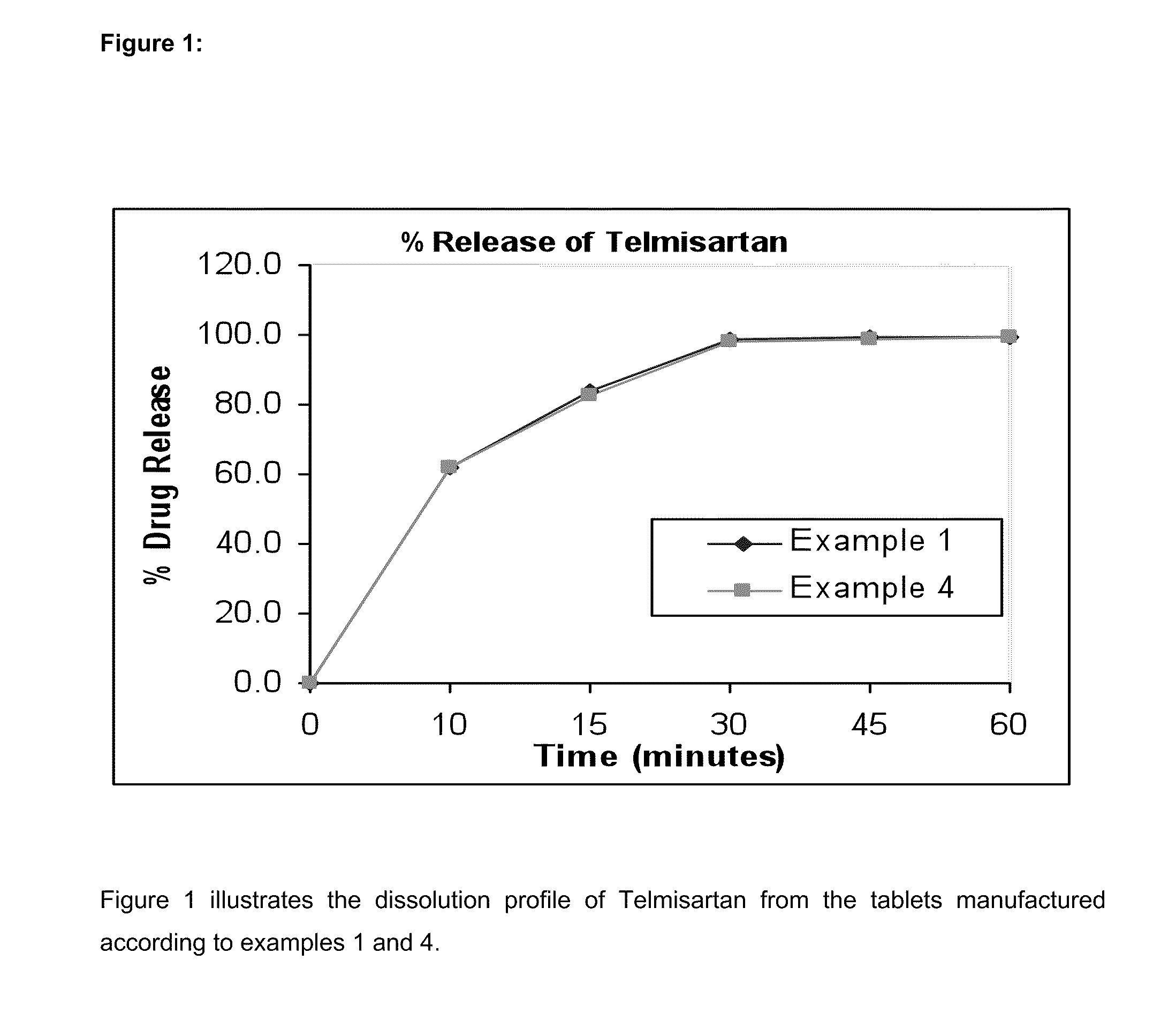
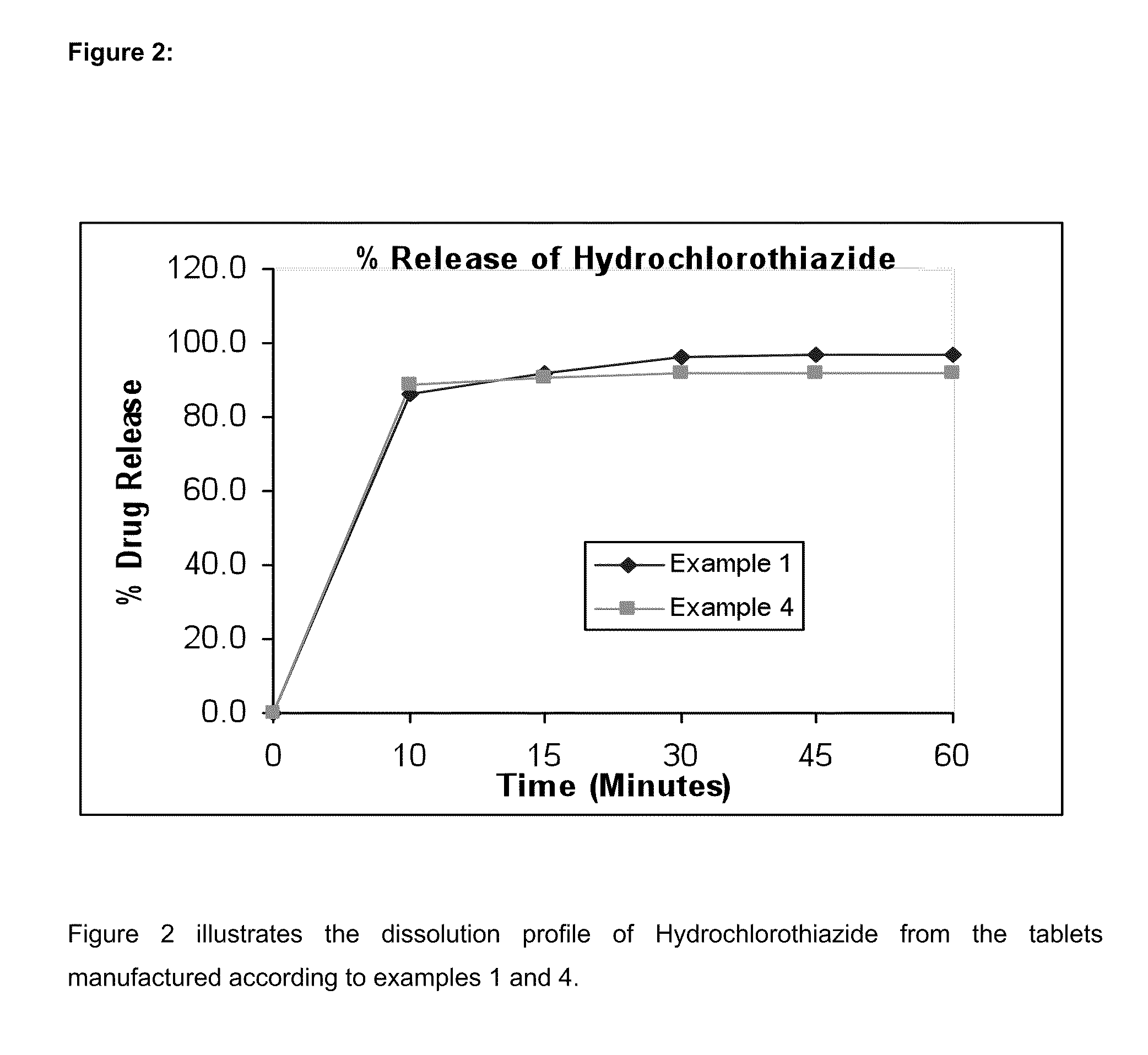
Bilayer pharmaceutical tablet comprising telmisartan and a diuretic and preparation thereof
InactiveUS20050089575A1Low dissolution rateOvercome problemsMaterial analysis by electric/magnetic meansPharmaceutical non-active ingredientsHydrochlorothiazideImmediate release
The present invention relates to a bilayer pharmaceutical tablet comprising a first layer formulated for immediate release of the angiotensin II receptor antagonist telmisartan from a dissolving tablet matrix which contains telmisartan in substantially amorphous form, and a second layer formulated for immediate release of a diuretic like hydrochlorothiazide from a fast disintegrating tablet matrix. A method of producing the bilayer tablet is also disclosed.
Owner:BOEHRINGER INGELHEIM PHARM KG
Treatment of Prevention of Unscheduled Bleeding in Women on Progestogen Containing Medication
InactiveUS20090197843A1Increased chronotropismIncreased inotropismBiocidePeptide/protein ingredientsPhysiologyProgestogen
The present invention relates to a method of treating or preventing unscheduled bleeding in women, the unscheduled bleeding being the result of repeated administration of a hormonal composition that contains a progestogen, wherein the method includes the administration of an effective amount of Renin Angiotensin System (RAS) suppressor selected from angiotensin converting enzyme inhibitors; angiotensin II receptor antagonists; renin inhibitors and combinations thereof. Other aspects of the invention relate to a pharmaceutical composition containing a RAS suppressor and a progestogen and to a pharmaceutical kit having a plurality of dosage units, wherein at least one dosage unit contains a progestogen; at least one dosage unit contains an estrogen; and at least one dosage unit contains a RAS suppressor.
Owner:PANTARHEI BIOSCI
Cosmetic and pharmaceutical compositions comprising ace inhibitors and/or angiotensin ii receptor antagonists
InactiveUS20090137556A1Reduced gene expressionIncrease gene expressionAntibacterial agentsBiocideDermatological disordersACE Inhibitor Fetopathy
In one aspect, the present invention relates to use of an ACE inhibitor and / or angiotensin II receptor antagonist for the preparation of a medicament for the treatment of a dermatological disorder, particularly by topical application of said ACE inhibitor and / or angiotensin II receptor antagonist. The present invention also provides cosmetic methods for improving and / or maintaining the skin tone of an individual suffering from, or at risk of suffering from, a dermatological disorder, said method comprising contacting the skin of said individual with an ACE inhibitor and / or angiotensin II receptor antagonist.
Owner:ACE APS
Pharmaceutical composition for treating hypertension and cardiovascular disease
InactiveCN1883478AImprove solubilityImprove bioavailabilityPill deliveryGranular deliveryVascular diseaseTreatment effect
Owner:CSPC OUYI PHARM CO LTD
Combined preparation for the treatment of cardiovascular diseases based on chronotherapy theory
InactiveUS20100047341A1Improve Medication AdherenceConstant controlBiocideAnimal repellantsCo administrationSide effect
The present invention relates to a functional combination preparation comprising a dihydropyridine-based calcium channel blocker such as amlodipine and an ARB (Angiotensin-2 receptor blocker) such as losartan. In particular, the present invention relates to a chronotherapeutical combination pharmaceutical formulations with controlled-release for the prevention or treatment of cardiovascular disease, which is formulated in accordance with xenobiotics and chronotherapy for enabling the two drugs to be chronotherapeutically released, thereby improving the therapeutic activity as compared to the co-administration of each drug in the form of a single pill, while reducing side effects and maintaining the therapeutic activity as high as possible at the time of day when the risk of a complication of cardiovascular disease is highest.
Owner:HANALL PHARMA CO LTD
Combination therapies for treatment of hypertension and complications in patients with diabetes or metabolic syndrome
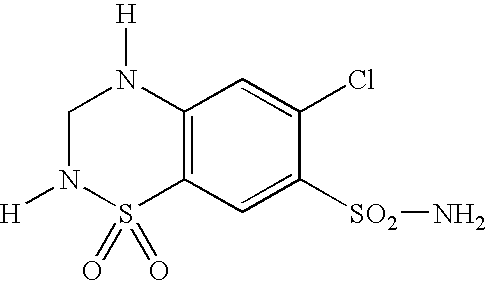
InactiveUS20050043391A1Treating and preventing nephropathyEliminate side effectsBiocideNervous disorderCombination therapyCicletanine
Preferred embodiments of the present invention are related to novel therapeutic drug combinations and methods for treating and / or preventing hypertension and complications in patients with diabetes and / or metabolic syndrome. More particularly, aspects of the present invention are related to using a combination of cicletanine and a second antihypertensive agent (preferably a calcium antagonist, an ACE inhibitor, or an angiotensin II receptor antagonist) for treating and / or preventing hypertension and complications in patients with diabetes and / or metabolic syndrome.
Owner:COTHERIX INC
Novel PPAR ligands that do not cause fluid retention, edema or congestive heart failure
Methods are provided for treating or prophylactically preventing metabolic disorders in humans without causing, promoting, or aggravating fluid retention, peripheral edema, pulmonary edema, or congestive heart failure, by administration of a therapeutically effective amount of a compound sufficient to partially or fully activate peroxisome proliferator activated receptors (PPARs) and partially or fully inhibit, antagonize or block the activity of angiotensin II type 1 receptors. Metabolic disorders that can be treated or prevented include but are not limited to type 2 diabetes, the metabolic syndrome, prediabetes, and other insulin resistance syndromes. Compounds are provided that antagonize or block the angiotensin II type 1 (AT1) receptor, function as partial or full activators of peroxisome proliferator activated receptors (PPARs), can be used to treat or prevent diseases known to be treatable or preventable by PPAR activators and were not previously recognized to be therapeutic targets for angiotensin II receptor antagonists.
Owner:BETHESDA PHARMA
Conjoint administration of morphogens and ACE inhibitors in treatment of chronic renal failure
InactiveUS20050272649A1Preventing delaying needReducing necessary frequencyBiocidePeptide/protein ingredientsRenal disorderMorphine
The present invention provides reagents and methods for the treatment, and pharmaceuticals for use in the prevention and / or treatment, of chronic renal failure and other renal disorders in subjects (particularly mammalian subjects) renal replacement therapy. The methods involve the conjoint administration of ACE (Angiotensin-Converting Enzyme) inhibitors or Angiotensin II Receptor Antagonists (AIIRAs) with one or more OP / BMP family of proteins (morphogens, or inducers of morphogens, or agonists of the corresponding morphogen receptors, etc.). The invention also provides methods for implantation of renal cells induced with the conjoint administration of ACE inhibitors or AIIRAs with those morphogens.
Owner:BARNES JEWISH HOSPITAL +1
PPAR Ligands that do not cause fluid retention, edema or congestive heart failure
Methods are provided for treating or prophylactically preventing metabolic disorders in humans without causing, promoting, or aggravating fluid retention, peripheral edema, pulmonary edema, or congestive heart failure, by administration of a therapeutically effective amount of a compound sufficient to partially or fully activate peroxisome proliferator activated receptors (PPARs) and partially or fully inhibit, antagonize or block the activity of angiotensin II type 1 receptors. Metabolic disorders that can be treated or prevented include but are not limited to type 2 diabetes, the metabolic syndrome, prediabetes, and other insulin resistance syndromes. Compounds are provided that antagonize or block the angiotensin II type 1 (AT1) receptor, function as partial or full activators of peroxisome proliferator activated receptors (PPARs), can be used to treat or prevent diseases known to be treatable or preventable by PPAR activators and were not previously recognized to be therapeutic targets for angiotensin II receptor antagonists.
Owner:BETHESDA PHARMA
Use of ace inhibitors and/or angiostensin ii receptor antagonists for the improving and/or maintaining the skin tone and for the treatment of skin ageing
InactiveUS20090143458A1Reduced gene expressionIncrease gene expressionCosmetic preparationsBiocideNK1 receptor antagonistDepressant
The present invention relates to use of an ACE inhibitor and / or angiotensin II receptor antagonist of the preparation of a medicament for the treatment of skin ageing or wrinkling. Furthermore, the present invention relates to use of an ACE inhibitor and / or angiotensin II receptor antagonist for the preparation of a cosmetic composition.
Owner:ACE APS
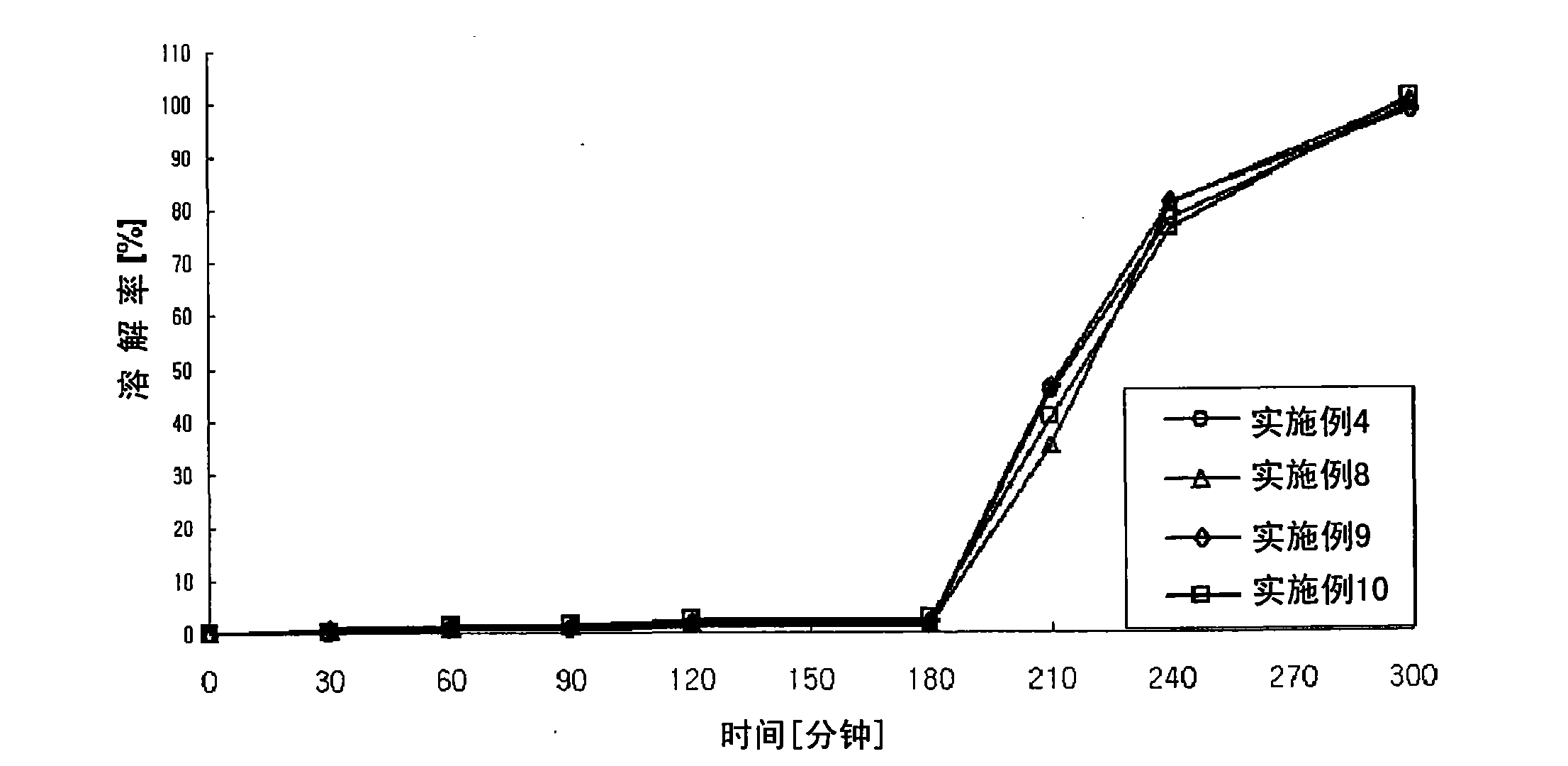
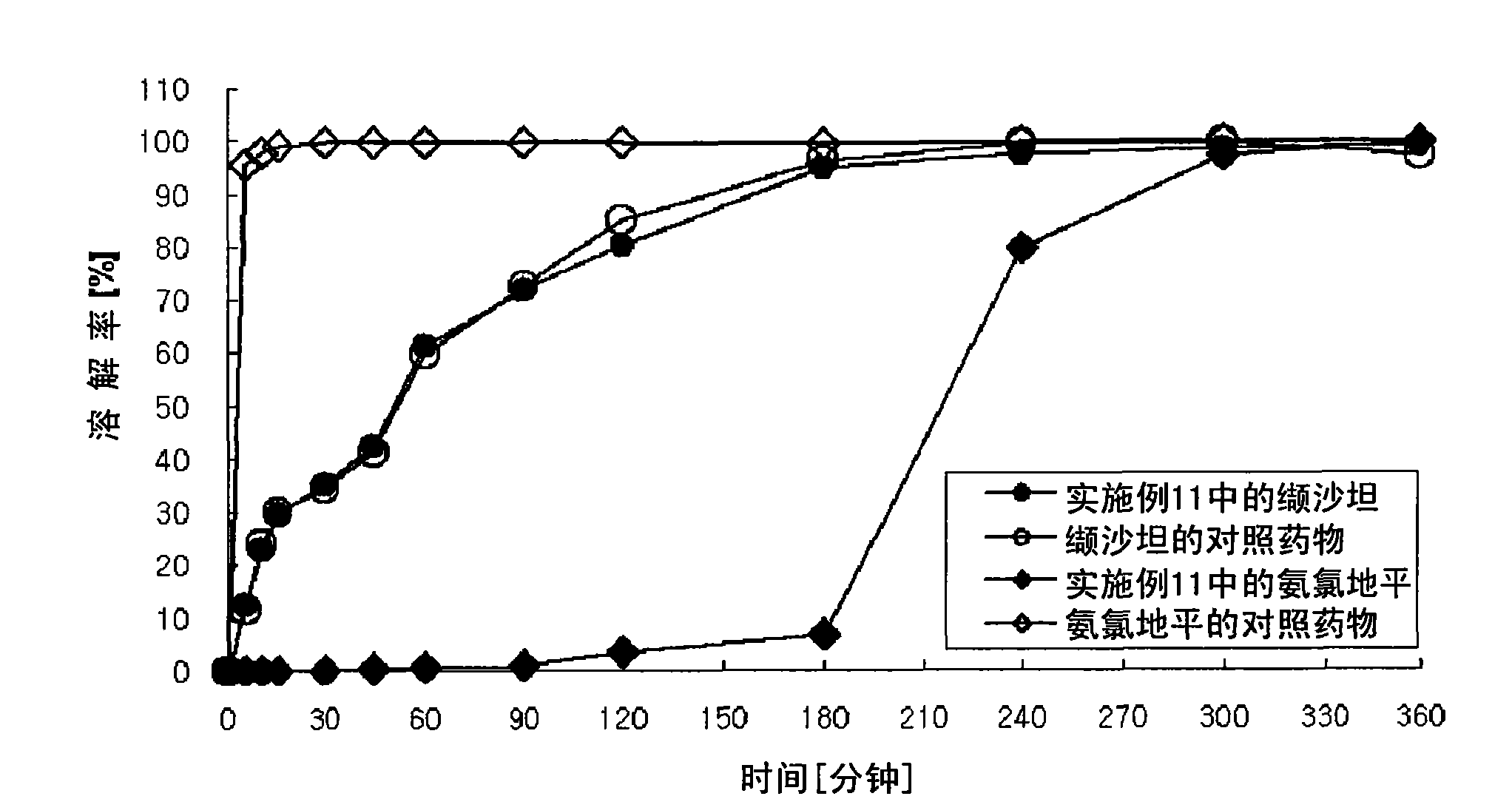
Pharmaceutical Preparation Containing an Angiotensin II Receptor Antagonist and a Calcium Channel Blocker
InactiveUS20080279942A1High dissolution ratePowder deliveryBiocideAcid substancesPharmaceutical formulation
A pharmaceutical preparation comprising an angiotensin II receptor antagonist, a calcium channel blocker and at least one substance selected from the group consisting of a hydrophilic polymer, an acidic substance and a fluidizing agent. The pharmaceutical preparation has improved dissolution properties.
Owner:DAIICHI SANKYO CO LTD
Composition for lowering blood pressure and application thereof
InactiveCN101890165AImprove compliancePrevent or delay damageOrganic active ingredientsMetabolism disorderTasosartanValsartan
The invention provides a pharmaceutical composition which comprises calcium channel blockers of a medicinal dose, angiotensin II receptor antagonists of a medicinal dose, one or more of B vitamins of a medicinal dose and pharmaceutically acceptable carriers, wherein the calcium channel blockers are selected from amlodipine, felodipine, israbipine, nicardipine, nifedipine, nisoldipine, nitrendipine, lacidipine, diltiazem or verapamil; the angiotensin II receptor antagonists are selected from candesartan, telmisartan, losartan, valsartan, irbesartan, eprosartan, tasosartan or olmesartan; and the B vitamins are selected from one or more of vitamin B6, vitamin B12, folic acid and calcium leucovorin. The pharmaceutical composition of the invention can improve the curative effect of the hypotensor, enhance the target organ protecting action of the hypotensor, and reduce the morbidity of complications of angina, myocardial infarction and the like.
Owner:北京奥萨医药研究中心有限公司 +1
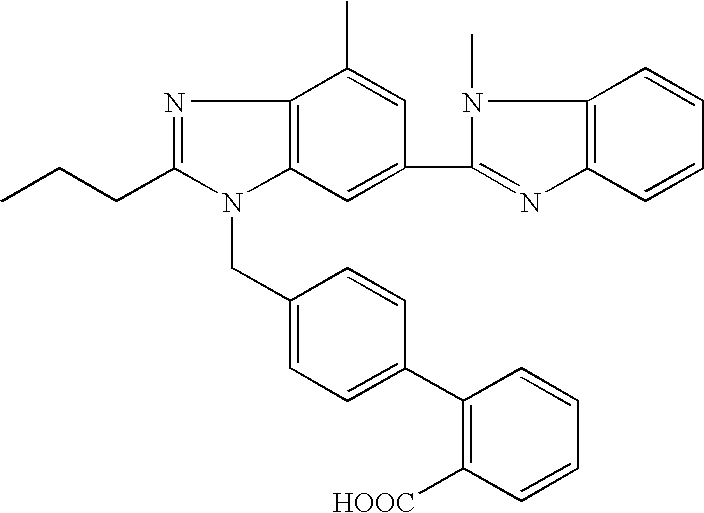
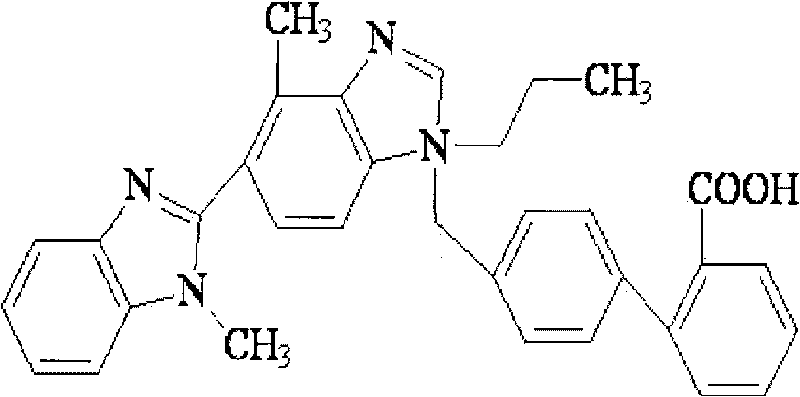
Telmisartan solid dispersion and preparation method thereof
InactiveCN102178642AImprove solubilityImprove bioavailability in vivoOrganic active ingredientsPharmaceutical delivery mechanismMass ratioDissolution
The invention relates to the technical field of medicines, in particular to a telmisartan solid dispersion as a specific angiotensin II receptor antagonist (ATI type) and a preparation method thereof. The telmisartan solid dispersion comprises medicine telmisartan, a carrier and an alkali matter, and also comprises a surfactant, wherein the carrier is a hydrophilic polymeric carrier, and the mass ratio of the medicine telmisartan to the hydrophilic polymeric carrier to the alkali matter to the surfactant is 1:(1-9):(0.1-0.5):(0.1-0.5). The telmisartan solid dispersion has better dissolution rate during the use, and is convenient for gastrointestinal tract absorption, thereby improving bioavailability.
Owner:SUZHOU UNIV
Pharmaceutical composition containing calcium blocker, AII receptor blocker and statins
InactiveCN101618215AReduce morbidityImprove complianceSenses disorderMetabolism disorderCandesartanLacidipine
The invention relates to a pharmaceutical composition containing a calcium channel blocker (CCB) or the mixture thereof, an angiotonin 3II receptor blocker (ARB) or the mixture thereof, statins or the mixture thereof and a pharmaceutically acceptable carrier, wherein the CCB is selected from l-amlodipine, amlodipine, lacidipine, nitrendipine or the mixture thereof; the angiotonin II receptor blocker is selected from telmisartan, losartan, irbesartan, candesartan or the mixture thereof; and the statins are selected from atorvastatin, simvastatin, ruishufatadine, fluvastatin or the mixture thereof. The pharmaceutical composition is used for treating various high blood pressures and preventing or treating cardiovascular and cerebrovascular diseases relevant to the hypertension, reduces the disease rate and / or mortality rate of the cardiovascular and cerebrovascular diseases and also improves the adaptability for a sufferer taking medicine.
Owner:王丽燕
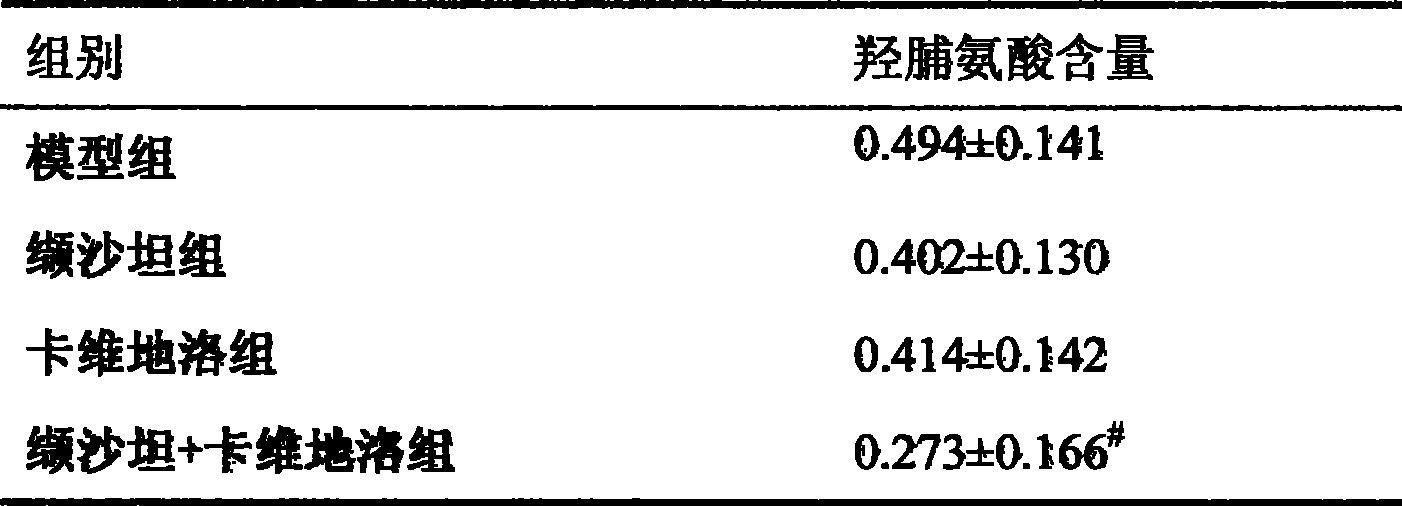
Use of medicine combination comprising carvedilol and angiotensin II recipient antagon in preparing medicine for treating kidney disease
ActiveCN101417132ASynergistic treatment effect is goodImpact on long-term survivalPowder deliveryMetabolism disorderNephrosisActive component
The invention provides the usages of a drug composition comprising the active components of carvedilol and an angiotensin II receptor antagonist for preparing a drug which can cure nephropathy. The cooperation effect of the carvedilol and the angiotensin II receptor antagonist are used for developing a method which is more effective in curing the nephropathy. The drug composition has remarkable effects when being used for curing nephropathy, diabetic nephropathy, hypertensive nephropathy, and the like.
Owner:LUNAN PHARMA GROUP CORPORATION
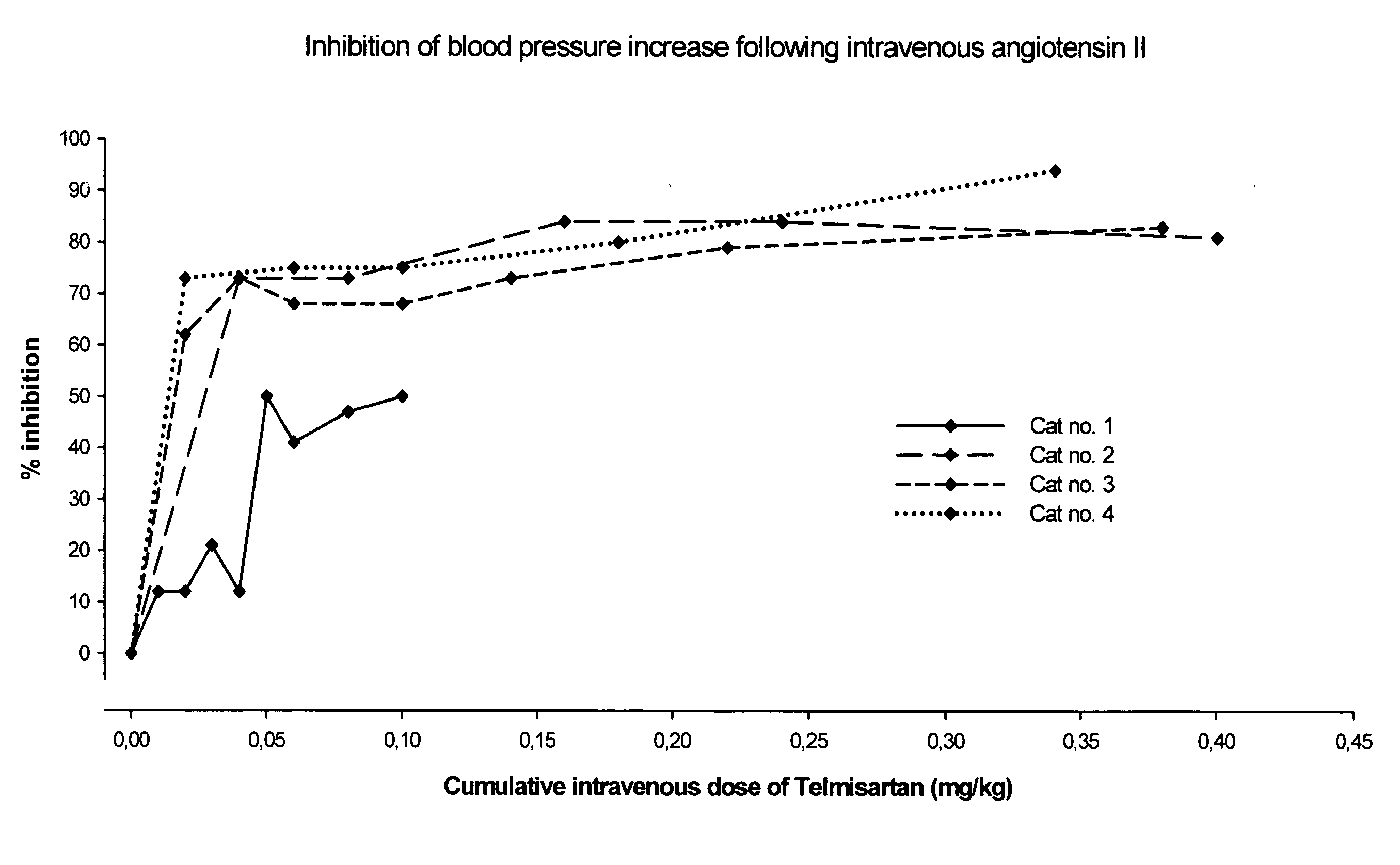
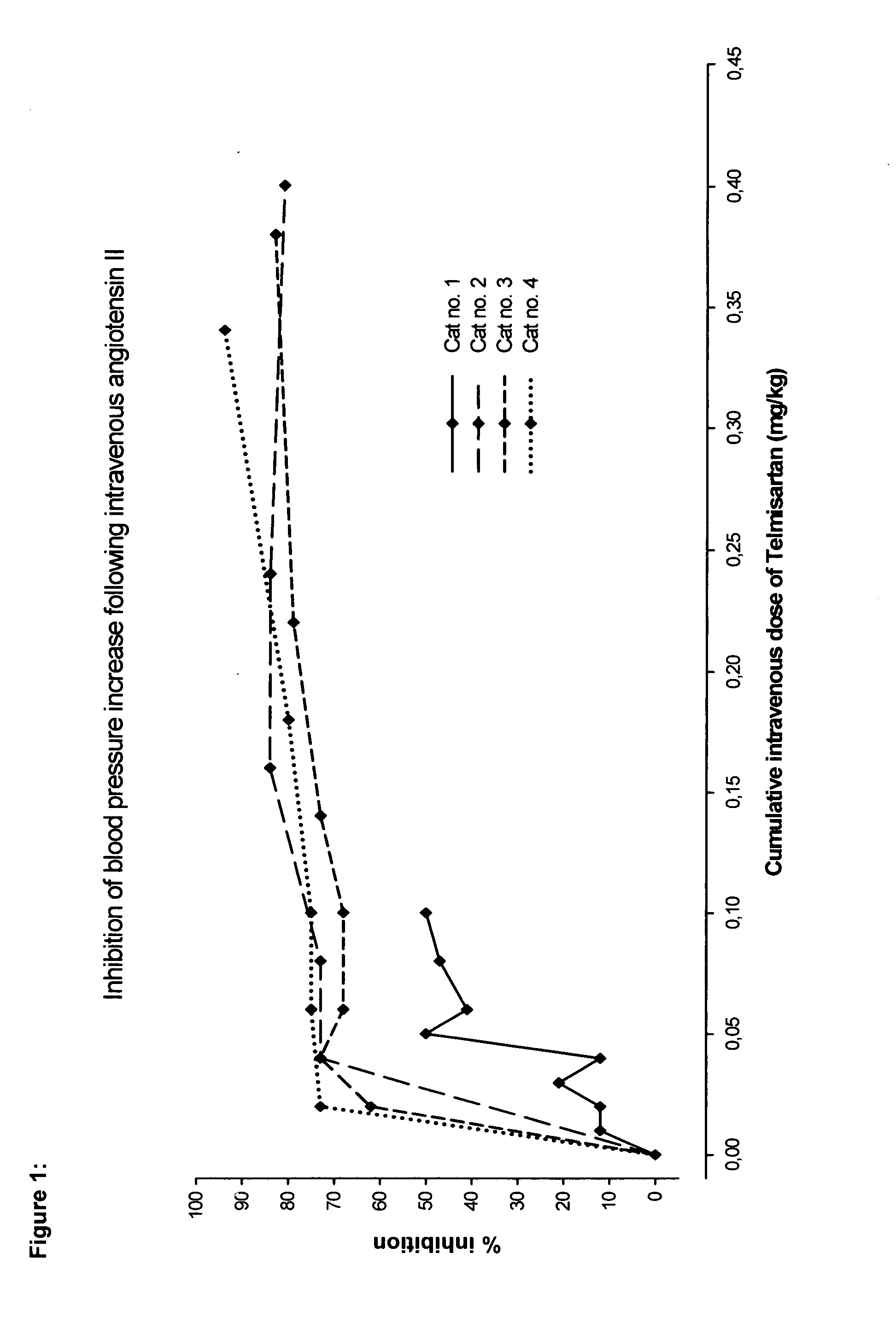
Angiotensin II receptor antagonist for the prevention or treatment of systemic diseases in cats
ActiveUS20080146543A1BiocideMetabolism disorderAngiotensin 2 Receptor BlockerAngiotensin II receptor antagonist
The present invention relates to a method of prophylaxis or treatment of systemic diseases in cats, wherein the method comprising administration of a therapeutically effective amount of angiotensin II receptor 1 (AT-1) antagonist (sartan) to a cat in need of such a treatment.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
Combined preparation for the treatment of cardiovascular diseases based on chronotherapy theory
InactiveCN101528203ASignificant blood pressure lowering effectExcellent suppression of side effectsOrganic active ingredientsAerosol deliverySide effectCo administration
The present invention relates to a functional combination preparation comprising a dihydropyridine-based calcium channel blocker such as amlodipine and an ARB (Angiotensin-2 receptor blocker) such as losartan. In particular, the present invention relates to a chronotherapeutical combination pharmaceutical formulations with controlled-release for the prevention or treatment of cardiovascular disease, which is formulated in accordance with xenobiotics and chronotherapy for enabling the two drugs to be chronotherapeutically released, thereby improving the therapeutic activity as compared to the co-administration of each drug in the form of a single pill, while reducing side effects and maintaining the therapeutic activity as high as possible at the time of day when the risk of a complication of cardiovascular disease is highest.
Owner:韩诺生物制约株式会社
Method to treat cardiofibrosis with a combination therapy of an angiotensin II antagonist and epoxymexrenone
A therapeutic method is described for treating cardiofibrosis or cardiac hypertrophy using a combination therapy comprising a therapeutically-effective amount of an angiotensin II receptor antagonist and a therapeutically-effective amount of expoxymexrenone.
Owner:GD SEARLE & CO
Oral suspension comprising telmisartan
ActiveUS20140364473A1Improve subjective overall impressionIncrease contact timeOrganic active ingredientsBiocideOral suspensionsAlcohol sugars
A pharmaceutical solution with a pH value of 10 or higher contains an angiotensin II receptor antagonist, where one or more sugar alcohols are present up to a total concentration of 40 wt. % to 70 wt. %.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
Novel composing prescription sustained-release preparation for treating high blood pressure and preparation method thereof
InactiveCN101185624AQuick-acting and long-actingReduce the frequency of takingPharmaceutical delivery mechanismHeterocyclic compound active ingredientsDissolutionDrug release
The invention relates to a sustained release preparation of a novel prescription for treating hypertension and the preparing method thereof. The novel prescription comprises a heart selective Beta1 receptor blocker metoprolol and an angiotensin II receptor antagonist (sartan drugs). The sustained release preparation consists of delayed release part and rapid release part, wherein the heart selective Beta1 receptor blocker metoprolol is the delayed release part, with first hour releasing 25-45%, fourth hour releasing 40-75% and eighth hour releasing over 75%; the angiotensin II receptor antagonist (sartan drugs) is the rapid release part, with 45 minutes dissolution over 75%. The composition has both rapid and prolonged action. The invention discloses in vitro drug release characteristics and preparation method thereof.
Owner:BEIJING RUNDEKANG MEDICAL TECH CO LTD
Pharmaceutical composition for treating cardiovascular diseases and preparation method and use thereof
The invention relates to a pharmaceutical composition containing eprosartan, amlodipine and hydrochlorothiazide and a prepration method and a use thereof, the composition consists of the eprosartan, the amlodipine and the hydrochlorothiazide, and the composition is used for treating patients with moderate hypertension, severe hypertension, coronary heart disease and angina and the patients with the hypertension, the coronary heart disease and the angina, wherein blood pressure of the patients can not be fully controlled after using an angiotensin II receptor antagonist or a calcium antagonist for treating.
Owner:GUANGXI FANGLUE GROUP LONGZHOU PHARMA
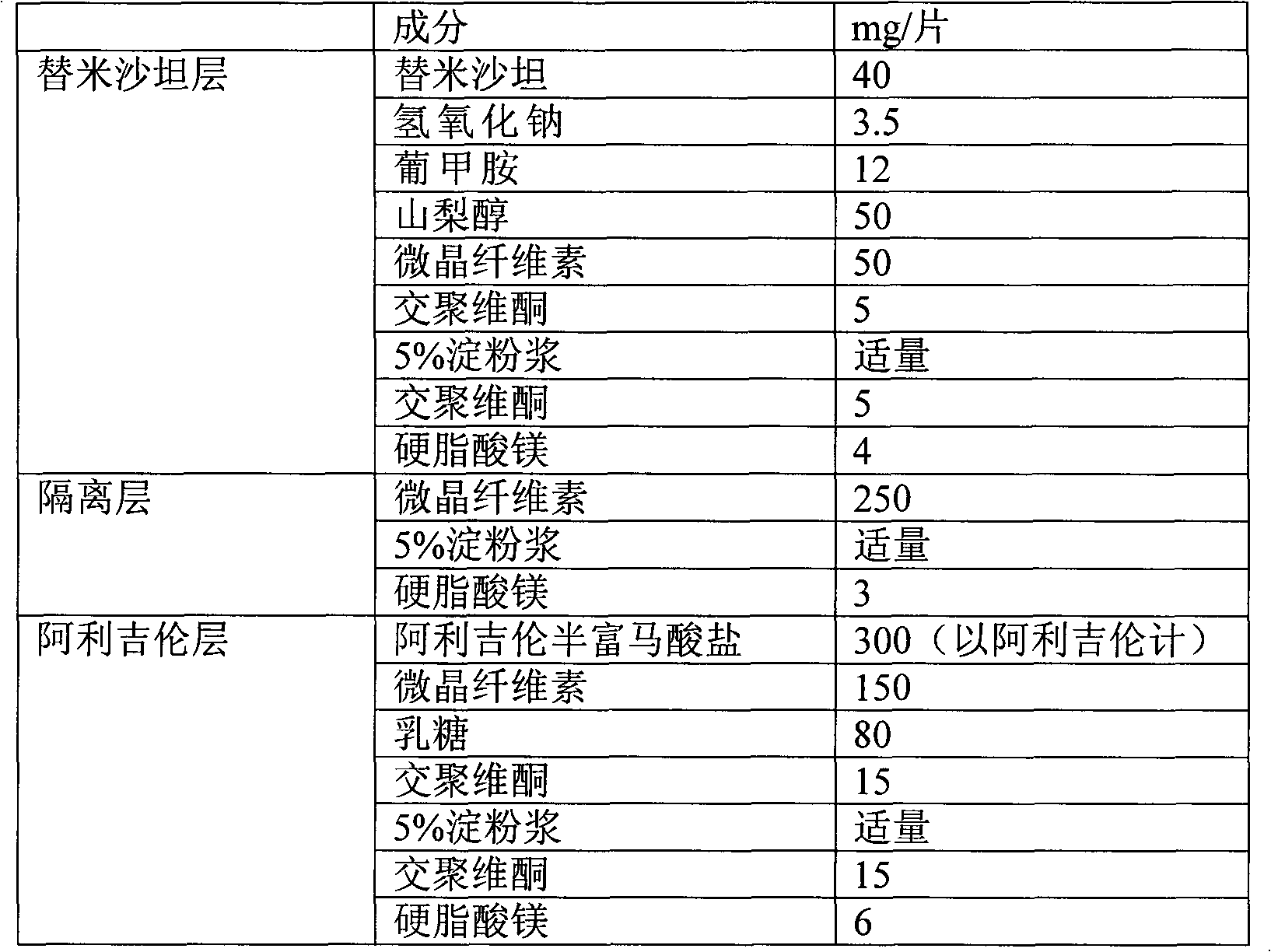
Combined medicament containing telmisartan and aliskiren and preparation method thereof
ActiveCN101926793AAvoid interactionGuaranteed stabilityOrganic active ingredientsPill deliveryPharmaceutical formulationTreatment hypertension
The invention relates to application of telmisartan and aliskiren or medicinal salts thereof in preparing a combined medicament for treating hypertension. The invention also provides the combined medicament for treating hypertension, wherein the combined medicament contains unit preparations of different specifications, and the preparations administrated simultaneously, respectively or in turn are prepared from telmisartan and aliskiren or medicinal salts thereof and pharmaceutically acceptable carriers. The invention also provides a preparation method and application of the combined medicament. The medicinal preparations are three-layer tablets so as to avoid interaction of the aliskiren and the telmisartan, improve the medicament stability and facilitate long-term storage. The composition preparations are used for treating medium and high hypertension patients and hypertension patients whose blood pressure cannot be fully controlled after being treated by angiotensin II receptor antagonists or renin inhibitors.
Owner:CHENGDU ZIHAO PHARMA
Medicine composite for treating and relieving high blood pressure
The invention discloses a medicine composite for treating and relieving high blood pressure and a preparation method thereof. The composite comprises 10-320mg of telmisartan and 1-20mg of felodipine slow-release preparation. The composite is used for treating patients with moderate and acute high blood pressure and patients whose blood pressure can not be adequately controlled after the high blood pressure is treated by an angiotensin II receptor antagonist or a calcium antagonist.
Owner:广西方略集团崇左制药有限公司
Process for the manufacture of organic compounds
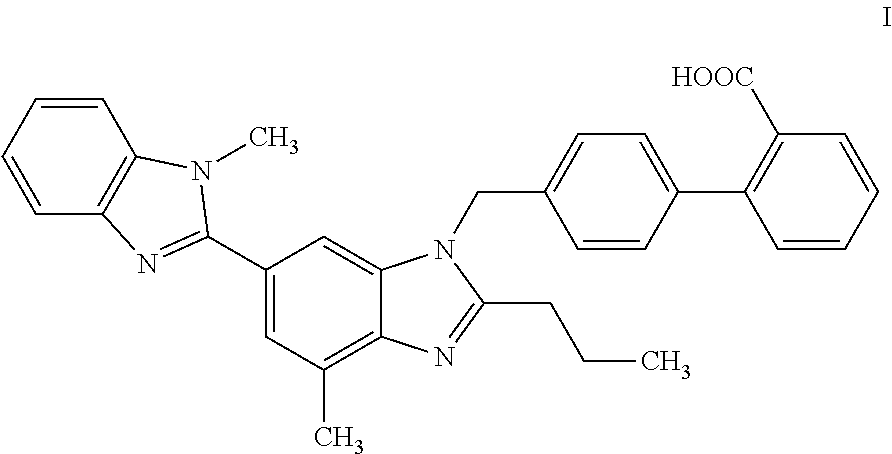
InactiveCN102596899AOrganic compound preparationCardiovascular disorderAngiotensin receptor iiOrganic compound
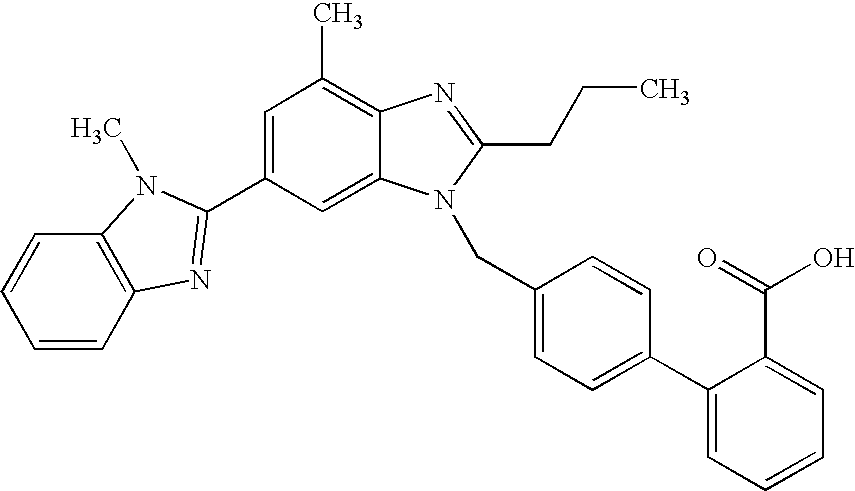
The present invention relates to processes for the manufacture of an angiotensin receptor blocker (ARB; also called angiotension II receptor antagonist or AT1 receptor antagonist) and salts thereof, to novel intermediates and process steps.
Owner:NOVARTIS AG
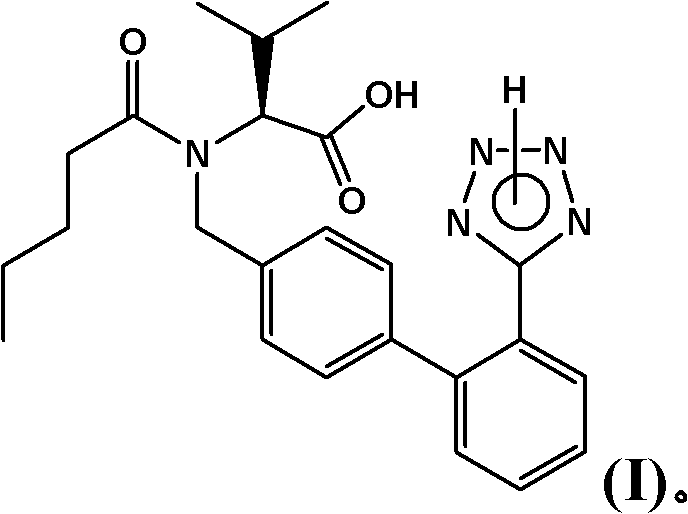
Refining method for enkephalin enzyme inhibitor and angiotensin II receptor antagonist eutectic compound
InactiveCN105646384AEfficient removalHigh purityCarboxylic acid amide separation/purificationEnkephalinase inhibitorDissolution
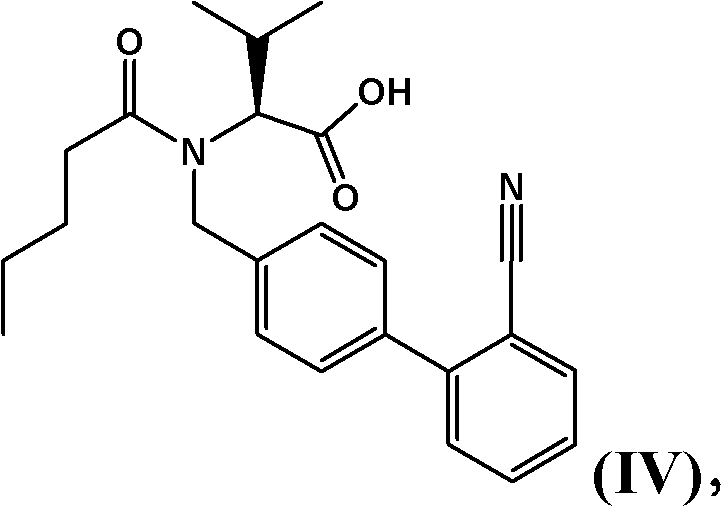
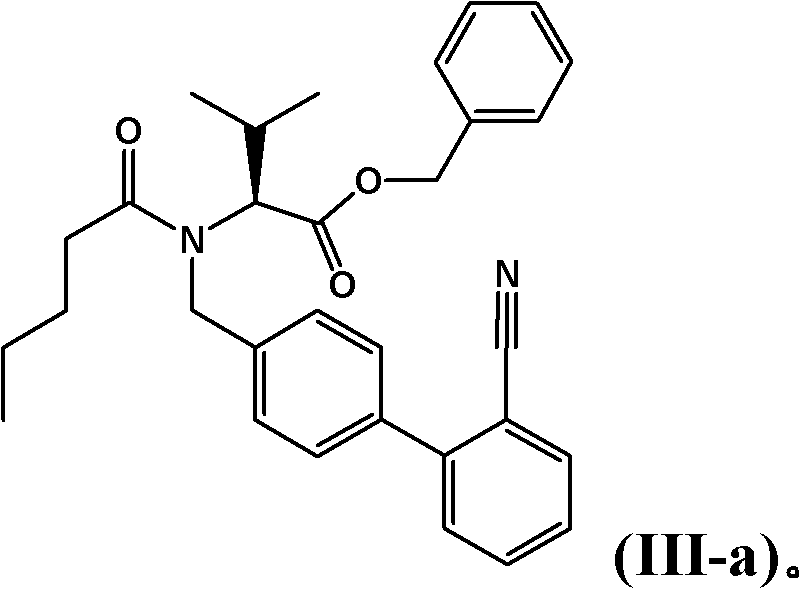
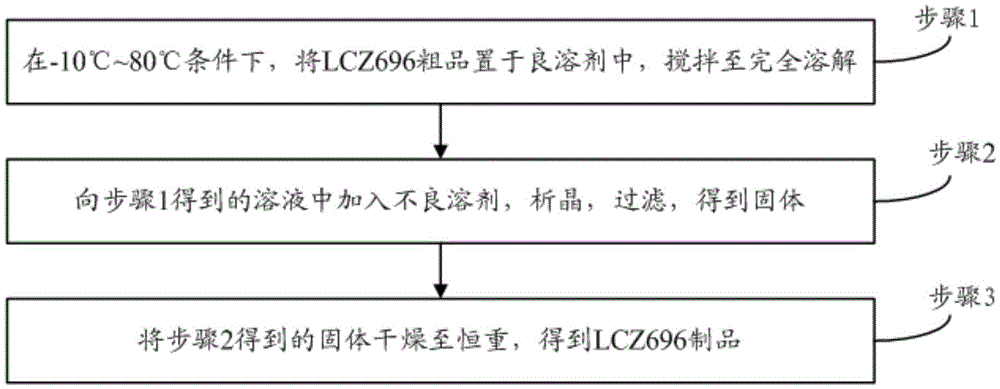
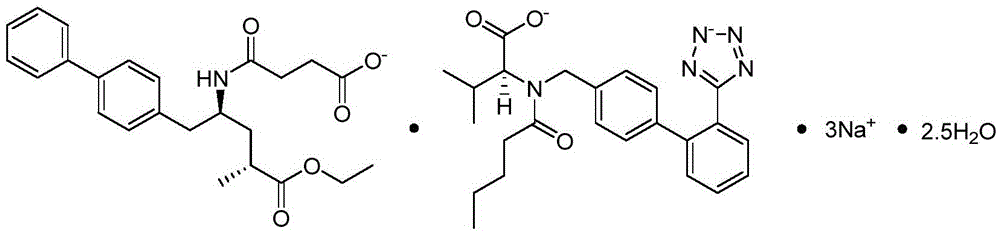
The invention provides a refining method for an enkephalin enzyme inhibitor and angiotensin II receptor antagonist eutectic compound. The refining method comprises the following steps that firstly, under the condition of minus 10-80 DEG C, an LCZ696 crude product is put into a good solvent and stirred to complete dissolution, wherein LCZ696 is the enkephalin enzyme inhibitor and angiotensin II receptor antagonist eutectic compound; secondly, the solution obtained in the first step is added with a poor solvent, crystallization and filtering are carried out, and a solid is obtained; thirdly, the solid obtained in the second step is dried to the constant weight, and an LCZ696 product is obtained. The refining method has the advantages that enkephalin enzyme inhibitor hydrolysis impurities and other impurities in the LCZ696 crude product can be effectively removed, the purity of the LCZ696 product can be improved, and thus the quality stability of LCZ696 can be ensured; in addition, the refining method is high in product yield and easy to implement.
Owner:BEIJING VOBAN PHARMA TECH CO LTD
Solid pharmaceutical composition comprising a non-peptide angiotensin ii receptor antagonist and a diuretic
The present invention relates to a solid pharmaceutical composition comprising at least two layers, wherein the first layer contains a non-peptide angiotensin II receptor antagonist or a pharmaceutically acceptable salt thereof in a dissolving matrix and the second layer contains a diuretic or a pharmaceutically acceptable salt thereof. The invention also provides methods for the production of said pharmaceutical compositions.
Owner:RATIOPHARM GMBH
Remedy for glomerular diseae
InactiveCN101010079AUrinary disorderHeterocyclic compound active ingredientsChronic glomerular diseaseGlomerular diseases
It is intended to provide a medicinal composition which is efficacious in preventing or treating a glomerular disease, in particular, a progressive chronic glomerular disease. A preventive / remedy for a glomerular disease containing pyridoxamine or its salt and an angiotensin II receptor antagonist.
Owner:KOWA CO LTD
Cosmetic and pharmaceutical compositions comprising ace inhibitors and/or angiotensin ii receptor antagonists
InactiveUS20120219514A1Reduce riskImprove skin toneAntibacterial agentsCosmetic preparationsPharmaceutical drugAngiotensin Receptor Antagonists
In one aspect, the present invention relates to use of an ACE inhibitor and / or angiotensin II receptor antagonist for the preparation of a medicament for the treatment of a dermatological disorder, particularly by topical application of said ACE inhibitor and / or angiotensin II receptor antagonist. The present invention also provides cosmetic methods for improving and / or maintaining the skin tone of an individual suffering from, or at risk of suffering from, a dermatological disorder, said method comprising contacting the skin of said individual with an ACE inhibitor and / or angiotensin II receptor antagonist.
Owner:ACE APS
Valsartan dispersible tablet and preparation method thereof
ActiveCN104042580AGood dispersionImprove drug dissolutionPharmaceutical product form changePill deliveryValsartanDrug Dissolution
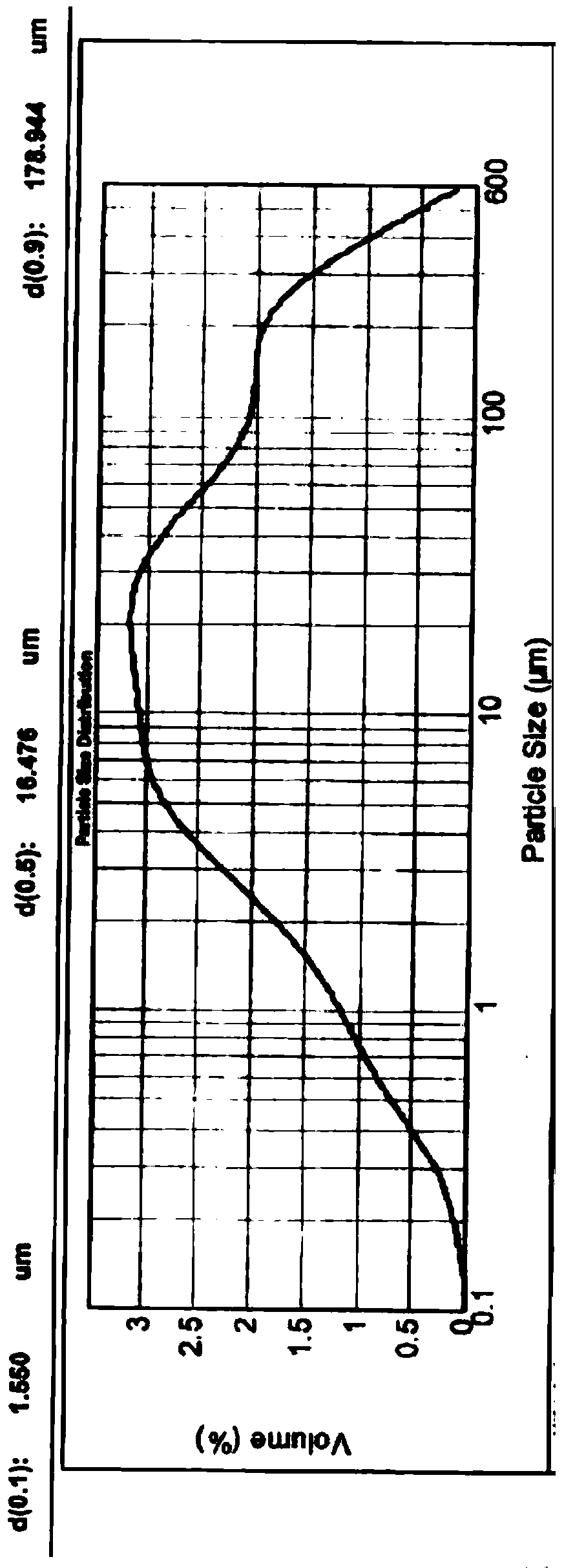
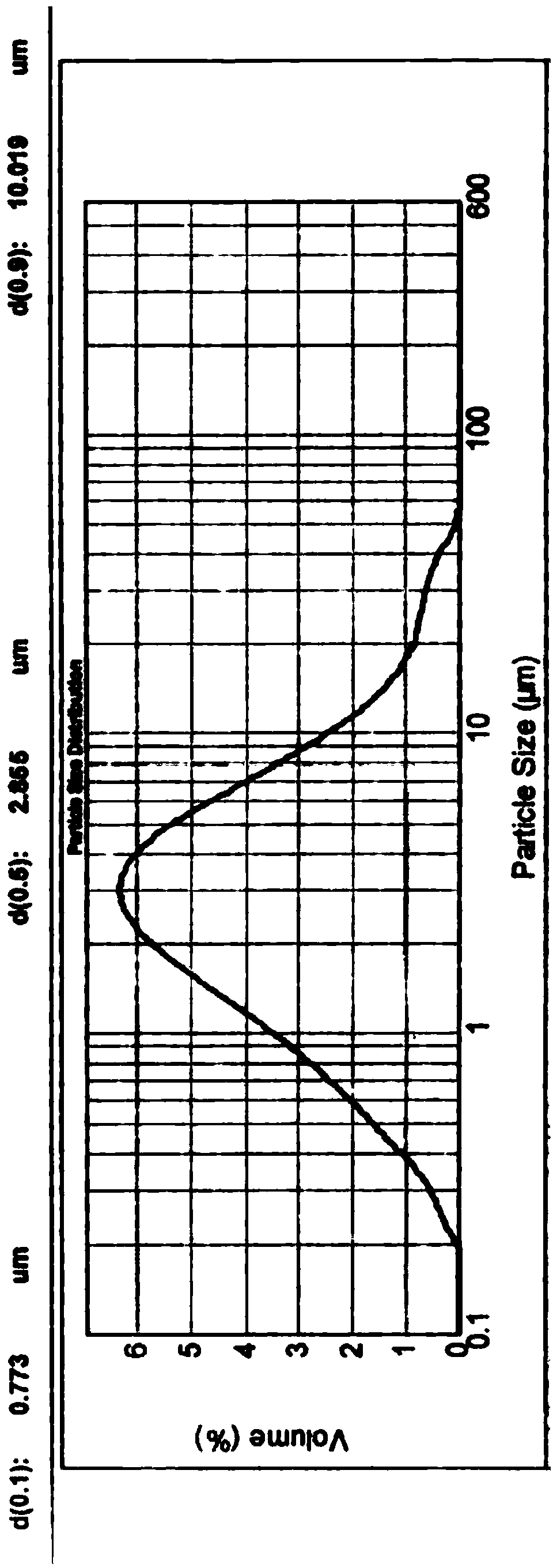
The invention relates to medicine field, and particularly relates to an angiotensin II receptor antagonist valsartan dispersible tablet and a preparation method thereof. The prescription of the product comprises pharmaceutical adjuvants acceptable in pharmacy like valsartan bulk drug, a filler, a disintegrant, an adhesive, flow aid, a lubricant, and corrigent, and the dispersible tablet is prepared by a wet granulation method. The valsartan bulk drug needs to be micronized, and the diameter 90 is less than 75mu m, preferably, the diameter 90 is less than 10mu m. The size distribution of the valsartan bulk drug directly affects the dispersibility of the dispersible tablet. The valsartan has polymorphy, different crystal forms of valsartan have different size distribution, and the dispersibility of the valsartan dispersible tablet is more different. The size distribution of the valsartan bulk drug is controlled by virtue of the micronization technology, the diameter 90 is less than 75mu m, preferably, when the diameter 90 is less than 10mu m, the difference of size distribution of different crystal forms of valsartan bulk drug is eliminated, the dispersibility of the valsartan dispersible tablet is improved obviously, the drug dissolution rate of the valsartan dispersible tablet is improved, and the product quality is controllable and is improved remarkably.
Owner:珠海润都制药股份有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com