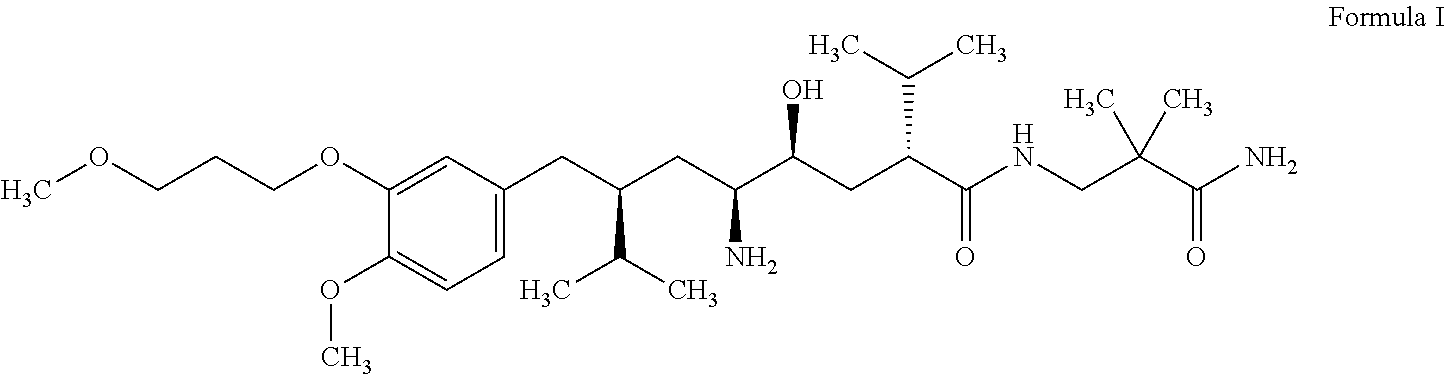
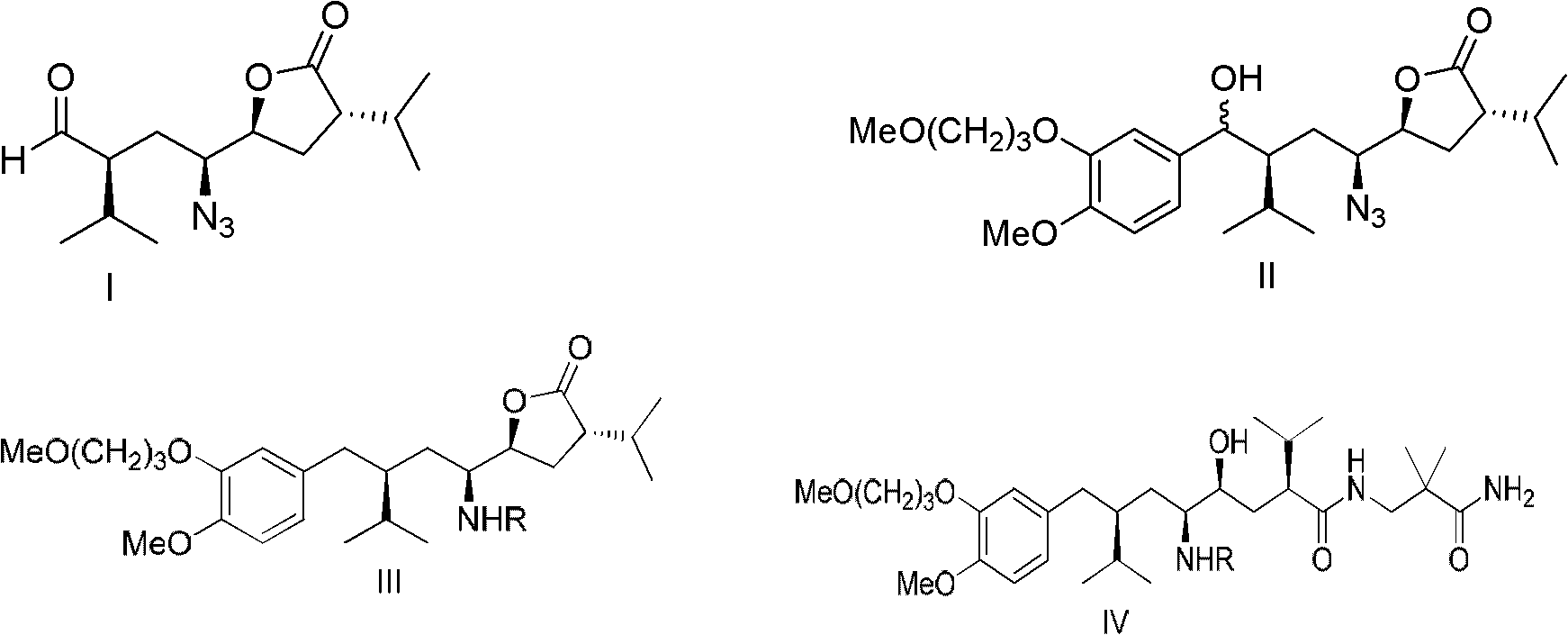
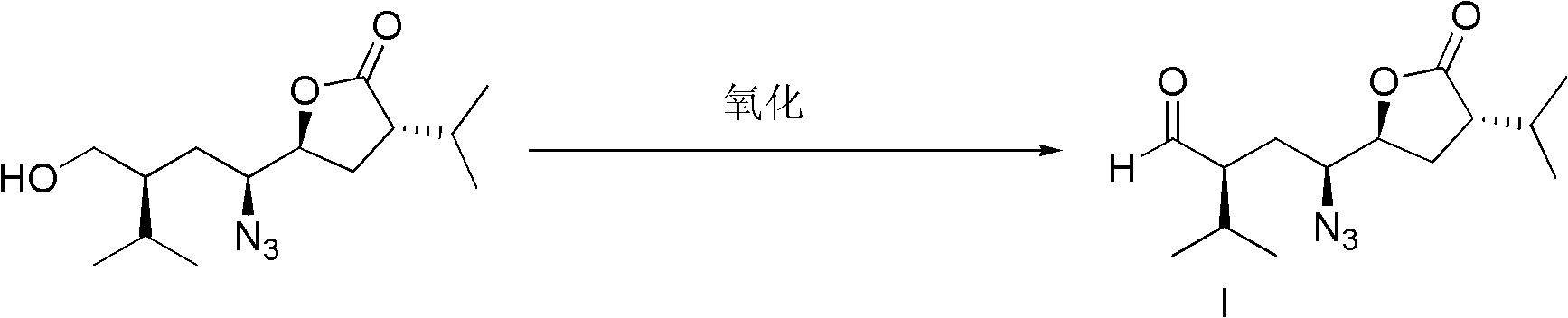
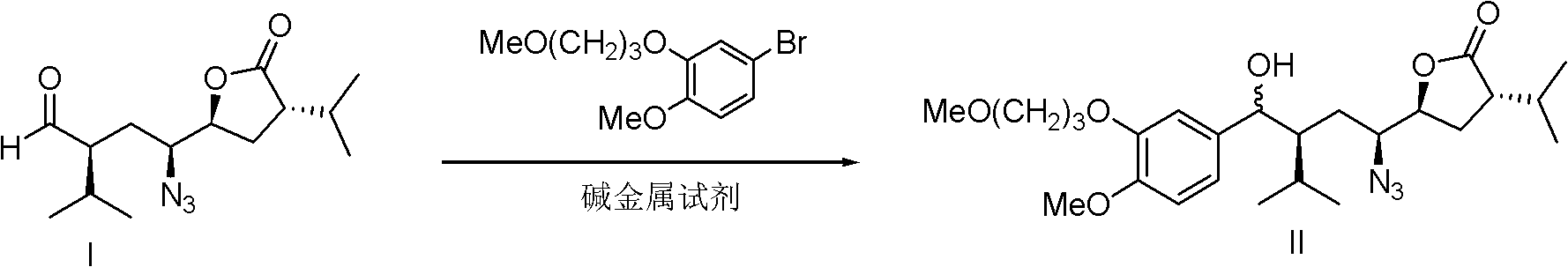
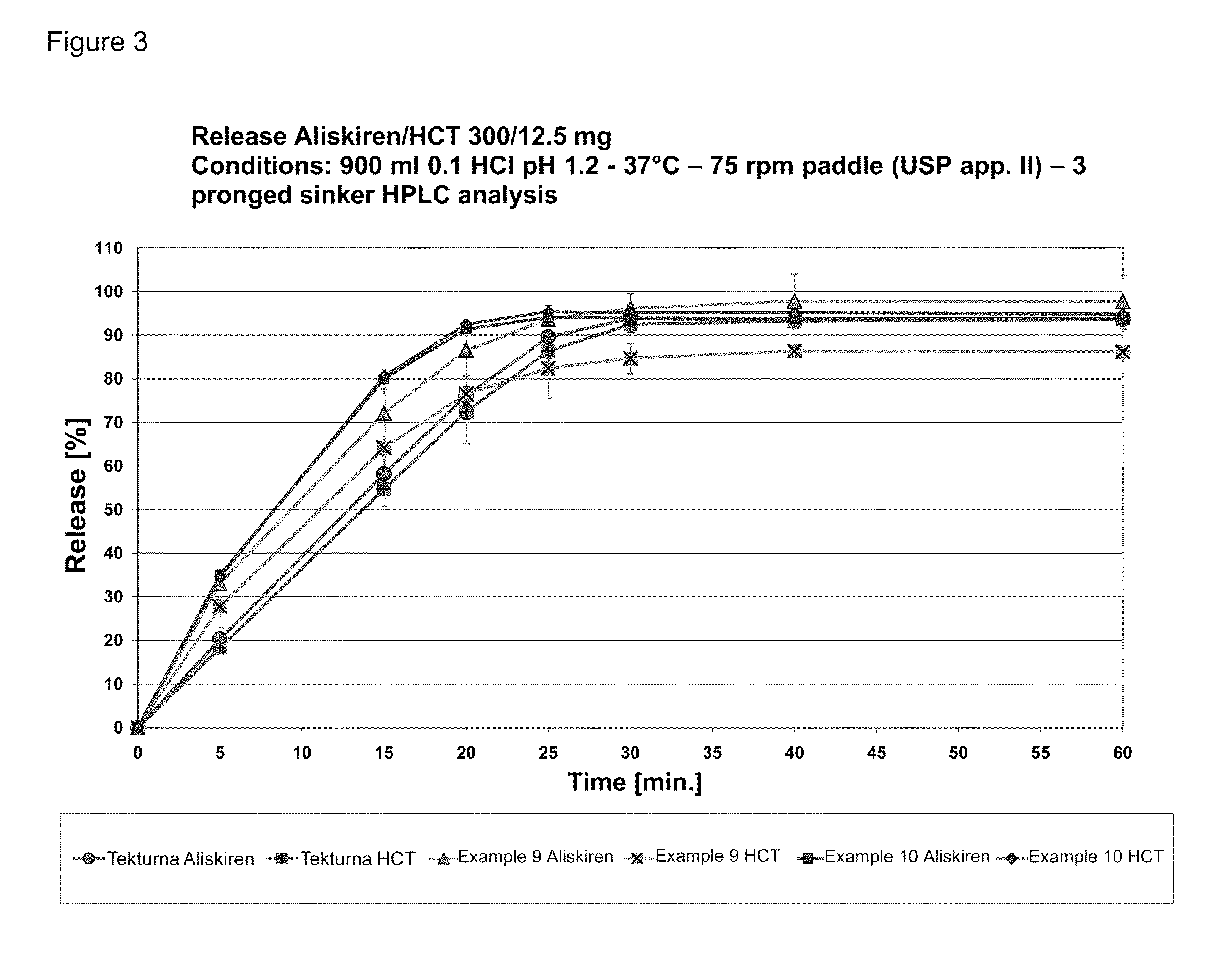
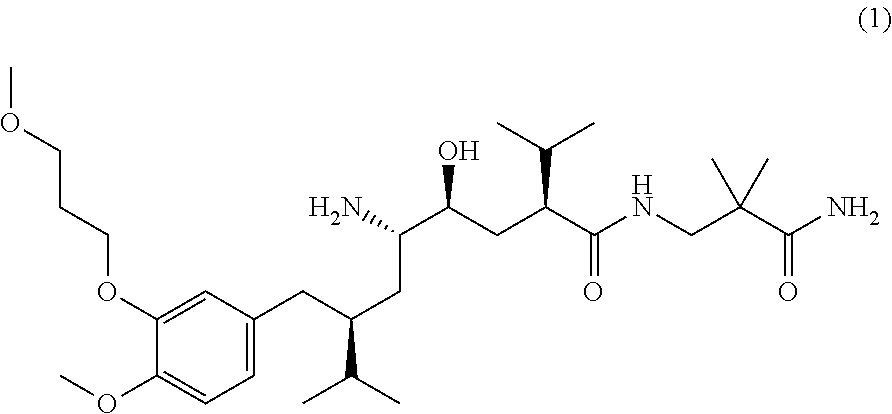
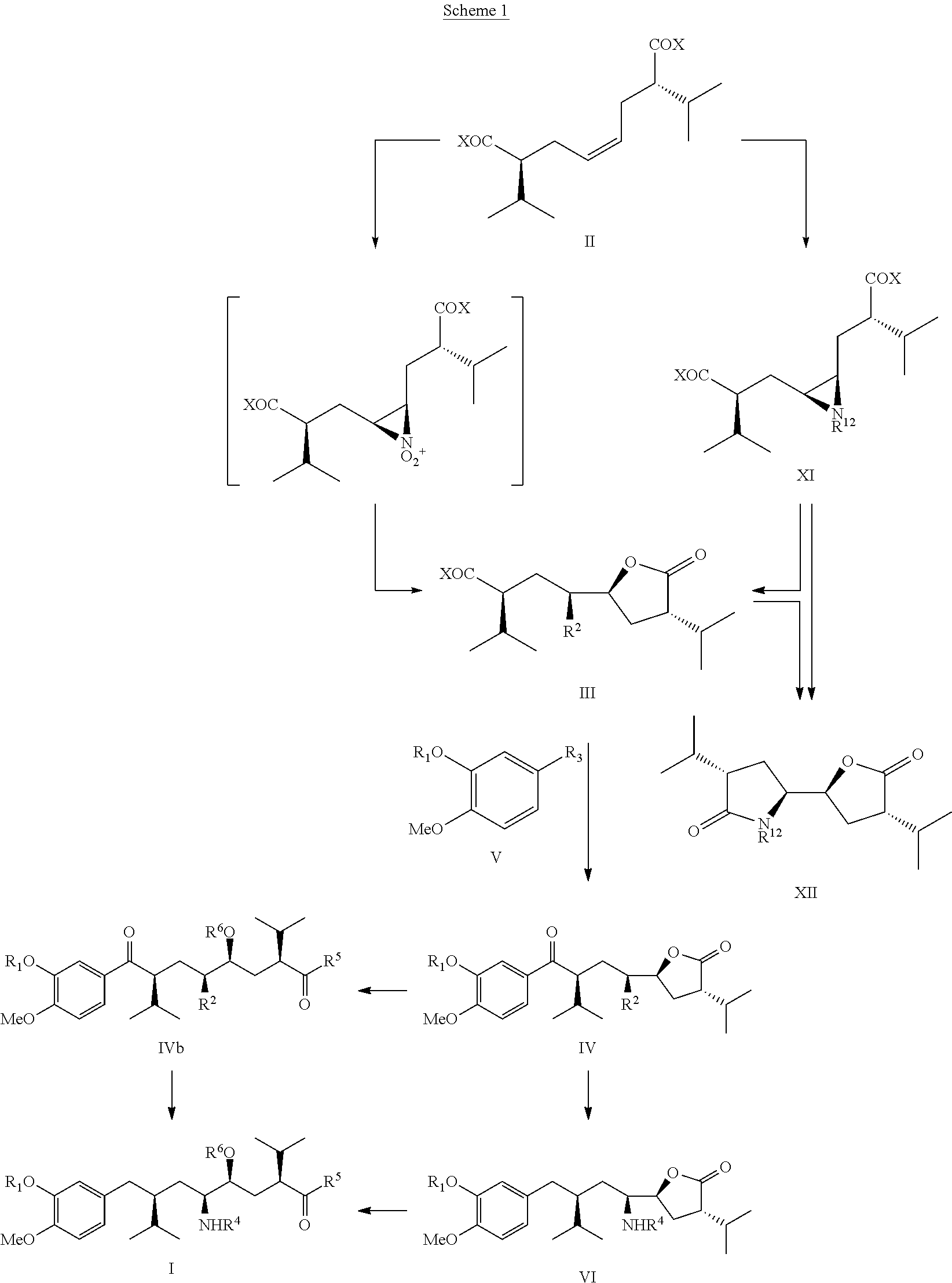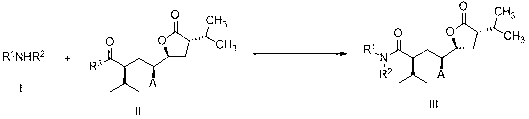Patents
Literature
119 results about "Aliskiren" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
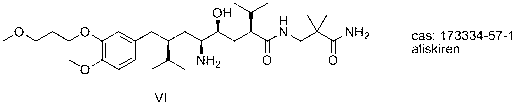
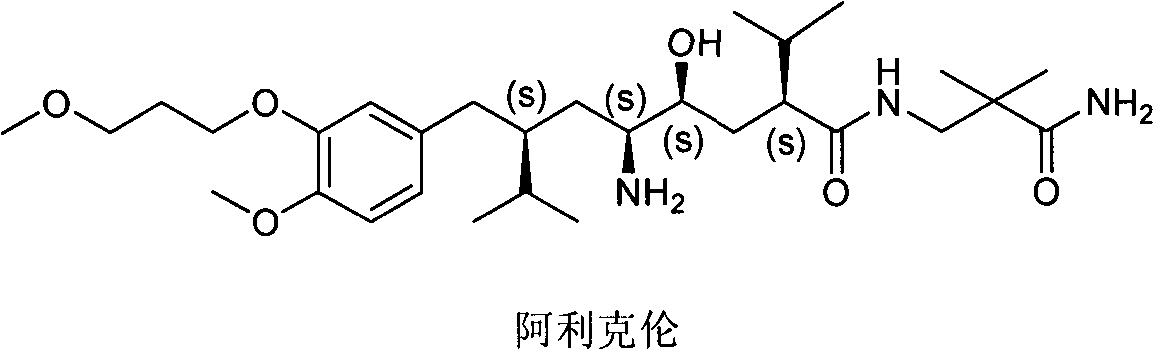
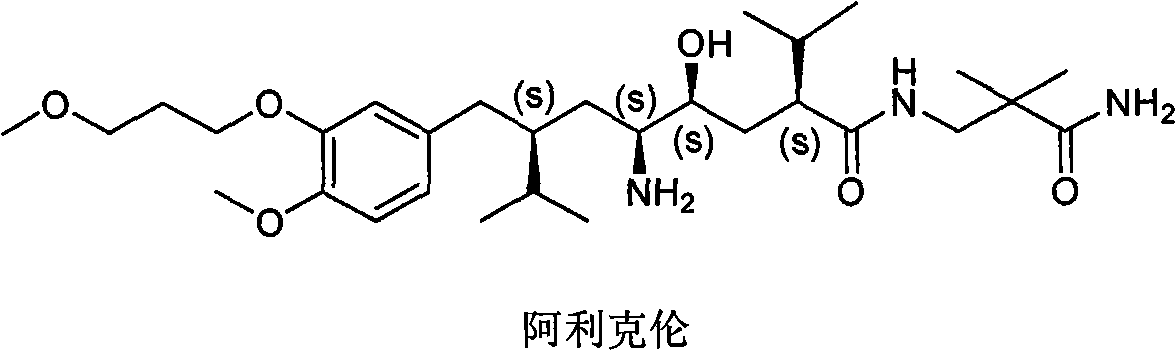
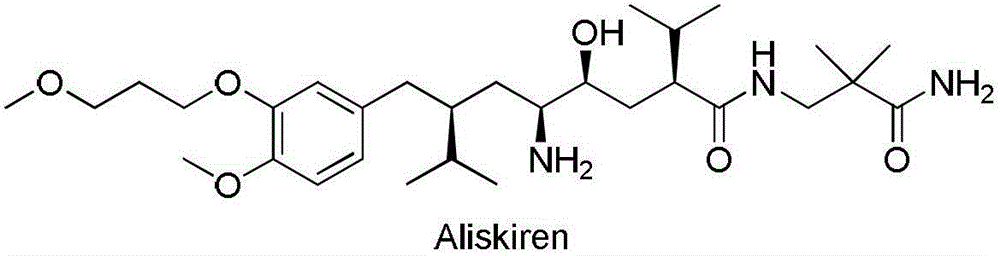
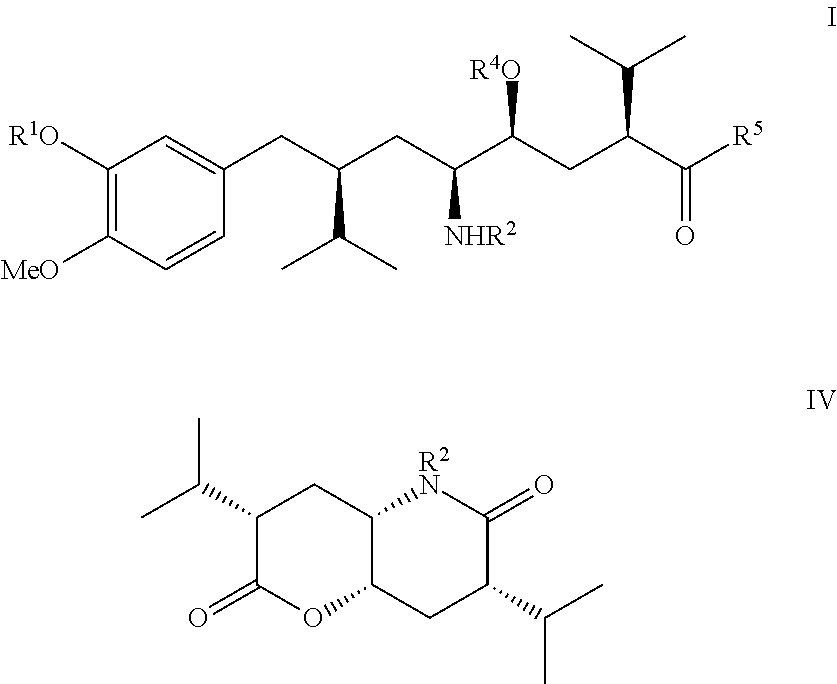
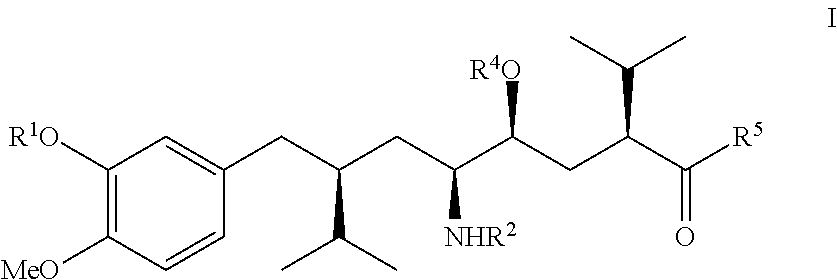
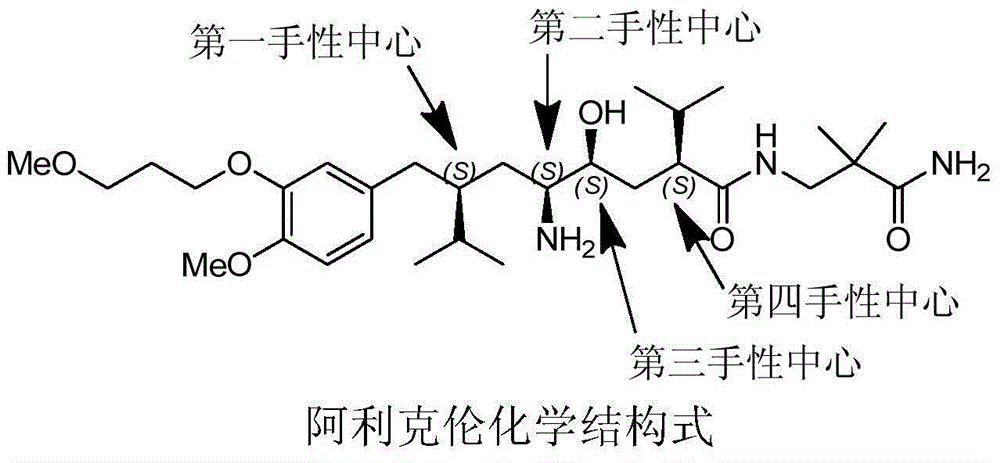
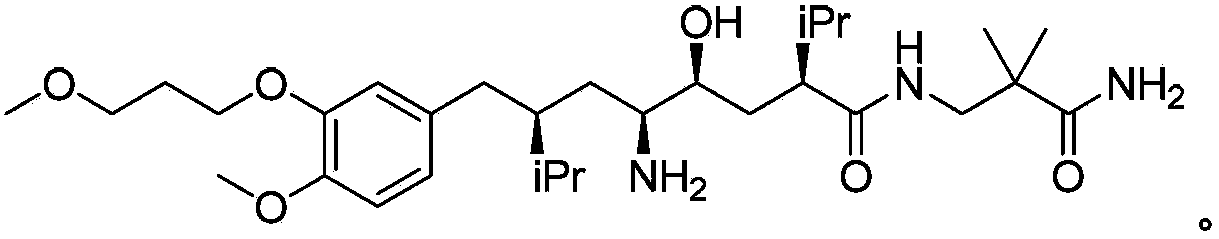
Aliskiren (trade names Tekturna and Rasilez) is the first in a class of drugs called direct renin inhibitors. It is used for essential (primary) hypertension. While used for high blood pressure, other better studied medications are typically recommended due to concerns of higher side effects and less evidence of benefit.
Practical synthesis method for feritin inhibitor aliskiren
InactiveCN101016253AReasonable choice of reaction processFew reaction stepsOrganic compound preparationCarboxylic acid amides preparationSynthesis methodsTert-Butyloxycarbonyl protecting group
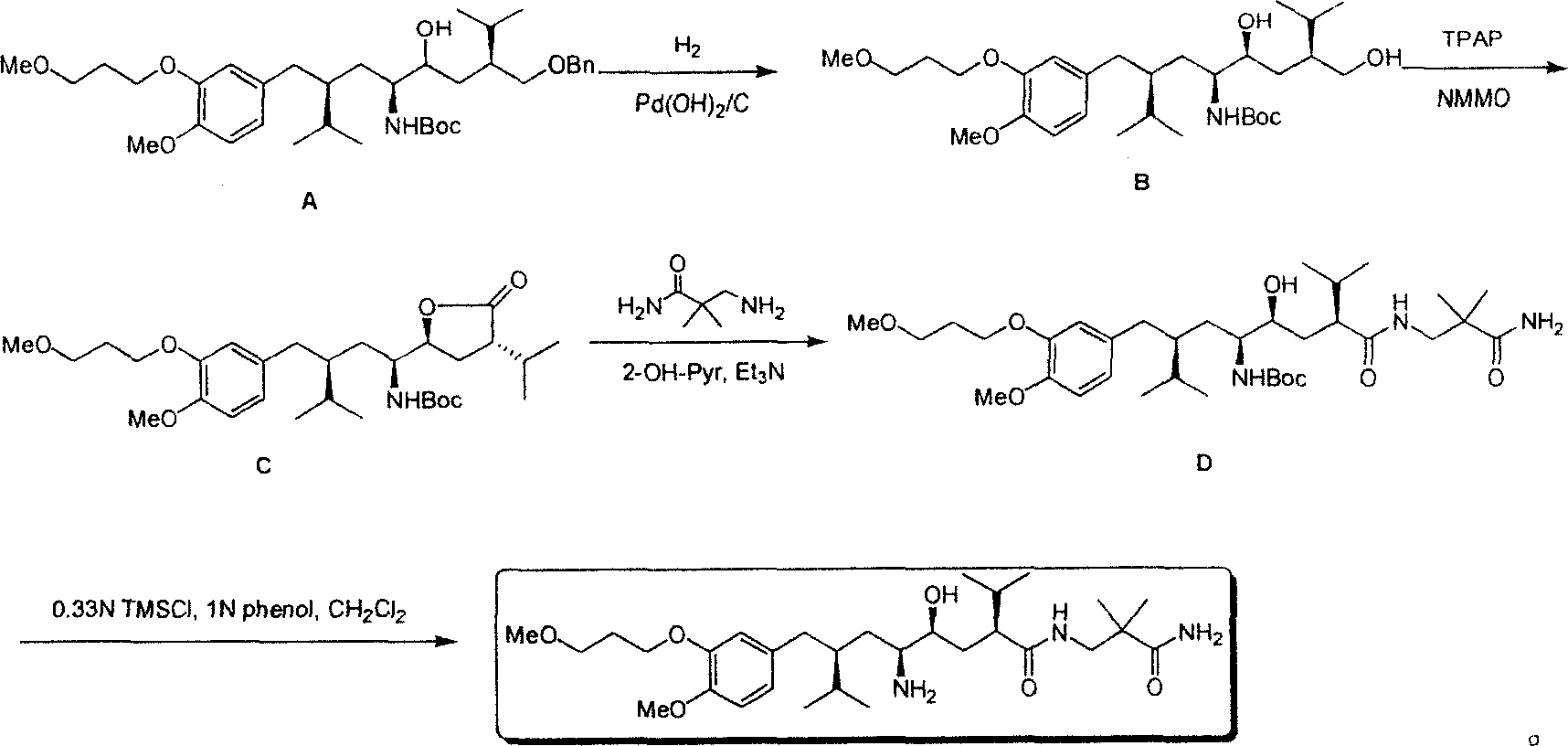
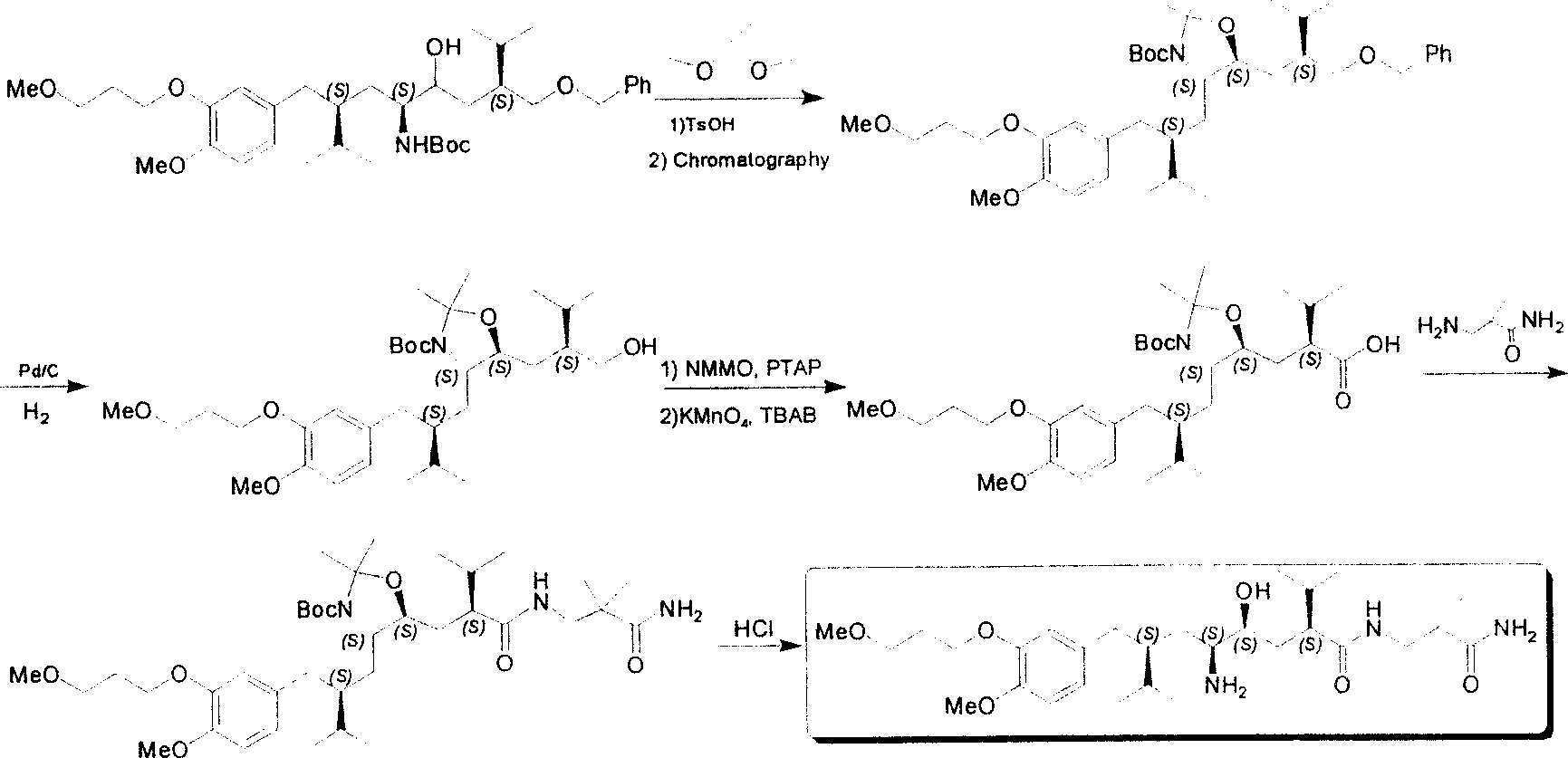
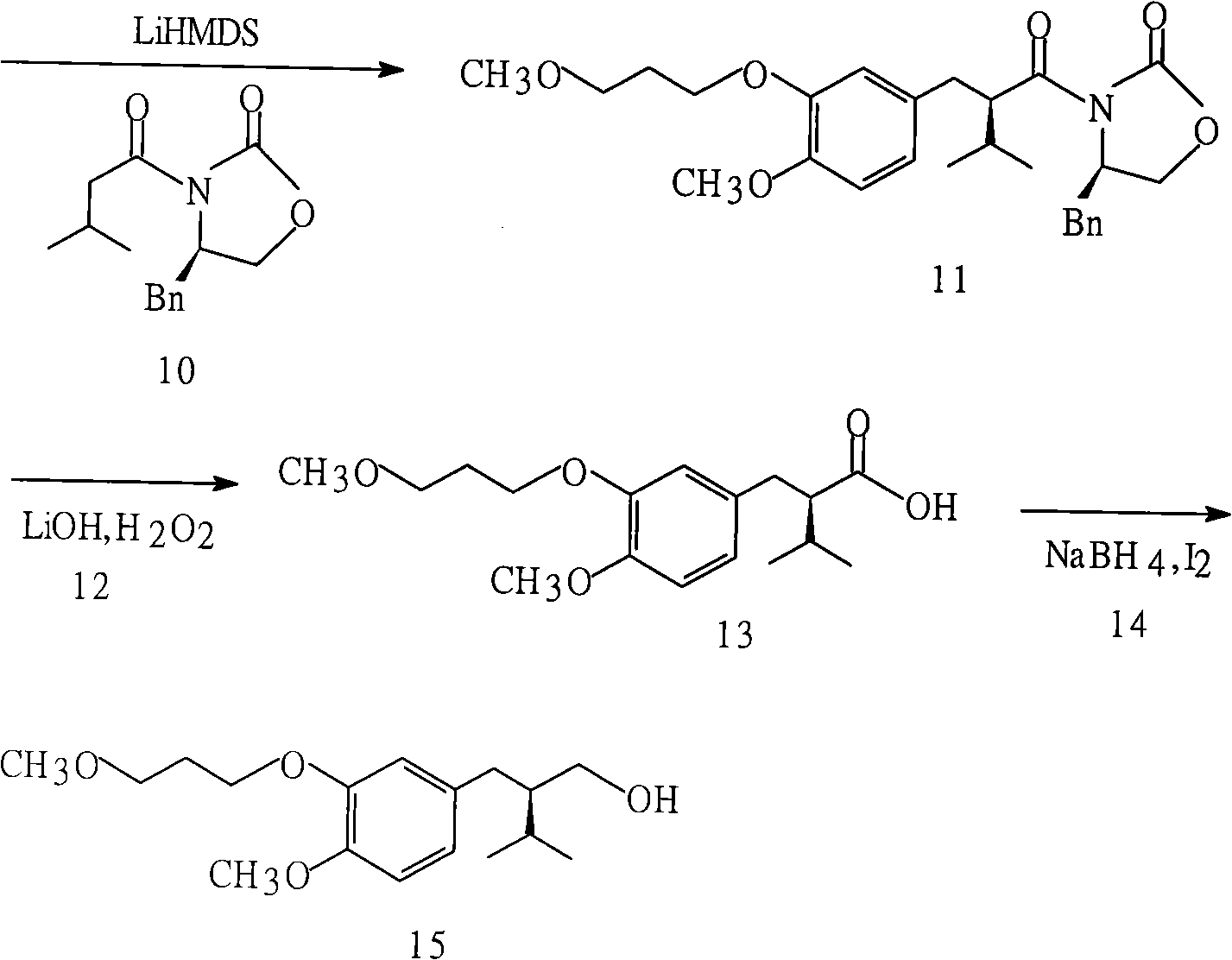
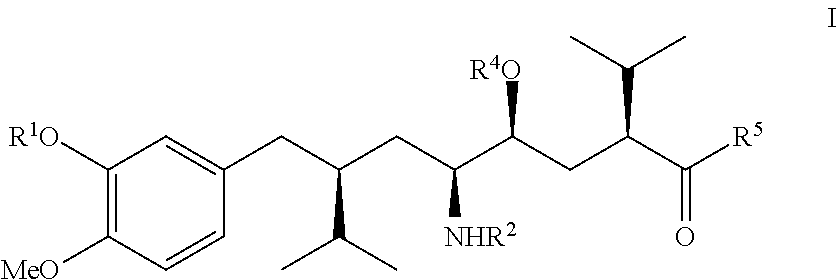
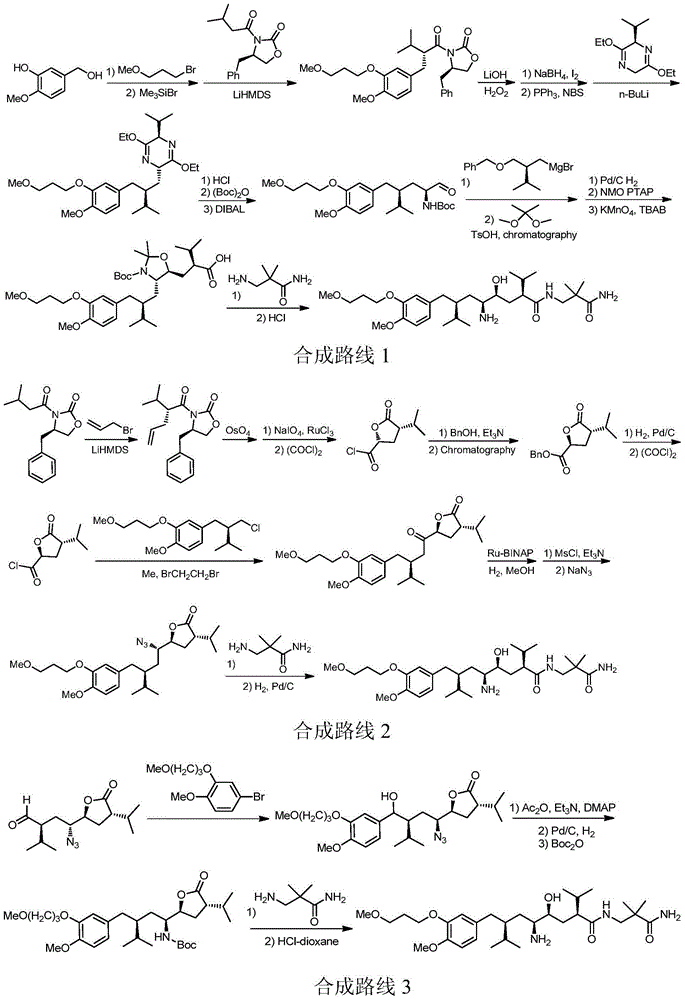
The invention discloses a synthesizing method of Alikelun of hypertension proteinogenase inhibitor, which comprises the following steps: adopting (1S, 4S, 2'S)-(4-benzyloxy methyl-2-hydroxy-1-{2-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-3-methyl-butyl}-5-methyl-hexyl)-carbonic tert-butyl as raw material; catalyzing; hydrogenating; removing benzyl; separating two R and S-typed diastereomers through column chromatography; obtaining (1S, 2S, 4S, 2'S)-(2-hydroxy-4-methylol-1-{2-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-3-methyl-butyl}-5-methyl-hexyl)-carbonic tert-butyl ester; oxidizing into (1S, 3S, 1'S, 4'S)-{1-(4-isopropyl-5-oxo-tetrahydrofuran-2-radical)-3-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-4-methyl-amyl}-carbonic tert-butyl ester; proceeding amide ester exchange to obtain (1 S, 3S, 1'S, 4'S)-(4-(2-amino formyl-2-methyl-propyl amino formyl)-2-hydroxy-1-{2-[4-methoxy-3-(3-methoxy-propoxy-benzyl]-3-methyl-butyl}-5-methyl-hexyl) carbonic tert-butyl ester; removing terbu-carbonyl to produce Aliskiren.
Owner:上海药明康德新药开发有限公司
Synthetic method for mainly intermediate compounds of anti-hypertensive drug aliskiren
InactiveCN101284769ARaw materials are easy to getThe synthesis steps are simpleOrganic chemistryOrganic compound preparationAlcoholBenzaldehyde
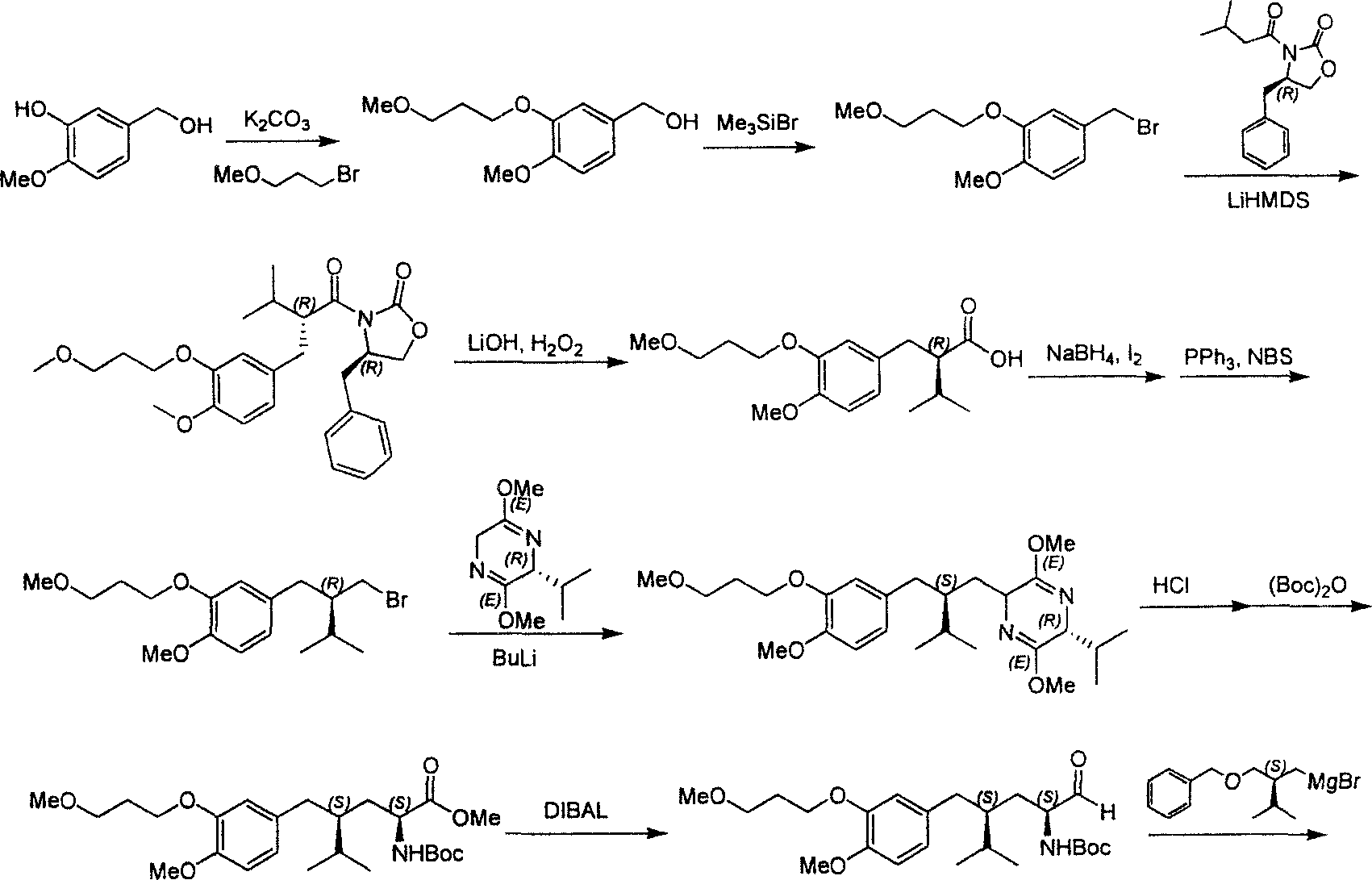
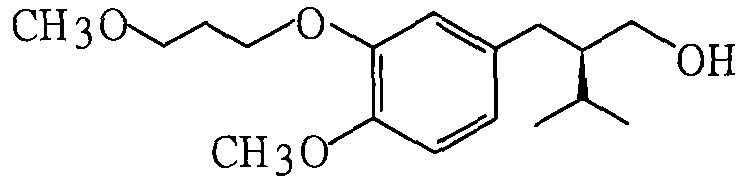
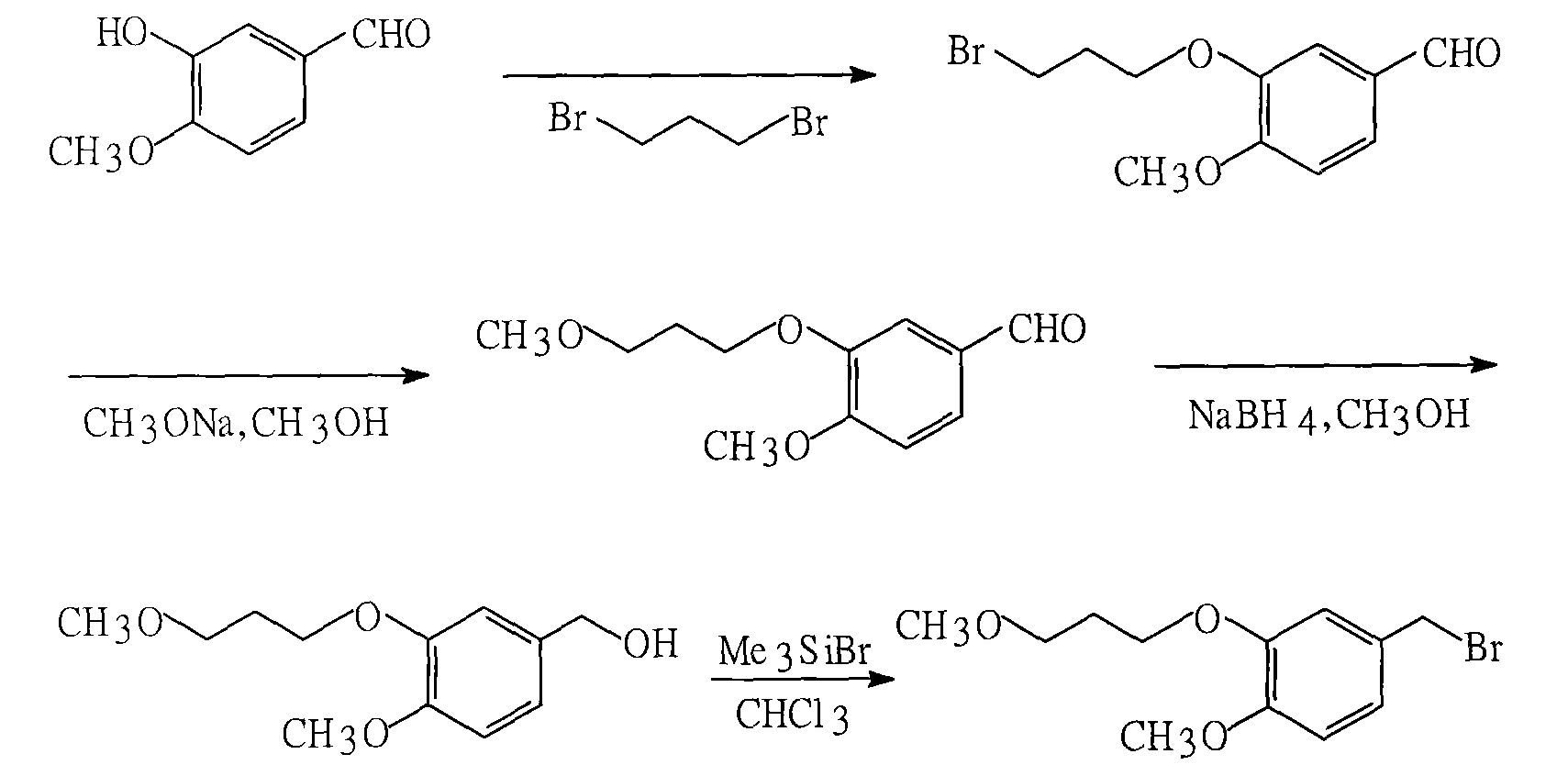
The invention provides a novel synthetic method for the main intermediate compound-(2R)-3-methy-2-(4 -methoxy-3-(3-methoxy-C) benzyl)-1-butyl alcohol of hypertension drug Aliskiren. The invention adopts the method with the steps that firstly the raw material of 3-hydroxy-4-methoxy benzaldehyde is etherified by 3-methoxy bromopropane (mol ratio of 1:1 to 1.2), and then is condensed with isopentyl aldehyde (in mol ratio of 1:1 to 1.3); catalytic hydrogenation is performed to the condensation product; then the main intermediate compound of the hypertension drug Aliskiren is obtained through resolution. The method has the advantages that the raw material and the reagent are easy to be obtained, the cost is low, the synthetic process is short, and only three steps are required to prepare the raceme of the main intermediate compound of the Aliskiren; when the enzyme method is utilized to perform the resolution of the raceme, the main intermediate compound-(2R)-3-methy-2-(4-methoxy-3-(3-methoxy-C) benzyl)-1-butyl alcohol of the hypertension drug Aliskiren can be obtained.
Owner:CHONGQING NANSONG CHEMI TECH
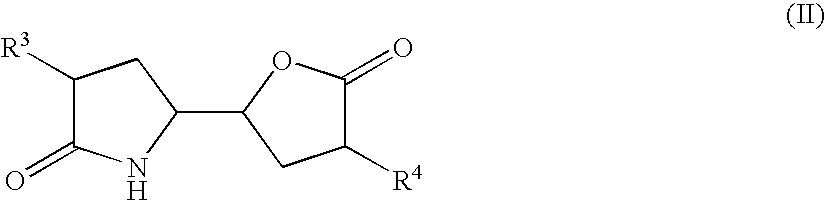
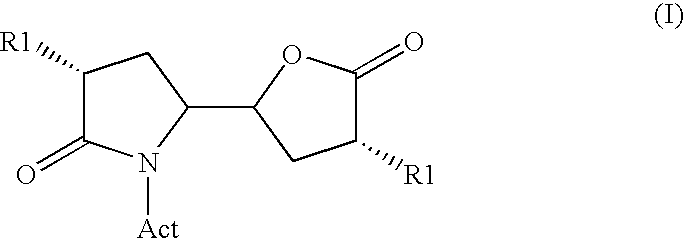
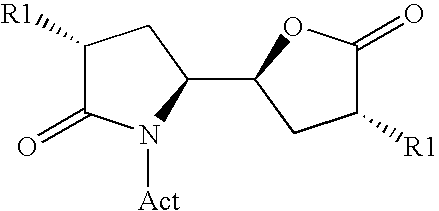
3-alkyl-5- (4-alkyl-5-oxo-tetrahydrofutran-2-yl) pyrrolidin-2-one derivatives as intermediates in the synthesis of renin inhibitors
ActiveUS7772405B2Simple manufacturing methodHigh purityCarbamic acid derivatives preparationOrganic compound preparationSimple Organic CompoundsKetone
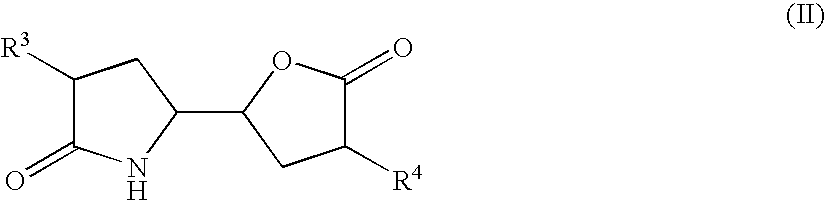
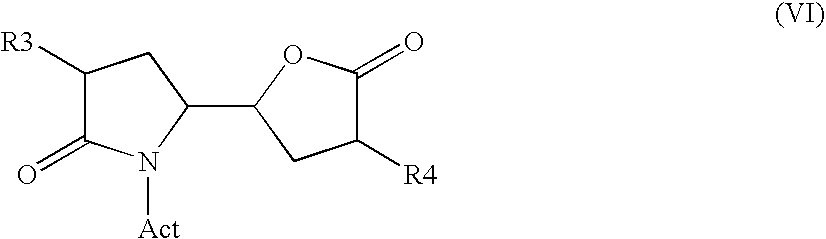
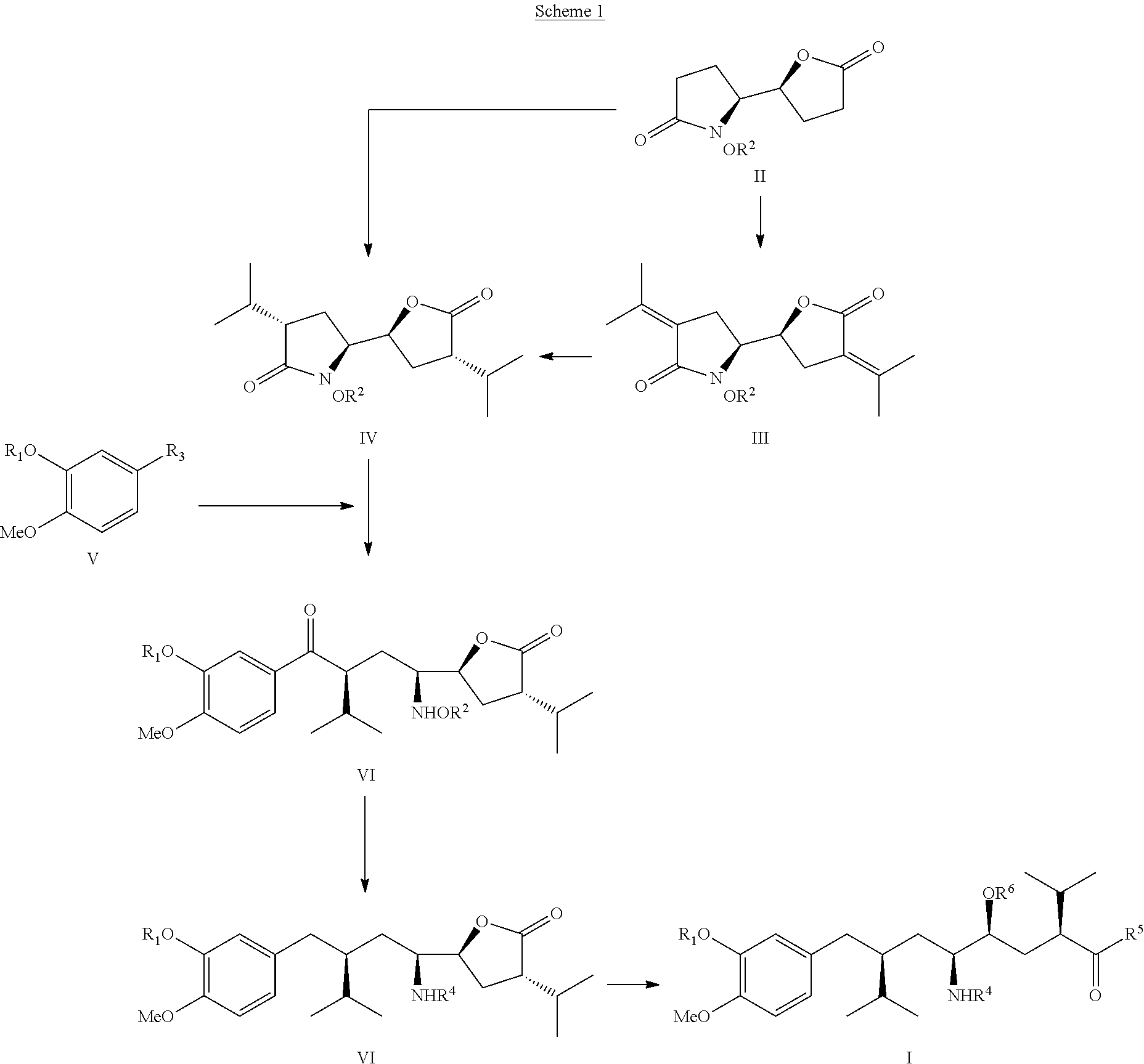
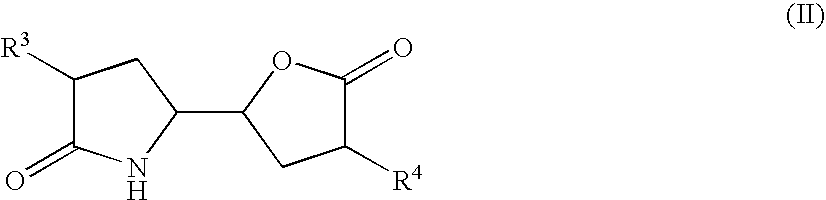
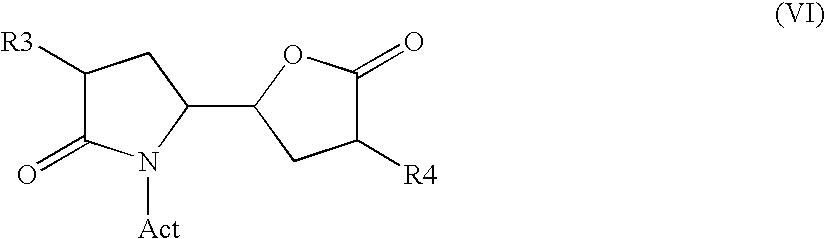
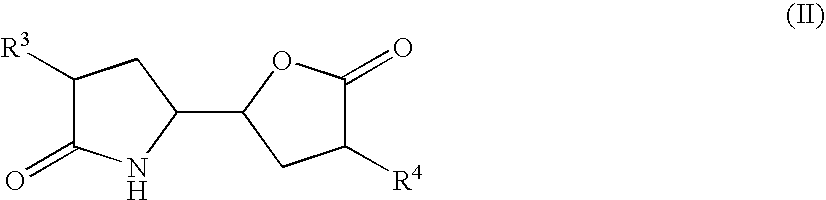
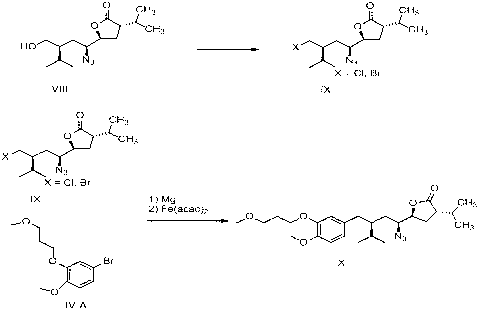
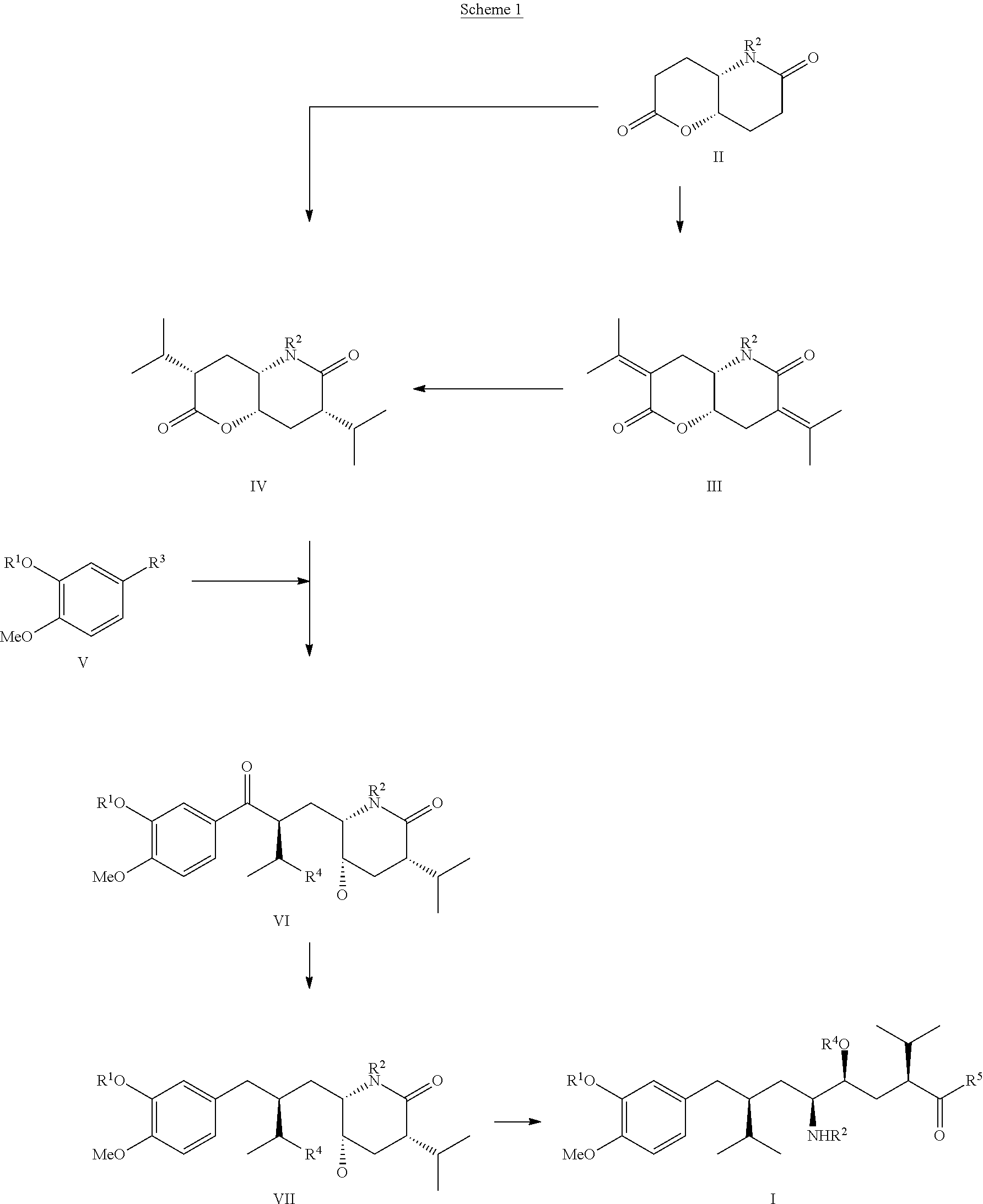
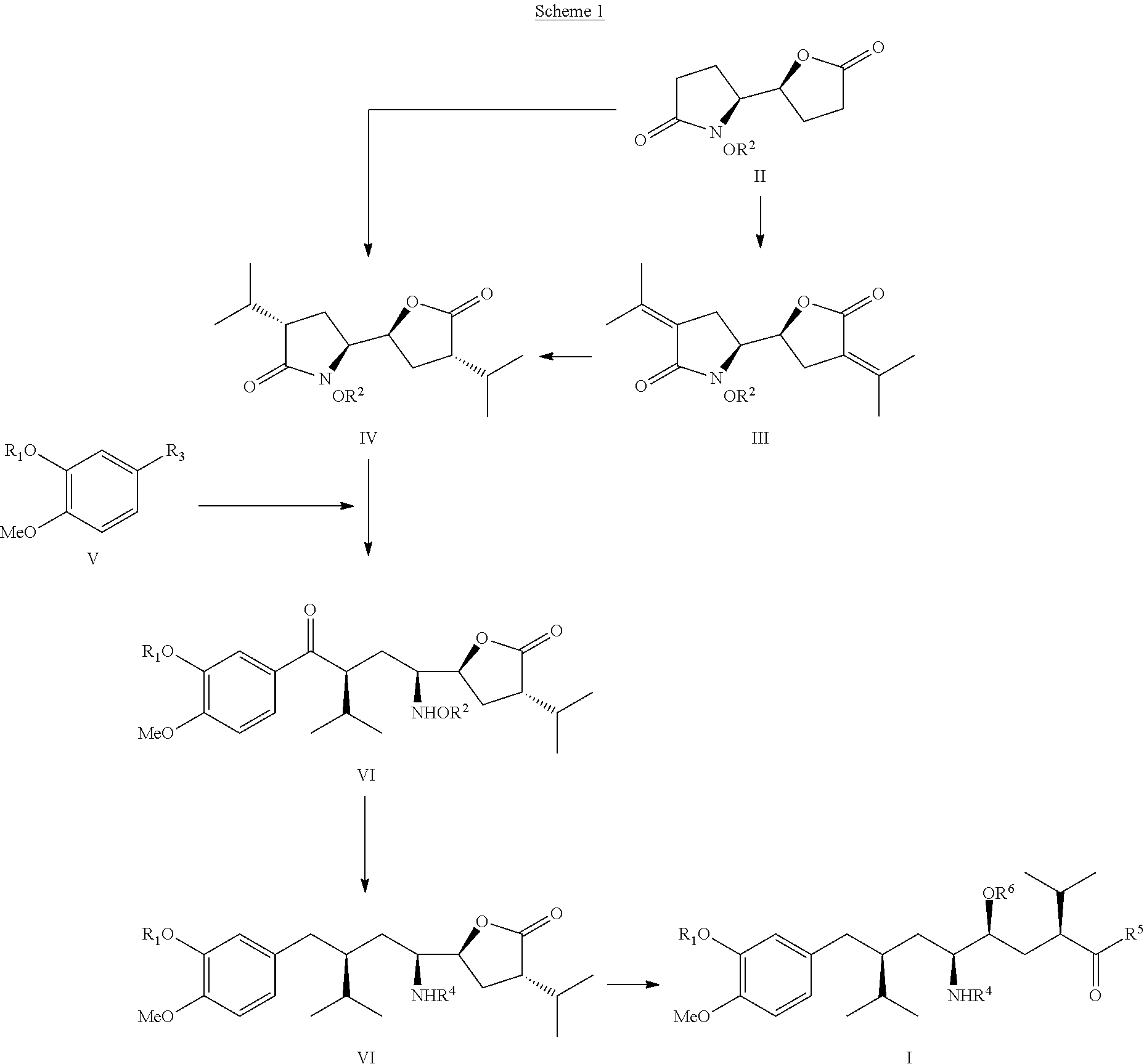
The invention related to a novel process, novel process steps and novel intermediates useful in the synthesis of pharmaceutically active compounds, especially renin inhibitors, such as Aliskiren. Inter alia, the invention relates to a process for the manufacture of a compound of the formula II,or a salt thereof, and a compound of formula VIor a salt thereof, wherein R3 and R4 as well as Act are as defined in the specification, and processes of manufacturing these.Additionally transformation of compounds (VI) with metallo organic compounds (VII) give rise to the new compounds (VIII) which are direct precursors for the preparation of Aliskiren.
Owner:NODEN PHARMA DAC
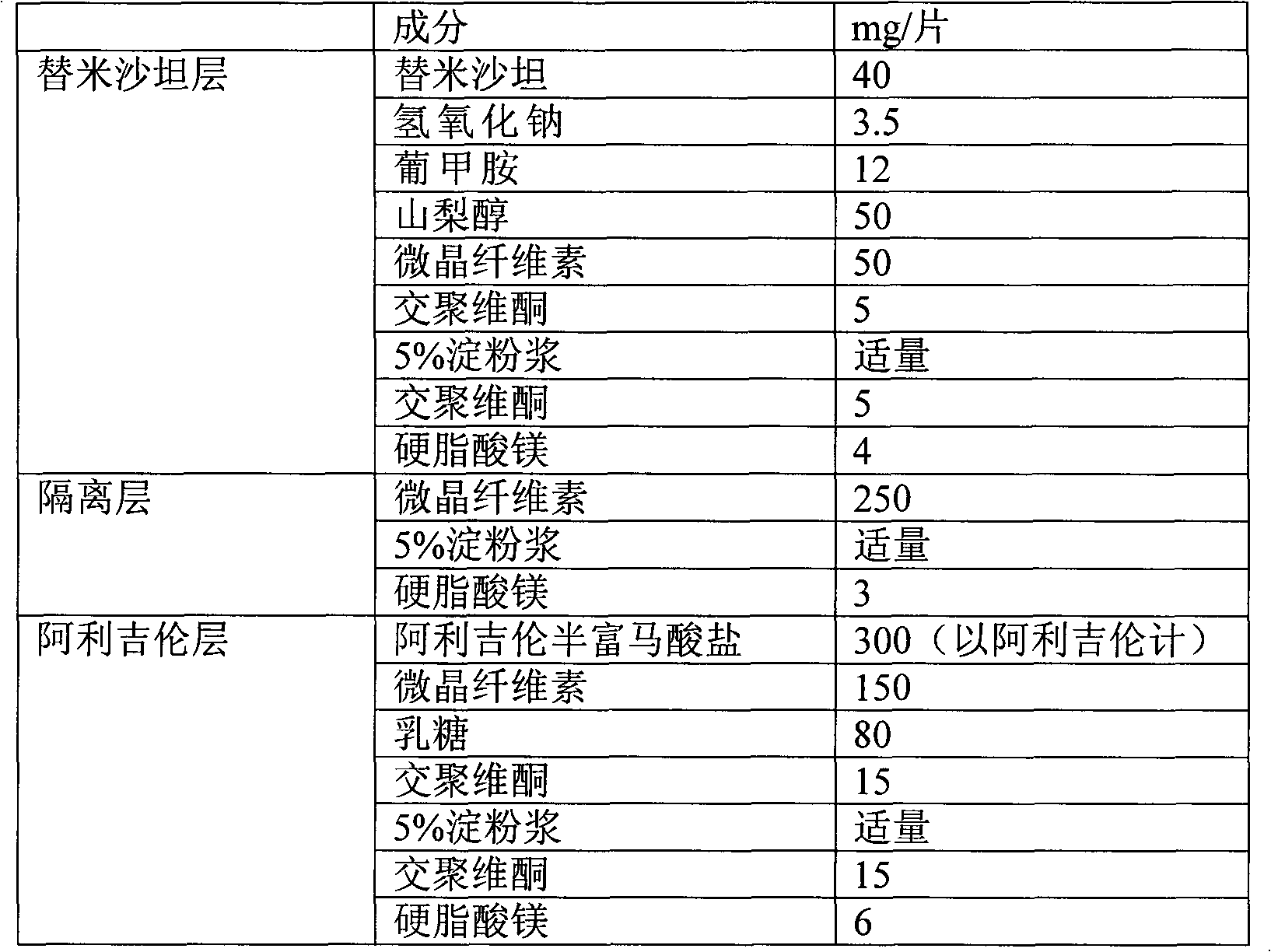
Combined medicament containing telmisartan and aliskiren and preparation method thereof
ActiveCN101926793AAvoid interactionGuaranteed stabilityOrganic active ingredientsPill deliveryPharmaceutical formulationTreatment hypertension
The invention relates to application of telmisartan and aliskiren or medicinal salts thereof in preparing a combined medicament for treating hypertension. The invention also provides the combined medicament for treating hypertension, wherein the combined medicament contains unit preparations of different specifications, and the preparations administrated simultaneously, respectively or in turn are prepared from telmisartan and aliskiren or medicinal salts thereof and pharmaceutically acceptable carriers. The invention also provides a preparation method and application of the combined medicament. The medicinal preparations are three-layer tablets so as to avoid interaction of the aliskiren and the telmisartan, improve the medicament stability and facilitate long-term storage. The composition preparations are used for treating medium and high hypertension patients and hypertension patients whose blood pressure cannot be fully controlled after being treated by angiotensin II receptor antagonists or renin inhibitors.
Owner:CHENGDU ZIHAO PHARMA
Novel blood pressure reducing composition
InactiveCN102247344AExplain the curative effect advantageHeterocyclic compound active ingredientsCardiovascular disorderAngiotensin-converting enzymeHydrochlorothiazide
The invention relates to a novel blood pressure reducing composition. The blood pressure reducing composition is a medicinal composition of which active ingredients are any one or two of aliskiren and a medicinal salt thereof, a hydrate, a calcium channel blocker (CCB), an angiotensin type 1 (AT1) receptor blocker (ARB), an angiotensin-converting enzyme inhibitor (ACEI), hydrochlorothiazide and the like. The composition can be prepared into oral tablets or capsules and is used for treating hypertension, and the curative effect is superior to the blood pressure reducing effect of the independent aliskiren.
Owner:FUKANGREN BIO PHARMA
Novel blood lipid lowering composition
InactiveCN102247345AMetabolism disorderEster active ingredientsSecondary hyperlipidemiaCurative effect
The invention discloses a novel blood lipid lowering composition, which is a medicinal composition comprising aliskiren and a medicinal salt thereof, a hydrate and 1-2 of statin, fibrate and nicotinic acid lipid lowering medicaments, serving as active ingredients. The composition can be prepared into oral tablets or capsules for treating hyperlipidemia; and the curative effect of the composition is superior to that of the conventional common lipid lowering medicament.
Owner:FUKANGREN BIO PHARMA
Manufacturing process for enantiomerically pure 8-Aryloctanoic acids as Aliskiren
InactiveUS20110105767A1Organic compound preparationCarboxylic acid amides preparationMedicinal chemistryEnzyme inhibitor
Owner:CARBODESIGN
3-Alkyl-5- (4-Alkyl-5-Oxo-Tetrahydrofutr An -2-Yl) Pyrrolidin-2-One Derivatives As Intermediates In The Synthesis Of Renin Inhibitors
ActiveUS20080262246A1High diastereomeric purityHigh enantiomeric purityCarbamic acid derivatives preparationLithium organic compoundsKetoneOrganic compound
The invention related to a novel process, novel process steps and novel intermediates useful in the synthesis of pharmaceutically active compounds, especially renin inhibitors, such as Aliskiren. Inter alia, the invention relates to a process for the manufacture of a compound of the formula II,or a salt thereof, and a compound of formula VIor a salt thereof, wherein R3 and R4 as well as Act are as defined in the specification, and processes of manufacturing these.Additionally transformation of compounds (VI) with metallo organic compounds (VII) give rise to the new compounds (VIII) which are direct precursors for the preparation of Aliskiren.
Owner:NODEN PHARMA DAC
Method for preparing aliskiren intermediate
InactiveCN104016947AThe process method is safe and reliableHigh yieldOrganic chemistryBromineCarboxylic acid
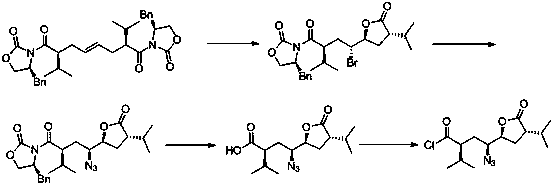
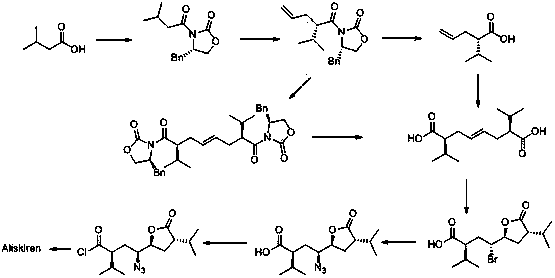
The invention discloses a method for preparing an aliskiren intermediate. The method comprises the following synthesis steps: (1) by adopting trans-1, 4-dibromo butene as a raw material, carrying out nucleophilic substitution with a chiral auxiliary agent under the action of strong alkali; (2) removing one molecule of S-benzyl oxazolidinone under the action of a bromo-reagent; (3) reacting with an azide reagent in the presence of a catalyst; (4) removing one molecule of S-benzyl oxazolidinone under the action of an alkali reagent to form a carboxylic acid compound; (5) reacting with a acyl chloride reagent and converting carboxylic acid into an anhydride compound under the action of the alkali reagent; (6) reducing ester group into alcoholic hydroxyl group under the action of a reducing reagent; (7) selectively oxidizing the alcoholic hydroxyl group into aldehyde under the action of an oxidizing agent to obtain the intermediate. The method disclosed by the invention has the beneficial effects that the process method is safe and reliable, high in yield, easy to operate and reasonable in cost and is more suitable for large-scale industrial production.
Owner:NANTONG CHANGYOO PHARMATECH CO LTD
Process for enantiomerically pure 8-Aryloctanoic acids as Aliskiren
InactiveUS20110137047A1Efficient preparationHigh stereoselectivityOrganic compound preparationCarboxylic acid amides preparationArylAziridine
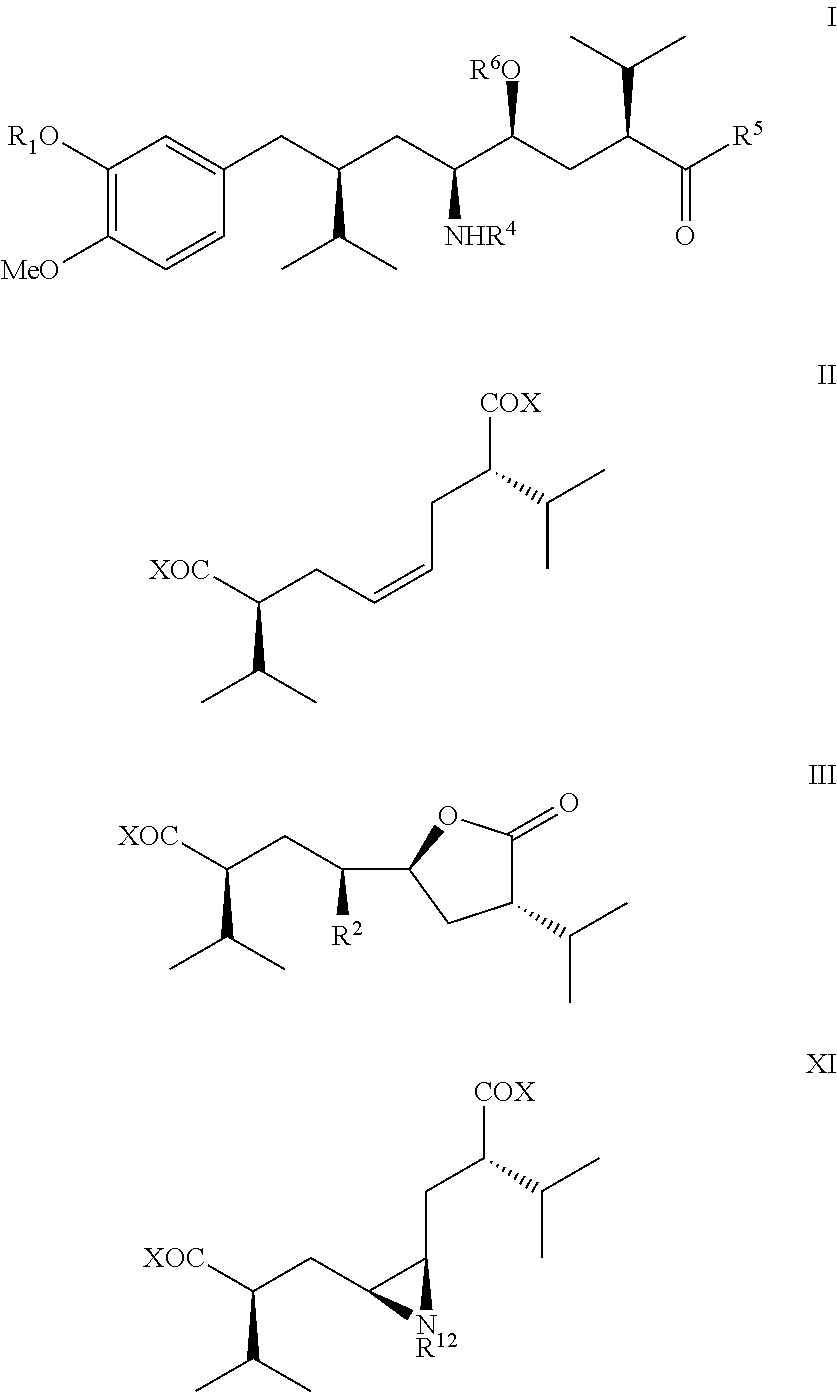
The present invention relates to a novel manufacturing process and novel intermediates useful in the synthesis of pharmaceutically active compounds, especially rennin inhibitors such as Aliskiren. The invention describes a preparation of enantiomerically pure 8-aryloctanoic acids of general formula I from readily available key intermediate, chiral cis-diacid of formula II, aziridine of formula XI and a monocyclic compound of formula III.
Owner:CARBODESIGN
Preparation method of Aliskiren intermediate 3-amino-2,2-dimethylpropionamide
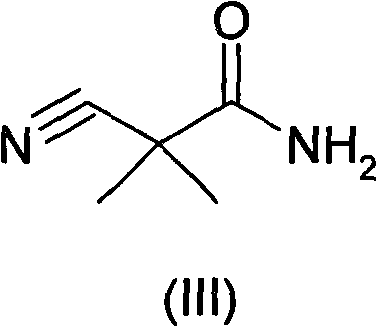
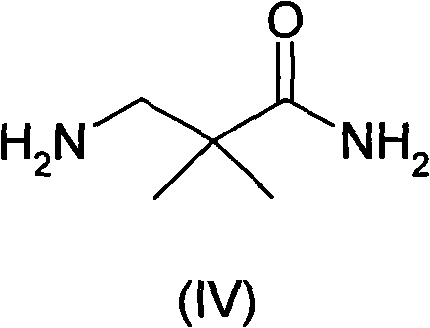
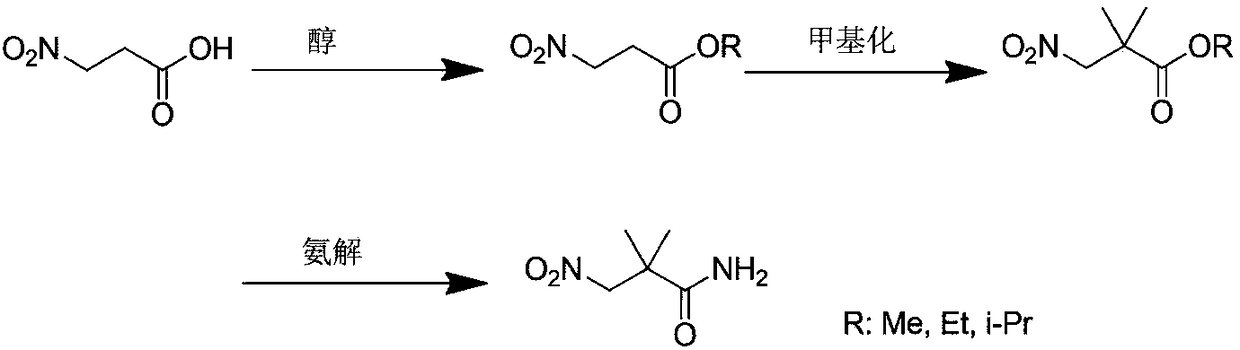
ActiveCN102140068ALow priceEasy post-processingOrganic compound preparationCarboxylic acid amides preparationAcetic acidCyanoacetic acid
The invention relates to an industrial preparation method of synthesizing a key intermediate 3-amino-2,2-dimethylpropionamide of an angiotonase inhibitor Aliskiren. In the method, 3-amino-2,2-dimethylpropionamide is obtained after a derivative of cyanoacetic acid is methylated, aminated and reduced. The method has the advantages of low cost, environmental friendliness, short reaction step, simple operation, high yield, high purity of product and the like, and is especially suitable for industrial production.
Owner:ZHEJIANG HUAHAI PHARMACEUTICAL CO LTD
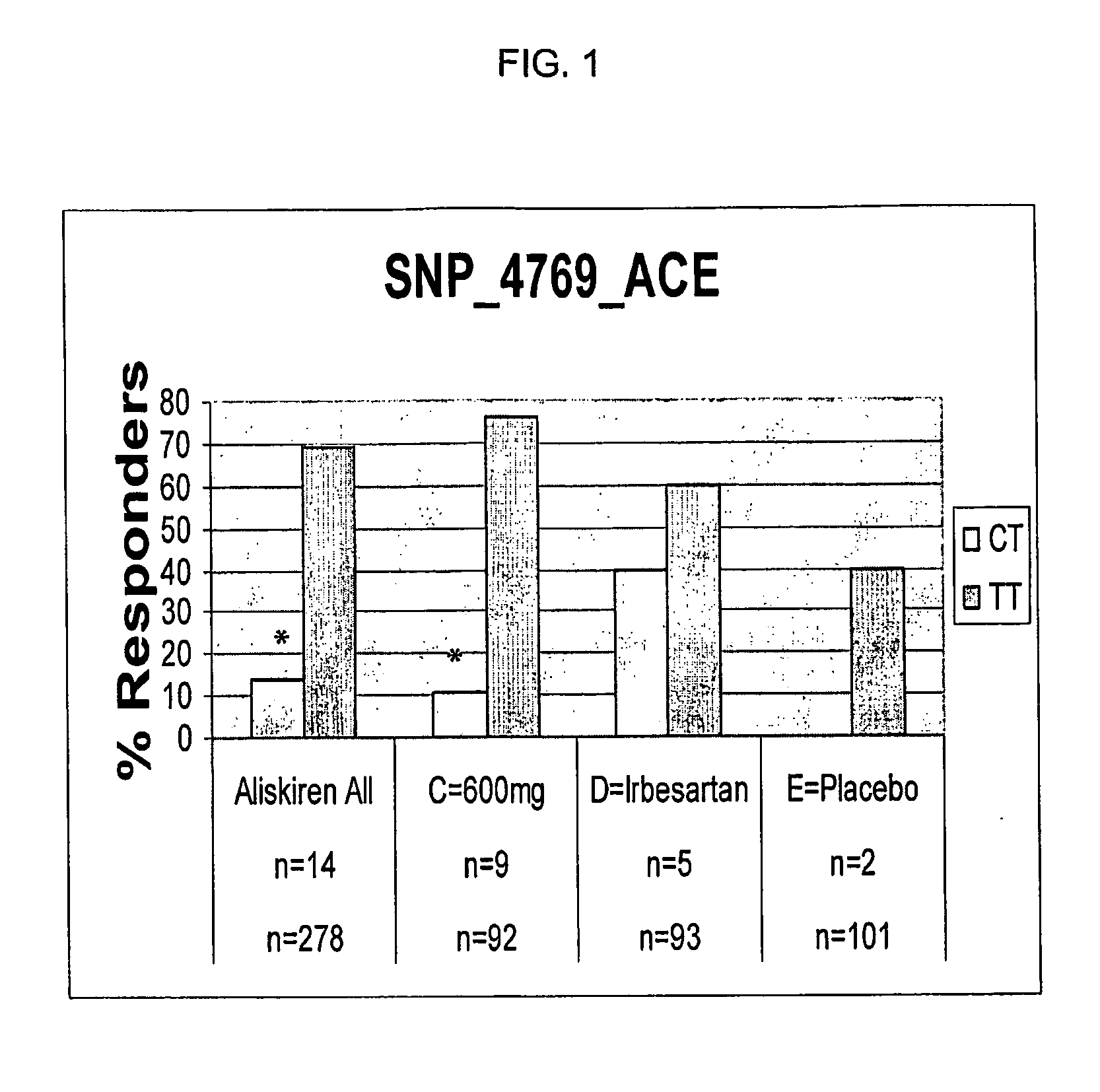
Biomarkers for Efficacy of Aliskiren as a Hypertensive Agent
A retrospective pharmacogenetic analysis was conducted in an attempt to evaluate potential association between genetic variation and outcome of a clinical trial of efficacy of aliskiren as an antihypertensive agent. Forty-eight polymorphisms were examined in twelve genes from the renin-angiotensin-aldosterone system (RAS) or previously implicated in blood pressure regulation. Significant associations were seen between one polymorphism in the angiotensin converting enzyme (ACE) gene, two polymorphisms in the angiotensin II type 2 receptor (AGTR2) gene, and clinical parameters of mean sitting diastolic and systolic blood pressure decrease. These effects were not found with irbesartan and placebo treatment, but instead were specific to aliskiren treatment.
Owner:NOVARTIS AG
Multicoated aliskiren formulations
InactiveUS20110268797A1Provide stabilityEasy to produceBiocideAmide active ingredientsPharmaceutical formulationOral drug preparation
An oral pharmaceutical formulation of aliskiren, or a pharmaceutically acceptable salt or polymorph thereof, having at least two coating layers.
Owner:SANOVEL ILAC SANAYI & TICARET ANONIM SIRKETI
Preparation method for aliskiren
ActiveCN102351734ARaw materials are cheap and easy to getShort reaction pathOrganic compound preparationCarboxylic acid amides preparationCatalytic oxidationKetone
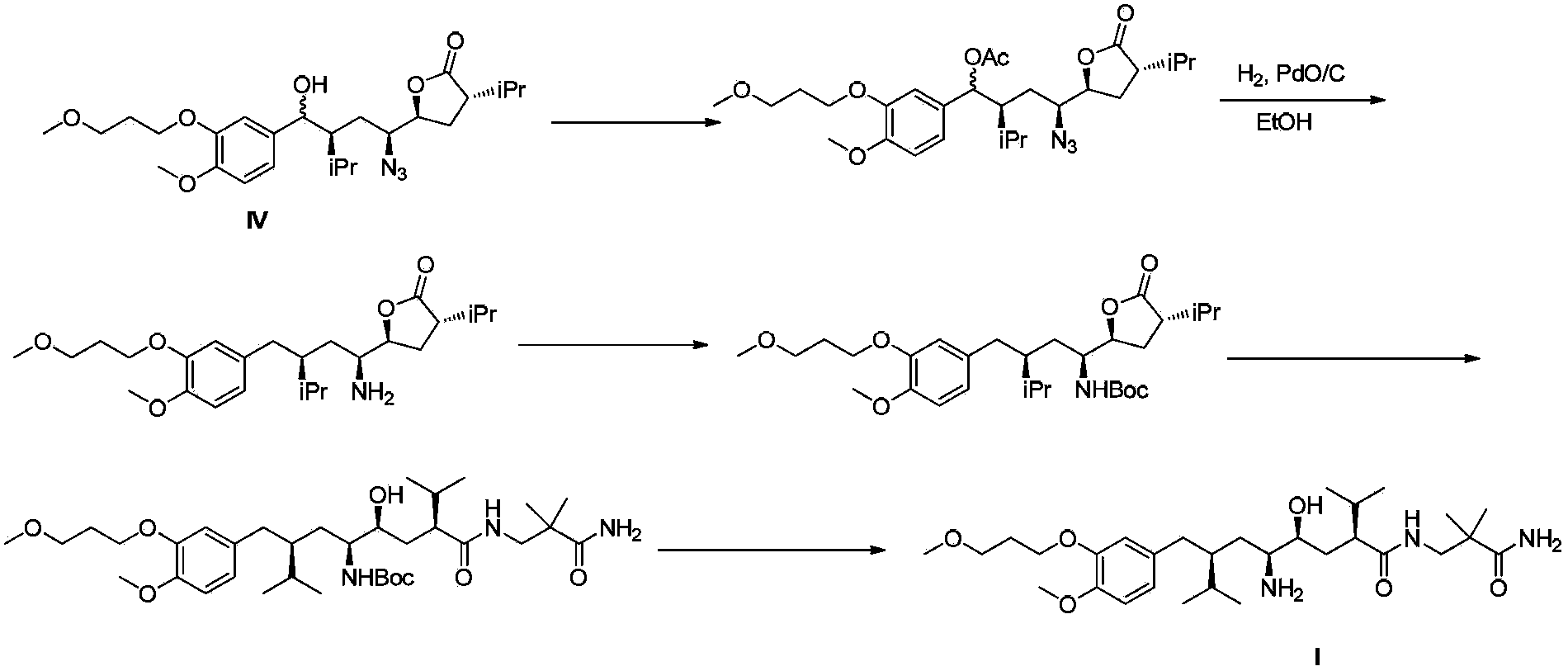
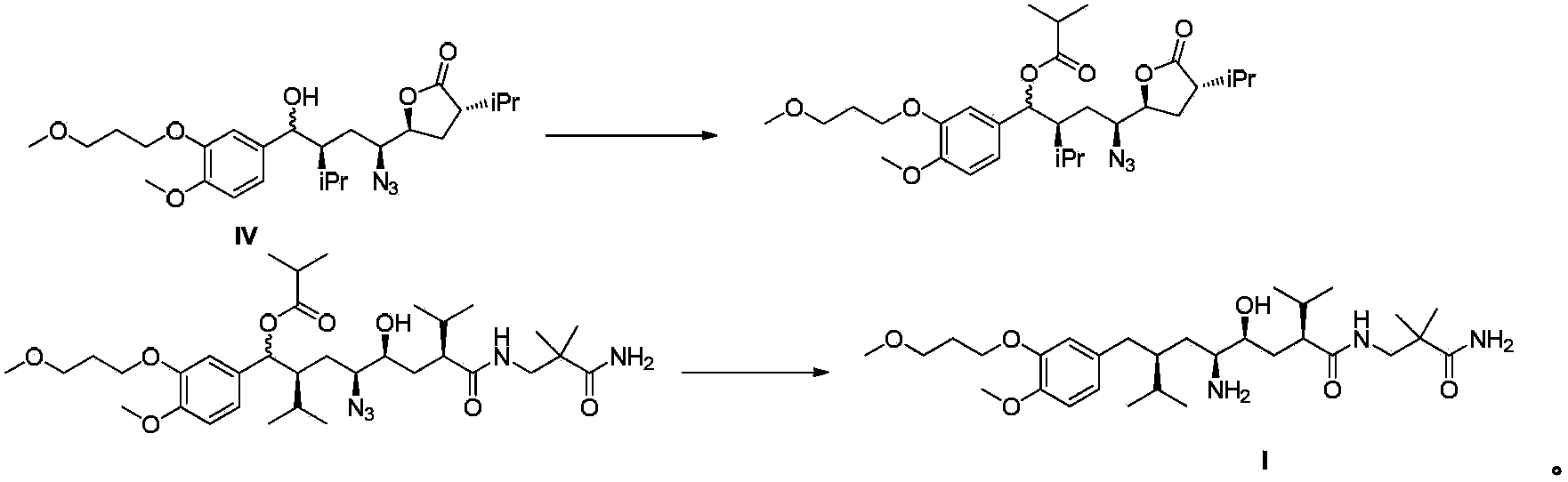
The invention discloses a preparation method for aliskiren. The preparation method comprises the following steps of: preparing a compound (I) from (3S, 5S, 1'S, 3'S)-5-(1'-azido-3'-hydroxymethyl-4'-methyl-amyl)-3-isopropyl-dihydrofuran-2-ketone through catalytic oxidation; performing addition with 4-methoxyl-3-(3-methoxyl propoxy)-bromobenzene under the action of a metal reagent to obtain a compound (II); then performing catalytic hydrogenation and amino group protection to obtain a lactone compound (III), serving an important intermediate of the aliskiren; and finally condensing with 3-amino-2,2-methacrylamide and removing an amino protecting group to obtain the aliskiren. Compared with the prior art, the preparation method has the beneficial effects of cheap and readily available raw materials, short reaction route, easiness and convenience in operation, no special requirement on equipment, high yield, low cost and small environmental protection pressure, is suitable for commercial production and has a higher industrial application value.
Owner:ZHEJIANG APELOA JIAYUAN PHARMA +1
Aliskiren intermediate, and preparation method and application thereof
InactiveCN103204834AEasy to manufactureEasy to purifyOrganic chemistryBulk chemical productionAliskirenCombinatorial chemistry
The invention relates to the organic pharmaceutical field, and especially relates to a synthesized renin inhibitor aliskiren intermediate, a synthetic method thereof and an application method of the synthesized aliskiren intermediate in a further reaction for forming an intermediate closer to aliskiren. The new aliskiren intermediate, the preparation method thereof and the application method of the aliskiren intermediate in the formation of the intermediate closer to aliskiren are provided to overcome the defects comprising high cost, complex technological route, many byproducts, long reaction time and the like of current synthetic technologies. A purpose that the aliskiren intermediate has the advantages of easy preparation and purification, low cost, high safety and suitableness for the industrialized production is realized.
Owner:NANJING OCEAN PHARMA TECH
Preparation method of aliskiren intermediate
ActiveCN102757399AHigh yieldImprove reaction efficiencyOrganic chemistryOrganic compound preparationOrganic solventBenzyl group
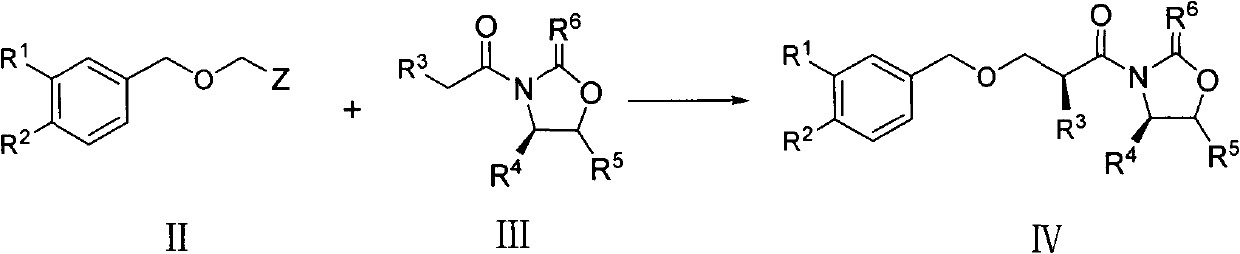
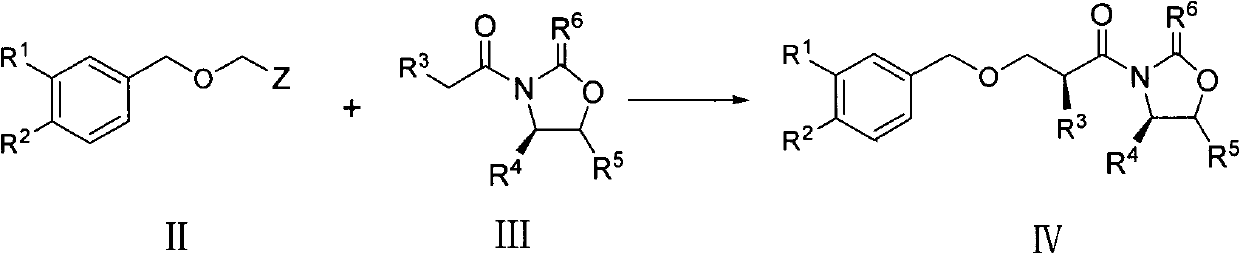
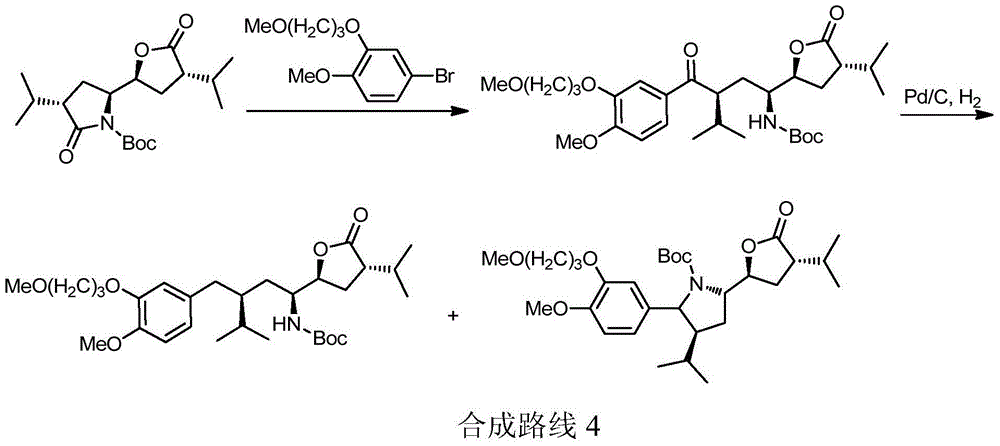
The invention discloses a preparation method of an aliskiren intermediate shown as a formula IV. The method comprises the following step of: reacting a compound II with a compound III under the protection of an inert gas in a dry organic solvent under the actions of titanium tetrachloride and dry DIPEA (Diisopropylethylamine), wherein Z is Cl, Br or I; R1, R2 and R5 independently refer to H, straight chain or branch chain alkyl with 1-3 carbon atoms, straight chain or branch chain alkoxyl with 1-3 carbon atoms, straight chain or branch chain alkyl with 1-3 carbon atoms which is substituted by straight chain or branch chain alkoxyl with 1-3 carbon atoms, or alkoxyl with 1-6 carbon atoms which is substituted by alkoxyl with 1-6 carbon atoms; R3 is straight chain or branch chain alkyl with 1-4 carbon atoms; R4 is benzyl or benzyl with a substituent on a benzene ring; and R6 is O, S or HN. According to the method, the reaction yield is increased on a large scale, the reaction cost is reduced, and the reaction efficiency is increased. The method is suitable for large-scale industrial production, and has a wide application prospect.
Owner:SHANGHAI INST OF PHARMA IND +1
Method for preparing aliskiren intermediate
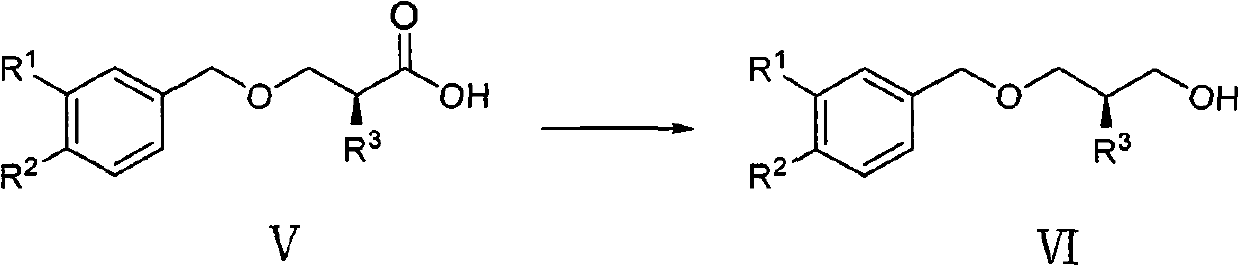
ActiveCN102757320AHigh yieldLow costOrganic chemistryOrganic compound preparationOrganic solventAlkoxy group
The invention discloses a method for preparing an aliskiren intermediate which is shown as a formula V. The method comprises the following step of: performing a reducing reaction on a compound V and NaBH4 and / or KBH4 in the presence of a boron trifluoride ether complex, KHSO4 or an ethylene glycol diethyl ether.hydrochloric acid compound in an organic solvent, wherein R1 and R2 independently refer to H, straight chain or branch chain alkyl with 1-3 carbon atoms, straight chain or branch chain alkoxyl with 1-3 carbon atoms, straight chain or branch chain alkyl with 1-3 carbon atoms which is substituted by straight chain or branch chain alkoxyl with 1-3 carbon atoms, or alkoxyl with 1-6 carbon atoms which is substituted by alkoxyl with 1-6 carbon atoms; and R3 is straight chain or branch chain alkyl with 1-4 carbon atoms. An aliskiren intermediate, i.e., (2S)-bromomethyl-3-methyl-butyl benzyl oxide and a derivative thereof can be prepared conveniently from a 3-hydroxypropyl benzyl oxide derivative. The method has the advantages of high yield, easiness and convenience for operating, low cost and suitability for large-scale industrial production.
Owner:SHANGHAI INST OF PHARMA IND +1
Method for preparing aliskiren key intermediate by enzyme method
InactiveCN108192933AProcess environment friendlySmall discharge of organic wasteFermentationNitroreductaseNitro reduction
The invention discloses a method for preparing an aliskiren key intermediate by an enzyme method. 2,2-dimethyl-3-amino propenamide is obtained by using 3-nitropropionic acid as raw materials through esterification, methylation, ammonolysis and nitro-reduction reaction, wherein the 2,2-dimethyl-3-amino propenamide is prepared through reduction reaction under the effects of nitroreductase, coenzymeand buffer solution by the enzyme method. Compared with the prior art, the method provided by the invention has the advantages that the environment-friendly effect is good; the organic three-waste (waste water, waste solid and waste gas) discharging quantity is small; the raw materials can be easily obtained; the yield is higher; the production cost is greatly reduced; good industrial applicationvalues are realized.
Owner:上海韶屹生物科技有限公司
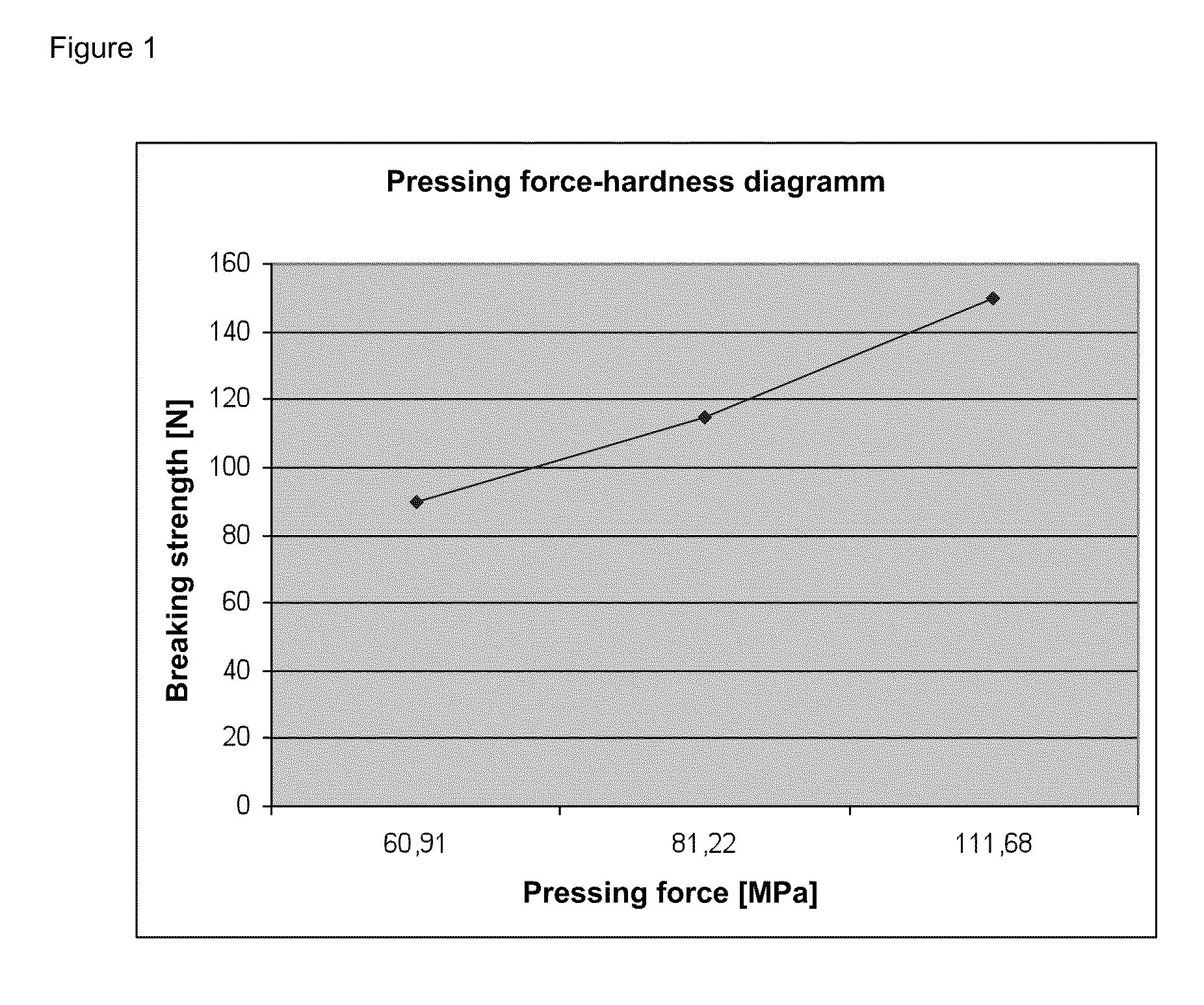
Directly pressed aliskiren tablets
InactiveUS20110165235A1Good compressibilityReadily be directly pressed into tabletsPowder deliveryBiocideActive agentPharmacology
The invention relates to pharmaceutical compositions which contain the active agent Aliskiren and are suitable for the production of tablets by dry pressing, so that prior wet granulation can be obviated. The invention also relates to tablets which can be obtained by dry pressing of these pharmaceutical compositions and to a method for producing these tablets. The invention furthermore relates to the use of the novel pharmaceutical compositions and tablets for treating hypertension and illnesses associated therewith.
Owner:RATIOPHARM GMBH
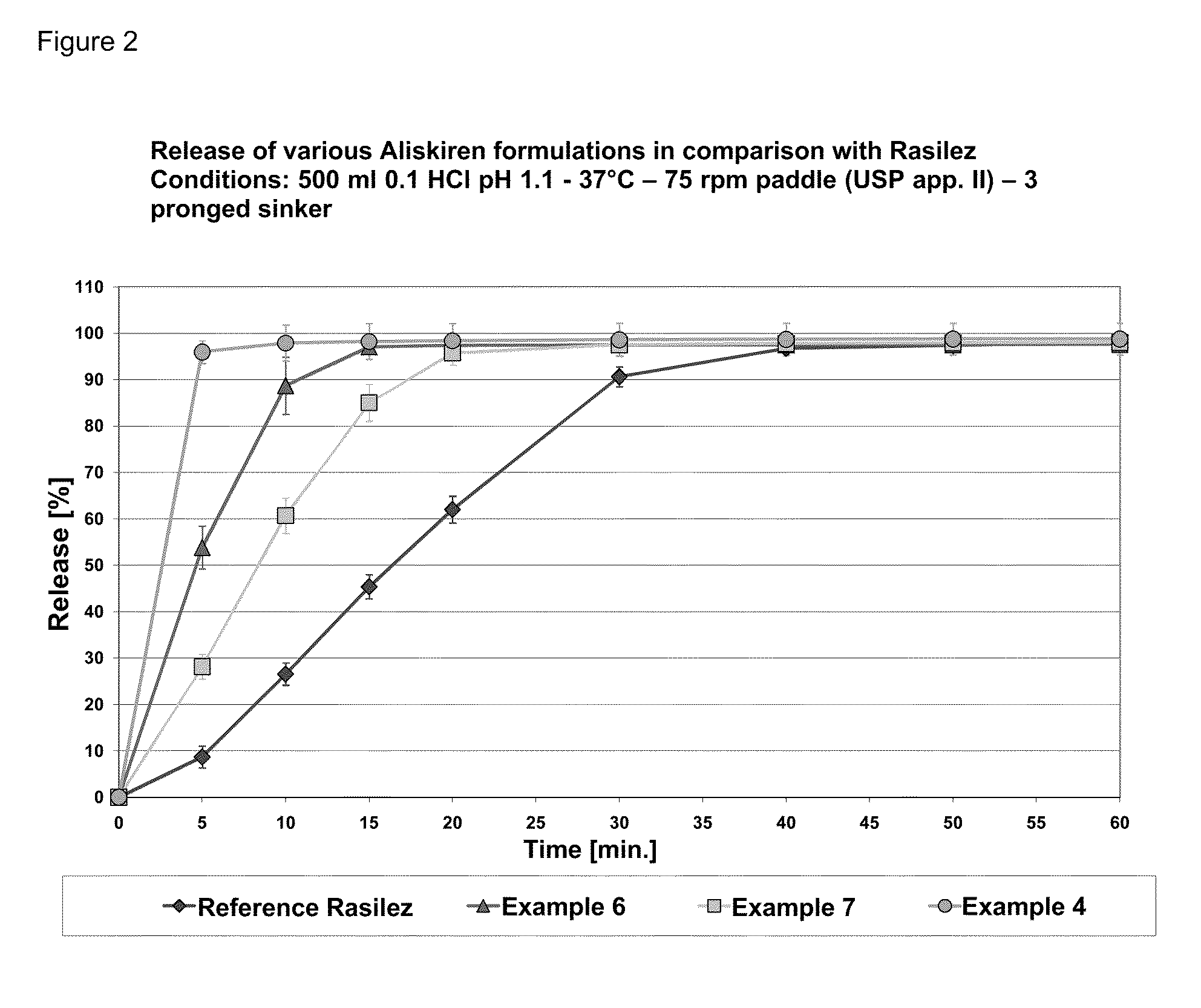
Galenical Formulations of a Fixed Dose Combination of Valsartan and Aliskiren
InactiveUS20120009257A1Solve the lack of hardnessSolve the lack of resistanceBiocideNervous disorderValsartanFixed-dose combination
The invention provides a pharmaceutical oral fixed dose combination of aliskiren and valsartan. Aliskiren is shown to slow the dissolution rate of valsartan and the resultant undesirable gelling of valsartan in the presence of aliskiren is overcome by the use of disintegrants.
Owner:NOVARTIS AG
Aliskiren enantiomer content detecting method
InactiveCN104034825ADetermination of contentSolve the problem of content determinationComponent separationCelluloseCarbamate
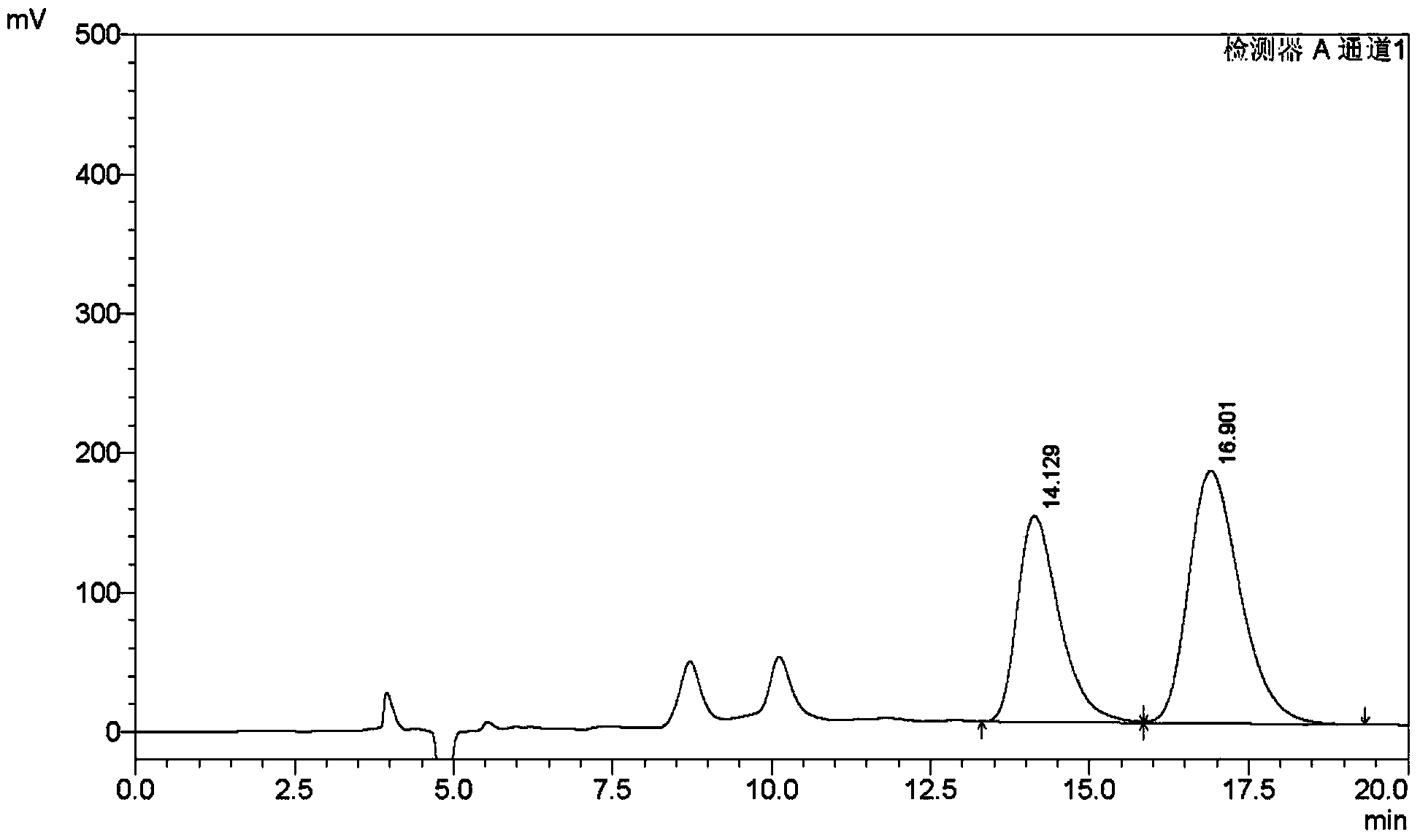
The invention discloses an aliskiren enantiomer content detecting method which is a high performance liquid chromatography. The aliskiren enantiomer content detecting method comprises the steps that the surface of silica gel is coated with a normal-phase chromatographic column with a cellulose-three[3,5-xylyl carbamate] chiral stationary phase as padding, the mixed solvent of the normal hexane, the ethyl alcohol and the organic amine polarity modifier as the mobile phase, the volume proportion of the normal hexane, the ethyl alcohol and the organic amine polarity modifier is 90-94:6-10:0-0.5, detection is carried out through an ultraviolet detector, the flow velocity is 0.6-1.0ml / min, and the temperature of the chromatographic column ranges from 25 DEG C to 40 DEG C. According to the method, the aliskiren and the enantiomer corresponding to the aliskiren can be well separated, and therefore the content of the enantiomer of the aliskiren can be accurately measured, and the method can be used for carrying out quality control on the studying and producing process of the raw aliskiren medicine and the preparations of the raw aliskiren medicine.
Owner:常州市亚邦医药研究所有限公司 +3
Preparation method of Aliskiren
ActiveCN102746182ARaw materials are easy to getSimple and fast operationOrganic compound preparationCarboxylic acid amides preparationKetoneAminolysis
The invention discloses a preparation method of the Aliskiren. The method includes that (3S,5S)-3-isopropyl-5-((2S,4S)-4-isopropyl-5-oxo-tetrahydrofuran-2-yl)-pyrrolidine-2-ketone (compound I) protected by amino serves as an initial material, subjecting the initial material to addition through 4-methoxy-3-(3-methoxy-propoxy)-bromobenzene and metal reagents, hydrogenation reduction, acid catalysis cyclization, 3-Amino-2,2-dimethylpropionamide aminolysis, reduction ring-opening and deprotection, and obtaining the Aliskiren. The preparation method of the Aliskiren has the advantages that raw materials are easy to obtain, the operation is simple and convenient, the production cost is low, the conversion rate is high, the device utilization rate is high, and the method is environment friendly and suitable for industrial production.
Owner:ZHEJIANG APELOA JIAYUAN PHARMA +1
Process for the manufacture of enantiomerically pure aryloctanoic acids as aliskiren
InactiveUS20110092706A1Organic compound preparationCarboxylic acid amides preparationArylMedicinal chemistry
The present invention relates to a novel manufacturing process and novel intermediates useful in the synthesis of pharmaceutically active compounds, especially rennin inhibitors such as Aliskiren. The invention describes a preparation of enantiomerically pure 8-aryloctanoic acids of general formula I from readily available key intermediate, a novel bicyclic compound of formula IV.
Owner:CARBODESIGN
Preparation method of aliskiren
ActiveCN105566150ASolving Problems Requiring Column Chromatographic SeparationSimple and fast operationOrganic compound preparationCarboxylic acid amides preparationChromatographic separationStereochemistry
The invention discloses a preparation method of aliskiren. Aliskiren is prepared from (R)-t-butylsulfenamide and a compound VIII. The chiral induction effect of the (R)-t-butylsulfenamide is used to construct a required second chiral center and solve the problem of column chromatographic separation needed in the construction of a second chiral center, a third chiral center and a fourth chiral center. The preparation method of aliskiren has the advantages of simple operation, high yield, low cost, high device utilization rate, and suitableness for industrial production.
Owner:ZHEJIANG LEPU PHARMA CO LTD
Preparation method for aliskiren
InactiveCN103570579AShort reaction pathEasy to operateOrganic compound preparationCarboxylic acid amides preparationSilanesFormate
The invention relates to a preparation method for aliskiren. The method includes: taking a known formula II compound as a raw material, subjecting the formula II compound and a reducing reagent to a reduction reaction in an organic solvent in the presence of a catalyst so as to generate aliskiren. Specifically, the reducing reagent is hydrogen, trialkyl substituted silane or formate; the catalyst is one of or a combination of several of palladium carbon, rhodium carbon, palladium carbon oxide, palladium hydroxide on carbon, palladium carbon, and platinum dioxide; the reduction reaction temperature is 0DEG C-100DEG C, and the pressure is 1-10bar. The method provided by the invention can prepare aliskiren by one-step reduction, and has the advantages of short reaction route, simple operation, good yield, and high product purity, thus being suitable for industrialized production.
Owner:SHANGHAI SYNCORES TECH INC
Amlodipine, aliskiren and pril compound antihypertensive medicament
The invention relates to an amlodipine, aliskiren and pril compound antihypertensive medicament, wherein effective components of the medicament comprise aliskiren or medical salt thereof, amlodipine or medical salt thereof and pril or medical salt in a weight ratio of (10-500):1:(2.5-100), preferably (15-120):1:(5-32), and more preferably 30:1:5. The medicinal composition has excellent antihypertensive effect, and has remarkably reduced toxic and side effects.
Owner:邬林祥 +1
Methods
InactiveUS20100130749A1Simple manufacturing methodHigh puritySilicon organic compoundsOrganic compound preparationMedicinal chemistryRenin inhibitor
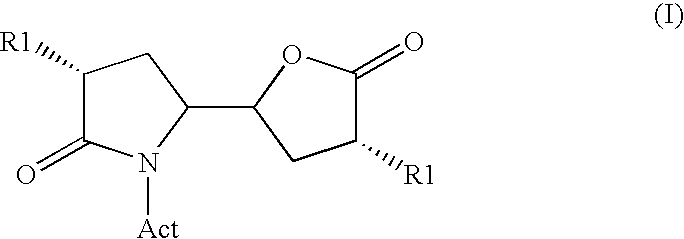
The invention related to a novel process, novel process steps and novel intermediates useful in the synthesis of pharmaceutically active compounds, especially renin inhibitors, such as Aliskiren Inter alia the invention relates to a process for the manufacture of a compound of the formula I.or a salt thereof, wherein R1 as well as Act are as defined in the specification, and processes of manufacturing this compound as well as intermediates in this process.
Owner:NODEN PHARMA DAC
A kind of western medicine compound and application for treating coronary heart disease
InactiveCN102283831ASignificant progressEffective treatmentOrganic active ingredientsCardiovascular disorderCoronary artery diseaseTypes diseases
The invention relates to a western medicine compound for curing coronary diseases and medical application thereof. The active ingredients of the western medicine compound comprise the following two medicines: (1) aliskiren or pharmaceutically acceptable salts thereof; and (2) statins, wherein the statins include simvastatin or rosuvastatin calcium. The compound medicine disclosed by the invention has obvious curable effects on myocardial ischemia type coronary diseases, myocardial infarction type diseases and angina type coronary diseases.
Owner:THE FIRST AFFILIATED HOSPITAL OF XINXIANG MEDICAL UNIV
Aliskiren composition capsule
ActiveCN103536576AImprove bioavailabilitySignificant blood pressure lowering effectOrganic active ingredientsPharmaceutical non-active ingredientsChitosan nanoparticlesMedicine
The invention provides an aliskiren composition capsule, and belongs to the field of medicine and medicine production technology. The aliskiren composition capsule comprises 75mg of aliskiren, 35.5mg of chitosan, 30mg of povidone K30, and 3mg of magnesium stearate. Beneficial effects of the aliskiren composition capsule are that:1) the composition with a ratio of aliskiren to chitosan nanoparticle being 1:0.5 is capable of increasing bioavailability of aliskiren, and prolonging medicine action time; 2) the composition is capable of improving blood pressure reduction effect of aliskiren significantly, reducing dosage of aliskiren in clinic, and reducing untoward effects of aliskiren; and 3) chitosan can be taken as a disintegrating agent of the aliskiren composition capsule, so that dissolution rate of aliskiren is increased.
Owner:HAINAN WEI KANG PHARMA QIANSHAN
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com