Preparation method for aliskiren
A compound, selected technology, applied in the preparation of organic compounds, carboxylic acid amide preparation, chemical instruments and methods, etc., can solve the problems of unfavorable industrial production and cumbersome routes, and achieve short reaction routes, simple operation and high product purity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1 is prepared formula IIa compound by formula IV compound
[0031]
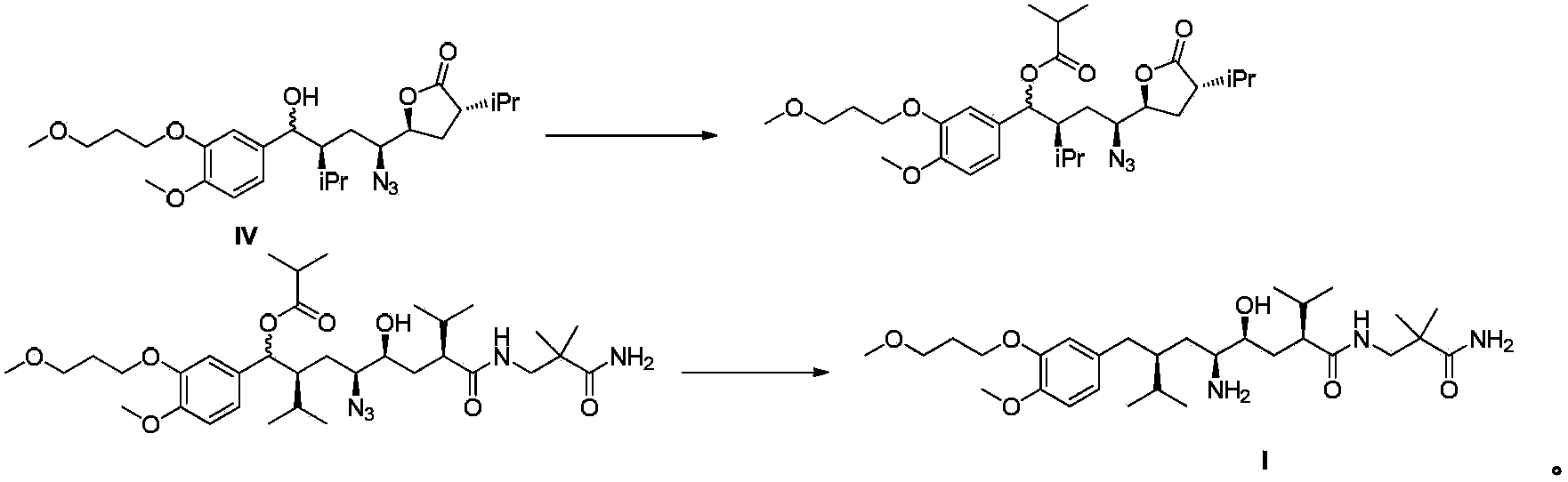
[0032] The specific preparation process is as follows: Formula IV compound (1.0g, 2.09mmol), 3-amino-2,2-dimethylpropanamide (1.4g, 12.1mmol), 2-hydroxypyridine (0.1g, 1.05mmol) and Triethylamine (5ml) was heated to 95~105°C and reacted for 24h. After the reaction was detected by HPLC, it was cooled to room temperature, methyl tert-butyl ether was added to dilute the reaction mixture, and then the organic phase was washed with water and saturated brine. After the organic phase was dried and concentrated, the residue was subjected to flash column chromatography to obtain 1.06g of the compound of formula IIa, MS (m / z): 576 (M-H 2 O+1), HPLC (peak area purity) 96%, yield 85%.
Embodiment 2
[0033] Embodiment 2 is prepared formula IId compound by formula IV compound
[0034]
[0035] Concrete preparation process is as follows:
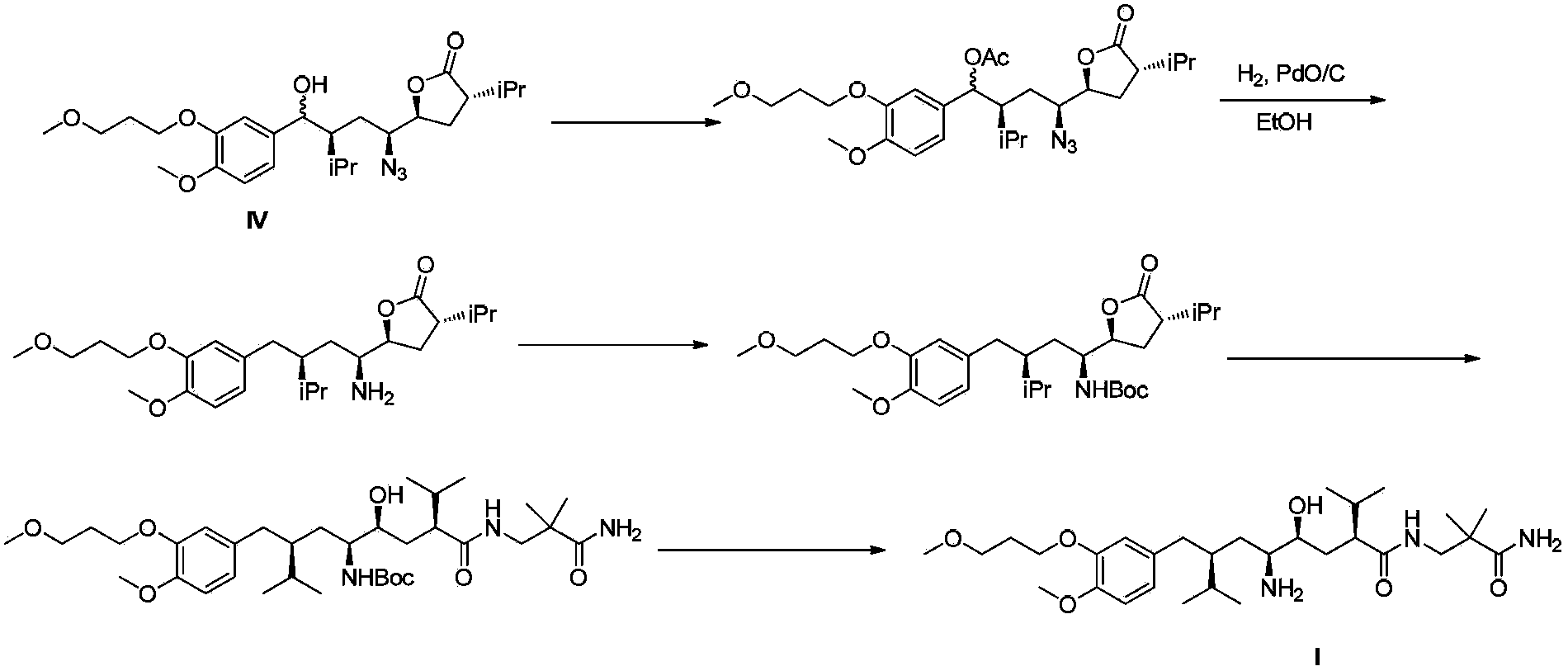
[0036] (1) Formula IV compound (1.0g, 2.09mmol), 3-amino-2,2-dimethylpropanamide (1.4g, 12.1mmol), 2-hydroxypyridine (0.1g, 1.05mmol) and triethyl Amine (5ml) was heated to 95~105°C and reacted for 24h. After the reaction was detected by HPLC, it was cooled to room temperature, methyl tert-butyl ether was added to dilute the reaction mixture, and then the organic phase was washed with water and saturated brine. After the organic phase was dried and concentrated, the residue was subjected to flash column chromatography to obtain 1.06 g of the compound of formula IIb, MS (m / z): 576 (M-H2O+1), HPLC (peak area purity) 96%, yield 85%.
[0037] (2) Dissolve the compound of formula IIb (1.0 g, 1.69 mmol) obtained in step (1) in 10 ml of ethanol, add 10% Pd / C (50 mg), and hydrogenate at 15-25°C under normal pressure. After the HPLC detection...
Embodiment 3
[0038] Example 3 Preparation of Aliskiren (compound of formula I) by formula IIa compound
[0039]
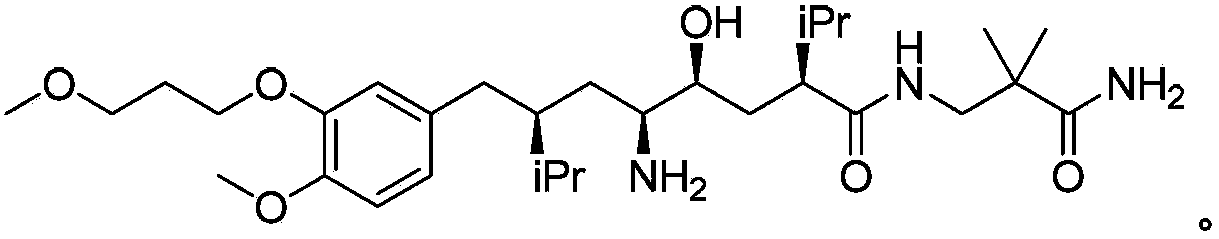
[0040] The specific preparation process is as follows: Dissolve the compound of formula IIa (1.0g, 1.69mmol) in 10ml of ethanol and 1ml of acetic acid, add 10% Pd / C (100mg), at 4bar pressure, 35-45 ℃, pass through hydrogen, carry out Reduction reaction, HPLC detection after the reaction is complete, filter to remove the catalyst, after the filtrate is concentrated, the residue is flash column chromatography to obtain Aliskiren 0.79g, MS (m / z): 552 (M+1), HPLC (peak area purity) 96%, yield 85%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com